Abstract
Introduction:
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first recognition during pregnancy.[1] Pregnancy is a unique situation in which there is a physiological temporary increase in insulin resistance (IR). The mechanisms responsible for the gestational-induced IR are not completely understood. The current study was undertaken to compare adiponectin levels during 24–28 weeks period of gestation in drug-naive newly diagnosed GDM women with a cohort of normoglycemic pregnant women.
Subjects and Methods:
A total of 47 pregnant women in the age group of 18–40 years were included in this cross-sectional study, of which 13 were GDM cases and 34 were normoglycemic controls. Serum adiponectin level was analyzed by enzyme-linked immunosorbent assay.
Results:
The mean adiponectin level was 16.92 ng/ml (standard deviation [SD] = 2.78) and 19.38 ng/ml (SD = 2.71) in case and control groups, respectively, and the difference was found to be statistically significant (P = 0.008).
Conclusion:
Our study demonstrated decreased serum adiponectin levels in women with GDM when compared with age- and body mass index-matched euglycemic pregnant women.
Key words: Adiponectin, antenatal screening, gestational diabetes mellitus
INTRODUCTION
The global prevalence of hyperglycemia in pregnant women (20–49 years) is 16.9%, and more than 90% cases of hyperglycemia in pregnancy are estimated to occur in low- and middle-income countries.[2] An Indian national survey conducted in 2004 and a hospital-based survey conducted in 2008 that studied the prevalence of gestational diabetes mellitus (GDM) in Indian women showed a prevalence of 16.55% and 21.6%, respectively.[3,4]
Insulin resistance (IR) during pregnancy is increased to about three times the resistance in the nonpregnant state. IR can be categorized as prereceptor, pertaining to insulin antibodies as in autoimmune diseases; receptor, with decreased receptors on the cell surface as seen in obesity; and postreceptor defects in the intracellular insulin signaling pathway. In pregnancy, decreased insulin sensitivity can be attributed to a postreceptor defect, resulting in the decreased ability of insulin to bring about GLUT4 mobilization from the interior cell to the cell surface.[5] This could be due to increase in the plasma levels of one or more of the pregnancy-associated hormones.[6]
There is an increase in the beta-cell mass as well as insulin levels throughout pregnancy. Failure to upregulate insulin production to overcome the degree of IR leads to hyperglycemia and GDM during pregnancy.[7]
Adipocyte-derived mediators such as nonesterified fatty acids and tumor necrosis factor-α have been proposed as factors that may link IR and beta-cell dysfunction in type 2 diabetes mellitus (T2DM). Another candidate is adiponectin, a novel adipocytokine with pleiotropic effects, including putative insulin sensitizing, antiatherogenic, and anti-inflammatory properties. Consistent with the low circulating levels of adiponectin observed in T2DM, adiponectin concentration is inversely related to both IR and central adiposity. Low adiponectin concentration at early stages can predict the development of IR while elevated baseline levels have been shown to be protective against development of T2DM.[8]
Screening for GDM and identifying the associated potential risks are important in all pregnant females. The current study was undertaken to correlate the relationship between adiponectin levels in drug-naive newly diagnosed GDM females with a cohort of normoglycemic pregnant females during 24–28 weeks of gestation.
SUBJECTS AND METHODS
After the institutional ethical clearance, the present study was undertaken in a tertiary care hospital in South India from June 2015 to August 2015.
Inclusion criteria
All pregnant women in the age group of 18–40 years, permanent residents of the city in 24–28 weeks of gestation, who underwent GDM screening either by one-step or by two-step ADA 2015 criteria, were included in the study. The controls were age matched with cases for ±2 years.
Exclusion criteria
The exclusion criteria included any preexisting medical condition such as (a) women with T1DM and T2DM, (b) those with preeclampsia, hypertension, polycystic ovary syndrome, and (c) women on treatment with drugs such as metformin, antihypertensives, and antidepressants.
Sample size
One of the previous studies in women with normal and pregnancies with complications has shown that the mean adiponectin levels were 8.2 mg/L (standard deviation [SD] = 2.88) in normoglycemic controls as compared to 5.2 mg/L (SD = 2.12) in GDM cases.[8] Based on the above findings and keeping the power of the study at 80% and α error at 5%, it is estimated that 11 women needed to be included in each study group. However, in the present study, 13 women with GDM as cases and 34 without GDM as controls were included in the ratio of 1:3 to have better precision. The sample size was calculated using n Master software (Biostatistics Resource and Training Centre, Vellore).
Baseline evaluation
Screening with either 50 g oral glucose challenge test followed by fasting 100 g oral glucose tolerance test (OGTT) or fasting 75 g OGTT was performed in all women between 24 and 28 weeks of gestation. Diagnosis of GDM was established according to the diagnostic criteria of the American Diabetes Association 2015.[9] Plasma glucose was measured by glucose oxidase method. Blood samples for serum adiponectin were collected, sera were separated and stored at −20°C, and subsequently, adiponectin levels were estimated by enzyme-linked immunosorbent assay (ELISA) method with RayBio® Human Adiponectin (ACRP30) ELISA Kit.
Clinical parameters recorded included (a) patient demographics; (b) information regarding current pregnancy including illnesses and medications; (c) personal, medical, and obstetric history and specific GDM risk factors assessed including age, prepregnancy weight, and anthropometric measurements of height (measured to nearest 0.5 cm) and weight (measured to nearest 0.25 kg).
Statistical analyses
Continuous measurements such as glucose, serum adiponectin level, and body mass index (BMI) were expressed as mean with SD and median with interquartile range. Categorical variables were presented as proportions. Differences in the mean adiponectin levels between the case and control groups were tested by Student's t-test, and in case of nonnormality, Mann–Whitney U-test was employed. All the analyses were carried out employing SPSS Inc. Released 2009. PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc. Probability value of ≤5% was considered statistically significant.
RESULTS
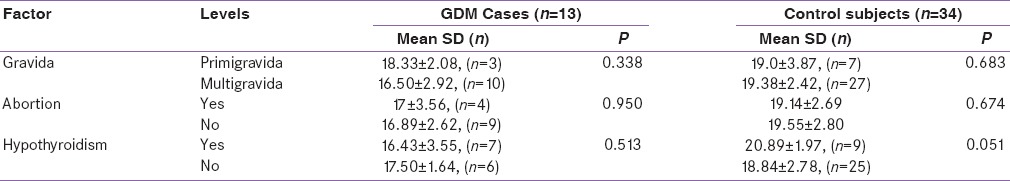
In the study, 47 pregnant women were enrolled, of which 13 were GDM (27.6%) and 34 (72.3%) were normoglycemic. The mean age of females with GDM was 28.9 years (SD = 3.64) as compared to 27.3 years (SD = 3.27) for the controls and was not statistically significant (P = 0.138). The mean adiponectin level was 16.92 ng/ml (SD = 2.78) and 19.38 ng/ml (SD = 2.71) in case and control groups, respectively, and the differences were statistically significant (P = 0.008) [Table 1]. The mean prepregnancy BMI was 24.6 kg/m2 (SD = 3.47) and 23.8 kg/m2 (SD = 4.79) for the case and control groups, respectively, and the difference was not statistically significant (P = 0.569). Similarly, it was also noted that 53.8% of the cases had a BMI >24.9 kg/m2 in comparison to 64.7% of the controls. It was also observed that 53.8% of the GDM group had a history of hypothyroidism as compared to 26.5% of the controls. The differences were statistically not significant (P = 0.096). Both groups were equally matched for parity with 76.9% multipara mothers in GDM group and 79.4% in the control group [Table 2].
Table 1.
Serum Adiponectin level in two groups
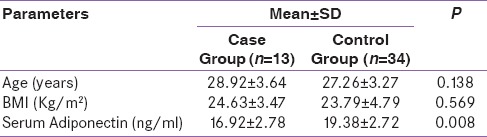
Table 2.
Serum Adiponectin Level Gravida, Abortion and Hypothyroidism
DISCUSSION
GDM in pregnancy is characterized by impaired glucose tolerance which can be first recognized as low serum adiponectin levels during screening.[10,11] β-cell reserve is insufficient to compensate for the increased IR that occurs during pregnancy. Changes in β-cell mass adapt to physiological needs and increased functional demands.[12] These β-cell mass changes can be achieved by neogenesis, hyperplasia, and hypertrophy. The β-cell proliferation during pregnancy is stimulated by placental lactogen, prolactin, and growth hormone, which have similar effects on β-cells and are also elevated during pregnancy.[13] Reduced serum adiponectin level inhibits insulin-stimulated amino acid transport across the placenta and has an important implication for placental nutrient transport and fetal growth in pregnancy.
In this study, we demonstrate that low serum adiponectin level is an independent correlate of beta-cell function in mid-pregnancy. Mean adiponectin level was significantly lower in GDM cases (16.92 ng/ml, SD = 2.78) compared to normoglycemic controls (19.38 ng/ml, SD = 2.71). Reduced adiponectin levels in GDM have been reported by previous studies.[14,15,16] A study from India has also shown lower adiponectin concentrations in women with gestational diabetes mellitus, compared to those having a normal pregnancy.[17] Previous studies have reported negative correlation between adiponectin level and BMI in both GDM and normoglycemic pregnant women.[10,18,19] In our study, similar correlation could not be seen between the two groups possibly due to small sample size.
With over a decade of data evaluating adiponectin, it emerges as an important factor potentially linking IR and beta-cell dysfunction in the pathogenesis of diabetes. In the present study age, parity and BMI factors were comparable with cases and controls, thereby making the groups more comparable. The possible reason for not observing statistically significant difference in the prevalence of hypothyroidism could be due to small numbers in the GDM group.
CONCLUSION
Our study has demonstrated decreased serum adiponectin levels in women with GDM when compared with age- and BMI-matched euglycemic pregnant women. The data suggest that low adiponectin levels in GDM may be an early event in the natural history of the disease and could be an important one-step analysis in antenatal screening for GDM.
Limitations
The study was of a small sample size.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21(Suppl 2):B161–7. [PubMed] [Google Scholar]
- 2.Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103:176–85. doi: 10.1016/j.diabres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Seshiah V, Balaji V, Balaji MS, Sanjeevi CB, Green A. Gestational diabetes mellitus in India. J Assoc Physicians India. 2004;52:707–11. [PubMed] [Google Scholar]
- 4.Swami SR, Mehetre R, Shivane V, Bandgar TR, Menon PS, Shah NS. Prevalence of carbohydrate intolerance of varying degrees in pregnant females in Western India (Maharashtra) – A hospital-based study. J Indian Med Assoc. 2008;106:712–4, 735. [PubMed] [Google Scholar]
- 5.Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction. 2010;140:365–71. doi: 10.1530/REP-10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62:263–71. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Noaemi MC, Shalayel MH. Pathophysiology of gestational diabetes mellitus: The past, the present and the future. Gestational Diabetes. 2011;6:91–114. [Google Scholar]
- 8.Cortelazzi D, Corbetta S, Ronzoni S, Pelle F, Marconi A, Cozzi V, et al. Maternal and foetal resistin and adiponectin concentrations in normal and complicated pregnancies. Clin Endocrinol (Oxf) 2007;66:447–53. doi: 10.1111/j.1365-2265.2007.02761.x. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes. 2015;33:97–111. doi: 10.2337/diaclin.33.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. Reduced adiponectin concentration in women with gestational diabetes: A potential factor in progression to type 2 diabetes. Diabetes Care. 2004;27:799–800. doi: 10.2337/diacare.27.3.799. [DOI] [PubMed] [Google Scholar]
- 11.Al-Badri MR, Zantout MS, Azar ST. The role of adipokines in gestational diabetes mellitus. Ther Adv Endocrinol Metab. 2015;6:103–8. doi: 10.1177/2042018815577039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballesteros M, Simón I, Vendrell J, Ceperuelo-Mallafré V, Miralles RM, Albaiges G, et al. Maternal and cord blood adiponectin multimeric forms in gestational diabetes mellitus: A prospective analysis. Diabetes Care. 2011;34:2418–23. doi: 10.2337/dc11-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doruk M, Ugur M, Oruç AS, Demirel N, Yildiz Y. Serum adiponectin in gestational diabetes and its relation to pregnancy outcome. J Obstet Gynaecol. 2014;34:471–5. doi: 10.3109/01443615.2014.902430. [DOI] [PubMed] [Google Scholar]
- 14.Worda C, Leipold H, Gruber C, Kautzky-Willer A, Knöfler M, Bancher-Todesca D. Decreased plasma adiponectin concentrations in women with gestational diabetes mellitus. Am J Obstet Gynecol. 2004;191:2120–4. doi: 10.1016/j.ajog.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 15.Kinalski M, Telejko B, Kuzmicki M, Kretowski A, Kinalska I. Tumor necrosis factor alpha system and plasma adiponectin concentration in women with gestational diabetes. Horm Metab Res. 2005;37:450–4. doi: 10.1055/s-2005-870238. [DOI] [PubMed] [Google Scholar]
- 16.Altinova AE, Toruner F, Bozkurt N, Bukan N, Karakoc A, Yetkin I, et al. Circulating concentrations of adiponectin and tumor necrosis factor-alpha in gestational diabetes mellitus. Gynecol Endocrinol. 2007;23:161–5. doi: 10.1080/09513590701227960. [DOI] [PubMed] [Google Scholar]
- 17.Saini V, Kataria M, Yadav A, Jain A. Role of leptin and adiponectin in gestational diabetes mellitus: A study in a North Indian tertiary care hospital. Internet J Med Update EJ. 2015;10:11–4. [Google Scholar]
- 18.Ranheim T, Haugen F, Staff AC, Braekke K, Harsem NK, Drevon CA. Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta Obstet Gynecol Scand. 2004;83:341–7. doi: 10.1111/j.0001-6349.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 19.Lowe LP, Metzger BE, Lowe WL, Jr, Dyer AR, McDade TW, McIntyre HD. HAPO Study Cooperative Research Group. Inflammatory mediators and glucose in pregnancy: Results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin Endocrinol Metab. 2010;95:5427–34. doi: 10.1210/jc.2010-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]


