Abstract
To gain insights into nutrient biomarkers and setting of dietary nutrient requirements, selenium biomarker levels and requirements in response to multiple graded levels of dietary selenium were compared between day-old turkeys and chickens versus weanling rats and mice and 2-d-old lambs supplemented with sodium selenite. In rodents, there was no significant effect of dietary selenium on growth, indicating that the minimum selenium requirement was <0.007 μg Se/g diet. In contrast, there was a significant effect in turkeys, chicks, and lambs, which showed selenium requirements for growth of 0.05, 0.025, and 0.05 μg Se/g diet, respectively. Liver glutathione peroxidase (GPX) 1 activity fell in all species to <4% of selenium-adequate levels, plasma GPX3 activity fell to <3% in all species except for mice, and liver GPX4 activity fell to <10% in avians but only to ∼50% of selenium-adequate levels in rodents. Selenium-response curves for these biomarkers reached well-defined plateaus with increasing selenium supplementation in all species, collectively indicating minimum selenium requirements of 0.06–0.10 μg Se/g for rats, mice, and lambs but 0.10–0.13 μg Se/g for chicks and 0.23–0.33 μg Se/g for turkeys. In contrast, increasing dietary selenium did not result in well-defined plateaus for erythrocyte GPX1 activity and liver selenium in most species. Selenium-response curves for GPX1 mRNA for rodents and avians had well-defined plateaus and similar breakpoints. GPX4 mRNA was not significantly regulated by dietary selenium in rodents, but GPX4 mRNA in avians decreased in selenium deficiency to ∼35% of selenium-adequate plateau levels. Notably, no selenoprotein activities or mRNA were effective biomarkers for supernutritional selenium status. Robust biomarkers, such as liver GPX1 and plasma GPX3 activity for selenium, should be specific for the nutrient, fall dramatically in deficiency, and reach well-defined plateaus. Differences in biomarker-response curves may help researchers better understand nutrient metabolism and targeting of tissues in deficiency, thus to better characterize requirements.
Keywords: glutathione peroxidase, mRNA, recommended dietary allowances, requirement, selenoprotein P, transcripts, vitamin E
Introduction
The series of DRIs for humans from the Institute of Medicine now include RDAs or Adequate Intakes for 15 elements (1–3). Scrutiny of the reports supporting these recommendations indicates that the majority of these recommendations are based on factorial modeling or analysis, balance data, median intake, average mineral content of foods, or energy intake, whereas only 2 recommendations—for selenium and copper—use metal-dependent enzyme activity as the key biomarkers for assessment of nutrient status. For animals, the estimated nutrient requirements recommended by the NRC are generally stated as minimum dietary requirements and given as dietary concentration provided on an as-fed basis (4). These animal requirements are largely based on dietary concentrations sufficient for maximal growth but also may involve a functional measure such as bone mineral content (calcium, phosphorus, magnesium), serum mineral concentration (magnesium, copper), and/or metalloprotein activity or concentration (copper, iron, molybdenum, selenium), with 4 functional measures cited for copper in rats (platelet cytochrome C oxidase, serum ceruloplasmin, plasma copper, copper-zinc–dependent superoxide dismutase). For selenium, levels of glutathione peroxidase (GPX), selenoprotein P (SEPP), and thyroxine deiodinase, as well as protection against sucrose-induced deterioration of the microvasculature of the retina, were cited as criteria for setting the minimum selenium requirement in rats (4). The bases for dietary upper limit/toxicity levels in both animals and humans are even more subjective. Collectively, these recommendations for both humans and animals suggest that these dietary requirement recommendations should be revisited to take advantage of both newly emerging biomarkers and the increased understanding of the homeostasis of essential elements.
For selenium, we conducted a series of studies that used multiple graded levels of dietary selenium in weanling rats and mice, in day-old turkeys and chickens, and in 2-d-old lambs, all focused on growth plus the use of biomarkers for assessment of selenium status and requirements. These biomarker studies initially used GPX activity in a variety of tissues to demonstrate biochemical selenium deficiency and to determine minimum dietary selenium requirements (5). As part of exploring the underlying mechanism for selenium regulation of GPX activity, we found that GPX protein could also be used as a biomarker for selenium status (6). That led us to investigate GPX mRNA level, which we surprisingly found to be also regulated by selenium status and which could be used as a good biomarker for selenium status (7). Studies in rodents found that both activity and mRNA levels of a second GPX, phospholipid hydroperoxide GPX (GPX4), were differentially regulated by selenium status, showing a hierarchy of selenium requirements depending on which biomarker is selected (8). Differential incorporation of selenium into selenoproteins, first described by Behne et al. (9), was also noted by other investigators as new selenoproteins were discovered (10–13). These additional selenoproteins expanded the array of both biochemical and transcript-based biomarkers of selenium status in our studies (14–18). The identification of the complete selenoproteome (19) further expanded the number of selenoprotein transcripts as “molecular biomarkers” for the assessment of selenium status and requirements in rodents (20–23) and is helping researchers to better understand the hierarchy of selenium incorporation into selenoproteins.
The apparent higher selenium requirement of the turkey than of rodents has been quantitatively documented for >30 y (24). To explore selenium requirements in turkeys, we recently completed the sequencing of the turkey selenoprotein transcriptome (25), and now have used these sequences to assess selenium status in turkey poults (26). We also conducted a parallel study in chicks at the same time to further explore species differences in selenium requirements and the hierarchy of selenium status (27).
Thus, presented here is a direct comparison of our studies in 5 species. The purpose is to show by direct comparison the differences and similarities of biomarker levels and selenium requirements in response to graded levels of dietary selenium, and to gain insights into the setting of dietary nutrient requirements with an emphasis on selenium.
Methods
These studies were conducted with the use of selenium-deficient torula-yeast semipurified diets of essentially identical composition and prepared from virtually the same components. Diets were supplemented with multiple graded levels of dietary selenium, provided as inorganic sodium selenite. In addition, the analyses of biomarkers were conducted by identical protocols or by methods shown to yield identical results.
Selenium biomarker data for rats are from the report of Barnes et al. (20), which used the basal selenium-deficient 30% torula-yeast diet containing 0.007 μg Se/g diet supplemented with graded levels of dietary selenium from 0 to 0.8 μg Se/g as Na2SeO3. Vitamin E was provided at 100 mg/kg diet. Weanling 21-d-old male rats were housed in hanging wire cages and fed these diets for 28 d. All other aspects of the study have been previously described in detail (20).
For mice, the selenium biomarker data are from the report of Ferguson-Kohout (28) and the preliminary report of Ferguson-Kohout and Sunde (29). This study used the basal selenium-deficient 30% torula-yeast diet containing 0.007 μg Se/g diet and supplemented with graded levels of dietary selenium from 0 to 0.5 μg Se/g as Na2SeO3. Vitamin E was provided at 100 mg/kg diet. For growth data, weanling 21-d-old male mice (Swiss-Webster, Hsd:ND4; Harlan Sprague Dawley) were housed in hanging wire cages and fed these diets for 28 d. To obtain data for selenium molecular biomarkers, wild-type mice congenic to the GPX1−/− deletion strain described by Ho et al. (30) were fed the same diets. These studies were conducted concurrently with a rat study, and the determinations of selenium concentrations in the liver and diet; of GPX1, GPX4, and GPX3 activities; and of GPX1, GPX4, and SEPP1 transcript levels were all conducted as previously described in detail (15). Additional data are from a study that used the same mouse strain supplemented with 0, 0.05, and 0.2 μg Se/g as Na2SeO3 (22).
Selenium biomarker data for turkey poults are from the report of Taylor and Sunde (26), which used a basal selenium-deficient 30% torula-yeast diet supplemented with 7% crystallized amino acids and with graded levels of dietary selenium from 0 to 1.0 μg Se/g as Na2SeO3. The diet provided ∼150% of the NRC recommendations for vitamins and minerals, with the exception of selenium and vitamin E. Vitamin E was provided at 150 mg/kg diet as all-rac-α-tocopheryl acetate, so changes in biomarkers were due only to changes in selenium status. Day-old male poults were housed in battery cages with raised wire floors and 24-h lighting and fed these diets for 28 d. All other aspects of the study were previously described in detail (26). Liver selenium concentration data are from our previous turkey study (31).
For chicks, the selenium biomarker data are from the report of Li and Sunde (27), which used the basal selenium-deficient 30% torula-yeast diet supplemented with 7% crystallized amino acids and with graded levels of dietary selenium from 0 to 1.0 μg Se/g as Na2SeO3. The only difference between the chick and turkey diets was a minor adjustment to the concentrations of calcium, zinc, and manganese to better match the NRC requirements (32). Day-old male chicks were housed in battery cages with raised wire floors and 24-h lighting and fed these diets for 29 d. All other aspects of the study were previously described in detail (27).
For lambs, the selenium biomarker data are from the report of Oh et al. (33), which used the basal selenium-deficient torula-yeast liquid diet (20% solids, with 60% torula-yeast on an air-dried solids basis) containing 0.01 μg Se/g diet and supplemented with graded levels of dietary selenium from 0 to 0.5 μg Se/g as Na2SeO3 on an air-dried solids basis. Vitamin E was provided at 33 mg/kg diet. Two-day-old male lambs were housed in galvanized steel cages and fed these diets for 56 d. The determinations of selenium concentration in the liver and diet, GPX1 activity in the liver and RBCs, and GPX3 activity in plasma were all conducted as previously described in detail (33); for presentation here, the activity values determined by using the stop-analyze assay for GPX in lambs (5) were converted to our coupled-assay units with the use of a factor of 5.5 coupled-assay/stop-analyze enzyme units (34).
Data are presented as means ± SEMs. For growth and enzyme analysis and for mRNA expression, n = 4–5 and 3–5 animals/dietary selenium treatment, respectively, were used. The detailed significance of biomarker differences within an experiment is described in the original publications; for each biomarker, the resulting ANOVA P values and the levels of dietary selenium that were significantly different from plateau values are given in Table 1. An “Se-response curve” was constructed by using sigmoidal or hyperbolic regression analysis (Sigma Plot; Jandel Scientific) with the use of all individual values at each dietary selenium treatment, as described previously (14, 18, 20). To allow direct comparison of species, selenium-response curves for selenium biomarkers that reached well-defined plateaus [indicated by nonsignificant differences in at least the highest 4 (2 for lambs) dietary selenium supplementation levels] were recalculated and redrawn with plateaus of zero slope at a level equal to the mean of individual values in treatment groups on the plateau. The “plateau breakpoint” for each selenium-response curve, defined as the intersection of the line tangent to the point of steepest slope and the plateau, was calculated as described previously (14, 18, 20) to estimate the “minimum dietary Se requirement” necessary to obtain the plateau response.
TABLE 1.
Minimum selenium requirements and level of biomarker expression in selenium deficiency for 5 species1
| Enzyme activity |
Liver transcript levels |
||||||||
| Growth rate | Liver Se | Plasma GPX3 | RBC GPX1 | Liver GPX1 | Liver GPX4 | GPX1 mRNA | GPX4 mRNA | SEPP1 mRNA | |
| Rat (20) | |||||||||
| Selenium requirement,2 μg Se/g | <0.007 | 0.08 | 0.06 | 0.08 | 0.09 | 0.06 | 0.07 | <0.01 | 0.04 |
| Selenium adequate,3 % | — | 2.8 (1.6) | 2.0 | 28.8 (14.6) | 2.0 | 47.0 | 10.0 | — | 52.5 |
| P4 | 0.88 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.1000 | 0.0008 |
| Signif. low selenium,5 μg Se/g | NS | ≤0.04 | ≤0.06 | ≤0.04 | ≤0.08 | ≤0.06 | ≤0.04 | NS | ≤0.02 |
| Mouse (22, 28, 29) | |||||||||
| Selenium requirement,2 μg Se/g | <0.007 | 0.11 | 0.05 | 0.05 | 0.09 | 0.06 | 0.04 | <0.01 | <0.01 |
| Selenium adequate,3 % | — | 9.9 | 37.1 | 55.8 (37.3) | 3.3 | 55.4 | 20.1 | — | — |
| P4 | 0.20 | <0.05 | 0.05 | <0.0001 | <0.0001 | <0.006 | 0.017 | 0.13 | 0.89 |
| Signif. low selenium,5 μg Se/g | NS | ≤0.08 | ≤0.02 | ≤0.02 | ≤0.08 | ≤0.02 | ≤0.02 | NS | NS |
| Turkey (26, 31) | |||||||||
| Selenium requirement,2 μg Se/g | 0.05 | 0.20 | 0.29 | 0.29 | 0.33 | 0.23 | 0.07 | 0.08 | 0.05 |
| Selenium adequate,3 % | 64.4 | 14.9 (10.4) | 2.3 | 37.0 | 3.2 | 6.7 | 35.0 | 29.06 | 36.06 |
| P4 | <0.05 | <0.0001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.04 | 0.13 | 0.18 |
| Signif. low selenium,5 μg Se/g | ≤0.025 | ≤0.10 | ≤0.20 | ≤0.10 | ≤0.20 | ≤0.10 | ≤0.03 | NS | NS |
| Chick (27) | |||||||||
| Selenium requirement,2 μg Se/g | 0.025 | — | 0.11 | 0.30 | 0.13 | 0.10 | 0.11 | 0.07 | 0.09 |
| Selenium adequate,3 % | 48.1 | — | 2.6 | 9.44 (5.56) | 1.8 | 9.7 | 39.4 | 35.5 | 44.7 |
| P4 | <0.05 | — | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0007 | 0.0001 | 0.013 |
| Signif. low selenium,5 μg Se/g | 0 | — | ≤0.08 | ≤0.20 | ≤0.10 | ≤0.20 | ≤0.10 | ≤0.08 | ≤0.05 |
| Lamb (33) | |||||||||
| Selenium requirement,2 μg Se/g | 0.05 | 0.10 | 0.10 | 0.10 | 0.10 | — | — | — | — |
| Selenium adequate,3 % | 61.2 | 26.8 (8.8) | 1.3 | 39.4 (28.1) | 1.0 | — | — | — | — |
| P4 | <0.01 | <0.01 | <0.05 | <0.05 | <0.05 | — | — | — | — |
| Signif. low selenium,5 μg Se/g | 0 | ≤0.10 | ≤0.05 | ≤0.10 | ≤0.05 | — | — | — | — |
The corresponding reference number(s) for original data are shown in parentheses after each species name. GPX, glutathione peroxidase; SEPP1, selenoprotein P1; Signif., significantly.
For each species (in rows) and each biomarker (in columns), the minimum selenium requirement determined as the breakpoint in the selenium-response curve as described in text.
Impact of selenium deficiency is expressed as a percentage of the selenium-adequate plateau; for biomarkers that did not reach a plateau, the value is a percentage of the expression level at the breakpoint and the value in parentheses is the percentage of the highest value.
Derived by using 1-factor ANOVA on the biomarker for all levels of selenium supplementation.
Dietary selenium levels resulting in biomarker values significantly different from selenium-adequate plateau values; NS indicates that the biomarkers were not significantly different from the selenium-adequate plateau at any level of selenium supplementation.
Selenium-deficient value is significantly different (t test) from the 0.4-μg Se/g value for liver GPX4 mRNA (P = 0.023) and SEPP1 mRNA (P = 0.029).
Results
Growth
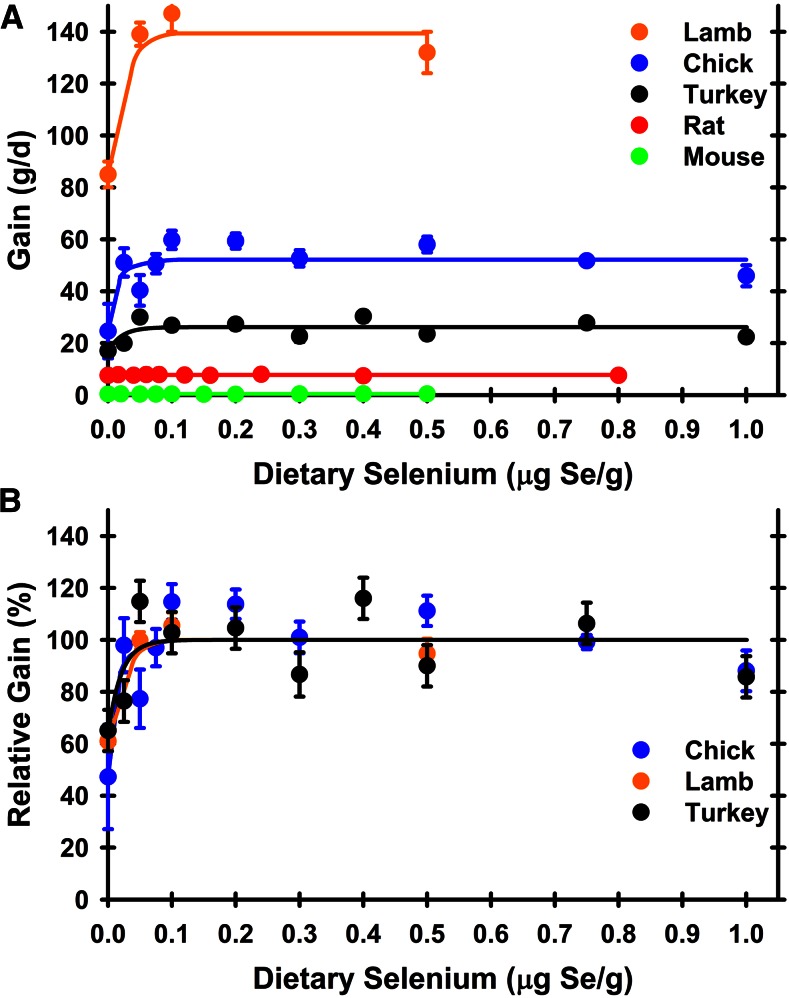
For these studies, offspring were obtained from dams supplemented with standard levels of dietary selenium, and thus with usual initial body stores. Rats and mice had mean gains of 7.7 and 0.41 g/d in these studies, respectively, over 28 d (Figure 1, Table 1), but there was no significant effect of dietary selenium supplementation on final body weight nor on daily gain (P = 0.88 and 0.20 for rats and mice, respectively), indicating that the minimum selenium requirement for growth was <0.007 μg Se/g diet for rodents. In contrast, there was a significant effect of dietary selenium level on growth for turkey poults and chicks (Figure 1). For turkey poults, there was no significant effect on final body weight after 28 d (P = 0.61), but for growth from day 7 to 28, poults fed 0 and 0.025 μg Se/g diet had a significantly lower rate of growth (17.0 and 19.9 g/d, respectively) compared with a mean growth of 26.4 g/d for all other groups, yielding a minimum dietary selenium requirement of 0.05 μg Se/g diet for male turkey poults. For chicks, supplementation with ≥0.25 μg Se/g diet resulted in a mean growth of 52 g/d, whereas chicks fed the selenium-deficient diet averaged 25 g/d; the minimum dietary selenium requirement was thus 0.025 μg Se/g for chicks. Lambs supplemented with ≥0.05 μg Se/g diet showed a mean gain of 139 g/d, whereas lambs fed the selenium-deficient diet gained only 85 g/d over 56 d, yielding a minimum dietary selenium requirement of 0.05 μg Se/g for lambs.
FIGURE 1.
Effect of dietary selenium on growth rate. (A) Daily weight gain is plotted for the indicated graded levels of dietary selenium in rats, mice, turkeys, chickens, and lambs, determined as described in the text. Selenium-response curves were calculated as described in the text. (B) Relative daily weight gain, expressed as a percentage of the selenium-adequate plateau level, is plotted for turkeys, chickens, and lambs. Values are means ± SEMs, n = 4–5 animals/dietary selenium treatment. Significance for each biomarker is shown in Table 1.
Selenium-adequate biomarker levels
Selenium-response curves for liver GPX1 activity, liver GPX4 activity, and plasma GPX3 activity in all species reached well-defined plateaus with increasing selenium supplementation (Figure 2). Comparison of the selenium-adequate plateau levels indicates that liver GPX1 levels in turkeys and chickens are ∼10% of levels in rodents, whereas liver GPX4 levels in avian liver are ∼6 times the levels in rodent liver (Table 2). Selenium-adequate levels of plasma GPX3 activity in mice and lambs are ∼50% of levels in rats, chickens, and turkeys. For selenium biomarkers with defined plateaus, selenium-response curves were drawn relative to plateau levels to allow direct comparison between species. The defined plateaus also clearly demonstrate that supernutritional selenium supplementation does not further increase these biomarkers.
FIGURE 2.
Effect of dietary selenium on relative liver GPX1 (A), plasma GPX3 (B), and liver GPX4 (C) activities. Selenium-response curves for liver GPX1, plasma GPX3, and liver GPX4 activity, expressed relative to the selenium-adequate plateau level, are plotted for the indicated graded levels of dietary selenium in rats, mice, turkeys, chickens, and lambs (only liver GPX1 and plasma GPX3). Values are means ± SEMs, n = 4–5 animals/dietary selenium treatment. Significance for each biomarker is shown in Table 1. Selenium-response curves were calculated as described in the text. GPX, glutathione peroxidase.
TABLE 2.
Biomarker expression levels in selenium-adequate animals for 5 species1
| Enzyme activity, EU/g protein |
|||||
| Species | Liver selenium, nmol/g | Plasma GPX3 | RBC GPX1 | Liver GPX1 | Liver GPX4 |
| Rat (20) | 6.3 ± 0.32 (12.6 ± 1.0) | 101 ± 4 | 219 ± 52 (434 ± 12) | 834 ± 53 | 9.8 ± 06 |
| Chick (27) | — | 121 ± 15 | 83.7 ± 5.82 (142 ± 7) | 127 ± 5 | 57.7 ± 5.9 |
| Lamb (33) | 2.3 ± 0.22 (7.1 ± 0.3) | 58.9 ± 3.6 | 390 ± 182 (547 ± 32) | 265 ± 56 | — |
| Turkey (26, 31) | 3.9 ± 0.32 (5.6 ± 0.2) | 112 ± 6 | 81.6 ± 4.9 | 89.9 ± 6.9 | 62.4 ± 3.8 |
| Mouse (22, 28, 29) | 12.6 ± 0.7 | 52.6 ± 11.9 | 195 ± 242 (291 ± 10) | 860 ± 43 | 9.5 ± 0.8 |
Values are mean ± SEM selenium-adequate plateau levels, n = 3–6 animals/dietary selenium treatment. The corresponding reference number(s) for original data are shown in parentheses after each species name. EU, enzyme units; GPX, glutathione peroxidase.
Biomarker that did not plateau with increasing selenium supplementation: expression level at plateau breakpoint of selenium-response curve; the maximum expression level is indicated in parentheses.
In contrast to selenium biomarkers in the liver and plasma, increasing selenium supplementation in these young animals did not result in well-defined plateaus for RBC GPX1 activity in rats, mice, chicks, or lambs (Figure 3). Instead, there was an initial steep increase with increasing dietary selenium, followed by a distinct transition (breakpoint) to a second phase where the selenium-response curve slope was ∼50% of the initial slope. At the breakpoint, RBC GPX1 activities in turkeys and chickens were ∼40% and 20% of RBC GPX1 activities in selenium-adequate rodents and lambs, respectively (Table 2). Selenium-response curves for liver selenium concentration also did not reach well-defined plateaus in rats, turkeys, or lambs. At the breakpoint, liver selenium concentration ranged from 2.3 to 12.6 nmol/g tissue. In animals fed 0.5 μg Se/g diet, the liver selenium concentration in turkeys and chickens was ∼50% of that in rodents.
FIGURE 3.
Effect of dietary selenium on RBC GPX1 activity (A) and liver selenium (B). Selenium-response curves for RBC GPX1 activity, expressed as EU/g protein, and liver selenium concentration, expressed as nmol/g wet weight, are plotted for the indicated graded levels of dietary selenium in rats, mice, turkeys, chickens, and lambs. Values are means ± SEMs, n = 4–5 animals/dietary selenium treatment. Significance for each biomarker is shown in Table 1. Selenium-response curves were calculated as described in text. EU, enzyme units; GPX, glutathione peroxidase; prot, protein.
Liver GPX1 activity.
Liver GPX1 activity was measured by using H2O2 as the oxidized substrate in all species, but in turkeys and chickens the specific GPX1 activity was corrected for the high level of GPX4 activity (31). Liver GPX1 activity fell uniformly to 1–3% of selenium-adequate levels in all species (Figure 2, Table 1). The selenium-response curves for all species except for mice were sigmoidal. Minimum dietary selenium requirements for liver GPX1 activity, as determined by breakpoint analysis, were uniformly 0.09–0.10 μg Se/g for rats, mice, and lambs, slightly higher at 0.13 μg Se/g for chicks, but 2–3 times higher than the other species at 0.33 μg Se/g for the turkey poults. Again, supernutritional selenium supplementation did not further increase liver GPX1 activity in all species.
Plasma GPX3 activity.
In all studies, plasma GPX3 activity was measured by using H2O2 as the substrate. In animals fed the selenium-deficient diet, plasma GPX3 activity fell to 1–3% of plateau levels in all species except for mice; mouse GPX3 activity in selenium deficiency only decreased to 37% of plateau levels (Figure 2, Table 1). Increasing supplemental selenium resulted in hyperbolic selenium-response curves for rats and turkeys, whereas definite sigmoidal selenium-response curves were observed for mice, chicks, and lambs. Notably, the breakpoint for turkey GPX3 activity was 0.29 μg Se/g diet compared with 0.11 and 0.10 for chicks and lambs and 0.06 and 0.05 for rats and mice, respectively. For all species, selenium supplementation above the breakpoint of the selenium-response curves did not further increase GPX3 activity.
Liver GPX4 activity.
GPX4 activity was assayed by using phosphatidylcholine hydroperoxide (PCOOH), which is not a substrate for GPX1. The impact of selenium status on liver GPX4 was distinctly different in rodents compared with avians. In rats and mice, liver GPX4 activity decreased only to ∼50% of selenium-adequate levels, whereas liver GPX4 activity fell to 7–10% of selenium-adequate levels in turkeys and chicks (Figure 2, Table 1). Liver GPX4 activity in the lamb study conducted in 1973 was not assayed. Minimum selenium requirements for liver GPX4 activity were 0.06–0.10 μg Se/g for rats, mice, and chicks, whereas the requirement of liver GPX4 activity in the turkey was more than double at 0.23 μg Se/g diet. Supernutritional selenium supplementation above the requirement also did not further increase liver GPX4 activity in rodents or avians.
RBC GPX1 activity.
GPX1 activity in freshly hemolyzed RBCs was assayed by using H2O2. In contrast to the other selenoenzyme activities, selenium-response curves for RBC GPX1 activity in these young animals did not reach well-defined plateaus with increasing selenium supplementation for rats, mice, chicks, or lambs (Figure 3, Table 1). For mice, rats, lambs, and turkeys, the selenium-response curves were hyperbolic, with an initial steep increase in GPX1 activity with increasing dietary selenium, followed by a distinct transition (breakpoint) to a second phase where the GPX1 activity slope was ∼50% of the initial slope. For chicks, the selenium-response curve was distinctly sigmoidal but was otherwise similar to the other species; in addition, RBC GPX1 activity in the selenium-deficient chicks was 9% of the activity at the breakpoint, whereas RBC GPX1 activities in selenium-deficient rats, turkeys, lambs and mice were 22–56% of the activity at the breakpoint. Selenium-response curve breakpoints indicated that the minimum dietary selenium requirement was 0.05–0.10 μg Se/g diet for mice, rats, and lambs, but 0.29–0.30 μg Se/g diet for turkeys and chicks.
Liver selenium concentration.
Liver selenium concentrations reached a well-defined plateau in young mice but did not reach a plateau with increasing selenium supplementation in rats, turkeys, and lambs (Figure 3, Table 1). The selenium-response curve in mice was hyperbolic with a well-defined breakpoint at 0.11 μg Se/g diet. For turkeys and lambs, liver selenium-response curves were also hyperbolic, but with breakpoints at 0.2 and 0.1 for turkeys and lambs, respectively, which was followed by slower increases in liver selenium. The rat selenium-response curve for liver selenium was sigmoidal, with a plateau from 0.1–0.24 μg Se/g diet, which was followed by a slower increase to a maximum selenium concentration at the same level as the plateau in the mouse liver selenium concentration.
Molecular biomarkers
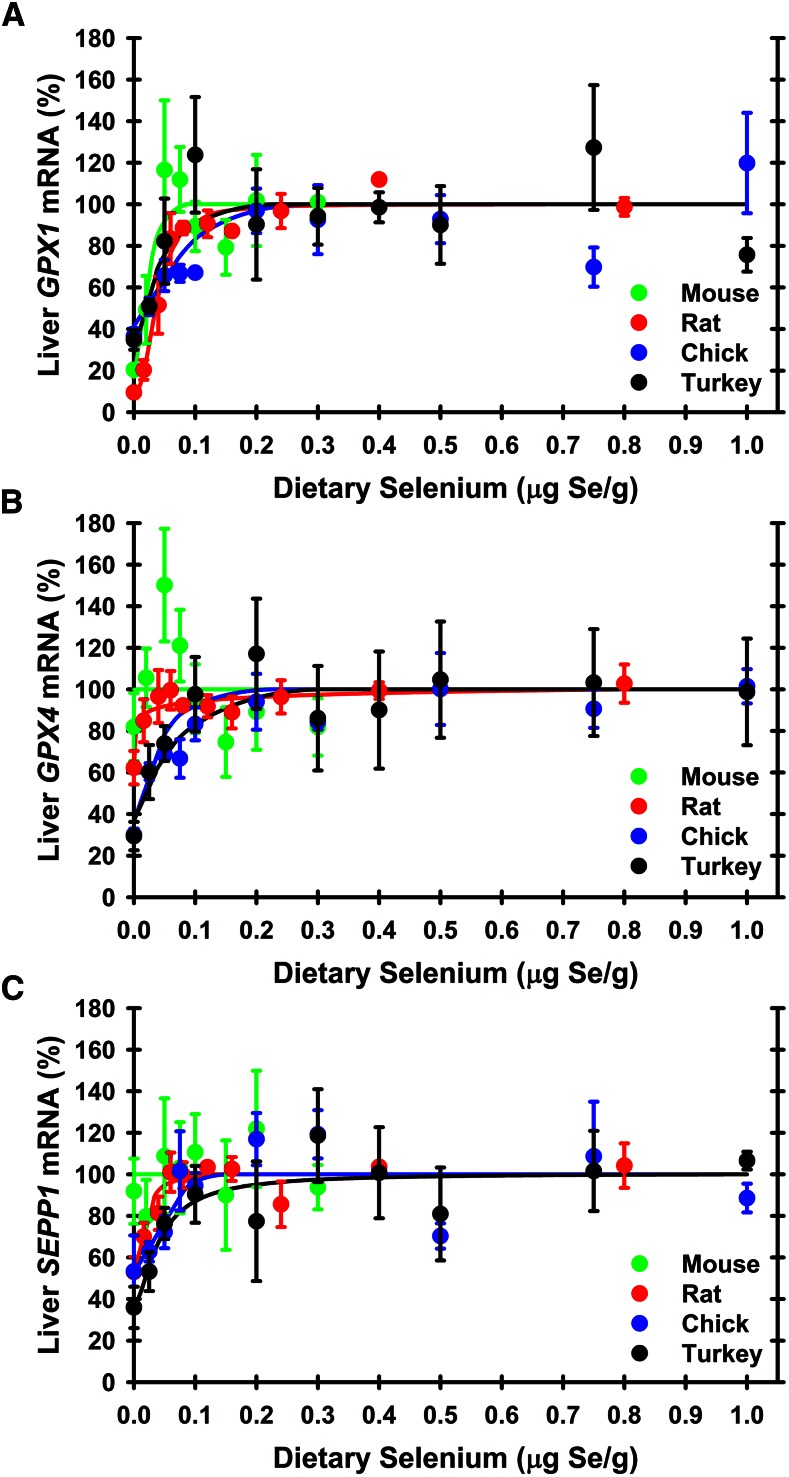
Levels of several selenoprotein transcripts (mRNA) are regulated by selenium status and can be used as molecular biomarkers of selenium status (35–37). The selenium-response curves for GPX1 mRNA are relatively similar for rodents and avians, with well-defined plateaus and similar breakpoints (Figure 4, Table 1). Liver GPX1 mRNA levels in selenium-deficient animals were 10%, 20%, 35%, and 49% of selenium-adequate levels in rats, mice, turkeys, and chicks, respectively, with minimum selenium requirements of 0.04–0.11 μg Se/g diet.
FIGURE 4.
Effect of dietary selenium on relative liver GPX1 (A), GPX4 (B), and SEPP1 (C) transcript levels. Selenium-response curves for liver GPX1, GPX4, and SEPP1 mRNA, expressed relative to the selenium-adequate plateau level, are plotted for the indicated graded levels of dietary selenium in rats, mice, turkeys, and chickens. Values are means ± SEMs, n = 3–5 animals/dietary selenium treatment. Significance for each biomarker is shown in Table 1. Selenium-response curves were calculated as described in text. GPX, glutathione peroxidase; SEPP, selenoprotein P.
In contrast to GPX1 transcripts, selenium-response curves for liver GPX4 mRNA levels are distinctly different for avians compared with rodents. GPX4 mRNA levels were not significantly regulated by selenium status in mice and rats. In avians, liver GPX4 mRNA in selenium-deficient chicks was significantly downregulated to 35% (P = 0.0001), with a breakpoint of 0.07 μg Se/g diet; and liver GPX4 mRNA in selenium-deficient turkeys was downregulated [P = 0.13 (ANOVA); 0 compared with 0.4 mg Se/g, P = 0.023 (t test)] to 29%, with a breakpoint of 0.08 μg Se/g diet.
The selenium regulation pattern for liver SEPP1 mRNA was similar to that for GPX4 mRNA. Liver SEPP1 mRNA was not significantly regulated by selenium status in mice, and only decreased to 53% of selenium-adequate plateau levels in rats. Selenium-response curves for SEPP1 mRNA in chicks also decreased moderately and significantly (P = 0.013) to 45% of selenium-adequate levels with a minimum selenium requirement of 0.09 μg Se/g diet; in turkeys, SEPP1 mRNA decreased [P = 0.18 (ANOVA); 0 compared with 0.4 μg Se/g, P = 0.029 (t test)] to 36% of selenium-adequate levels with a minimum selenium requirement of 0.05 μg Se/g diet.
Discussion
Robust biomarkers—liver GPX1 and plasma GPX3 activity.
This series of studies clearly show that there are major differences in selenium metabolism and requirements between mammals and birds, as well as distinct differences within these clades (Table 1). In these studies, liver GPX1 activity fell dramatically in selenium deficiency in all species and reached distinct plateaus with supplemental selenium. By using this biomarker, avians showed distinctly higher dietary selenium requirements than mammals; the turkey selenium requirement is 3 times the mammalian selenium requirement and the chicken selenium requirement is 1.3–1.4 times the mammalian selenium requirement, whereas the rat, mouse, and lamb requirements are very similar. A similar hierarchy of selenium requirements was observed on the basis of plasma GPX3 activity, which also fell dramatically with selenium deficiency in all species except for mice and which reached distinct plateaus for all species. Applying the selenium-response curve approach to studies conducted by Lei et al. (38) in 4- to 6-wk-old weanling pigs fed 0–0.5 μg Se/g yielded level selenium-adequate plateaus for liver GPX1 and plasma GPX3 activity with minimum selenium requirements of 0.2 and 0.1 μg Se/g diet, respectively. Collectively, these selenoenzyme biomarkers—liver GPX1 and plasma GPX3—were the most robust indicators (see below) of selenium status and selenium requirements among the biomarkers evaluated here.
GPX4 activity.
The other conventional biomarkers studied here were less robust. Liver GPX4 activity, assayed by using the specific substrate PCOOH, also reached distinct plateaus in rodents and birds and fell to <10% of plateau activity in selenium-deficient birds, but only decreased to ∼50% of plateau activity in rodents. Underlying these differences, liver GPX4 activity was 6-fold higher in birds compared with rodents, and liver GPX4 mRNA levels were not downregulated significantly by selenium deficiency in rodents.
Liver selenium.
Liver selenium and RBC GPX1 activity as primary selenium biomarkers are more problematic. In young animals, liver selenium continued to increase with supplemental selenium above the breakpoints on the selenium-response curves for all species except for mice. This occurs even in monogastric rodents that were supplemented with inorganic selenium (selenite) to prevent nonspecific incorporation of organic selenium (selenomethionine) into protein (39). In addition, only in rat liver—the organ targeted by selenium deficiency in the rat—did liver selenium fall to <5% of selenium-adequate levels. Although rats and mice have comparable levels of GPX1 and GPX4 activity, liver selenium in selenium-deficient mice only decreased to 10% of selenium-adequate levels. The turkey minimum selenium requirement, based on the breakpoint in the liver selenium-response curve, was double that of rodents, indicating that proportionately less selenium is retained in liver in turkeys relative to rodents when dietary selenium is limiting (Table 1).
RBC GPX1 activity.
RBC GPX1 activity as a selenium biomarker is also problematic, because it continued to increase with supplemental selenium above the selenium-response curve breakpoints for all species except for turkeys. For all species, apparent RBC GPX1 activities also decreased less dramatically than liver GPX1 activities in selenium deficiency; this is most likely due in part because other non-GPX1 enzyme activities contribute to the activity measured by using H2O2 as the oxidized substrate. Despite these limitations, apparent selenium requirements based on the selenium-response curve breakpoints for RBC GPX1 activity in turkeys and chickens were 3 times the mammalian selenium requirements.
Plasma SEPP1.
Plasma SEPP1 also has potential as a selenium biomarker. Studies that repleted selenium-deficient humans in China found that the selenium requirement based on plasma SEPP1 was 50% higher than the requirement based on plasma GPX3 activity (40). Liver is the major source of plasma SEPP1 in rodents and humans (41, 42). In rats, plasma SEPP1 levels as well as plasma GPX3 and liver GPX1 activity all decreased to <5% of selenium-adequate levels when rats were fed a selenium-deficient diet (43). Selenium-response curves, with a linear dietary selenium axis from 0 to 2 μg Se/g diet (as selenate), indicates that the dietary selenium requirement in these rats was 0.1 μg Se/g diet on the basis of plasma SEPP1 concentration as well as plasma GPX3 and liver GPX1 activities. Plasma SEPP1 levels at 2 μg Se/g diet, however, were 140% of levels at 0.1 and 0.5 μg Se/g diet, whereas plasma GPX3 and liver GPX1 activities did not increase, showing that plasma SEPP1 protein does not reach a plateau, at least in rats (43). The lack of dramatic downregulation of SEPP1 transcripts in selenium deficiency, as well as the potential to incorporate variable quantities of selenium (1–10 selenocysteines) per SEPP1 molecule (44), may further restrict the value of plasma SEPP1 as a selenium biomarker. Additional studies are needed to better understand the impact of dietary selenium per se on selenium metabolism and the accompanying selenium biomarkers.
Resulting insights.
The selenium-response curves for the above 5 biomarkers illustrate necessary components for robust nutrient biomarkers for assessing deficiency and minimum dietary requirements. 1) The biomarker must be specific for the nutrient; clearly, this is the case for selenium-dependent enzymes as well as tissue selenium concentrations. 2) In truly deficient subjects, biomarker levels should fall substantially below adequate levels to allow for precision in distinguishing adequate from deficient status; this is also the case for the above 5 biomarkers. 3) Importantly, well-defined selenium-adequate plateaus provide important robustness for a biomarker, because level plateaus indicate that nutrient availability is no longer the factor limiting expression and are defined by the animal’s innate metabolism rather than the investigator. Plasma GPX3 activity, liver GPX1 activity, and liver GPX4 activity met all 3 of these criteria. Biomarkers that decrease in deficiency but continue to increase with increasing dietary supplementation instead rely on arbitrary decisions made by the investigator rather than innately by the animal. 4) Last, although not illustrated by the studies presented here, the biomarker must be shown to be efficacious throughout the life cycle, in health and disease. For instance, levels of liver GPX1 mRNA and activity in selenium-adequate pregnant rats declined to ∼50% of levels in nonpregnant rats, illustrating the need to fully characterize biomarker expression at all stages of the life cycle (18). Similarly, thyroxine deiodinase activity is also regulated by iodine status (via thyroid stimulating factor), at least in thyroid (45), complicating the use of thyroxine deiodinase as a robust selenium biomarker.
Growth and tissue stores.
The selenium requirements based on growth in these young animals were distinctly less than requirements based on selenoenzyme activity. For weanling rodents allowed to nurse on selenium-adequate dams for 21 d, the resulting selenium stores were sufficiently high so that there was no impact of selenium deficiency on growth. Note that second-generation selenium-deficient rats showed a selenium requirement for growth (16). In the study in pigs cited earlier (38), the weanling pigs were fed a selenium-deficient diet for 10 d before the start of the study, which may have elevated the resulting selenium requirements. These differences also illustrate the importance of initial nutrient stores, and of starting dietary requirement studies in animals with adequate nutrient status; otherwise, the apparent requirement is for repletion, not maintenance.
Molecular biomarkers.
Subsets of the selenoprotein transcripts can be used as molecular biomarkers in rodents (20) and in avians (26, 27). In selenium deficiency, GPX1 transcript levels fell to 10–20% of selenium-adequate levels in rodents and to 35–39% in birds (Figure 4, Table 1). The resulting minimum selenium requirements based on the GPX1 transcript levels were spread closely over a ∼2-fold range, extending from 0.04 μg Se/g diet for mice to 0.11 μg Se/g diet for chickens; in all cases, the minimum selenium requirement based on GPX1 transcript level was less than the requirement based on GPX1 activity in liver. GPX4 mRNA was not significantly regulated in rodents but was regulated by selenium status in birds (with higher levels of GPX4 activity). At least for rodents and avians, selenoenzyme transcript levels can serve as molecular biomarkers that can be used to identify selenium-deficient animals, but the resulting minimum selenium requirements are lower than those determined by using selenoenzyme activity.
Supernutritional biomarkers.
One of the underlying objectives of our studies was to identify biomarkers for supernutritional and toxic selenium status. The resulting selenium-response curves, which extend to 0.8, 1.0, and 1.0 μg Se/g diet for the rat, turkey, and chicken studies described here, however, found that most selenoprotein biomarkers reached well-defined plateaus (described here: GPX1 and GPX4 activity in liver; GPX3 activity in plasma; GPX1, GPX4, and SEPP1 mRNA in liver). Additional studies in rats fed up to 5 μg Se/g diet also failed to identify good selenoprotein-based biomarkers for supernutritional and toxic selenium status. The continued increase in RBC GPX1 activity above the breakpoint does not appear to reach a well-defined plateau in young rodents and chickens, nor in adult rats (5), further complicating the use of RBC GPX1 activity as a primary biomarker for high selenium status. Thus, selenoprotein biomarkers cannot be used effectively as indicators of high selenium status. By using transcriptomics, however, we identified in rats a limited number of nonselenoprotein transcripts that have potential as biomarkers for supernutritional and toxic selenium status (23, 46).
Multiple graded selenium levels.
A number of other studies in various species reported that >75% of selenoprotein transcripts examined were downregulated significantly by selenium deficiency when just a selenium-deficient and a selenium-adequate group were compared (47–50), and several reported that the majority of selenoprotein transcripts examined were downregulated significantly by selenium deficiency when 3 groups (selenium-deficient, selenium-adequate, high-selenium) were studied (51, 52). In contrast, when multiple levels of selenium supplementation were used, resulting in well-defined biomarker plateaus, far fewer selenoprotein transcripts were significantly altered by selenium deficiency (20), especially in avian studies that used commercial (outbred) strains (26, 27). This shows that statistical significance may not equate with biological significance and shows the importance of using multiple graded levels of selenium to establish whether or not a parameter will be a good biomarker for selenium status.
Tissue selenium and selenomethionine.
The slow increase in liver selenium concentrations above the breakpoint in the selenium-response curves indicates that liver selenium is not a robust indicator of high selenium status. The nature of the additional selenium accumulating in monogastric animals fed inorganic selenium is unclear because selenoprotein selenium reaches a plateau in most tissues. In animals fed selenomethionine, or in ruminants, a major component of tissue selenium is selenomethionine incorporated nonspecifically into proteins in place of methionine, increasing tissue selenium concentrations but without further increases in levels of catalytic selenoproteins (39). The incorporation of selenomethionine is also adversely affected by the level of dietary methionine (39, 53), thus further confounding the value of tissue selenium as a biomarker.
Homeostasis.
The gap between the dietary selenium level required for optimal growth and the requirement for plateau levels of the selenoenzymes shows that maximum levels of at least these selenoproteins are not required for growth, for support of accompanying metabolism, and for protection from acute disease, at least under the dietary and husbandry conditions used in these studies. Clearly, homeostatic mechanisms are in play that direct sufficient selenium to the proteins critical for normal metabolism and growth. First, this suggests that biology has thus already incorporated a “safety factor” in evolutionarily setting the selenium-adequate expression of these selenoproteins. The following corollary is that the addition of a safety factor, as used in establishing RDA requirements for humans (1), may be an unnecessary overadjustment.
Second, the regulation of some but not all selenoprotein transcripts by selenium status is certainly a mechanism that directs limiting selenium to selected tissues and for incorporation into key selenoproteins. In rodents, liver SEPP1 transcripts [expressed at levels similar to GPX1 transcripts in selenium-adequate liver (54)] appear to be little regulated by selenium deficiency in mice and only moderately regulated in rats, supporting continued selenium incorporation into SEPP1 for export. Receptors in brain and testes, in turn, facilitate targeted and preferential uptake of selenium in SEPP1 into these tissues, maintaining presumably critical selenium functions (41). In turkeys and chickens, liver SEPP1 transcripts [expressed at 12- and 4-times GPX1 transcripts in selenium-adequate liver (26, 27)] are also modestly downregulated by selenium deficiency, sustaining selenium export to other tissues for proteins critical for normal metabolism and growth. High levels of SEPP1 transcript expression in avian liver may underlie the higher selenium requirements in avians.
Third, the variation in the first-affected organ in selenium deficiency (liver necrosis in rats, multiple organ necrosis in mice, gizzard myopathy in turkeys, pancreatic atrophy in chickens, and white muscle disease in lambs) appears to compensate for decreases in the expression of 1 or more selenoprotein in selenium deficiency by maintenance of expression of other overlapping protective proteins or processes that maintain organ integrity. In the targeted tissue, in contrast, the decrease in 1 or more selenoprotein is not fully compensated for by other protective processes, resulting in the disease. For instance, the very high expression of GPX1 accompanied by the relatively low expression of GPX4 in rat liver may potentiate the targeting of liver in selenium deficiency. In contrast, GPX1 expression in turkey liver is ∼10% of levels in rat liver and GPX4 expression is 6 times the level in rat liver, which suggests that evolution has balanced selenoprotein expression with the expression of other, non– selenium-dependent proteins to provide parallel metabolism or pathways that protect against disease.
In summary, comparison of these studies shows that the minimum dietary selenium requirement for young mammals lies over a narrow range, whereas the requirement for chicks is slightly higher, and turkey requirements are 3 times those of mammals. Notably, no selenoprotein activities or mRNA were effective biomarkers for supernutritional selenium status. Differences in these biomarker selenium-response curves may help researchers understand selenium metabolism and targeting of tissues in selenium deficiency, and thus help to better characterize selenium requirements.
Acknowledgments
RAS designed the research and had primary responsibility for final content; and RAS, J-LL, and RMT conducted the research, analyzed data, and wrote the manuscript. All authors read and approved the final manuscript.
References
- 1.Food and Nutrition Board. Dietary Reference Intakes for vitamin C, vitamin E, selenium and carotenoids. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 2.Food and Nutrition Board. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academies Press; 2001. [PubMed] [Google Scholar]
- 3.Food and Nutrition Board. Dietary Reference Intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington (DC): National Academies Press; 1999. [PubMed] [Google Scholar]
- 4.National Research Council. Nutrient requirements of laboratory animals. 4th ed Washington (DC): National Academies Press; 1995. [PubMed] [Google Scholar]
- 5.Hafeman DG, Sunde RA, Hoekstra WG. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr 1974;104:580–7. [DOI] [PubMed] [Google Scholar]
- 6.Knight SAB, Sunde RA. The effect of progressive selenium deficiency on anti-glutathione peroxidase antibody reactive protein in rat liver. J Nutr 1987;117:732–8. [DOI] [PubMed] [Google Scholar]
- 7.Saedi MS, Smith CG, Frampton J, Chambers I, Harrison PR, Sunde RA. Effect of selenium status on mRNA levels for glutathione peroxidase in rat liver. Biochem Biophys Res Commun 1988;153:855–61. [DOI] [PubMed] [Google Scholar]
- 8.Lei XG, Evenson JK, Thompson KM, Sunde RA. Glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase are differentially regulated in rats by dietary selenium. J Nutr 1995;125:1438–46. [DOI] [PubMed] [Google Scholar]
- 9.Behne D, Scheid S, Kyriakopoulos A, Hilmert H. Subcellular distribution of selenoproteins in the liver of the rat. Biochim Biophys Acta 1990;1033:219–25. [DOI] [PubMed] [Google Scholar]
- 10.Hill KE, Lyons PR, Burk RF. Differential regulation of rat liver selenoprotein mRNAs in selenium deficiency. Biochem Biophys Res Commun 1992;185:260–3. [DOI] [PubMed] [Google Scholar]
- 11.Bermano G, Nicol F, Dyer JA, Sunde RA, Beckett GJ, Arthur JR, Hesketh JE. Tissue-specific regulation of selenoenzyme gene expression during selenium deficiency in rats. Biochem J 1995;311:425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurer S, Friedrich C, Leist M, Maiorino M, Brigelius-Flohe R. Attempt to differentiate between individual glutathione peroxidases in biological samples. Z Ernahrungswiss 1998;37(Suppl 1):110–3. [PubMed] [Google Scholar]
- 13.Köhrle J. Thyroid hormone deiodinases—a selenoenzyme family acting as gate keepers to thyroid hormone action. Acta Med Austriaca 1996;23:17–30. [PubMed] [Google Scholar]
- 14.Weiss SL, Evenson JK, Thompson KM, Sunde RA. The selenium requirement for glutathione peroxidase mRNA level is half of the selenium requirement for glutathione peroxidase activity in female rats. J Nutr 1996;126:2260–7. [DOI] [PubMed] [Google Scholar]
- 15.Weiss SL, Evenson JK, Thompson KM, Sunde RA. Dietary selenium regulation of glutathione peroxidase mRNA and other selenium-dependent parameters in male rats. J Nutr Biochem 1997;8:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson KM, Haibach H, Evenson JK, Sunde RA. Liver selenium and testes phospholipid hydroperoxide glutathione peroxidase are associated with growth during selenium repletion of second-generation Se-deficient male rats. J Nutr 1998;128:1289–95. [DOI] [PubMed] [Google Scholar]
- 17.Hadley KB, Sunde RA. Selenium regulation of thioredoxin reductase activity and mRNA levels in rat liver. J Nutr Biochem 2001;12:693–702. [DOI] [PubMed] [Google Scholar]
- 18.Sunde RA, Evenson JK, Thompson KM, Sachdev SW. Dietary selenium requirements based on glutathione peroxidase-1 activity and mRNA levels and other selenium parameters are not increased by pregnancy and lactation in rats. J Nutr 2005;135:2144–50. [DOI] [PubMed] [Google Scholar]
- 19.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, Gladyshev VN. Characterization of mammalian selenoproteomes. Science 2003;300:1439–43. [DOI] [PubMed] [Google Scholar]
- 20.Barnes KM, Evenson JK, Raines AM, Sunde RA. Transcript analysis of the selenoproteome indicates that dietary selenium requirements in rats based on selenium-regulated selenoprotein mRNA levels are uniformly less than those based on glutathione peroxidase activity. J Nutr 2009;139:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schriever SC, Barnes KM, Evenson JK, Raines AM, Sunde RA. Selenium requirements are higher for glutathione peroxidase-1 mRNA than GPX1 activity in rat testis. Exp Biol Med (Maywood) 2009;234:513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sunde RA, Raines AM, Barnes KM, Evenson JK. Selenium status highly-regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci Rep 2009;29:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raines AM, Sunde RA. Selenium toxicity but not deficient or super-nutritional selenium status vastly alters the transcriptome in rodents. BMC Genomics 2011;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott ML, Olson G, Krook L, Brown WR. Selenium-responsive myopathies of myocardium and of smooth muscle in the young poult. J Nutr 1967;91:573–83. [DOI] [PubMed] [Google Scholar]
- 25.Sunde RA, Sunde GR, Sunde CM, Sunde ML, Evenson JK. Cloning, sequencing, and expression of selenoprotein transcripts in the turkey (Meleagris gallopavo). PLoS One 2015;10:e0129801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor RM, Sunde RA. Selenoprotein transcript level and enzyme activity as biomarkers for selenium status and selenium requirements of turkeys (Meleagris gallopavo). PLoS One 2016;11:e0151665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li JL, Sunde RA. Selenoprotein transcript level and enzyme activity as biomarkers for selenium status and selenium requirements of chickens (Gallus gallus). PLoS One 2016;11:e0152392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferguson-Kohout N. Glutathione peroxidase expression and selenium parameters. Columbia (MO): University of Missouri; 1998. [Google Scholar]
- 29.Ferguson-Kohout N, Sunde RA. Dietary selenium regulation of glutathione peroxidase (GPX1) and phospholipid hydroperoxide glutathione peroxidase (GPX4) in mice. FASEB J 1997;11:A359. [Google Scholar]
- 30.Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, Funk CD. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem 1997;272:16644–51. [DOI] [PubMed] [Google Scholar]
- 31.Sunde RA, Hadley KB. Phospholipid hydroperoxide glutathione peroxidase (GPX4) is highly regulated in male turkey poults and can be used to determine dietary selenium requirements. Exp Biol Med (Maywood) 2010;235:23–31. [DOI] [PubMed] [Google Scholar]
- 32.National Research Council. Nutrient requirements of poultry. 9th ed Washington (DC): National Academies Press; 1994. [Google Scholar]
- 33.Oh SH, Sunde RA, Pope AL, Hoekstra WG. Glutathione peroxidase response to selenium intake in lambs fed a torula yeast-based, artificial milk. J Anim Sci 1976;42:977–83. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence RA, Sunde RA, Schwartz GL, Hoekstra WG. Glutathione peroxidase activity in rat lens and other tissues in relation to dietary selenium intake. Exp Eye Res 1974;18:563–9. [DOI] [PubMed] [Google Scholar]
- 35.Sunde RA, Thompson KM, Evenson JK, Thompson BM. Blood glutathione peroxidase-1 mRNA levels can be used as molecular biomarkers to determine selenium requirements in rats. Exp Biol Med (Maywood) 2009;234:1271–9. [DOI] [PubMed] [Google Scholar]
- 36.Sunde RA. Molecular biomarker panels for assessment of selenium status in rats. Exp Biol Med (Maywood) 2010;235:1046–52. [DOI] [PubMed] [Google Scholar]
- 37.Sunde RA. mRNA transcripts as molecular biomarkers in medicine and nutrition. J Nutr Biochem 2010;21:665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lei XG, Dann HM, Ross DA, Cheng WH, Combs GF, Roneker KR. Dietary selenium supplementation is required to support full expression of three selenium-dependent glutathione peroxidases in various tissues of weanling pigs. J Nutr 1998;128:130–5. [DOI] [PubMed] [Google Scholar]
- 39.Waschulewski IH, Sunde RA. Effect of dietary methionine on tissue selenium and glutathione peroxidase activity in rats fed selenomethionine. Br J Nutr 1988;60:57–68. [DOI] [PubMed] [Google Scholar]
- 40.Xia Y, Hill KE, Li P, Xu J, Zhou D, Motley AK, Wang L, Byrne DW, Burk RF. Optimization of selenoprotein P and other plasma selenium biomarkers for assessment of the selenium nutritional requirement. A placebo-controlled double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am J Clin Nutr 2010;92:525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burk RF, Hill KE. Selenoprotein P—expression, functions, and roles in mammals. Biochim Biophys Acta 2009;1790:1441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burk RF, Hill KE, Boeglin ME, Ebner FF, Chittum HS. Plasma selenium in patients with cirrhosis. Hepatology 1998;27:794–8. [DOI] [PubMed] [Google Scholar]
- 43.Yang JG, Hill KE, Burk RF. Dietary selenium intake controls rat plasma selenoprotein P concentration. J Nutr 1989;119:1010–2. [DOI] [PubMed] [Google Scholar]
- 44.Hill KE, Lloyd RS, Yang JG, Read R, Burk RF. The cDNA for rat selenoprotein P contains 10 TGA codons in the open reading frame. J Biol Chem 1991;266:10050–3. [PubMed] [Google Scholar]
- 45.Köhrle J, Gartner R. Selenium and thyroid. Best Pract Res Clin Endocrinol Metab 2009;23:815–27. [DOI] [PubMed] [Google Scholar]
- 46.Sunde RA, Raines AM. Selenium regulation of the selenoprotein and non-selenoprotein transcriptomes in rodents. Adv Nutr 2011;2:138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang JQ, Li DL, Zhao H, Sun LH, Xia XJ, Wang KN, Luo X, Lei XG. The selenium deficiency disease exudative diathesis in chicks is associated with downregulation of seven common selenoprotein genes in liver and muscle. J Nutr 2011;141:1605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu CP, Fu J, Lin SL, Wang XS, Li S. Effects of dietary selenium deficiency on mRNA levels of twenty-one selenoprotein genes in the liver of layer chicken. Biol Trace Elem Res 2014;159:192–8. [DOI] [PubMed] [Google Scholar]
- 49.Zhao X, Yao H, Fan R, Zhang Z, Xu S. Selenium deficiency influences nitric oxide and selenoproteins in pancreas of chickens. Biol Trace Elem Res 2014;161:341–9. [DOI] [PubMed] [Google Scholar]
- 50.Yao H, Zhao W, Zhao X, Fan R, Khoso PA, Zhang Z, Liu W, Xu S. Selenium deficiency mainly influences the gene expressions of antioxidative selenoproteins in chicken muscles. Biol Trace Elem Res 2014;161:318–27. [DOI] [PubMed] [Google Scholar]
- 51.Zhou JC, Zhao H, Li JG, Xia XJ, Wang KN, Zhang YJ, Liu Y, Zhao Y, Lei XG. Selenoprotein gene expression in thyroid and pituitary of young pigs is not affected by dietary selenium deficiency or excess. J Nutr 2009;139:1061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosse AC, Pallauf J, Hommel B, Sturm M, Fischer S, Wolf NM, Mueller AS. Impact of selenite and selenate on differentially expressed genes in rat liver examined by microarray analysis. Biosci Rep 2010;30:293–306. [DOI] [PubMed] [Google Scholar]
- 53.Sunde RA, Gutzke GE, Hoekstra WG. Effect of dietary methionine on the biopotency of selenite and selenomethionine in the rat. J Nutr 1981;111:76–88. [DOI] [PubMed] [Google Scholar]
- 54.Weiss Sachdev S, Sunde RA. Selenium regulation of transcript abundance and relative translational efficiency of glutathione peroxidase 1 and 4 in rat liver. Biochem J 2001;357:851–8. [DOI] [PMC free article] [PubMed] [Google Scholar]