Abstract
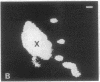
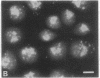
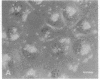
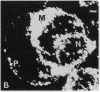
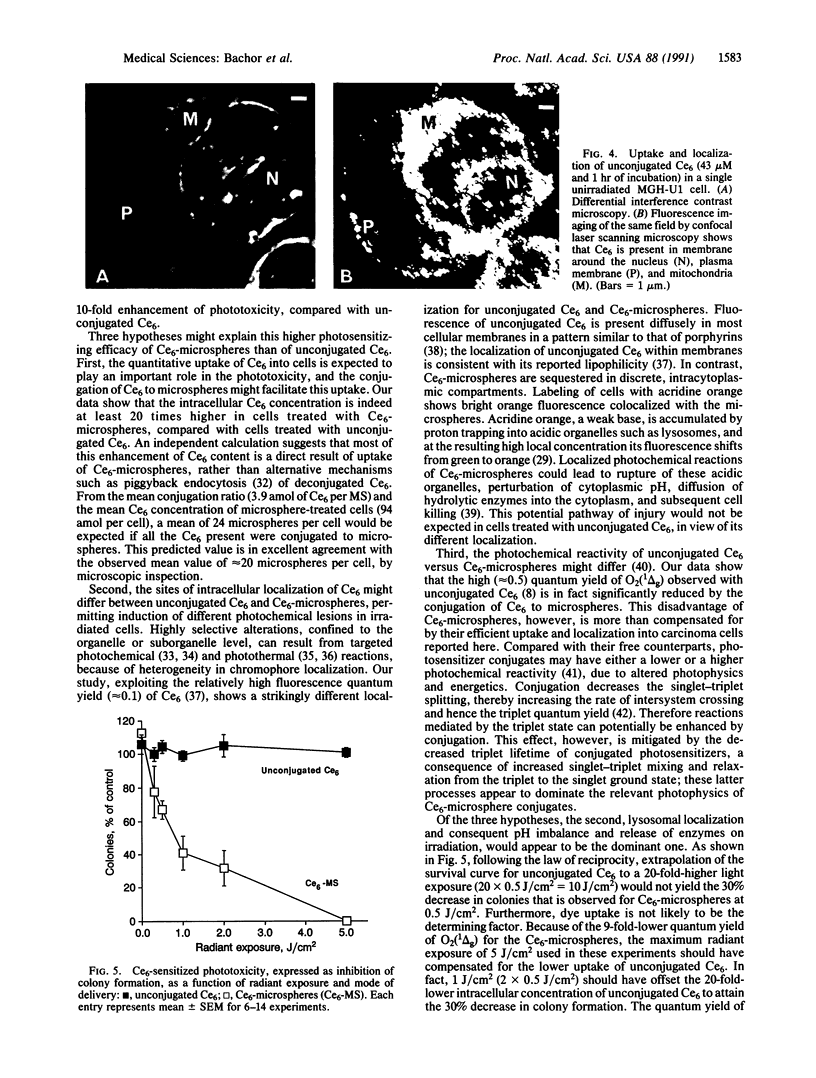
A photosensitizer conjugate, chlorin e6 (Ce6) covalently bound to 1-micron-diameter polystyrene microspheres, has been investigated in the photodynamic destruction of MGH-U1 human bladder carcinoma cells in vitro. The microspheres were taken up avidly by the carcinoma cells; confocal laser scanning fluorescence microscopy showed them to be localized in the cytoplasm, apparently within lysosomes, visualized by labeling with acridine orange. In contrast, fluorescence of unconjugated Ce6 was present within most cellular membranes. Use of Ce6-microsphere conjugates led to a 20-fold-higher mean intracellular concentration, compared with unconjugated Ce6. Cells incubated in the presence of Ce6-microsphere conjugates (0.43 microM equivalent) and subsequently irradiated at 659 nm with a dye laser pumped by an argon-ion laser showed dose-dependent phototoxicity, leading to total inhibition of colony formation at a radiant exposure of 5J/cm2; in contrast, cells incubated with either unconjugated Ce6 (0.43 microM) or unconjugated microspheres before laser irradiation were unaffected. Cells pretreated with Ce6-microsphere conjugates and irradiated in the presence of 90% 2H2O showed significantly increased phototoxicity, an effect consistent with an important role for excited-state singlet oxygen in the mechanism of injury. In solution, however, photosensitized generation of singlet oxygen with Ce6-microsphere conjugates was 9 times less efficient than with unconjugated Ce6. The markedly greater phototoxicity of Ce6-microsphere conjugates compared to unconjugated Ce6 was therefore a consequence of the high intracellular Ce6 concentration attained by phagocytosis of the conjugates and their particular sites of intracellular localization. Thus, these conjugates are an efficient system for the delivery of photosensitizing drugs to carcinoma cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. R., Parrish J. A. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983 Apr 29;220(4596):524–527. doi: 10.1126/science.6836297. [DOI] [PubMed] [Google Scholar]
- Bernal S. D., Lampidis T. J., McIsaac R. M., Chen L. B. Anticarcinoma activity in vivo of rhodamine 123, a mitochondrial-specific dye. Science. 1983 Oct 14;222(4620):169–172. doi: 10.1126/science.6623064. [DOI] [PubMed] [Google Scholar]
- Cannistraro S., Calberg-Bacq C. M., Van de Vorst A. Photosensitizing efficiency of proflavine bound to polyadenylic acid as studied by EPR. Photochem Photobiol. 1976 Feb;23(2):137–139. doi: 10.1111/j.1751-1097.1976.tb06786.x. [DOI] [PubMed] [Google Scholar]
- Dayhaw-Barker P., Truscott T. G. Direct detection of singlet oxygen sensitized by nalidixic acid: the effect of pH and melanin. Photochem Photobiol. 1988 May;47(5):765–767. doi: 10.1111/j.1751-1097.1988.tb02777.x. [DOI] [PubMed] [Google Scholar]
- Devanathan S., Dahl T. A., Midden W. R., Neckers D. C. Readily available fluorescein isothiocyanate-conjugated antibodies can be easily converted into targeted phototoxic agents for antibacterial, antiviral, and anticancer therapy. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2980–2984. doi: 10.1073/pnas.87.8.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J. Photosensitizers: therapy and detection of malignant tumors. Photochem Photobiol. 1987 Jun;45(6):879–889. doi: 10.1111/j.1751-1097.1987.tb07898.x. [DOI] [PubMed] [Google Scholar]
- Hasan T., Khan A. U. Phototoxicity of the tetracyclines: photosensitized emission of singlet delta dioxygen. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4604–4606. doi: 10.1073/pnas.83.13.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan T., Kochevar I. E., McAuliffe D. J., Cooperman B. S., Abdulah D. Mechanism of tetracycline phototoxicity. J Invest Dermatol. 1984 Sep;83(3):179–183. doi: 10.1111/1523-1747.ep12263531. [DOI] [PubMed] [Google Scholar]
- Hasan T., Lin C. W., Lin A. Laser-induced selective cytotoxicity using monoclonal antibody-chromophore conjugates. Prog Clin Biol Res. 1989;288:471–477. [PubMed] [Google Scholar]
- Hirn-Scavennec J., Brailly H., Maraninchi D., Mannoni P., Mawas C., Delaage M. Elimination of leukemia cells from human bone marrow using floating beads. Transplantation. 1988 Oct;46(4):558–563. doi: 10.1097/00007890-198810000-00018. [DOI] [PubMed] [Google Scholar]
- Jay D. G. Selective destruction of protein function by chromophore-assisted laser inactivation. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5454–5458. doi: 10.1073/pnas.85.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C., Burton M. A., Gray B. N. Enhanced in vivo activity of adriamycin incorporated into controlled release microspheres. Br J Cancer. 1989 May;59(5):743–745. doi: 10.1038/bjc.1989.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jori G., Reddi E., Cozzani I., Tomio L. Controlled targeting of different subcellular sites by porphyrins in tumour-bearing mice. Br J Cancer. 1986 May;53(5):615–621. doi: 10.1038/bjc.1986.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J. R., Van Dyke R. W., Scharschmidt B. F. Acidic vesicles in cultured rat hepatocytes. Identification and characterization of their relationship to lysosomes and other storage vesicles. Gastroenterology. 1987 May;92(5 Pt 1):1251–1261. [PubMed] [Google Scholar]
- Langer R. New methods of drug delivery. Science. 1990 Sep 28;249(4976):1527–1533. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- Lee P. C., Rodgers M. A. Laser flash photokinetic studies of rose bengal sensitized photodynamic interactions of nucleotides and DNA. Photochem Photobiol. 1987 Jan;45(1):79–86. doi: 10.1111/j.1751-1097.1987.tb08407.x. [DOI] [PubMed] [Google Scholar]
- Lin C. W., Lin J. C., Prout G. R., Jr Establishment and characterization of four human bladder tumor cell lines and sublines with different degrees of malignancy. Cancer Res. 1985 Oct;45(10):5070–5079. [PubMed] [Google Scholar]
- Marin-Padilla M. Erythrophagocytosis by epithelial cells of a breast carcinoma. Cancer. 1977 Mar;39(3):1085–1089. doi: 10.1002/1097-0142(197703)39:3<1085::aid-cncr2820390312>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Mew D., Lum V., Wat C. K., Towers G. H., Sun C. H., Walter R. J., Wright W., Berns M. W., Levy J. G. Ability of specific monoclonal antibodies and conventional antisera conjugated to hematoporphyrin to label and kill selected cell lines subsequent to light activation. Cancer Res. 1985 Sep;45(9):4380–4386. [PubMed] [Google Scholar]
- Moan J., Johannessen J. V., Christensen T., Espevik T., McGhie J. B. Porphyrin-sensitized photoinactivation of human cells in vitro. Am J Pathol. 1982 Nov;109(2):184–192. [PMC free article] [PubMed] [Google Scholar]
- Moan J., Peng Q., Evensen J. F., Berg K., Western A., Rimington C. Photosensitizing efficiencies, tumor- and cellular uptake of different photosensitizing drugs relevant for photodynamic therapy of cancer. Photochem Photobiol. 1987 Nov;46(5):713–721. doi: 10.1111/j.1751-1097.1987.tb04837.x. [DOI] [PubMed] [Google Scholar]
- Mosley S. T., Goldstein J. L., Brown M. S., Falck J. R., Anderson R. G. Targeted killing of cultured cells by receptor-dependent photosensitization. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5717–5721. doi: 10.1073/pnas.78.9.5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. A., Frenz D. A., Hasegawa E., Akiyama S. K. Matrix-driven translocation: dependence on interaction of amino-terminal domain of fibronectin with heparin-like surface components of cells or particles. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4791–4795. doi: 10.1073/pnas.84.14.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseroff A. R., Ohuoha D., Hasan T., Bommer J. C., Yarmush M. L. Antibody-targeted photolysis: selective photodestruction of human T-cell leukemia cells using monoclonal antibody-chlorin e6 conjugates. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8744–8748. doi: 10.1073/pnas.83.22.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrochi J. J., Mamelak A. N., Madison R. D., Macklis J. D., Hobson J. A. Mapping neuronal inputs to REM sleep induction sites with carbachol-fluorescent microspheres. Science. 1989 Sep 1;245(4921):984–986. doi: 10.1126/science.2475910. [DOI] [PubMed] [Google Scholar]
- Rakestraw S. L., Tompkins R. G., Yarmush M. L. Antibody-targeted photolysis: in vitro studies with Sn(IV) chlorin e6 covalently bound to monoclonal antibodies using a modified dextran carrier. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4217–4221. doi: 10.1073/pnas.87.11.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi E., Lo Castro G., Biolo R., Jori G. Pharmacokinetic studies with zinc(II)-phthalocyanine in tumour-bearing mice. Br J Cancer. 1987 Nov;56(5):597–600. doi: 10.1038/bjc.1987.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. G., Berns M. W. In vitro photosensitization I. Cellular uptake and subcellular localization of mono-L-aspartyl chlorin e6, chloro-aluminum sulfonated phthalocyanine, and photofrin II. Lasers Surg Med. 1989;9(2):90–101. doi: 10.1002/lsm.1900090203. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., SHIRLEY W., BARDAWIL W. A. 'Piggy-back' phagocytosis. Nature. 1962 Apr 21;194:255–256. doi: 10.1038/194255a0. [DOI] [PubMed] [Google Scholar]
- Sakas D. E., Moskowitz M. A., Wei E. P., Kontos H. A., Kano M., Ogilvy C. S. Trigeminovascular fibers increase blood flow in cortical gray matter by axon reflex-like mechanisms during acute severe hypertension or seizures. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1401–1405. doi: 10.1073/pnas.86.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea C. R., Hefetz Y., Gillies R., Wimberly J., Dalickas G., Hasan T. Mechanistic investigation of doxycycline photosensitization by picosecond-pulsed and continuous wave laser irradiation of cells in culture. J Biol Chem. 1990 Apr 15;265(11):5977–5982. [PubMed] [Google Scholar]
- Shea C. R., Sherwood M. E., Flotte T. J., Chen N., Scholz M., Hasan T. Rhodamine 123 phototoxicity in laser-irradiated MGH-U1 human carcinoma cells studied in vitro by electron microscopy and confocal laser scanning microscopy. Cancer Res. 1990 Jul 1;50(13):4167–4172. [PubMed] [Google Scholar]
- Shea C. R., Whitaker D., Murphy G. F., Hasan T. Ultrastructure and dynamics of selective mitochondrial injury in carcinoma cells after doxycycline photosensitization in vitro. Am J Pathol. 1988 Nov;133(2):381–388. [PMC free article] [PubMed] [Google Scholar]
- Sieber F., Spivak J. L., Sutcliffe A. M. Selective killing of leukemic cells by merocyanine 540-mediated photosensitization. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7584–7587. doi: 10.1073/pnas.81.23.7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. H., Tebbi C. K., Freeman A. I., Green D. M., Brecher M. L., Barcos M. Malignant histiocytosis. Complete remission in two pediatric patients. Cancer. 1987 May 1;59(9):1566–1570. doi: 10.1002/1097-0142(19870501)59:9<1566::aid-cncr2820590906>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Spikes J. D. Chlorins as photosensitizers in biology and medicine. J Photochem Photobiol B. 1990 Jul;6(3):259–274. doi: 10.1016/1011-1344(90)85096-f. [DOI] [PubMed] [Google Scholar]
- Spivak J. L. Phagocytic tumour cells. Scand J Haematol. 1973;11(3):253–256. doi: 10.1111/j.1600-0609.1973.tb00126.x. [DOI] [PubMed] [Google Scholar]
- Valenzeno D. P. Photomodification of biological membranes with emphasis on singlet oxygen mechanisms. Photochem Photobiol. 1987 Jul;46(1):147–160. doi: 10.1111/j.1751-1097.1987.tb04749.x. [DOI] [PubMed] [Google Scholar]
- Van Peteghem M. C., Mareel M. M., De Bruyne G. K. Phagocytic capacity of invasive malignant cells in three-dimensional culture. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;34(2):193–204. doi: 10.1007/BF02892417. [DOI] [PubMed] [Google Scholar]
- Widder K. J., Morris R. M., Poore G., Howard D. P., Jr, Senyei A. E. Tumor remission in Yoshida sarcoma-bearing rts by selective targeting of magnetic albumin microspheres containing doxorubicin. Proc Natl Acad Sci U S A. 1981 Jan;78(1):579–581. doi: 10.1073/pnas.78.1.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemul S., Berger C., Estabrook A., Suarez S., Edelson R., Bayley H. Selective killing of T lymphocytes by phototoxic liposomes. Proc Natl Acad Sci U S A. 1987 Jan;84(1):246–250. doi: 10.1073/pnas.84.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdolsek J. M., Olsson G. M., Brunk U. T. Photooxidative damage to lysosomes of cultured macrophages by acridine orange. Photochem Photobiol. 1990 Jan;51(1):67–76. doi: 10.1111/j.1751-1097.1990.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Zee-Cheng R. K., Cheng C. C. Delivery of anticancer drugs. Methods Find Exp Clin Pharmacol. 1989 Jul-Aug;11(7-8):439–529. [PubMed] [Google Scholar]