Abstract
The present study explored the effects of supplementing male rats with either choline, omega-3 fatty acids, or phytoestrogens, from weaning into early adulthood, on emotionality and hippocampal plasticity. Because of the neuroprotective properties of these nutrients, we hypothesized that they would positively affect both behavior and hippocampal function when compared to non-supplemented control rats. To test this hypothesis, male Sprague Dawley rats were assigned to one of four nutrient conditions after weaning: 1) control (normal rat chow); 2) choline (supplemented in drinking water); 3) omega 3 fatty acids (daily oral supplements); or 4) phytoestrogens (supplemented in chow). After 4 weeks on their respective diets, a subset of rats began 3 weeks of behavioral testing, while the remaining behaviorally naïve rats were sacrificed after 6 weeks on the diets to assess numbers of adult-born hippocampal neurons using the immature neuron marker, doublecortin. The results revealed that choline supplementation affected emotional functioning; compared to rats in other diet conditions, rats in this group were less anxious in an open field and after exposure to predator odor and showed less behavioral despair after forced swimming. Similar behavioral findings were evident following supplementation with omega-3 fatty acids and phytoestrogens supplementation, though not on all tests and not to the same magnitude. Histological findings followed a pattern consistent with the behavioral findings: choline supplementation, followed by omega-3 fatty acid supplementation, but not phytoestrogen supplementation, significantly increased the numbers of new-born hippocampal neurons. Choline and omega −3 fatty acids have similar biological functions—affecting cell membranes, growth factor levels, and epigenetically altering gene transcription. Thus, the present findings suggest that targeting nutrients with these effects may be a viable strategy to combat adult psychopathologies.
Keywords: anxiety, despair, predator odor, adult hippocampal neurogenesis, choline, omega 3 fatty acids, phytoestrogens
1. Introduction
The developing mammalian brain exhibits significant plasticity and many internal and environmental variables can influence its organization during this time [1–5]. For example, optimal levels of certain nutrients during the prenatal and postnatal periods are critical for normal brain development [6] and contribute to variety in behavioral phenotypes [7,8], like shaping adult emotional and affective behavior [9]. During adolescence, disturbances in emotion, including symptoms of anxiety and depression, become more pronounced in at-risk youth [10,11] and, in the general population, there are correlations between nutrient intake and cognitive and emotional functioning [12,13]. In the present study, emotional behaviors were examined in young adult male rats that were supplemented with one of three specific nutrients from weaning: choline, omega-3 fatty acids, and phytoestrogens. Previous research suggests that these nutrients are neuroprotective [13], particularly in the hippocampus [14,15], a brain region that contributes to normal cognitive and emotional functions [16,17]. The hippocampus is markedly sensitive to stress and is adversely affected by it: chronic stress atrophies hippocampal neurons [18] and decreases adult hippocampal neurogenesis [19, but see 20]. These kinds of morphological changes are associated with numerous psychopathologies of mood, including anxiety-related disorders, depression, and schizophrenia [21, 22]. Thus, we sought evidence that, in addition to affecting emotional behaviors on tests that index anxiety, fear, and despair, the nutrient supplements may also increase neural plasticity, indexed by numbers of newborn neurons in the dentate gyrus of the hippocampus.
The dietary nutrients under investigation in the present study have related functions, but distinct mechanisms of action. Choline is an essential nutrient with various biological roles critical to healthy brain function: it is the precursor to the neurotransmitter, acetylcholine [23,24], promotes cell membrane integrity through its conversion to phosphatidylcholine [23,25], participates in signaling pathways at the cell membrane [26], and is an important source of methyl groups, which are critical mediators of epigenetic modifications to gene expression [23,27]. Prenatal choline availability is integral in normal brain development, both during neural tube closure in early gestation, and later during the development of the basal forebrain-hippocampal cholinergic system [26,28]. Choline supplementation during late pregnancy increases the expression of neurotropic factors [29,30], alters the expression of genes through methylation reactions [23, 31,32], and increases hippocampal neurogenesis prenatally [33] and in adulthood [29]. Our recent work also suggest that choline may modulate emotional behaviors; female rats supplemented with choline prenatally (gestation day 10 to birth) or postnatally (during the period after weaning and extending past adolescence: postnatal days 25–50) had less anxiety- and depressive-like behaviors [9]. These behaviors are often accompanied by alterations in hippocampal neurogenesis [34], suggesting it may be a biological basis of these alterations. Thus, there is compelling evidence that increased choline availability offers the brain protection from environmental insults, including stress, [35].
Like choline, omega-3 fatty acids also contribute to the integrity of neuronal plasma membranes, increase the expression of neurotropic factors [36], and alter DNA methylation patterns integral for gene expression [37]. The omega-3 fatty acid, docasahexaenoic acid (DHA), helps maintain ionic permeability of the membrane and thus synaptic function [36], and, consistent with the neuroprotective properties of omega-3 fatty acids, normalizes dysregulation of neurotrophic factors by traumatic brain injury [38]. Omega-3 fatty acids also regulate cognitive and emotional functioning in humans [36,39–41] and in animal models [42], and, like choline, may exert these effects through actions on hippocampal plasticity. The biological functions of phytoestrogens differ from those of choline and omega-3 fatty acids; their primary mechanism of action is via estrogen receptor binding. In this way, they affect not only the reproductive system, but also the hypothalamic-pituitary-adrenal (HPA) axis, and the many brain regions rich in estrogen receptors, including the hippocampus [43]. Much nutritional phytoestrogen research employs soy, as it contains isoflavones, a sub-class of phytoestrogens [44]. As a common source of dietary protein, isoflavones are frequently found in commercial rodent chows and in many processed human foods, making them an especially physiologically relevant phytoestrogen [44].
Unlike research on choline and omega-3 fatty acids, work examining the behavioral and neural impacts of phytoestrogens has produced contradictory findings. For example, one study found that male rats fed a soy-rich diet, compared with rats fed a soy-free diet, were more anxious in a social interaction test and on an elevated plus maze, and had increased levels of corticosterone and vasopressin [44]; another study found that male rats fed a soy-rich diet were less anxious than controls in the elevated plus maze [45]. In contrast to these conflicting findings of phytoestrogen supplementation in males, phytoestrogen supplementation appears to have a reliable anxiolytic effect on females, both ovariectomized and intact [45–47]. This sexually dimorphic response to phytoestrogens also occurs on tests of learning and memory: phytoestrogen supplementation improved female rats’ performance on the radial arm maze for visual-spatial memory, but disrupted male performance in the same task [46]. Research at the molecular level has also yielded mixed results. Phytoestrogens may activate estrogen receptors α and β (ERα and ERβ), but the β receptor is strongly activated by isoflavone phytoestrogens found in soy [46]. Administration of the ERβ agonist diarylpropionitrile (DPN), subcutaneously or orally, had no effect on measures of anxiety in one study [48], while a separate study found that injecting ERβ agonists, including DPN, directly into the hippocampus decreased anxiety-related behaviors in rats [49]. Therefore, it is evident that the effects of phytoestrogens on emotional behavior and hippocampal function is uncertain and the lack of congruent data in the literature warrants further research to better characterize the role of phytoestrogens in the regulation of emotional behaviors and hippocampal plasticity.
In order to better understand the impact of essential dietary nutrients on specific emotional behaviors, the present study compared the effects of nutrient supplementation of either choline, omega-3 fatty acids, phytoestrogens with regular rat chow. The diets began right after weaning and continued into young adulthood. A subset of adult rats from each diet condition underwent behavioral tests and the remaining rats served as cage controls for assays of hippocampal plasticity. The emotional behaviors under investigation were 1) anxiety, assessed in the open field and on a test of predator odor; 2) fear, assessed in the presence of predator odor; and 3) despair, assessed in the forced swim test. Hippocampal plasticity was assessed with unbiased stereological procedures to estimate numbers of new immature neurons, marked by immunostaining for the microtubule-associated protein doublecortin (DCX). Overall, our findings support choline’s role in buffering against anxiogenic experiences and provide compelling evidence that phytoestrogens and omega-3 fatty acids may also possess similar anxiolytic properties. It is expected that this work will open a line of research surrounding the potential for nutritional therapies to be used as putative interventions and preventions for emotional disorders.
2. Material and methods
2.1. Animals and Diets
The subjects were 60 male Sprague Dawley rats (CD strain; Charles River Breeders, Raleigh, NC) that arrived in the colony post-weaning on postnatal day (PD) 23. Rats were housed in individually ventilated clear polycarbonate cages (30.8 × 30.8 × 18.7 cm; Thoren Caging Systems, Hazleton, PA) in a colony with a 12:12 h light-dark cycle with lights on at 08:00 h; the colony temperature was 21±2 °C with 40–60% humidity. After acclimating to colony conditions for 2 days, rats were placed on one of 4 dietary nutrient protocols that continued for the duration of the study. The protocols were: 1) CONTROL (n=14)—commercially available rat chow (2016 Teklad Global 16% Protein Rodent Diet; Harlan Laboratories) without alfalfa or soybean meal, and thus little to no phytoestrogen content and non-detectable to 20 mg/kg isoflavone concentrations, was available to rats ad libitum along with free access to drinking water flavored with 50 mM saccharin; 2) CHOLINE (n=15)—rat chow, as described for the CONTROL protocol, was available ad libitum along with choline-supplemented drinking water containing 25 mM choline chloride and 50 mM saccharin; 3) OMEGA (n=15)— rat chow and saccharin-sweetened drinking water was available ad libitum, as described for the CONTROL rats, and was combined with daily oral supplements of fish oil (3 g/kg; Sigma Aldrich Chemical Co., St. Louis, MI) containing 20–31% omega-3 fatty acids as triglycerides; and 4) PHYTO (n=16)—commercially available rat chow (8640 Teklad 22/5 Rodent Diet; Harlan Laboratories), containing 350–650 mg/kg isoflavone concentration and thus rich in phytogestrogens, was available ad libitum along with free access to saccharin-sweetened drinking water.
2.2. Experimental Design and Timeline
To clearly distinguish the effects of these diet protocols on brain and behavior, each group was further divided into two sub-groups: 1) a behaviorally tested group and 2) a cage control group that did not undergo behavioral testing but was used for neurogenesis immunohistochemistry. Thus, for behavioral testing there were n=8 CONTROL, n=10 CHOLINE, n=8 OMEGA, and n=10 PHYTO rats and for neurogenesis measures there were n=6 CONTROL, n=5 CHOLINE, n=6 OMEGA, and n=7 PHYTO rats; assignment to experimental groups was random. Behavioral testing began after rats were on their respective diet protocols for 4 weeks and they were all tested on each of the 3 tests described below, one test per week for a total of 3 weeks. To gather evidence of the effects of the diet protocols on neurogenesis after the same approximate duration of diets, we aimed for the center of the behavioral test period to sacrifice the cage controls. Thus, they were sacrificed after being on the diet protocols for 6 weeks. All procedures were carried out during the light phase and were evaluated and approved by Colby College’s Institutional Animal Care and Use Committee in line with federally regulated standards.
2.3. Behavioral Tests
Each of the tests described below were selected as excellent indicators of rats’ emotional reactivity to novel places and stimuli and with each test there was a general increase in the robustness of the emotional response rats typically have in them [9]. Emotional behaviors under investigation were exploration, anxiety, fear, and despair. As mentioned above, behavioral tests began for a subset of rats after 4 weeks on the diet protocols, during PD 51–72, and diets were continued throughout the testing period.
2.3.1. Open field
This is a widely used assay of activity levels and anxiety-like behavior in rats. The field was a large, 100 × 100 cm arena constructed of wood and painted black. A CCD camera was ceiling-mounted above the center of the maze and attached to a laptop computer running HVS Image 2020 software (HVS Image Ltd., Buckingham, UK). Rats were placed in the field, one at a time, and allowed to freely explore the field for 5 minutes. The software tracked and analyzed each rat’s path within a 4 × 4 grid digitally overlaid on the field, creating 16 equal-sized squares. General activity was gauged by the overall number of squares rats entered whereas anxiety-like behavior was gauged by the number of entries rats made into the 4 center squares.
2.3.2. Predator odor
The purpose of this test was twofold: 1) to assess the reaction of rats to a noxious, fear-inducing stimulus (cat odor from a collar), and 2) to assess the reaction of rats to the same environment in the absence of the cat collar. This test was therefore conducted over a two-day period. The test area was a novel 70 × 70 cm open field with 60 cm high walls constructed of wood and painted beige. In one corner of the field there was a small wooden shelter, approximately 10 cm3, with a small opening on the side facing into the center of the field. In the opposite corner was a small petri dish (6 cm diameter). On the first day each rat was placed in the field, individually, starting in the shelter. On this day the petri dish in the opposite corner contained a 1 cm segment of a worn cat collar. The collars were worn by a domestic house cat for 2 weeks and upon removal were segmented into small 1cm pieces, placed in sealed plastic containers, and stored at −20°C until used in the test. The latency to the first time the rat poked its head out of the shelter, or ‘head-out’, and the latency to emerge from the shelter were the primary dependent measures. On the second day, each rat was placed in the shelter in the field again, except this time the petri dish contained a 1 cm segment of cat collar that had not been worn by a cat. These collars were treated exactly the same in all other regards. Once again, the latency to the first head-out and the latency to emerge from the shelter were recorded. Additionally, on both days, numbers of head-outs and the time spent proximal to the collar segments (within 5 cm) was recorded.
2.3.3. Forced swim
The forced swim test is used to gauge behavioral despair in rats by exposing them to an inescapable column of water. This test was conducted using a glass cylinder that was 25 cm in diameter and 75 cm high. The cylinder was filled with 25°C water to a height of 40 cm. All rats received two experiences in the water column as described by Porsolt et al., 1978 [50]. The first experience was considered the ‘induction phase’: the rat was placed in the cylinder of water, from which there was no escape, for 10 minutes. This experience was intended to induce a state of despair in the rats and the extent to which it was psychologically traumatic was then indexed in the second experience in the water column. The second experience was considered the ‘assessment phase’: 24 hours after the first phase rats were placed in the water column again, this time for 5 minutes. In this test an altered mood state, or despair, is generally expected to appear in the assessment phase and is indexed by the emergence and extent of immobility displayed by the rats. The criterion for immobility was a minimum of 3 seconds without movement except for small movements made by the rat to keep its head above the water. All swim sessions were videotaped and an experimenter, blind to the diet condition of the rats, coded behaviors of rats in the water columns from the digital records.
For both the induction and assessment phases of the test, we recorded the latency to the first instance of immobility meeting the criterion; this measure allowed us to evaluate how quickly rats ‘gave up’ in each phase of the test. We then recorded the amount of time each rat spent immobile during each minute of each test; this allowed us to study the progression of immobility over the course of each test. Using the data from each minute of each phase of the test we were also able to obtain the total amount of time rats spent immobile during induction and assessment.
2.4. Doublecortin Immunohistochemistry
As previously mentioned, the rats that were not behaviorally tested were used for immunohistochemistry. Because behavioral testing was conducted during weeks 5, 6, and 7, the cage-control rats were sacrificed after 6 weeks on the diet protocols (PD 65) to gather evidence regarding the effects of the diets on hippocampal neurogenesis at about the same time that behaviors in their matched cohort were assessed. Rats were deeply anesthetized with isoflurane delivered in 1.5% oxygen and decapitated. Brains were rapidly extracted and post-fixed in 4% paraformaldehyde for 2 weeks prior to being transferred to 0.1% sodium azide for storage until processing.
Every 4th 60-µm section through the rostral-caudal extent of the hippocampus in one hemisphere, obtained using a vibratome, was retained for doublecortin (DCX) immunohistochemistry, as described in Glenn et al., 2007 [29]. DCX is a microtubule-associated protein that is preferentially expressed in immature, newly born neurons that are still in the process of migrating and extending axons. This protein is highly expressed during development but is still evident in adult neurogenic regions, like the dentate gyrus of the hippocampus. To mark for it, free-floating sections were rinsed in Tris-buffered saline (TBS; pH 7.3), treated with 0.6% hydrogen peroxide for 30 minutes, and incubated in a blocking solution containing 0.1% Triton X-100 (TTX; Sigma-Aldrich) and 3% normal horse serum (NHS; Vector Laboratories, Burlingame, CA) in TBS for 30 minutes at room temperature. Tissue were then incubated with the primary antibody—an affinity purified polyclonal goat antibody raised against a peptide mapping at the carboxy terminus of human DCX (1:200; Santa Cruz Biotechnology, Santa Cruz, CA)—overnight at 4°C. The next day the tissue was rinsed in TBS and incubated with the secondary antibody (biotinylated horse anti-goat 1:200; Vector Laboratories) for 2 hours at room temperature. After rinsing again in TBS, tissue was incubated in an avidin-biotin complex (ABC, Vector Laboratories) for 1 hour at room temperature, rinsed again in TBS, and developed with vector grey (Vector Laboratories).
2.5. Unbiased Stereology
Numbers of new neurons in the dentate gyrus of the hippocampus were estimated using unbiased stereological techniques [51,52] and this methodology is described in detail in Glenn et al., 2007 [29]. In brief, five sections through the rostral-caudal extent of the dorsal hippocampus of each rat were sampled. Contours were drawn around the full extent of the dorsal and ventral blades of the dentate gyrus in each section for each rat using StereoInvestigator (Microbrightfield Inc. Williston, VT). The software then systematically sampled through the outlined region and an experimenter blind to the conditions of the rats marked cells positive for DCX. We used an 80 × 80 µm counting frame and analyzed 20–40 sites per section, yielding 100–200 frames per rat. These parameters resulted in suitable numbers of DCX+ neurons for sampling. For analysis, an optical dissector height of 20 µm with 2 µm guard zones was used and cells were identified at 400× magnification. The volume of the dentate gyrus region sampled was derived from the size and spacing of the contours [51] and numbers of DCX+ neurons were determined per volume of dentate gyrus in each rat to overcome potential individual or group differences in contour and region size.
2.6. Statistical Analyses
Mean values and standard error of the mean were calculated for dependent measures and are displayed in figures. The dependent measures were analyzed using one-way analyses of variance (ANOVA), with Diet as the between-subjects factor (CONTROL, CHOLINE, OMEGA, AND PHYTO). Each forced swim session was analyzed using mixed factorial ANOVAs with Diet as the between-subjects factor and Minute of the session as the within-subjects factor. When ANOVAs were statistically significant, differences amongst the four groups were investigated using the post-hoc tests of Least Significant Difference (LSD). Based on the statistical models outlined by Keppel & Wickens, 2004 [53], we conducted planned comparisons to address a priori predictions about group differences when ANOVAs were not statistically significant. We did this to pursue our a priori hypotheses that each diet-manipulated rats would be different from control-fed rats; for each comparison the F is calculated based on the comparison mean square and the omnibus error term from the overall ANOVA [53]. As these a priori predictions pertain only to the difference between each diet group and the CONTROL group (we did not have predictions for how, or whether, the diet groups would differ from each other), just three planned comparisons per analysis out of eight possible comparisons were conducted. Accordingly, the risk of inflating type 1 error rates with multiple comparisons was considered modest and tests were therefore uncorrected and, for all statistical tests, statistical significance was set at p<0.05.
3. Results
3.1. Behavioral Tests
3.1.1. Open field
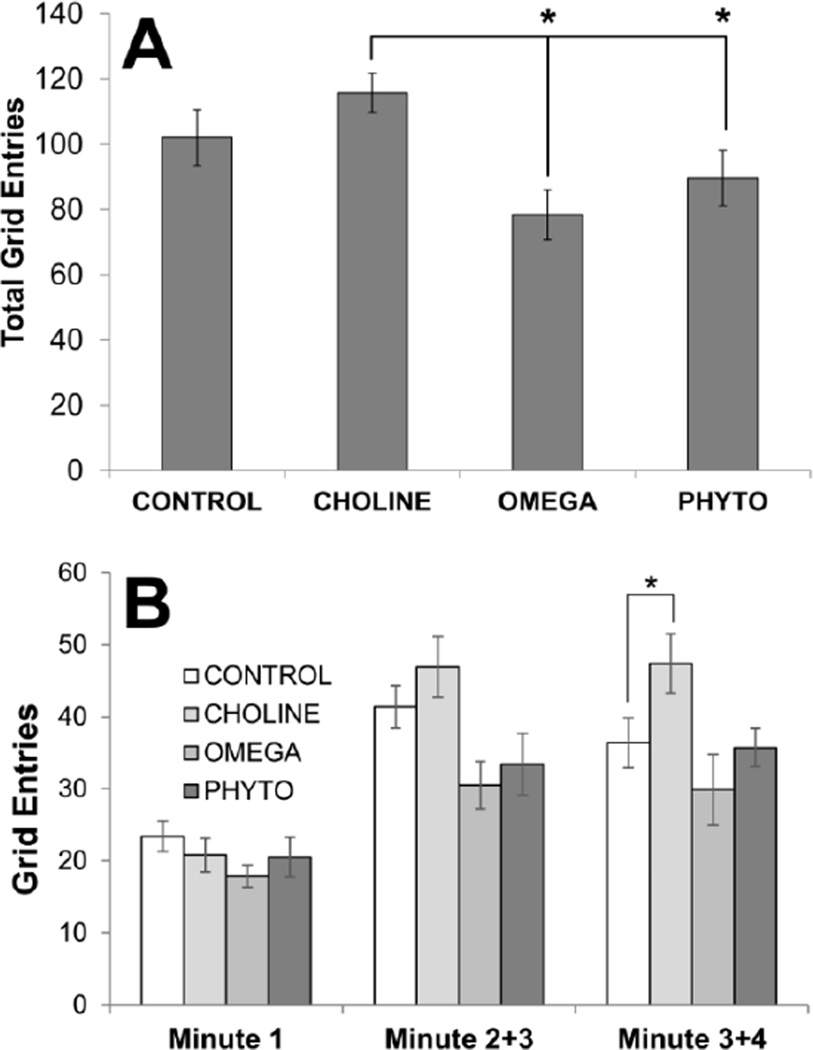
Figures 1 and 2 show the results of the 5-minute open field test. An ANOVA conducted on total numbers of grid entries in the field was statistically significant (F[3,32]=4.115, p = 0.014; Fig. 1A) and posthoc tests revealed that rats in the CHOLINE group entered significantly more squares than rats in the OMEGA and PHYTO groups (p=0.002 and p=0.020, respectively). CHOLINE and PHYTO rats were not significantly different from CONTROL rats (p’s>0.05) but there was a tendency for OMEGA rats to enter fewer squares than CONTROL rats, though it was not statistically significant (p=0.056). To more closely pursue these patterns, numbers of grid entries were gathered over different parts of the 5-minute test: the first minute was analyzed separately and the last 4 minutes were analyzed together in blocks of 2 minutes. As can be seen in Fig. 1B, all experimental groups had fewer grid entries during the first minute and there were no statistically significant differences among them (F[3,32]<1). A mixed factorial ANOVA revealed no significant difference between the first and second blocks of 2 minutes (F[1,32]<1) and no interaction between blocks and diet (F[3,32]=1.132, p=0.347). However, as evident in Fig. 1B, there was an increase in the number of grid entries for CONTROL and CHOLINE rats compared to OMEGA and PHYTO rats in the second and third minutes and the maintenance of this increase in the CHOLINE rats (p=0.032 compared to CONTROL) in the fourth and fifth minutes.
Figure 1.
Overall activity of rats in the open field as a function of dietary condition. A 4×4 grid was digitally superimposed on the field; the total number of grid entries (A) was used to gauge the general activity levels of the rats in the field. Numbers of grid entries in the first minute, the second and third minutes combined, and the fourth and fifth minutes combined (B) are also shown. Bars are ±SEM. *p<0.05
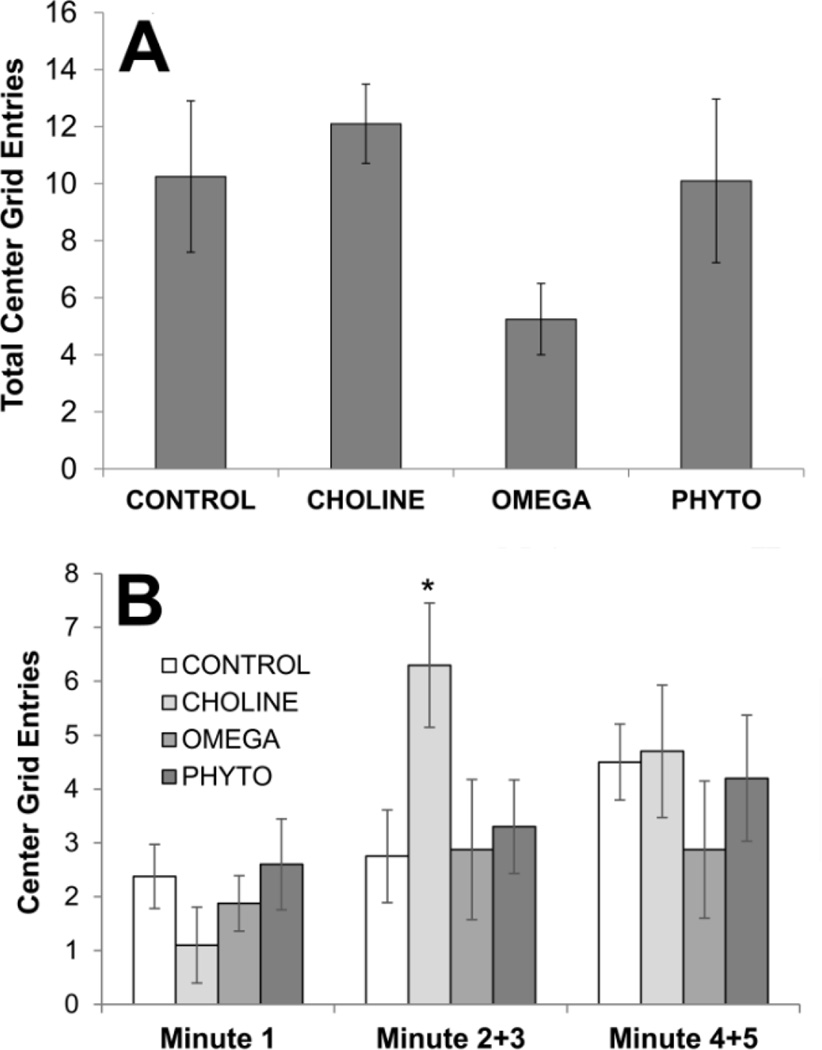
Figure 2.
Anxious-like behavior of rats in the open field as a function of dietary condition. A 4×4 grid was digitally superimposed on the field; the total number of center grid entries (A) was used to gauge the anxiety levels of the rats in the field. Numbers of center grid entries in the first minute, the second and third minutes combined, and the fourth and fifth minutes combined (B) are also shown. Bars are ±SEM. *p<0.05 compared to CONTROL
An ANOVA conducted on numbers of entries into the center squares only was not statistically significant (F[3,32]=1.880, p=0.153; Fig. 2A). However, as can be seen in Fig. 2A, the OMEGA rats entered fewer center squares than all the other groups. As for total grid entries, rats’ entries into center grids was also analyzed across the 5-minute test (Fig. 2B). There were no significant differences amongst the groups during the first minute (F[3,32]<1), the later minute blocks significantly different (F[1,32]<1), and the interaction between blocks and diets was not significant (F[3,32]=2.159, p=0.112). However, as is plain from Fig. 2B, the CHOLINE rats displayed significantly more center grid entries during the second and third minutes of the test (p=0.016 compared to CONTROL).
3.1.2. Predator odor
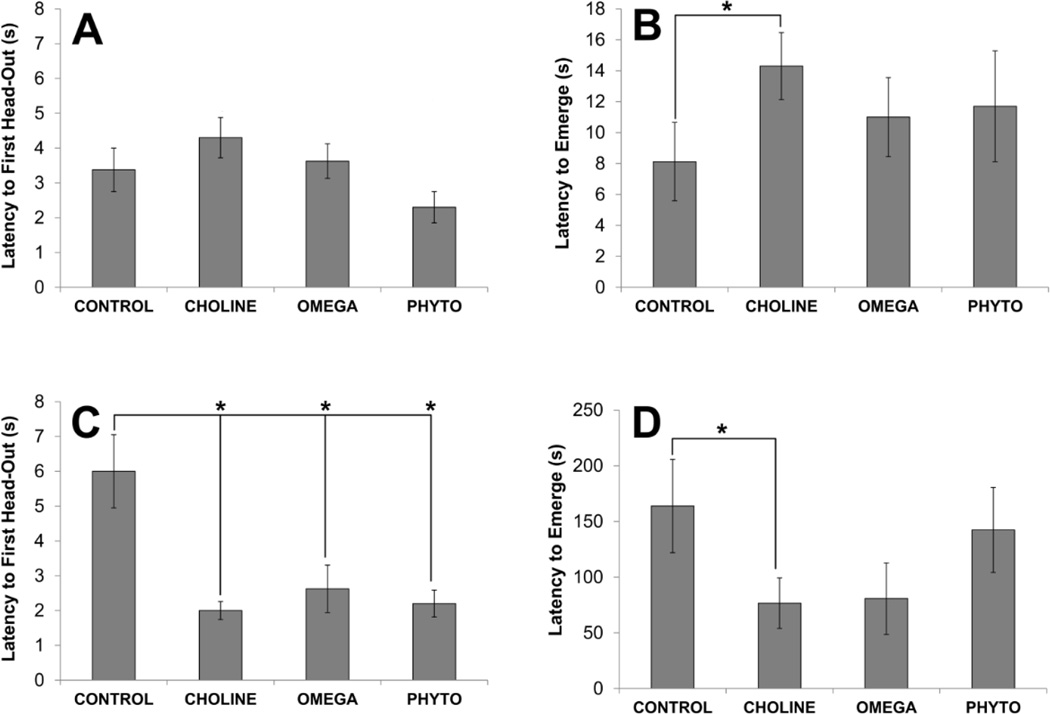
Figures 3 and 4 show the results of the predator odor test. An ANOVA conducted on the latency to the first head-out on the first day of predator odor testing, when cat odor was present in the apparatus, was not statistically significant (F[3,32]=2.618, p=0.068; Fig. 3A). However, as can be seen in Fig. 3A, there was a tendency for CHOLINE rats to take longer to make their first head-out, but it was not significantly longer than CONTROL rats (p>0.05). An ANOVA conducted on the latency to emerge from the shelter on the first day was also not statistically significant (F[3,32]<1, p=0.504; Fig. 3B); however, again, CHOLINE rats had a tendency to take longer to emerge and this was significantly different from the emergence latency of CONTROL rats (p=0.040). On the second day of predator odor testing, when cat odor was not present in the apparatus, an ANOVA conducted on latency to the first head-out was statistically significant (F[3,32]=8.860, p<0.001; Fig. 3C). Posthoc tests revealed that each diet-manipulated group was significantly faster to make the first head-out than the CONTROL group (versus CONTROL: all p’s<0.001). There were no significant differences among the diet-manipulated groups. An ANOVA conducted on the latency for rats to emerge from the shelter was also not statistically significant (F[3,32]=1.622, p=0.204; Fig. 3D), though CHOLINE rats emerged significantly faster than CONTROL rats (p=0.041).
Figure 3.
Behavior of rats in response to predator odor as a function of dietary condition. The top panels show the latency of rats to the first head-out of (A) and latency to emerge from (B) a safe area in the environment on the first day of the test when predator odor (a collar worn by a domestic cat) was present. The bottom panel shows the latency to the first head-out (C) and to emerge (D) on the second day when there was no predator odor. Bars are ±SEM. *p<0.05
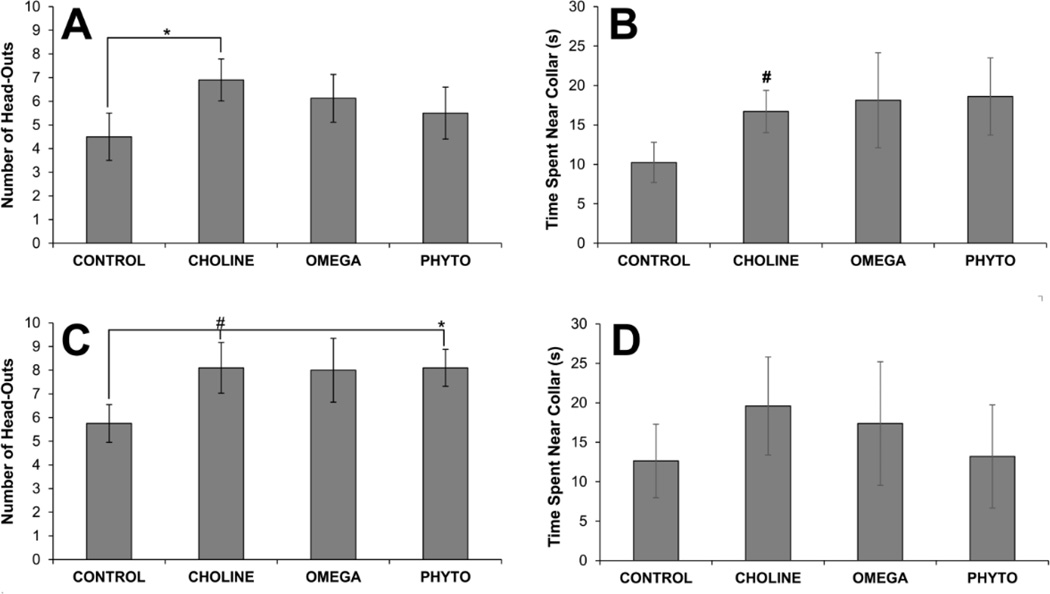
Figure 4.
Behavior of rats in response to predator odor as a function of dietary condition. The top panels show the number of head-outs of (A) and the amount of time rats spent near the collar (B) on the first day of the test when predator odor (a collar worn by a domestic cat) was present. The bottom panel shows the number of head-outs (C) and time near the collar (D) on the second day when there was no predator odor. Bars are ±SEM. *p<0.05; #p<0.06
Additional measures of number of head-outs and time spent within 5 cm of the collar segments on both days of testing yielded a similar pattern of results. Though all diet-treated groups, compared to CONTROL, had more head-outs in the presence of cat odor, the ANOVA results were not statistically significant (F[3,32]<1). Additionally, CHOLINE rats did have significantly more head-outs on this test than CONTROL rats (p=0.046; Fig. 4A). A similar pattern emerged for time spent near the cat collar: CONTROL rats spent the least amount of time near the cat collar but the ANOVA was not statistically significant (F[3,32]<1). In this case, CHOLINE rats were not significantly different from CONTROL rats, though the test result approached statistical significance (p=0.053; Fig. 4B). On the second day, without the cat odor, all diet-treated groups again displayed more head-outs than the CONTROL group but failed to reach statistical significance (F[3,32]=1.213, p=0.321). In this case, the difference between CHOLINE and CONTROL rats only approached significance (p=0.056), whereas the difference between PHYTO and CONTROL rats was significant (p=0.027; Fig. 4C). An ANOVA on time near the collar segment on the second day of testing was significant (F[3,32]<1) and there were no significant differences between CONTROL rats and any diet-treated groups (all ps<0.05; Fig. 4D).
3.1.3. Forced swim
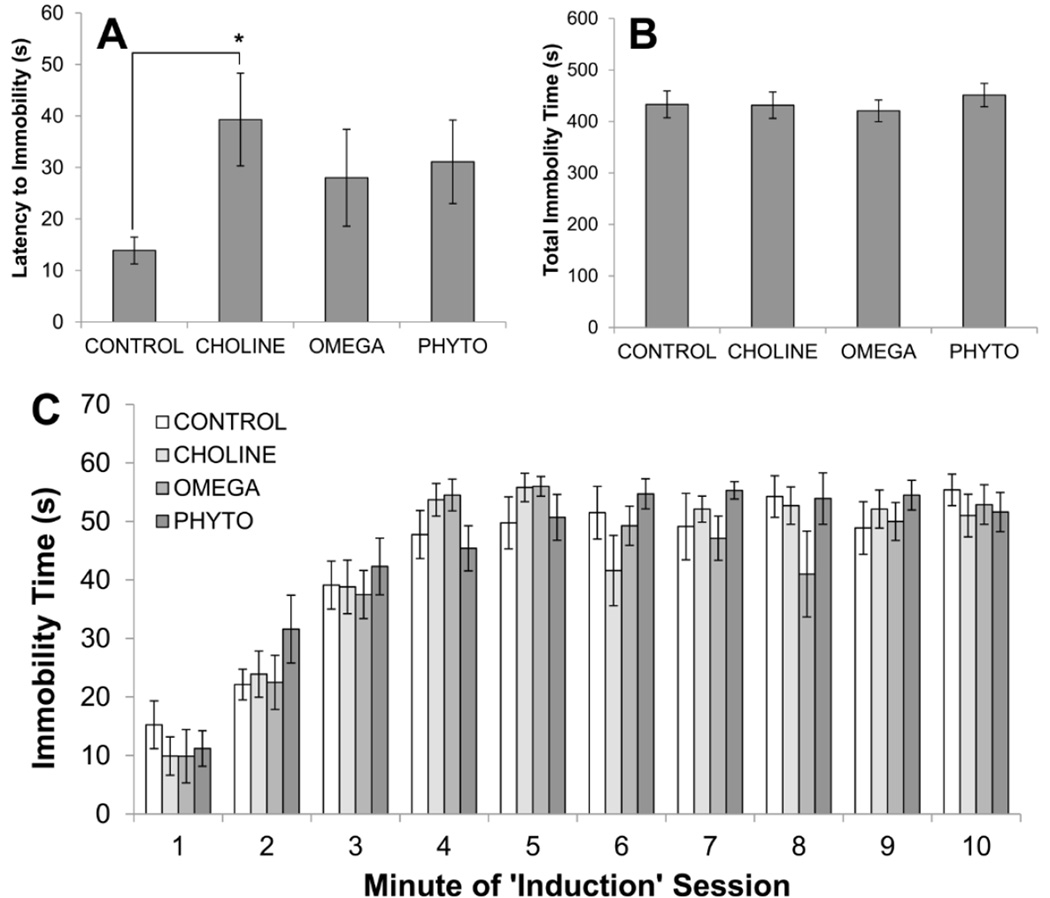
Figure 5 shows the results of the ‘induction’ phase of the forced swimming procedures. An ANOVA conducted on the latencies of rats to display immobility during induction was not statistically significant (F[3,32]=1.694, p=0.188; Fig. 5A). As can be seen in Fig. 5A though, the diet-treated rats all took longer than CONTROL rats to become immobile. Planned comparisons revealed that this difference was only statistically significant for CHOLINE rats (p=0.017; all other p’s>0.05). A 4×10 mixed factorial ANOVA on immobility during each minute of the 10-minute session revealed a statistically significant main effect of Minute (F[9,288]=75.196, p<0.001; Fig. 5B). Neither the main effect of Diet (F[3,32]<1; Fig. 5C) nor the interaction between Diet and Minute (F[27,288]=1.471, p=0.066; Fig. 3C) was statistically significant.
Figure 5.
Response of rats to the first forced swimming exposure (induction) as a function of dietary condition. Top panels show the latency to the first instance of immobility (A) and the total amount of time spent immobile during the 10-minute test (B). The bottom panel (C) shows the time spent immobile during each minute of the 10-minute session. Bars are ±SEM. *p<0.05
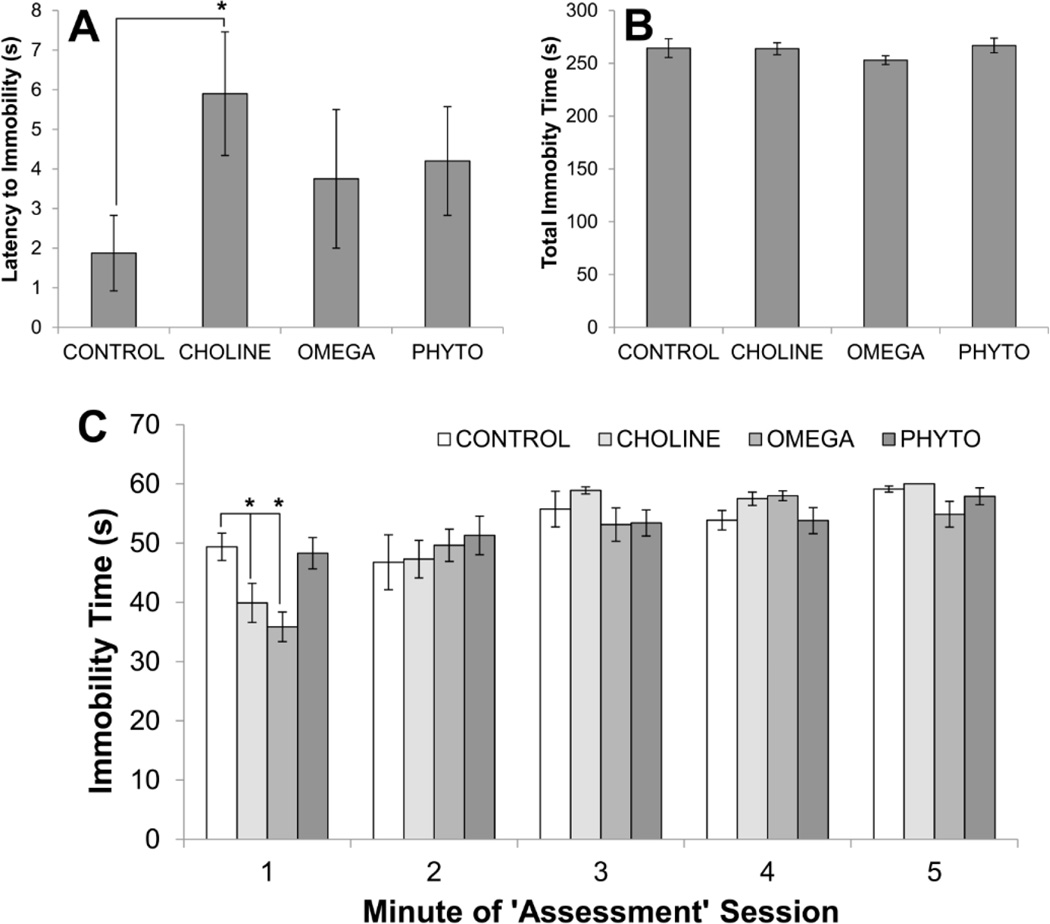
Figure 6 shows the results of the ‘assessment’ phase of the forced swimming procedures. An ANOVA conducted on the latencies of rats to display immobility during assessment was not statistically significant (F[3,23]=1.275, p=0.300; Fig. 6A). However, as seen during induction, all diet-treated groups took longer to become immobile than CONTROL rats during assessment. Also as in induction, this was difference was only statistically significant for CHOLINE rats (p=0.030; all other p’s>0.05). A 4×5 mixed factorial ANOVA on immobility during each minute of the 5-minute session revealed a significant main effect of Minute (F[4,128]=29.605, p<0.001) and a significant interaction between Diet and Minute (F[12,128]=2.831, p=0.002; Fig. 6C). As can be seen in Fig. 6C, CHOLINE (p=0.020) and OMEGA (p<0.001), but not PHYTO (p=0.385), rats showed significantly less immobility during the first minute of the assessment when compared to CONTROL rats. Overall, however, the main effect of Diet was not statistically significant (F[3,32]<1; Fig. 6B).
Figure 6.
Response of rats to the second forced swimming exposure (assessment) as a function of dietary condition. Top panels show the latency to the first instance of immobility (A) and the total amount of time spent immobile during the 10-minute test (B). The bottom panel (C) shows the time spent immobile during each minute of the 10-minute session. Bars are ±SEM. *p<0.05
3.2. Unbiased stereology
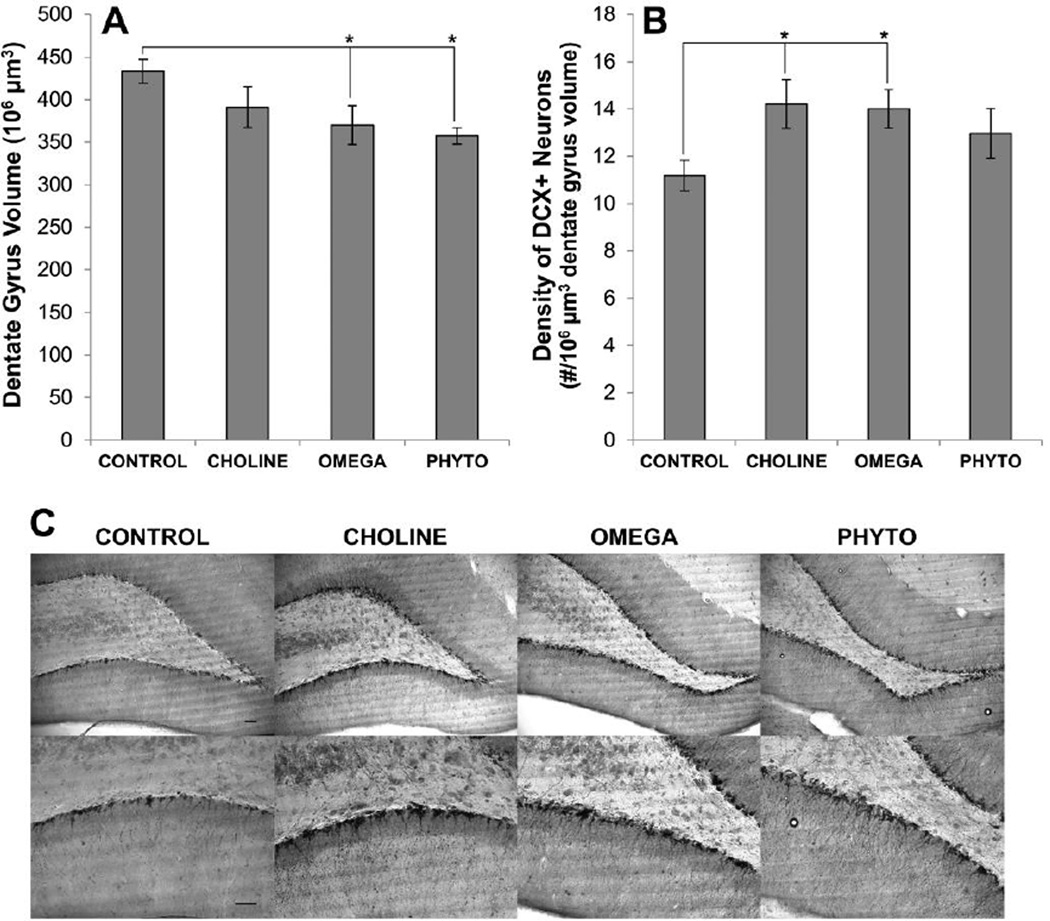
Figure 7 shows the histological results, including volume estimates and the density of DCX+ neurons per 106 µm3 volume of dentate gyrus as a function of the diet treatments. An ANOVA on the volume estimates was statistically significant (F[3,20]=3.735, p=0.028; Fig. 7A) and post hoc tests revealed significantly less volume of dentate gyrus in OMEGA and PHYTO rats compared to CONTROL rats (p=0.006 and p=0.014, respectively), but the volume of dentate gyrus in CHOLINE rats was not significantly different from CONTROL rats (p=0.103). An ANOVA on the density of DCX+ neurons was also statistically significant (F[3,20]=2.998, p=0.05; Fig. 7B). As seen in Fig. 7B, CHOLINE (p=0.019) and OMEGA (p=0.016), but not PHYTO (p=0.101), rats had significantly higher densities of DCX+ neurons than CONTROL rats.
Figure 7.
Adult hippocampal neurogenesis as a function of dietary condition. Top panels show the average volume of the region of dentate gyrus sampled (A) and the density of neurons stained positively for the immature neuron marker, doublecortin (B). Bars are ±SEM. *p<0.05 The bottom panel (C) shows photomicrographs of DCX-labeling in the dentate gyrus from a representative rat from each diet condition; top photomicrographs are 100× magnification and bottom photomicrographs are 200× magnification. Bars in CONTROL images represent 25 µm and apply to all images in the corresponding row.
4. Discussion
The main objective of the present study was to examine emotional functioning in young adult rats that received supplements of the essential dietary nutrients choline, omega-3 fatty acids, or phytoestrogens beginning just prior to adolescence and continuing into early adulthood. These nutrients were selected for their putative neuroprotective properties and were expected to attenuate adverse reactions of rats to a series of anxiety-provoking situations. Overall, the findings provided evidence for this hypothesis: in general, supplemented rats displayed a phenotype of enhanced emotional functioning, but some nutrients exerted larger behavioral and neural effects than others. For example, choline consistently exerted the largest change to emotional behavior, followed by omega-3 fatty acids. Phytoestrogens, however, exerted only modest effects on behavior. Additionally, omega-3 fatty acids and phytoestrogens sometimes yielded mixed effects in the different tests. We also sought evidence to support the hypothesis that periadolescent nutrient availability increases hippocampal neurogenesis in adult rats. This hypothesis emerged in light of putative links between psychopathologies linked to stress, emotional functioning, and hippocampal neural plasticity. Data gathered to test this hypothesis were collected in a matched cage-control cohort of behaviorally naïve rats and were consistently in line with the behavioral findings; specifically, choline and omega-3 supplements produced a significant increase in the number of newly born hippocampal neurons, while supplements of phytoestrogens did not. Taken together, the present behavioral and neurological findings offer novel insight into the ways in which these specific nutrients may be neuroprotective against the emergence of debilitating psychopathologies.
The positive effects on emotional functioning and hippocampal plasticity seen in the choline-supplemented rats in the present study fit well with past research on this essential dietary nutrient at both prenatal and perinatal windows of sensitivity, suggesting both preventative and curative roles. Prenatal choline supplementation may protect the brain from various assaults, like advanced age, neurotoxin exposure, and seizures, through its actions on neural plasticity and growth factor expression [29,30,54,55]. Neonatal choline supplementation is also neuroprotective, effectively reversing many of the adverse effects resulting from prenatal trauma, such as ethanol exposure [56] and stress to the mother during pregnancy [35]. Previous work clearly shows that supplementing rats with dietary choline perinatally enhances overall cognitive function and slows normal age-related cognitive decline [57,58]. Importantly, its specific enhancement of hippocampal neurogenesis and BDNF expression likely underlie our recent findings indicating that prenatal and adolescent choline supplementation results in antidepressant [9] and antischizophrenic [35] properties in adulthood.
Consistent with these previously reported effects with prenatal choline supplementation [9] are the present that choline-supplementation later in life increased activity in the open field, enhanced vigilance to predator odor with reductions in persistent anxiety, and less despair in the forced swim test. Furthermore, these behavioral effects were accompanied by increases in hippocampal neurogenesis. It should be noted that the behavioral effects in the present study were more modest than previously reported [9]; however, the previous report utilized female subjects. Male and female rats, even when standard-fed, display differences in emotional functioning; male rats are reportedly more anxious and reactive in tests like those utilized here [59]. Even so, the difference in the magnitude of choline’s effects is important and suggests that the robustness of choline’s neuroprotection may be sexually dimorphic, which is unsurprising, as dietary need for choline intake is sexually dimorphic [60]. Additionally, though choline supplemented rats in the present were more active in the perimeter and center of the open field compared to control-fed rats, this difference was only statistically significant during the second and third minutes of the test for center activity and the fourth and fifth minutes of the test for overall activity. However, the choline supplemented rats were clearly more active than the other diet groups in this test and the combination of this pattern with the findings in the other tests adds to our interpretation of their decreased emotional reactivity.
In particular, during predator odor testing, the choline supplemented rats were slower to emerge from the shelter in the presence of the cat odor and faster to emerge in its absence. Thus, one interpretation is that these rats were better able to detect the odor and thus regulated their behavior accordingly. However, CHOLINE rats also displayed an increase in head-outs in the presence of cat odor and more time in proximity to the cat odor. These measures are similarly impacted by anxiolytic drugs [79], further supporting the phenotype of decreased anxiety in the CHOLINE rats. Nonetheless, it is possible that enhanced odor discrimination and decreased emotionality contributed to their performance. Given that choline supplementation increases adult hippocampal neurogenesis, it is also possible that it increases olfactory bulb neurogenesis. This may be a key mechanism underlying enhanced odor discrimination [80] and altered reactivity to the predator odor and would be an important next step in this work.
While the findings from the choline supplemented rats fit well with our hypotheses and past research, the findings from the rats supplemented with omega-3 fatty acids were not as well defined. These rats tended to be less active in the open field than all other groups, suggesting an anxiogenic effect on this test. However, this was mainly on overall activity in the field and primarily on the second and third minutes of the test; fewer differences were evident on center activity suggesting that these groups were not displaying more anxious-like behavior. Additionally, these rats displayed decreased despair and less anxiety in the context previously associated with predator odor. In particular, the finding that omega-3 fatty acid supplementation produced decreased despair in the forced swim test is well supported by previous reports that deficiencies in omega-3 fatty acids are linked with increased incidences of depression. For example, depressed suicide victims have lower DHA than healthy (i.e. no cardiovascular disease) controls [61], and major depressive disorder (MDD) patients have low evening DHA levels [62]. Low levels of DHA are also linked with other behavioral disorders, such as attention deficit hyperactivity [63] and autism spectrum disorder [64]. Disturbances in emotional behaviors are consistent with these disorders, thus the present finding of decreased anxiety in the predator odor test in omega-3 fatty acid supplemented rats suggests that it may have broader efficacy in stabilizing mood in humans. Accordingly, increased DHA levels have improved behavioral disorders: an improvement of major depression has occurred with increased levels of erythrocyte DHA [65], and supplementation improved impairment of social interaction in humans with ASD [66].
Like choline supplementation and consistent with the present behavioral findings and past reports [66], omega-3 fatty acid supplementation increased numbers of newborn neurons in the hippocampus. Given that the most pronounced behavioral and physiological changes were in our choline- and omega-3 fatty acid- supplemented rats, it is not surprising that these nutrients have several over-lapping functions. Both choline [5,31,67] and omega-3 fatty acids [68] can epigenetically regulate gene expression, act as structural components of cell membranes, and serve as methyl donors in the one-carbon cycle. An interesting recent study found that maternal deficiency in B12, another one-carbon metabolite similar to choline [69], significantly reduced antioxidant defense enzymes while omega-3 supplementation partially rescued this effect [70]. This result suggests that the mechanism by which choline and omega-3 fatty acids exert their neuroprotective effects has some, but not perfect, overlap. Nonetheless, these similarities in basic function may underlie their shared behavioral and neural effects.
Another shared metabolic mechanism of choline and omega-3 fatty acids that may have contributed to their effects on behavior and brain seen in the present study is their actions on growth factor expression [71,72]. In addition to their antidepressant properties [9,66], both choline and omega-3 fatty acids show promise as potential nutrient preventatives against the onset of other neurological disorders, such as schizophrenia [35, 73]. In a recent study using an MK-801-induced model of schizophrenia, a choline supplemented diet in early adolescence (i.e. after weaning, for 25 days) elevated cognitive abilities to levels of controls (i.e., no MK-801; [35]). Similarly, omega-3 fatty acid supplementation prevented the onset of ketamine-induced schizophrenia-like symptoms in Wistar rats [73]. Additionally, supplementation with choline [55] or omega-3 fatty acids [74] also contributed to the prevention of physiological changes associated with epilepsy. Choline and omega-3 fatty acid’s similar mechanisms, biological changes, and behavioral effects demonstrate the importance of both nutrients in maintaining mental health.
In contrast to rats treated with choline and omega-3 fatty acid supplementation, rats in the present study that were supplemented with phytoestrogens, when compared to control-fed rats, exhibited fewer differences on behavioral tests and had similar rates of hippocampal neurogenesis. These results were not surprising given the discrepancies in the current literature regarding phytoestrogens’ effects on mental health [46] but are consistent with past research findings that this nutrient has little impact on emotional behaviors [44,48]. In general, based on these findings, it is unlikely that increasing amounts of phytoestrogens in the human will adversely impact emotional function, but it may do less to protect against psychopathologies.
The link between emotional behaviors and hippocampal neurogenesis is well described: all known effective antidepressant drugs decrease emotional reactivity and increase neurogenesis [75]. Supporting this, Santarelli and colleagues [76] found that antidepressant drugs failed to exert positive behavioral outcomes in tests of emotionality when their neurogenic effects on the hippocampus were blocked. Additionally, chronic stress decreases neurogenesis, increases emotional reactivity, and is a potent contributing factor in mood disorders [77,78]. We provide further support to these patterns: in the present study, those diet manipulations with the biggest positive effects on emotional behaviors also significantly increased hippocampal neurogenesis. We purposefully chose not to conduct our behavioral and neural assays in the same groups of rats, as behavioral experiences are likely to affect hippocampal plasticity themselves. Thus, the design of the present work allowed us to determine whether choline and omega-3 fatty acid supplementation increased hippocampal neurogenesis during the time of behavioral testing. The significant decreases in dentate volume observed in rats supplemented with phytoestrogens and omega-3 fatty acids, compared to the control-fed rats, was an unexpected finding that stands in contrast to the increases in numbers of new born neurons in that region in all groups, though only statistically significant for choline- and omega-3 fatty acid supplemented rats. The volume measure, however, was dependent on the user-defined contour setting and thus may not accurately reflect the true volume of the region. It was, though, instrumental to our use of a density measure for DCX+ neurons. Nonetheless, that there may be significant structural changes is a possibility that warrants further investigation.
5. Conclusions
Overall, this study highlights the potential for choline and omega-3 supplementation to positively modulate emotional function, particularly when supplementation begins during sensitive developmental periods, and that they may have long-lasting beneficial effects extending into adulthood. Though the effects reported here were modest, the patterns were consistent over multiple tests and measures. Indeed, these modest effects are noteworthy given that only a single nutrient was manipulated in each group and these manipulations began long after the prenatal and early postnatal period, when the brain is exquisitely sensitive to the environmental factors. These results suggest that the brain continues to be sensitive to these effects later in life, in this case during adolescence. Future studies should focus on identifying which of the shared metabolic roles underlies these alterations in behavior and whether these effects are sensitive to amount of supplementation and specific developmental periods.
Research Highlights.
Postweaning nutrient supplementation reduced emotional reactivity in adult male rats
Choline and omega-3 fatty acid supplementation reduced anxiety and despair
Phytoestrogen supplementation yielded mixed results on behavioral tests of emotion
Choline and omega-3 fatty acid supplementation increased hippocampal neurogenesis
Phytoestrogen supplementation did not increase hippocampal neurogenesis
Acknowledgments
The authors would like to thank Amanda Kimball for her comments and editorial assistance on earlier drafts of this manuscript and Logan Hunter for his contribution to the collection of the behavioral data. This project was supported by grants to MJG from the National Center for Research Resources (5P20RR016463-12) and the National Institute of General Medical Sciences (8P20GM103423-12) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caspi A, Williams B, Kim-Cohen J, Craig IW, Milne BJ, Poulton R, et al. Moderation of breastfeeding effects on the IQ by genetic variation in fatty acid metabolism. Proc Natl Acad Sci USA. 2007;104:18860–18865. doi: 10.1073/pnas.0704292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conner JM, Franks KM, Titterness AK, Russell K, Merrill DA, Christie BR, et al. NGF is essential for hippocampal plasticity and learning. J Neurosci. 2009;29:10883–10889. doi: 10.1523/JNEUROSCI.2594-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 4.Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, denate gyrus, and subiculum. Proc Natl Acad Sci USA. 2012;109:E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeisel SH. Diet-gene interactions underlie metabolic individuality and influence brain development: implications for clinical practice derived from studies on choline metabolism. Ann Nutr Metab. 2012;60(Suppl 3):19–25. doi: 10.1159/000337310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgane PJ, Austin-LaFrance R, Bronzine J, Tonkiss J, Diaz-Cintra S, Cintra L, et al. Prenatal malnutrion and development of the brain. Neurosci Biobehav Rev. 1993;17:91–128. doi: 10.1016/s0149-7634(05)80234-9. [DOI] [PubMed] [Google Scholar]
- 7.Long SJ, Benton D. Effects of vitamin and mineral supplementation on stress, mild psychiatric symptoms, and mood in nonclinical samples: a meta-analysis. Psychosom Med. 2013;75:144–153. doi: 10.1097/PSY.0b013e31827d5fbd. [DOI] [PubMed] [Google Scholar]
- 8.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 9.Glenn MJ, Adams RS, McClurg L. Supplemental dietary choline during development exerts antidepressant-like effects in adult female rat. Brain Res. 2012;1443:52–63. doi: 10.1016/j.brainres.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zisook S, Lesser I, Stewart JW, Wisniewski SR, Balasubramani GK, Fava M, et al. Effect of age at onset on the course of major depressive disorder. Am J Psychiatry. 2007;164:1539–1546. doi: 10.1176/appi.ajp.2007.06101757. [DOI] [PubMed] [Google Scholar]
- 12.Dauncy MJ. Recent advances in nutrition, genes and brain health. Proc Nutr Soc. 2012;71:581–591. doi: 10.1017/S0029665112000237. [DOI] [PubMed] [Google Scholar]
- 13.Morris MS, Fava M, Jacques PF, Selhub, Rosenburg IH. Depression and folate status in the US Population. Psychother Psychosom. 2003;72:80–87. doi: 10.1159/000068692. [DOI] [PubMed] [Google Scholar]
- 14.Denis I, Potier B, Vancassel S, Heberden C, Lavaille M. Omega-3 fatty acids and brain resistance to ageing and stress: Body of evidence and possible mechanisms. Ageing Res Rev. 2013;12:579–594. doi: 10.1016/j.arr.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Wong-Goodrich SJ, Glenn MJ, Mellot TJ, Blusztajn JK, Meck WH, Williams CL. Spatial memory and hippocampal plasticity are differentially sensitive to the availability of choline in adulthood as a function of choline supply in utero. Brain Res. 2008;27:1237:153–166. doi: 10.1016/j.brainres.2008.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenzie S, Robinson NT, Herrera L, Churchill JC, Eichenbaum H. Learning Causes Reorganization of Neuronal Firing Patterns to Represent Related Experiences within a Hippocampal Schema. J Neurosci. 2013;33:10243–10256. doi: 10.1523/JNEUROSCI.0879-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray JA, McNaughton N. The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. 2nd. Oxford: Oxford University Press; 2003. [Google Scholar]
- 18.McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry. 2000;48:721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- 19.Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 20.Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233:12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Min GL, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci. 2008;28:11785–11791. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- 23.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blusztajn JK, Wurtman RJ. Choline and cholinergic neurons. Science. 1983;221:614–620. doi: 10.1126/science.6867732. [DOI] [PubMed] [Google Scholar]
- 25.Blusztajn JK, Mellot TJ. Choline nutrition programs brain development via DNA and histone methylation. Cent Nerv Syst Agents Med Chem. 2012;12:82–94. doi: 10.2174/187152412800792706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders LM, Zeisel SH. Choline: dietary requirements and role in brain development. Nutri Today. 2007;42:181–186. doi: 10.1097/01.NT.0000286155.55343.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nut. 2002;132:2333S–2335S. doi: 10.1093/jn/132.8.2333S. [DOI] [PubMed] [Google Scholar]
- 28.Monk BR, Leslie FM, Thomas JD. The effects of perinatal choline supplementation on hippocampal cholinergic development in rats exposed to alcohol during the brain growth spurt. Hippocampus. 2012;22:1750–1757. doi: 10.1002/hipo.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glenn MJ, Gibson EM, Kirby ED, Mellot TJ, Blusztajn KJ, Williams CL. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur J Neurosci. 2007;25:2473–2482. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glenn MJ, Kirby ED, Gibson EM, Wong-Goodrich SJ, Mellott TJ, Blusztajn JK, et al. Age-related declines in exploratory behavior and markers of hippocampal plasticity are attenuated by prenatal choline supplementation in rats. Brain Res. 2008;1237:110–123. doi: 10.1016/j.brainres.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehedint MG, Zeisel SH. Choline’s role in maintaining liver function: new evidence for epigenetic mechanisms. Curr Opin Clin Nutr Metab Care. 2013;16:339–345. doi: 10.1097/MCO.0b013e3283600d46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006;20:43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craciunescu CN, Albright CD, Mar MH, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J Nutr. 2003;133:3614–3618. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338:72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corriveau JA, Glenn MJ. Postnatal choline levels mediate cognitive deficits in a rat model of schizophrenia. Pharmacol Biochem Behav. 2012;103:60–68. doi: 10.1016/j.pbb.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9:568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhobale M, Joshi S. Altered maternal micronutrients (folic acid, vitamin B(12)) and omega 3 fatty acids through oxidative stress may reduce neurotrophic factors in preterm pregnancy. J Matern Fetal Neonatal Med. 2012;25:317–323. doi: 10.3109/14767058.2011.579209. [DOI] [PubMed] [Google Scholar]
- 38.Wu A, Ying Z, Gomez-Pinilla F. The salutary effects of DHA dietary supplementation on cognition, neuroplasticity, and membrane homeostasis after brain trauma. J Neurotrauma. 2011;28:2113–2122. doi: 10.1089/neu.2011.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 40.Hibbeln JR. Fish consumption and major depression. Lancet. 1998;18:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- 41.Moriguchi T, Greiner RS, Salen N., Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- 42.Vines A, Delattre AM, Lima MM, Rodrigues LS, Suchecki D, Machado RB, et al. The role of 5-HT1A receptors in fish oil-mediated increased BDNF expression in the rat hippocampus and cortex: a possible antidepressant mechanism. Neuropharmacology. 2012;62:184–191. doi: 10.1016/j.neuropharm.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 43.Walf AA, Frye CA. Rapid and estrogen receptor beta mediated actions in the hippocampus mediate some functional effects of estrogen. Steriods. 2008;338:72–75. doi: 10.1016/j.steroids.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartley DE, Edwards JE, Spiller CE, Alom N, Tucci S, Seth P, et al. The soya isoflavone content of rat diet can increase anxiety and stress hormone release in the male rat. Psychopharmacology (Berl) 2003;167:46–53. doi: 10.1007/s00213-002-1369-7. [DOI] [PubMed] [Google Scholar]
- 45.Lund TD, Lephart ED. Dietary soy phytoestrogens produce anxiolytic effects in the elevated plus-maze. Brain Res. 2001;913:180–184. doi: 10.1016/s0006-8993(01)02793-7. [DOI] [PubMed] [Google Scholar]
- 46.Lephart ED, West TD, Weber KS, Rhees RW, Setchell KD, Adlercreutz H, et al. Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol Teratol. 2002;24:5–16. doi: 10.1016/s0892-0362(01)00197-0. [DOI] [PubMed] [Google Scholar]
- 47.Rodríguex-Landa JF, Hernández-Figueroa JD, Hernández-Calerón Bdel C, Saavedra M. Anxiolytic-like effect of phytoestrogen genistein in rats with long-term absense of ovarian hormones in the black and white model. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:367–372. doi: 10.1016/j.pnpbp.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 48.Patisaul HB, Burke KT, Hinkle RE, Adewale HB, Shea D. Systemic administration of diarylpropionitirle (DPN) or phytoestrogens does note affect anxiety-related behaviors in gonodally intact male rats. Horm Behav. 2009;55:319–328. doi: 10.1016/j.yhbeh.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walf AA, Frye CA. Administration of estrogen receptor beta-specific selective estrogen receptor modulators to the hippocampus decrease activity and depressive behavior of overiectomized rats. Pharmacol Biochem Behav. 2007;86:417–414. doi: 10.1016/j.pbb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioral despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 51.Mouton PR, Gokhale AM, Ward NL, West MJ. Stereological length estimation using spherical probes. J Microsc. 2002;206:54–64. doi: 10.1046/j.1365-2818.2002.01006.x. [DOI] [PubMed] [Google Scholar]
- 52.West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- 53.Keppel G, Wickens TD. Design and Analysis: A Researcher’s Handbook. 4th. New Jersey: Pearson-Prentice Hall; 2004. [Google Scholar]
- 54.Guo-Ross SX, Clark S, Montoya DA, Jones KH, Obernier J, Shetty AK, et al. Prenatal choline supplementation protects against postnatal neurotoxicity. J Neurosci. 2002;22:RC195. doi: 10.1523/JNEUROSCI.22-01-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong-Goodrich SJ, Mellott TJ, Glenn MJ, Blusztajn JK, Williams CL. Prenatal choline supplementation attenuates neuropathological response to status epilepticus in the adult rat hippocampus. Neurobiol Dis. 2008;30:255–269. doi: 10.1016/j.nbd.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas JD, Idrus NM, Monk BR, Dominguez HD. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res A Clin Mol Teratol. 2010;88:827–837. doi: 10.1002/bdra.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 58.Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Front Integr Neurosci. 2007 doi: 10.3389/neuro.07.007.2007. Epub 2007 Sept 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gray JA. Sex differences in emotional behaviour in mammals including man: endocrine bases. Acta Psychol (Amst) 1971;35:29–46. doi: 10.1016/0001-6918(71)90029-1. [DOI] [PubMed] [Google Scholar]
- 60.Fischer LM, daCosta KA, Kwock L, Stewart PW, Lu TS, Stabler SP, et al. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr. 2007;85:1275–1285. doi: 10.1093/ajcn/85.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McNamara RK, Jandacek R, Tso P, Dwivedi Y, Ren X, Pandey GN. Lower docosahexaenoic acid concentrations in the postmortem prefrontal cortex of adult depressed suicide victims compared with controls without cardiovascular disease. J Psychiatr Res. 2013 doi: 10.1016/j.jpsychires.2013.05.007. Epub 2013 Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mocking RJ, Ruhé HG, Assies J, Lok A, Koeter MW, Visser I, et al. Relationship between the hypothalamic-pituitary-adrenal-axis and fatty acid metabolism in recurrent depression. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.01.013. Epub 2013 Feb 25. [DOI] [PubMed] [Google Scholar]
- 63.Gow RV, Matsudaira T, Taylor E, Rubia K, Crawford M, Ghebremeskel K, et al. Total red blood cell concentrations of omega-3 fatty acids are associated with emotion-elicited neural activity in adolescent boys with attention-deficit hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids. 2009;80:151–156. doi: 10.1016/j.plefa.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Yui K, Koshiba M, Nakamura S, Kobayashi Y. Effects of large doses of arachidonic acid added to docosahexaenoic acid on social impairment in individuals with autism spectrum disorders: a double-blind, placebo-controlled, randomized trial. J Clin Psychopharmacol. 2012;32:200–206. doi: 10.1097/JCP.0b013e3182485791. [DOI] [PubMed] [Google Scholar]
- 65.Meyer BJ, Grenyer BF, Crowe T, Owen AJ, Grigonis-Deane EM, Howe PR. Improvement of Major Depression is Associated with Increased Erythrocyte DHA. Lipids. 2013 doi: 10.1007/s11745-013-3801-7. Epub 2013 Jun 4. [DOI] [PubMed] [Google Scholar]
- 66.Beltz BS, Tlusty MF, Benton JL, Sandeman DC. Omega-3 fatty acids upregulate adult neurogenesis. Neurosci Lett. 2007;415:154–158. doi: 10.1016/j.neulet.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeisel SH. Dietary choline deficiency causes DNA strand breaks and alters epigenetic marks on DNA and histones. Mutat Res. 2012;733:34–38. doi: 10.1016/j.mrfmmm.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sadli N, Ackland ML, De Mel D, Sinclair AJ, Suphioglu C. Effects of zinc and DHA on the epigenetic regulation of human neuronal cells. Cell Physiol Biochem. 2012;29:87–98. doi: 10.1159/000337590. [DOI] [PubMed] [Google Scholar]
- 69.Zeisel SH. Is maternal diet supplementation beneficial? Optimal development of infant depends on mother's diet. Am J Clin Nutr. 2009;89:685S–687S. doi: 10.3945/ajcn.2008.26811F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roy S, Sable P, Khaire A, Randhir K, Kale A, Joshi S. Effect of maternal micronutrients (folic acid and vitamin B12) and omega 3 fatty acids on indices of brain oxidative stress in the offspring. Brain Dev. 2013 doi: 10.1016/j.braindev.2013.03.004. Epub 2013 Apr 23. [DOI] [PubMed] [Google Scholar]
- 71.Wu A, Ying Z, Gomez-Pinilla F. J Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. Neurotrauma. 2004;21:1457–1467. doi: 10.1089/neu.2004.21.1457. [DOI] [PubMed] [Google Scholar]
- 72.Rao JS, Ertley RN, Lee HJ, DeMar JC, Jr, Arnold JT, Rapoport SI, Bazinet RP. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- 73.Gama CS, Canever L, Panizzutti B, Gubert C, Stertz L, Massuda R, et al. Effects of omega-3 dietary supplement in prevention of positive, negative and cognitive symptoms: a study in adolescent rats with ketamine-induced model of schizophrenia. Schizophr Res. 2012;141:162–167. doi: 10.1016/j.schres.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 74.Ferrari D, Cysneiros RM, Scorza CA, Arida RM, Cavalheiro EA, de Almeida AC, et al. Neuroprotective activity of omega-3 fatty acids against epilepsy-induced hippocampal damage: Quantification with immunohistochemical for calcium-binding proteins. Epilepsy Behav. 2008;13:36–42. doi: 10.1016/j.yebeh.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 75.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 76.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 77.de Andrade JS, Céspedes IC, Abrão RO, Dos Santos TB, Diniz L, Britto LR, et al. Chronic unpredictable mild stress alters an anxiety-related defensive response, Fos immunoreactivity and hippocampal adult neurogenesis. Behav Brain Res. 2013;250:81–90. doi: 10.1016/j.bbr.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 78.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 79.Dielenberg RA, McGregor IS. Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev. 2001;25:597–609. doi: 10.1016/s0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- 80.Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]