Abstract
Single cell suspensions, prepared from brain stem, cerebellum, and forebrain parenchyma of embryonic and adult mice, were plated on monolayers of an astroglial cell line derived from a spontaneously immortalized mouse cerebellar culture, the D19 clone. A few of the brain cells adhering to the D19 monolayers were immunoreactive to the Mac-1 antibody, which labels all cells of the monocytic and granulocytic lineages. The Mac-1-positive cells proliferated vigorously and later most of them acquired the F4/80 epitope specific for macrophages and microglia cells. Studies in clonal conditions allowed development of large colonies of about 2 x 10(5) cells that expressed typical microglia markers. Bone marrow Mac-1-positive cells cocultured on D19 monolayers were also induced to proliferate, whereas peritoneal macrophages were not. D19 astrocytes express macrophage colony-stimulating factor (CSF-1) activity at a high level, and their conditioned media induced the proliferation of brain and bone marrow Mac-1-positive cells. A specific anti-CSF-1 antiserum completely blocked bone marrow macrophage progenitor proliferation and significantly reduced the multiplication of microglial precursors induced by the D19-conditioned medium. These data indicate that the embryonic and adult mouse brain parenchyma contains potential progenitors for microglial cells.
Full text
PDF



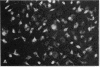
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alliot F., Delhaye-Bouchaud N., Geffard M., Pessac B. Role of astroglial cell clones in the survival and differentiation of cerebellar embryonic neurons. Brain Res Dev Brain Res. 1988 Dec 1;44(2):247–257. doi: 10.1016/0165-3806(88)90223-4. [DOI] [PubMed] [Google Scholar]
- Alliot F., Pessac B. Astrocytic cell clones derived from established cultures of 8-day postnatal mouse cerebella. Brain Res. 1984 Jul 23;306(1-2):283–291. doi: 10.1016/0006-8993(84)90377-9. [DOI] [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett P. F. Pluripotential hemopoietic stem cells in adult mouse brain. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2722–2725. doi: 10.1073/pnas.79.8.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biguet N. F., Buda M., Lamouroux A., Samolyk D., Mallet J. Time course of the changes of TH mRNA in rat brain and adrenal medulla after a single injection of reserpine. EMBO J. 1986 Feb;5(2):287–291. doi: 10.1002/j.1460-2075.1986.tb04211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O., Birnberg N., Herbert E. Detection and quantitation of pro-opiomelanocortin mRNA in pituitary and brain tissues from different species. J Biol Chem. 1982 Jun 25;257(12):6783–6787. [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Giulian D., Baker T. J. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986 Aug;6(8):2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber M. B., Tetzlaff W., Streit W. J., Kreutzberg G. W. Microglial cells but not astrocytes undergo mitosis following rat facial nerve axotomy. Neurosci Lett. 1988 Mar 10;85(3):317–321. doi: 10.1016/0304-3940(88)90585-x. [DOI] [PubMed] [Google Scholar]
- Hetier Emmanuelle, Ayala Jésus, Bousseau Anne, Denèfle Patrice, Prochiantz Alain. Amoeboid Microglial Cells and not Astrocytes Synthesize TNF-alpha in Swiss Mouse Brain Cell Cultures. Eur J Neurosci. 1990;2(9):762–768. doi: 10.1111/j.1460-9568.1990.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I., Alliot F., Pessac B., Robel P., Baulieu E. E. Localisation immunocytochimique du cytochrome P-450scc dans les oligodendrocytes de rat en culture. C R Acad Sci III. 1989;308(6):165–170. [PubMed] [Google Scholar]
- Morstyn G., Burgess A. W. Hemopoietic growth factors: a review. Cancer Res. 1988 Oct 15;48(20):5624–5637. [PubMed] [Google Scholar]
- Perry V. H., Hume D. A., Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985 Jun;15(2):313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Pringle N., Mosley M. J., Westermark B., Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988 Apr 22;53(2):309–319. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- Risau W., Wolburg H. Development of the blood-brain barrier. Trends Neurosci. 1990 May;13(5):174–178. doi: 10.1016/0166-2236(90)90043-a. [DOI] [PubMed] [Google Scholar]
- Sachs L. The molecular control of blood cell development. Science. 1987 Dec 4;238(4832):1374–1379. doi: 10.1126/science.3317831. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]