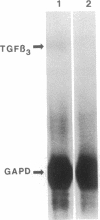
Abstract
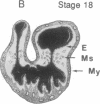
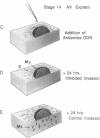
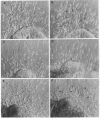
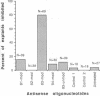
During early cardiac development, the progenitor cells of the heart valves and membranous septa undergo an epithelial-mesenchymal transformation. Previous studies have shown that this transformation depends on the activity of a transforming growth factor beta (TGF beta) molecule produced by the heart. In the present study, we have used modified antisense oligodeoxynucleotides generated to nonconserved regions of TGF beta 1, -2, -3, and -4 to examine the possible roles of these members in this transformation. A phosphoramidate-modified oligonucleotide complementary to TGF beta 3 mRNA was capable of inhibiting normal epithelial-mesenchymal transformation by 80%. Unmodified oligonucleotides to TGF beta 3, modified oligonucleotides to TGF beta 1, -2, and -4, and two modified control oligonucleotides were unable to inhibit the transformation. These data demonstrate that a specific member of the TGF beta family, TGF beta 3, is essential for the epithelial-mesenchymal cell transformation.
Full text
PDF

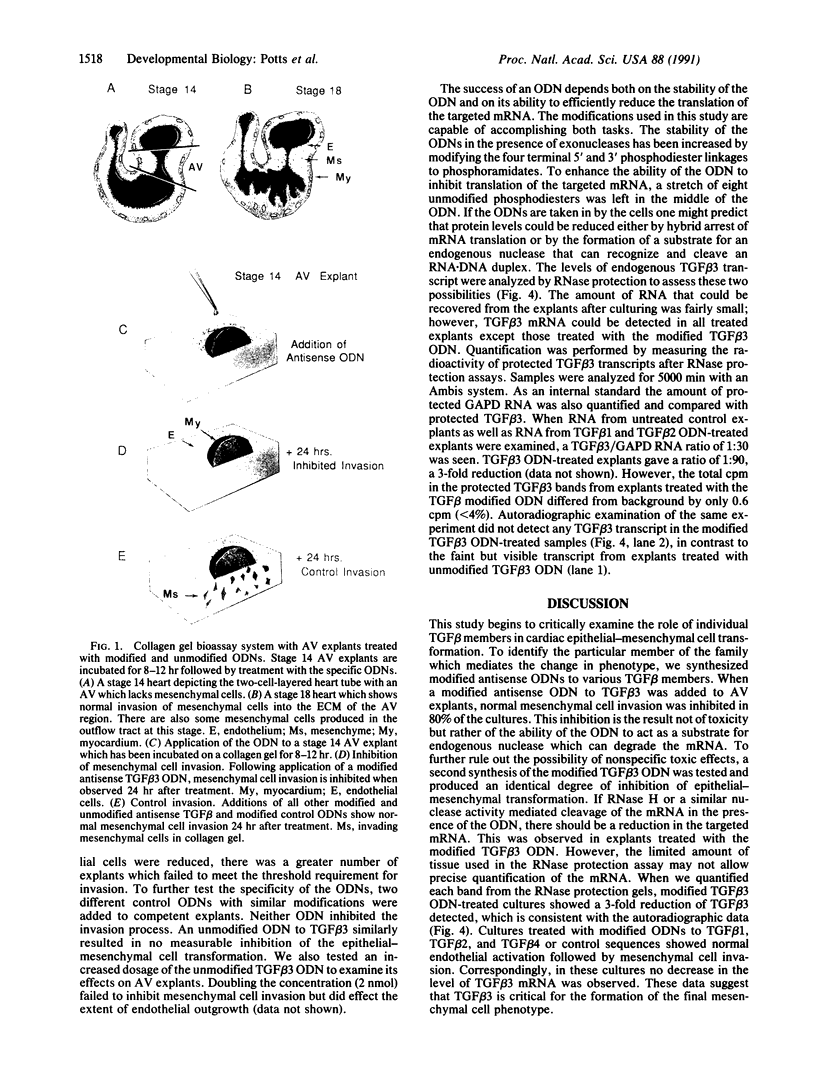
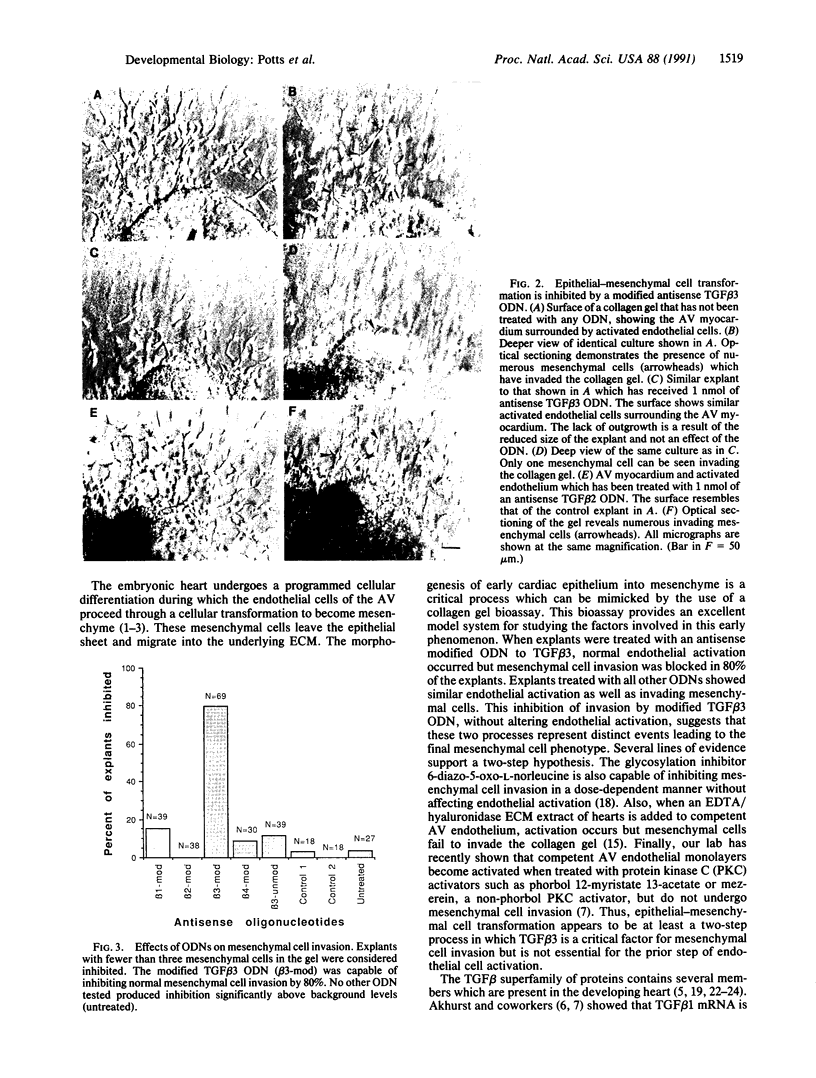

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhurst R. J., Lehnert S. A., Faissner A., Duffie E. TGF beta in murine morphogenetic processes: the early embryo and cardiogenesis. Development. 1990 Apr;108(4):645–656. doi: 10.1242/dev.108.4.645. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dagle J. M., Walder J. A., Weeks D. L. Targeted degradation of mRNA in Xenopus oocytes and embryos directed by modified oligonucleotides: studies of An2 and cyclin in embryogenesis. Nucleic Acids Res. 1990 Aug 25;18(16):4751–4757. doi: 10.1093/nar/18.16.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders K. C., Thompson N. L., Cissel D. S., Van Obberghen-Schilling E., Baker C. C., Kass M. E., Ellingsworth L. R., Roberts A. B., Sporn M. B. Transforming growth factor-beta 1: histochemical localization with antibodies to different epitopes. J Cell Biol. 1989 Feb;108(2):653–660. doi: 10.1083/jcb.108.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehler B. C., Ng P. G., Matteucci M. D. Synthesis of DNA via deoxynucleoside H-phosphonate intermediates. Nucleic Acids Res. 1986 Jul 11;14(13):5399–5407. doi: 10.1093/nar/14.13.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine U., Munoz E. F., Flanders K. C., Ellingsworth L. R., Lam H. Y., Thompson N. L., Roberts A. B., Sporn M. B. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol. 1987 Dec;105(6 Pt 2):2861–2876. doi: 10.1083/jcb.105.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakowlew S. B., Dillard P. J., Kondaiah P., Sporn M. B., Roberts A. B. Complementary deoxyribonucleic acid cloning of a novel transforming growth factor-beta messenger ribonucleic acid from chick embryo chondrocytes. Mol Endocrinol. 1988 Aug;2(8):747–755. doi: 10.1210/mend-2-8-747. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Krug E. L., Runyan R. B., Markwald R. R. Protein extracts from early embryonic hearts initiate cardiac endothelial cytodifferentiation. Dev Biol. 1985 Dec;112(2):414–426. doi: 10.1016/0012-1606(85)90414-2. [DOI] [PubMed] [Google Scholar]
- Lehnert S. A., Akhurst R. J. Embryonic expression pattern of TGF beta type-1 RNA suggests both paracrine and autocrine mechanisms of action. Development. 1988 Oct;104(2):263–273. doi: 10.1242/dev.104.2.263. [DOI] [PubMed] [Google Scholar]
- Lyons K. M., Pelton R. W., Hogan B. L. Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A). Development. 1990 Aug;109(4):833–844. doi: 10.1242/dev.109.4.833. [DOI] [PubMed] [Google Scholar]
- Markwald R. R., Fitzharris T. P., Bank H., Bernanke D. H. Structural analyses on the matrical organization of glycosaminoglycans in developing endocardial cushions. Dev Biol. 1978 Feb;62(2):292–316. doi: 10.1016/0012-1606(78)90218-x. [DOI] [PubMed] [Google Scholar]
- Markwald R. R., Fitzharris T. P., Manasek F. J. Structural development of endocardial cushions. Am J Anat. 1977 Jan;148(1):85–119. doi: 10.1002/aja.1001480108. [DOI] [PubMed] [Google Scholar]
- Markwald R. R., Funderburg F. M. Use of 6-diazo-5-oxo-L-norleucine to study interaction between myocardial glycoconjugate secretion and endothelial activation in the early embryonic chick heart. Dev Biol. 1983 Oct;99(2):395–407. doi: 10.1016/0012-1606(83)90289-0. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt C. H., Lepera R. C., Markwald R. R. Myocardial specificity for initiating endothelial-mesenchymal cell transition in embryonic chick heart correlates with a particulate distribution of fibronectin. Dev Biol. 1987 Jan;119(1):59–67. doi: 10.1016/0012-1606(87)90206-5. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt C. H., Markwald R. R. Induction of an epithelial-mesenchymal transition by an in vivo adheron-like complex. Dev Biol. 1989 Nov;136(1):118–128. doi: 10.1016/0012-1606(89)90135-8. [DOI] [PubMed] [Google Scholar]
- Potts J. D., Runyan R. B. Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor beta. Dev Biol. 1989 Aug;134(2):392–401. doi: 10.1016/0012-1606(89)90111-5. [DOI] [PubMed] [Google Scholar]
- Runyan R. B., Markwald R. R. Invasion of mesenchyme into three-dimensional collagen gels: a regional and temporal analysis of interaction in embryonic heart tissue. Dev Biol. 1983 Jan;95(1):108–114. doi: 10.1016/0012-1606(83)90010-6. [DOI] [PubMed] [Google Scholar]
- Runyan R. B., Potts J. D., Sharma R. V., Loeber C. P., Chiang J. J., Bhalla R. C. Signal transduction of a tissue interaction during embryonic heart development. Cell Regul. 1990 Feb;1(3):301–313. doi: 10.1091/mbc.1.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. L., Flanders K. C., Smith J. M., Ellingsworth L. R., Roberts A. B., Sporn M. B. Expression of transforming growth factor-beta 1 in specific cells and tissues of adult and neonatal mice. J Cell Biol. 1989 Feb;108(2):661–669. doi: 10.1083/jcb.108.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmé J. J., Hélène C. Antimessenger oligodeoxyribonucleotides: an alternative to antisense RNA for artificial regulation of gene expression--a review. Gene. 1988 Dec 10;72(1-2):51–58. doi: 10.1016/0378-1119(88)90127-8. [DOI] [PubMed] [Google Scholar]
- Walder J. Antisense DNA and RNA: progress and prospects. Genes Dev. 1988 May;2(5):502–504. doi: 10.1101/gad.2.5.502. [DOI] [PubMed] [Google Scholar]