Abstract
Cytoadhesion of Plasmodium falciparum infected erythrocytes to gC1qR has been associated with severe malaria, but the parasite ligand involved is currently unknown. To assess if binding to gC1qR is mediated through the P. falciparum erythrocyte membrane protein 1 (PfEMP1) family, we analyzed by static binding assays and qPCR the cytoadhesion and var gene transcriptional profile of 86 P. falciparum isolates from Mozambican children with severe and uncomplicated malaria, as well as of a P. falciparum 3D7 line selected for binding to gC1qR (Pf3D7gC1qR). Transcript levels of DC8 correlated positively with cytoadhesion to gC1qR (rho = 0.287, P = 0.007), were higher in isolates from children with severe anemia than with uncomplicated malaria, as well as in isolates from Europeans presenting a first episode of malaria (n = 21) than Mozambican adults (n = 25), and were associated with an increased IgG recognition of infected erythrocytes by flow cytometry. Pf3D7gC1qR overexpressed the DC8 type PFD0020c (5.3-fold transcript levels relative to Seryl-tRNA-synthetase gene) compared to the unselected line (0.001-fold). DBLβ12 from PFD0020c bound to gC1qR in ELISA-based binding assays and polyclonal antibodies against this domain were able to inhibit binding to gC1qR of Pf3D7gC1qR and four Mozambican P. falciparum isolates by 50%. Our results show that DC8-type PfEMP1s mediate binding to gC1qR through conserved surface epitopes in DBLβ12 domain which can be inhibited by strain-transcending functional antibodies. This study supports a key role for gC1qR in malaria-associated endovascular pathogenesis and suggests the feasibility of designing interventions against severe malaria targeting this specific interaction.
Author Summary
Plasmodium falciparum sequesters in vital organs. This phenomenon mediated by cytoadhesion of infected-erythrocytes to host receptors in the microvasculature, contributes to the development of severe malaria. Although cytoadhesion to Endothelial Protein-C Receptor has a central role in severe malaria, other host receptors are also likely to be involved. Our results generated by the analysis of P. falciparum isolates from Mozambican patients and laboratory parasite lines indicate that a specific domain (DBLβ12) from DC8-type PfEMP1s can bind to the human receptor gC1qR, previously associated with severe malaria. Our findings revealed that antibodies against PfEMP1 could provide strain-transcending inhibition of gC1qR-binding. Overall, these results support a key role for the adhesion to gC1qR in malaria-associated endovascular pathogenesis and the feasibility of new interventions targeting this specific interaction.
Introduction
Case fatality rates for severe malaria (SM) remain unacceptably high even after administration of effective anti-malarial drugs [1]. There is an urgent need to develop novel interventions against life-threatening malaria. However, the mechanisms underlying the clinical heterogeneity and spectrum of malaria [2] remain largely unknown. The general state of health and physiological condition of the host, in particular variations in host immunity, together with genetic predisposition and parasite factors involved in the virulence of the infection, might influence the progression of malaria towards a life-threatening outcome. Sequestration of infected erythrocytes (IE) in vital organs is believed to constitute a key pathogenic event in P. falciparum SM [3], eventually leading to hemorrhages, thrombi formation and pathological inflammation [4], all at the basis of microvascular obstruction [4–6]. Strategies to inhibit or prevent parasite sequestration thus have the potential to reduce the high fatality rate in SM.
Surface proteins at the interface of malaria parasites and the human host contribute to sequestration through the cytoadhesion of IEs to the vascular endothelium, to uninfected erythrocytes to form rosettes [7] and to IEs through platelet binding to form agglutinates (Platelet-mediated [PM]-agglutination) [8]. Cytoadhesion is primarily mediated by interactions between Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) [9] and host receptors such as CD36 [10], ICAM-1 [11], CSA [12], heparin [7], EPCR [13] and gC1qR [8,14]. PfEMP1 is a family of highly diverse antigens located on the surface of mature stage IEs that contain 2–9 adhesion domains termed DBL (Duffy binding-like) and CIDR (cysteine-rich interdomain region). Each parasite contains ∼60 different var genes per haploid genome that encode PfEMP1s, which subvert acquisition of protective immunity [15] through constant transcriptional switching [16] and mutually exclusive expression [17]. Antibodies to PfEMP1 that occur after natural infections or after immunization with recombinant PfEMP1 domains are predominantly variant- and strain-specific, as expected for highly variable parasite antigens [18–20]. However, epidemiological observations that children acquire immunity to non-cerebral severe malaria after a small number of infections [21] suggest that strain-transcending antibodies recognizing conserved epitopes on PfEMP1 may occur [19,22], or that the parasites that cause severe malaria are of restricted antigenic types [23,24].
PfEMP1s can be classified into three major groups (A, B and C) and two intermediate groups (B/A and B/C), based on motifs in non-coding sequences and locus position [25]. Whereas most group B and C PfEMP1 proteins appear to be under selection to bind CD36 [26] and tend to be associated with uncomplicated and asymptomatic malaria [27,28], groups A and B/A are often expressed in young children with limited malaria immunity [23] and in those with SM [28–31]. A subset of these A and B/A PfEMP1 variants that contain a combination of adhesion domains, termed domain cassettes 8 and 13 (DC8 and DC13) [32–34], can bind through their CIDRα1.1/4/5/7 domains to Endothelial Protein C Receptor (EPCR) [13]. It has been suggested that EPCR-mediated parasite cytoadhesion could interfere with activation of cytoprotective and anti-inflammatory pathways, which in turn may contribute to severe malaria pathology [13]. However, adhesion to human cell lines is likely to be mediated by interaction with several receptors [35]. Indeed other domains of DC8 and DC13 PfEMP1 variants have been shown to bind avidly to endothelial cells from different tissues through unknown host receptors [36]. These data highlight the heterogeneity of receptors used by IEs in different vascular beds and the importance of identifying other receptors involved in host-parasite interactions.
P. falciparum IEs use gC1qR as a receptor for both cytoadhesion to human cells and platelet-mediated clumping [14], a cytoadhesion phenotype which has been associated with SM in Mozambican children [8]. Human gC1qR is a multi-functional cellular protein expressed on a wide range of tissues and cell types including endothelial cells, lymphocytes, dendritic cells and platelets [37]. In addition to modulating the activation of complement through binding to C1q [38], gC1qR can serve as a receptor for diverse pro-inflammatory ligands [39] and functional antigens of viral and bacterial origin to promote pathogen attachment and/or entry [40]. However, the protein used by malaria parasites to mediate cytoadhesion of IEs to gC1qR is currently unknown.
Selection of IT/FCR3 parasite lines for binding to human brain microvascular endothelial cells (HBMEC) was associated with an up-regulation of DC8- and DC13-PfEMP1 and an increase in binding to gC1qR [33]. Based on this observation, we hypothesized that PfEMP1s containing DC combinations associated with SM may mediate binding to gC1qR. To address this, we assessed the var expression patterns and gC1qR cytoadhesion profile of P. falciparum isolates collected from Mozambican children [8] and in a P. falciparum 3D7 line selected in vitro for binding to gC1qR. The relationship of var transcript levels with disease severity, previous malaria exposure and antibody-mediated recognition of IEs was also analyzed. Our results demonstrate that transcript abundance of DC8 in field isolates is associated with binding of IEs to gC1qR and that DBLβ12 from the DC8-type PFD0020c mediates such interaction in the P. falciparum 3D7 line selected for binding to gC1qR. The successful induction of strain-transcending antibodies against DBLβ12 with activity to inhibit binding to gC1qR by field isolates suggests shared surface epitopes amongst heterologous gC1qR-binding PfEMP1 variants and the feasibility to designing interventions to prevent severe malaria.
Results
Study population and clinical outcomes
Blood samples from 132 malaria patients were used in the study, 111 from Manhiça, Mozambique (86 children and 25 adults) and 21 from European travelers (Table 1). Among the Mozambican children, 43 had uncomplicated malaria (UM) and 43 had SM, defined as severe anemia, acidosis or respiratory distress, multiple seizures, prostration, cerebral malaria or hypoglycemia (Table 1) [8]. Among the 43 cases of severe malaria, 19 (44%) had a single criteria of malaria severity and the rest overlapping symptoms (13 [30%] had two and 11 [26%] three or more). Prostration was observed in 34 (79%) of the children, acidosis/respiratory distress in 17 (39%), severe anemia in 13 (30%) and multiple seizures in 11 (26%), whereas cerebral malaria and hypoglycemia was observed only in 3 and 2 of the children, respectively (Table 1). European travelers were coming from Western Africa (Ghana, Republic of Côte d'Ivoire, The Gambia, Guinea, Equatorial Guinea, Togo, Senegal and Burkina Faso), Middle Africa (Cameroon, Congo and Central African Republic) and Eastern Africa (Mozambique and Madagascar), with none of them presenting SM at recruitment. Parasitemia, quantified by qPCR, was the highest in Mozambican adults, followed by SM and UM cases, with travelers showing the lowest levels of parasitemia (P = 0.022). No differences were observed in the multiplicity of infection (MOI) between groups (P = 0.106).
Table 1. Characteristics of the patients with malaria included in the study.
| Spain | Mozambique | ||||
|---|---|---|---|---|---|
| Patient characteristics | Travelers (n = 21) | SM (n = 43) | UM (n = 43) | Adults (n = 25) | P |
| Age (years), median (IQR) | 34 (29–40) | 2.4 (1.3–3.6) | 2.6 (1.3–3.6) | 36 (30–46) | 0.791 |
| qPCR Parasite density*, median(IQR) | 724(322–9973) | 9060(2290–31982) | 3618(1050–13623) | 10260(2267–38327) | 0.022 |
| MOI, median (IQR) | 2 (1–3) | 3 (3–5) | 3 (2–4) | 2 (2–3) | 0.106 |
| Males, n (%) | 16 (76) | 28 (65) | 28 (65) | 16 (64) | 1.000 |
| Clinical manifestation of SM a (n) | |||||
| Cerebral malaria | - | 3 | - | - | |
| Severe anaemia | - | 13 | - | - | |
| Multiple seizures | - | 11 | - | - | |
| Prostration | - | 34 | - | - | |
| Hypoglicemia | - | 2 | - | - | |
| Acidosis/Respiratory Distress | - | 17 | - | - | |
IQR, Interquartile range; SM, severe malaria; UM, uncomplicated malaria; MOI, multiplicity of infection.
*, Expressed as parasites per μL.
a, Nineteen (44%) out of the 43 SM cases had a single criterion of malaria severity and the rest overlapping symptoms (13 [30%] had two and 11 [26%] three or more).
Transcript level of DC8 and DC11 var genes correlate with gC1qR cytoadhesion in Mozambican isolates
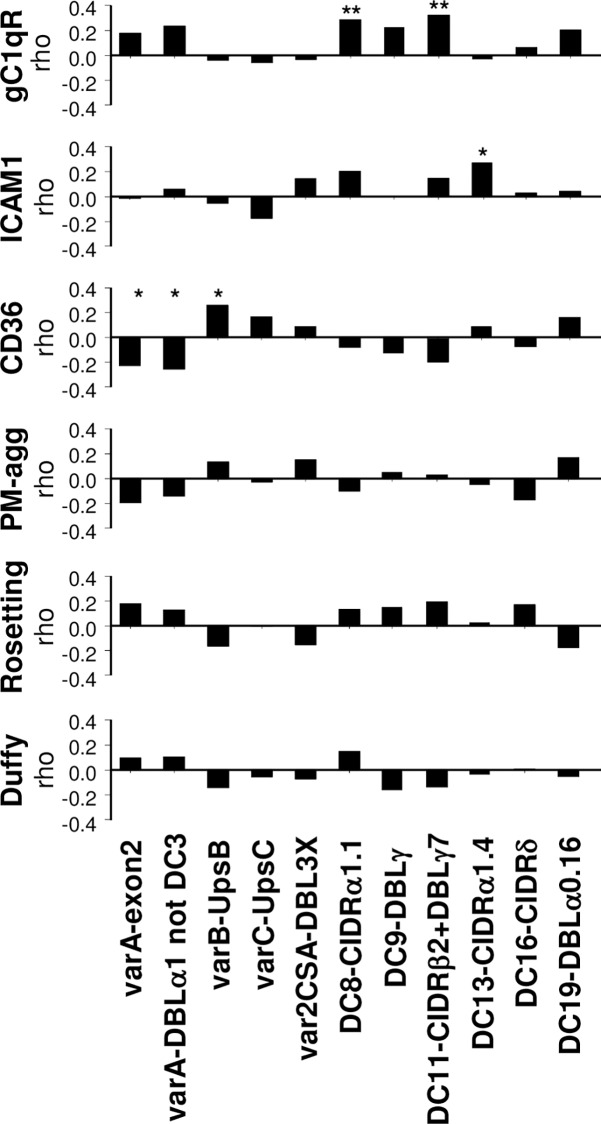
The relationship between cytoadhesion and var/DCs transcript levels was assessed among P. falciparum isolates collected from Mozambican children (n = 86; Fig 1). Adhesion to CD36 was the most frequent cytoadhesion phenotype (76/86 [88%]; median binding of 180 IEs/mm2, IQR[101–353]), followed by PM-agglutination (57/86 [66%]; median of 7%, IQR[2–22]), adhesion to gC1qR (38/86 [44%]; median binding of 60 IEs/mm2, IQR[45–155]), ICAM1 (37/86 [43%]; median binding of 55 IEs/mm2, IQR[39–105]) and rosetting (31/86 [36%]; median of 2%, IQR[1–5]; Table A in SI Text). The percentage of isolates expressing var/DCs ranged from 41% (35/86) for DC13-CIDRα1.4 to 100% for varA-exon2, varB-UpsB and DC11-CIDRβ2+DBLγ7 (Table B in SI Text). Adhesion to gC1qR correlated positively with DC8 transcript levels (targeted by DC8-CIDRα1.1, rho: 0.287, P = 0.007) and with DC11 (rho: 0.324, P = 0.002). Adhesion to ICAM1 showed a positive correlation with transcript levels of DC13 (rho: 0.273, P = 0.011). Adhesion to CD36 correlated positively with varB (rho: 0.259, P = 0.016) and negatively with varA (rho = -0.228, P = 0.035) and varA-notDC3 (rho: -0.256, P = 0.018). No association was found between var transcript levels, PM-agglutination, rosetting or binding to the negative control Duffy receptor (Fig 1).
Fig 1. Correlation between var transcript levels and cytoadhesive phenotypes of P. falciparum isolates from Mozambican children.
The relationship between adhesion and transcript levels of var/DCs was assessed by Spearman correlation analysis, with * indicating P<0.05 and ** if statistically significant after Benjamini-Hochberg correction for the six adhesive phenotypes tested. PM-agg: platelet-mediated agglutination.
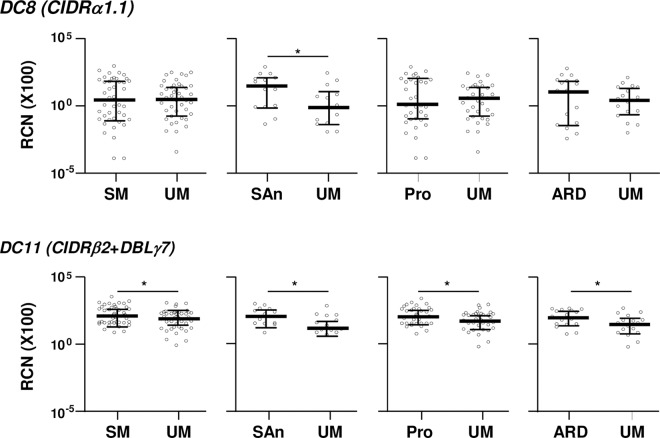
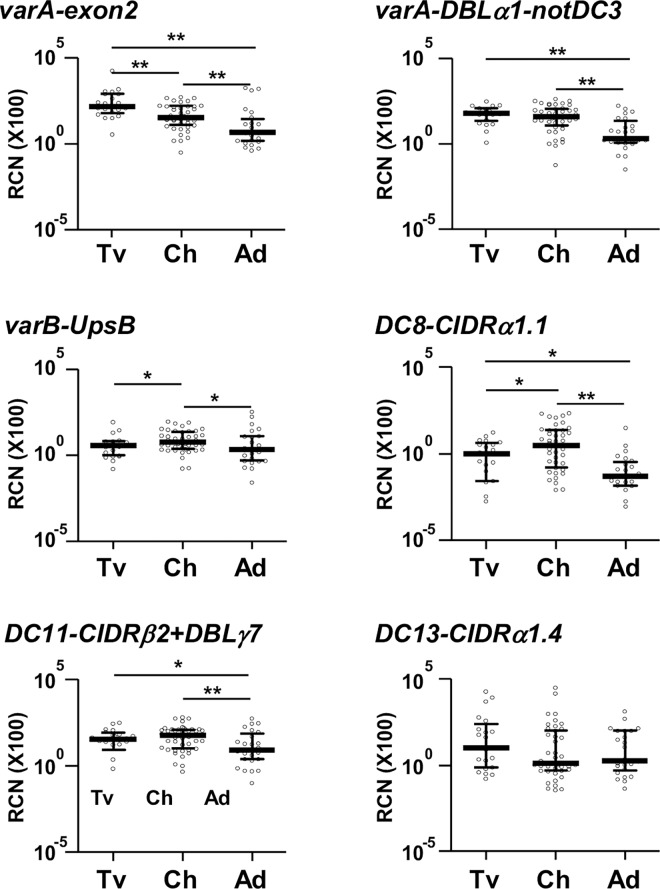
DC11 transcript levels were lower in isolates from children with UM than in those from SM children (P = 0.043), severe anemia (P = 0.022), prostration (P = 0.050) and acidosis/respiratory distress (P = 0.044, Fig 2 and Table C in SI Text). Similarly, transcript levels of DC8 were higher in children with severe anemia compared to their UM pairs (P = 0.030, Fig 2). Both DCs were transcribed at similar levels by isolates from travelers and children, being the lowest in isolates from Mozambican adults (Fig 3).
Fig 2. Transcript levels of DC8 and DC11 by severe malaria symptoms in Mozambican children.
Transcript levels (y axis) correspond to relative copy number of target genes relative to seryl-tRNA synthetase gene copies (X100). Bars represent the median and interquartile range. Transcript levels were compared between matched case/control pairs by Sign-test, with * indicating P<0.05. RCN: Relative copy number; DC: Domain Cassette; SM: severe malaria; UM: uncomplicated malaria; SAn: severe anemia; Pro: prostration; ARD: acidosis or respiratory distress.
Fig 3. Transcript levels of var/DCs in P. falciparum isolates from travelers, children with uncomplicated malaria and adults.
Transcript levels (y axis) correspond to relative copy number relative to seryl-tRNA synthetase gene copies (X100). Bars represent the median and interquartile range. Transcript levels were compared between groups by Mann-Whitney test, with * indicating P<0.05 and ** if statistically significant after Benjamini-Hockberg correction. RCN: Relative copy number; DC: Domain Cassette; Tv: travelers, Ch: Mozambican children, Ad: Mozambican adults.
Increased IgG recognition of isolates highly transcribing DC8 var genes
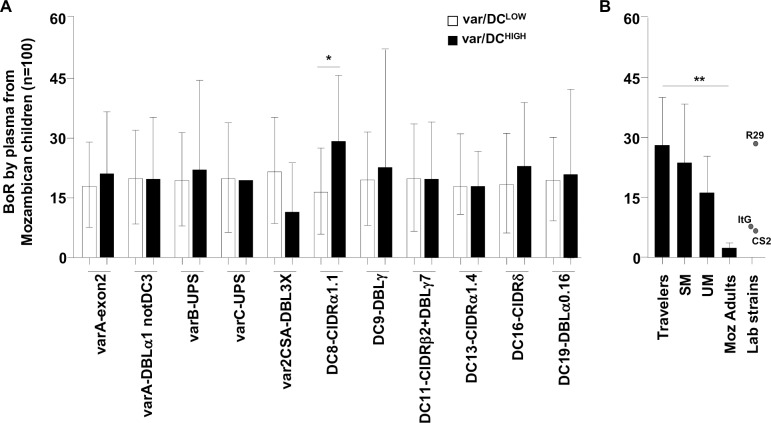
Isolates transcribing DC8 at high levels (i.e., copy number ≥0.5-fold of Seryl-tRNA-synthetase copy number) were more often recognized by plasmas from Mozambican children (n = 100; mean breadth of recognition: 29%, Standard Deviation (SD) 16) than those transcribing DC8 at low levels (16%, SD 11; incidence rate ratio = 2.3, 95% CI [1.2–4.5], P = 0.019; Fig 4A). No differences were observed for other DCs. Breadth of IgG recognition of parasites transcribing DC8 at high levels was higher among the Mozambican adult population (76%, SD 18) than among children (p = 0.010 by Signrank test). However, no difference was observed in the breadth among children with SM (p = 0.969). Finally, recognition by plasma from Mozambican children was the highest for IEs from travelers (mean breadth of recognition: 28%; SD 12), followed by isolates from SM (24%, SD 14) and UM (16%; SD 9), being the lowest for parasites from Mozambican adults (3%, SD 1; test for tend, P≤0.001; Fig 4B).
Fig 4. Breadth of IgG recognition of P. falciparum isolates according to var transcript levels and origin.
Geometric Mean Fluorescence Intensity (GMFI) values from each parasite/plasma combination were scored in relation to the threshold of positivity (GMFI of negative controls plus two standard deviations), with a score of 0 assigned if GMFI values were below the cut-off; 1 if the value was between one- and two-fold the cut-off; 2 if the value was between two- and three-fold the cut-off; and so on until a maximum score of 5. Breadth of IgG recognition (BoR) was calculated as the sum of scores obtained for each parasite and expressed as percentage of the maximum score possible. BoR was compared between A) isolates transcribing var/DCs at low- or high- levels by negative binomial regression models adjusted by age and B) between isolates collected from travelers (n = 3), severe malaria (SM, n = 23), uncomplicated malaria (UM, n = 15) children and adults (Moz adults, n = 4) by test for trend across ordered groups. Bars represent the mean of BoR and standard deviation. * indicates P<0.05 and ** P≤0.001.
DBLβ12 domain from the DC8-type PFD0020c in 3D7 is associated with gC1qR cytoadhesion
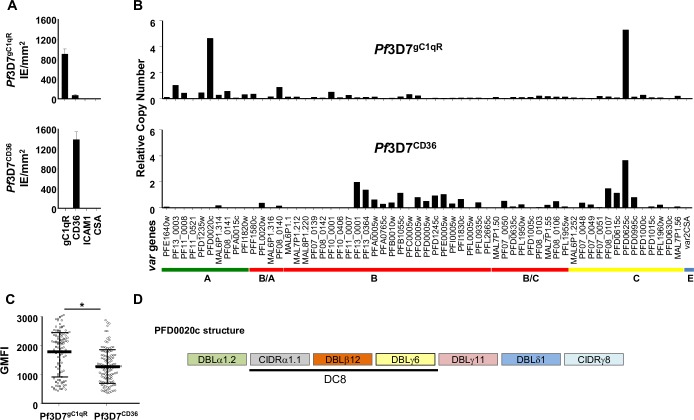
After two rounds of in vitro selection for binding to gC1qR followed by a limiting dilution cloning, a P. falciparum 3D7 clone was obtained (Pf3D7gC1qR) that showed high binding to gC1qR (mean: 900 parasites/mm2, SD 101) and low to CD36 (71 IEs/mm2, SD 2. In contrast, the unselected 3D7 clone (Pf3D7CD36) showed high levels of adhesion to CD36 (1400 IEs/mm2, SD 159) but no adhesion to gC1qR (Fig 5A). The selection for binding to gC1qR was associated with a 1.3-fold increase in the levels of IgG recognition by plasmas from Mozambican children (Geometric Mean Fluorescence Intensity [GMFI) for Pf3D7gC1qR of 1679, SD: 765 vs GMFI for Pf3D7CD36 of 1266, SD: 576, P≤0.001; Fig 5C). P. falciparum 3D7gC1qR transcribed mostly the DC8-PFD0020c (4.7-fold seryl-tRNA synthetase gene), as well as PFD0625c (5.3-fold seryl-tRNA synthetase gene), whereas Pf3D7CD36 mostly expressed PFD0625c (3.7-fold seryl-tRNA synthetase gene, Fig 5B). The fold ratio of PFD0020c transcript levels in gC1qR-selected Pf3D7 line compared with that in unselected parasites was 4656, and 1.45 for PFD0625c.
Fig 5. Phenotypical and molecular characterization of Pf3D7gC1qR and Pf3D7CD36.
A) Binding assays of Pf3D7gC1qR and Pf3D7CD36 over gC1qR, CD36, ICAM1 and CSA receptors. B) Transcriptional analysis of the var gene repertoire of Pf3D7. Transcript levels of var genes were determined by qPCR using primers specific for each of the P. falciparum 3D7 var genes and were expressed as copy number relative to the seryl-tRNA synthetase gene. C) Levels of IgG recognition by plasma from Mozambican children were compared by Wilcoxon matched pair test, with * indicating P≤0.001. Bars represent mean and standard deviation of geometric mean fluorescence intensity (GMFI). D) Domain structure of PFD002c.
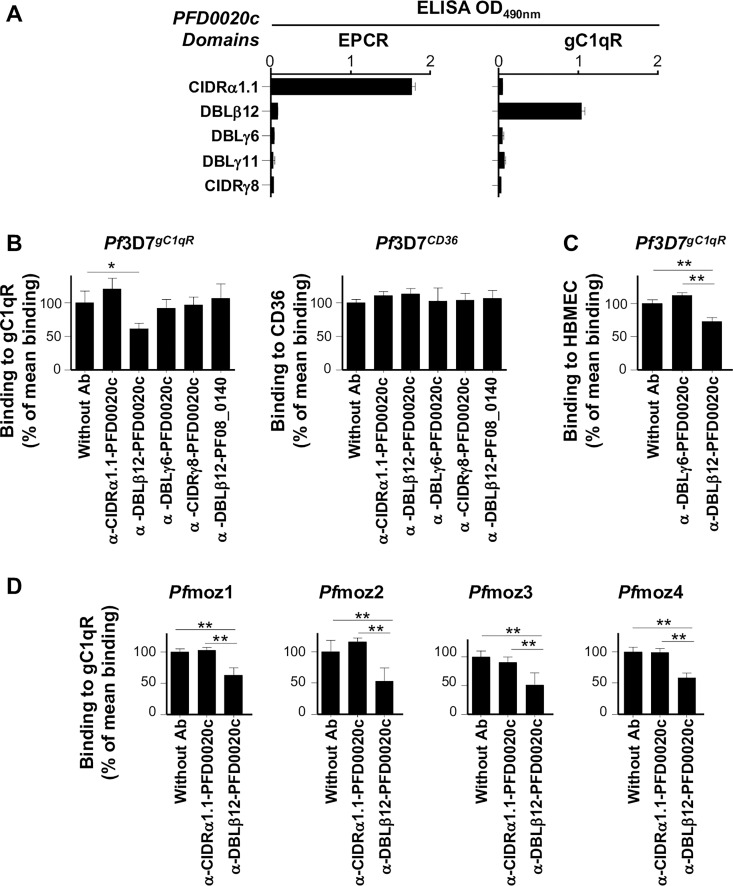
To identify the domain(s) mediating binding to gC1qR, we assessed the ability of recombinant constructs representing extracellular domains of PFD0020c (Fig 5D) to bind to gC1qR by ELISA-binding assays. The DBLβ12 from PFD0020c, but not CIDRα1.1, DBLγ6, DBLγ11 and CIDRγ8 was shown to interact with gC1qR (Fig 6A). We further confirmed the gC1qR-binding specificity through a bead-suspension technology (Luminex) in which the beads were coupled with the seven PFD0020c domains and tested for binding to gC1qR, allowing us to confirm that only DBLβ12 was able to bind to gC1qR (Fig A in SI Text). In contrast, only CIDRα1.1 from PFD0020c reacted with rEPCR but not the other domains tested, DBLβ12, DBLγ6, DBLγ11 and CIDRγ8. Moreover, purified polyclonal IgG generated in rabbit against domain DBLβ12PFD0020c, at concentrations of 300 μg/mL, were able to inhibit binding of Pf3D7gC1qR to gC1qR by ∼40% (SD 8) compared with binding of Pf3D7gC1qR to gC1qR in absence of antibodies (P = 0.029). Antibodies against the other PFD0020c domains tested and antibodies against DBLβ12PF08_0140 did not inhibit the binding of Pf3D7gC1qR to gC1qR (Fig 6B). IgGs against DBLβ12-PFD0020c inhibited binding of Pf3D7gC1qR to HBMEC cells, known to express gC1qR on their surface [14], by 46% compared to the control antibody (α -DBLγ6-PFD0020c; Fig 6C). We also tested inhibition of gC1qR binding by four P. falciparum isolates collected from Mozambican children which transcribed DC8, as targeted by DC8-CIDRα1.1, or DBLβ12&DBLβ3/5 domains, at high levels (Fig B in SI Text). In all the four isolates, binding to gC1qR was also reduced by ∼50% in the presence of antibodies against DBLβ12PFD0020c (Fig 6D). IgG against domain DBLβ12PFD0020c did not affect binding to EPCR nor CD36 (Fig B in SI Text). Finally, we show that DBLβ12, together with DBLγ6, DBLγ11, DBLδ1 and CIDRγ8, exhibited the highest increase in IgG recognition among malaria-infected Mozambican children compared to never-exposed Spanish individuals, as well as the highest increase with age of Mozambican children (more than 2.5 years versus less than 2.5 years of age; Fig C in SI Text).
Fig 6. DBLβ12 domain of PFD0020c is involved in the interaction with gC1qR.
A) Reactivity of PFD0020c domains against gC1qR and EPCR by ELISA-based binding assays. Antibody-mediated inhibition of B) binding to gC1qR or CD36 of P. falciparum Pf3D7 gC1qR, Pf3D7 CD36; C) binding to human brain endothelial cells (HBMEC) of Pf3D7 gC1qR and D) binding to gC1qR of four P. falciparum Mozambican isolates. The gC1qR binding levels in absence of antibodies were 299 IEs/mm2 (SD 53) for Pf3D7gC1qR; 202 IEs/mm2 (SD 11) for Pfmoz1; 92 IEs/mm2 (SD 17) for Pfmoz2; 74 IEs/mm2 (SD 8) for Pfmoz3; and 81 IEs/mm2 (SD 7) for Pfmoz4. The CD36 binding level in absence of antibodies was 615 IEs/mm2 (SD 27) for Pf3D7CD36. Binding is expressed as the percentage of mean binding in absence of antibodies. Bars represent the mean and standard deviation. * indicates P<0.05 and **P<0.001.
Discussion
The combined analysis of P. falciparum isolates from malaria infected Mozambique patients and an in vitro selected P. falciparum 3D7 line shows a relationship between cytoadhesion to gC1qR and transcription of DC8-type var genes. The clinical relevance of such a phenotype has been suggested in a field study conducted in Mozambique which showed that prevalence of parasite isolates exhibiting adhesion to gC1qR was associated with multiple seizures [8], although binding levels only tended to be higher compared with isolates from children with severe malaria. In the present study, the use of primer sets targeting the most clinically-relevant DCs [28,34,41] allowed us firstly to correlate the cytoadhesion to gC1qR with abundance of DC8 var transcripts in Mozambican isolates. Secondly, selection of P. falciparum 3D7 line for binding to gC1qR showed the up-regulation of the DC8-PFD0020c. Recombinant DBLβ12PFD0020c bound to gC1qR in ELISA assays and antibodies against this domain were able to inhibit binding of Pf3D7gC1qR and P. falciparum Mozambican isolates to gC1qR by 50%. Overall, these results point to the DBLβ12 domain present in DC8-PfEMP1 variants as the domain that mediates cytoadhesion to gC1qR.
Cytoadhesion to gC1qR by Mozambican isolates correlated positively with their transcript levels of DC8 which, in line with previous studies [34,42,43], were higher in parasites collected from Mozambican children with severe anemia than in those with UM. Moreover, DC8 was transcribed at higher levels by isolates from individuals with limited antimalarial immunity (i.e., Mozambican children and first-time infected travelers) compared to isolates from Mozambican adults with life-long exposure to malaria. As adults and children included in this study come from the same region in Mozambique, it is unlikely that differences observed are due to spatial heterogeneities in the DC8-expressing profile of parasite populations, especially when all parasite genomes appear to have similar repertoires globally [44]. The results rather suggest an exhaustion of the var gene repertoire mediating cytoadhesion and severe malaria with increasing immunity. Alternatively, antigenic variants different to DC8 may increase through ectopic recombination in chronic infections [45] which are expected to be more frequent among semi-immune adults. Also, parasites transcribing DC8 at high levels were more often recognized by plasma from malaria-exposed children than parasites with low DC8 transcription. This is in line with the observation that malaria-exposed Tanzanian population acquires antibodies to EPCR-binding CIDR domains more rapidly than antibodies to other CIDR domains [46]. Although isolates highly transcribing DC8 were better recognized by plasmas from semi-immune Mozambican adults than by children, no difference was observed between plasmas from children with severe and uncomplicated malaria. This latter observation, in line with previous studies conducted in the same area [47], might be attributed to difficulties in disentangling the role of antibodies as markers of exposure and protection among infected population. Overall, these results point towards the contribution of DC8 to gC1qR binding and severe malaria, the antigenic conservation of these PfEMP1 variants, their preferential transcription by malaria parasites infecting individuals who have still not developed antimalarial immunity [34,48,49] and the need to perform longitudinal studies to assess the role of antibodies against DC8 in reducing the risk of severe malaria.
Selection of P. falciparum 3D7 line for binding to gC1qR [14] was accompanied by a marked increase in the expression of a single varA gene, PFD0020c, whose transcript levels were 4656-fold higher than in the unselected line, as well as by an increase in the IgG recognition of IEs by plasmas from exposed children. In contrast, the unselected line, which bound to CD36 in static assays, transcribed B and C var genes at relatively low levels. Similarly to previous in vitro studies with P. falciparum 3D7 [50,51], PFD0625c was also detected in the selected and unselected 3D7 line, which may be due to some degree of relaxed transcription in 3D7 [52]. The PFD0020c specifically up-regulated in Pf3D7gC1qR is a PfEMP1 variant characterized by having three of the four domains usually found in DC8 (CIDRα1.1, DBLβ12, DBLγ4/6), differing only in the first DBLα domain. We were not able to show binding of Pf3D7gC1qR to recombinant EPCR, as would have been expected by the expression of a DC8-containing var gene, although we did not assess this binding specificity to endothelial cells [13]. The up-regulation of a var gene containing DC8 after selection of P. falciparum 3D7 for gC1qR binding fits well with previous in vitro studies showing the transcription of PFD0020c’s orthologs, IT4var19/IT4var07 and HB3var03, after selecting parasites lines IT4 and HB3 for binding to HBMEC cells [32,33]. Importantly, up-regulation of the PFD0020c ortholog IT4var19 after selection of IT for binding to HBMEC was associated with an increased binding to gC1qR as observed in static assays [33].
Recombinant DBLβ12 from PFD0020c, but not other domains from PFD0020c (DBLα1.2, CIDRα1.1, DBLγ4/6, DBLδ1 and CIDRγ8) and from the var type 3 PFI1820w (consisting in domains DBLα1.3-DBLε8), showed binding to gC1qR in ELISA- and Luminex-based binding assays. Moreover, antibodies against DBLβ12 from PFD0020c were able to inhibit the binding to gC1qR by ∼40% at antibody concentrations of 300 μg/mL. This inhibition was not observed in the CD36-binder Pf3D7CD36, which suggest that DBLβ12 is the domain with the ability to bind gC1qR. DBLβ12, which consists in 149 aa and 19 homologous blocks, is present in 12 of the 399 PfEMP1s present in the genomes of seven P. falciparum laboratory strains [44], 9 of them belonging to DC8-PfEMP1 and sharing 56% of similarity at the amino acid level. DBLβ12 was shown to be among the PFD0020c domains most immunogenic in natural infections, as shown by the increase in IgG recognition by malaria-infected Mozambican children compared to never-exposed Spanish individuals, as well as the increase in IgG levels with age of Mozambican children. Importantly, antibodies against DBLβ12 from PFD0020c raised in animal models were able to inhibit binding of Pf3D7gC1qR to HBMEC cells by 46% compared to the control antibody (antibodies against DBLγ6-PFD0020c), demonstrating the gC1qR-dependent adhesion of IEs to endothelial cells through the DBLβ12 domain. Finally, polyclonal antibodies against DBLβ12 from PFD0020c showed cross-inhibitory activity against all the 4 Mozambique clinical isolates sharing the same gC1qR adhesion in vitro, reduced binding by 50%. Three of the four Mozambican field isolates analyzed transcribed DC8, as targeted by DC8-CIDRα1.1. However, one of the isolates (Pfmoz2) did not transcribe DC8, but transcribed DBLβ12&DBLβ3/5 at high levels, suggesting that DBLβ12-containing DC8-like PfEMP1s may share the ability to bind gC1qR. Overall, these data show that parasites with a virulence-associated adhesion phenotype such as gC1qR share PfEMP1 epitopes that can be targeted by strain-transcending functional antibodies to PfEMP1. The existence of shared surface epitopes amongst functionally similar disease-associated P. falciparum parasite isolates suggests the feasibility of developing therapeutic interventions against severe malaria
This study also shows that binding level of IEs to CD36 correlated positively with transcript levels of group B genes and negatively with varA levels, confirming the earlier findings that parasite ligands for CD36 are PfEMP1 variants encoded by var genes belonging to groups B and C [26,53,54]. In contrast to other studies showing up-regulation of DC13 in children with SM [34,42], this DC was not found associated with SM in children in our study, probably due to the low prevalence (7%) of cerebral malaria in the study population. However, transcript levels of DC13 were positively correlated with binding levels to ICAM1. Although DC13 does not have a conserved DBLβ domain with a proven ICAM-1 binding capability [55] most of DC13s are flanked by DBLβ domains, and thus this DC might be associated with a ICAM1-binding DBLβ domain type yet to be described. In fact, the DBLβ domain following DC13 in PF11_0521 [44] has been shown to bind ICAM1 [56]. Importantly, results of this study provide evidences of the potential involvement of DC11 in the pathophysiology of severe malaria. DC11 transcripts were found at higher levels in parasites collected from children with SM than UM as well as in isolates from Mozambican children and first exposed individuals (travelers) compared to isolates from Mozambican adults with life-long exposure to malaria. This DC11 has been involved in rosetting mediated by IgM [57], which has been suggested as the most clinically important rosetting phenotype [58]. However, transcript levels of DC11 were not associated with rosetting in the Mozambican isolates tested. Similarly, platelet-mediated agglutination, previously associated with binding to gC1qR [14], did not show correlation with any var DC. These results suggest that other receptors may be involved in IE rosetting and platelet-mediated agglutination and point towards the relevance of DC11 in the physiopathology of SM. However, further work will be necessary to elucidate the role of DC11 in the severity of the malaria disease.
This study has several limitations. First, more than half of the 43 children with severe malaria included in this study (n = 24; 56%) had two or more criteria for malaria severity [59]. Such a high degree of overlap in severe symptoms, which is otherwise common in endemic areas [60], together with the limited sample size of the cerebral malaria group, may have hampered the identification of molecular correlates that are particular to a clinical form of SM. Second, the degenerate primers used in qPCR assays have incomplete coverage of the global var gene repertoire. Moreover, the parasite populations obtained from peripheral blood may only partially represent the sequestering parasite population. Third, the conditions of the binding assay may not allow for 100% inhibition as has been shown for other receptors [32,61]. Alternatively, residual binding may be supported by other domains, for example those present in DC11, that may also mediate gC1qR adhesion. Fourth, given limited amounts of RNA and cryopreserved IEs available from P. falciparum field isolates included in the study, we focused the transcriptional analysis on domain cassettes previously associated with severe malaria [34,57] and the binding phenotypes on those receptors previously analyzed [8]. Fifth, gC1qR binding assays were performed on five of the 7 PFD0020c domains, but we did not test multiple domains constructs potentially involved in the binding phenotype. Finally, the fact that levels of transcripts encoding certain PfEMP1 domains types associates with the cognate parasites receptor binding capability does not necessarily mean that the particular domain mediates that receptor-binding, and other PfEMP1 domains or structures (i.e., DC11) could convey parasites this binding phenotype. More studies are needed to assess the relationship between expression of DC11, binding to gC1qR and malaria severity.
In summary, the positive correlation between gC1qR cytoadhesion by P. falciparum field isolates and their DC8 transcript levels, the overexpression of DC8-PFD0020c after selection of P. falciparum 3D7 for binding to gC1qR, and the inhibition of gC1qR binding by antibodies against DBLβ12PFD0020c, supports that DC8-PfEMP1s mediate binding to gC1qR through a conserved motif present in the DBLβ12 domain. Overall, our findings suggest that binding to gC1qR, mediated by interactions with DBLβ12, constitutes one of the three different host receptors suggested by protease-treatment assays of IT4 [36]. Moreover, the successful induction of strain-transcending antibodies against DBLβ12 domain from the PfEMP1 variant PFD0020c capable of inhibiting binding to gC1qR by field isolates suggests shared surface epitopes amongst heterologous gC1qR-binding PfEMP1 variants and the feasibility of designing interventions to prevent severe malaria. DC8 may thus facilitate binding to endothelial cells [32,33] via the interactions with gC1qR, known to be expressed in a wide range of human cells [14], in concert with binding to EPCR [13]. Further studies are needed to assess the relationship between DC8 expression, EPCR and gC1qR cytoadhesion, and their influence on malaria disease. Similarly to EPCR, gC1qR has been implicated in inflammatory processes such as the modulation of the complement cascade [40] and suggested to mediate bacterial cell adhesion to sites of vascular injury and thrombosis [62]. Moreover, up-regulation of gC1qR in bone marrow endothelial cells through inflammatory mediators [63] could contribute to sequestration of asexual late stages observed in ex vivo studies [64,65]. The results of this study support the possibility of a role for gC1qR in malaria-associated endovascular pathogenesis.
Methods
Study population
The study was conducted at the Manhiça District Hospital (MDH) in Southern Mozambique, a malaria endemic area where transmission of P. falciparum is perennial with some seasonality and moderate intensity [66], and at the Tropical Medicine Unit in Hospital Clinic of Barcelona (HCB), Spain. Between April and November 2006, 86 children 1 to 5 years of age [8] were recruited at MDH with P. falciparum clinical malaria, defined as the presence of fever (axillary temperature ≥37.5°C) and an asexual parasitemia of P. falciparum ≥500 parasites/μL on thin blood film examination [67]. Children with SM were those presenting with at least one of the following clinical definitions: cerebral malaria, severe anemia, acidosis or respiratory distress, prostration, hypoglycemia or multiple seizures [8]. Children with clinical malaria not showing any of the mentioned signs of severity and able to take oral medication (uncomplicated malaria; UM) were sex and age (+/-3 months) matched to SM cases. All cases and controls were reviewed by the study pediatrician to confirm that malaria was the sole or principal cause of the disease. Children with concomitant positive bacteremia were excluded from the study. Non-pregnant Mozambican adults (women and men) with life-long exposure to P. falciparum (n = 25) presenting clinical malaria at MDH were recruited between 2004 and 2005 [41]. European adults presenting a first episode of malaria after a travel to malaria endemic areas (n = 21), were recruited between 2005 and 2009 at HCB (Spain) [68]. Before treatment, peripheral blood was collected by venipuncture and 2 drops were spotted onto filter paper. Following centrifugation, plasma and 300 μL of the red blood cell pellet resuspended in 3 mL of Trizol reagent (Invitrogen) were stored at -80°C. The remaining red blood cell pellet was cryopreserved in liquid nitrogen [8].
Ethical considerations
The study protocol was approved by the National Mozambican Ethics Review Committee and the Hospital Clínic of Barcelona Ethics Review Committee. All patients were included into the study after written informed consent was given by them or their parents/guardians and were treated following national guidelines of Mozambique or Spain at the time of the study.
Parasite densities and msp1/msp2 genotyping
Total genomic DNA was extracted from filter papers using QIAmp DNA Mini Kit (Qiagen). Parasitemia was measured by real-time quantitative PCR (qPCR) targeting the P. falciparum 18S ribosomal RNA gene [69]. The number of concurrent infections (multiplicity of infection, MOI) was estimated as the highest number of msp-1 or msp-2 alleles detected in the sample by nested-PCR genotyping [70].
var/DC transcriptional profile
Total RNA prepared in Trizol reagent was extracted using PureLink Micro-to-Midi RNA purification kit (Ambion). RNA was treated with DNaseI (Invitrogen) for 1.5h at 37°C. After discarding the presence of gDNA by PCR-based amplification of P. falciparum tubulin (PF10_0084) [41] or Seryl-tRNA synthetase genes (PF07_0073) [71], reverse transcription was performed using the Super Script III First Strand synthesis system (Invitrogen) with random hexamers primers. Complementary DNA (cDNA) synthesis was confirmed by PCR-based amplification of P. falciparum tubulin or seryl-tRNA synthetase genes. Then, the transcript levels of var subgroups was determined by qPCR using degenerated primers targeting varA-exon2, varB group (varB-UTR region), varC group (varC-UTR region) [28], varA-DBLα1 (varA-notDC3) [34] and var2CSA (DBL3X domain) [41]. DC transcript levels were assessed by qPCR using a set of primers targeting semi-conserved domains belonging to DC8 (CIDRα1.1), DC9 (DBLγ), DC11 (CIDRβ2+DBLγ7; Forward: TTRGTHACAGCAAAATAYGAAGGTG and reverse: CTCTTACRATATCWCCTATATCKGCA), DC13 (CIDRα1.4), DC16 (CIDRδ) and DC19 (DBLα0.16) [34]. Seryl-tRNA-synthetase gene was used as the reference gene [71]. Individual 20 μL qPCR reactions were performed in duplicate using ABI Prism 7500 Real-Time system (Applied Biosystems) containing 10 μL of Power SYBR Green Master Mix (Applied Biosystems), 4 μL of cDNA and primer concentration of 1μM with cycling conditions of 50°C for 2 min, 95°C for 10 min followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Data were analyzed using the 7500 System SDS software v1.4. PCR efficiencies of each primer pair were calculated on a standard curve from 7 log dilutions of P. falciparum 3D7 or P. falciparum ItG gDNA by the formula (E = 10−1/m), where m is the slope. Specificity of amplification was assessed by melting-curve analysis of final products. Non-template controls were tested in every plate. Samples with fluorescence detected over the 40 cycles were considered positive. If Ct value felt out of the linear range of the standard curve, a Ct value of 41 was assigned. Ct values were converted to copy numbers [72] using the formula C/E ΔCt, where E is the efficiency of the PCR, C is the number of copies of the gene in the P. falciparum 3D7 or ItG genome [34,72] and ΔCt is the difference in Ct values between a sample and P. falciparum 3D7 or ItG reference gDNA loaded in each plate [73]. Relative copy number of target genes was calculated by dividing the target gene copies by Seryl-tRNA synthetase gene copies. Transcript levels of var/DCs were considered as high if the copy number was ≥0.5-fold of Seryl-tRNA-synthetase copy numbers and low if copy number was <0.5-fold.
Cytoadhesion profiling
Adhesion of P. falciparum pediatric isolates to gC1qR (Creative BioMart), CD36, ICAM-1 (R&D Systems) and Duffy-Fc [74], as well as rosetting and PM-agglutination was assessed as previously described [8] and expressed as IEs/mm2, percentage of IEs forming rosettes and percentage of IEs in a clump, respectively. Adhesion to purified receptors was considered positive if the number of IEs bound per mm2 was higher than the mean binding plus 2 standard deviations to Duffy-Fc coated Petri dishes (19.5 IE/mm2) [8], rosetting if frequency of rosettes was higher than 2% [75] and PM-agglutination if frequency of clumps was higher in presence of platelets than in buffer-control [8].
Antigenic profiling of infected erythrocytes
Forty five P. falciparum isolates were tested for IgG recognition by plasma from 50 children with SM and 50 with UM, as well as 22 adults recruited in the same study area [8]. After thawing and washing erythrocytes in incomplete RPMI 1640 medium, parasites were matured at 37°C for 18–36 hours until late-stages. Fifty μL of plasmas at 1/10 dilution, previously depleted of antibodies reacting against uninfected A/B-erythrocytes, were mixed with 50 μL of erythrocyte suspension at 1% hematocrit and 0.5–2.2% parasitemia in PBS-1% BSA for 1 hour at room temperature. After sequential incubations with 100 μL of polyclonal rabbit anti-human IgG (DakoCytomation; 1/200 dilution) and 100 μL of Alexa Fluor 488-conjugated donkey anti-rabbit IgG (Invitrogen; 1/1,000) plus 10 μg/mL of ethidium bromide, data from 1,000 ethidium bromide positive events were acquired with a Becton Dickinson LSR Fortessa flow cytometer. Reactivity against IEs was expressed as the difference between the geometric mean fluorescence intensity (GMFI) of IEs and the GMFI of uninfected erythrocytes. A pool of plasma samples from immune Mozambican adults and six plasma samples from non-exposed European adults were included as positive and negative controls, respectively. To allow comparability between isolates, GMFI values from each parasite/plasma combination were scored in relation to the threshold of positivity for each isolate defined as the GMFI of negative controls plus two standard deviations (cut-off). A score of 0 was assigned if GMFI values were below the cut-off; 1 if the value was between one- and two-fold the cut-off; 2 if the value was between two- and three-fold the cut-off; and so on until a maximum score of 5. Breadth of IgG recognition (BoR) was calculated as the sum of scores obtained for each parasite and expressed as percentage of the maximum score possible.
var profiling of P. falciparum 3D7 selected for cytoadhesion to gC1qR
To select for binding to gC1qR, a P. falciparum 3D7 culture synchronized in trophozoite/schizont stages was incubated for 1 h in bacteriological Petri plates coated with 2 mL of recombinant gC1qR diluted in PBS (50 μg/mL) [14]. Unbound parasites were collected using a pipette and separated from bound parasites. Both unbound and bound parasites were cultured, with the latter being subjected to a second round of selection for binding to gC1qR. After a limiting dilution cloning, a selected and unselected clone were expanded and tested for binding to gC1qR, CD36, ICAM-1, CSA (Chondroitin sulfate A sodium salt from bovine trachea Sigma-Aldrich) and BSA (Bovine Serum Albumin, Santa Cruz Biotechnology), following standard procedures [8]. The var genes transcription profile was determined for both clones by individual qPCR performed in duplicate using primers covering the P. falciparum 3D7 var gene repertoire [71,76].
Binding between recombinant PfEMP1 domains and gC1qR
Recombinant PFD0020c domains produced in insect or Escherichia coli cells [13] were screened for binding against recombinant human EPCR or gC1qR by ELISA (CIDRα1.1, DBLβ12, DBLγ6, DBLγ11, CIDRγ8) and Luminex (DBLα1.2, CIDRα1.1, DBLβ12, DBLγ6, DBLγ11, DBLδ1 and CIDRγ8) in duplicate. For the ELISA assays, MaxiSorp immunoplates (Nunc) were coated overnight at 4°C with 50 μL per well of recombinant human EPCR and gC1qR at 3 μg/mL in PBS pH 7.4. After blocking with PBS 3%-skimmed milk and washing three times with PBS-0.05% TweenR20, PFD0020c domains were added at a concentration of 5 μg/mL in PBS 1%-skimmed milk and incubated for 1 h at 37°C. Secondary anti-V5-HRP antibody diluted in PBS 1%-skimmed milk at 1:3000 was added to each well and incubated for 1 hour at room temperature with gentle shaking. Plates were developed using 100 μL per well of a phosphate solution with o-phenylenediamine. The colorimetric reaction was stopped with 100 μL of 3 M H2SO4 after 10 minutes and the optical density (OD) was measured at 490 nm. For the Luminex assays, gC1qR was coupled at 50 μg/107 beads to MagPlex-C magnetic carboxylated microspheres (Luminex Corporation) following manufacturer’s instructions. Two thousand coupled beads were incubated with the recombinant PFD0020c domains (DBLα1.2, CIDRα1.1, DBLβ12, DBLγ6, DBLγ11, DBLδ1 and CIDRγ8) at 1ug/ml in incubation buffer (IB; 1% Skim Milk in PBS), overnight at 4°C. After 3 washes with washing buffer (PBS + 0.5% Tween20 + 0.25% skim milk), the beads were incubated with anti-V5 from mouse (ThermoFisher, R960-25) at 1/2500 in IB at room temperature for 1 hour, followed by an incubation with anti-mouse biotin conjugated antibody (Sigma, B7401) at 1/10000 in IB for 1 hour at RT, and streptavidin-R-phycoerythrin (Sigma, 42280) at 1/1000 in IB for 30 minutes at RT, with 3 washes after each incubation. Median Fluorescence Intensity was obtained using the Luminex 100/200 System (Luminex Corp., Austin, Texas).
Inhibition of P. falciparum-infected erythrocyte binding to gC1qR and Human Brain Microvascular Endothelial Cells
Anti-sera against domains belonging to PFD0020c (α-CIDRα1.1PFD0020c, α-DBLβ12PFD0020c, α-DBLγ6PFD0020c, α-CIDRγ8PFD0020c)), PF08_0140 (α-DBLβ12PF08_0140) and PFI1820w (α-PFI1820w) were produced in rabbit [13]. After depleting rabbit sera of antibodies against human erythrocytes, IgGs were purified by Affi-Gel Protein-A MAPS II Kit (Bio-Rad, Richmond, CA) and quantified using EPOCH spectrophotometer. To test their ability to inhibit binding of P. falciparum to recombinant gC1qR, 20 μL pellet of P. falciparum 3D7 pigmented trophozoite (≥2% parasitaemia, 1% hematocrit) were incubated in duplicate for 1.5 h at 37°C with 300 μg/mL rabbit IgGs diluted in PBS and used for a standard adhesion assay in Petri dishes [8]. Similar procedures were used to test inhibition of gC1R binding by 4 Mozambican P. falciparum isolates (Pfmoz 1–4).
Human Brain Microvascular Endothelial Cells (HBMEC; Innoprot) were seeded on flat-bottomed Nunclon Δ Surface (Nunc cat number: 150628) 12-well plates 3 to 4 days before assays and allowed to growth to 30–40% confluence in endothelial cell medium (Innoprot). Prior to the adhesion assay, HBMECs were washed once with PBS followed by addition of 20 μl 2% FCS in RPMI/well. For binding inhibition, IgG-purified anti-PfEMP1 rabbit antibodies and PBS alone were added to 2% parasitemia and 2% hematocrit late-stage IEs at a final concentration of 300 μg/ml incubated for 1.5 h at 37°C. 300 μl of the IE suspension were added to each well and co-incubated on a rocking table for 1 hour at room temperature. Unbound infected erythrocytes were removed by several gentle washes. Wells were then fixed in 2% glutaraldehyde over night at room temperature and stained with Giemsa for 10 min. Binding was quantified by determining the number of IEs adhering per endothelial cells nuclei in 50 random fields under 400× magnification. All binding assays were done in triplicate. The percentage of binding was expressed relative to binding in the absence of antibodies.
IgG measurement in human plasma samples
IgG reactivity against the recombinant PFD0020c domains (DBLα1.2, CIDRα1.1, DBLβ12, DBLγ6, DBLγ11, DBLδ1 and CIDRγ8) was assessed in 135 malaria-infected Mozambican children (67 with severe malaria and 68 with uncomplicated malaria) and 18 Spanish adults never exposed to malaria. PfEMP1 domains or BSA (Sigma, A7030, as background control) were coupled at 50 μg/107 beads to MagPlex-C magnetic carboxylated microspheres (Luminex Corporation) following manufacturer’s instructions. Multiplexed beads were incubated with plasma samples (1/50 dilution) and antibody levels were detected as described elsewhere [77]. Positive, negative and background controls were added to each plate. Median Fluorescence Intensity (MFI) was obtained from the InVitrogen Luminex platform (xPONENT Software, at least 100 counts/analyte) and normalized for inter-plate variability by multiplying MFIs by the median value of a positive control from all plates and dividing by each plate’ value.
Statistical analysis
Correlations between variables were assessed by Spearman’s rank coefficient, with Benjamini-Hochberg correction for multiple comparisons. Continuous data were compared between matched case/control pairs by Sign-test and between non-paired groups by Mann-Whitney test. BoR was compared between groups by a Test for trend across ordered groups and between isolates transcribing var/DCs at low- or high-levels by negative binomial regression models adjusted by age. Mean ratio of IgGs and 95% confidence intervals between Mozambican children and Spanish adults, as well as between Mozambican children older than 2.5 years of age and less than 2.5 years were calculated in linear regression models, with log-transformed MFIs. Statistical analysis was performed with Stata/SE software (version 12.0; StataCorp).
Supporting Information
Table A. Percentage of isolates from Mozambican children (n = 86) showing cytoadherence, and cytoadherence levels. Table B. Prevalence of isolates expressing the target var/DC genes tested. Table C. Transcript levels of var/DCs by severe malaria symptoms. Fig A. Binding of recombinant PFD0020c domains (DBLα1.2, CIDRα1.1, DBLβ12, DBLγ6, DBLγ11, DBLδ1 and CIDRγ8) to gC1qR as assessed by Luminex assay. Fig B. Percentage of inhibition of P. falciparum cytoadhesion by purified antibodies against PFD0020c domains to EPCR or CD36 receptors. Fig C. IgG recognition of PFD0020c domains by plasmas from malaria-infected Mozambican children and never-exposed individuals from Spain.
(DOCX)
Acknowledgments
We thank the children and their parents/guardians for their participation in the study, and the staff of the Manhiça District Hospital and the Centro de Investigação em Saúde de Manhiça (CISM) for their work and dedication.
Data Availability
Confidential patient data are not available for public deposition due to privacy concerns. The patient data are available from the ISGlobal Institutional Data Access for researchers who meet the criteria for access to confidential data. Data use and transfer is monitored by ISGlobal’s Data Management and Biostatistics Unit (contact e-mail: ubioesdm@isglobal.org).
Funding Statement
This work was supported by the Instituto de Salud Carlos III (PI13/01478 cofunded by the Fondo Europeo de Desarrollo Regional [FEDER], CES10/021-I3SNS to AM and CP11/00269 from the Miguel Servet program to QB). The Manhiça Health Research Centre receives core support from the Spanish Agency for International Cooperation and Development. AM, JM and JG are supported by the Departament d’Universitats i Recerca de la Generalitat de Catalunya (AGAUR; grants 2014SGR26 and 2014SGR263). AMT received financial support from the Secretaría Nacional de Ciencia, Tecnología e Innovación (Instituto para la Formación y Aprovechamiento de los Recursos Humanos, República de Panamá) and BDLS from Estrategia de Sostenibilidad 2014–2015 CODI-Universidad de Antioquia, Colombia. TL and LT received support from Danish Council of Independent research, Lundbeck Foundation and University of Copenhagen. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, et al. (2005) Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 376: 1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mackintosh CL, Beeson JG, Marsh K (2004) Clinical features and pathogenesis of severe malaria. Trends Parasitol 20: 597–603. 10.1016/j.pt.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 3. Hanson J, Lam SW, Mahanta KC, Pattnaik R, Alam S, et al. (2012) Relative contributions of macrovascular and microvascular dysfunction to disease severity in falciparum malaria. J Infect Dis 206: 571–579. 10.1093/infdis/jis400 [DOI] [PubMed] [Google Scholar]
- 4. Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, et al. (2004) Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med 10: 143–145. 10.1038/nm986 [DOI] [PubMed] [Google Scholar]
- 5. Dondorp AM, Ince C, Charunwatthana P, Hanson J, van Kuijen A, et al. (2008) Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis 197: 79–84. 10.1086/523762 [DOI] [PubMed] [Google Scholar]
- 6. Beare NA, Harding SP, Taylor TE, Lewallen S, Molyneux ME (2009) Perfusion abnormalities in children with cerebral malaria and malarial retinopathy. J Infect Dis 199: 263–271. 10.1086/595735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heddini A, Pettersson F, Kai O, Shafi J, Obiero J, et al. (2001) Fresh isolates from children with severe Plasmodium falciparum malaria bind to multiple receptors. Infect Immun 69: 5849–5856. 10.1128/IAI.69.9.5849-5856.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mayor A, Hafiz A, Bassat Q, Rovira-Vallbona E, Sanz S, et al. (2011) Association of severe malaria outcomes with platelet-mediated clumping and adhesion to a novel host receptor. PLoS One 6: e19422 10.1371/journal.pone.0019422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller LH, Baruch DI, Marsh K, Doumbo OK (2002) The pathogenic basis of malaria. Nature 415: 673–679. 10.1038/415673a [DOI] [PubMed] [Google Scholar]
- 10. Barnwell JW, Asch AS, Nachman RL, Yamaya M, Aikawa M, et al. (1989) A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J Clin Invest 84: 765–772. 10.1172/JCI114234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ochola LB, Siddondo BR, Ocholla H, Nkya S, Kimani EN, et al. (2011) Specific receptor usage in Plasmodium falciparum cytoadherence is associated with disease outcome. PLoS One 6: e14741 10.1371/journal.pone.0014741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fried M, Duffy PE (1996) Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272: 1502–1504. [DOI] [PubMed] [Google Scholar]
- 13. Turner L, Lavstsen T, Berger SS, Wang CW, Petersen JE, et al. (2013) Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 498: 502–505. 10.1038/nature12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biswas AK, Hafiz A, Banerjee B, Kim KS, Datta K, et al. (2007) Plasmodium falciparum uses gC1qR/HABP1/p32 as a receptor to bind to vascular endothelium and for platelet-mediated clumping. PLoS Pathog 3: 1271–1280. 10.1371/journal.ppat.0030130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bull PC, Lowe BS, Kortok M, Molyneux CS, Newbold CI, et al. (1998) Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med 4: 358–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scherf A, Lopez-Rubio JJ, Riviere L (2008) Antigenic variation in Plasmodium falciparum. Annu Rev Microbiol 62: 445–470. 10.1146/annurev.micro.61.080706.093134 [DOI] [PubMed] [Google Scholar]
- 17. Guizetti J, Scherf A (2013) Silence, activate, poise and switch! Mechanisms of antigenic variation in Plasmodium falciparum. Cell Microbiol 15: 718–726. 10.1111/cmi.12115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bull PC, Lowe BS, Kortok M, Marsh K (1999) Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect Immun 67: 733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marsh K, Howard RJ (1986) Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science 231: 150–153. [DOI] [PubMed] [Google Scholar]
- 20. Is Vigan-Womas, Guillotte M, Juillerat A, Vallieres C, Lewit-Bentley A, et al. (2011) Allelic Diversity of the Plasmodium falciparum Erythrocyte Membrane Protein 1 Entails Variant-Specific Red Cell Surface Epitopes. PLoS ONE 6: e16544 10.1371/journal.pone.0016544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C (1999) Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med 5: 340–343. 10.1038/6560 [DOI] [PubMed] [Google Scholar]
- 22. Elliott SR, Payne PD, Duffy MF, Byrne TJ, Tham WH, et al. (2007) Antibody recognition of heterologous variant surface antigens after a single Plasmodium falciparum infection in previously naive adults. Am J Trop Med Hyg 76: 860–864. [PubMed] [Google Scholar]
- 23. Bull PC, Kortok M, Kai O, Ndungu F, Ross A, et al. (2000) Plasmodium falciparum-infected erythrocytes: agglutination by diverse Kenyan plasma is associated with severe disease and young host age. J Infect Dis 182: 252–259. 10.1086/315652 [DOI] [PubMed] [Google Scholar]
- 24. Nielsen MA, Staalsoe T, Kurtzhals JA, Goka BQ, Dodoo D, et al. (2002) Plasmodium falciparum variant surface antigen expression varies between isolates causing severe and nonsevere malaria and is modified by acquired immunity. J Immunol 168: 3444–3450. [DOI] [PubMed] [Google Scholar]
- 25. Smith JD, Gamain B, Baruch DI, Kyes S (2001) Decoding the language of var genes and Plasmodium falciparum sequestration. Trends Parasitol 17: 538–545. [DOI] [PubMed] [Google Scholar]
- 26. Robinson BA, Welch TL, Smith JD (2003) Widespread functional specialization of Plasmodium falciparum erythrocyte membrane protein 1 family members to bind CD36 analysed across a parasite genome. Mol Microbiol 47: 1265–1278. [DOI] [PubMed] [Google Scholar]
- 27. Kaestli M, Cortes A, Lagog M, Ott M, Beck HP (2004) Longitudinal assessment of Plasmodium falciparum var gene transcription in naturally infected asymptomatic children in Papua New Guinea. J Infect Dis 189: 1942–1951. Epub 2004 Apr 1929. 10.1086/383250 [DOI] [PubMed] [Google Scholar]
- 28. Rottmann M, Lavstsen T, Mugasa JP, Kaestli M, Jensen AT, et al. (2006) Differential expression of var gene groups is associated with morbidity caused by Plasmodium falciparum infection in Tanzanian children. Infect Immun 74: 3904–3911. 10.1128/IAI.02073-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bull PC, Berriman M, Kyes S, Quail MA, Hall N, et al. (2005) Plasmodium falciparum Variant Surface Antigen Expression Patterns during Malaria. PLoS Pathogens 1: e26 10.1371/journal.ppat.0010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kyriacou HM, Stone GN, Challis RJ, Raza A, Lyke KE, et al. (2006) Differential var gene transcription in Plasmodium falciparum isolates from patients with cerebral malaria compared to hyperparasitaemia. Molecular and Biochemical Parasitology 150: 211–218. 10.1016/j.molbiopara.2006.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Warimwe GM, Keane TM, Fegan G, Musyoki JN, Newton CRJC, et al. (2009) Plasmodium falciparum var gene expression is modified by host immunity. Proceedings of the National Academy of Sciences 106: 21801–21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Avril M, Tripathi AK, Brazier AJ, Andisi C, Janes JH, et al. (2012) A restricted subset of var genes mediates adherence of Plasmodium falciparum-infected erythrocytes to brain endothelial cells. Proceedings of the National Academy of Sciences of the United States of America 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Claessens A, Adams Y, Ghumra A, Lindergard G, Buchan CC, et al. (2012) A subset of group A-like var genes encodes the malaria parasite ligands for binding to human brain endothelial cells. Proceedings of the National Academy of Sciences of the United States of America 109: E1772–1781. 10.1073/pnas.1120461109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lavstsen T, Turner L, Saguti F, Magistrado P, Rask TS, et al. (2012) Plasmodium falciparum erythrocyte membrane protein 1 domain cassettes 8 and 13 are associated with severe malaria in children. Proceedings of the National Academy of Sciences of the United States of America 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gillrie MR, Avril M, Brazier AJ, Davis SP, Stins MF, et al. (2015) Diverse functional outcomes of Plasmodium falciparum ligation of EPCR: potential implications for malarial pathogenesis. Cell Microbiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Avril M, Brazier AJ, Melcher M, Sampath S, Smith JD (2013) DC8 and DC13 var Genes Associated with Severe Malaria Bind Avidly to Diverse Endothelial Cells. PLoS Pathog 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ghebrehiwet B, Peerschke EI (2004) cC1q-R (calreticulin) and gC1q-R/p33: ubiquitously expressed multi-ligand binding cellular proteins involved in inflammation and infection. Mol Immunol 41: 173–183. 10.1016/j.molimm.2004.03.014 [DOI] [PubMed] [Google Scholar]
- 38. Ghebrehiwet B, Lim BL, Peerschke EI, Willis AC, Reid KB (1994) Isolation, cDNA cloning, and overexpression of a 33-kD cell surface glycoprotein that binds to the globular "heads" of C1q. J Exp Med 179: 1809–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ghebrehiwet B, Peerschke EI (1998) Structure and function of gC1q-R: a multiligand binding cellular protein. Immunobiology 199: 225–238. 10.1016/S0171-2985(98)80029-6 [DOI] [PubMed] [Google Scholar]
- 40. Ghebrehiwet B, Lim BL, Kumar R, Feng X, Peerschke EI (2001) gC1q-R/p33, a member of a new class of multifunctional and multicompartmental cellular proteins, is involved in inflammation and infection. Immunol Rev 180: 65–77. [DOI] [PubMed] [Google Scholar]
- 41. Rovira-Vallbona E, Dobaño C, Bardají A, Cisteró P, Romagosa C, et al. (2011) Transcription of var Genes Other Than var2csa in Plasmodium falciparum Parasites Infecting Mozambican Pregnant Women. Journal of Infectious Diseases 204: 27–35. 10.1093/infdis/jir217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bertin GI, Lavstsen T, Guillonneau F, Doritchamou J, Wang CW, et al. (2013) Expression of the Domain Cassette 8 Plasmodium falciparum Erythrocyte Membrane Protein 1 Is Associated with Cerebral Malaria in Benin. PLoS One 8: e68368 10.1371/journal.pone.0068368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Almelli T, Nuel G, Bischoff E, Aubouy A, Elati M, et al. (2014) Differences in gene transcriptomic pattern of Plasmodium falciparum in children with cerebral malaria and asymptomatic carriers. PLoS One 9: e114401 10.1371/journal.pone.0114401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rask TS, Hansen DA, Theander TG, Gorm Pedersen A, Lavstsen T (2010) Plasmodium falciparum erythrocyte membrane protein 1 diversity in seven genomes—divide and conquer. PLoS Comput Biol 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Claessens A, Hamilton WL, Kekre M, Otto TD, Faizullabhoy A, et al. (2014) Generation of Antigenic Diversity in Plasmodium falciparum by Structured Rearrangement of Var Genes During Mitosis. PLoS Genet 10: e1004812 10.1371/journal.pgen.1004812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Turner L, Lavstsen T, Mmbando BP, Wang CW, Magistrado PA, et al. (2015) IgG antibodies to endothelial protein C receptor-binding cysteine-rich interdomain region domains of Plasmodium falciparum erythrocyte membrane protein 1 are acquired early in life in individuals exposed to malaria. Infect Immun 83: 3096–3103. 10.1128/IAI.00271-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rovira-Vallbona E, Moncunill G, Bassat Q, Aguilar R, Machevo S, et al. (2012) Low antibodies against Plasmodium falciparum and imbalanced pro-inflammatory cytokines are associated with severe malaria in Mozambican children: a case-control study. Malar J 11: 181 10.1186/1475-2875-11-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cham GK, Turner L, Kurtis JD, Mutabingwa T, Fried M, et al. (2010) Hierarchical, domain type-specific acquisition of antibodies to Plasmodium falciparum erythrocyte membrane protein 1 in Tanzanian children. Infect Immun 78: 4653–4659. 10.1128/IAI.00593-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cham GKK, Turner L, Lusingu J, Vestergaard L, Mmbando BP, et al. (2009) Sequential, Ordered Acquisition of Antibodies to Plasmodium falciparum Erythrocyte Membrane Protein 1 Domains. J Immunol 183: 3356–3363. 10.4049/jimmunol.0901331 [DOI] [PubMed] [Google Scholar]
- 50. Wang CW, Lavstsen T, Bengtsson DC, Magistrado PA, Berger SS, et al. (2012) Evidence for in vitro and in vivo expression of the conserved VAR3 (type 3) Plasmodium falciparum erythrocyte membrane protein 1. Malar J 11: 129 10.1186/1475-2875-11-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berger SS, Turner L, Wang CW, Petersen JE, Kraft M, et al. (2013) Plasmodium falciparum Expressing Domain Cassette 5 Type PfEMP1 (DC5-PfEMP1) Bind PECAM1. PLoS One 8: e69117 10.1371/journal.pone.0069117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Joergensen L, Bengtsson DC, Bengtsson A, Ronander E, Berger SS, et al. (2010) Surface co-expression of two different PfEMP1 antigens on single plasmodium falciparum-infected erythrocytes facilitates binding to ICAM1 and PECAM1. PLoS Pathog 6: e1001083 10.1371/journal.ppat.1001083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Janes JH, Wang CP, Levin-Edens E, Vigan-Womas I, Guillotte M, et al. (2011) Investigating the host binding signature on the Plasmodium falciparum PfEMP1 protein family. PLoS Pathog 7: e1002032 10.1371/journal.ppat.1002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smith JD, Kyes S, Craig AG, Fagan T, Hudson-Taylor D, et al. (1998) Analysis of adhesive domains from the A4VAR Plasmodium falciparum erythrocyte membrane protein-1 identifies a CD36 binding domain. Mol Biochem Parasitol 97: 133–148. [DOI] [PubMed] [Google Scholar]
- 55. Bengtsson A, Joergensen L, Rask TS, Olsen RW, Andersen MA, et al. (2013) A novel domain cassette identifies Plasmodium falciparum PfEMP1 proteins binding ICAM-1 and is a target of cross-reactive, adhesion-inhibitory antibodies. J Immunol 190: 240–249. 10.4049/jimmunol.1202578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gullingsrud J, Saveria T, Amos E, Duffy PE, Oleinikov AV (2013) Structure-function-immunogenicity studies of PfEMP1 domain DBL2betaPF11_0521, a malaria parasite ligand for ICAM-1. PLoS One 8: e61323 10.1371/journal.pone.0061323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ghumra A, Semblat JP, Ataide R, Kifude C, Adams Y, et al. (2012) Induction of strain-transcending antibodies against Group A PfEMP1 surface antigens from virulent malaria parasites. PLoS Pathog 8: e1002665 10.1371/journal.ppat.1002665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rowe JA, Shafi J, Kai OK, Marsh K, Raza A (2002) Nonimmune IgM, but not IgG binds to the surface of Plasmodium falciparum-infected erythrocytes and correlates with rosetting and severe malaria. Am J Trop Med Hyg 66: 692–699. [DOI] [PubMed] [Google Scholar]
- 59.WHO (2004) Management of severe malaria: a practical handbook. Second ed.
- 60. Maitland K, Marsh K (2004) Pathophysiology of severe malaria in children. Acta Tropica 90: 131–140. 10.1016/j.actatropica.2003.11.010 [DOI] [PubMed] [Google Scholar]
- 61. Nielsen MA, Pinto VV, Resende M, Dahlback M, Ditlev SB, et al. (2009) Induction of adhesion-inhibitory antibodies against placental Plasmodium falciparum parasites by using single domains of VAR2CSA. Infect Immun 77: 2482–2487. 10.1128/IAI.00159-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nguyen T, Ghebrehiwet B, Peerschke EI (2000) Staphylococcus aureus protein A recognizes platelet gC1qR/p33: a novel mechanism for staphylococcal interactions with platelets. Infect Immun 68: 2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Guo WX, Ghebrehiwet B, Weksler B, Schweitzer K, Peerschke EI (1999) Up-regulation of endothelial cell binding proteins/receptors for complement component C1q by inflammatory cytokines. J Lab Clin Med 133: 541–550. [DOI] [PubMed] [Google Scholar]
- 64. Aguilar R, Magallon-Tejada A, Achtman AH, Moraleda C, Joice R, et al. (2014) Molecular evidence for the localization of Plasmodium falciparum immature gametocytes in bone marrow. Blood 123: 959–966. 10.1182/blood-2013-08-520767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Aguilar R, Moraleda C, Achtman AH, Mayor A, Quinto L, et al. (2014) Severity of anaemia is associated with bone marrow haemozoin in children exposed to Plasmodium falciparum. Br J Haematol 164: 877–887. 10.1111/bjh.12716 [DOI] [PubMed] [Google Scholar]
- 66. Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, et al. (2004) Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 364: 1411–1420. 10.1016/S0140-6736(04)17223-1 [DOI] [PubMed] [Google Scholar]
- 67. Saute F, Aponte J, Almeda J, Ascaso C, Abellana R, et al. (2003) Malaria in southern Mozambique: malariometric indicators and malaria case definition in Manhica district. Trans R Soc Trop Med Hyg 97: 661–666. [DOI] [PubMed] [Google Scholar]
- 68. Moncunill G, Mayor A, Jimenez A, Nhabomba A, Puyol L, et al. (2013) Cytokine and antibody responses to Plasmodium falciparum in naive individuals during a first malaria episode: effect of age and malaria exposure. PLoS One 8: e55756 10.1371/journal.pone.0055756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hermsen CC, Telgt DSC, Linders EHP, van de Locht LATF, Eling WMC, et al. (2001) Detection of Plasmodium falciparum malaria parasites in vivo by real-time quantitative PCR. Molecular and Biochemical Parasitology 118: 247–251. [DOI] [PubMed] [Google Scholar]
- 70. Snounou G, Zhu X, Siripoon N, Jarra W, Thaithong S, et al. (1999) Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Trans R Soc Trop Med Hyg 93: 369–374. [DOI] [PubMed] [Google Scholar]
- 71. Salanti A, Staalsoe T, Lavstsen T, Jensen ATR, Sowa MPK, et al. (2003) Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol 49: 179–191. [DOI] [PubMed] [Google Scholar]
- 72. Kaestli M, Cockburn Ian A, Cortés A, Baea K, Rowe JA, et al. (2006) Virulence of Malaria Is Associated with Differential Expression of Plasmodium falciparum var Gene Subgroups in a Case-Control Study. The Journal of Infectious Diseases 193: 1567–1574. 10.1086/503776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucl Acids Res 29: e45–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Choe H, Moore MJ, Owens CM, Wright PL, Vasilieva N, et al. (2005) Sulphated tyrosines mediate association of chemokines and Plasmodium vivax Duffy binding protein with the Duffy antigen/receptor for chemokines (DARC). Mol Microbiol 55: 1413–1422. 10.1111/j.1365-2958.2004.04478.x [DOI] [PubMed] [Google Scholar]
- 75. Rowe JA, Handel IG, Thera MA, Deans AM, Lyke KE, et al. (2007) Blood group O protects against severe Plasmodium falciparum malaria through the mechanism of reduced rosetting. Proc Natl Acad Sci U S A 104: 17471–17476. 10.1073/pnas.0705390104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dahlback M, Lavstsen T, Salanti A, Hviid L, Arnot DE, et al. (2007) Changes in var gene mRNA levels during erythrocytic development in two phenotypically distinct Plasmodium falciparum parasites. Malaria Journal 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Campo JJ, Dobano C, Sacarlal J, Guinovart C, Mayor A, et al. (2011) Impact of the RTS,S malaria vaccine candidate on naturally acquired antibody responses to multiple asexual blood stage antigens. PLoS One 6: e25779 10.1371/journal.pone.0025779 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A. Percentage of isolates from Mozambican children (n = 86) showing cytoadherence, and cytoadherence levels. Table B. Prevalence of isolates expressing the target var/DC genes tested. Table C. Transcript levels of var/DCs by severe malaria symptoms. Fig A. Binding of recombinant PFD0020c domains (DBLα1.2, CIDRα1.1, DBLβ12, DBLγ6, DBLγ11, DBLδ1 and CIDRγ8) to gC1qR as assessed by Luminex assay. Fig B. Percentage of inhibition of P. falciparum cytoadhesion by purified antibodies against PFD0020c domains to EPCR or CD36 receptors. Fig C. IgG recognition of PFD0020c domains by plasmas from malaria-infected Mozambican children and never-exposed individuals from Spain.
(DOCX)
Data Availability Statement
Confidential patient data are not available for public deposition due to privacy concerns. The patient data are available from the ISGlobal Institutional Data Access for researchers who meet the criteria for access to confidential data. Data use and transfer is monitored by ISGlobal’s Data Management and Biostatistics Unit (contact e-mail: ubioesdm@isglobal.org).