ABSTRACT
Flaviviruses, such as Zika virus, yellow fever virus, dengue virus, and West Nile virus (WNV), are a serious concern for human health. Flaviviruses produce an abundant noncoding subgenomic flavivirus RNA (sfRNA) in infected cells. sfRNA results from stalling of the host 5′-3′ exoribonuclease XRN1/Pacman on conserved RNA structures in the 3′ untranslated region (UTR) of the viral genomic RNA. sfRNA production is conserved in insect-specific, mosquito-borne, and tick-borne flaviviruses and flaviviruses with no known vector, suggesting a pivotal role for sfRNA in the flavivirus life cycle. Here, we investigated the function of sfRNA during WNV infection of Culex pipiens mosquitoes and evaluated its role in determining vector competence. An sfRNA1-deficient WNV was generated that displayed growth kinetics similar to those of wild-type WNV in both RNA interference (RNAi)-competent and -compromised mosquito cell lines. Small-RNA deep sequencing of WNV-infected mosquitoes indicated an active small interfering RNA (siRNA)-based antiviral response for both the wild-type and sfRNA1-deficient viruses. Additionally, we provide the first evidence that sfRNA is an RNAi substrate in vivo. Two reproducible small-RNA hot spots within the 3′ UTR/sfRNA of the wild-type virus mapped to RNA stem-loops SL-III and 3′ SL, which stick out of the three-dimensional (3D) sfRNA structure model. Importantly, we demonstrate that sfRNA-deficient WNV displays significantly decreased infection and transmission rates in vivo when administered via the blood meal. Finally, we show that transmission and infection rates are not affected by sfRNA after intrathoracic injection, thereby identifying sfRNA as a key driver to overcome the mosquito midgut infection barrier. This is the first report to describe a key biological function of sfRNA for flavivirus infection of the arthropod vector, providing an explanation for the strict conservation of sfRNA production.
IMPORTANCE Understanding the flavivirus transmission cycle is important to identify novel targets to interfere with disease and to aid development of virus control strategies. Flaviviruses produce an abundant noncoding viral RNA called sfRNA in both arthropod and mammalian cells. To evaluate the role of sfRNA in flavivirus transmission, we infected mosquitoes with the flavivirus West Nile virus and an sfRNA-deficient mutant West Nile virus. We demonstrate that sfRNA determines the infection and transmission rates of West Nile virus in Culex pipiens mosquitoes. Comparison of infection via the blood meal versus intrathoracic injection, which bypasses the midgut, revealed that sfRNA is important to overcome the mosquito midgut barrier. We also show that sfRNA is processed by the antiviral RNA interference machinery in mosquitoes. This is the first report to describe a pivotal biological function of sfRNA in arthropods. The results explain why sfRNA production is evolutionarily conserved.
INTRODUCTION
Viruses from the genus Flavivirus (family Flaviviridae), such as the highly pathogenic dengue virus (DENV) (1), the emerging Zika virus (2), and West Nile virus (WNV) (3, 4), are important threats to human health and are collectively responsible for millions of annual reported cases of infection (5). Flaviviruses are positive-sense single-stranded RNA (ssRNA) viruses that can infect many different hosts, including insects, ticks, birds, and mammals (6). They can be further divided based on their vector species into mosquito-borne flaviviruses (MBFs), tick-borne flaviviruses (TBFs), and insect-specific flaviviruses (ISFs) and flaviviruses with no known vector (NKVFs). The epidemic potential of flaviviruses was clearly demonstrated when WNV was first detected in New York City in 1999, reaching the West Coast of the United States only 4 years later, causing the largest outbreak of WNV-induced neuroinvasive disease to date (7). In southern Europe, lineage I WNV outbreaks were reported as early as the 1960s (8), but more recently, a novel pathogenic lineage II WNV isolate was identified (4, 9, 10). In 2010, in Greece, an exceptionally severe outbreak of this lineage II WNV strain resulted in 197 patients with neuroinvasive disease and 33 deaths (4). Understanding the determinants of transmission of these flaviviruses is important to evaluate their epidemic potential and is the first step in designing novel ways to break the flavivirus transmission cycle.
The overall capacity of a mosquito to become orally infected after an infectious blood meal and to transmit the virus to the next vertebrate host is expressed as the vector competence (11). For a successful infection of the mosquito vector, arboviruses have to overcome the midgut infection and escape barriers, as well as the salivary gland barriers (12). After ingestion of a viremic blood meal, the midgut epithelial cells have to become infected. These cells have a strong RNA interference (RNAi) response that restricts virus infection and acts as a bottleneck on virus population diversity (13, 14). Next, the virus has to replicate in the midgut cells, produce progeny virus, and escape from the midgut cells through the basal lamina into the hemolymph. Subsequently, the virus disseminates to other organs and ultimately infects the salivary glands. After sufficiently high virus titers in the saliva have been reached, viruses can be transmitted to a new vertebrate host via a bite by the infected mosquito.
Vector competence is influenced by viral, environmental (e.g., temperature and the microbiome), and vector-related factors, including vector genetics and antiviral responses (11). In arthropods, antiviral responses are predominantly mediated by RNAi (13, 15), and also by Toll, IMD, and JAK/STAT signaling pathways and apoptosis (16, 17). Three distinct insect small-RNA pathways can be discriminated: the 21-nucleotide (nt) small interfering RNA (siRNA) response, the ∼21- to 22-nt microRNA (miRNA) pathway, and the 25- to 30-nt PIWI-interacting RNA (piRNA) response (18–20). In Culex mosquitoes—the predominant group of WNV vectors—virus-specific siRNAs are highly abundant, whereas viral piRNAs (vpiRNAs) appear to be absent (14, 21). For flaviviruses, three putative viral suppressors of RNAi (VSRs) have been identified in vitro: the NS4B and NS3 proteins of DENV type 2 (DENV-2) and the noncoding subgenomic flavivirus RNA (sfRNA) for WNV (22–24).
During viral replication in both vertebrate and invertebrate host cells, flaviviruses produce a highly abundant noncoding sfRNA approximately 0.5 kb in size. sfRNA is produced via a unique mechanism involving degradation of the viral genomic RNA (vgRNA) by the host 5′→3′ exoribonuclease XRN1/Pacman (25). XRN1 stalls at stem-loop (SL) and dumbbell (DB) RNA structures within the flaviviral 3′ untranslated region (UTR), resulting in the accumulation of sfRNA (26–29). The stalling of XRN1 occurs due to steric hindrance caused by interactions of pseudoknots (PK) and other tertiary RNA structures (30). During WNV infection, three species of sfRNA, named sfRNA1, -2, and -3, are produced by stalling of XRN1 on SL-II, SL-IV, and DB-1 (26, 29). The formation of sfRNA is important for replication in insect cells and several types of vertebrate cells, since sfRNA mutants that produce only sfRNA3 have attenuated replication rates, even in Dicer-2-deficient C6/36 cells (25, 28, 29, 31). In mammalian cells, sfRNA is essential for flavivirus-induced cytopathicity and pathogenicity (25). Importantly, sfRNA acts as an antagonist of both interferon- and retinoic acid-inducible gene-I-like receptor-dependent innate immune responses (31, 32). Accordingly, WNV mutants deficient in sfRNA production are attenuated in mice (25, 29).
Despite the clear functions of sfRNA in flavivirus pathogenesis in vertebrates, the role of sfRNA in the arthropod vector is poorly understood. sfRNA production is conserved for all MBFs and TBFs, and although recent RNA structure analysis of ISF 3′ UTRs failed to identify XRN1-resistant structures (33, 34), it was previously reported that ISFs and NKVFs have conserved SL structures with putative pseudoknot structures in their 3′ UTRs (35). Indeed, sfRNA production has been experimentally shown for several NKVFs and the ISF cell-fusing agent virus (36). The conservation of sfRNA production by MBFs, TBFs, and ISFs emphasizes the putative importance of sfRNA production in arthropods (35). So far, it has been demonstrated that sfRNA acts as a suppressor of miRNA- and siRNA-mediated RNAi in vitro in both mammalian and arthropod cells (23, 37). The highly abundant sfRNA most likely acts as a decoy substrate for Dicer to prevent it from cleaving other double-stranded RNA (dsRNA) molecules. Recently, sfRNA has been demonstrated to mildly suppress the RNAi pathway in vivo, but the biological significance of this suppression requires more detailed studies (38). Based on the high abundance of sfRNA in infected arthropod cells and the conservation of sfRNA in most, if not all, flavivirus genomes known to date (35), including those restricted to replication in arthropods, we hypothesize that sfRNA is a crucial factor for flavivirus transmission by the arthropod vector.
In this study, the importance of sfRNA for dissemination and transmission of WNV by Culex pipiens mosquitoes was investigated. By using a lineage II WNV infectious clone deficient in sfRNA formation, we investigated replication kinetics in mammalian cells and both RNAi-competent and -deficient mosquito cell lines. The putative role of sfRNA in modulating the RNAi response in vivo was determined by a deep-sequencing approach using viral small RNAs (vsRNAs). Notably, the importance of sfRNA in WNV infection and transmission by Culex mosquitoes was studied in vivo, and the role of the midgut epithelium was assessed by comparison of infections via the blood meal and via intrathoracic injections. The outcomes of this research highlight the importance of sfRNA for flavivirus transmission by mosquitoes and provide a biological explanation as to why sfRNA production is strictly conserved in the genus Flavivirus.
MATERIALS AND METHODS
Cell culture.
Aedes albopictus U4.4 and C6/36 (ATCC CRL-1660) mosquito cells were cultured in Leibovitz L-15 medium (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco), 2% tryptose phosphate broth (Gibco), and 1% nonessential amino acids (Gibco). Culex tarsalis Cx.t cells (39) (CDC, Fort Collins, CO) were cultured in Schneider's medium (Gibco) supplemented with 10% FBS. All the mosquito cells were maintained as a monolayer in T25 cell culture flasks at 28°C. African green monkey kidney Vero E6 (ATCC CRL-1586) cells were cultured in Dulbecco's modified Eagle medium containing HEPES (DMEM-HEPES) (Gibco) supplemented with 10% FBS, penicillin (100 U/ml; Sigma-Aldrich), and streptomycin (100 μg/ml; Sigma-Aldrich). The cells were maintained as a monolayer in T25 cell culture flasks at 37°C and 5% CO2.
Generation of infectious clones and virus stocks.
The wild-type WNV lineage 2 isolate used in this study was isolated in southeastern Europe (GenBank KC496015.1) and is referred to below as WNVGR. The infectious clone of WNVGR, based on the same virus isolate, was supplied by Tamás Bakonyi from the Department of Microbiology and Infectious Diseases, Szent István University, Budapest, Hungary (40). The infectious clone is referred to below as WNVIC. A subclone of the virus was made by PCR amplification of the NS4-3′ UTR region with Phusion polymerase (New England BioLabs), using primers FW (5′-TGGCTGAAGTCCCAGGAACGA-3′) and RV (5′-TGGAAGTCCGAGCTCATCGCT-3′). The resulting amplicon was cloned into pJET1.2 (Fermentas), and site-directed mutagenesis was used to produce pJET1.2/subGR10ΔSF1, with mutations in the pseudoknot site of SL-II, with Phusion PCR using primers FW (5′-GAAGCTCACTAGACGGTGCTGCCTGCG-3′) and RV (5′-CTAGTGAGCTTCCGGTGGCAGCATTAATC-3′). Site-directed mutagenesis of pJET1.2/subGR10ΔSF1 with primers FW (5′-CTCTAGTGTGGCACTCTGCGGAGAGTGCAG-3′) and RV (5′-CGCAGAGTGCCACACTAGAGTGTGGTCTGAC-3′) was used to produce pJET1.2/subGR10ΔSF1+2 with mutations in SL-II and SL-IV. The NS4-3′ UTR region of pJET1.2/subGR10ΔSF1 or pJET1.2/subGR10ΔSF1+2 was cloned using SphI-HF (New England BioLabs) and Pfl23II (Fermentas) into WNVIC to produce WNVΔSF1 and WNVΔSF1+2. Virus stocks of WNVIC, WNVΔSF1, and WNVΔSF1+2 were produced by transfecting Vero cell monolayers with 800 ng infectious clone DNA using 2 μl of Lipofectamine 2000 (Invitrogen) in 6-well plates. Three days posttransfection (dpt), the supernatant was harvested and used to inoculate a T75 flask of C6/36 cells seeded 1 day in advance. Six days postinfection (dpi), the supernatant of C6/36 cells was harvested and stored at −80°C. All the viruses used in this study are from a second passage (P2).
Cell viability assay.
Cell monolayers were washed with 1× phosphate-buffered saline (PBS) and lysed using passive lysis buffer (PLB) (Promega) for 10 min at room temperature. Subsequently, CellTiter-Glo 2.0 reagent (Promega) was mixed with the PLB in a 1:1 ratio and incubated in the dark for 10 min at room temperature before measuring the luminescence using a Fluostar Optima microplate reader (BMG Labtech).
Virus titrations.
The 50% tissue culture infectious dose (TCID50) was determined using an endpoint dilution assay (EPDA) on Vero cells, as described previously (41). Briefly, Vero cell monolayers were detached using trypsin (Gibco) and diluted in DMEM supplemented with 10% FBS, penicillin, and streptomycin. Tenfold dilutions of the virus samples were made in culture media, and a Vero cell suspension (1.0 × 105 cells) was added in a 1:1 ratio. For each dilution, 10 μl was plated at 6-fold in a 60-well Micro-Well plate (Nunc) and scored 3 dpi for the presence of WNV by either cytopathic effect (CPE) or immunostaining using anti-E monoclonal antibodies.
Virus growth curves.
Monolayers of the indicated cell types were infected in a 6-well plate at a multiplicity of infection (MOI) of 1 by adding 1 ml of diluted virus to the cells. The cells were incubated in the presence of virus for 2 h and washed three times with 1× PBS. Two milliliters of fresh cell culture medium was added, and the cells were incubated at either 37°C with 5% CO2 (Vero cells) or at 28°C (mosquito cells). At the indicated times postinfection, 30 μl of samples of cell culture medium were frozen at −80°C until further processing. Virus samples were titrated on Vero cells by EPDA.
RNA isolation and Northern blotting.
Total RNA was isolated from cell monolayers using TRIzol reagent (Invitrogen) following the manufacturer's protocol. For RNA isolations performed on mosquito bodies, the bodies were homogenized in a Bullet blender using zirconium beads (Next Advance, New York, NY) for 2 min at maximum speed. DMEM (100 μl) was added, samples were centrifuged for 2 min at 12,000 × g, and the pellet was homogenized in 500 μl TRIzol reagent. An additional 75% ethanol wash step was included to ensure a clean RNA pellet. The isolated RNA was quantified with a NanoDrop UV photospectrometer and stored at −80°C. Five micrograms of total RNA was separated by denaturing gel electrophoresis in a 6% polyacrylamide–7 M urea–0.5× Tris-borate-EDTA (TBE) gel for 1.45 h at a constant voltage of 150 V. The RNA was semidry blotted on a Hybond-N membrane (Amersham Biosciences) for 60 min at a constant amperage of 0.2 A, UV cross-linked by 1 min of UV light exposure, and hybridized overnight with digoxigenin (DIG)-labeled probes in modified Church (0.36 M Na2HPO4, 0.14 M NaH2PO4, 1 mM EDTA, 7% [wt/vol] SDS)-10% formamide buffer at 50°C. DIG-labeled probes were made by PCR using Phusion polymerase (New England BioLabs) with DIG-labeled nucleotides (Roche) (FW, 5′-GCAGTCTGCGACAGTGCC-3′; RV, 5′-GTTGTGCAGAGCAGAAGATC-3′) using WNVIC as a template. The blots were stained with allophycocyanin (AP)-labeled anti-DIG (α-DIG) antibodies for 30 min (11093274910; Roche) and developed with nitroblue tetrazolium (NBT)-BCIP (5-bromo-4-chloro-3-indolylphosphate) solution (Roche) until a sufficient signal was achieved. The blots were scanned with a Bio-Rad Gel Doc scanner. Quantification of band signal intensities was performed in ImageJ by transforming the image into an 8-bit format, followed by analyzing the band intensity using the measure function. After deducting the background signal from the sfRNA1 and sfRNA2 band intensities, the sfRNA1/sfRNA2 ratio was determined.
RT-PCR and qRT-PCR.
First-strand synthesis was performed with 500 to 1,000 ng total RNA per reaction mixture using Superscript III (Invitrogen) and random hexamers (Roche), following the manufacturer's protocol. Reverse transcription (RT)-PCRs were performed on 0.2 μl cDNA using GoTaq green (Promega) according to the manufacturer's protocol. Quantitative RT (qRT)-PCR was performed with SYBR Select master mix (Applied Biosystems) on a Rotagene 3000 (Corbett Research). Briefly, the cDNA was diluted 5-fold in double-distilled H2O (ddH2O), and 1 μl was used as a template with primers targeting either the vgRNA (FW, 5′-TGCACTCCTAGTCCTAGTGTTTGGG-3′; RV, 5′-TCTTGAATGTAGCCATGAGTGCC-3′) or the C. pipiens 40S ribosomal protein S7 (rpS7) RNA (FW, 5′-ATGGTTTTCGGATCAAAGGT-3′; RV, 5′-GACGTGCTTGCCGGAGAAC-3′) at an annealing temperature of 57°C.
Deep sequencing of small RNAs.
Total RNA was isolated as described above, and 15 μg of RNA per sample per lane was separated by gel electrophoresis on 15% polyacrylamide-7 M urea-0.5×TBE gels. For the sequencing of small RNAs from WNVIC- and WNVΔSF-infected northern European mosquitoes, the 19- to 24-nt RNAs were cut from the gel using radioactively labeled RNA oligonucleotides and loaded in adjacent lanes of the gel as rulers. Subsequently, the gel slices were crushed using gel breaker tubes, and small RNAs were eluted in 800 μl 0.3 M sodium acetate (NaOAc), precipitated with 80% ethanol (EtOH), and dissolved in 11 μl H2O. Small-RNA deep-sequencing libraries were prepared using the TruSeq small-RNA kit (15016914; Illumina) according to the manufacturer's protocol, as described previously (21). Briefly, after adapter ligation, reverse transcription, and PCR amplification, the cDNA libraries were gel purified from 6% polyacrylamide-1× TBE gels, eluted in 500 μl 0.3 M NaOAc, precipitated with 80% EtOH, and dissolved in 11 μl 10 mM Tris-HCl, pH 8.5. The small-RNA library was sequenced on an Illumina HiSeq 2500 (Baseclear, Leiden, The Netherlands), and single-end FASTQ reads were generated using the Illumina Casava pipeline version 1.8.3. Initial quality assessments were performed with Baseclear using in-house filtering protocols and the FASTQC quality control tool version 0.10.0. Further sequence analysis was performed on the Galaxy server (41). Adapter sequences were removed from each read using the Clip tool version 1.0.1 and mapped to either the WNVIC or WNVΔSF1 genome with Bowtie version 1.1.2 (41). Size profiles of the viral small RNAs were retrieved from all the mapped reads, allowing 1 mismatch. For genome profiles, the 5′ ends of the 21-nt virus reads were mapped along the viral genome.
Mosquito rearing.
A C. pipiens colony originating from Brummen, The Netherlands, was established in 2010 and maintained at 23°C and 60% relative humidity (RH) on a 16-h/8-h light-dark (L-D) cycle. The mosquitoes were maintained in Bugdorm cages and provided with 6% glucose solution ad libitum. Hemotek PS5 feeders (Discovery Workshops) filled with bovine or chicken whole blood (KemperKip, Uden, The Netherlands) were used to administer a blood meal for egg production. Egg rafts were allowed to hatch in tap water supplemented with Liquifry no. 1 (Interpet Ltd., Dorking, United Kingdom). The larvae were fed with a 1:1:1 mixture of ground koi food, ground rabbit food, and bovine liver powder (Sigma-Aldrich, Zwijndrecht, The Netherlands).
In vivo infections. (i) Infectious blood meal.
Two days before blood feeding, the mosquitoes were starved by providing them with a tissue soaked in water. One milliliter of whole chicken blood (Kemperkip, Uden, The Netherlands) was mixed with 1 ml virus solution in Leibovitz-L15 supplemented with 10% FBS to a final concentration of 4.0 × 107 TCID50/ml. Two- to 7-day-old mosquitoes were allowed to feed ad libitum through a Parafilm membrane using a Hemotek feeder in a controlled dark room at 24°C and 70% RH for 1 h. After blood feeding, the mosquitoes were anesthetized with 100% CO2, and engorged females were selected on a CO2 pad.
(ii) Intrathoracic injections.
Two- to 5-day-old female mosquitoes were anesthetized with 100% CO2 and placed on a CO2 pad, and ∼7 × 103 or ∼7 × 102 TCID50 of virus was injected in a total volume of 69 nl using a Drummond Nanoject II Auto-Nanoliter Injector (Drummond Scientific Company). Blood-fed or injected mosquitoes were incubated at 28°C on a 16-h/8-h L-D cycle and fed with 6% sugar water during the course of the experiment. At 14 dpi (or as indicated), mosquitoes were anesthetized with 100% CO2, and the legs and wings were removed from the mosquito bodies. Saliva from the mosquitoes was collected by putting the proboscis in 5 μl of 3% sugar water, 50% FBS for 45 min. After salivating, the mosquito bodies were added to a 1.5-ml Eppendorf tube containing 0.5-mm zirconium beads (Next Advance, New York, NY) and frozen at −80°C. Fifty-five microliters of HEPES-buffered DMEM–10% FBS–penicillin/streptomycin–gentamicin was added to the saliva samples. The mosquito homogenates and saliva samples were stored at −80°C until further processing.
WNV infectivity assay.
WNV infectivity assays were performed as described previously (42). Briefly, mosquito bodies were homogenized in a Bullet blender (Next Advance, New York, NY; two rounds of 2 min each at maximum speed); 100 μl of HEPES-buffered DMEM–10% FBS–PS–gentamicin was added to the mosquito homogenates, and 30 μl mosquito homogenate was used to inoculate Vero cell monolayers in 96-well plates. For saliva samples, 30 μl saliva in DMEM-10% FBS-PS-gentamicin was used to inoculate a Vero cell monolayer in a 96-well plate. At 3 dpi, the wells were scored for the presence of virus by CPE and immunostaining using anti-E monoclonal antibodies (43, 44).
RNA structure modeling in silico.
Secondary structures were folded using the Mfold Web server with standard settings and flat exterior loop type (45). The secondary RNA structure was visualized using the VARNA RNA editing package (46). Tertiary-structure folding was performed using the RNAComposer Web server with standard settings (47). The tertiary RNA structure was visualized using the PyMOL viewer (48).
Statistics.
All statistical analyses were performed in GraphPad Prism 5. Significant differences between virus infection and transmission rates were determined by Fisher's exact test (α = 0.05). t tests were performed using a 95% confidence interval. The D'Agostino and Pearson omnibus normality test was used to check for Gaussian distributions. In cases where the data did not follow a Gaussian distribution, a Mann-Whitney U test was used (α = 0.05) to replace the t test. Statistics on the virus growth curves were done using a two-way analysis of variance (ANOVA).
RESULTS
An sfRNA1-deficient clone of WNV is not attenuated in vitro.
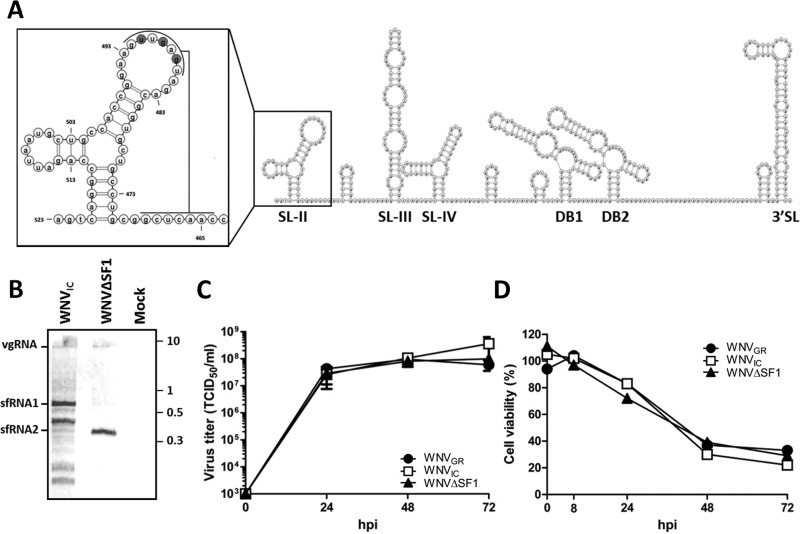
WNV mutants that are deficient in the formation of full-length sfRNA (sfRNA1) cause progressively less CPE in cell culture when only shorter and/or less abundant sfRNA species can be produced (25, 29). To study the effects of sfRNA on transmission independent of effects on replication and cytopathicity, we used a mutant that is deficient only in sfRNA1. This mutant has previously been shown to have little effect on the replication rate and cytopathicity of WNV, as opposed to sfRNA1-sfRNA2-deficient mutants, which are attenuated in both insect and invertebrate cell lines (25, 29, 31). An infectious clone of WNV (WNVIC) was compared to an sfRNA1-deficient infectious clone of WNV (WNVΔSF1) that was generated by mutating the top loop of SL-II (Fig. 1A). The wild-type virus (WNVGR) was used for comparison in the growth curve and cell viability experiments. As expected, Northern blot analysis of RNA isolated from Vero cells using a 3′ UTR-specific probe indicated that WNVIC predominantly produced sfRNA1. Conversely, WNVΔSF1 produced only sfRNA2 as a result of mutated SL-II, resulting in XRN1 stalling on SL-IV (Fig. 1B). An unexpected additional band was observed for WNVIC between sfRNA1 and sfRNA2, which was not produced in cells infected with WNVΔSF1 (Fig. 1B). Comparison with the flaviviruses Usutu virus (USUV) and WNV New York 1999 (WNVNY99) demonstrated that the size of full-length sfRNA from WNVIC was similar, confirming that the top band is sfRNA1 (data not shown). One-step growth curves in Vero cells demonstrated that WNVΔSF1 had growth kinetics similar to those of WNVIC and the wild-type WNVGR virus isolate (Fig. 1C). In addition, WNVΔSF1 caused a level of CPE comparable to that of WNVGR and WNVIC, as observed by a steady decrease in cell viability between 1 and 3 dpi (Fig. 1D). These results validate WNVΔSF1 as a model to study the effects of sfRNA without interference from altered cytopathicity and/or replication.
FIG 1.
Generation and characterization of an sfRNA1-deficient WNV. (A) Two-dimensional (2D) RNA structure model of the 3′ UTR/sfRNA of WNVGR. The lines indicate pseudoknot interaction; the nucleotides mutated in WNVΔSF1 are shaded. (B) Northern blot of RNA harvested from Vero cells infected with WNVIC or WNVΔSF1 or mock treated. Numbers at right are kilobases. (C) Vero cells were infected with WNVGR, WNVIC, or WNVΔSF1 at an MOI of 1. Supernatants were taken at 0, 1, 2, 3, and 4 dpi and titrated by EPDA. Shown are the mean titers of two independent experiments ± standard errors of the mean (SEM). Statistics were done using a two-way ANOVA. (D) Vero cells were infected with WNVGR10, WNVIC, or WNVΔSF1 at an MOI of 5, and at the indicated times, the cell viability was measured by CellTiter-Glo assay. hpi, hours postinfection.
An sfRNA1-deficient WNV replicates efficiently in RNAi-competent mosquito cells.
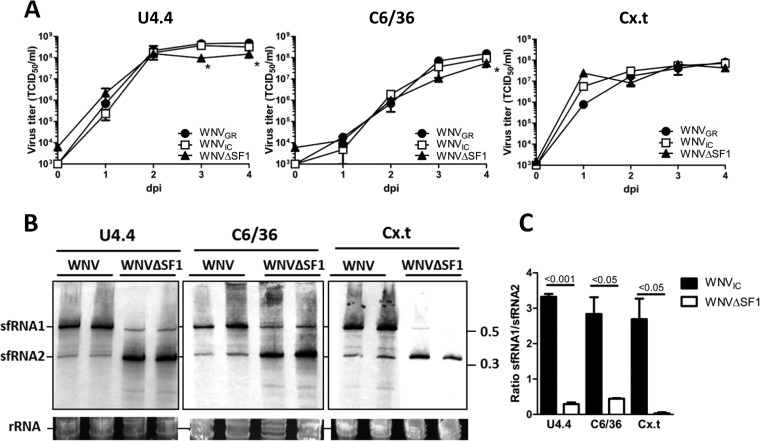
To investigate whether sfRNA1 is required for replication of WNV in mosquito cells, one-step virus growth curves were performed on C. tarsalis Cx.t, A. albopictus U4.4, and Dicer2-deficient A. albopictus C6/36 cells (49). WNVGR, WNVIC, and WNVΔSF1 replicated similarly in all three mosquito cell lines, with maximum titers reaching ∼4 × 108 TCID50/ml at 3 dpi in U4.4 cells and ∼1 × 108 TCID50/ml at 4 dpi in both C6/36 and Cx.t cells (Fig. 2A); however, WNVΔSF1 reached slightly lower titers at 3 and 4 dpi in U4.4 and 4 dpi in C6/36 cells. These results indicate that sfRNA1 is not required for efficient WNV replication in mosquito cells, consistent with the observations in mammalian cells (Fig. 1C). Furthermore, the similar virus titers in RNAi-competent Cx.t and U4.4 cells compared to the RNAi-deficient C6/36 cells indicate that there is no obvious effect of an active RNAi response on either WNVIC or WNVΔSF1 production. Northern blot analysis indicated that abundant levels of sfRNA1, but low levels of sfRNA2, are produced in WNVIC-infected U4.4, C6/36, and Cx.t cells (Fig. 2B). However, cells infected with WNVΔSF1 produced large amounts of sfRNA2 and much lower levels of sfRNA1, particularly in the Cx.t cells. Quantification of sfRNA1 and sfRNA2 signal intensities demonstrated an sfRNA1/sfRNA2 ratio of ∼3 for WNVIC-infected versus 0 to 0.4 for WNVΔSF1-infected cells (Fig. 2C). These results indicate that WNVΔSF1 is largely defective in sfRNA1 production and can successfully replicate in RNAi-competent mosquito cells.
FIG 2.
One-step growth curves and sfRNA production in mosquito cells. (A) A. albopictus U4.4 and C6/36 and C. tarsalis Cx.t cells were infected with WNVGR, WNVIC, or WNVΔSF1 at an MOI of 1. Supernatants were taken at 0, 1, 2, 3, and 4 dpi and titrated by EPDA. Shown are the mean titers ± SEM from two independent experiments. The statistics were done using a two-way ANOVA. *, P < 0.05. (B) Northern blot of RNA isolated 4 dpi from U4.4, C6/36, or Cx.t cells using a 3′ UTR-specific probe. Numbers at right are kilobases. (C) Mean sfRNA1/sfRNA2 signal ratio. Shown are the mean results and SEM from two independent experiments. The statistics were performed using a two-tailed unpaired t test.
Viral siRNAs derived from the 3′ UTR are produced in NA and NWE C. pipiens mosquitoes infected with WNVGR.
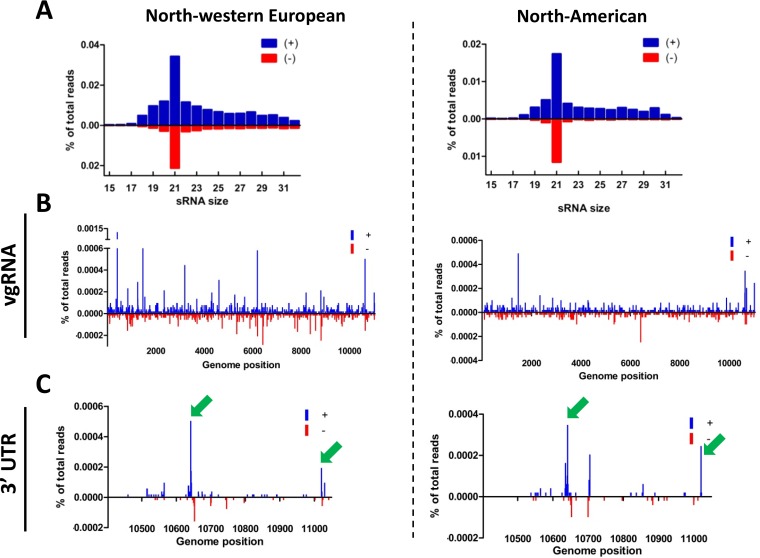
While sfRNA suppresses reporter-based RNAi in mosquito cells (23, 37) and in vivo in mosquitoes and interacts with the RNAi machinery in mammalian cell lines (38), it is still unclear whether during an in vivo infection in mosquitoes the 3′ UTR sfRNA is processed by the RNAi machinery. To investigate if WNVGR 3′ UTR-derived small RNAs are produced in C. pipiens mosquitoes, we analyzed the vsRNA profiles generated in a set of independent experiments with a wild-type WNV lineage II isolate (WNVGR) in northwestern European (NWE) and North American (NA) C. pipiens strains (Fig. 3) (21). Size distributions of vsRNAs showed that 21-nt viral siRNAs (vsiRNAs) were highly abundant and that no obvious >25-nt vsRNAs were produced (Fig. 3A), indicating that no WNV-specific piRNAs are produced in C. pipiens mosquitoes. A small shoulder of 25- to 32-nt sRNAs was present; however, these sRNAs did not have the 10A-biased piRNA signature (21). Hot- and cold-spot analysis of the 21-nt vsRNAs along the WNV genome indicated that vsiRNAs were produced across the whole length of the genome (Fig. 3B). NA mosquitoes produced smaller amounts of vsiRNAs and mapped differentially across the genome, indicating that different mosquito populations can influence the sRNA profile. Detailed hot- and cold-spot analysis of the viral 3′ UTR showed that in both NWE and NA mosquitoes two hot spots were present in SL-III and the 3′ SL, respectively, while NA mosquitoes also displayed a hot spot in SL-IV (Fig. 3C). These results show that the 3′ UTR of the WNVGR gRNA is targeted by the RNAi machinery and processed into 21-nt vsRNA, indicating that sfRNA might be processed by the RNAi machinery in vivo.
FIG 3.
Deep sequencing of siRNAs from WNVGR-infected North American and northwestern European mosquitoes that target the 3′ UTR. (A) Size distributions of 15- to 32-nt small RNAs mapping to the viral genome of WNVGR normalized to the total number of clipped reads in the library. (B) Twenty-one-nucleotide small-RNA reads were aligned to the viral genome and normalized against the total number of clipped reads in the library. Positive-strand reads are displayed in blue and negative-strand reads in red. (C) Detailed view of the 21-nt small-RNA distribution on the 3′ UTR. The arrows indicate the vsiRNA hot spots that target the 3′ UTR in both mosquito colonies.
C. pipiens mosquitoes mount an active RNAi response against wild-type and sfRNA-deficient WNV.
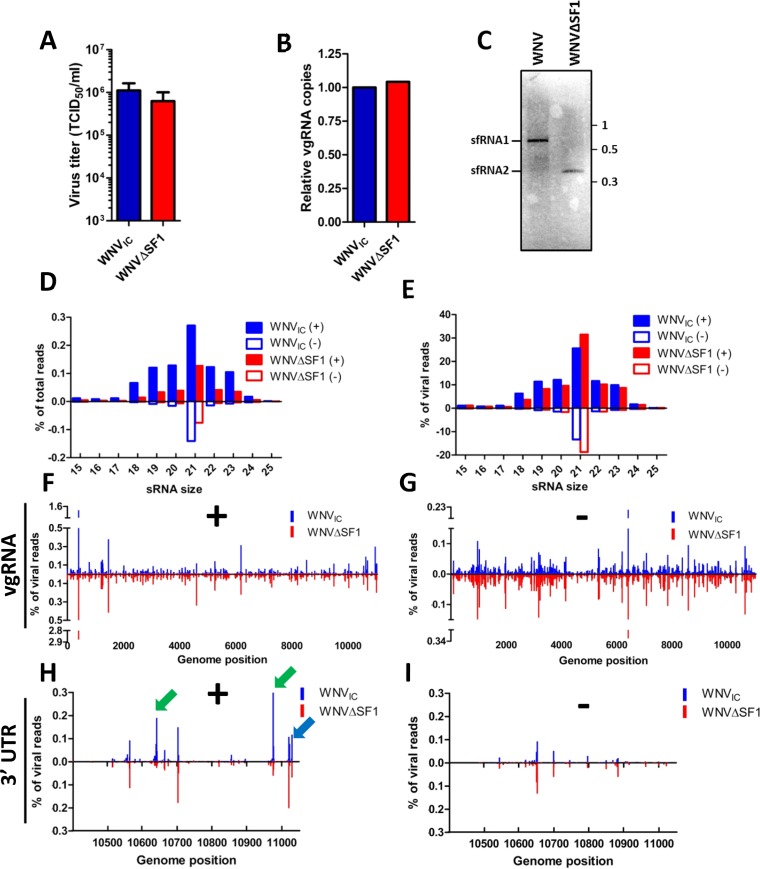
Our experiments clearly show that an sfRNA-deficient mutant replicates efficiently in RNAi-competent mosquito cells, although the 3′ UTR appears to be processed by the RNAi machinery in vivo. To investigate if sfRNA is a substrate for Dicer and/or influences the mosquito RNAi response in vivo, 19- to 24-nt small-RNA populations were sequenced from pools (n > 12) of WNVIC or WNVΔSF1 blood meal-infected mosquitoes at 14 dpi (Fig. 4). RNAs larger than 25 nt were excluded from deep sequencing, since it was previously shown that Culex mosquitoes do not produce 25- to 30-nt vpiRNAs (Fig. 3A) (14, 21). To rule out potential effects of differentiation between the two mosquito pools, we first verified the virus titers and vgRNA levels at 14 dpi. The virus titers in the two pools of mosquitoes were marginally different, with a slightly but not significantly higher titer for WNVIC (1.1 × 106 versus 6.3 × 105 TCID50/ml; P = 0.272) (Fig. 4A), while qPCR on the vgRNA demonstrated that the numbers of viral genome copies were similar (Fig. 4B). Northern blot analysis of total RNA isolated from pools of WNV-positive mosquitoes confirmed that in mosquitoes, as well, WNVIC predominantly produced sfRNA1 while WNVΔSF1 produced only sfRNA2 (Fig. 4C). The vsRNA size distributions were very similar in WNVIC- and WNVΔSF1-infected mosquitoes (Fig. 4D and E). A distinctive peak of 21-nt vsRNAs was observed for both WNVIC and WNVΔSF1 (Fig. 4D and E). This 21-nt vsRNA population was present on both the positive and negative strands, indicating that they can be regarded as vsiRNAs. WNVIC produced slightly higher total levels of vsiRNAs than WNVΔSF1 (Fig. 4D), although when normalized to the number of viral reads (Fig. 4E), there was no difference in the percentages of 21-nt reads. This indicates that the difference in percentages of total reads can be explained by the slightly elevated titer of WNVIC, although the numbers of viral genome copies were similar (Fig. 4A and B). Next, we investigated whether sfRNA affected the genome distribution of vsRNAs by mapping the 21-nt reads along the vgRNA for the positive (Fig. 4F) and negative (Fig. 4G) strands. The vsiRNA genome distributions were highly similar between WNVIC and WNVΔSF1 on both the positive and negative strands, as illustrated by the mirrored hot- and cold-spot plots (Fig. 4F and G), providing no direct evidence that sfRNA1 interferes with the antiviral RNAi response in vivo.
FIG 4.
Small-RNA sequences of WNVIC or WNVΔSF1 blood-fed mosquitoes. (A) WNV titers of mosquito bodies used for small-RNA sequencing. The statistics were performed using an unpaired t test. The error bars indicate SEM. (B) WNV genome copies in RNA samples used for small-RNA sequencing determined by qRT-PCR. (C) Northern blot from the same RNA sample that was used for deep sequencing using a 3′ UTR-specific probe. Numbers at right are kilobases. (D and E) Size distributions of small-RNA reads mapping to the viral genome of WNVIC or WNVΔSF1 blood-fed mosquitoes normalized to the total number of reads (D) or the total number of viral reads (E) in the library. (F and G) The 5′ ends of the reads were aligned to the viral genome and normalized against the number of viral reads. The positive-strand (F) and negative-strand (G) reads were mapped for WNVIC and WNVΔSF1. (H and I) Detailed view of the 21-nt RNA distribution on the 3′ UTR for the positive strand (H) and the negative strand (I). The green arrows indicate siRNA hot spots that are sfRNA specific. The blue arrow indicates the presence of KUN-miR-1.
sfRNA-derived vsiRNAs should be positive-strand biased due to the nature of sfRNA biogenesis (25). Indeed, two distinct hot spots that occur on the positive strand in WNVIC-infected mosquitoes, but not on the positive strand of WNVΔSF1-infected mosquitoes (Fig. 4H) or on the negative strands of both viruses, were identified (Fig. 4I). Hot spots 10642 and 10976 were present only in WNVIC-infected mosquitoes and not in mosquitoes infected with WNVΔSF1, indicating that the vsiRNAs from these hot spots are derived from sfRNA1. Interestingly, these sfRNA-specific hot spots cooccurred with predicted secondary structures of SL-III (hot spot 10642) and the 3′ SL (hot spot 10976) (Fig. 4H and 5A). Finally, Kunjin virus miR-1 (KUN-miR-1), previously discovered in vitro (50), was also found at position 11030, indicating that KUN-miR-1 could have a potential function in vivo in mosquitoes. These results show for the first time that sfRNA is processed by the mosquito RNAi machinery and that an sfRNA1-defective WNV mutant has a differential vsiRNA profile in the viral 3′ UTR.
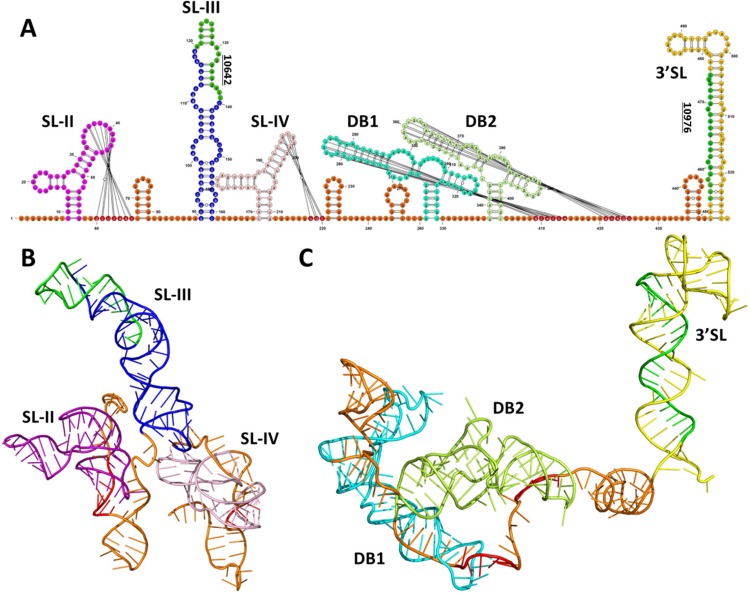
FIG 5.
Three-dimensional RNA structure model of sfRNA revealing that SL-III and the 3′ SL are more accessible to the mosquito RNAi machinery. (A) Schematic presentation of the WNVGR sfRNA secondary RNA structure with projections of the vsiRNA hot spots. Pseudoknot interactions are indicated by lines, and pseudoknot-interacting bases are colored red. sfRNA-specific vsiRNAs are indicated in green, and each SL and DB was given a separate color. (B and C) 3D RNA structure model of the first 240 nt (B) and last 288 nt (C) of WNV sfRNA. The colors match the color scheme of the secondary structures in panel A.
3D modeling of WNV sfRNA reveals SL-III and the 3′ SL as accessible dsRNA substrates.
Our small-RNA deep-sequencing data clearly show that sfRNA is processed by the RNAi machinery into vsiRNAs but do not explain why vsiRNA hot spots occur in certain RNA structures. To better understand why some regions of the 3′ UTR/sfRNA are preferentially targeted by RNAi, a three-dimensional (3D) model of the WNVGR 3′ UTR/sfRNA was created using RNAComposer (47) (Fig. 5). Each secondary structure was color coded, and the two most significant vsiRNA hot spots are colored green in Fig. 5A. The pseudoknot interactions in the XRN1/Pacman stalling structures SL-II and SL-IV result in very tight tertiary folding (Fig. 5B), potentially making these structures inaccessible to processing by host nucleases. In contrast, SL-III sticks out of the 3′ UTR coil and thus could present an accessible dsRNA substrate for Dicer and/or other nucleases. Similarly tight tertiary folding occurs for the dumbbell RNA structures DB1 and DB2 (Fig. 5C), which also display tight folding via pseudoknot interactions with the main coil of the 3′ UTR/sfRNA. The 3′ SL, however, sticks out of the 3′ UTR coil and thus could also present an accessible dsRNA substrate. These results provide insight into the tertiary fold of the 3′ UTR/sfRNA and explain why vsiRNAs are mostly derived from SL-III and the 3′ SL.
sfRNA1 determines WNV transmission by mosquitoes.
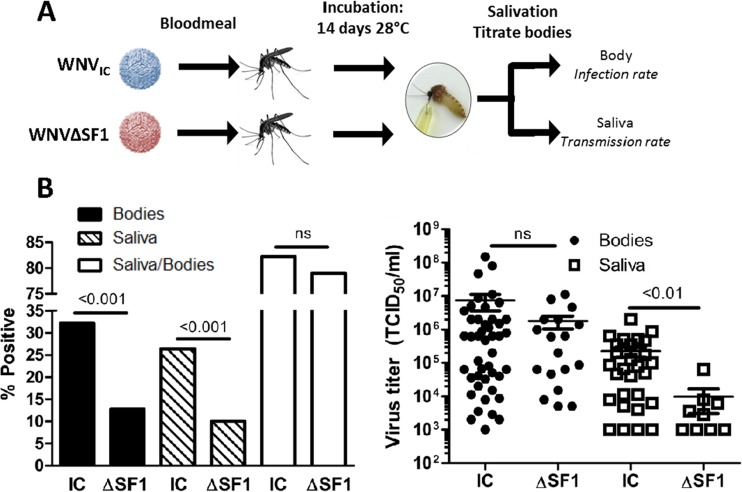
The conservation of sfRNA production in all flaviviruses suggests an important biological function in both mammalian and insect hosts. Importantly, the sequence of the flaviviral 3′ UTR can be phylogenetically divided based on the virus vector species (33), indicating a role for sfRNA in infection and/or transmission of the arthropod vector. To investigate if sfRNA is important for dissemination and transmission of WNV, C. pipiens mosquitoes were allowed to feed for 1 h on a blood meal containing either WNVIC or WNVΔSF1. After 14 days, the titer of WNV in the mosquito bodies was determined by EPDA, and the presence of WNV in the saliva was determined by inoculating Vero cells (Fig. 6A). Importantly, the infection rate, as determined by the percentage of engorged mosquitoes with a measurable virus titer, was significantly lower for WNVΔSF1 than for WNVIC (13% versus 32%; P < 0.001) (Fig. 6B and Table 1). In addition, the transmission rate, as determined by the percentage of saliva-positive mosquitoes out of the total number of engorged mosquitoes, was significantly lower for mosquitoes infected with WNVΔSF1 than for mosquitoes infected with WNVIC (10% versus 26%; P < 0.001) (Fig. 6B and Table 1), indicating that sfRNA1 production was important for transmission when mosquitoes were orally infected. Dissemination rates, as determined by the percentage of saliva-positive mosquitoes out of the number of virus-positive mosquitoes, was not affected by sfRNA1 (82% versus 79%; P = 1.000) (Fig. 6B and Table 1). This indicates that sfRNA1 mainly affects infection of the midgut and consequently increases the transmission rate. The viral titers of mosquito bodies that were positive for the presence of virus by EPDA were not significantly different between WNVIC and WNVΔSF1 (P = 0.360) (Fig. 6C). However, the viral titers in the saliva were significantly higher for WNVIC-infected than for WNVΔSF-infected mosquitoes (2.2 × 105 versus 8.0 × 103; P < 0.01) (Fig. 6C). These results suggest that sfRNA1 determines WNV transmission by increasing the overall infection rates and by boosting viral titers in the mosquito saliva.
FIG 6.
Transmission and infection rates of WNVIC and WNVΔSF1 in C. pipiens mosquitoes after an infectious blood meal. (A) Schematic overview of the experimental procedure. (B) Infection (Bodies), transmission (Saliva), and dissemination (Saliva/Bodies) rates of C. pipiens mosquitoes infected through an infectious blood meal containing WNVIC (n = 140) or WNVΔSF1 (n = 149). The infection, transmission, and dissemination rates are presented as percentages of the total number of mosquitoes. The data represent cumulative numbers from four independent experiments. The statistics were performed using Fisher exact tests. ns, not significant. (C) Titers of WNV-positive mosquito bodies and saliva samples of WNVIC or WNVΔSF1 blood meal-infected mosquitoes. Shown are the mean titers ± SEM. The statistics were performed using a two-tailed unpaired Mann-Whitney U test.
TABLE 1.
Four replicate blood-feeding experiments demonstrate that sfRNA determines the infection and transmission rates of WNV in C. pipiens mosquitoesa
| Virus | Replicate | n | Positive bodies (no.) | Positive saliva (no.) | Infection (%) | Transmission (%) | Dissemination (%) |
|---|---|---|---|---|---|---|---|
| WNVIC | 1 | 33 | 11 | 11 | 33 | 33 | 100 |
| 2 | 39 | 20 | 14 | 51 | 36 | 70 | |
| 3 | 24 | 5 | 4 | 21 | 17 | 80 | |
| 4 | 44 | 9 | 8 | 20 | 18 | 89 | |
| Total | 140 | 45 | 37 | 32 | 26 | 82 | |
| WNVΔSF1 | 1 | 37 | 8 | 6 | 22 | 16 | 75 |
| 2 | 40 | 3 | 3 | 8 | 8 | 100 | |
| 3 | 23 | 2 | 2 | 9 | 9 | 100 | |
| 4 | 49 | 6 | 4 | 12 | 8 | 67 | |
| Total | 149 | 19 | 15 | 13 | 10 | 79 |
Shown are infection, transmission, and dissemination rates of C. pipiens mosquitoes orally infected with either WNVIC or WNVΔSF1. In addition, the sample size (n) as the number of engorged females after a blood meal and absolute numbers of positive bodies and saliva samples are shown for each replicate.
sfRNA1 is a key driver to overcome the mosquito midgut infection barrier.
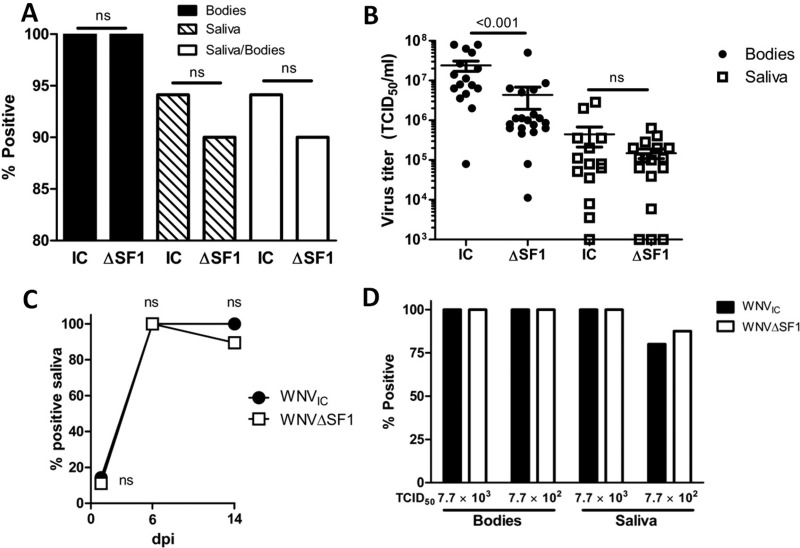
The infection rate of mosquitoes and the subsequent dissemination to the salivary glands are important parameters for arbovirus transmission (11). Clearly, WNVΔSF1 displayed significantly reduced transmission rates, which could be (i) the result of reduced virus dissemination through an effect of sfRNA1 on a postmidgut barrier or (ii) the consequence of reduced initial infection rates. Although our data suggest that the main effect of sfRNA occurs in the midgut, we discriminated between these two possibilities by intrathoracic injections of C. pipiens mosquitoes with 7 × 103 TCID50 of either WNVIC or WNVΔSF1. In this way, the midgut barriers are bypassed to allow the study of postmidgut virus dissemination and transmission. At 14 dpi, an infection rate of 100% was achieved for mosquitoes injected with either WNVIC or WNVΔSF1 (Fig. 7A and Table 2), suggesting that the two viruses are equally capable of establishing a systemic, fully disseminated infection when the midgut infection barrier is bypassed. Next, the saliva of all mosquitoes was tested for the presence of WNV. There was no significant difference in transmission rates (94% versus 90%; P = 0.696) (Fig. 7A and Table 2) or dissemination rates (94% versus 90%; P = 0.696) (Fig. 7A) between WNVIC and WNVΔSF1, despite a significant difference in average body titers (WNVIC, 2.4 × 107; WNVΔSF1, 4.4 × 106; P < 0.001) (Fig. 7B). Titration of the mosquito saliva showed no significant differences (WNVIC, 4.4 × 105; WNVΔSF1, 1.0 × 106; P = 0.873). Additionally, at 1, 6, and 14 dpi, no significant difference between the transmission rates of WNVIC and WNVΔSF1 was observed (1 dpi, 14% versus 11% [P = 1.000]; 6 dpi, 100% versus 100% [P = 1.000]; 14 dpi, 100% versus 86% [P = 0.492]) (Fig. 7C). To rule out the possibility that the lack of difference between WNVIC and WNVΔSF1 was due to a viral dose that was too high, we repeated the experiment with a 10-fold-lower injection dose of 7 × 102 TCID50 per mosquito and scored the infection and transmission rates at an earlier time point of 7 dpi (Fig. 7D). Again, no difference between WNVIC and WNVΔSF1 was observed in either the infection or transmission rate, indicating that there is no clear postmidgut effect of sfRNA1 on transmission. These results demonstrate that sfRNA1 does not play a determining role in postmidgut dissemination to the salivary glands. Instead, sfRNA1 is vital to efficiently overcome the midgut infection and/or escape barrier, resulting in enhanced virus dissemination and subsequent transmission.
FIG 7.
Intrathoracic injections of C. pipiens mosquitoes with WNVIC and WNVΔSF1. (A) Infection (Bodies), transmission (Saliva), and dissemination (Saliva/Bodies) rates of WNVIC (n = 34) and WNVΔSF1 (n = 50) in C. pipiens mosquitoes after intrathoracic injections with 7.7 × 103 TCID50 per mosquito. The data represent cumulative numbers from three independent experiments. The statistics were performed using Fisher exact tests. (B) Titers of WNV-positive mosquito bodies and saliva samples from mosquitoes injected with WNVIC or WNVΔSF1. Shown are the mean titers ± SEM. The statistics were performed using a two-tailed unpaired Mann-Whitney U test. (C) Transmission rates in C. pipiens mosquitoes of WNVIC or WNVΔSF1 after intrathoracic injection at 1, 6, and 14 dpi. The statistics were performed using Fisher exact tests. (D) Infection and transmission rates at 7 dpi in C. pipiens mosquitoes after intrathoracic injection of a low dose (7.7 × 102 TCID50 versus 7.7 × 103 TCID50) of WNVIC or WNVΔSF1. The statistics were performed using Fisher exact tests.
TABLE 2.
Three replicate intrathoracic injection experiments indicate no effect of sfRNA on postmidgut dissemination of WNV in C. pipiens mosquitoesa
| Virus | Replicate | n | Positive bodies (no.) | Positive saliva (no.) | Infection (%) | Transmission (%) | Dissemination (%) |
|---|---|---|---|---|---|---|---|
| WNVIC | 1 | 15 | 15 | 15 | 100 | 100 | 100 |
| 2 | 13 | 13 | 11 | 100 | 85 | 85 | |
| 3 | 6 | 6 | 6 | 100 | 100 | 100 | |
| Total | 34 | 34 | 32 | 100 | 94 | 94 | |
| WNVΔSF1 | 1 | 19 | 19 | 17 | 100 | 89 | 89 |
| 2 | 21 | 21 | 18 | 100 | 86 | 86 | |
| 3 | 10 | 10 | 10 | 100 | 100 | 100 | |
| Total | 50 | 50 | 45 | 100 | 90 | 90 |
Shown are infection, transmission, and dissemination rates of C. pipiens mosquitoes that were intrathoracically injected with either WNVIC or WNVΔSF1. In addition, the sample size (n) and absolute numbers of positive bodies and saliva samples are shown for each replicate.
An sfRNA1-sfRNA2-deficient WNV has attenuated replication in Culex cells and decreased transmission by Culex mosquitoes.
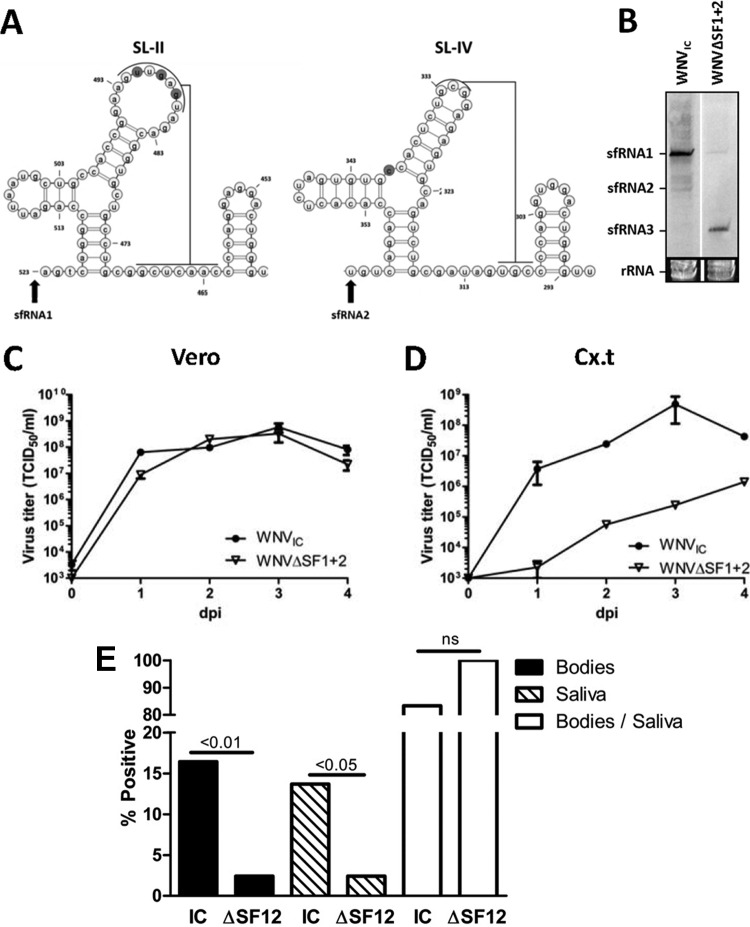
We showed that sfRNA1 is required for successful passing of the mosquito midgut barrier, but WNVΔSF1 still produces the shorter sfRNA2. To investigate whether combined abrogation of both sfRNA1 and sfRNA2 production would further attenuate WNV, we generated an infectious clone with mutations in both SL-II and SL-IV (WNVΔSF1+2) (Fig. 8A). The mutation introduced in SL-IV resides in the conserved three-way-junction that was previously shown to be important for XRN1 resistance (30). Northern blot analysis of RNA isolated from C6/36 mosquito cells infected with WNVIC, or WNVΔSF1+2 demonstrated that the double mutant indeed fails to produce sfRNA1 and sfRNA2 (Fig. 8B). The effect of sfRNA1-sfRNA2 deletion on replication was tested by performing growth curves on Cx.t and Vero cells with WNVIC and WNVΔSF1+2. No difference in replication was observed in Vero cells, although WNVΔSF1+2 presented slightly lower titers (Fig. 8C). However, in Cx.t cells, WNVΔSF1+2 was severely attenuated in virus growth, indicating that sfRNA is important for successful replication in Culex (Fig. 8D). Since we showed that deletion of sfRNA1 production results in decreased transmission rates in C. pipiens after an infectious blood meal, we hypothesized that combined abrogation of sfRNA1 and sfRNA2 production further attenuates WNV in mosquitoes. To test this hypothesis, C. pipiens mosquitoes were given an infectious blood meal with 5.0 × 106 TCID50/ml of either WNVIC or WNVΔSF1+2 (Fig. 8E) (Table 3). The titer of this blood meal was slightly lower than in the previous experiments due to a lower plateau titer of WNVΔSF1+2 in C6/36 cells. WNVIC-infected mosquitoes reached an infection rate of 16% and an ∼14% transmission rate, consistent with the data in Fig. 6. However, the WNVΔSF1+2 blood-fed mosquitoes reached significantly lower infection (2%; P = 0.004) and transmission (2%; P = 0.04) rates, indicating that sfRNA1 and sfRNA2 are vital for infection of mosquitoes. Sequencing of the 3′ UTR of the WNVΔSF1+2-infected mosquitoes showed that the mutations in SL-II and SL-IV were still present, indicating that no reversions occurred (data not shown). In conclusion, these results provide the first evidence that sfRNA plays an important role in mosquito transmission of WNV, which could underpin the strict conservation of sfRNA production by flaviviruses.
FIG 8.
Transmission and infection rates of C. pipiens infected with WNVIC and WNVΔSF1+2. (A) 2D RNA structure model of SL-II and SL-IV of WNVGR. Pseudoknot interaction is indicated by the lines; the nucleotides mutated in WNVΔSF1+2 are shaded. The arrows indicate the starting points of sfRNA1 and sfRNA2. (B) Northern blot of RNA harvested from C6/36 cells infected with WNVIC or WNVΔSF1+2. (C and D) Vero (C) or Cx.t (D) cells were infected with WNVIC or WNVΔSF1+2 at an MOI of 1. Supernatants were taken at 0, 1, 2, 3, and 4 dpi and titrated by EPDA. Shown are the mean titers of two replicate samples ± SEM. (E) Infection (Bodies), transmission (Saliva), and dissemination (Saliva/Bodies) rates of C. pipiens mosquitoes infected through an infectious blood meal containing WNVIC (n = 73) or WNVΔSF1+2 (n = 83). The infection, transmission, and dissemination rates are presented as percentages of the total number of engorged female mosquitoes. The data represent cumulative numbers from three independent experiments. The statistics were performed using Fisher exact tests.
TABLE 3.
Three replicate blood feeding experiments demonstrate that deficiency in sfRNA1 and sfRNA2 results in decreased WNV infection and transmission rates in C. pipiensa
| Virus | Replicate | n | Positive bodies (no.) | Positive saliva (no.) | Infection (%) | Transmission (%) | Dissemination (%) |
|---|---|---|---|---|---|---|---|
| WNVIC | 1 | 23 | 8 | 7 | 35 | 30 | 88 |
| 2 | 28 | 3 | 2 | 11 | 7 | 67 | |
| 3 | 22 | 1 | 1 | 5 | 5 | 100 | |
| Total | 73 | 12 | 10 | 16 | 14 | 83 | |
| WNVΔSF1 + 2 | 1 | 18 | 0 | 0 | 0 | 0 | 0 |
| 2 | 36 | 2 | 2 | 6 | 6 | 100 | |
| 3 | 29 | 0 | 0 | 0 | 0 | 0 | |
| Total | 83 | 2 | 2 | 2 | 2 | 100 |
Shown are infection, transmission, and dissemination rates of orally infected C. pipiens mosquitoes with either WNVIC or WNVΔSF1+2. In addition, the sample size (n) as the number of engorged females after a blood meal and absolute numbers of positive bodies and saliva samples are shown for each replicate.
DISCUSSION
Flavivirus epidemics occur as a result of a complex three-way interplay between virus, host, and vector and are influenced by a range of environmental factors. Where and when flavivirus outbreaks will occur cannot be readily predicted, but it is clear that the ability of a mosquito population to transmit a certain flavivirus is essential for efficient viral spread from one vertebrate host to another. This vector competence is primarily dictated by the specific virus-vector combination, yet the viral determinants of vector competence are not well defined. For WNV02, it has been reported that a single amino acid change in the envelope glycoprotein E resulted in increased mosquito transmission rates compared to the original NY99 strain (51). This example illustrates that flaviviral products (i.e., viral proteins and viral RNA) may determine vector competence by overcoming infection barriers to establish a transmissible infection in the mosquito (52).
Here, we have identified a novel determinant of WNV vector competence, a noncoding viral RNA whose abundant production in infected cells is widely conserved in the large group of flaviviruses. This sfRNA, as well as the flavivirus 3′ UTR it is derived from, contains various conserved RNA structures that may influence virus transmission by the arthropod vector (35, 53). sfRNA production is highly conserved among all members of the genus Flavivirus, including the NKVFs, ISFs, TBFs, and MBFs (25, 35, 36). Despite the many functions of sfRNA described in mammalian cells, the biological significance for sfRNA production in the arthropod vector has remained unknown (35). We now demonstrate that sfRNA determines WNV transmission by C. pipiens mosquitoes. An sfRNA1-deficient WNV mutant, WNVΔSF1, was attenuated in the mosquito and had significantly decreased infection and transmission rates. A mutant deficient in sfRNA1 and sfRNA2, WNVΔSF1+2, was also attenuated for replication in Culex cells and demonstrated decreased transmission and infection rates in C. pipiens mosquitoes after an infectious blood meal. From these studies, we conclude that sfRNA is produced abundantly in the mosquito to facilitate efficient transmission to the next host.
It has been firmly established that infection of and escape from midgut epithelial cells present crucial barriers for arbovirus transmission (recently reviewed in reference 12). Our results show that sfRNA affects the transmission rate at the level of the mosquito midgut, since WNV transmission was negatively affected by sfRNA deficiency when the virus was administered through an infectious blood meal but not after intrathoracic injection. After administration through intrathoracic injections, the infection and transmission rates between WNVIC and WNVΔSF1 were not significantly different at all tested virus doses and time points, although we did observe a slight difference in the overall virus titer in the mosquito bodies. This may be an indication of a potential additional postmidgut effect of sfRNA on virus dissemination, which is supported by the lower titers in the saliva for WNVΔSF1 after infection via an infectious blood meal. However, the transmission rates of wild-type and sfRNA-defective WNV in the injection experiment were always close to 100%, and no significant difference in the virus titer in the saliva was observed.
Several intracellular mechanisms could be involved in establishing the mosquito midgut barrier against arbovirus infection, such as RNAi, Toll/IMD/JAK-STAT signaling pathways, or apoptosis. The importance of antiviral RNAi in midgut cells for virus transmission and population diversity has been shown convincingly in Culex mosquitoes (13, 14). We aimed to determine the strength of the mosquito RNAi response by analyzing the vsiRNA reads relative to the viral genome in a direct comparison between WNVIC and WNVΔSF1. The abundance of vsiRNA is dependent on (i) the efficiency of the putative viral RNAi suppressor function of sfRNA and (ii) the amount of available viral dsRNA substrate in the mosquito body, which is a function of viral RNA replication and thus dependent on the success of RNAi suppression. In our study, we found no direct correlation between the expression of sfRNA1 and the abundance of (v)siRNAs produced in vivo. We cannot formally exclude the possibility that sfRNA1 facilitates WNV replication by RNAi suppression at an early stage so that later more viral dsRNA can be processed into vsiRNA, but the similar levels of WNVIC and WNVΔSF1 viral genomic RNAs in infected mosquitoes do not support this.
The effect of sfRNA on midgut infection most likely occurs during initial infection, which takes place in a small number of cells (54). This “single-cell” interaction requires very high-resolution sequence data to detect potential RNAi suppression by sfRNA, which can explain why we did not find this correlation in our experiments. Sequencing of individual midguts of WNVIC and WNVΔSF1 blood-fed mosquitoes could provide more detail on the mechanism of interplay between the RNAi response and sfRNA production in the mosquito midgut. A recent study showed that sfRNA mildly suppressed the dsRNA-induced silencing in Culex quinquefasciatus mosquitoes infected with Kunjin virus (38). The silencing suppression was demonstrated to be significant, although the difference between the wild-type and the sfRNA-deficient virus was not large. Since complete knockdown of RNAi is associated with increased mosquito mortality upon arbovirus infection, it is quite possible that sfRNA only mildly modulates the RNAi response to facilitate virus transmission without negatively affecting mosquito fitness (38).
Thus, because vsiRNA abundance cannot provide conclusive evidence for RNAi suppression by sfRNA, we reasoned that analyzing the distribution of the vsiRNA on the viral genome would be more informative to reveal potential differences between WNVIC and WNVΔSF1. However, sfRNA also did not affect the genome distribution of vsiRNAs on either the positive or negative strand of the WNV genome. Despite the clear importance of sfRNA in WNV transmission by the mosquito, WNVΔSF1 replicated equally efficiently in RNAi-competent Cx.t and U4.4 cells, suggesting that the observed effect of sfRNA on transmission in vivo may be independent of the RNAi response.
sfRNA was previously demonstrated to be a substrate for Dicer in vitro and able to suppress dsRNA- and short hairpin RNA (shRNA)-induced RNAi silencing in A. albopictus U4.4 and Drosophila melanogaster S2 cells (37). Detailed analysis of the vsiRNAs that mapped to the 3′ UTR region of WNVIC and WNVΔSF1 now show for the first time that sfRNA is processed into vsiRNAs in vivo in mosquitoes, as demonstrated by two sfRNA-specific hot spots in the 3′ UTR. The hot spot in SL-III of the 3′ UTR was consistently found in independent experiments in two different mosquito colonies. However, the same hot spot was not found in USUV-infected C. pipiens mosquitoes (reference 21 and data not shown), even though SL-III is present in the 3′ UTR of USUV. Analysis of the WNVGR 3′ UTR sequence by VMir did not predict either of the two hot spots as potential miRNAs, while it did predict the presence of the previously discovered KUN-miR-1 that is derived from the 3′ SL (50, 55, 56) (data not shown). Additionally, alignment of the SL-III regions from the 3′ UTRs of several flaviviruses from the Japanese encephalitis virus (JEV) serogroup (including WNV, USUV, JEV, and Kunijn virus) showed that the sequence of this hot spot is poorly conserved (data not shown). This indicates that the SL-III-derived viral small RNA is unlikely to have an important biological function as an miRNA/siRNA but leaves open the possibility that processing of SL-III by Dicer may ultimately have an effect on virus dissemination in the mosquito midgut, e.g., via an RNA decoy mechanism. The hot spot in the 3′ SL occurred only in WNVIC-infected mosquitoes and not in WNVΔSF1-infected mosquitoes, indicating that this small RNA is derived from sfRNA1 even though sfRNA2 also contains the 3′ SL and is produced by WNVΔSF1. Perhaps the absence of sfRNA1 decreases the amount of substrate to be processed into small RNAs. Alternatively, the different folding of the shorter sfRNA2 compared to that of sfRNA1 results in different processing by the RNAi machinery.
When the 3D RNA structure of sfRNA is modeled, both SL-III and the 3′ SL are clearly sticking out of the compact fold of the predicted sfRNA tertiary structure. These exposed stem-loops could potentially make them easily accessible substrates for Dicer and/or other host nucleases. Further studies are required to fully understand the molecular interplay of sfRNA, and the RNA structures within the molecule, with RNAi and the relative importance of the interaction for flavivirus transmission in vivo.
Our finding that sfRNA is very important in mosquito infection is in line with recent research that studied the mutation rate of flavivirus 3′ UTR RNA sequences in the insect host. It was demonstrated for DENV-2 that the XRN1/Pacman-resistant RNA structures SL-I and SL-II (equivalent to SL-II and SL-IV, respectively, for WNV) evolve differently depending on the origin of the host cell. In mosquito cells, SL-II incorporated mutations very rapidly compared to the upstream SL-I, which was conserved in mosquito cells but mutated more frequently during infection of mammalian cells (34). This suggests that the XRN1/Pacman-resistant SL-I is under strong selective pressure to be maintained in the insect host, most likely to safeguard production of sfRNA1. Consistent with this notion, it was recently reported that sfRNA plays a role in flavivirus transmission during a DENV epidemic. A DENV clade from Puerto Rico was outcompeted by a new clade that produced higher sfRNA/vgRNA ratios, indicating that sfRNA production correlates with increased epidemiological fitness (32). Our results support and extend this hypothesis by demonstrating for the first time that sfRNA is required for successful flavivirus transmission by mosquitoes.
In conclusion, we have shown that sfRNA is a key driver to overcome the mosquito midgut infection barrier to establish a transmissible infection. Moreover, we provide novel evidence that sfRNA is processed by the RNAi machinery in vivo in mosquitoes. Our results provide the first biological explanation for the highly conserved nature of sfRNA among all flaviviruses, foremost those that replicate in mosquitoes, which may offer novel targets to interfere with the complex flavivirus transmission cycle in nature.
ACKNOWLEDGMENTS
We thank Aaron Brault for providing the Cx.t cells and Tamás Bakonyi for providing us the WNV lineage II infectious clone.
Funding Statement
This work was supported by the European Community's Seventh Framework Programme (FP7 VECTORIE project 261466), a Ph.D. fellowship from Radboud UMC to P.M., and a Consolidator Grant from the European Research Council under the European Union's Seventh Framework Programme (ERC grant 615680) to R.P.V.R.
REFERENCES
- 1.Guzman MG, Harris E. 2015. Dengue. Lancet 385:453–465. doi: 10.1016/S0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- 2.Musso D, Cao-Lormeau VM, Gubler DJ. 2015. Zika virus: following the path of dengue and chikungunya? Lancet 386:243–244. doi: 10.1016/S0140-6736(15)61273-9. [DOI] [PubMed] [Google Scholar]
- 3.Asnis DS, Conetta R, Teixeira AA, Waldman G, Sampson BA. 2000. The West Nile virus outbreak of 1999 in New York: the flushing hospital experience. Clin Infect Dis 30:413–418. doi: 10.1086/313737. [DOI] [PubMed] [Google Scholar]
- 4.Danis K, Papa A, Theocharopoulos G, Dougas G, Athanasiou M, Detsis M, Baka A, Lytras T, Mellou K, Bonovas S, Panagiotopoulos T. 2011. Outbreak of West Nile virus infection in Greece, 2010. Emerg Infect Dis 17:1868–1872. doi: 10.3201/eid1710.110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackenzie JS, Gubler DJ, Petersen LR. 2004. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 10:S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 6.Brinton MA. 2002. The molecular biology of West Nile virus: a new invader of the western hemisphere. Annu Rev Microbiol 56:371–402. doi: 10.1146/annurev.micro.56.012302.160654. [DOI] [PubMed] [Google Scholar]
- 7.Lanciotti ARS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe E, Crabtree MB, Scherret JH, Hall RA, Mackenzie JS, Cropp CB, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Stone W, McNamara T, Gubler DJ. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 8.Lanciotti RS, Ebel GD, Deubel V, Kerst AJ, Murri S, Meyer R, Bowen M, McKinney N, Morrill WE, Crabtree MB, Kramer LD, Roehrig JT. 2002. Complete genome sequences and phylogenetic analysis of West Nile virus strains isolated from the United States, Europe, and the Middle East. Virology 298:96–105. doi: 10.1006/viro.2002.1449. [DOI] [PubMed] [Google Scholar]
- 9.Barzon L, Pacenti M, Franchin E, Pagni S, Lavezzo E, Squarzon L, Martello T, Russo F, Nicoletti L, Rezza G, Castilletti C, Capobianchi MR, Salcuni P, Cattai M, Cusinato R, Palù G. 2013. Large human outbreak of West Nile virus infection in north-eastern Italy in 2012. Viruses 5:2825–2839. doi: 10.3390/v5112825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakonyi T, Ivanics E, Erdélyi K, Ursu K, Ferenczi E, Weissenböck H, Nowotny N. 2006. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg Infect Dis 12:618–623. doi: 10.3201/eid1204.051379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenney JL, Brault AC. 2014. The role of environmental, virological and vector interactions in dictating biological transmission of arthropod-borne viruses by mosquitoes. Adv Virus Res 89:39–83. doi: 10.1016/B978-0-12-800172-1.00002-1. [DOI] [PubMed] [Google Scholar]
- 12.Franz A, Kantor A, Passarelli A, Clem R. 2015. Tissue barriers to arbovirus infection in mosquitoes. Viruses 7:3741–3767. doi: 10.3390/v7072795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair CD. 2011. Mosquito RNAi is the major innate immune pathway controlling arbovirus infection and transmission. Future Microbiol 6:265–277. doi: 10.2217/fmb.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brackney DE, Beane JE, Ebel GD. 2009. RNAi targeting of West Nile virus in mosquito midguts promotes virus diversification. PLoS Pathog 5:e1000502. doi: 10.1371/journal.ppat.1000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bronkhorst AW, Van Rij RP. 2014. The long and short of antiviral defense: small RNA-based immunity in insects. Curr Opin Virol 7:19–28. doi: 10.1016/j.coviro.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Sim S, Jupatanakul N, Dimopoulos G. 2014. Mosquito immunity against arboviruses. Viruses 6:4479–4504. doi: 10.3390/v6114479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clem RJ. 2016. Arboviruses and apoptosis: the role of cell death in determining vector competence. J Gen Virol 97:1033–1036. doi: 10.1099/jgv.0.000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Rij RP, Saleh MC, Berry B, Foo C, Houk A, Antoniewski C, Andino R. 2006. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev 20:2985–2995. doi: 10.1101/gad.1482006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnettler E, Donald CL, Human S, Watson M, Siu RWC, McFarlane M, Fazakerley JK, Kohl A, Fragkoudis R. 2013. Knockdown of piRNA pathway proteins results in enhanced Semliki Forest virus production in mosquito cells. J Gen Virol 94:1680–1689. doi: 10.1099/vir.0.053850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hussain M, Walker T, O'Neill SL, Asgari S. 2013. Blood meal induced microRNA regulates development and immune associated genes in the Dengue mosquito vector, Aedes aegypti. Insect Biochem Mol Biol 43:146–152. doi: 10.1016/j.ibmb.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Fros JJ, Miesen P, Vogels CB, Gaibani P, Sambri V, Martina BE, Koenraadt CJ, van Rij RP, Vlak JM, Takken W, Pijlman GP. 2015. Comparative Usutu and West Nile virus transmission potential by local Culex pipiens mosquitoes in north-western Europe. One Heal 1:31–36. doi: 10.1016/j.onehlt.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakumani PK, Ponia SS, Sood S RKV, Chinnappan M, Banerjea AC, Medigeshi GR, Malhotra P, Mukherjee SK, Bhatnagar RK. 2013. Role of RNA interference (RNAi) in dengue virus replication and identification of NS4B as an RNAi suppressor. J Virol 87:8870–8883. doi: 10.1128/JVI.02774-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnettler E, Tykalová H, Watson M, Sharma M, Sterken MG, Obbard DJ, Lewis SH, McFarlane M, Bell-Sakyi L, Barry G, Weisheit S, Best SM, Kuhn RJ, Pijlman GP, Chase-Topping ME, Gould EA, Grubhoffer L, Fazakerley JK, Kohl A. 2014. Induction and suppression of tick cell antiviral RNAi responses by tick-borne flaviviruses. Nucleic Acids Res 42:9436–9446. doi: 10.1093/nar/gku657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kakumani PK, Rajgokul KS, Ponia SS, Kaur I, Mahanty S, Medigeshi GR, Banerjea AC, Chopra AP, Malhotra P, Mukherjee SK, Bhatnagar RK. 2015. Dengue NS3, an RNAi suppressor, modulates the human-miRNA pathways through its interacting partner. Biochem J 471:89–99. doi: 10.1042/BJ20150445. [DOI] [PubMed] [Google Scholar]
- 25.Pijlman GP, Funk A, Kondratieva N, Leung J, Torres S, van der Aa L, Liu WJ, Palmenberg AC, Shi P-Y, Hall RA, Khromykh AA. 2008. A highly structured, nuclease-resistant, noncoding RNA produced by flaviviruses is required for pathogenicity. Cell Host Microbe 4:579–591. doi: 10.1016/j.chom.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Chapman EG, Moon SL, Wilusz J, Kieft JS. 2014. RNA structures that resist degradation by Xrn1 produce a pathogenic Dengue virus RNA. eLife 3:e01892. doi: 10.7554/eLife.01892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kieft JS, Rabe JL, Chapman EG. 2015. New hypotheses derived from the structure of a flaviviral Xrn1-resistant RNA: conservation, folding, and host adaptation. RNA Biol 12:1169–1177. doi: 10.1080/15476286.2015.1094599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva PAGC, Pereira CF, Dalebout TJ, Spaan WJM, Bredenbeek PJ. 2010. An RNA pseudoknot is required for production of yellow fever virus subgenomic RNA by the host nuclease XRN1. J Virol 84:11395–11406. doi: 10.1128/JVI.01047-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Funk A, Truong K, Nagasaki T, Torres S, Floden N, Balmori Melian E, Edmonds J, Dong H, Shi P-Y, Khromykh AA. 2010. RNA structures required for production of subgenomic flavivirus RNA. J Virol 84:11407–11417. doi: 10.1128/JVI.01159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapman EG, Costantino DA, Rabe JL, Moon SL, Wilusz J, Nix JC, Kieft JS. 2014. The structural basis of pathogenic subgenomic Flavivirus RNA (sfRNA) production. Science 344:307–310. doi: 10.1126/science.1250897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuessler A, Funk A, Lazear HM, Cooper DA, Torres S, Daffis S, Jha BK, Kumagai Y, Takeuchi O, Hertzog P, Silverman R, Akira S, Barton DJ, Diamond MS, Khromykh AA. 2012. West Nile virus noncoding subgenomic RNA contributes to viral evasion of the type I interferon-mediated antiviral response. J Virol 86:5708–5718. doi: 10.1128/JVI.00207-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manokaran G, Finol E, Wang C, Gunaratne J, Bahl J, Ong EZ, Tan HC, Sessions OM, Ward AM, Gubler DJ, Harris E, Garcia-Blanco MA, Ooi EE. 2015. Dengue subgenomic RNA binds TRIM25 to inhibit interferon expression for epidemiological fitness. Science 350:217–221. doi: 10.1126/science.aab3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villordo SM, Carballeda JM, Filomatori CV, Gamarnik AV. 2016. RNA structure duplications and flavivirus host adaptation. Trends Microbiol 24:270–283. doi: 10.1016/j.tim.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villordo SM, Filomatori CV, Sánchez-Vargas I, Blair CD, Gamarnik AV. 2015. Dengue virus RNA structure specialization facilitates host adaptation. PLoS Pathog 11:e1004604. doi: 10.1371/journal.ppat.1004604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roby JA, Pijlman GP, Wilusz J, Khromykh AA. 2014. Noncoding subgenomic flavivirus RNA: multiple functions in West Nile virus pathogenesis and modulation of host responses. Viruses 6:404–427. doi: 10.3390/v6020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva PAGC. 2011. Functions and requirements of conserved RNA structures in the 3′ untranslated region of Flaviviruses. Ph.D thesis. Leiden University, Leiden, The Netherlands. [Google Scholar]
- 37.Schnettler E, Sterken MG, Leung JY, Metz SW, Geertsema C, Goldbach RW, Vlak JM, Kohl A, Khromykh AA, Pijlman GP. 2012. Noncoding flavivirus RNA displays RNA interference suppressor activity in insect and mammalian cells. J Virol 86:13486–13500. doi: 10.1128/JVI.01104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moon SL, Dodd BJT, Brackney DE, Wilusz CJ, Ebel GD, Wilusz J. 2015. Flavivirus sfRNA suppresses antiviral RNA interference in cultured cells and mosquitoes and directly interacts with the RNAi machinery. Virology 485:322–329. doi: 10.1016/j.virol.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Main OM, Hardy JL, Reeves WC. 1977. Growth of arboviruses and other viruses in a continuous line of Culex-tarsalis cells. J Med Entomol 14:107–112. doi: 10.1093/jmedent/14.1.107. [DOI] [PubMed] [Google Scholar]
- 40.Szentpáli-Gavallér K, Lim S, Dencső L, Bányai K, Koraka P, Osterhaus A, Martina B, Bakonyi T, Bálint A. 2016. In vitro and in vivo evaluation of mutations in the NS region of Lineage 2 West Nile virus associated with neuroinvasiveness in a mammalian model. Viruses 8:E49. doi: 10.3390/v8020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goecks J, Nekrutenko A, Taylor J. 2010. Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol 11:R86. doi: 10.1186/gb-2010-11-8-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fros JJ, Geertsema C, Vogels CB, Roosjen PP, Failloux A-B, Vlak JM, Koenraadt CJ, Takken W, Pijlman GP. 2015. West Nile virus: high transmission rate in north-western European mosquitoes indicates its epidemic potential and warrants increased surveillance. PLoS Negl Trop Dis 9:e0003956. doi: 10.1371/journal.pntd.0003956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adams SC, Broom AK, Sammels LM, Hartnett AC, Howard MJ, Coelen RJ, Mackenzie JS, Hall RA. 1995. Glycosylation and antigenic variation among Kunjin virus isolates. Virology 206:49–56. doi: 10.1016/S0042-6822(95)80018-2. [DOI] [PubMed] [Google Scholar]
- 44.Hall RA, Burgess GW, Kay BH, Clancy P. 1991. Monoclonal antibodies to Kunjin and Kokobera viruses. Immunol Cell Biol 69:47–49. doi: 10.1038/icb.1991.7. [DOI] [PubMed] [Google Scholar]
- 45.Zuker M. 2003. Mfold web server for Nucleic acid folding and hybridization prediction. Nucleic Acids Res 31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darty K, Denise A, Ponty Y. 2009. VARNA: interactive drawing and editing of the RNA secondary structure. Bioinformatics 25:1974–1975. doi: 10.1093/bioinformatics/btp250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Popenda M, Szachniuk M, Antczak M, Purzycka KJ, Lukasiak P, Bartol N, Blazewicz J, Adamiak RW. 2012. Automated 3D structure composition for large RNAs. Nucleic Acids Res 40:e112. doi: 10.1093/nar/gks339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schrödinger LLC. 2015. The PyMOL molecular graphics system, version 1.8. Schrödinger LLC, New York, NY. [Google Scholar]
- 49.Brackney DE, Scott JC, Sagawa F, Woodward JE, Miller NA, Schilkey FD, Mudge J, Wilusz J, Olson KE, Blair CD, Ebel GD. 2010. C6/36 Aedes albopictus cells have a dysfunctional antiviral RNA interference response. PLoS Negl Trop Dis 4:e856. doi: 10.1371/journal.pntd.0000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hussain M, Torres S, Schnettler E, Funk A, Grundhoff A, Pijlman GP, Khromykh AA, Asgari S. 2012. West Nile virus encodes a microRNA-like small RNA in the 3′ untranslated region which up-regulates GATA4 mRNA and facilitates virus replication in mosquito cells. Nucleic Acids Res 40:2210–2223. doi: 10.1093/nar/gkr848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. 2008. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog 4:e1000092. doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goertz GP, Pijlman GP. 2015. Dengue non-coding RNA: TRIMmed for transmission. Cell Host Microbe 18:133–134. doi: 10.1016/j.chom.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Gritsun TS, Gould EA. 2007. Origin and evolution of 3′UTR of flaviviruses: long direct repeats as a basis for the formation of secondary structures and their significance for virus transmission. Adv Virus Res 69:203–248. [DOI] [PubMed] [Google Scholar]
- 54.Whitfield SG, Frederick AM, Sudia WD. 1973. St. Louis Encephalitis virus: an ultrastructural vector of infection in a mosquito. Virology 56:70–87. [DOI] [PubMed] [Google Scholar]
- 55.Grundhoff A, Sullivan CS, Ganem D. 2006. A combined computational and microarray-based approach identifies novel microRNAs encoded by human gamma-herpesviruses. RNA 12:733–750. doi: 10.1261/rna.2326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sullivan CS, Grundhoff A. 2007. Identification of viral microRNAs. Methods Enzymol 427:3–23. [DOI] [PubMed] [Google Scholar]