Abstract
Background:
Aerobic fitness and muscle bioenergetic capacity decline with age; whether such declines explain age-related slowing of walking speed is unclear. We hypothesized that muscle energetics and aerobic capacity are independent correlates of walking speed in simple and challenging performance tests and that they account for the observed age-related decline in walking speed in these same tests.
Methods:
Muscle bioenergetics was assessed as postexercise recovery rate of phosphocreatine (PCr), k PCr, using phosphorus magnetic resonance spectroscopy (31P-MRS) in 126 participants (53 men) of the Baltimore Longitudinal Study of Aging aged 26–91 years (mean = 72 years). Four walking tasks were administered—usual pace over 6 m and 150 seconds and fast pace over 6 m and 400 m. Separately, aerobic fitness was assessed as peak oxygen consumption (peak VO2) using a graded treadmill test.
Results:
All gait speeds, k PCr, and peak VO2 were lower with older age. Independent of age, sex, height, and weight, both k PCr and peak VO2 were positively and significantly associated with fast pace and long distance walking but only peak VO2 and not k PCr was significantly associated with usual gait speed over 6 m. Both k PCr and peak VO2 substantially attenuated the association between age and gait speed for all but the least stressful walking task of 6 m at usual pace.
Conclusion:
Muscle bioenergetics assessed using 31P-MRS is highly correlated with walking speed and partially explains age-related poorer performance in fast and long walking tasks.
Keywords: Lower extremity performance, Walking speed, Bioenergetics, Muscle, Magnetic resonance spectroscopy
Walking is a complex task of fundamental importance for functional independence (1). Age-related decline in gait speed is associated with mobility impairment and strongly predicts adverse health outcomes, including disability in activities of daily living, and death (2–4). Walking requires integration and co-ordination of multiple physiological systems, including the central and peripheral nervous systems, the cardiopulmonary system, musculoskeletal system, and sensory systems (1). Although many risk factors for mobility loss have been previously described, the extent to which each of these systems contributes independently to age-related mobility loss is not fully understood. Recent data from longitudinal studies suggest that neuromuscular impairments and aerobic fitness both play prominent roles (5,6). The relationship between muscle function and walking is of particular interest, because effective muscle contraction is essential for walking, and aging is associated with a decline in both muscle strength and mass; the decline in muscle strength exceeds that expected by changes in muscle mass (7–9). The causes of decline in muscle mass and strength remain the subject of active investigation. One leading hypothesis attributes the decline in strength to a primary defect in energy metabolism, possibly linked to impaired mitochondrial function. Under this hypothesis, parameters of muscle bioenergetics and of fitness should be more strongly related to performance in more demanding walking tasks (10). Indeed, Bohannon (11) and Ko and colleagues (12) reported that the age-related decline of gait speed measured at maximum pace is steeper than for gait speed measured at normal pace. In addition, Cunningham and colleagues found that aerobic capacity is more strongly associated with gait speed measured at a faster pace than at normal pace (13).
In a sample of young- and middle-aged individuals, Fleischman and colleagues demonstrated that mitochondrial function assessed by phosphorus magnetic resonance spectroscopy of muscle declines with age. These authors did not find any independent association between mitochondrial function and muscle maximal voluntary contraction and did not investigate walking performance (14). In previous small studies, both whole-body aerobic capacity (13,15) assessed as peak oxygen consumption (peak VO2) and skeletal muscle bioenergetics assessed by 31-phosphorus magnetic resonance spectroscopy (31P-MRS) have been associated with walking speed. VO2 and 31P-MRS can be conceptualized as measures of aerobic capacity that globally assess oxygen transport to the tissues and the mitochondrial rate of oxidative phosphorylation (15). However, performance on a treadmill test for measuring peak VO2 is strongly affected by additional factors such as osteoarthritis and balance impairment. In contrast, assessing the rate of maximum in vivo oxidative capacity of skeletal muscle by 31P-MRS in not affected by postural stability or balance, and therefore represents a more direct measure of muscle mitochondrial function (16,17). Our implementation of 31P-MRS bioenergetic assessment consists of a brief period of forceful alternating knee flexion and extension exercise that significantly depletes PCr, followed by a postexercise rest period during which the rate of recovery of PCr is assessed. There are minimal energy demands during this rest period, so that this rate largely reflects the maximal capacity of muscle mitochondria to synthesize ATP (18–21).
The primary objectives of this study were to evaluate the separate and combined associations of muscle bioenergetics and global fitness with walking speeds at usual and fast pace over short and long duration and to determine the extent to which energetics and fitness explain age-related slowing of walking. We hypothesized that (i) recovery rate of phosphocreatine (k PCr) and peak VO2 would be associated with walking speed independent of age, sex, height, and weight, (ii) the associations would be stronger for more challenging walking tasks, (iii) k PCr would contribute independently to challenging walking, and (iv) after accounting for k PCr and peak VO2, the association between age and gait performance would be largely attenuated.
Methods
Participants
Data on 31P-MRS postexercise recovery rate (k PCr) were collected in 181 Baltimore Longitudinal Study of Aging (BLSA) participants from April to December 2013. Of these, 126 had complete data from a physical examination, a completed health history questionnaire, whole-body aerobic capacity (peak VO2) measurement, and gait speed measurement for the four walking tasks considered here. Trained technicians administered all assessments following standardized protocols. Height and weight were objectively assessed using a stadiometer, and the average of three measures was used in the analysis. The institutional internal review board approved the study protocol (BLSA – 03-AG-0325), and all participants provided written informed consent.
Gait Speed Metrics
The usual and rapid short-course gait speed measurements were performed over a 6-m course with participants asked to walk at their “normal walking pace” and “as fast as possible” for two trials each, with the fastest trial used in the analyses. The longer usual and rapid pace walks were modeled after the Long Distance Corridor Walk (22,23) where the participant walks back and forth over a 20-m course in an uncarpeted corridor. The first walk (UGS-150s) is performed at the participant’s “usual, comfortable pace” for 2.5 minutes (150 seconds). The second walk (RGS-400m) follows immediately and covers 400 m and was performed “as quickly as possible.”
31P-MRS
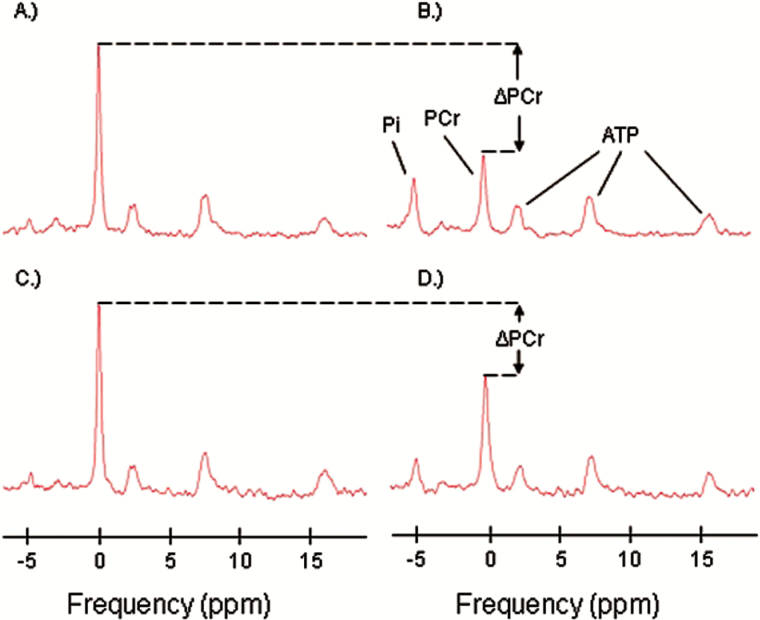
In vivo 31P-MRS measurements of phosphorus-containing metabolites were obtained from the quadriceps muscles using a 3T Philips Achieva MR scanner (Philips, Best, The Netherlands). Participants were positioned supine upon the bed of the scanner, with a foam wedge placed underneath the knee to maintain slight flexion, and with ankles, thighs, and hips secured with straps to reduce movement during exercise. After placement of the participant within the bore of the magnet, participants performed a rapid and intense ballistic knee extension exercise, similar to that described by Coen and colleagues (15). A series of pulse-acquire 31P-MRS acquisitions were obtained before, during, and after this exercise protocol, using a 10-cm 31P-tuned, flat surface coil (PulseTeq, Surrey, United Kingdom) secured over the vastus lateralis muscle of the left thigh. Spectra were obtained using adiabatic radio frequency (RF) excitation pulses with a 90° flip angle, 1.5-second repetition time, and four signal averages, resulting in an effective time resolution of 6 seconds between measurements. A total of 75 spectra were obtained over a total acquisition time of 7 minutes 30 seconds. All spectra were visually examined and were found of acceptable quality. Example of spectra used in the analysis is shown in Figure 1. The duration of exercise was controlled to achieve depletion in PCr signal amplitude to a value within 33% to 67% of the resting amplitude. Participants practiced the exercise before entering the magnet. Spectra were processed using jMRUI (version 5.0) and quantified using a nonlinear least square algorithm (AMARES) (24–26).
Figure 1.
Representative 31P spectra with inorganic phosphate (Pi), phosphocreatine (PCr), and ATP resonances indicated in panel B. Panels (A) and (B) show example baseline and postexercise spectra representing a typical level of exercise-induced PCr depletion (ΔPCr). Panels (C) and (D) show example baseline and postexercise spectra representing a minimally acceptable level of PCr depletion, with ΔPCr ~ 33%.
Skeletal Muscle Oxidative ATP Resynthesis Rate Determined by 31P-MRS
Postexercise PCr recovery rate was calculated by fitting the time-dependent changes in PCr peak area to the monoexponential recovery function:
where PCr0, the PCr signal amplitude immediately following in-magnet exercise, is the initial condition for the recovery phase of the protocol, ΔPCr = (PCrbaseline − PCr0) is the decrease in PCr signal amplitude observed during in-magnet exercise from the baseline value PCrbaseline to the end-exercise value PCr0, τ is the PCr recovery time constant, and k PCr is the PCr recovery rate constant determined as 1/τ. [PCrbaseline], the initial concentration of PCr at rest, was estimated using a fully relaxed 31P MR spectrum through normalization of the PCr peak by γ-ATP, under the assumption that [ATP] = 5.5 mmol/kg wet weight at rest. From this, ATPmax, a measure of mitochondrial capacity largely independent of exercise intensity, was estimated as [PCrbaseline]*k PCr (27).
In addition, we estimated the initial slope of the recovery of PCr post exercise from a linear fit of the three initial postexercise values of PCr signal amplitude, representing data acquired over the first 12 seconds after the cessation of exercise. This slope was then normalized by the percent decrease in PCr (28). Note that for the ideal exponential model, the resulting value would be equal to k PCr; estimation of initial slope therefore accounts for a possible deviation of the PCr recovery curve from the ideal monoexponential model. The corresponding ATPmax estimate was again calculated from these derived values of k PCr.
In light of work suggesting a significant influence of resting creatine concentration on mitochondrial respiration (29), we estimated [Cr] using a method similar to that described in (28), which is based on the resting concentration of PCr and the proportion of unphosphorylated creatine to total creatine. Given the wide range of reported values for this proportion (30), we performed our analysis using the value of 15% (28), as well as values of 30% and 45% in order to examine the sensitivity of the relationship between walking speed and mitochondrial function to this parameter.
Peak VO2
Whole-body aerobic capacity was determined by continuous measurement of oxygen consumption using a modified version of the Balke protocol (31). Treadmill speed was determined by age and sex and did not vary during testing. The initial grade was 0% and was progressively increased by 3% at each stage until voluntary exhaustion. The first incline change was implemented at 45 seconds, and all subsequent increases occurred every 3 minutes. Expired O2 and CO2 concentrations were measured with an O2 and CO2 analyzer (Ultima C2, MedGraphics, St. Paul, MN). Oxygen consumption was calculated every 30 seconds with the highest value determining peak VO2, expressed in milliliters per kilogram per minute (mL/kg/min). Collected peak VO2 was the measure of aerobic capacity used in this study.
Statistical Analysis
Exploratory analyses
We performed several exploratory regression models with walking speed as outcome in order to compare the alternative metrics of mitochondrial function estimated either by the exponential fit of the full recovery curve (k PCr,exponential, ATPmaxexponential) or by fitting the first three points of the recovery curve (k PCr,3points, ATPmax3points). We also included %PCr depletion during exercise and estimated [PCrbaseline] and [Cr] as additional independent variables in the regression analysis. For all four walking tests, k PCr provided a marginally better fit than ATPmax. In addition, regression models that used estimates from the full recovery curve provided a nonsignificantly better fit compared with those that used estimates from the initial slope, except for the task of walking 6 m at usual pace where the parameters estimated from the initial slope performed slightly, although nonsignificantly, better than those estimated from the full recovery curve. Including %PCr depletion as an additional independent variable in the model led to a slight improvement of the fit to the model, whereas the introduction of [PCrbaseline] and [Cr] had no substantial effect. Therefore, in regression models that included k PCr as one of the independent variables, we also included %PCr depletion. Possible nonlinearity in the relationship between mitochondrial function and walking speed was excluded by adding to the final models a quadratic term for k PCr; this quadratic term was not found to be statistically significant in any of the analyses.
Main analyses
Descriptive population characteristics are reported as means (SD) or values (percentages by age < 65 years vs ≥ 65 years). p Values were computed using t tests or chi-square tests as appropriate. In addition, we computed Pearson correlation coefficients and corresponding p values between age, k PCr, and peak VO2.
We fit four hierarchical linear regression models testing the relationship of muscle energetics and fitness with outcomes from each of the four gait tasks, with additional adjustment for sex, height, and weight. Model 1 estimated the coefficient linking age to walking speed for the different walking tasks; Model 2 also incorporated age, k PCr, and %PCr depletion. This model tested whether mitochondrial function is a significant independent correlate of walking speed and, when compared with Model 1, whether changes with age in mitochondrial function account for the decline of walking speed with aging; Model 3 added peak VO2 to Model 1 and tests whether aerobic fitness is an independent correlate of walking speed and, when compared with Model 1, whether changes with age in aerobic fitness account for the decline of walking speed with aging; Model 4 included age, k PCr, %PCr depletion, and peak VO2 to test whether mitochondrial function estimated by MRS and fitness estimated by treadmill testing are independent correlates of walking speed. A sensitivity analysis did not find strong evidence for interactions of sex with age, peak VO2, and k PCr for any of the four gait measures (p > .25 for all). Covariate-adjusted associations between age and gait speed were displayed graphically using adjusted variable plots. The y-axis of the adjusted variable plot was computed as the residual of a linear regression of gait speed on covariates, excluding age, which was added to the sample mean of gait speed. The x-axis of the adjusted variable plot was computed as the residual of a linear regression of age on covariates, which was added to the sample mean of age. The resulting slope of the best-fit regression line is equivalent to that between age and gait speed, adjusted for the corresponding covariates included in Models 1–4. A p value less than .05 was considered statistically significant for all analyses.
Results
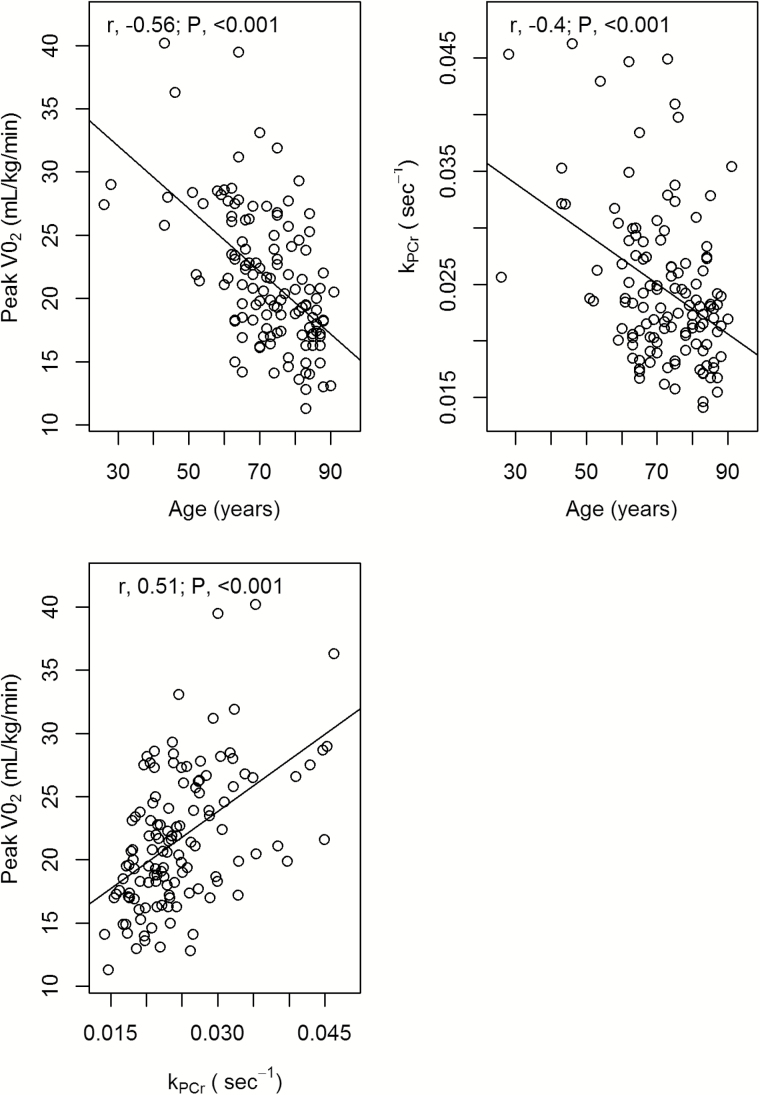
Baseline characteristics, including sex, average height, and weight, did not significantly differ by age group. Average k PCr, peak VO2, and speed for all four gait tasks were significantly lower in older versus younger adults (p value < .005 for all) (Table 1). Figure 2 further shows that age was negatively correlated with k PCr (r = −.56, p value < .001) and peak VO2 (r = −.40, p value < .001) and that k PCr and peak VO2 were positively correlated with each other (r = .51, p value < .001).
Table 1.
Characteristics of the 126 Participants From the Baltimore Longitudinal Study of Aging Evaluated in This Study
| Characteristic | Age < 65 y, (n = 30) | Age ≥ 65 y, (n = 96) | p Value |
|---|---|---|---|
| Mean (SD) or No. (%) | Mean (SD) or No. (%) | ||
| Female sex | 19 (63.3) | 54 (56.2) | .64 |
| Height, cm | 168.9 (9.9) | 165.7 (8.3) | .12 |
| Weight, kg | 78.5 (17.2) | 73.6 (15.2) | .17 |
| k PCr, s−1 | 0.029 (0.008) | 0.023 (0.006) | .001 |
| %PCr depletion | 26.67 (8.27) | 29.31 (9.33) | .166 |
| Initial PCr | 24.61 (4.88) | 20.81 (4.73) | <.001 |
| Peak VO2, mL/kg/min | 26.6 (5.6) | 20.0 (4.3) | <.001 |
| Usual gait speed (6 m), m/s | 1.3 (0.2) | 1.2 (0.2) | .001 |
| Usual gait speed (150s), m/s | 1.3 (0.1) | 1.2 (0.1) | .002 |
| Rapid gait speed (6 m), m/s | 2.0 (0.3) | 1.7 (0.3) | <.001 |
| Rapid gait speed (400 m), m/s | 1.7 (0.2) | 1.4 (0.2) | <.001 |
Notes: k PCr = phosphocreatine recovery rate; VO2 = oxygen consumption.
p values were derived using t tests for continuous variables and using chi-square tests for binary variables.
Figure 2.
Scatterplots of relationships between phosphocreatine recovery rate (k PCr), peak oxygen consumption (peak VO2), and age.
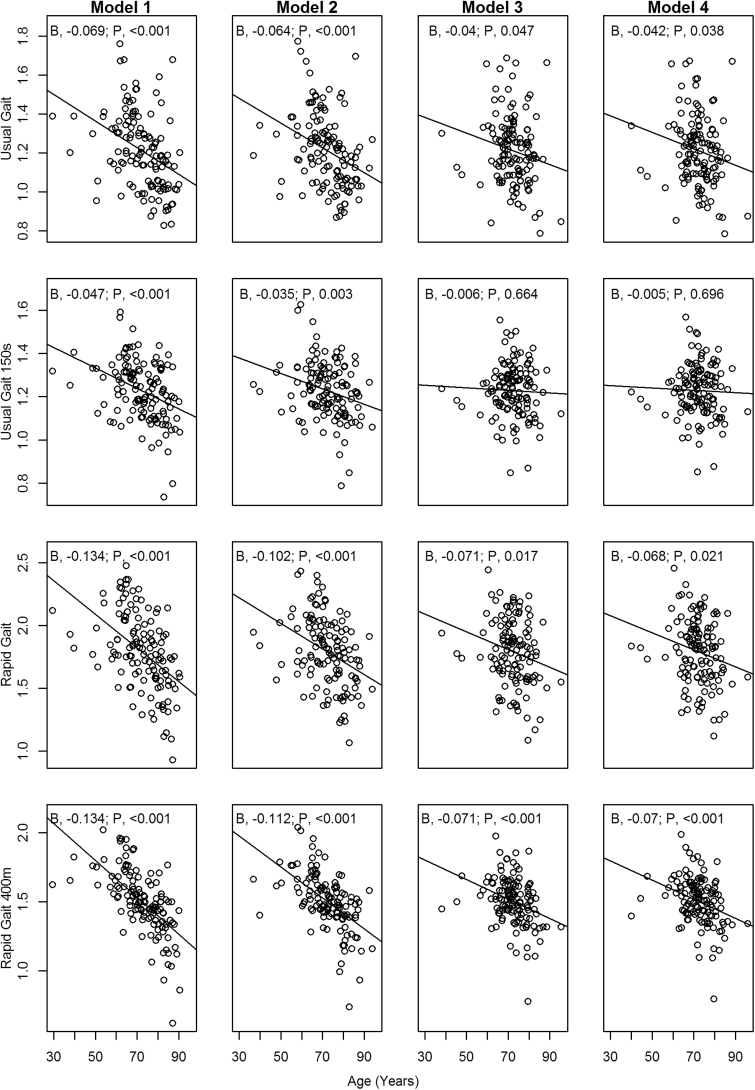
After adjustment for sex, height, and weight, older age was significantly associated with slower UGS-6m, RGS-6m, and RGS-400m irrespective of adjustment for k PCr and %PCr depletion or peak VO2 (p value < .05) (Table 2). Older age was additionally associated with slower UGS-150s with and without adjustment for k PCr (p value < .005; Models 1 and 2) but not after adjustment for peak VO2 (p value = .66, Model 3; p value = .70, Model 4). Additionally, higher k PCr was associated with faster UGS-150s, RGS-6m, and RGS-400m after adjustment for age, sex, height, weight, and %PCr depletion (p value < .05; Model 2) but not with UGS-6m. After adjustment for peak VO2, k PCr remained significantly associated with RGS-6m (p value = .02; Model 4) and approached significant association with RGS-400m (p value = .059; Model 4). As expected, because peak VO2 is measured during treadmill exercise, higher peak VO2 was significantly associated with faster speed for all gait tests (p value < .05). However, after adjustment for k PCr, peak VO2 remained independently associated with walking speed only in the long-distance tasks (UGS-150s and RGS-400m). Inclusion of k PCr after adjustment for peak VO2 (Model 4 vs Model 3) resulted in a negligible impact on R 2 except for RGS-6m (.36 vs .33).
Table 2.
Regression Models Testing Associations of k PCr and VO2 With Gait Speed in the Four Gait Tasks
| Age (per 10 y) | k PCr (s−1) | PCr depletion (*10%) | Peak VO2 (mL/kg/min) | Model R 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Model | Beta (95% CI) | p Value | Beta (95% CI) | p Value | Beta (95% CI) | p Value | Beta (95% CI) | p Value | |
| Usual gait speed (6 m), m/s | Model 1 | −0.069 (−0.098, −0.039) | <.001 | .194 | ||||||
| Model 2 | −0.064 (−0.096, −0.032) | <.001 | 2.495 (−3.257, 8.247) | .392 | 0.035 (−0.004, 0.073) | .077 | .216 | |||
| Model 3 | −0.040 (−0.080, −0.001) | .047 | 0.009 (0.001, 0.018) | .042 | .222 | |||||
| Model 4 | −0.042 (−0.082, −0.002) | .038 | 0.301 (−5.915, 6.517) | .924 | −0.027 (−0.012, 0066) | .171 | 0.008 (−0.001, 0.019) | .082 | .236 | |
| Usual gait speed (150s), m/s | Model 1 | −0.047 (−0.068, −0.026) | <.001 | .169 | ||||||
| Model 2 | −0.035 (−0.058, −0.013) | .003 | 5.238 (1.163, 9.314) | .012 | −0.018 (−0.009, 0.045) | .188 | .213 | |||
| Model 3 | −0.006 (−0.033, 0.021) | .664 | 0.013 (0.007, 0.019) | <.001 | .286 | |||||
| Model 4 | −0.005 (−0.033, 0.022) | .696 | 2.158 (−2.197, 5.536) | .315 | −0.008 (−0.019, 0.034) | .57 | 0.012 (0.005, 0.018) | <.001 | .292 | |
| Rapid gait speed (6 m), m/s | Model 1 | −0.134 (−0.178, −0.090) | <.001 | .271 | ||||||
| Model 2 | −0.102 (−0.148, −0.056) | <.001 | 14.299 (6.007, 22.252) | .001 | −0.043 (−0.012, 0.099) | .124 | .337 | |||
| Model 3 | −0.071 (−0.129, −0.013) | .017 | 0.020 (0.007, 0.033) | .002 | .326 | |||||
| Model 4 | −0.068 (−0.125, −0.010) | .021 | 10.798 (0.605, 19.733) | .018 | −0.031 (−0.025, 0.088) | .271 | 0.013 (−0.001, 0.027) | .054 | .358 | |
| Rapid gait speed (400 m), m/s | Model 1 | −0.134 (−0.163, −0.104) | <.001 | .431 | ||||||
| Model 2 | −0.112 (−0.143, −0.081) | <.001 | 9.839 (4.321, 15.356) | .001 | −0.036 (−0.001, 0.073) | .056 | .487 | |||
| Model 3 | −0.071 (−0.108, −0.034) | <.001 | 0.020 (0.012, 0.028) | <.001 | .528 | |||||
| Model 4 | −0.070 (−0.106, −0.033) | <.001 | 5.478 (−0.222, 11.178) | .059 | −0.021 (−0.015, 0.057) | .249 | 0.017 (0.008, 0.026) | <.001 | .543 | |
Notes: CI = confidence interval; k PCr = phosphocreatine recovery rate; VO2 = oxygen consumption.
All models additionally adjusted for sex, height, and weight. Model 1 estimated the coefficient linking age to walking speed; Model 2 also incorporated age, k PCr, and %PCr depletion. This model tested whether mitochondrial function is a significant independent correlate of walking speed and, when compared with Model 1, whether changes with age in mitochondrial function account for the decline of walking speed with aging; Model 3 added peak VO2 to Model 1 and tests whether aerobic fitness is an independent correlate of walking speed and, when compared with Model 1, whether changes with age in aerobic fitness account for the decline of walking speed with aging; Model 4 included age, k PCr, %PCr depletion, and peak VO2 to test whether mitochondrial function estimated by magnetic resonance spectroscopy and fitness estimated by treadmill testing, are independent correlates of walking speed.
Figure 3 shows the attenuation of the relationship of age with gait speed after adjustment for k PCr or peak VO2. The slopes in Model 4 were less steep than the slopes for the corresponding gait test in Model 1, especially for UGS-150s (slope = −0.005, Model 4 vs slope = −0.047, Model 1).
Figure 3.
Covariate-adjusted associations between age and gait speed displayed graphically using adjusted variable plots (see statistical analysis for explanation). The slopes in Model 4 were less steep than the slopes for the corresponding gait test in Model 1, especially for UGS-150s.
Discussion
We investigated the relationship between a direct MR spectroscopy–based measure of muscle bioenergetics (k PCr) and several measures of walking speed in a population ranging in age from 26 to 91 years. Independent of age, sex, height, and weight, this measure was associated with walking tasks performed at fast speed or covering relatively long distances but not with a short (6 m) walking test at usual speed. As expected, aerobic fitness was a dominant correlate of walking speed, because it was measured as peak VO2 consumption during a treadmill protocol, that is, during a stressful walking task. Peak VO2 was expected to, and in fact did, account for a substantial portion of the association between age and walking speed in most tests. Overall, these results suggest that muscle bioenergetics is a limiting factor for walking performance in more challenging tasks but not for shorter walking tasks at usual pace.
Our findings are consistent with those of Coen and colleagues who reported that k PCr in the quadriceps was associated with preferred gait speed as well as aerobic capacity in a small number (n = 30) of older adults (15). In the current study, we performed a similar analysis in a larger cohort (n = 126) spanning a greater age range (26 to 91 years). Further, we incorporated four gait speed metrics, representing a more extended and varied range of mobility assessments. In particular, this permitted us to evaluate the contributors to walking speed across a range from a brief, slow walking task to a much more challenging walking task.
An additional finding was that muscle oxidative capacity is captured in large part by tests of aerobic capacity. In fact, after adjusting for peak VO2, the size of the regression coefficient for k PCr was substantially reduced and statistically significant only for the RGS-6m test. This finding can be readily interpreted in view of the fact that a walking-based measure of peak VO2 captures a multitude of factors such as pain, co-ordination, proprioception, pulmonary function and balance in addition to muscle bioenergetics, and all these factors affect gait speed; in other words, the treadmill test is itself a test of walking capacity. Therefore, its correlation with walking speed is to be expected. In contrast, our measure of k PCr is minimally influenced by factors other than muscle oxidative capacity. These findings highlight the importance of noninvasive, nonwalking, measures of muscle bioenergetics such as those provided by 31P-MRS.
Understanding the mechanisms by which decline in physical function with aging often manifests as slower gait speed is a central focus of aging research. Although it is generally accepted that the causal pathway leading to mobility loss in older persons is multifactorial, identifying the specific causal contributions of particular mechanisms remains a challenge. In this study, we evaluated muscle oxidative capacity by measuring k PCr with 31P-MRS. Our findings are consistent with the concept that early-stage impairments in specific physiological systems may become evident only under challenging conditions. Specifically, the role of mitochondrial function in mobility may become manifest primarily during the performance of more challenging tasks, reflecting a decline in bioenergetic reserve (32). Indeed, with the exception of usual gait speed over 6 m, the least demanding walking task, the independent associations between gait speed and k PCr were robust.
The present work represents the largest study to date in which 31P-MRS outcome measures, aerobic capacity, and several tests of walking speed were assessed in well-characterized individuals covering a broad age range. The use of several walking tests representing different degrees of challenge provides a dynamic range of functional outcomes that permits inferences regarding the role of muscle oxidative capacity and associated mitochondrial function on walking performance in aging.
This study has certain limitations. First, the BLSA cohort comprises relatively healthy and educated volunteers and may not accurately represent the general population of a similar age range. However, although inclusion of less functionally robust individuals may have broadened our findings, these individuals would have been excluded from several of the core measures used, especially assessment of peak VO2. Further, our analysis did not consider thigh muscle mass as one of the possible mediators (or confounders) of the relationship between muscle bioenergetics and walking speed. An MRI-based measure of muscle mass was introduced later in the BLSA and was not available for most of the participants considered in this study. The effect of muscle mass will be incorporated into future analyses as more measurements are obtained. In addition, in our analysis, we did not adjust for chronic diseases that may independently contribute, in a particular individual, to the causal pathway from reduced aerobic capacity to mobility loss with aging. Indeed, it would be of significant interest to explore the role of specific chronic diseases, such as diabetes, on muscle oxidative capacity and mitochondrial function. In addition, factors aside from muscle bioenergetics may impact overall muscle quality and performance; muscle performance is only one of several elements affecting walking. However, in spite of this complexity, we have demonstrated that muscle oxidative capacity emerges as a strong predictor of walking speed; our findings suggest that age-related decline in oxidative phosphorylation has observable functional consequences. Finally, because this study was cross-sectional, the possibility of reverse causality, namely low mobility leading to reduced muscle oxidative capacity, cannot be ruled out. Establishing underlying causal relations would require additional performance of longitudinal studies testing the hypothesis that poor muscle oxidative capacity predicts future loss of mobility with aging.
Overall, our results indicate that, in a large, well-characterized study population, intrinsic muscle bioenergetics is an important determinant of mobility and may exert its impact early in the pathway leading to mobility loss. Interventions that potentially improve muscle mitochondrial function are currently available. Testing whether these interventions may improve mitochondrial function in older persons and, in turn, prevent mobility loss may represent an important further line of research in an effort to maintain functional status in the aging population.
Funding
This work was funded by the Intramural Research Program of the National Institute on Aging, National Institute of Health, Baltimore, MD.
References
- 1. Halter J, Ouslander J, Tinetti M, Studenski S, High K, Asthana S. Hazzard’s Geriatric Medicine and Gerontology. 6th ed New York: McGraw-Hill Education; 2009. [Google Scholar]
- 2. Schrack JA, Simonsick EM, Chaves PH, Ferrucci L. The role of energetic cost in the age-related slowing of gait speed. J Am Geriatr Soc. 2012;60:1811–1816. doi:10.1111/j.1532-5415.2012.04153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi:10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. [DOI] [PubMed] [Google Scholar]
- 5. Fiser WM, Hays NP, Rogers SC, et al. Energetics of walking in elderly people: factors related to gait speed. J Gerontol A Biol Sci Med Sci. 2010;65:1332–1337. doi:10.1093/gerona/glq137 [DOI] [PubMed] [Google Scholar]
- 6. Reinders I, Murphy RA, Koster A, et al. Muscle quality and muscle fat infiltration in relation to incident mobility disability and gait speed decline: the Age, Gene/Environment Susceptibility-Reykjavik Study. J Gerontol A Biol Sci Med Sci. 2015;70:1030–1036. doi:10.1093/gerona/glv016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hayashida I, Tanimoto Y, Takahashi Y, Kusabiraki T, Tamaki J. Correlation between muscle strength and muscle mass, and their association with walking speed, in community-dwelling elderly Japanese individuals. PLoS One. 2014;9:e111810. doi:10.1371/journal.pone.0111810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moore AZ, Caturegli G, Metter EJ, et al. Difference in muscle quality over the adult life span and biological correlates in the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 2014;62:230–236. doi:10.1111/jgs.12653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stenholm S, Shardell M, Bandinelli S, Guralnik JM, Ferrucci L. Physiological factors contributing to mobility loss over 9 years of follow-up-results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2015;70:591–597. doi:10.1093/gerona/glv004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzalez-Freire M, de Cabo R, Bernier M, et al. Reconsidering the role of mitochondria in aging. J Gerontol A Biol Sci Med Sci. 2015;70:1334–1342. doi:10.1093/gerona/glv070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bohannon RW. Comfortable and maximum walking speed of adults aged 20–79 years: reference values and determinants. Age Ageing. 1997;26:15–19. [DOI] [PubMed] [Google Scholar]
- 12. Ko SU, Hausdorff JM, Ferrucci L. Age-associated differences in the gait pattern changes of older adults during fast-speed and fatigue conditions: results from the Baltimore longitudinal study of ageing. Age Ageing. 2010;39:688–694. doi:10.1093/ageing/afq113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cunningham DA, Rechnitzer PA, Pearce ME, Donner AP. Determinants of self-selected walking pace across ages 19 to 66. J Gerontol. 1982;37:560–564. [DOI] [PubMed] [Google Scholar]
- 14. Fleischman A, Makimura H, Stanley TL, et al. Skeletal muscle phosphocreatine recovery after submaximal exercise in children and young and middle-aged adults. J Clin Endocrinol Metab. 2010;95:E69–E74. doi:10.1210/jc.2010-0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coen PM, Jubrias SA, Distefano G, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68:447–455. doi:10.1093/gerona/gls196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102:5618–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edwards LM, Kemp GJ, Dwyer RM, et al. Integrating muscle cell biochemistry and whole-body physiology in humans:(31)P-MRS data from the InSight trial. Sci Rep. 2013;3:1182. doi:10.1038/srep01182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edwards LM, Tyler DJ, Kemp GJ, et al. The reproducibility of 31-phosphorus MRS measures of muscle energetics at 3 Tesla in trained men. PloS One. 2012;7:e37237 doi:10.1371/journal.pone.0037237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Broskey NT, Greggio C, Boss A, et al. Skeletal muscle mitochondria in the elderly: effects of physical fitness and exercise training. J Clin Endocrinol Metab. 2014;99:1852–1861. doi:10.1210/jc.2013-3983 [DOI] [PubMed] [Google Scholar]
- 21. Amara CE, Marcinek DJ, Shankland EG, Schenkman KA, Arakaki LS, Conley KE. Mitochondrial function in vivo: spectroscopy provides window on cellular energetics. Methods. 2008;46:312–318. doi:10.1016/j.ymeth.2008.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49:1544–1548. [DOI] [PubMed] [Google Scholar]
- 23. Simonsick EM, Fan E, Fleg JL. Estimating cardiorespiratory fitness in well-functioning older adults: treadmill validation of the long distance corridor walk. J Am Geriatr Soc. 2006;54:127–132. [DOI] [PubMed] [Google Scholar]
- 24. Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001;31:269–286. [DOI] [PubMed] [Google Scholar]
- 25. Naressi A, Couturier C, Devos JM, et al. Java-based graphical user interface for the MRUI quantitation package. Magma. 2001;12:141–152. [DOI] [PubMed] [Google Scholar]
- 26. Vanhamme L, Van Huffel S, Van Hecke P, van Ormondt D. Time-domain quantification of series of biomedical magnetic resonance spectroscopy signals. J Magn Reson. 1999;140:120–130. [DOI] [PubMed] [Google Scholar]
- 27. McCully KK, Fielding RA, Evans WJ, Leigh JS, Jr, Posner JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol. 1993;75:813–819. [DOI] [PubMed] [Google Scholar]
- 28. Smith SA, Montain SJ, Zientara GP, Fielding RA. Use of phosphocreatine kinetics to determine the influence of creatine on muscle mitochondrial respiration: an in vivo 31P-MRS study of oral creatine ingestion. J Appl Physiol. 2004;96:2288–2292. [DOI] [PubMed] [Google Scholar]
- 29. Walsh B, Tonkonogi M, Soderlund K, Hultman E, Saks V, Sahlin K. The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J Physiol. 2001;537:971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boska M. ATP production rates as a function of force level in the human gastrocnemius/soleus using 31P MRS. Magn Reson Med. 1994;32:1–10. [DOI] [PubMed] [Google Scholar]
- 31. Fleg JL, Morrell CH, Bos AG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. [DOI] [PubMed] [Google Scholar]
- 32. Schrack JA, Simonsick EM, Ferrucci L. The relationship of the energetic cost of slow walking and peak energy expenditure to gait speed in mid-to-late life. Am J Phys Med Rehabil. 2013;92:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]