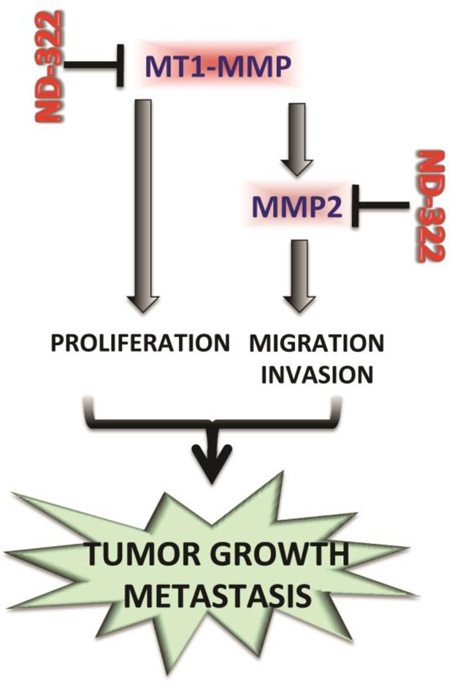
Abstract
MT1-MMP and MMP2 have been implicated as pro-tumorigenic and pro-metastatic factors in a wide variety of cancers including melanoma. We have previously demonstrated that MT1-MMP is highly expressed in melanoma where it promotes melanoma cell invasion and metastasis in part through the activation of its target MMP2. Given the accessibility of MMPs, as they are either secreted (e.g. MMP2) or membrane-tethered (e.g. MT1-MMP), they represent ideal targets for specific inhibition via small molecules. Here we show that the novel small-molecule inhibitor ND-322 with high selectivity for MT1- MMP and MMP2, effectively inhibits MT1-MMP and MMP2 activity resulting in reduced in vitro melanoma cell growth, migration and invasion. Importantly, these inhibitory effects lead to significant reduction of melanoma tumor growth and metastasis. We further show that while cell migration and invasion could be similarly hampered by specific inhibition of either MT1-MMP or MMP2 via shRNAs, the growth inhibitory activity of ND-322 could only be mirrored by specific inhibition of MT1-MMP. These data support ND-322 as a novel effective inhibitor capable of counteracting both MT1-MMP and MMP2, two key proteases involved in melanoma growth and metastasis. ND-322 may therefore represent a new inhibitor in the repertoire of treatments against melanoma.
Keywords: melanoma; growth; invasion; migration; metastasis; MT1-MMP, MMP2, ND-322
Graphical Abstract
Introduction
Metastatic melanoma is the leading cause of death among skin cancer patients, with a five-year survival rate of only 15%[1]. The ability to degrade and pass through the extracellular matrix (ECM) is a critical step in cancer cell invasion and metastasis. A group of enzymes known as matrix metalloproteinases (MMPs) are the primary agents responsible for ECM degradation and their aberrant expression has been linked to the progression of many cancers including melanoma[2, 3]. Indeed the abnormal expression of many MMPs has been linked to poor prognosis and an increase in metastatic incidence [4, 5]. However, not all MMPs have detrimental roles [6–8]. For example, MMP8, MMP3, and MMP12 have been shown to possess antitumor effects in knockout mouse models of cancer and are therefore considered drug antitargets, i.e. molecules that exert normal roles in cells and tissues where they perform physiological functions [8, 9].
The inhibition of the “good” MMPs and the severe side effects such as musculoskeletal pain and inflammation that were observed in clinical trials, are among the problems encountered with broadspectrum MMP inhibitors that led to their failure a decade ago [10, 11]. Hence, the use of more selective MMP inhibitors capable of only inhibiting the target molecule and sparing the antitargets, and therefore reduce or even eliminate side effects, may be highly beneficial.
Evidence from our laboratory has highlighted a key role of MT1-MMP in melanoma growth and progression. MT1-MMP, a membrane bound MMP, is often over expressed in many types of cancer. We have demonstrated that MT1-MMP increases as melanoma progress toward a more invasive, metastatic phenotype and that its expression inversely correlates with melanoma patient survival[12]. MT1-MMP contributes to melanoma through a variety of mechanisms: i) by promoting invasion and migration through activation of MMP2 and RAC1[12]; ii) by inhibiting the tumor suppressor protein SPRY4[13]; and by promoting melanoma cell growth through the cleavage and activation of Notch1[14]. Given the multiplicity of functions that MT1-MMP modulates in melanoma and the fact that it is located on the plasma-membrane, it may be an ideal target for therapeutic intervention.
ND-322 is a member of the thiirane class of selective MMP inhibitors, which are potent mechanism-based inhibitors of gelatinases (MMP2 and MMP9) and MT1-MMP, inhibiting these proteinases with inhibition constants (Ki) of 24 ±15 nM, 870 ± 110 nM, and 210 ± 20 nM, respectively[15]. The glutamate in the active site of these proteinases carries out a reaction that opens up the thiirane ring to the corresponding thiolate, resulting in picomolar tight-binding inhibition[16] and in long residence times (the time the inhibitor is bound to the target proteinases). For instance, the residence time of ND-322 bound to MMP2 is 23.4 min, which is substantially longer than MMP2 bound to tissue inhibitor of matrix metalloproteinase (TIMP)1 of 6.9 min and MMP2 bound to TIMP2 of 6.7 min[17]. This mechanism of inhibition is at the root of the selectivity of the thiirane inhibitors towards the gelatinases and MT1- MMP; ND-322 does not inhibit or poorly inhibits other MMPs [15]. Here we show that ND-322, used at concentrations that do not affect MMP9, inhibits growth, migration and invasion of melanoma cells. Importantly, ND-322 inhibits tumor growth and delays metastasis to lungs in a melanoma orthotopic mouse model. Interestingly, inhibition of MMP2 by specific shRNAs mirrors the anti-migratory and invasive capacity of ND-322 in melanoma cells. However, only inhibition of MT1-MMP can both impinge upon migration/invasion and growth of melanoma cells. These data support ND-322 as a selective inhibitor capable of counteracting melanoma growth and metastasis by the simultaneous inhibition of MMP2 and MT1-MMP.
Materials and Methods
Syntheses of ND-322
ND-322 was synthesized as described previously[15]. ND-322 was dissolved in DMSO at a concentration of 10µM and maintained in aliquots at −80°C. ND-322 was used in vitro at 0.16 and 0.32µM final concentration. For in vivo studies, the stock of ND-322 was dissolved in sterile PBS at a final concentration of 7.5 mg/ml. The dosing solutions were sterilized by filtration through a 0.2μm Acrodisc syringe filter (Pall Life Sciences, Ann Arbor, MI, USA). A 100µl aliquot of this solution was administered subcutaneously once a day to mice, which corresponded to a dose of 25mg/Kg.
MT1-MMP activity assay
Membrane proteins were extracted from 106 WM266-4 cells by three cycles of freeze-thaw in dry ice/ethanol/37°C baths. Cells were previously treated O/N by either ND-322 (0.32µM), 1 μg/ml neutralizing antibody (Millipore, LEM-2/15.8[18]), 0.5 μg/ml recombinant MT1-MMP (Anaspec, Fremont, CA, USA), or were expressing a specific shMT1-MMP (Sigma, San Louis, MO). Lysates were sonicated for 3s, and the membranes were pelleted by centrifugation (30 min, 13 000 g4°C) and resuspended in PBS. Equal protein amounts per sample were incubated with a fluorogenic MT1-MMP substrate (Mca-PLGL-Dap(Dnp)-AR-NH2) provided by the manufacturer (Anaspec, Fremont, CA, USA). Fluorescence intensity was measured at Ex/Em = 490 + 20 nm/520 + 20 nm using a SpectraMax M2 Elisa reader (Molecular Devices, Sunnyvale, CA, USA).
Gelatin Zymography
Conditioned serum-free culture supernatants from WM266-4 cells melanoma cells treated overnight with 0.32µM ND-322 or from cells expressing shMT1-MMP or shMMP2, were collected and concentrated using a microtube device with a cutoff of 30K (Pall Life Science, Ann Arbor, MI). Protein (10 μg) was resolved on an 8% (w/v) standard SDS-polyacrylamide gel containing 2 mg/ml gelatin as a substrate. Gels were washed once in 2.5% (v/v) Triton X-100 to remove SDS and then washed in 50 mM Tris-HCl, 5 mM CaCl2and 0.1% Triton X-100 (pH 7.8) and incubated overnight at 37°C in the same buffer with gentle agitation. Zymograms were stained for 45 min with 0.25% (w/v) Coomassie Brilliant Blue R250 dissolved in 40% methanol and 10% glacial acetic acid and de-stained in the same solution without Coomassie Blue.
shRNAs and cell lines
shRNAs against human MT1-MMP (TRCN0000050855) and MMP2 (TRCN0000051526) were purchased from Sigma and were previously described[12, 13]. The primary melanoma cells line WM115, the syngeneic metastatic WM266–4, and the metastatic melanoma cell lines V2387 and K457 were originally purchased from ATCC or a gift or Dr. Marianne Broome Powell (Stanford University, Stanford, CA). Cells were maintained in DMEM supplemented with 10% FCS, 1% glutamine, and 1% penicillin-streptomycin.
Western blotting
Cell seeding, collection of protein and Western blot methods were as previously described[12, 13]. Membranes were probed with the following antibodies: anti-MT1-MMP (Millipore, LEM-2/15.8); anti–β-actin (Santa Cruz Biotechnology, CA).
Real-time PCR
cDNA was synthesized from total RNA using SuperScript first-strand synthesis system for RT-PCR (Invitrogen, Carlsbad, CA), then used for PCR amplification with SYBR Green PCR master mix (Roche, Florence, SC). The following primer sets were used to amplify specific target genes: human GAPDH forward: 5′-CGCTCTCTGCTCCTCCTGTT-3′; reverse: 5′-CCATGGTGTCTGAGCGATGT-3′; human MT1-MMP forward: 5′-CTCCCTCGGCTCGGCCCAAA-3′; reverse: 5′-CGCCTCATGGCCTTCATGGTGTCT-3′; human MMP2: forward: 5′-TGATCTTGACCAGAATACCATTGA-3′; reverse: 5′-GGCTTGCGAGGGAAGAAGTT-3′. Relative quantification of mRNA expression levels (fold change) was normalized by GAPDH.
Cell Proliferation Assays
Cells (initial seeding density 16,000/cm2) were plated in 96-well plates in triplicate. At each time point cells were fixed with 10% buffered formalin and subjected to the crystal violet assay as described previously[19].
Migration and invasion assays
WM266-4 cells were plated in a confluent monolayer in duplicate and treated with 0.16 and 0.32µM ND-322. A scratch was produced using a pipette tip, and the detached cells were gently washed away with PBS. Plates were incubated under a time-lapse microscope for 24 h. Pictures were taken every hour for the duration of the experiment. Frames were aligned, and the distance from one of the edges of the wound in the first frame (considered time zero) to the migration front was calculated for the time points indicated in Figures. For the transwell invasion assay, a suspension of 25 × 104 cells was added to an 8- μm-pore-size insert, either uncoated (control) or coated with a Matrigel growth factor reduced basement membrane matrix (BD Biosciences, San Jose, CA). The cell suspension was treated with 0.16 or 0.,32µM ND-322. DMEM containing 5% fetal bovine serum (FBS) was added to the lower chamber (24 – well plate) as a chemoattractant. After a 24-h incubation, cells that had migrated through the control inserts or Matrigel were collected on the bottom membrane, fixed with 4% formaldehyde, and stained with Coomassie blue. Each treatment was carried out in triplicates. Membranes were incubated in 10% acetic acid to extract the Coomassie stain, and the color intensity was quantified at 295 nm. The percentage of invasion was calculated as (mean reads of Matrigel)/(mean reads of control insert) × 100.
RAC1 activity assay
Cells at 70% confluency were serum starved in DMEM containing 0.5% serum for 24 h followed by a further 24 h-starvation in serum-free media. Cell lysis was carried out in the lysis buffer provided by the manufacturer in the G-Lisa Rac activation assay kit (Cytoskeleton, Denver, CO). Equal amounts of protein lysates were added to the Rac1-GTP-binding wells and RAC1 activity measured as per manufacturer's instructions at an OD of 490 nm.
In vivo tumor growth
Male NOD/SCID mice (5 – 8 weeks old) were supplied by the Athymic Animal Facility at Case Western Reserve University. All experimental protocols were approved by the Administrative Panel on Laboratory Animal Care of Case Western Reserve University. Cells (2 × 106) were injected intradermally in the dorsal flanks of mice for a total of nine and eighteen tumors per control and treated groups, respectively. Mice were subjected to survival surgery to remove the primary tumor 42 days after cell inoculation. Tumors were measured, and tumor volume was calculated as [(w2 × l) × 0.52], in which w and l represent width and length, respectively[20]. After surgery, mice were observed twice a week and were euthanized when signs of morbidity were observed. At the end-time point (30 weeks post-surgery), lungs and thymi were collected and stained with S100.
Results
ND-322 is a small molecular inhibitor of MMP2 and MT1-MMP
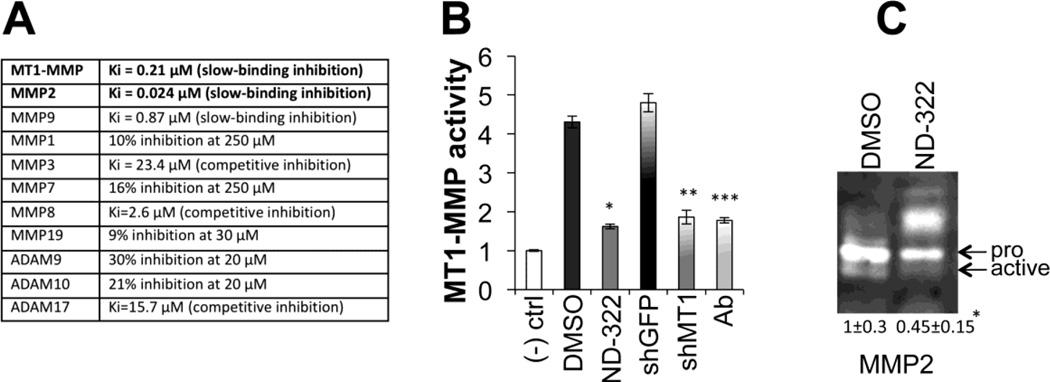
ND-322 is an effective and selective inhibitor of gelatinases (MMP2 and MMP9) and of the membrane tethered matric metalloproteinase MT1-MMP, as shown in figure 1A by its in vitro Ki values. To test the effectiveness of ND-322 in melanoma cells, we first determined whether the inhibitor could indeed affect MT1-MMP and MMP2 activity. An MT1-MMP activity assay was employed[12] and cells treated with ND-322 overnight were compared to cells expressing a specific shMT1-MMP and cells treated with an MT1-MMP neutralizing antibody (Fig. 1B). ND-322 proved as effective as antibody neutralization and knockdown at a concentration (0.32 µM) that is 2.7-fold below the Ki for MMP9. Additionally, ND-322 also inhibited MMP2 activation as assessed by gelatin zymography (Fig. 1C). Together, these data suggest ND-322 can effectively inhibit MT1-MMP and MMP2, two major proteases involved in melanoma pathogenesis.
Figure 1. ND-322 inhibits MT1-MMP and MMP2 activity.
A Ki values of ND-322 for a variety of MMPs. B MT1-MMP activity. Cells (WM266-4) were either treated for three days with DMSO (vehicle), ND322 (0.32µM) or a neutralizing MT1-MMP moAb (1 µg/ml); or were expressing shGFP (control) and shMt1- MMP. Data are the average between three independent experiments. *,**,***p< 0.001, Student’s t test. C Gelatin zymography of serum-free condition media of WM266-4 treated with DMSO or ND-322 (0.32 µM) for three days. Inactive and active MMP2 are indicated. Numbers represent the relative intensity of the ratio between active and inactive MMP2 and are the average ± STDEV of four independent assays. The decrease in active MMP2 between DMSO and ND-322 is statistically significant (*p<0.01, Student’s t test).
ND-322 inhibits melanoma cell growth, migration, and invasion
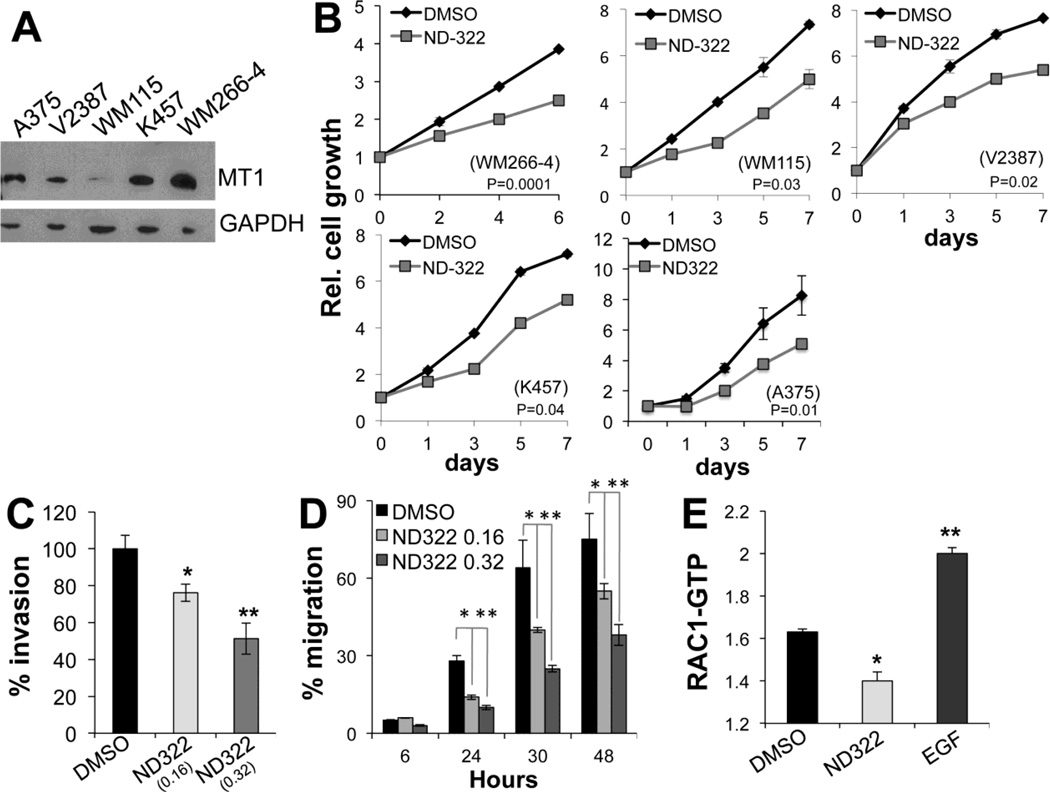
We have previously shown that melanoma cell growth is in part controlled by MT1-MMP[14], whereas cell migration and invasion is a function of an MT1-MMP/MMP2 dependent pathway[12, 13]. Given that ND-322 behaves as a suitable MT1-MMP and MMP2 dual inhibitor, we sought to determine whether ND-322 could hinder these cell functions. Indeed, ND-322 inhibited cell growth of several melanoma cell lines (Fig. 2A), again at a dose (0.32 µM) that is 2.7-fold below the Ki for MMP9. Moreover, it inhibited significantly the motility and invasion capacity of WM266-4 melanoma cells in a dose dependent manner (Fig. 2B, C). Interestingly, ND-322 was able to reduce the activity of the small GTPase RAC1 (Fig. 2D), which we have previously shown to play a critical role in modulating melanoma cell motility downstream of MT1-MMP/MMP2[12].
Figure 2. ND-322 inhibits cell growth, migration and invasion.
A Relative cell growth of four melanoma cell lines treated with 0.32 µM ND-322 for the indicated times. Differences in growth are significant at all time points with the exception of day 1 of A375. p values in each figure are relative to the last time point (Student’s t test). B Invasion through matrigel gels of WM266-4 cells treated for 24 hours with ND-322 or DMSO. C Migration of cells treated with two doses of ND-322 for the indicated times. 24 hours: DMSO vs 0.16µM ND-322; p< 0.01; DMSO vs 0.32µM ND-322: p<0.0001; 30 hours: DMSO vs 0.16µM ND-322; p< 0.001; DMSO vs 0.32µM ND-322: p<0.0001; 48 hours: DMSO vs 0.16µM ND-322; p< 0.0001; DMSO vs 0.32µM ND-322: p<0.0001 (Student’s t test). D RAC1 activity of WM266-4 cells treated for 24 hours with ND-322 (0.32 µM). *p=0.004. EGF (10 ng/ml for 3 min) was used as positive control.
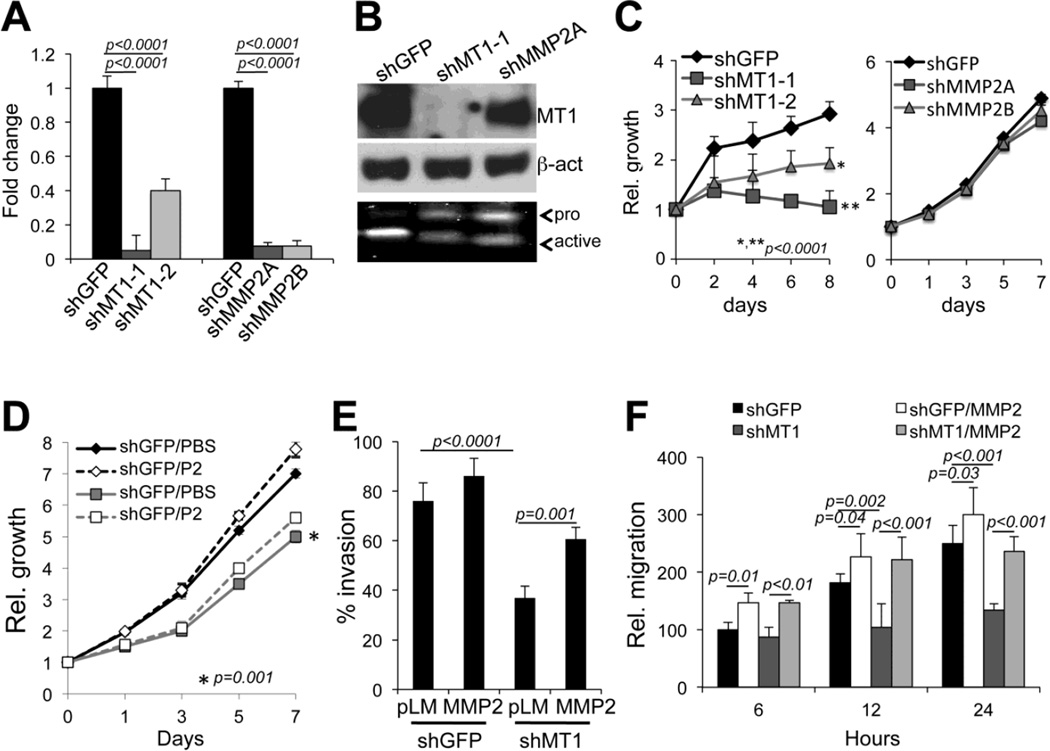
To further dissect the role of MT1-MMP and MMP2 in these biological functions, cells were stably transduced with specific shRNAs against MT1-MMP or MMP2[12] (Fig. 3A, B). Effective knock down was confirmed by diminished mRNA for each factor (Fig. 3A), and by diminished expression of MT1-MMP protein (Fig. 3B, upper panel). Additionally, both MT1-MMP and MMP2 down regulation inhibited MMP2 activation as assessed by gelatin zymography (Fig. 3B, lower panel). This is expected, as MMP2 activation is dependent on MT1-MMP[21]. We have previously shown that MT1-MMP inhibits both invasion and migration via MMP2[12]. We further confirmed these previous data in two different cell lines (WM266-4 and A375) by showing that indeed inhibition of MT1-MMP and MMP2 similarly inhibit both invasion and migration (Suppl. Figure 2). Interestingly, however, only MT1-MMP knock down was able to reduce cell growth, while MMP2 inhibition did not (Fig. 3C and suppl. Fig. 2A). Furthermore, while addition of recombinant active MMP2 to cells expressing shMT1-MMP rescued cell invasion and motility (Fig. 3E, F), it did not rescue cell growth (Fig. 3D). Finally, to further differentiate between MT1- MMP and MMP2 in modulating distinctive cell functions, an MMP2 inhibitor that does not affect MT1- MMP activity was used[22] (Suppl. Fig. 1). ND-378 similarly to shMMP2, inhibited cell migration and invasion but did not affect growth.
Figure 3. MT1-MMP and MMP2 differentially regulate cell growth, migration and invasion.
A qRT-PCR of MT1-MMP and MMP2 mRNA in WM266-4 cells expressing shGFP, shMT1-MMP (shMT1-1 and shMT1- 2) or shMMP2 (shMMP2A, shMMP2B. B Expression of MT1-MMP and activity of MMP2 (zymogram, bottom panel) of the cells in A (only cells expressing shMT1-1 and shMMP2A are shown). β-actin was used as loading control. C Relative cell growth of WM266-4 cells expressing either shRNAs against MT1- MMP (left panel) or against MMP2 (right panel) versus control cells (shGFP). D Relative cell growth of WM266-4 cells expressing shGFP or shMT1-1 and treated with recombinant MMP2 (P2 - 25ng/ml). *p<0.001, Student’s t test. E Invasion through matrigel gels of WM266-4 cells expressing shGFP or shMT1-1 and treated with recombinant MMP2. F Migration over 24 hours of the cells in D. MMP2 indicates recombinant MMP2 added at 25ng/ml.
Together, these data indicate that ND-322, by simultaneously inhibiting both MT1-MMP and MMP2 impinges upon cell growth, migration and invasion, all key pro-tumorigenic features of melanoma cells.
ND322 inhibits tumor growth and metastasis
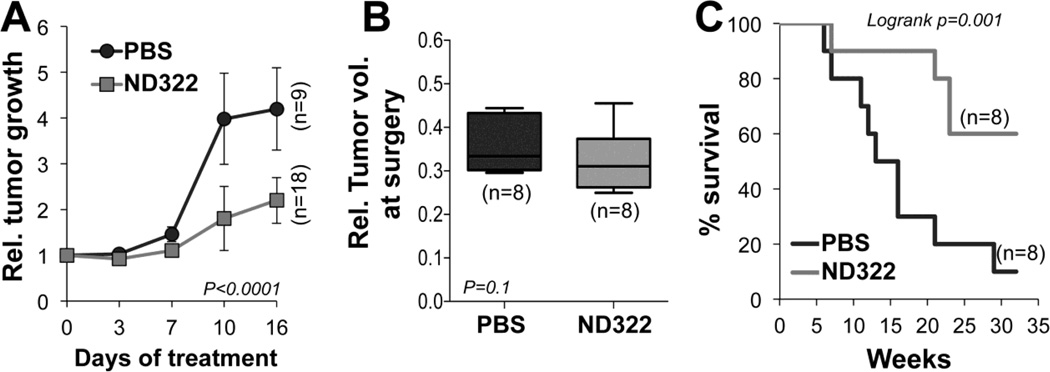
To more conclusively confirm the anti-tumor activity of ND-322, WM266-4 cells stably transduced with a luciferase construct were implanted intradermally into NOD/SCID mice. This cell line was chosen because of its ability to form metastasis in an orthotopic mouse melanoma model, as we have previously shown[12], and because it expresses elevated levels of MT1-MMP/MMP2[12]. Tumors were allowed to grow to comparable sizes (120mm3 on average) before dividing the animals into two groups: a control (PBS) group and a treatment (25 mg/Kg ND-322/day) group. Daily treatment with ND-322 reduced tumor growth significantly compared to controls as shown in Fig. 4A. The treatment was well tolerated and mice maintained their weight over time (not shown). At the end-time point, the treatment was stopped and mice bearing tumors of similar sizes (Fig. 4B) were subjected to survival surgery to remove the primary growth. Mice were then examined twice weekly for the presence of bioluminescence in the lungs, as done previously[12]. We found that treatment with ND-322 significantly reduced the number of mice showing lung metastasis within 30 weeks post-surgery, time at which 90% of control animals presented with tumor cells in the lungs versus 40% of the treated group (Fig. 4C).
Figure 4. ND-322 inhibits melanoma tumor growth and delays metastases to lungs.
A) WM266-4 cells (2x106) were implanted intradermally in SCID mice. Treatment with ND-322 (25mg/Kg/day) started when tumors in each animal reached 120mm3 on average (time 0). The averaged volumes of control and treated tumors at time 0 were arbitrarily set at 1. Tumor growth rates were calculated by normalizing mean tumor volumes at each time point to the mean tumor volume at time 0 for each treatment group. B) Relative volumes of tumors excised from mice at time of surgery. Only mice with similar tumor sizes were utilized to assay metastatic dissemination. C) % Survival of the mice in B. Mice were sacrificed when lung metastasis were observed by bioluminescence.
These data show that inhibition of MT1-MMP/MMP2 effectively inhibits melanoma tumor growth and metastatic dissemination.
Discussion
In previous studies we demonstrated that MT1-MMP correlates negatively with the survival of melanoma patients and that its expression increases in metastatic melanoma[12]. We also showed that MT1-MMP is required for melanoma metastasis[12]. MT1-MMP therefore acts as an oncogene in melanoma and allows for tumor cells to escape to other organs in part by promoting invasion and migration via an MT1-MMP/MMP2 signaling pathway[12, 13]. Hence, blockade of MT1-MMP/MMP2 may represent an effective means to prevent/inhibit metastatic melanoma. Here we show that a specific MT1-MMP/MMP2 inhibitor delays melanoma tumor growth and reduces metastasis to lungs in an orthotopic mouse melanoma model. This results somewhat differ from previous work in which we demonstrated that inhibition of MT1-MMP in tumor cells, while it did not alter their growth in vivo, it did inhibit metastasis[12].It is possible that systemic delivery of ND322 affects melanoma growth because it not only inhibits MT1-MMP/MMP2 in the tumor but might be affecting these proteases also in the stroma and in the vascular compartment, leading to a more global anti-tumor effects[23–25].
ND-322 is a novel class of selective gelatinase (MMP2 and MMP9) and MT1-MMP inhibitors capable of targeting only a few MMPs, with poor inhibition towards several other MMPs, including those regarded as anti-targets. Anti-targets MMPs, such as MMP3, MMP8 and MMP12 are molecules that exert normal roles in cells and tissues where they perform positive functions. Some of them, such as MMP8 have also been shown to possess anti-cancer properties [8, 9].
ND-322, at concentrations that are 2.7-fold below the Ki for MMP9, was able to inhibit numerous melanoma cell functions including growth, migration and invasion. Interestingly, migration and invasion appeared to be dependent on MMP2, as inhibition of this protease by a specific shRNA mirrored the inhibitory effects observed with ND-322. Furthermore, addition of recombinant active MMP2 was sufficient to rescue migration and invasion in cells in which MT1-MMP was knock down. These data further confirmed previous findings in which we showed that MT1-MMP promotes melanoma metastasis through the activation of MMP2, which in turn stimulates cell migration by activating RAC1[12]. Of note, ND-322 was able to hamper RAC1 activity, suggesting that part of its inhibitory function is through the inhibition of an MMP2/RAC1 axis.
Interestingly, however, cell growth was mostly a function of MT1-MMP. Only cells in which MT1-MMP was knock down by a specific shRNA showed slower growth. On the other hand, no effect was observed in cells expressing a specific shMMP2. Additionally, treatment with recombinant active MMP2 did not rescue cell growth that was inhibited by the knock down of MT1-MMP. Similarly, a different small molecule inhibitor (ND-378), also in the thiirane class of selective MMP inhibitors, with high selectivity for MMP2 but virtually inactive on MT1-MMP, affected cell motility and invasion but did not inhibit cell growth.
SB-3CT, an analog of ND-322, was previously shown to effectively prevent bone metastasis in a xenograft model of prostate cancer[26]. SB-3CT and ND-322 have also been shown to cross the blood/brain barrier[27, 28], indicating that ND-322 could potentially be used for the treatment of melanoma brain metastasis, which affects up to 40% of metastatic melanoma patients[29]. Finally, in our study ND-322 was well tolerated by mice, similarly to what was previously shown in a model of wound healing[30] This aspect is important as earlier trials with broad-spectrum MMP inhibitors failed mostly due to severe side effects such as musculoskeletal pain and inflammation, accompanied by negligible anti-cancer effects [10, 11].
In conclusion, given the key roles played by MT1-MMP and MMP2 in melanoma, specific targeting of these proteases with ND-322, likely in combination with other established therapies such as anti BRAF/MEK drugs, might represent an intriguing new avenue in treating aggressive melanoma.
Supplementary Material
Acknowledgments
This work was supported by: NIH-NCI grant R21CA187695.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest associated with the research described in this manuscript
Bibliography
- 1.Howlader N, N A, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2008, National Cancer Institute. 2011. [Google Scholar]
- 2.Bartolome RA, et al. The chemokine receptor CXCR4 and the metalloproteinase MT1-MMP are mutually required during melanoma metastasis to lungs. Am J Pathol. 2009;174(2):602–612. doi: 10.2353/ajpath.2009.080636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moro N, Mauch C, Zigrino P. Metalloproteinases in melanoma. Eur J Cell Biol. 2014;93(1–2):23–29. doi: 10.1016/j.ejcb.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Ren F, et al. Overexpression of MMP Family Members Functions as Prognostic Biomarker for Breast Cancer Patients: A Systematic Review and Meta-Analysis. PLoS One. 2015;10(8):e0135544. doi: 10.1371/journal.pone.0135544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadler-Olsen E, Winberg JO, Uhlin-Hansen L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumour Biol. 2013;34(4):2041–2051. doi: 10.1007/s13277-013-0842-8. [DOI] [PubMed] [Google Scholar]
- 6.Zucker S, Cao J. Selective matrix metalloproteinase (MMP) inhibitors in cancer therapy: ready for prime time? Cancer Biol Ther. 2009;8(24):2371–2373. doi: 10.4161/cbt.8.24.10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hua H, et al. Matrix metalloproteinases in tumorigenesis: an evolving paradigm. Cell Mol Life Sci. 2011;68(23):3853–3868. doi: 10.1007/s00018-011-0763-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dufour A, Overall CM. Missing the target: matrix metalloproteinase antitargets in inflammation and cancer. Trends Pharmacol Sci. 2013;34(4):233–242. doi: 10.1016/j.tips.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Morrison CJ, et al. Matrix metalloproteinase proteomics: substrates, targets, and therapy. Curr Opin Cell Biol. 2009;21(5):645–653. doi: 10.1016/j.ceb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295(5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 11.Pavlaki M, Zucker S. Matrix metalloproteinase inhibitors (MMPIs): the beginning of phase I or the termination of phase III clinical trials. Cancer Metastasis Rev. 2003;22(2–3):177–203. doi: 10.1023/a:1023047431869. [DOI] [PubMed] [Google Scholar]
- 12.Shaverdashvili K, et al. MT1-MMP modulates melanoma cell dissemination and metastasis through activation of MMP2 and RAC1. Pigment Cell Melanoma Res. 2014;27(2):287–296. doi: 10.1111/pcmr.12201. [DOI] [PubMed] [Google Scholar]
- 13.Shaverdashvili K, et al. MT1-MMP dependent repression of the tumor suppressor SPRY4 contributes to MT1-MMP driven melanoma cell motility. Oncotarget. 2015;6(32):33512–33522. doi: 10.18632/oncotarget.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma J, et al. Noncanonical Activation of Notch1 Protein by Membrane Type 1 Matrix Metalloproteinase (MT1-MMP) Controls Melanoma Cell Proliferation. J Biol Chem. 2014;289(12):8442–8449. doi: 10.1074/jbc.M113.516039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gooyit M, et al. Selective water-soluble gelatinase inhibitor prodrugs. J Med Chem. 2011;54(19):6676–6690. doi: 10.1021/jm200566e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forbes C, et al. Active site ring-opening of a thiirane moiety and picomolar inhibition of gelatinases. Chem Biol Drug Des. 2009;74(6):527–534. doi: 10.1111/j.1747-0285.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson MW, et al. Kinetic analysis of the binding of human matrix metalloproteinase-2 and-9 to tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2. J Biol Chem. 1997;272(47):29975–29983. doi: 10.1074/jbc.272.47.29975. [DOI] [PubMed] [Google Scholar]
- 18.Aplin AC, et al. Vascular regression and survival are differentially regulated by MT1-MMP and TIMPs in the aortic ring model of angiogenesis. Am J Physiol Cell Physiol. 2009;297(2):C471–C480. doi: 10.1152/ajpcell.00019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang K, et al. A Notch1-neuregulin1 autocrine signaling loop contributes to melanoma growth. Oncogene. 2012;31(43):4609–4618. doi: 10.1038/onc.2011.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arbiser JL, et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci U S A. 1997;94(3):861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deryugina EI, et al. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res. 2001;263(2):209–223. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- 22.Gao M, et al. Selective Inhibition of MMP-2 Does Not Alter Neurological Recovery after Spinal Cord Injury. ACS Chem Neurosci. 2016 doi: 10.1021/acschemneuro.6b00217. [DOI] [PubMed] [Google Scholar]
- 23.Genis L, et al. MT1-MMP: universal or particular player in angiogenesis? Cancer Metastasis Rev. 2006;25(1):77–86. doi: 10.1007/s10555-006-7891-z. [DOI] [PubMed] [Google Scholar]
- 24.Sounni NE, et al. MT-MMPS as Regulators of Vessel Stability Associated with Angiogenesis. Front Pharmacol. 2011;2:111. doi: 10.3389/fphar.2011.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taniwaki K, et al. Stroma-derived matrix metalloproteinase (MMP)-2 promotes membrane type 1-MMP-dependent tumor growth in mice. Cancer Res. 2007;67(9):4311–4319. doi: 10.1158/0008-5472.CAN-06-4761. [DOI] [PubMed] [Google Scholar]
- 26.Bonfil RD, et al. Inhibition of human prostate cancer growth, osteolysis and angiogenesis in a bone metastasis model by a novel mechanism-based selective gelatinase inhibitor. Int J Cancer. 2006;118(11):2721–2726. doi: 10.1002/ijc.21645. [DOI] [PubMed] [Google Scholar]
- 27.Song W, et al. Water-soluble mmp-9 inhibitor prodrug generates active metabolites that cross the blood-brain barrier. ACS Chem Neurosci. 2013;4(8):1168–1173. doi: 10.1021/cn400077d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gooyit M, et al. Selective gelatinase inhibitor neuroprotective agents cross the blood-brain barrier. ACS Chem Neurosci. 2012;3(10):730–736. doi: 10.1021/cn300062w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tazi K, et al. Survival of melanoma patients with brain metastases treated with ipilimumab and stereotactic radiosurgery. Cancer Med. 2015;4(1):1–6. doi: 10.1002/cam4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao M, et al. Acceleration of diabetic wound healing using a novel protease-anti-protease combination therapy. Proc Natl Acad Sci U S A. 2015;112(49):15226–15231. doi: 10.1073/pnas.1517847112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.