Abstract
Objective:
Although our understanding of viral transmission among people who inject drugs (PWID) has improved, we still know little about when and how many times each injector transmits HIV throughout the duration of infection. We describe HIV dynamics in PWID to evaluate which preventive strategies can be efficient.
Design:
Due to the notably scarce interventions, HIV-1 spread explosively in Russia and Ukraine in 1990s. By studying this epidemic between 1995 and 2005, we characterized naturally occurring transmission dynamics of HIV among PWID.
Method:
We combined publicly available HIV pol and env sequences with prevalence estimates from Russia and Ukraine under an evolutionary epidemiology framework to characterize HIV transmissibility between PWID. We then constructed compartmental models to simulate HIV spread among PWID.
Results:
In the absence of interventions, each injector transmits on average to 10 others. Half of the transmissions take place within 1 month after primary infection, suggesting that the epidemic will expand even after blocking all the post–first month transmissions. Primary prevention can realistically target the first month of infection, and we show that it is very efficient to control the spread of HIV-1 in PWID. Treating acutely infected on top of primary prevention is notably effective.
Conclusion:
As a large proportion of transmissions among PWID occur within 1 month after infection, reducing and delaying transmissions through scale-up of harm reduction programmes should always form the backbone of HIV control strategies in PWID. Growing PWID populations in the developing world, where primary prevention is scarce, constitutes a public health time bomb.
Keywords: epidemiology, harm reduction, HIV, people who inject drugs, phylodynamics, recent infection
Introduction
HIV in people who inject drugs (PWID) has, to date, posed a secondary burden worldwide after sexual transmission, with the striking exceptions of eastern European and central Asian countries [1]. Much work has been done to study how HIV spreads in PWID, and now we have interventions that proved to be effective to slow down the HIV epidemic in this group [2]. Still, research to date has not measured forward transmission (i.e. number of transmissions that can be attributed to an infected person), and our knowledge is limited to the force of infection (i.e. the probability of PWID getting infected over time) [3].
With almost 1 million HIV-infected individuals and a PWID population of around 2 million people [1], Russia and Ukraine provide a unique opportunity for studying HIV transmission dynamics in this group. The epidemic in these countries has been accompanied by very limited harm reduction services [4], making it a large-scale ‘natural experiment’ of HIV spread among PWID. By studying the epidemiological dynamics of the largest HIV epidemic among PWID, we might understand epidemics emerging in countries of the Middle East and northern Africa [5,6], southeastern Asia [7], Europe [8,9], and the USA [10]. Critically, although harm reduction approaches among PWID since the 1980s have had significant effects in the developed world [11], it remains unclear whether these preventive strategies should be prioritized over newer approaches such as treatment as prevention (TasP) [12] in resource-limited settings.
Here, we combined the evolutionary epidemiology approach with simple mathematical modelling to describe transmission dynamics of the HIV epidemic among PWID in Ukraine and Russia that started in the mid-1990s and assess factors mitigating HIV epidemics in PWID.
Methods
Nucleotide sequences
We compiled three HIV-1 sequence alignment datasets with sequences available from the Los Alamos HIV sequence database (http//:hiv.lanl.gov) (Supplementary Table 2): a reference dataset A of 2199 pol sequences; dataset B with 418 pol sequences from Russia and Ukraine sampled in 1997–2013; dataset C with 92 env sequences from Russia and Ukraine sampled in 1993–2011. All sequence alignments were performed using MEGA 6.0 software [13] and then manually edited. We built Bayesian Skyline Plots with BEAST 1.8.1 [14,15].
Estimates of epidemiological parameters
To obtain estimations of some of the epidemiological parameters, we used our previously described methods [16]. We estimated the number of secondary infections per primary infection in a completely susceptible population as
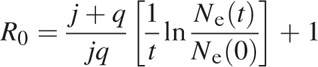 |
where Ne(t) is the effective population size at time t, N(0) is the effective population size at the baseline of the exponential growth phase, j is the rate of progression from the recent to long-term infection (1/0.5 a year, 2.0), and q is the overall mortality rate of HIV-infected individuals (in the absence of prevention assumed to average 1/10 years, 0.1).
We assume that R0,a is the mean number of secondary infections per transmitter (per injector) for our epidemiological settings:
where u is the proportion of PWID in the HIV-infected population.
We estimate the generation time T (the expected time from initial HIV infection to transmission to other individuals) as
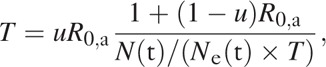 |
where Ne(t) × T is estimated from the Bayesian Skyline Plot reconstructed from the HIV sequences (env gene).
Compartmental modelling
We used a compartmental Susceptible – Recently Infected – Chronically Infected (Susceptibles – individuals who are not infected, but are at risk of infection; Recently Infected – individuals who acquired infection within the last 6 months; and Chronically Infected – individuals who acquired infection longer than 6 months ago) model to describe dynamics of HIV epidemic among PWID in Russia and Ukraine (Supplementary Fig. 1). We further advanced this model to include the compartment of treated individuals and take into account the uncertainty of our estimates.
More details on the methods can be found in Supplementary text. A description of the model parameters is provided in Supplementary Table 1.
Results
Phylodynamics and epidemiologic parameters estimates
The Bayesian Skyline Plots of the HIV population in Ukraine and Russia (Fig. 1) estimated from the pol and env alignments closely followed the epidemiological estimates of HIV prevalent cases: the epidemic grew rapidly between 1995 and 2000, and slower growth continued until 2005. Using the skyline plot and the estimated number of prevalent cases, we calculated R0,a to be 6–15 for corresponding values of the duration of infectivity (p) 5–15 years (Supplementary Fig. 2A). A plausible duration of infectivity for untreated HIV-infected PWID is unlikely to be more than 8–10 years [17], suggesting that the epidemic in Russia and Ukraine had an R0,a of 8–10. We calculated T using different values of the proportion of transmitters, u, approximated by the proportion of PWID among the HIV infected population. T was estimated to be 25 days when R0,a is around 10 and u = 70% (Supplementary Fig. 2B); T would exceed 3 months if u was less than 30%.
Fig. 1.
Bayesian Skyline Plot estimated from pol (dark blue line) and env (cyan line).
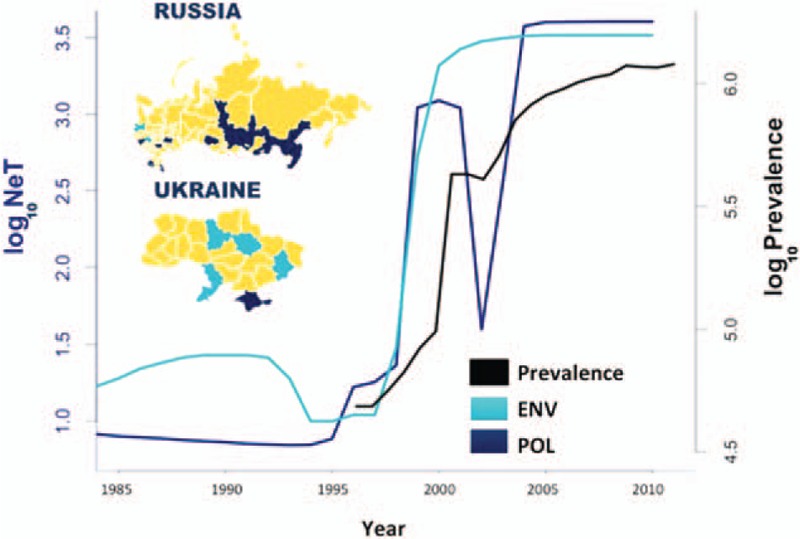
Maps show representation of sequences by geographical regions within the countries. The UNAIDS estimates of HIV prevalent cases are shown by the black line (prevalence).
Modelling the establishment of HIV epidemics in people who inject drugs
In the natural epidemic model without treatment or prevention, HIV prevalence among PWID was predicted to reach 86% [credibility interval (CI) 81–88%] after 20 years (Supplementary Fig. 3). The proportion of transmissions attributed to recently infected individuals rapidly decreased over the first years of the epidemic and stabilized at around 20% after 7 years (Supplementary Fig. 4).
In absence of prevention, but with treatment, the model showed that HIV prevalence would still reach 83% (CI 73–87%) after 20 years if 50% of long-term infected individuals receive treatment on average 4 years after becoming infected (Fig. 2a). If treatment was provided to a small proportion (25%) of recently infected individuals, after 20 years we did not observe a dramatic reduction in prevalence (72%, CI 62–80%) (Fig. 2b). We next investigated the scenario where R0 was reduced from the very beginning of an outbreak by scaling up harm reduction interventions and changing risky behaviours. By reducing R0,a by 60% (studies show that harm reduction programmes can help to reduce risky practices by 60% [18,19] and incidence in PWID by 80% [20]) to R0,a = 4, we were able to prevent the epidemic from establishing, provided that treatment was offered to at least 25% of recently infected PWID (Fig. 2d). There was no intervention scenario to prevent the epidemic from being established when treatment was not provided to recently infected individuals (Fig. 2c). For the scenarios with the well established epidemic (30% baseline HIV prevalence) (Fig. 2e–h), the best case scenario was also to treat at least a small proportion of recently infected individuals while scaling up prevention.
Fig. 2.
Results of the compartmental model representing HIV spread under different treatment scenarios.
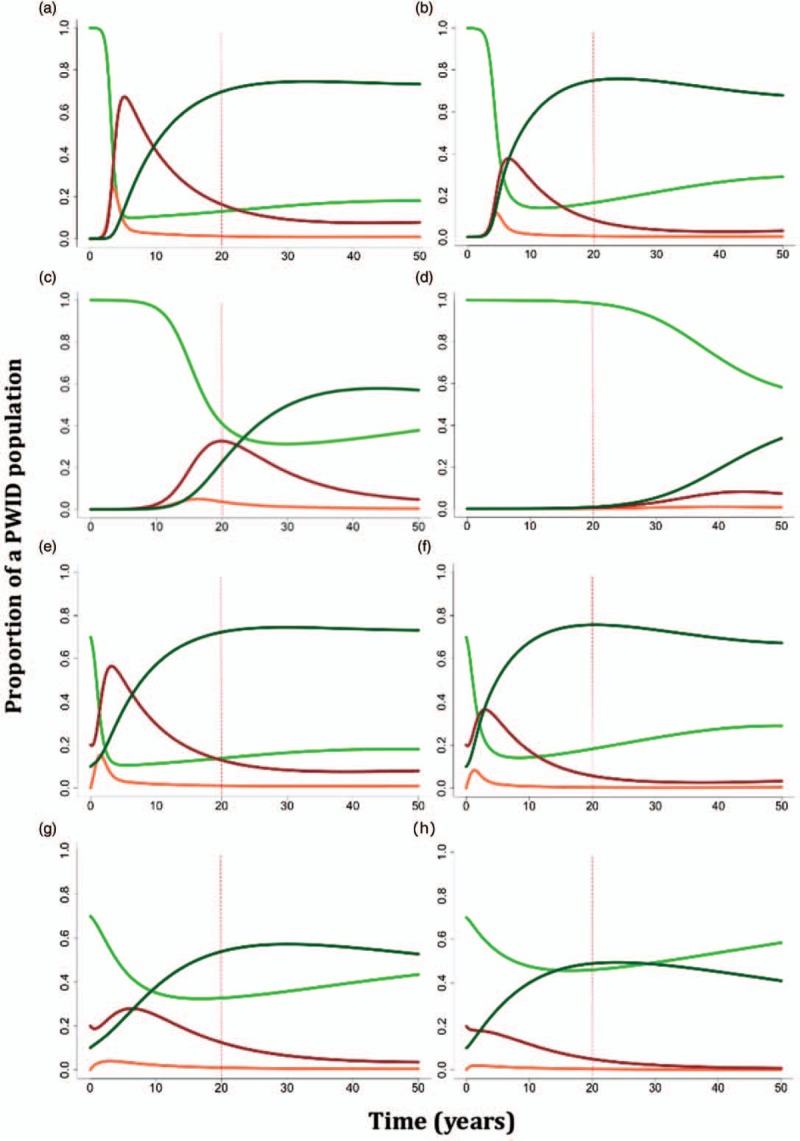
(a)–(d) HIV spread during an outbreak. (a) and (b) Scenarios when 0 and 25% of recently infected individuals, respectively, and 50% of long-term infected individuals (for both a and b) are provided with treatment, while no other preventive services are suggested. (c) and (d) Scenarios when, in addition to providing treatment, other harm reduction interventions are introduced, so that R0,a is reduced to 4. (e)–(h) HIV spread in a well established epidemic. (e) and (f) Scenarios when 0 and 25% of recently infected individuals, respectively, and 50% of long-term infected individuals (for both e and f) are provided with treatment, while no other preventive services are suggested. (g) and (h) Scenarios when, in addition to providing treatment, other harm reduction interventions are introduced, so that R0,a is reduced to 4.
Discussion
We analysed the uncontrolled and extensive HIV epidemic in Russia and Ukraine that began in the mid-1990s with an evolutionary epidemiology approach. We estimated that at the time, each injector transmitted to at least five individuals within the first month after getting infected. We thus showed that in the absence of prevention, HIV transmissions in PWID occur rapidly after primary infection.
Can we realistically target the first month of the infection? With primary prevention, such as syringe exchange programmes (SEPs) and safe practice training, we intervene from the very first day of infections. As the infectivity drops after the first month as a result of lower viral load and established immune responses, if we were to increase generation time, we would reduce the probability of infection per contact; we would then have more time to intervene with treatment.
Indeed, we show that TasP has a place in controlling an outbreak only when we introduce large-scale primary prevention and appeared to be more effective when we targeted recently infected individuals. Other studies show the importance of recently infected individuals in sexually driven HIV epidemics [21–23], but no consensus was reached on whether aiming HIV prevention efforts specifically at the recently infected group would be game-changing [24,25]. While such evidence for PWID is largely missing, we show that prevention of early transmissions provides major gains when we reduce transmissibility by reducing risky injecting practices.
Our findings provide insights on the dynamics of recently emerging HIV outbreaks such as those in Athens [8], Romania [9], or Indiana [26]. Crucially, HIV epidemics in PWID have been recently emerging in resource-limited countries in the Middle East and north Africa (particularly Afghanistan, Egypt, and Morocco) [5], as well as in some southeast Asian countries (e.g. Indonesia and Philippines) [7,27]. PWID populations in these locations were historically small, but their growth created the potential for new HIV outbreaks. Our study suggests that, if primary prevention is not introduced in countries with increasing prevalence of drug use, the epidemics in this group will expand rapidly when started and remain uncontrolled until sufficient primary prevention is put in place. Given that intravenous drug use is increasing faster than preventive responses in the developing world, it is a matter of time that these public health time bombs will explode again.
Changes in drug-taking behaviour might also influence transmission dynamics and introduce new pools of susceptible individuals within PWID populations. In our results, the drop of NeT around 2002 might be partly attributed to the shift in the Russian–Ukrainian drug scene towards stimulant drugs (associated with risky injecting behaviours [28]). Thus, settings with changing drug scenes, including changes in drug of choice or demographic changes, should also be addressed with basic prevention scale-up.
Our modelling suggested that increasing SEP coverage and reducing risky injecting practices in addition to treatment provision to half of the infected PWID will yield an additional 15% decrease in HIV prevalence after 20 years. Another modelling study that was informed by data from Ukraine showed that a 40% reduction in HIV prevalence within 10 years period should be expected if at least 60% of the unmet needs of harm reduction services (including SEPs and opioid substitution treatment) and treatment were covered [29], but this study did not model differences in transmissibility among recently and chronically infected. Our modelling suggested that such a steep decrease in HIV prevalence can be obtained only if we additionally provide at least 25% of recently infected individuals with treatment.
Apart from epidemiological reasons, early treatment initiation provides clear benefits for the patients themselves [30,31]. Thus, a combination of the expansion of existing harm reduction interventions and the development of new approaches to identify and treat recently infected people should be applied to settings with PWID-driven HIV epidemics [32]. Unfortunately, reaching those recently infected is extremely challenging, especially in settings in which preventive responses have been limited due to the sociopolitical context in which the outbreak arises. In the 1990s, social stigmatization of PWID in Russia and Ukraine was reflected in governmental and healthcare sectors, which limited the implementation of harm reduction services [4,33]. Other developing countries experiencing HIV outbreaks among PWID face similar problems; for example, despite decriminalization of drug use, police harassment of PWID in Indonesia undermines the success of SEPs [34]. Political support to reduce stigmatization of PWID could significantly increase access to health services and mitigate HIV spread in this vulnerable group.
Acknowledgements
T.I.V. and G.M. developed the research question, T.I.V., G.M., J.L., and T.K. contributed to the data analysis. T.I.V., S.R.F., J.L., S.G., A.H., O.G.P., A.K., P.S., T.K., D.P., and G.M. wrote the manuscript. T.I.V. is supported by the Clarendon Fund and Hertford College of the University of Oxford, S.R.F. is supported by the NIH NIDA (Grant number DP1DA034989). G.M. is supported by an MRC Clinician Scientist Fellowship (MR/K010565/1).
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
References
- 1.Joint United Nations Programme on HIV/AIDS. UNAIDS report 2014: the Gap report. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2014. [Google Scholar]
- 2.MacArthur GJ, van Velzen E, Palmateer N, Kimber J, Pharris A, Hope V, et al. Interventions to prevent HIV and hepatitis C in people who inject drugs: a review of reviews to assess evidence of effectiveness. Int J Drug Policy 2014; 25:34–52. [DOI] [PubMed] [Google Scholar]
- 3.Thomas MSD, Raj Y, Pandey A. A probability model for estimating the force of transmission of HIV infection and its application. Am J Math Stat 2014; 4:171–177. [Google Scholar]
- 4.Harm Reduction International. The global state of Harm Reduction. Towards an integrated response. In. Edited by Stoicescu C. London: Harm Reduction International; 2012. [Google Scholar]
- 5.Mumtaz GR, Weiss HA, Thomas SL, Riome S, Setayesh H, Riedner G, et al. HIV among people who inject drugs in the Middle East and North Africa: systematic review and data synthesis. PLoS Med 2014; 11:e1001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghasemian A, Mostafavi SKS, Vardanjani A-RK. Human immunodeficiency virus (HIV) distribution in Middle East region. Avicenna J Clin Microb Infect 2016; 3:e35965. [Google Scholar]
- 7.Afriandi I, Aditama TY, Mustikawati D, Oktavia M, Alisjahbana B, Riono P. HIV and injecting drug use in Indonesia: epidemiology and national response. Acta Med Indones 2009; 41 suppl 1:75–78. [PubMed] [Google Scholar]
- 8.Paraskevis D, Nikolopoulos G, Tsiara C, Paraskeva D, Antoniadou A, Lazanas M, et al. HIV-1 outbreak among injecting drug users in Greece, 2011: a preliminary report. Euro Surveill 2011; 16:16. [DOI] [PubMed] [Google Scholar]
- 9.Niculescu I, Paraschiv S, Paraskevis D, Abagiu A, Batan I, Banica L, et al. Recent HIV-1 outbreak among intravenous drug users in Romania: evidence for cocirculation of CRF14_BG and subtype F1 strains. AIDS Res Hum Retroviruses 2014; 31:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conrad C, Bradley HM, Broz D, Buddhal S, Chapman EL, Galang RR, et al. Community outbreak of HIV infection linked to injection drug use of oxymorphone – Indiana, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:443–444. [PMC free article] [PubMed] [Google Scholar]
- 11.Wodak A, McLeod L. The role of harm reduction in controlling HIV among injecting drug users. AIDS 2008; 22 suppl 2:S81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 2013; 30:2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 2007; 7:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drummond AJ, Ho SY, Phillips MJ, Rambaut A. Relaxed phylogenetics and dating with confidence. PLoS Biol 2006; 4:e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magiorkinis G, Sypsa V, Magiorkinis E, Paraskevis D, Katsoulidou A, Belshaw R, et al. Integrating phylodynamics and epidemiology to estimate transmission diversity in viral epidemics. PLoS Comput Biol 2013; 9:e1002876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longini IM, Jr, Clark WS, Byers RH, Ward JW, Darrow WW, Lemp GF, et al. Statistical analysis of the stages of HIV infection using a Markov model. Stat Med 1989; 8:831–843. [DOI] [PubMed] [Google Scholar]
- 18.Huo D, Ouellet LJ. Needle exchange and injection-related risk behaviors in Chicago: a longitudinal study. J Acquir Immune Defic Syndr 2007; 45:108–114. [DOI] [PubMed] [Google Scholar]
- 19.Bluthenthal RN, Kral AH, Gee L, Erringer EA, Edlin BR. The effect of syringe exchange use on high-risk injection drug users: a cohort study. AIDS 2000; 14:605–611. [DOI] [PubMed] [Google Scholar]
- 20.Des Jarlais DC, Marmor M, Friedmann P, Titus S, Aviles E, Deren S, et al. HIV incidence among injection drug users in New York City, 1992–1997: evidence for a declining epidemic. Am J Public Health 2000; 90:352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenner BG, Roger M, Routy JP, Moisi D, Ntemgwa M, Matte C, et al. High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis 2007; 195:951–959. [DOI] [PubMed] [Google Scholar]
- 22.Powers K, Ghani A, Miller W, Hoffman I, Pettifor A, Hosseinipour M, et al. The contribution of HIV-discordant couples to HIV transmission in Lilongwe, Malawi. J Int AIDS Soc 2012; 15:79–179. [Google Scholar]
- 23.Pinkerton SD. How many sexually-acquired HIV infections in the USA are due to acute-phase HIV transmission?. AIDS 2007; 21:1625–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eaton JW, Hallett TB. Why the proportion of transmission during early-stage HIV infection does not predict the long-term impact of treatment on HIV incidence. Proc Natl Acad Sci U S A 2014; 111:16202–16207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasylyeva TI, Friedman SR, Magiorkinis G. Prevention of early HIV transmissions might be more important in emerging or generalizing epidemics. Proc Natl Acad Sci U S A 2015; 112:E1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rich JD, Adashi EY. Ideological anachronism involving needle and syringe exchange programs: lessons from the Indiana HIV outbreak. JAMA 2015; 314:23–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farr AC, Wilson DP. An HIV epidemic is ready to emerge in the Philippines. J Int AIDS Soc 2010; 13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Booth RE, Lehman WE, Kwiatkowski CF, Brewster JT, Sinitsyna L, Dvoryak S. Stimulant injectors in Ukraine: the next wave of the epidemic?. AIDS Behav 2008; 12:652–661. [DOI] [PubMed] [Google Scholar]
- 29.Strathdee SA, Hallett TB, Bobrova N, Rhodes T, Booth R, Abdool R, et al. HIV and risk environment for injecting drug users: the past, present, and future. Lancet 2010; 376:268–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Group TAS, Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–822. [DOI] [PubMed] [Google Scholar]
- 31.Group ISS, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasylyeva TI, Friedman SR, Smyrnov P, Bondarenko K. A new approach to prevent HIV transmission: project protect intervention for recently infected individuals. AIDS Care 2015; 27:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Csete J. Human Rights Watch (Organization). Lessons not learned: human rights abuses and HIV/AIDS in the Russian Federation. New York, NY: Human Rights Watch; 2004. [Google Scholar]
- 34.Mboi N. Indonesia. A consummate insider pushes ideas from outside Indonesia. Science 2014; 345:162–163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


