Abstract
Malnutrition is the result of an inadequate balance between energy intake and energy expenditure that ultimately leads to either obesity or undernutrition. Several factors are associated with the onset and preservation of malnutrition. One of these factors is the gut microbiota, which has been recognized as an important pathophysiologic factor in the development and sustainment of malnutrition. However, to our knowledge, the extent to which the microbiota influences malnutrition has yet to be elucidated. In this review, we summarize the mechanisms via which the gut microbiota may influence energy homeostasis in relation to malnutrition. In addition, we discuss potential therapeutic modalities to ameliorate obesity or undernutrition.
Keywords: malnutrition, obesity, undernutrition, gut microbiota, energy homeostasis, appetite, gut-brain axis, probiotics, prebiotics, fecal transplantation
Introduction
Malnutrition is a broad term that encompasses many different manifestations of inadequate nutrition, including both undernutrition and obesity. It is characterized by an imbalance in energy intake and energy expenditure (1). In 2014, >600 million people worldwide were obese, and >1 billion people suffered from undernutrition (2, 3).
Obese individuals experience increased feelings of hunger despite the large amounts of stored energy in adipose tissue (4). It is an ongoing paradox why obese individuals show this strong urge to eat. The most accepted hypothesis is that they have an increased resting energy expenditure (REE)8 that corresponds to an increased energy need. On the contrary, undernourished individuals such as patients with anorexia nervosa (AN) seem to have an opposite imbalance. These individuals experience a loss of appetite despite the fact that REE remains the same (5, 6). This suggests that both obese and anorectic individuals lose the tight connection between food intake and REE that is normally found in healthy individuals.
Despite the increasing insight into the pathophysiology of obesity and undernutrition, the currently available treatment modalities are largely ineffective (7–9), suggesting that certain biological systems are not adequately restored. A new player in this field of research might be the composition of our indwelling bacterial species: the gut microbiota.
The gut microbiota (the collective genomic content of microorganisms) in humans contains ∼40 trillion microorganisms. Until recently, most studies stated that the bacteria residing in the human intestinal tract outnumbered human cells by a ratio of 10:1. However, Sender et al. (10) recalculated this ratio and concluded that the ratio of microbial cells is much closer to equal numbers of human cells (1:1). The 2 dominating phyla in humans, accounting for 90% of the gut microbiota, are Firmicutes and Bacteroidetes. There are currently >274 genera within the Firmicutes phylum, including Bacillus, Lactobacillus, Mycoplasma, and Clostridium. Bacteroidetes includes ∼20 genera, of which the most abundant genus in the human gastrointestinal tract is Bacteroides (11).
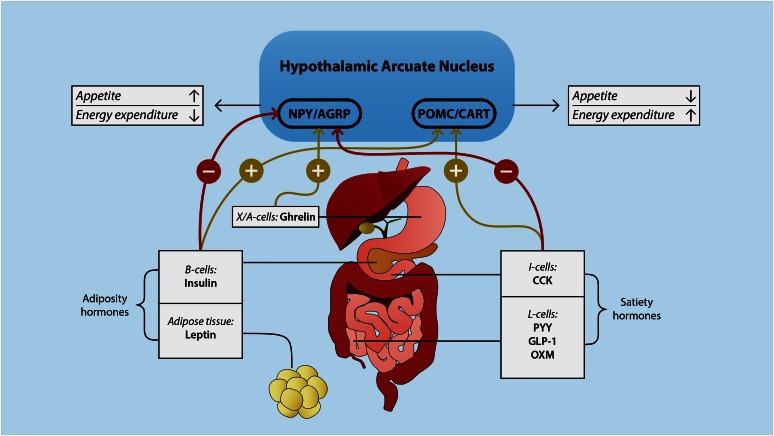
The gut microbiota plays an important role in the absorption, storage, and expenditure of energy obtained from dietary intake (12–15). Furthermore, recent animal studies have shown that the gut microbiota is also involved in the regulation of food intake by affecting hormones that influence metabolic function and areas in the brain associated with eating behavior (16). This so-called microbiota gut-brain axis represents a bidirectional signaling axis that regulates body weight by balancing appetite, storage, and energy expenditure (Figure 1) (14).
FIGURE 1.
The gut-brain axis. Satiety and adiposity signals are secreted in the gastrointestinal tract and adipose tissue. These hormones directly or indirectly signal to NPY/AGRP- and POMC/CART-containing neurons located in the hypothalamic arcuate nucleus. This arcuate nucleus plays a key role in the regulation of appetite and energy expenditure. The activation of NPY/AGRP neurons has an orexegenic effect, whereas the activation of POMC/CART neurons has an anorexigenic effect. AGRP, agouti-related protein; CART, cocaine- and amphetamine-regulated transcript; CCK, cholecystokinin; GLP-1, glucagon-like peptide 1; NPY, neuropeptide Y; OXM, oxyntomodulin; POMC, pro-opiomelanocortin; PYY, peptide tyrosine tyrosine.
In this regard, obesity and undernutrition share an important biological factor: alterations in the composition and diversity of the gut microbiota compared to healthy individuals (1, 17–20). This disruption in the microbial composition, a phenomenon known as dysbiosis, is associated with altered bodyweight and fat storage. Whether the dysbiosis is a cause or consequence of obesity and undernutrition has to our knowledge yet to be determined.
Improved understanding of how the gut microbiota is involved in energy homeostasis and appetite regulation can eventually lead to novel therapeutics, such as probiotics and fecal microbiota transplantation (FMT), that potentially modulate the gut microbiota in a more effective way than the current treatment modalities.
Therefore, in this review, we focus on the influence of the gut microbiota on energy homeostasis and appetite regulation. We discuss alterations of the gut microbiota known so far in obesity and undernutrition first and then provide insight into the potential value of novel therapeutic strategies such as probiotics and FMT.
Gut Microbiota and Energy Homeostasis
In humans, nutrient digestion and absorption mainly occur in the stomach and proximal small intestine. In healthy individuals, ∼66–95% of proteins, 85% of carbohydrates, and ∼95% of fats are absorbed before entering the large intestine (21). The highest density of gastrointestinal microorganisms is found in the cecum and proximal colon (12). The gut microbiota is predominantly involved in the fermentation of indigestible carbohydrates into SCFAs, which have been found to exert multiple effects on energy homeostasis and are crucial for intestinal health (22). The most abundant SCFAs are acetate, butyrate, and propionate; these SCFAs comprise >95% of the SCFA content (22).
There is growing evidence from human and animal studies that support a link between the gut microbiota, SCFAs, and obesity (17, 23–25). Several animal and human studies have found increased SCFA fecal concentration (in particular propionate) in obese individuals compared to lean individuals, suggesting that increased fecal concentrations of SCFAs are associated with obesity (23, 26). In apparent contrast, some animal studies have shown that treatment with SCFAs reduces weight gain and adiposity (27, 28). However, note that the fecal content of SCFAs does not directly correlate with the rate at which acetate, propionate, or butyrate are metabolized (18).
Furthermore, studies in mouse models have shown that gut-derived SCFAs are actively metabolized and that propionate, butyrate, and acetate play an important role as substrates for glucose metabolism (29). In addition, propionate and butyrate have the capacity to activate intestinal gluconeogenesis (30). In obese mice, the administration of oral sodium butyrate has been shown to reduce body weight by increasing fat oxidation and energy expenditure (27). Another study indicated that administering oral acetate, propionate, and butyrate to mice fed a high-fat diet improved insulin sensitivity and reduced body weight without changing food intake or physical activity rate (31).
In conclusion, although reports on the composition of the gut microbiota in obese individuals are not uniform, reduced microbial diversity seems to be a recurrent finding. These alterations are thought to be associated with altered SCFA composition, energy homeostasis, and inflammation. However, a causal relation between gut microbiota composition and energy homeostasis is complex, and contributory variables such as genes, age, and diet substantially affect the function of gut microbiota (32).
The Gut-Brain Axis
The central nervous system constantly responds to the various neural and chemical signals that monitor an individual’s energy state. Most of these signals are thought to be produced in the gastrointestinal tract and are collectively referred to as the gut-brain axis (14, 33). The gut-brain axis can be influenced by multiple factors, including diet, genes, and anatomy (e.g., effect of bariatric surgery), and recently the gut microbiota has been implicated (12). The pathways between the indwelling gut microbiota and regulation of appetite are far from elucidated, however. To comprehend the mechanisms through which the gut microbiota might influence appetite, we first summarize some basic knowledge about the gut-brain axis.
Food intake induces the release of numerous satiety hormones, causing a feeling of fullness that reduces appetite. Although taste perception is an important player in the regulation of food intake (34), in this review we focus on the more peripheral gastrointestinal signals and their relation to the gut microbiota. After taste, the second signal triggered by food intake is generated by mechanoreceptors in the stomach (35). Gastric distention induces vagal afferent firing, causing a negative-feedback signal in the brain (rhombencephalon) that evokes the feeling of fullness. Although gastric distension causes a quick feeling of fullness, specialized endocrine cells located within the gastrointestinal tract are thought to play a greater role in the regulation of appetite. These so-called enteroendocrine cells express chemosensors on their apical surfaces that respond to the preabsorptive nutrients that release several hormones involved in many physiological processes, including ghrelin, cholecystokinin, glucagon-like peptide 1 (GLP-1), peptide tyrosine tyrosine (PYY), and leptin (Figure 1) (36). These hormones activate vagal and spinal afferents directly and indirectly, initiating the gut-brain axis. In the nucleus solitary tract of the brainstem, both vagal– and spinal gut–derived signals are integrated, inducing a signal in the hypothalamic arcuate nucleus (ARC) (37, 38). The hypothalamus plays a central role in the regulation of energy homeostasis by affecting both appetite and energy expenditure. Two different types of neurons in the hypothalamus are responsible for the interpretation of these peripheral signals. The appetite-suppressing (anorexigenic) neurons pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript in the lateral side of the ARC express α-melanocyte–stimulating hormone (39). Melanocortins such as α-melanocyte–stimulating hormone promote negative energy balance (40). These peptides are synthesized in response to increased adipose tissue. The medial part of the ARC contains orexigenic neurons that express neuropeptide Y (NPY) and the agouti-related protein (AGRP) (14). NPY and AGRP are the main appetite-stimulating central neurotransmitters. They stimulate appetite and reduce energy expenditure through the release of orexin and melanocortin-releasing hormone and the inhibition of POMC (40).
In summary, the gut-brain axis is activated upon food intake through the release of gut hormones that activate the nucleus solitary tract and then the hypothalamus, which is the central regulator for appetite and energy expenditure. Based on their importance for weight regulation, we discuss the major gastrointestinal and pancreatic hormones and their proposed association with the gut microbiota.
Satiety hormones
Ghrelin.
Ghrelin is the only hunger-inducing or orexigenic hormone known so far and is predominantly produced by the enteroendocrine cells located in the stomach (X/A-like cells) (33). Ghrelin secretion is reduced during distension of the stomach and increased during the fasting state (41, 42). This hormone is involved in many physiologic processes, including prolactin and adrenocorticotropic hormone release, secretion of growth hormone secretagogue receptors, glucose metabolism, and hunger response (43). It enhances gastric motility and emptying and regulates appetite (44). Ghrelin acts both on the vagus nerve as well as the direct stimulation of NPY/AGRP neurons through their growth hormone secretagogue receptors (38). Wren et al. (44) demonstrated this by administering ghrelin intracerebroventricularly or peripherally in rats, resulting in an increase of appetite. Ghrelin also inhibits insulin release, suggesting its involvement in glucose and lipid metabolism (45).
The association between gut microbiota composition and ghrelin concentrations has been evaluated in a rat study. In this study, Queipo-Ortuño et al. (46) looked at the effect of exercise and food restriction on ghrelin concentrations in relation to gut microbiota composition. They found a significant negative correlation with ghrelin concentrations in individuals with increased numbers of Bifidobacterium and the B. coccoides/Eubacterium rectale and Lactobacillus groups. Of note, the latter bacterial species is used as a probiotic strain in humans, but the effects on ghrelin have never been studied to our knowledge.
Cholecystokinin.
Cholecystokinin, a 27-amino-acid polypeptide, is produced in the proximal small intestine by I and K cells in response to fat- and protein-containing meals. After food intake, cholecystokinin concentrations peak in ∼25 min and remain increased for ∼3 h (47). In animals, cholecystokinin is found to be involved in appetite regulation by reducing both meal size and meal duration (48). In addition to reducing appetite, cholecystokinin plays important roles in gastrointestinal motility, energy expenditure, and the secretion of pancreatic enzymes and gastric acid (49–51). Cholecystokinin was found to be the first gastrointestinal hormone to influence food intake in both obese and lean individuals (47). Interestingly, obese individuals are found to be less sensitive to cholecystokinin release, which might promote overeating and weight gain (52).
GLP-1.
The ingestion of carbohydrates, proteins, and lipids induce the release of GLP-1 from L cells located in the distal small intestine and reduces appetite (53). Animal and human studies have shown that the central and peripheral administration of GLP-1 induces slower gastric emptying, reduces appetite, and prolongs the period of postprandial satiety (54, 55). Ablation of the vagal-brainstem-hypothalamic pathway in rodents reduces this anorectic function, which emphasizes the importance of this pathway in regulating appetite (56). GLP-1 has also been shown to play a crucial role in glucose homeostasis in mice studies, inducing glucose-dependent insulin release and increasing β cell growth in the pancreas (57).
PYY.
PYY is a 36-amino-acid peptide that belongs to the pancreatic polypeptide family and is synthesized by the L cell in the distal gut (33). Studies in rodents have shown that PYY has an inhibitory effect on gastric motility and NPY, resulting in reduced appetite. A few studies have suggested that PYY plays a role in the pathogenesis of obesity. For example, Batterham et al. (58) showed that the peripheral administration of PYY at dose-mimicking postprandial concentrations markedly decreased appetite and food intake and increased satiety. Furthermore, Dakin et al. (59) showed that intravenous PYY affected metabolism by increasing postprandial thermogenesis and REE.
The composition of the intestinal microbiota (and dysbiosis) may affect the orchestration of these hormones involved in food intake and thus might be an underlying pathophysiologic factor present in malnutrition. Breton et al. (60) examined how intestinally infusing Escherichia coli proteins in mice and rats affected food intake and meal pattern, together with plasma GLP-1 and PYY. Indeed, they found increased plasma PYY concentrations and decreased food intake. These results suggest that alterations in the composition of the gut microbiota, resulting in a high or low abundance of gram-negative bacteria such as E. coli, may influence food intake via the incretin system.
SCFAs are also found to influence satiety hormones through the activation of G-protein–coupled cell-surface receptors G-protein–coupled receptor 41 (GPR41) and G-protein–coupled receptor 43 (GPR43) (61). Several in vitro studies have shown with the use of intestinal cell lines that SCFAs stimulate the secretion of PYY and GLP-1 from L cells through the activation of GPR41 and GPR43 (62–64).
Studies in rodents have shown increased plasma concentrations of PYY and GLP-1 after diets containing fermentable carbohydrates (e.g., oligofructose and resistant starch) (65–67), suggesting a link between the gut microbiota, production of SCFAs, and appetite regulation. These data led to an increase in studies examining the effects of fermentable carbohydrates on the release of GLP-1 and PYY in relation to body weight. For example, one study (58) found lower hunger scores in healthy humans (measured by visual analog scales) after dietary supplementation with oligofructose; these lower hunger scores were associated with increased plasma concentrations of GLP-1 and PYY. Moreover, in overweight subjects [BMI (in kg/m2) >25), intake of oligofructose for 12 wk led to a significant weight loss (1.03 kg; P < 0.01) (69).
Adiposity signals
Plasma concentrations of hormones such as insulin and leptin are directly related to the amount and degree of adipose tissue inflammation. Adipose tissue is the most important reservoir of energy in the body. In mammals, there are 2 types of adipose tissue: white (located subcutaneously and intra-abdominally) and brown adipose tissue (located between the scapulae and along the spinal tract). These tissues are directly regulated by the autonomic nervous system (70). The innervation of the sympathetic nervous system is mainly related to catabolic activities, such as lipolysis (70). The parasympathetic activation is mainly involved in anabolic activities, such as glucose and FA uptake through insulin (70). Because of caloric restriction or overeating, plasma insulin and leptin concentrations change in parallel, reflecting altered signals to the brain in the so-called adiposity negative-feedback model (37, 38, 40). These adiposity signals activate POMC neurons and inhibit NPY/AGRP neurons and, consequently, promote weight loss (71). For example, during caloric restriction, adipose signaling to the brain is reduced, resulting in decreased sensitivity to satiety hormones such as cholecystokinin. This compensatory mechanism induces an increased feeling of appetite that causes craving for food until body weight (and insulin or leptin concentrations) return to the state before the start of caloric restriction.
Insulin.
The anorexigenic hormone insulin is released by the pancreatic β cells after oral food intake. It stimulates the transport of glucose into the peripheral cells and provides a direct signal to the liver to convert glucose into glycogen for glucose storage (72). Glycogen synthesis is suppressed when the liver is saturated with glycogen (∼5% of liver mass) and excess glucose is used for the synthesis of FAs, which are transported in the form of TGs by lipoproteins to the peripheral tissues, including adipose tissue. In obesity, insulin resistance is present in multiple tissues. Insulin resistance is associated with low-grade inflammatory changes. The underlying pathophysiology of this low-grade inflammation and the role of the gut microbiota are discussed in the next paragraph.
Leptin.
The adiposity hormone leptin is mainly produced in white adipocytes (small amounts also come from the stomach and other tissues). Concentrations of leptin in plasma therefore link directly to the number of adipocytes and fat content (73). The anorexigenic effect of leptin occurs mainly through the inhibition of NPY secretion and stimulation of POMC secretion (74, 75). In a mouse study, SCFAs were found to stimulate the production of leptin in adipocytes through the activation of GPR41 (76). Intravenous administration of sodium propionate nearly doubled the plasma concentration of leptin (76). In addition, these stimulatory activities of SCFAs were inhibited when suppressing GPR41 expression by RNA interference, confirming the direct link between SCFAs and GPR41 (76).
Resistin.
Resistin is a small cysteine-rich protein that is also secreted by adipose tissue. It plays a role in insulin sensitivity, lipid metabolism, and inflammatory processes (77). Resistin has been proposed to be the link between obesity and insulin resistance. It influences glucose metabolism in the liver and skeletal muscles by reducing insulin sensitivity (78); however, the relation between intestinal microbes and resistin is not known.
In summary, regulating appetite is a complex interplay between peripheral signals and the central nervous system. Imbalances between these signals result in an inappropriate regulation of appetite and energy expenditure in obese and undernourished individuals. SCFAs may affect the orchestration of these hormones involved in food intake and thus might be an underlying pathophysiologic factor present in both obesity and undernutrition. However, whether these SCFAs are really physiologically relevant and a cause for malnutrition has yet to be determined.
Gut Microbiota in Obesity
Changes in lifestyle and the excessive availability of food are 2 important contributors to the increasing obesity epidemic. Enhanced consumption of high-fat and high-sugar diets have been shown to change microbial ecology, leading to the notion that gut microbiota may function as an “environmental” factor that results in increased energy harvest and obesity (79).
Metagenomic studies in humans have generated inconsistent findings with respect to the gut microbiota in obese compared to lean individuals. Which specific bacteria are present or absent and contribute to the development of obesity still has yet to be elucidated. Some studies have shown an increased proportion of Firmicutes and reduced concentrations of Bacteroidetes in obese compared to lean humans and mice (11), others have found no notable changes in microbial composition between the two groups, and some have even reported inverse findings (23). Ley et al. (11) showed a decrease in the Firmicutes:Bacteroidetes ratio in obese humans after weight loss after a diet. However, whether these alterations of the gut microbiota are a secondary phenomenon in obesity or truly causal remains to be determined.
Bäckhed et al. (79) also found an association between the gut microbiota and obesity by colonizing germ-free mice with gut microbiota harvested from the distal intestines of conventionally raised mice. Compared to the intake before the colonization, the total body fat content and epididymal fat weight of the germ-free mice increased 57% and 61%, respectively, within 10–14 d despite a decrease in food intake. Ridaura et al. (80) reproduced these findings by colonizing germ-free mice with feces of obese humans and showed identical effects on adipose tissue. Furthermore, the prevalence of obesity was lower in subjects with higher microbial diversity (81).
Other evidence indicating a correlation between the gut microbiota and obesity has been found in obese individuals who underwent Roux-en-Y gastric bypass (RYGB) surgery (82). The gut microbiota changed considerably after the procedure, resulting in increased gut microbiota richness. This suggests that the weight reduction and improved metabolic profile achieved through RYGB is possibly partly caused by a change in the gut microbiota. To test this notion, Liou et al. (83) performed FMT with feces harvested from RYGB-treated mice into germ-free mice and observed substantial weight loss and a decrease in fat mass compared to the mice that received microbiota from mice that underwent a sham procedure. However, considering the major anatomic changes resulting from the RYGB operation, we cannot conclude that all the beneficial effects of RYGB were caused by alterations of the gut microbiota.
In addition to the role of the gut microbiota in energy regulation, mouse studies have linked the gut microbiota to the pathogenesis of insulin resistance and inflammation in obesity. It is well known that obesity is associated with chronic low-grade inflammation and insulin resistance (84–86). White adipose tissue is metabolically the most important adipose tissue. It plays a central role in this inflammatory state, expressing proinflammatory cytokines such as TNF-α and IL-1, IL-6, IL-10, and IL-12 (87). In obesity, there is increased cytokine production in white adipose tissue and then an infiltration of macrophages (88, 89). This infiltration subsequently enhances proinflammatory cytokines and in turn induces insulin resistance (90). A contributing factor to the onset of this chronic low-grade inflammation is thought to be alterations in the composition of the gut microbiota induced by a high-fat diet. These alterations result in increased gut permeability—otherwise known as gut barrier dysfunction (91). Gut barrier dysfunction causes low-grade inflammation by either directly translocating gram-negative intestinal bacteria or increasing LPSs (92). LPSs originate from the outer membrane of gram-negative bacteria and induce metabolic endotoxemia, which in turn generates a low-grade inflammation (79, 91, 93). This phenomenon is still relatively unexplored and currently a topic of extensive research.
In conclusion, although reports on the composition of the gut microbiota in obese individuals are not uniform, reduced microbial diversity seems to be a recurrent finding. These alterations are thought to be associated with altered SCFA composition, energy homeostasis, and inflammation. However, a causal relation between gut microbiota composition and energy homeostasis is complex, and contributory variables such as genes, age, and diet substantially affect gut microbiota function (55).
Gut Microbiota in Undernutrition
The role of the gut microbiota in obesity reported in early studies provided a strong rationale for evaluating the role of gut bacterial species in undernutrition. Undernutrition is defined as a deficiency of calories or a shortage of ≥1 essential nutrient. It may develop because of difficulties in obtaining, eating, or absorbing food or a considerably increased need for calories.
To date, there is a lack of studies that have investigated the gut microbiota in malnourished adults. Most studies that have evaluated its role in undernutrition have focused on children because undernutrition in children is a major health problem, accounting for >3 million deaths/y (94). It is associated with numerous adverse outcomes, including reduced immune function, persistent stunting (reduced growth rate), and neurocognitive deficits (95). To investigate the role of the gut microbiota in severe acute malnutrition (kwashiorkor) in children, Smith et al. (96) studied 317 Malawian twin pairs from birth to 3 y of age. During this period, 50% of the twins remained well nourished, whereas 43% became discordant and 7% manifested concordance for acute malnutrition. Thereafter, fecal microbiota samples from 3 discordant pairs were transplanted into germ-free mice. In 2 of the 3 twin pairs, the combination of the kwashiorkor microbiome and Malawian diet resulted in marked weight loss in the recipient mice, along with disruptions in carbohydrate and amino acid metabolism (96).
Blanton et al. (97) also performed fecal transplants with the use of feces of malnourished Malawian children to colonize the intestines of germ-free mice. These mice were fed a nutrient-poor diet that reflected a standard Malawian diet. After a few weeks, the mice harboring a gut microbiota from malnourished donors gained substantially less weight and showed impaired growth compared to the control group that received microbiota from healthy children. The researchers found that 2 bacterial species, Ruminococcus gnavus and Clostridium symbiosum, were responsible for this effect. Introducing these species into germ-free mice together with the microbiota from the malnourished mice showed a considerable weight gain (95).
Another disorder resulting in severe malnutrition is AN. AN is characterized by a distorted body image and extreme dieting that leads to severe weight loss (BMI below the 10th BMI percentile) with a pathologic anxiety of becoming obese (98). Although AN is seemingly an entirely different disorder than childhood undernutrition, compelling evidence shows that key features of AN, including altered appetite regulation and energy homeostasis, are also associated with an altered composition of the gut microbiota (99).
A first study that evaluated the composition of gut microbiota in AN patients found an increased concentration of Methonobrevibacter smithii, a methane-producing archaeon, in 9 patients with AN compared to obese and normal-weight participants (99). Patients harboring this archaeon showed a negative correlation (r = −20) between BMI and M. smithii concentrations.
Morita et al. (100) compared the fecal microbiota composition of patients with AN (n = 25) to those of age-matched healthy controls. AN patients had markedly lower amounts of total bacteria and obligate anaerobes (C. coccoides group, B. fragilis, C. leptum, and Streptococcus). A recently published study (101) explored the potential role of the gut microbiota in AN by evaluating fecal microbiota composition and SCFA profiles in patients with AN before (n = 55) and after weight gain (n = 44) compared to normal-weight participants (n = 55). Patients with AN showed profound microbial perturbations compared to normal-weight participants, with reduced concentrations of the butyrate-producing Roseburia spp. and higher concentrations of mucin-degrading bacteria (Verrucomicrobia, mainly Akkermansia muciniphila), as well as members of Clostridium clusters I, X1, and XVIII. Strikingly, after weight gain microbial diversity increased, but perturbations in the gut microbiota composition and fecal SCFA profiles did not improve.
Therapeutics: Prebiotics, Probiotics, and FMT
Previous insights into the role of the gut microbiota in weight regulation have revealed the potential niche in therapeutic options for obese and undernourished individuals. The gut microbiota composition can be modified with the use of several tools, e.g., live bacteria (probiotics), specific nutrients that act as a fertilizer for bacteria (prebiotics), antibiotics, or FMT (102). In this section, we focus on the effects of probiotics, prebiotics, and FMT in malnutrition.
Probiotics
Probiotics are live microorganisms that can influence the gut microbiota and contain promising therapeutic utilities for patients with disorders caused or worsened by imbalances in the gut microbiota (103).
In animals, probiotics are excessively used in the farming industry to induce weight gain. Feed animals are predominantly given gram-positive species such as Enterococcus, Bacillus, Bifidobacterium, Pediococcus, Lactobacillus, and Streptococcus spp. (104).
In humans, probiotics have been shown to induce weight gain in children with severe malnutrition (105, 106). For example, the probiotic B. breve has been associated with substantial weight gain, especially in malnourished children (107–109). However, it is important to mention that related probiotic strains often vary considerably at functional and structural concentrations, such as the genus Lactobacillus (110). A meta-analysis on the effects of certain Lactobacillus spp. showed that L. fermentum, L. ingluviei, and L. acidophilus are associated with weight gain, whereas the administration of L. gasseri and L. plantarum promotes weight loss in obese animals and humans (107). Furthermore, the enrichment of gut microbiota with L. reuteri in glucose-tolerant humans induced a minor increase of insulin secretion, possibly because of an augmented release of incretins. L. gasseri and L. plantarum have also been shown to reduce weight, although only limited well-designed studies to our knowledge have exhibited their effects (107).
Regarding inflammation, growing evidence indicates that components of the gut microbiota might be involved in the regulation of the gut barrier function and in turn reduce inflammation. For example, A. muciniphila, which is found in the mucus layer of healthy humans, has been associated with restored gut barrier function, reduced endotoxemia concentrations, and improved metabolic function (111). This improved gut barrier function is striking because A. muciniphila is known to be a mucin-degrading bacterium (112). In obese mice, oral gavage with A. mucinphila reduced fat mass gain and adipose tissue inflammation and enhanced the gut barrier function. In humans, a high abundance of A. mucinphila is also associated with a healthier metabolic status and blood cholesterol concentrations (111).
Prebiotics
Prebiotics consist of complex carbohydrates that are nondigestible for humans and can be used as substrates for the microbiota. For instance, oligofructose intake has been shown to promote the growth of Bifidobacterium and Lactobacilus and reduce body fat in obese individuals (69). These results were associated with a suppressed postprandial ghrelin release and increased PYYA concentrations and a considerable reduction of appetite. Indeed, supplementing oligofructose in a high-fat diet increased the number of intestinal Bifidobacterium spp. and reduced obesity and symptoms of metabolic syndrome (67, 113). Therefore, bifidobacteria were thought to facilitate the oligofructose-induced effects in obesity and metabolic syndrome. However, Woting et al. (114) showed beneficial effects of oligofructose in mice on body weight, body fat accumulation, and glucose tolerance independently of the microbial status.
FMT
FMT has emerged as an effective treatment for recurrent C. difficile infection. Recent studies have suggested that FMT might also play a role in treating other gastrointestinal and nongastrointestinal diseases, including obesity, insulin resistance, and metabolic syndrome (115). The transfer of the fecal microbiota from human twins discordant for obesity into germ-free mice led to greater adiposity and body mass in mice transplanted with the obese microbiota (80). Interestingly, when the obese-transplanted mice were cohoused with the lean-transplanted mice, the obese-transplanted mice were protected from developing the increased adiposity and body mass (116). A metagenomic analysis of feces derived from the obese mice revealed a decreased number of genes involved in SCFA production but an enrichment of those that were involved in branched-chain amino-acid metabolism compared to their lean counterparts. In a small, double-blind, randomized controlled study, Vriese et al. (117) found that FMT from lean to obese (with metabolic syndrome) individuals resulted in improved insulin sensitivity, increased gut microbial diversity, and increased butyrate-producing bacteria (R. intestinalis) in obese recipients. Whether this effect is caused by changes in the composition of the gut microbiota or in certain gram-negative bacterial species (thus less endotoxemia) is unknown to our knowledge and currently under investigation.
Although there is a growing interest in the effects of FMT on obesity and metabolic syndrome, so far no studies on FMT and undernutrition in humans to our knowledge have been performed. There is, however, anecdotal evidence that supports the hypothesis that FMT can increase body weight. Indeed, a lean subject was reported to rapidly and unintentionally gain weight after receiving obese donor feces for a C. difficile infection (118). This case stimulates further investigation on the link between the gut microbiota, metabolism, and malnutrition.
In summary, these studies provide a potential proof of principle for future FMT studies on the treatment of obesity and undernutrition. Novel studies might help to identify bacterial strains involved in host energy metabolism that can possibly be isolated and developed as probiotics. However, patience is essential because regulatory (good manufacturing practice and stability of strains) and production hurdles (e.g., culturing these anaerobic bacterial strains in large quantities) preclude rapid translation into clinical practice.
Conclusions
The gut microbiota seems to be an important player in the regulation of energy homeostasis in humans. However, it remains difficult to prove causality in the interaction between gut microbiota and weight-regulatory mechanisms. Current studies mainly focus on the role of the gut microbiota in obese individuals. We feel that other metabolic disorders such as undernutrition in adults should also be taken into account. Therefore, future studies should not only focus on obesity but also try to mine the gut microbiota in undernutrition for novel probiotics and further examine the direct interaction between nutrient intake, energy homeostasis, and the gut microbiota.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AGRP, agouti-related protein; AN, anorexia nervosa; ARC, arcuate nucleus; GLP-1, glucagon-like peptide 1; GPR41, G-protein–coupled receptor 41; GPR43, G-protein–coupled receptor 43; NPY, neuropeptide Y; POMC, pro-opiomelanocortin; PYY, peptide tyrosine tyrosine; REE, resting energy expenditure; RYGB, Roux-en-Y gastric bypass.
References
- 1.Gordon JI, Dewey KG, Mills DA, Medzhitov RM. The human gut microbiota and undernutrition. Sci Transl Med 2012;4:137ps12. [DOI] [PubMed] [Google Scholar]
- 2.WHO. World health statistics 2010 [Internet]. [cited 2016 Jul 14]. Available from: http://www.who.int/gho/publications/world_health_statistics/2010/en.
- 3.WHO. Obesity and overweight fact sheet [Internet]. [cited 2016 Jul 11]. Available from: www.who.int/mediacentre/factsheets/fs311/en.
- 4.Caudwell P, Finlayson G, Gibbons C, Hopkins M, King N, Naslund E, Blundell JE. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. Am J Clin Nutr 2013;97:7–14. [DOI] [PubMed] [Google Scholar]
- 5.Gorwood P, Blanchet-Collet C, Chartrel N, Duclos J, Dechelotte P, Hanachi M, Fetissov S, Godart N, Melchior J-C, Ramoz N, et al. New insights in anorexia nervosa. Front Neurosci 2016;10:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleiman SC, Carroll IM, Tarantino LM, Bulik CM. Gut feelings: a role for the intestinal microbiota in anorexia nervosa? Int J Eat Disord 2015;48:449–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 1995;332:621–8. [DOI] [PubMed] [Google Scholar]
- 8.Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes 2015;39:1188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbate-Daga G, Amianto F, Delsedime N, De-Bacco C, Fassino S. Resistance to treatment in eating disorders: a critical challenge. BMC Psychiatry 2013;13:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 2016;164:337–40. [DOI] [PubMed] [Google Scholar]
- 11.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 2005;102:11070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duca FA, Lam TKT. Gut microbiota, nutrient sensing and energy balance. Diabetes Obes Metab 2014;16:68–76. [DOI] [PubMed] [Google Scholar]
- 13.Bakker GJ, Zhao J, Herrema H, Nieuwdorp M. Gut microbiota and energy expenditure in health and obesity. J Clin Gastroenterol 2015;49:S13–9. [DOI] [PubMed] [Google Scholar]
- 14.Bauer PV, Hamr SC, Duca FA. Regulation of energy balance by a gut-brain axis and involvement of the gut microbiota. Cell Mol Life Sci 2015;73:737–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaut M. Gut microbiota and energy balance: role in obesity. Proc Nutr Soc 2015;74:227–34. [DOI] [PubMed] [Google Scholar]
- 16.Kairupan TS, Amatani H, Cheng KC, Runtuwene J, Asakawa A, Inui A. Role of gastrointestinal hormones in feeding behavior and obesity treatment. J Gastroenterol 2016;51:93–103. [DOI] [PubMed] [Google Scholar]
- 17.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–31. [DOI] [PubMed] [Google Scholar]
- 18.Ley RE, Turnbaugh P, Klein S, Gordon J. Microbial ecology: human gut microbes associated with obesity. Nature 2006;444:1022–3. [DOI] [PubMed] [Google Scholar]
- 19.Tremaroli V, Kovatcheva-Datchary P, Bäckhed F. A role for the gut microbiota in energy harvesting? Gut 2010;59:1589–90. [DOI] [PubMed] [Google Scholar]
- 20.Genton L, Cani PD, Schrenzel J. Alterations of gut barrier and gut microbiota in food restriction, food deprivation and protein-energy wasting. Clin Nutr 2015;34:341–9. [DOI] [PubMed] [Google Scholar]
- 21.Chacko A, Cummings JH. Nitrogen losses from the human small bowel: obligatory losses and the effect of physical form of food. Gut 1988;29:809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummings JH. Short chain fatty acids in the human colon. Gut 1981;22:763–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos N, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010;18:190–5. [DOI] [PubMed] [Google Scholar]
- 24.Hartstra AV, Bouter KEC, Bäckhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care 2015;38:159–65. [DOI] [PubMed] [Google Scholar]
- 25.Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol 2013;11:639–47. [DOI] [PubMed] [Google Scholar]
- 26.Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TMS, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes 2014;4:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009;58:1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun 2014;5:3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.den Besten G, Lange K, Havinga R, van Dijk TH, Gerding A, van Eunen K, Müller M, Groen AK, Hooiveld GJ, Bakker BM, et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am J Physiol Gastrointest Liver Physiol 2013;305:G900–10. [DOI] [PubMed] [Google Scholar]
- 30.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014;156:84–96. [DOI] [PubMed] [Google Scholar]
- 31.den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, Oosterveer MH, Jonker JW, Groen AK, Reijngoud D-J, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes 2015;64:2398–408. [DOI] [PubMed] [Google Scholar]
- 32.Murphy EF, Cotter PD, Healy S, Marques TM, O’Sullivan O, Fouhy F, Clarke SF, O’Toole PW, Quigley EM, Stanton C, et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut 2010;59:1635–42. [DOI] [PubMed] [Google Scholar]
- 33.Reinehr T, Roth CL. The gut sensor as regulator of body weight. Endocrine 2015;49:35–50. [DOI] [PubMed] [Google Scholar]
- 34.Loper HB, La Sala M, Dotson C, Steinle N. Taste perception, associated hormonal modulation, and nutrient intake. Nutr Rev 2015;73:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berthoud H-R, Earle T, Zheng H, Patterson LM, Phifer C. Food-related gastrointestinal signals activate caudal brainstem neurons expressing both NMDA and AMPA receptors. Brain Res 2001;915:143–54. [DOI] [PubMed] [Google Scholar]
- 36.Ahlman H, Nilsson O. The gut as the largest endocrine organ in the body. Ann Oncol 2001;12(Suppl 2):S63–8. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661–71. [DOI] [PubMed] [Google Scholar]
- 38.Woods SC, D’Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab 2008;93:S37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bewick GA, Dhillo WS, Darch SJ, Murphy KG, Gardiner JV, Jethwa PH, Wing MK, Ghatei MA, Bloom SR. Hypothalamic cocaine- and amphetamine-regulated transcript (CART) and agouti-related protein (AgRP) neurons coexpress the NOP1 receptor and nociceptin alters CART and AgRP release. Endocrinology 2005;146:3526–34. [DOI] [PubMed] [Google Scholar]
- 40.Morton G, Schwartz M. Central nervous system control of food intake and body weight. Nat Rev 2006;443:289–95. [DOI] [PubMed] [Google Scholar]
- 41.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav 2006;89:71–84. [DOI] [PubMed] [Google Scholar]
- 42.Blom WA, Lluch A, Vinoy S, Stafleu A, van den Berg R, Holst JJ, Kok FJ, Hendriks HF. Effects of gastric emptying on the postprandial ghrelin response. Am J Physiol Endocrinol Metab 2006;290:E389–95. [DOI] [PubMed] [Google Scholar]
- 43.Garin MC, Burns CM, Kaul S, Cappola AR. The human experience with ghrelin administration. J Clin Endocrinol Metab 2013;98:1826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DGA, Ghatei MA, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 1997;138:5079–82. [DOI] [PubMed] [Google Scholar]
- 45.Ledderose C, Kreth S, Beiras-Fernandez A. Ghrelin, a novel peptide hormone in the regulation of energy balance and cardiovascular function. Recent Pat Endocr Metab Immune Drug Discov 2011;5:1–6. [DOI] [PubMed] [Google Scholar]
- 46.Queipo-Ortuño MI, Seoane LM, Murri M, Pardo M, Gomez-Zumaquero JM, Cardona F, Casanueva F, Tinahones FJ. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One. 2013;8(5). doi:10.1371/journal.pone.0065465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mishra AK, Dubey V. Obesity: an overview of possible role(s) of gut hormones, lipid sensing and gut microbiota. Metabolism 2016;65:48–65. [DOI] [PubMed] [Google Scholar]
- 48.Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature 2013;503:111–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chandra R, Liddle RA. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes 2007;14:63–7. [DOI] [PubMed] [Google Scholar]
- 50.Grider JR. Role of cholecystokinin in the regulation of gastrointestinal motility. J Nutr 1994;124(8 Suppl):1334S–1339S. [DOI] [PubMed] [Google Scholar]
- 51.Raybould HE. Mechanisms of CCK signaling from gut to brain. Curr Opin Pharmacol 2007;7:570–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012;490:55–60. [DOI] [PubMed] [Google Scholar]
- 53.Dubé PE, Brubaker PL. Nutrient, neural and endocrine control of glucagon-like peptide secretion. Horm Metab Res 2004;36:755–60. [DOI] [PubMed] [Google Scholar]
- 54.Näslund E, Gutniak M, Skogar S, Rössner S, Hellström PM. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr 1998;68:525–30. [DOI] [PubMed] [Google Scholar]
- 55.Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996;379:69–72. [DOI] [PubMed] [Google Scholar]
- 56.Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JRC, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY 3–36 and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 2005;1044:127–31. [DOI] [PubMed] [Google Scholar]
- 57.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–57. [DOI] [PubMed] [Google Scholar]
- 58.Batterham RL, Le Roux CW, Cohen MA, Park AJ, Ellis SM, Patterson M, Frost GS, Ghatei MA, Bloom SR. Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab 2003;88:3989–92. [DOI] [PubMed] [Google Scholar]
- 59.Dakin CL, Small CJ, Park AJ, Seth A, Ghatei MA, Bloom SR. Repeated ICV administration of oxyntomodulin causes a greater reduction in body weight gain than in pair-fed rats. Am J Physiol Endocrinol Metab 2002;283:E1173–7. [DOI] [PubMed] [Google Scholar]
- 60.Breton J, Tennoune N, Lucas N, Francois M, Legrand R, Jacquemot J, Goichon A, Guérin C, Peltier J, Pestel-Caron M, et al. Gut commensal E. coli proteins activate host satiety pathways following nutrient-induced bacterial growth. Cell Metab 2016;23:1–11. [DOI] [PubMed] [Google Scholar]
- 61.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 2003;278:11312–9. [DOI] [PubMed] [Google Scholar]
- 62.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012;61:364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond) 2015;39:424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res 2006;324:353–60. [DOI] [PubMed] [Google Scholar]
- 65.Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, Danna SC, Tripathy S, Hegsted M, Keenan MJ. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab 2008;295:E1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou J, Hegsted M, McCutcheon KL, Keenan MJ, Xi X, Raggio AM, Martin RJ. Peptide YY and proglucagon mRNA expression patterns and regulation in the gut. Obesity 2006;14:683–9. [DOI] [PubMed] [Google Scholar]
- 67.Cani PD, Neyrinck AM, Maton N, Delzenne NM. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like peptide-1. Obes Res 2005;13:1000–7. [DOI] [PubMed] [Google Scholar]
- 68.Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, De Backer F, Neyrinck AM, Delzenne NM. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr 2009;90:1236–43. [DOI] [PubMed] [Google Scholar]
- 69.Parnell JA, Reimer RA. Weight loss during oligofructose supplemen- tation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr 2009;89:1751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pénicaud L, Cousin B, Leloup C, Lorsignol A, Casteilla L. The autonomic nervous system, adipose tissue plasticity, and energy balance. Nutrition 2000;16:903–8. [DOI] [PubMed] [Google Scholar]
- 71.Schwartz MW, Gelling RW. Rats lighten up with MCH antagonist. Nat Med 2002;8:779–81. [DOI] [PubMed] [Google Scholar]
- 72.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab 2007;5:237–52. [DOI] [PubMed] [Google Scholar]
- 73.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol 2008;8:923–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioural and neural responsivity to CCK. Am J Physiol 1997;276:R145–9. [DOI] [PubMed] [Google Scholar]
- 75.Stocker CJ, Cawthorne MA. The influence of leptin on early life programming of obesity. Trends Biotechnol 2008;26:545–51. [DOI] [PubMed] [Google Scholar]
- 76.Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA 2004;101:1045–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakata M, Okada T, Ozawa K, Yada T. Resistin induces insulin resistance in pancreatic islets to impair glucose-induced insulin release. Biochem Biophys Res Commun 2007;353:1046–51. [DOI] [PubMed] [Google Scholar]
- 78.Pravenec M, Kazdová L, Landa V, Zídek V, Mlejnek P, Jansa P, Wang J, Qi N, Kurtz TW. Transgenic and recombinant resistin impair skeletal muscle glucose metabolism in the spontaneously hypertensive rat. J Biol Chem 2003;278:45209–15. [DOI] [PubMed] [Google Scholar]
- 79.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 2004;101:15718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau L, Griffi NW, Lombard V, Henrissat B, Bain JR, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice gut microbiota from twins metabolism in mice. Science 2013;341:1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto J-M, Kennedy S, et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013;500:541–6. [DOI] [PubMed] [Google Scholar]
- 82.Kong LC, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL, Zucker J, Doré J, Clément K. Gut microbiota after gastric bypass in human obesity:\rincreased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98:16–24. [DOI] [PubMed] [Google Scholar]
- 83.Liou AP, Paziuk M, Luevano J-M, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 2013;5:178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davis MP, Dreicer R, Walsh D, Lagman R, LeGrand SB. Appetite and cancer-associated anorexia: a review. J Clin Oncol 2004;22:1510–7. [DOI] [PubMed] [Google Scholar]
- 85.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 2013;339:172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011;11:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Batista ML, Peres SB, McDonald ME, Alcantara PSM, Olivan M, Otoch JP, Farmer SR, Seelaender M. Adipose tissue inflammation and cancer cachexia: possible role of nuclear transcription factors. Cytokine 2012;57:9–16. [DOI] [PubMed] [Google Scholar]
- 88.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 2012;18:363–74. [DOI] [PubMed] [Google Scholar]
- 91.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009;137:1716–24.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Olden C, Groen AK, Nieuwdorp M. Role of intestinal microbiome in lipid and glucose metabolism in diabetes mellitus. Clin Ther 2015;37:1172–7. [DOI] [PubMed] [Google Scholar]
- 93.Han J, Lin H. Intestinal microbiota and type 2 diabetes: from mechanism insights to therapeutic perspective. World J Gastroenterol 2014;20:17737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, et al. Maternal and child undernutrition and overweight in low income and middle-income countries. The Lancet 382;9890:427–51. [DOI] [PubMed] [Google Scholar]
- 95.Blanton LV, Barratt MJ, Charbonneau MR, Ahmed T, Gordon JI. Childhood undernutrition, the gut microbiota, and microbiota-directed therapeutics. Science 2016; 352:1533. [DOI] [PubMed] [Google Scholar]
- 96.Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, Kau AL, Rich SS, Concannon P, Mychaleckyj JC, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 2013;339:548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, Subramanian S, Manary MJ, Trehan I, Jorgensen JM, et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016;351:aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.American Psychiatric Association. Feeding and eating disorders [Internet]. [cited 2016 Jul 5]. Available from: http://www.dsm5.org/documents/eating%20disorders%20fact%20sheet.pdf.
- 99.Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in lactobacillus in obese patients and methanogens in anorexic patients. PLoS One 2009;4:e7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morita C, Tsuji H, Hata T, Gondo M, Takakura S, Kawai K, Yoshihara K, Ogata K, Nomoto K, Miyazaki K, et al. Gut dysbiosis in patients with anorexia nervosa. PLoS One 2015; 10:e0145274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mack I, Cuntz U, Grämer C, Niedermaier S, Pohl C, Schwiertz A, Zimmermann K, Zipfel S, Enck P, Penders J. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci Rep 2016;6:26752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bindels LB, Delzenne NM. Muscle wasting: the gut microbiota as a new therapeutic target? Int J Biochem Cell Biol 2013;45:2186–90. [DOI] [PubMed] [Google Scholar]
- 103.Foxx-Orenstein AE, Chey WD. Manipulation of the gut microbiota as a novel treatment strategy for gastrointestinal disorders. Am J Gastroenterol Suppl 2012;1:41–6. [Google Scholar]
- 104.Angelakis E, Merhej V, Raoult D. Related actions of probiotics and antibiotics on gut microbiota and weight modification. Lancet Infect Dis 2013;13:889–99. [DOI] [PubMed] [Google Scholar]
- 105.Kerac M, Bunn J, Seal A, Thindwa M, Tomkins A, Sadler K, Bahwere P, Collins S. Probiotics and prebiotics for severe acute malnutrition (PRONUT study): a double-blind efficacy randomised controlled trial in Malawi. Lancet 2009;374:136–44. [DOI] [PubMed] [Google Scholar]
- 106.Agarwal KN, Bhasin SK. Feasibility studies to control acute diarrhoea in children by feeding fermented milk preparations Actimel and Indian Dahi. Eur J Clin Nutr 2002;56(Suppl 4):S56–9. [DOI] [PubMed] [Google Scholar]
- 107.Million M, Angelakis E, Paul M, Armougom F, Leibovici L, Raoult D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog 2012;53:100–8. [DOI] [PubMed] [Google Scholar]
- 108.Kitajima H, Sumida Y, Tanaka R, Yuki N, Takayama H, Fujimura M. Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 1997;76:F101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vendt N, Grunberg H, Tuure T, Malminiemi O, Wuolijoki E, Tillmann V, Sepp E, Korpela R. Growth during the first 6 months of life in infants using formula enriched with Lactobacillus rhamnosus GG: double blind, randomized trial. J Hum Nutr Diet 2006;19:51–8. [DOI] [PubMed] [Google Scholar]
- 110.Canchaya C, Claesson MJ, Fitzgerald GF, van Sinderen D, O’Toole PW. Diversity of the genus Lactobacillus revealed by comparative genomics of five species. Microbiology 2006;152:3185–96. [DOI] [PubMed] [Google Scholar]
- 111.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 2013;110:9066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia municiphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 2004;54:1469–76. [DOI] [PubMed] [Google Scholar]
- 113.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007;50:2374–83. [DOI] [PubMed] [Google Scholar]
- 114.Woting A, Pfeiffer N, Hanske L, Loh G, Klaus S, Blaut M. Alleviation of high fat diet-induced obesity by oligofructose in gnotobiotic mice is independent of presence of Bifidobacterium longum. Mol Nutr Food Res 2015;59:2267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smits LP, Bouter KEC, De Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology 2013;145:946–53. [DOI] [PubMed] [Google Scholar]
- 116.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 2009;1:6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012;143:913–6.e7. [DOI] [PubMed] [Google Scholar]
- 118.Alang N, Kelly CR. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis 2015;2:ofv004. [DOI] [PMC free article] [PubMed] [Google Scholar]