Abstract
Metabolic diseases are associated with an increased risk of developing cardiovascular disease. The features comprising metabolic diseases include obesity, insulin resistance, hyperglycemia, hyperlipidemia, and hypertension. Recent evidence has emerged showcasing a role for cytochrome P450 epoxygenases, soluble epoxide hydrolase, and epoxyeicosatrienoic acids (EETs) in the development and progression of metabolic diseases. This review discusses the current knowledge related to the modulation of cytochrome P450 epoxygenases and soluble epoxide hydrolase to alter concentrations of biologically active EETs, resulting in effects on insulin resistance, lipid metabolism, obesity, and diabetes. Future areas of research to address current deficiencies in the understanding of these enzymes and their eicosanoid metabolites in various aspects of metabolic diseases are also discussed.
Keywords: cytochrome P450 (CYP) epoxygenase, epoxyeicosatrienoic acid, soluble epoxide hydrolase, insulin resistance, lipid, obesity, type 2 diabetes
Introduction
Metabolic diseases consist of a group of interrelated features, including central obesity, hyperlipidemia, hypertension, insulin resistance, and diabetes. The presence of these features is linked to an increased risk of cardiovascular disease. The incidence of metabolic diseases is increasing and is a major public health and clinical problem worldwide (1). Evidence from various studies suggests a role for enzymes involved in arachidonic acid (AA)6 (20:4n−6) metabolism, including cytochrome P450 (CYP) epoxygenases and soluble epoxide hydrolase (sEH), and their eicosanoid metabolites [epoxyeicosatrienoic acids (EETs)], in various aspects of metabolic diseases (2, 3).
AA is released from plasma membranes by phospholipase A2. AA can be converted to bioactive eicosanoids through the actions of 3 classes of enzymes: cyclooxygenases, lipoxygenases, and CYP monooxygenases (4, 5). The CYP epoxygenases, primarily members of the CYP2C and CYP2J families, catalyze the formation of 4 EET regioisomers: 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET (5). Each regioisomer consists of a mixture of R/S and S/R enantiomers (5). However, the relative quantity of EET regioisomers and enantiomers varies depending on the specific CYP epoxygenases present in particular tissues. In most cell types and organs, EETs can be hydrolyzed to dihydroxyeicosatrienoic acids (DHETs) by sEH (6). DHETs are more stable and less bioactive than EETs (7) and are easily detected in biological samples and tissues (4).
Over the past several decades, research has shown that EETs exhibit cardiovascular effects and the cellular signaling mechanisms of EETs have been comprehensively reviewed elsewhere (4, 6, 8–10). There is evidence that EETs interact with G protein–coupled receptors to activate intracellular signaling cascades (11–13); however, an EET receptor has yet to be identified. The inhibition of sEH results in increased EETs (4). sEH inhibitors (sEHIs) have been shown to protect against a variety of diseases, including hypertension-induced end-organ damage, atherosclerosis, aortic aneurysm, cardiac hypertrophy, arrhythmia, stroke, heart attack, pulmonary hypertension, chronic obstructive pulmonary disease, and renal failure in various animal models (14).
Dietary modulation is the cornerstone for the treatment of metabolic diseases. Recent data suggest that CYP epoxygenases and EETs are involved in the homeostasis of metabolic diseases, including obesity and diabetes (15, 16). Previous studies have also shown that sEH is expressed in adipose tissue (17–19), hepatocytes (17, 18), and pancreatic islets (2, 19–21). At least in part, it is speculated that EETs play an important role in the treatment of diet-associated metabolic diseases. This review article highlights current research related to the role of CYP epoxygenases, sEH, and EETs in metabolic diseases. Evidence related to the role of these enzymes and their eicosanoid metabolites in insulin resistance, lipid metabolism, obesity, diabetes, and diabetic complications is thoroughly discussed. Clinical trials of sEHIs and possible research areas for future studies are also addressed.
Insulin Resistance
Insulin decreases blood glucose by promoting glucose uptake in insulin target tissues, such as skeletal muscle, adipose tissue, and liver. Insulin also inhibits glucose production in the liver, kidney, and small intestine. Insulin resistance is a condition whereby insulin-induced glucose uptake is impaired and is one of the key risk factors in metabolic diseases (1).
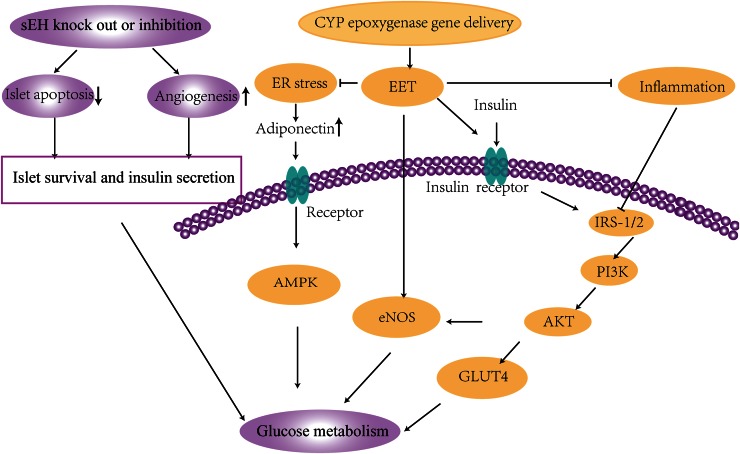
The CYP epoxygenase-EET-sEH system plays important roles in the development and progression of insulin resistance. Our previous study indicated that, in addition to lowering blood pressure, CYP2J3 overexpression improved insulin resistance in rats treated with fructose and in db/db diabetic mice. This improvement in insulin resistance was associated with the activation of insulin receptor signaling and adiponectin-mediated AMP-activated protein kinase (AMPK) signaling pathways (3, 15). CYP2J3 gene delivery markedly reversed insulin resistance via upregulated AMPK signaling, which was associated with decreased endoplasmic reticulum stress response in adipose tissue (15). Our data showed that CYP2J3-derived EETs alleviate insulin resistance, at least in part through upregulated endothelial nitric oxide synthase expression in rats treated with fructose (22), which was associated with activation of MAPK and protein kinase C signaling pathways (23). Genetic disruption or pharmacologic inhibition of sEH led to enhanced insulin signaling and sensitivity, increased islet size and vasculature, and decreased plasma glucose (21). sEH knockout or inhibition not only attenuated insulin resistance in diabetes but also enhanced glucose-stimulated insulin secretion from islet cells and decreased islet cell apoptosis (2, 20). The roles of CYP epoxygenases, sEH, and EETs in promoting glucose metabolism to protect against insulin resistance are summarized in Figure 1 and Table 1.
FIGURE 1.
Signaling mechanisms in glucose metabolism that protect against insulin resistance. EETs activate eNOS and insulin receptor downstream signaling pathways to promote glucose metabolism. EETs alleviate ER stress, leading to activation of adiponectin signaling, inflammation, and upregulation of adiponectin expression and signaling. EETs also reduce inflammation, leading to the activation of PI3K/AKT signaling pathways to increase glucose metabolism. Genetic disruption and/or pharmacologic inhibition of sEH increases EETs and increases islet survival and insulin secretion to promote glucose metabolism. AKT, protein kinase B; AMPK, AMP-activated protein kinase; CYP, cytochrome P450; EET, epoxyeicosatrienoic acid; eNOS, endothelial nitric oxide synthase; ER, endoplasmic reticulum; GLUT4, glucose transporter type 4; IRS-1/2, insulin receptor substrate-1/2; PI3K, phosphatidylinositol 3-kinase; sEH, soluble epoxide hydrolase.
TABLE 1.
Summary of signaling mechanisms of EETs in glucose metabolism evaluated in animal models of disease1
| Condition or disease | Experimental model | Treatment (reference) |
| Insulin resistance | Rats | CYP2J3 gene delivery (3, 22) |
| Type 2 diabetes | Mice | CYP2J3 gene delivery (3) |
| Obesity | Rats | CYP2J3 gene delivery (15) |
| Type 2 diabetes | Mice | CYP2J2 gene delivery (16) |
| Type 1 diabetes | Mice | CYP2J2 gene delivery (24, 25) |
| Insulin resistance | Mice | Knockout or inhibition of sEH (2, 19) |
| Type 1 diabetes | Mice | Knockout or inhibition of sEH (21) |
| NAFLD | Mice | Knockout of sEH (26) |
CYP, cytochrome P450; EET, epoxyeicosatrienoic acid; NAFLD, nonalcoholic fatty liver disease; sEH, soluble epoxide hydrolase.
Lipid Metabolism
Lipid metabolism is closely connected to metabolic diseases. Alterations in lipid metabolism can affect the development and progression of cardiovascular disease. Dyslipidemia is also related to obesity with increased TGs, reduced HDL-C and altered LDL-C. A component of metabolic diseases, muscle-specific insulin resistance, is also thought to play a role in the development and progression of hepatic lipogenesis, nonalcoholic fatty liver disease (NAFLD), and atherogenic dyslipidemia (27).
In vitro, EETs markedly lowered cholesterol concentrations in the human hepatoma cell line HepG2 (22). In contrast, HepG2 cells stably expressing human sEH showed elevated cholesterol (28). Overexpression of sEH in the liver increased TGs and enhanced the inflammatory response (17). A decrease of ∼25% in plasma cholesterol was observed in sEH knockout male mice compared with wild-type male mice. Consistent with plasma cholesterol concentrations, liver expression of β-hydroxy-β-methylglutaryl coenzyme A reductase, an enzyme integral for the production of cholesterol, was found to be ∼50% lower in sEH knockout male mice. Furthermore, the sEHI AR9276 significantly lowered cholesterol in an animal model of abdominal aortic aneurysm (29).
The sEHI trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (t-AUCB) ameliorated metabolic disease characteristics in rats fed a high-carbohydrate, high-fat diet (30). In addition, in adipocytes, t-AUCB enhanced the CD36-mediated recognition and degradation of oxidized LDL and improved cholesterol efflux via the upregulation of ATP-binding cassette transporter A1 (ABCA1) in a PPAR-γ–dependent manner (31). Our recent study indicated that endothelium-specific CYP2J2 overexpression [in tyrosine kinase 2 (Tie2)-CYP2J2-transgenic (Tr) mice] significantly attenuated high-fat diet–induced fatty liver progression that was associated with inhibition of NF-κB/c-Jun N-terminal kinase (JNK) signaling and activation of the antioxidant defense system (32). Furthermore, Tie2-CYP2J2-Tr mice showed significantly lower plasma and liver TGs and reduced high-fat diet–induced lipid accumulation compared with wild-type mice. CYP2J2 overexpression prevented peroxisome proliferator-activated receptor-α (PPAR-α) downregulation induced by a high-fat diet, activated the hepatic AMPKα subunit, increased acetyl-CoA carboxylase (ACC) phosphorylation, and increased the expression of carnitine palmitoyltransferase 1 (CPT-1) (26). These results highlight the role of CYPs, sEH, and EETs in lipid metabolism.
Obesity
Visceral obesity is an important element of metabolic diseases that contributes to the development of insulin resistance, diabetes, and cardiovascular disease. Adipogenesis, the differentiation of preadipocytes to mature adipocytes, is an integral component of obesity. Sustained activation of this process results in adipocyte dysfunction, glucose intolerance, and ultimately, the development of insulin resistance and type 2 diabetes (T2D) (33, 34). sEH activity is higher in the fat pads of mice fed a Western diet for 20 wk than in normal feed pellet–fed mice (35). Upon differentiation of 3T3-L1 preadipocytes, sEH is upregulated. PPAR-γ agonists can increase sEH expression in mature 3T3-L1 adipocytes in vitro and in adipose tissue in vivo. In addition, decreased EETs were associated with an increase in adipocyte stem cell differentiation and increased inflammatory cytokines (36). The EET agonist 12-(3-hexylureido)dodec-8(Z)-enoic acid decreased mesenchymal stem cell–derived adipocyte stem cell differentiation via upregulation of heme oxygenase (HO) 1–adiponectin–protein kinase B (AKT) signaling and inhibition of PPAR-γ, CCAAT/enhancer binding protein α (C/EBPα), and FA synthase (37). In vitro and in vivo induction of adipogenesis resulted in the dramatic suppression of adipose-derived EETs. In high-fat diet–fed mice, the administration of an EET analog elicited potent antiadipogenic effects via inhibition of the early phase of adipogenesis, resulting in significantly mitigated weight gain, adipose tissue expansion, proadipogenic gene expression, and glucose intolerance (38). In addition, the overexpression of CYP2J2 in endothelial cells attenuated adipogenesis due to decreased adipose tissue inflammation, oxidative stress, PPAR-γ, C/EBPα, mesoderm-specific transcript, and adipocyte 2 expression along with increased uncoupling protein 1 and 2 expression (39).
Numerous studies have shown that EETs exert beneficial effects on obesity-associated diseases (30). An epidemiologic study indicated that obesity was significantly associated with low plasma EETs and 14,15-EET to 14,15-DHET ratios. Age, diabetes, and cigarette smoking were also associated with CYP epoxygenase and sEH metabolic activity in the study (40). Rats fed a high-fat diet and an sEHI exhibited increased renal vascular dilation and reduced sodium retention, resulting in amelioration of hypertension (41). Rats with diet-induced hypertension and increased body weight showed reduced expression of CYP2C23 in renal tubules as well as a reduction in EETs (42). An EET agonist inhibited the development of the metabolic disease phenotype in HO-2–null mice by preventing weight gain, increasing insulin sensitivity, and decreasing inflammation (43). In rats fed a high-fat diet, the EET agonist (S)-2-[11-(nonyloxy)undec-8(Z)-enamido]succinic acid (NUDSA) prevented adiposity via a decrease in basic leucine zipper transcription factor 1 (Bach1) and an increase in HO-1 concentrations (44). Consistent with these results, the sEHI AR9281 promoted weight loss by reducing appetite and increasing metabolic rate in high-fat diet–treated mice (45). The sEHI t-AUCB alleviated diet-induced characteristics of metabolic diseases, including glucose, insulin, and lipid abnormalities, as well as pancreatic and liver structural changes (30). sEH deficiency or inhibition also attenuated diet-induced endoplasmic reticulum stress in liver and adipose tissue (18).
Obesity is characterized by increased lipid storage and decreased lipid removal. Adipose tissue is the main site of storage and mobilization of lipids. Adipocyte lipolysis is primarily regulated by adipose TG lipase (ATGL) and hormone-sensitive lipase (HSL) (46). ATGL enzyme activity is induced by PPAR-α and AMPK activation, whereas HSL enzyme activity can be induced by β-adrenergic stimulation, protein kinase A (PKA), AMPK, and extracellular signal–regulated kinase (ERK)–catalyzed phosphorylation (46, 47). It is known that EETs can activate PKA, AMPK, ERK, and PPAR-α signaling pathways (4). Our previous study showed that CYP2J2 gene delivery alleviated high-fat diet–induced hyperlipidemia and lipid accumulation in vivo by activating the AMPK and PPAR-α signaling pathways (26). Future studies are needed to determine if EETs exhibit direct effects on ATGL and HSL activity. Whether the CYP epoxygenase-sEH-EET effects on obesity development and progress are due to changes in lipolysis are also unknown. Adipocyte-specific overexpression of CYP epoxygenases or tissue-specific disruption of sEH will be informative to further elucidate these issues.
NAFLD is associated with increased cardiovascular and hepatic-related mortality. NAFLD ranges from TG accumulation in hepatocytes to inflammatory and hepatocellular ballooning injury that eventually leads to fibrosis and cirrhosis (48). In humans, obesity is associated with an increased risk of NAFLD (49). Genetic disruption or pharmacologic inhibition of sEH alleviated high-fat diet–induced hepatic steatosis, which was associated with a reduced systemic inflammatory response (17). Endothelium-specific expression of CYP2J2 significantly decreased liver lipid accumulation and improved liver function through anti-inflammatory and antioxidant effects (32). Importantly, the signaling mechanisms regulating these effects on liver steatosis still need to be investigated.
Diabetes
Type 1 diabetes (T1D) results from the destruction of pancreatic β cells through an autoimmune process and is characterized by a deficiency in insulin production. In contrast, type 2 diabetes (T2D) is a metabolic disease characterized by insulin resistance, β cell dysfunction, and elevated hepatic glucose output (50). Pancreatic islets in patients with T2D are characterized by β cell loss, β cell dysfunction, and formation of amyloid deposits derived from islet amyloid polypeptide (51). Multiple studies have investigated the role of EETs as well as the enzymes involved in controlling EET concentrations, CYP epoxygenases, and sEH in diabetes.
CYP2J2 is expressed in the pancreas and is highly localized to all islet of Langerhans cells, especially the glucagon-producing α cells in humans (52). This expression of CYP2J2 also correlated with pancreatic EET concentrations in human tissue (52). Our recent study indicated that CYP2J2 gene delivery attenuated the diabetic phenotype via inhibition of MAPK and NF-κB signaling pathways through the activation of PPAR-γ (16). Genetic analysis indicated that the CYP2J2 G-50T polymorphism may contribute to the pathogenesis of T2D in patients with a younger onset, partially through effects on insulin resistance. Indeed, stable EET metabolites were lower in plasma from younger-onset individuals with this polymorphism (53).
Alterations in sEH have also been implicated in diabetes. Several studies have shown that the disruption of sEH enhanced islet glucose-stimulated insulin secretion through AMPK signaling and decreased islet cell apoptosis in diabetes (2, 20, 54). Genetic disruption or pharmacologic inhibition of sEH prevents hyperglycemia in a T1D model induced by streptozotocin administration. Inhibiting sEH activity with t-AUCB provided significant protection against islet β cell damage and improved glucose homeostasis in streptozotocin-induced diabetes (2). sEH inhibition did not affect pancreatic or plasma EET-to-DHET ratios (2), indicating that the beneficial effects from sEH inhibition on glucose homeostasis and β cell survival may not be due to changes in EETs; however, this needs to be further investigated. Previous data showed that genetic disruption of sEH attenuated prostaglandin E2 (PGE2) as well as 5-hydroxy-eicosatetraenoic acid, but not prostaglandin D2 (PGD2) in response to LPS challenge (55). Therefore, it is speculated that the protective effects of sEH disruption may be due to changes in other products from the metabolism of AA by ω-hydroxylases, lipoxygenases, and/or cyclooxygenases.
Several aspects of diabetes have been shown to be directly affected by EETs. 5,6-EET directly stimulates the release of insulin but has no effect on glucagon release. In contrast, 8,9-EET, 11,12-EET, and 14,15-EET increase glucagon release without affecting insulin secretion (56). Vasopressin-induced glycogenolysis was mediated by EETs, in particular 14,15-EET, in isolated rat hepatocytes (57). Although evidence indicates that EETs exert beneficial effects on T1D and T2D, the precise signaling mechanisms need to be explored. Mouse models with β cell–specific overexpression of CYP epoxygenases and/or disruption of sEH could be used. Experiments to understand the contribution of EETs and how they alter relevant signaling pathways would be beneficial to understand the role of EETs in diabetes.
Complications of Diabetes
The vascular complications of diabetes are among the most serious clinical manifestations of the disease. Atherosclerosis, the formation of plaques consisting of fatty material in vessel walls, is the main reason for reduced life expectancy in patients with diabetes. Diabetic nephropathy and retinopathy are the primary contributors to end-stage renal disease and blindness, respectively (58). Recent studies (24) showed that CYP epoxygenases, EETs, and sEH play critical roles in the prevention and treatment of diabetic complications.
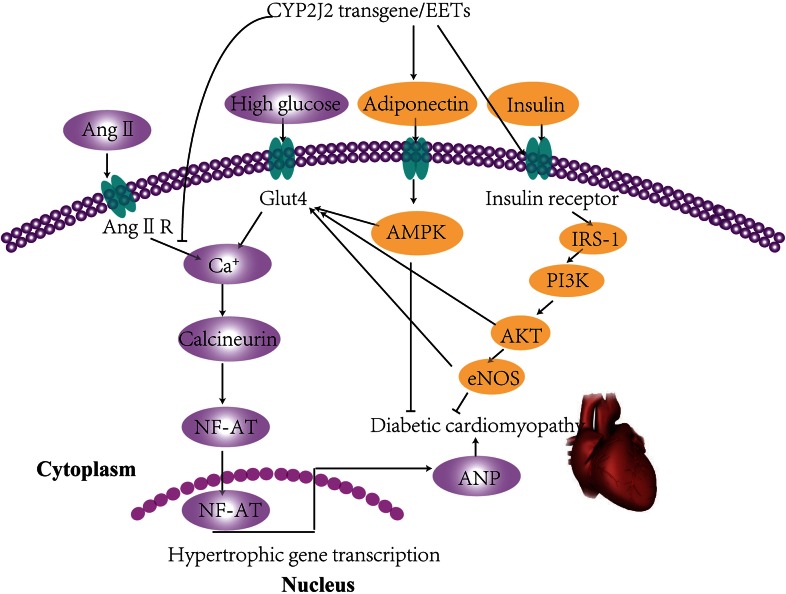
Research has shown that the inhibition of sEH protects against vascular complications in diabetes. sEH inhibition prevented endothelial dysfunction in diabetic conditions (30). The use of the sEHI 1-(1-acetypiperidin-4-yl)-3-adamantanylurea (APAU) resulted in slower progression of hyperglycemia, protection against changes in myocyte structure, and reduced Ca2+ dysregulation and sarcoplasmic reticulum Ca2+-ATPase (SERCA) remodeling in hyperglycemic rats (59) sEH inhibition reduced infarct size after middle cerebral artery occlusion in type 1 and type 2 diabetic mice (60, 61). EETs have been shown to reduce cardiac dysfunction after ischemia or reperfusion events in diabetic rats (62). A previous study from our laboratory indicated that cardiac-specific overexpression of CYP2J2, leading to increased EETs, protected against diabetic cardiomyopathy that was associated with improved cardiac insulin resistance and reversal of cardiac hypertrophy (24). The mechanisms involved in this process are detailed in Figure 2. Collectively, these data suggested that EETs exert cardioprotective effects in diabetic conditions and that sEH may be a potential therapeutic target for diabetes-related cardiovascular diseases.
FIGURE 2.
Signaling mechanisms involved in cardiovascular complications of insulin resistance. High glucose and Ang II increase intracellular calcium, promote NF-AT into the nucleus, and enhance transcription leading to cardiac hypertrophy. CYP2J2 overexpression increases the transfer of Glut4 to the cell membrane and also activates IRS-1/PI3K/AKT eNOS and upregulates adiponectin/AMPK signaling pathways, thus reversing the process of cardiac hypertrophy. AKT, protein kinase B; AMPK, AMP-activated protein kinase; Ang II, angiotensin II; Ang II R, angiotensin II receptor; ANP, atrial natriumtic peptide; CYP2J2, cytochrome P450 2J2; EET, epoxyeicosatrienoic acid; eNOS, endothelial nitric oxide synthase; Glut4, glucose transporter type 4; IRS-1, insulin receptor substrate-1; NF-AT, nuclear factor of activated T cells; PI3K, phosphatidylinositol 3-kinase. Adapted from reference 24 with permission.
Diabetic nephropathy is characterized by a progressive increase in proteinuria, a decline in glomerular filtration rate, hypertension, and a high risk of cardiovascular morbidity and mortality. Hyperglycemia results in increased transforming growth factor-β1 (TGF-β1) expression, but decreased EET production in the glomeruli, which may be factors that contribute to glomerular damage in the early stage of diabetic nephropathy (63). The inhibition of EETs enhanced hypertrophy and extracellular matrix accumulation in high glucose–treated proximal tubular epithelial cells (64). Endothelium-specific overexpression of CYP2J2 prevented renal damage characterized by reduced urinary albumin and glomerulosclerosis. These effects were associated with the inhibition of TGF-β–mothers against decapentaplegic homolog (Smad) signaling in the kidney (25). In contrast, reduced blood urea nitrogen and urinary albumin excretion was observed in sEH-deficient mice treated with streptozotocin. These mice also showed decreased renal tubular apoptosis and fibrosis due to the activation of the phosphatidylinositol 3-kinase–AKT–nitric oxide synthase 3 (PI3K-AKT-NOS3) and AMPK signaling cascades. In addition, sEH gene inhibition as well as exogenous EETs markedly protected human proximal tubule cells, HK-2 cells, against TNF-α–induced apoptosis in vitro (26) Taken together, these data suggest that EETs exerted beneficial effects in diabetic nephropathy, including improvement in glucose metabolism and direct inhibition of renal pathology.
Although studies have shown that EETs exert a myriad of beneficial effects on vascular complications associated with diabetes, the precise molecular mechanisms involved need to be further investigated. The role of EETs in diabetic retinopathy also needs to be explored in future studies.
Conclusions
AA metabolism by CYP epoxygenases results in the production of EETs. Modulating the concentration of CYP epoxygenases and/or sEH alters the concentration of biologically active EETs. This review summarizes current literature on the role of CYP epoxygenases, sEH, and EETs in the pathophysiology of metabolic diseases and their complications. Exogenous EETs as well as sEH disruption protect against obesity development and progression associated with reduced adipogenesis. Increased CYP epoxygenase expression or sEH inhibition decreased plasma cholesterol and TGs as well as liver steatosis due to effects on the inflammatory response and lipid metabolism. In addition, sEH disruption and/or overexpression of CYP epoxygenases exert beneficial effects on T1D and T2D due to increased islet survival and improved insulin resistance. EETs have been shown to exert beneficial effects on diabetic vascular complications, including improvement in diabetes-related endothelial dysfunction, enhanced cardioprotection, and alleviation of diabetic nephropathy. In contrast, CYP4A proteins were upregulated in livers of mice with genetically induced and diet-induced diabetes. The inhibition of CYP4A in mice reduced hepatic endoplasmic reticulum stress, apoptosis, insulin resistance, and steatosis (65). In addition, CYP2E1 deletion protects mice against high-fat diet–induced insulin resistance with improved glucose homeostasis in vivo (66).
On the basis of the in vivo and in vitro data described in this review, EET analogs and sEHIs may have the potential to help treat metabolic diseases including obesity and diabetes. However, the results of animal studies cannot be applied in humans. A clinical trial of the sEHI 1-(1-acetyl-piperidin-4-yl)-3-adamantan-1-yl-urea, designed for the treatment of hypertension and impaired glucose tolerance, was terminated in November 2009, because it did not appear to have any effect in phase II studies (67). Another sEHI, GSK2256294, has entered a phase I clinical trial for the treatment of chronic obstructive pulmonary disease (clinicaltrials.gov; ID: NCT01762774). Although clinical trials of sEHIs have not yielded positive results, sEH as a therapeutic target remains promising. Future studies are needed to further define the precise signaling mechanisms affected by EETs in these diseases. Animal models with specific expression and disruption of CYPs and sEH in β cells and adipocytes will be important to facilitate future work to address shortcomings in our understanding of particular signaling mechanisms affected by the CYP epoxygenase-sEH-EET pathway in diabetic and obese conditions. In addition, many mechanistic studies are needed to enable the extrapolation of animal results to humans, and a series of human genetic studies are also needed to determine the correlation between sEH polymorphisms and human diseases.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AA, arachidonic acid; ABCA1, ATP-binding cassette transporter A1; ACC,acetyl-CoA carboxylase; AKT, protein kinase B; AMPK, AMP-activated protein kinase; APAU,1-(1-acetypiperidin-4-yl)-3-adamantanylurea; ATGL, adipose TG lipase; Bach1, basic leucine zipper transcription factor 1; C/EBP⍺, CCAAT/enhancer binding protein a⍺; CPT-1, carnitine palmitoyltransferase 1; CYP, cytochrome P450; DHET, dihydroxyeicosatrienoic acid; EET,epoxyeicosatrienoic acid; ERK, extracellular signal–regulated kinase; HO, heme oxygenase; HSL,hormone-sensitive lipase; JNK, c-Jun N-terminal kinase; NAFLD, nonalcoholic fatty liver disease;NOS3, nitric oxide synthase 3; NUDSA, (S)-2-[11-(nonyloxy)undec-8(Z)-enamido]succinic acid;PGD2, prostaglandin D2; PGE2, prostaglandin E2; PI3K, phosphatidylinositol 3-kinase; PKA, proteinkinase A; PPAR-⍺, peroxisome proliferator-activated receptor-⍺; sEH, soluble epoxide hydrolase;sEHI, soluble epoxide hydrolase inhibitor; SERCA, sarcoplasmic reticulum Ca2+-ATPase; Smad, mothersagainst decapentaplegic homolog; t-AUCB, trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid; Tie2, tyrosine kinase 2; Tr, ; T1D, type 1 diabetes; T2D, type 2 diabetes.
References
- 1.Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol 2014;220:T1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luria A, Bettaieb A, Xi Y, Shieh GJ, Liu HC, Inoue H, Tsai HJ, Imig JD, Haj FG, Hammock BD. Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc Natl Acad Sci USA 2011;108:9038–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu X, Zhao CX, Wang L, Tu L, Fang X, Zheng C, Edin ML, Zeldin DC, Wang DW. Increased CYP2J3 expression reduces insulin resistance in fructose-treated rats and db/db mice. Diabetes 2010;59:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu X, Zhang XA, Wang DW. The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases. Adv Drug Deliv Rev 2011;63:597–609. [DOI] [PubMed] [Google Scholar]
- 5.Bellien J, Joannides R. Epoxyeicosatrienoic acid pathway in human health and diseases. J Cardiovasc Pharmacol 2013;61:188–96. [DOI] [PubMed] [Google Scholar]
- 6.Capdevila J, Wang W. Role of cytochrome P450 epoxygenase in regulating renal membrane transport and hypertension. Curr Opin Nephrol Hypertens 2013;22:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov 2009;8:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elmarakby AA. Reno-protective mechanisms of epoxyeicosatrienoic acids in cardiovascular disease. Am J Physiol Regul Integr Comp Physiol 2012;302:R321–30. [DOI] [PubMed] [Google Scholar]
- 9.Imig JD. Epoxyeicosatrienoic acids, 20-hydroxyeicosatetraenoic acid, and renal microvascular function. Prostaglandins Other Lipid Mediat 2013;104–105:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol 2007;292:C996–1012. [DOI] [PubMed] [Google Scholar]
- 11.Yang W, Holmes BB, Gopal VR, Kishore RV, Sangras B, Yi XY, Falck JR, Campbell WB. Characterization of 14,15-epoxyeicosatrienoyl-sulfonamides as 14,15-epoxyeicosatrienoic acid agonists: use for studies of metabolism and ligand binding. J Pharmacol Exp Ther 2007;321:1023–31. [DOI] [PubMed] [Google Scholar]
- 12.Ding Y, Frömel T, Popp R, Falck JR, Schunck WH, Fleming I. The biological actions of 11,12-epoxyeicosatrienoic acid in endothelial cells are specific to the R/S-enantiomer and require the G(s) protein. J Pharmacol Exp Ther 2014;350:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang C, Kwan YW, Au AL, Poon CC, Zhang Q, Chan SW, Lee SM, Leung GP. 14,15-Epoxyeicosatrienoic acid induces vasorelaxation through the prostaglandin EP(2) receptors in rat mesenteric artery. Prostaglandins Other Lipid Mediat 2010;93:44–51. [DOI] [PubMed] [Google Scholar]
- 14.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol 2013;53:37–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X, Tu L, Feng W, Ma B, Li R, Zheng C, Li G, Wang DW. CYP2J3 gene delivery up-regulated adiponectin expression via reduced endoplasmic reticulum stress in adipocytes. Endocrinology 2013;154:1743–53. [DOI] [PubMed] [Google Scholar]
- 16.Li R, Xu X, Chen C, Wang Y, Gruzdev A, Zeldin DC, Wang DW. CYP2J2 attenuates metabolic dysfunction in diabetic mice by reducing hepatic inflammation via the PPARgamma. Am J Physiol Endocrinol Metab 2015;308:E270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Taeye BM, Morisseau C, Coyle J, Covington JW, Luria A, Yang J, Murphy SB, Friedman DB, Hammock BD, Vaughan DE. Expression and regulation of soluble epoxide hydrolase in adipose tissue. Obesity (Silver Spring) 2010;18:489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Dang H, Li D, Pang W, Hammock BD, Zhu Y. Inhibition of soluble epoxide hydrolase attenuates high-fat-diet-induced hepatic steatosis by reduced systemic inflammatory status in mice. PLoS One 2012;7:e39165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bettaieb A, Nagata N, AbouBechara D, Chahed S, Morisseau C, Hammock BD, Haj FG. Soluble epoxide hydrolase deficiency or inhibition attenuates diet-induced endoplasmic reticulum stress in liver and adipose tissue. J Biol Chem 2013;288:14189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enayetallah AE, French RA, Barber M, Grant DF. Cell-specific subcellular localization of soluble epoxide hydrolase in human tissues. J Histochem Cytochem 2006;54:329–35. [DOI] [PubMed] [Google Scholar]
- 21.Luo P, Chang HH, Zhou Y, Zhang S, Hwang SH, Morisseau C, Wang CY, Inscho EW, Hammock BD, Wang MH. Inhibition or deletion of soluble epoxide hydrolase prevents hyperglycemia, promotes insulin secretion, and reduces islet apoptosis. J Pharmacol Exp Ther 2010;334:430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X, Tu L, Wang L, Fang X, Wang DW. CYP2J3 gene delivery reduces insulin resistance via upregulation of eNOS in fructose-treated rats. Cardiovasc Diabetol 2011;10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Lin L, Jiang J, Wang Y, Lu ZY, Bradbury JA, Lih FB, Wang DW, Zeldin DC. Up-regulation of endothelial nitric-oxide synthase by endothelium-derived hyperpolarizing factor involves mitogen-activated protein kinase and protein kinase C signaling pathways. J Pharmacol Exp Ther 2003;307:753–64. [DOI] [PubMed] [Google Scholar]
- 24.Ma B, Xiong X, Chen C, Li H, Xu X, Li X, Li R, Chen G, Dackor RT, Zeldin DC, et al. . Cardiac-specific overexpression of CYP2J2 attenuates diabetic cardiomyopathy in male streptozotocin-induced diabetic mice. Endocrinology 2013;154:2843–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen G, Wang P, Zhao G, Xu G, Gruzdev A, Zeldin DC, Wang DW. Cytochrome P450 epoxygenase CYP2J2 attenuates nephropathy in streptozotocin-induced diabetic mice. Prostaglandins Other Lipid Mediat 2011;96:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen G, Xu R, Zhang S, Wang Y, Wang P, Edin ML, Zeldin DC, Wang DW. CYP2J2 overexpression attenuates nonalcoholic fatty liver disease induced by high-fat diet in mice. Am J Physiol Endocrinol Metab 2015;308:E97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 2014;371:2237–8. [DOI] [PubMed] [Google Scholar]
- 28.Enayetallah A, Cao L, Grant DF. Novel role of soluble epoxide hydrolase in regulating cholesterol in mammalian cells. Open Drug Metab J 2007;1:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.EnayetAllah AE, Luria A, Luo B, Tsai HJ, Sura P, Hammock BD, Grant DF. Opposite regulation of cholesterol levels by the phosphatase and hydrolase domains of soluble epoxide hydrolase. J Biol Chem 2008;283:36592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang LN, Vincelette J, Cheng Y, Mehra U, Chen D, Anandan SK, Gless R, Webb HK, Wang YX. Inhibition of soluble epoxide hydrolase attenuated atherosclerosis, abdominal aortic aneurysm formation, and dyslipidemia. Arterioscler Thromb Vasc Biol 2009;29:1265–70. [DOI] [PubMed] [Google Scholar]
- 31.Iyer A, Kauter K, Alam MA, Hwang SH, Morisseau C, Hammock BD, Brown L. Pharmacological inhibition of soluble epoxide hydrolase ameliorates diet-induced metabolic syndrome in rats. Exp Diabetes Res 2012;2012:758614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen L, Peng H, Zhao S, Xu D. A potent soluble epoxide hydrolase inhibitor, t-AUCB, modulates cholesterol balance and oxidized low density lipoprotein metabolism in adipocytes in vitro. Biol Chem 2014;395:443–51. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S, Chen G, Li N, Dai M, Chen C, Wang P, Tang H, Hoopes SL, Zeldin DC, Wang DW, et al. . CYP2J2 overexpression ameliorates hyperlipidemia via increased fatty acid oxidation mediated by the AMPK pathway. Obesity (Silver Spring) 2015;23:1401–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest 2011;121:2094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 2013;339:172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burgess A, Vanella L, Bellner L, Schwartzman ML, Abraham NG. Epoxyeicosatrienoic acids and heme oxygenase-1 interaction attenuates diabetes and metabolic syndrome complications. Prostaglandins Other Lipid Mediat 2012;97:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim DH, Vanella L, Inoue K, Burgess A, Gotlinger K, Manthati VL, Koduru SR, Zeldin DC, Falck JR, Schwartzman ML, et al. . Epoxyeicosatrienoic acid agonist regulates human mesenchymal stem cell-derived adipocytes through activation of HO-1-pAKT signaling and a decrease in PPARgamma. Stem Cells Dev 2010;19:1863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zha W, Edin ML, Vendrov KC, Schuck RN, Lih FB, Jat JL, Bradbury JA, DeGraff LM, Hua K, Tomer KB, et al. . Functional characterization of cytochrome P450-derived epoxyeicosatrienoic acids in adipogenesis and obesity. J Lipid Res 2014;55:2124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abraham NG, Sodhi K, Silvis AM, Vanella L, Favero G, Rezzani R, Lee C, Zeldin DC, Schwartzman ML. CYP2J2 targeting to endothelial cells attenuates adiposity and vascular dysfunction in mice fed a high-fat diet by reprogramming adipocyte phenotype. Hypertension 2014;64:1352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Theken KN, Schuck RN, Edin ML, Tran B, Ellis K, Bass A, Lih FB, Tomer KB, Poloyac SM, Wu MC, et al. . Evaluation of cytochrome P450-derived eicosanoids in humans with stable atherosclerotic cardiovascular disease. Atherosclerosis 2012;222:530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang H, Morisseau C, Wang J, Yang T, Falck JR, Hammock BD, Wang MH. Increasing or stabilizing renal epoxyeicosatrienoic acid production attenuates abnormal renal function and hypertension in obese rats. Am J Physiol Renal Physiol 2007;293:F342–9. [DOI] [PubMed] [Google Scholar]
- 42.Wang MH, Smith A, Zhou Y, Chang HH, Lin S, Zhao X, Imig JD, Dorrance AM. Downregulation of renal CYP-derived eicosanoid synthesis in rats with diet-induced hypertension. Hypertension 2003;42:594–9. [DOI] [PubMed] [Google Scholar]
- 43.Sodhi K, Inoue K, Gotlinger KH, Canestraro M, Vanella L, Kim DH, Manthati VL, Koduru SR, Falck JR, Schwartzman ML et al. , Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmacol Exp Ther 2009;331:906–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sodhi K, Puri N, Inoue K, Falck JR, Schwartzman ML, Abraham NG. EET agonist prevents adiposity and vascular dysfunction in rats fed a high fat diet via a decrease in Bach 1 and an increase in HO-1 levels. Prostaglandins Other Lipid Mediat 2012;98:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.do Carmo JM, da Silva AA, Morgan J, Jim Wang YX, Munusamy S, Hall JE. Inhibition of soluble epoxide hydrolase reduces food intake and increases metabolic rate in obese mice. Nutr Metab Cardiovasc Dis 2012;22:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F. Fat signals—lipases and lipolysis in lipid metabolism and signaling. Cell Metab 2012;15:279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis—a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res 2011;50:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, Kellum J, Warnick R, Contos MJ, Sanyal AJ. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab 2012;15:665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330–44. [DOI] [PubMed] [Google Scholar]
- 50.Coughlan KA, Valentine RJ, Ruderman NB, Saha AK. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes 2014;7:241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shigihara N, Fukunaka A, Hara A, Komiya K, Honda A, Uchida T, Abe H, Toyofuku Y, Tamaki M, Ogihara T, et al. . Human IAPP-induced pancreatic beta cell toxicity and its regulation by autophagy. J Clin Invest 2014;124:3634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeldin DC, Foley J, Boyle JE, Moomaw CR, Tomer KB, Parker C, Steenbergen C, Wu S. Predominant expression of an arachidonate epoxygenase in islets of Langerhans cells in human and rat pancreas. Endocrinology 1997;138:1338–46. [DOI] [PubMed] [Google Scholar]
- 53.Wang CP, Hung WC, Yu TH, Chiu CA, Lu LF, Chung FM, Hung CH, Shin SJ, Chen HJ, Lee YJ. Genetic variation in the G-50T polymorphism of the cytochrome P450 epoxygenase CYP2J2 gene and the risk of younger onset type 2 diabetes among Chinese population: potential interaction with body mass index and family history. Exp Clin Endocrinol Diabetes 2010;118:346–52. [DOI] [PubMed] [Google Scholar]
- 54.Luo P, Wang MH. Eicosanoids, beta-cell function, and diabetes. Prostaglandins Other Lipid Mediat 2011;95:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu JY, Yang J, Inceoglu B, Qiu H, Ulu A, Hwang SH, Chiamvimonvat N, Hammock BD. Inhibition of soluble epoxide hydrolase enhances the anti-inflammatory effects of aspirin and 5-lipoxygenase activation protein inhibitor in a murine model. Biochem Pharmacol 2010;79:880–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falck JR, Manna S, Moltz J, Chacos N, Capdevila J. Epoxyeicosatrienoic acids stimulate glucagon and insulin release from isolated rat pancreatic islets. Biochem Biophys Res Commun 1983;114:743–9. [DOI] [PubMed] [Google Scholar]
- 57.Yoshida S, Hirai A, Tamura Y, Yoshida S. Possible involvement of arachidonic acid metabolites of cytochrome P450 monooxygenase pathway in vasopressin-stimulated glycogenolysis in isolated rat hepatocytes. Arch Biochem Biophys 1990;280:346–51. [DOI] [PubMed] [Google Scholar]
- 58.Rask-Madsen C, King GL. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab 2013;17:20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guglielmino K, Jackson K, Harris TR, Vu V, Dong H, Dutrow G, Evans JE, Graham J, Cummings BP, Havel PJ, et al. . Pharmacological inhibition of soluble epoxide hydrolase provides cardioprotection in hyperglycemic rats. Am J Physiol Heart Circ Physiol 2012;303:H853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jouihan SA, Zuloaga KL, Zhang W, Shangraw RE, Krasnow SM, Marks DL, Alkayed NJ. Role of soluble epoxide hydrolase in exacerbation of stroke by streptozotocin-induced type 1 diabetes mellitus. J Cereb Blood Flow Metab 2013;33:1650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zuloaga KL, Krasnow SM, Zhu X, Zhang W, Jouihan SA, Shangraw RE, Alkayed NJ, Marks DL. Mechanism of protection by soluble epoxide hydrolase inhibition in type 2 diabetic stroke. PLoS One 2014;9:e97529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yousif MH, Benter IF, Roman RJ. Cytochrome P450 metabolites of arachidonic acid play a role in the enhanced cardiac dysfunction in diabetic rats following ischaemic reperfusion injury. Auton Autacoid Pharmacol 2009;29:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo P, Zhou Y, Chang HH, Zhang J, Seki T, Wang CY, Inscho EW, Wang MH. Glomerular 20-HETE, EETs, and TGF-beta1 in diabetic nephropathy. Am J Physiol Renal Physiol 2009;296:F556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eid S, Maalouf R, Jaffa AA, Nassif J, Hamdy A, Rashid A, Ziyadeh FN, Eid AA. 20-HETE and EETs in diabetic nephropathy: a novel mechanistic pathway. PLoS One 2013;8:e70029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park EC, Kim SI, Hong Y, Hwang JW, Cho GS, Cha HN, Han JK, Yun CH, Park SY, Jang IS, et al. . Inhibition of CYP4A reduces hepatic endoplasmic reticulum stress and features of diabetes in mice. Gastroenterology 2014;147:860–9. [DOI] [PubMed] [Google Scholar]
- 66.Zong H, Armoni M, Harel C, Karnieli E, Pessin JE. Cytochrome P-450 CYP2E1 knockout mice are protected against high-fat diet-induced obesity and insulin resistance. Am J Physiol Endocrinol Metab 2012;302:E532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen HC, Hammock BD. Discovery of inhibitors of soluble epoxide hydrolase: a target with multiple potential therapeutic indications. J Med Chem 2012;55:1789–808. [DOI] [PMC free article] [PubMed] [Google Scholar]