Abstract
Osteoarthritis, a degenerative disease of the load-bearing joints, greatly reduces quality of life for millions of Americans and places a tremendous cost on the American healthcare system. Due to limitations of current treatments, tissue engineering of articular cartilage may provide a promising therapeutic option to treat cartilage defects. However, cartilage tissue engineering has yet to recapitulate the functional properties of native tissue. During normal joint loading, cartilage tissue experiences variations in osmolarity and subsequent changes in ionic concentrations. Motivated by these known variations in the cellular microenvironment, this study sought to improve the mechanical properties of neocartilage constructs via the application of hyperosmolarity and transient receptor potential vanilloid 4 (TRPV4) channel activator 4α-Phorbol 12,13-didecanoate (4αPDD). It was shown that 4αPDD elicited significant increases in compressive properties. Importantly, when combined, 4αPDD positively interacted with hyperosmolarity to modulate its effects on tensile stiffness and collagen content. Thus, this study supports 4αPDD-activated channel TRPV4 as a purported mechanosensor and osmosensor that can facilitate the cell and tissue level responses to improve the mechanical properties of engineered cartilage. To our knowledge, this study is the first to systematically evaluate the roles of hyperosmolarity and 4αPDD on the functional (i.e., mechanical and biochemical) properties of self-assembled neotissue. Future work may combine 4αPDD-induced channel activation with other chemical and mechanical stimuli to create robust neocartilages suitable for treatment of articular cartilage defects.
Keywords: neocartilage, cartilage tissue engineering, dynamic loading, self-assembly, scaffold-free
1. Introduction
Osteoarthritis is characterized by degenerative joint changes that greatly reduce quality of life. Unfortunately, articular cartilage’s self-healing is limited. Common techniques to treat cartilage defects may delay the onset of total joint arthroplasty, but therapies restoring long-term cartilage mechanical properties remain lacking. Cartilage tissue engineering, thus, seeks to fill the gap between techniques providing temporary repair and total joint replacement by producing a functional replacement tissue capable of durable repair under the high loads of articulating joints.
The self-assembling process in tissue engineering is a scaffold-free technique that promotes cell-cell recognition to develop a cartilage protein-rich extracellular matrix (ECM) (Ofek et al., 2008). Chemical and mechanical stimuli can be applied to self-assembled neocartilage to enhance their properties (Elder and Athanasiou, 2009; Murphy et al., 2013). Self-assembled neocartilages exhibit compressive and tensile properties on the order of or approaching native immature bovine cartilage (Eleswarapu and Athanasiou, 2013; Makris et al., 2013).
Stimuli for neocartilage engineering are inspired by the physiological conditions of the joint. Dynamic loading causes matrix deformation and interstitial fluid flow, leading to depth-dependent, temporary changes in tissue osmolarity and increases in fixed charge density (FCD) (Oswald et al., 2008). The interaction of these fixed charges with a mobile ionic phase plays a major role in the tissue’s functionality in compression (Chen et al., 2001). As a result of tissue deformation, temporary increases in osmolarity and changes in FCD can induce physiological changes on the extracellular (Villanueva et al., 2010) and cellular level, including alterations in cell volume (Korhonen and Herzog, 2008), intracellular calcium levels (Sánchez and Wilkins, 2004), and gene expression (Palmer et al., 2001). Related to these findings, our group has previously altered intracellular sodium and calcium levels via ion channel modulation to positively affect neocartilage tensile properties (Natoli et al., 2010). Informed by the physiology of normal joint function, recapitulating the in vivo environment of the joint by application of osmotic and ionic agents in neocartilage engineering may enhance the properties of engineered constructs toward those of native tissues.
Motivated by recent work, this study sought to improve neocartilage properties by examining the effect of hyperosmolarity (450mOsm) and 4α-Phorbol 12,13-didecanoate (4αPDD), an agonist of the calcium-permeable, transient receptor potential vanilloid 4 (TRPV4) channel recently identified as a chondrocyte mechanotransducer (O'Conor et al., 2014). Cartilage tissue engineering studies are traditionally performed in an osmotic environment of 350mOsm. However, native articular cartilage’s osmolarity is closer to 450mOsm (Maroudas and Evans, 1972; Oswald et al., 2008). At the tissue level, osmotic pressure is hypothesized to drive fluid out of the tissue, increasing FCD and concentrating growth factors. At the cellular level, osmotic pressure drives fluid out of cells, reducing cell volume and leading to various intracellular effects (Finan and Guilak, 2010). Thus, when applied in neocartilage engineering, osmotic pressure may drive ECM synthesis and, ultimately, improve tissue properties.
As an agonist of the TRPV4 channel, 4αPDD induces extracellular calcium flux into cells. This study builds upon our previous study using 4αPDD to produce constructs with increased tensile stiffness and collagen content (Eleswarapu and Athanasiou, 2013). The present study, thus, seeks to apply this channel activator in combination with hyperosmolarity, in the presence of both standard in vitro culture medium (1.8mM) and physiological (4.0mM) (Maroudas, 1979) levels of calcium, motivated by the hypothesis that these agents would positively interact to elicit ECM synthesis more effectively to improve neocartilage properties than each factor alone. While previous studies have investigated these agents independently primarily for elucidating cellular response mechanisms, to our knowledge, this study is the first to systematically investigate the combination of hyperosmolarity and 4αPDD towards enhancing the functional properties (i.e., mechanical and biochemical) of engineered neocartilage.
2. Materials and Methods
2.1 Media formulations
This study employed a chondrogenic medium with a standard in vitro culture (1.8mM) calcium level (CHG), a hyperosmotic medium (Hyp+CHG), a medium at a physiological (4mM) calcium level (PC), and a hyperosmotic medium at a physiological calcium level (Hyp+PC). CHG consisted of: high glucose Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco); 100nM dexamethasone (Sigma); 0.1mM non-essential amino acids (Gibco); 1% ITS+ (insulin, transferrin, and selenous acid; BD Biosciences); 1% penicillin-streptomycin-fungizone (Lonza BioWhittaker); 50µg/mL ascorbate-2-phosphate (Sigma); 40µg/mL L-proline (Sigma); and 100µg/mL sodium pyruvate (Sigma). Hyperosmotic medium (450mOsm) consisted of CHG supplemented with 100mM sucrose; CHG possesses an osmolarity of 350mOsm. Osmolarity was measured by a VAPRO 5520 vapor pressure osmometer (Wescor). Calcium chloride (CaCl2) was added to CHG (1.8mM Ca2+) to create PC (4mM Ca2+), with no significant change in medium. Supplementation of 4αPDD was at 10µM.
2.2 Cell isolation
Chondrocytes were harvested from the stifle joints of eight juvenile calves (Research 87, MA) via 0.2% collagenase II (Worthington) digestion. Cells were frozen at −80°C until use.
2.3 Chondrocyte self-assembly and osmotic loading
Non-adherent, 5mm-diameter agarose wells were fabricated by inserting custom-made well-makers into molten agarose (2% wt/vol). On day 0, 4.5×106 chondrocytes/100µL CHG were seeded into each well and self-assembled for 4hrs before receiving additional CHG. From day 1, constructs received either CHG or PC. During days 10–14, hyperosmolarity and 4αPDD treatments were applied for 1 hr/day to constructs in CHG or PC; after loading, constructs were returned to CHG or PC. The treatment duration was motivated by previous work identifying days 10–14 as an optimal treatment window (Elder and Athanasiou, 2009). In addition, continuous hyperosmotic loading of neocartilage resulted in deleterious effects (Supplemental Figure 1A–I). In this study, PC was applied continuously. Hyperosmolarity, 4αPDD, and PC were applied in a full-factorial design.
2.4 Gross morphology and histology
After 28 days, construct wet weights (WW) were taken and dimensions were measured via ImageJ (National Institutes of Health). Constructs were divided for mechanical, biochemical, and histological analysis. Histology samples (14µm) were fixed in formalin and stained with picrosirius red and Safranin-O/Fast Green for collagen and glycosaminoglycan visualization, respectively.
2.5 Compressive testing
A creep indentation apparatus was used to evaluate construct compressive properties using the linear biphasic model (Mow et al., 1989), yielding the aggregate modulus (HA), permeability (k), and Poisson’s ratio (ν). Samples were affixed to a stainless steel platen and equilibrated in PBS. A 0.7g mass was applied with a 0.8mm-diameter flat, porous indenter tip until samples reached equilibrium displacement. Specimen thickness was measured via ImageJ.
2.6 Tensile testing
Dog -one specimens were glued to paper tabs outside the gauge length (1.45mm) and loaded in a uniaxial materials testing system (Test Resources). Specimens were pulled at 1% of the gauge length per second until failure, producing force-displacement curves. Cross-sectional area was determined by measuring sample width and thickness in ImageJ, and used to generate stress-strain curves. Tensile stiffness (Young’s modulus, EY) was determined by a least-squares fit of the linear region of the stress-strain curve, and the maximum stress determined the ultimate tensile strength (UTS).
2.7 Biochemical testing
Biochemical samples were weighed wet, frozen, then lyophilized. Dry weights (DW) were taken after lyophilization to determine water content. Samples were digested in 125µg/mL papain (Sigma) (18 hrs, 65°C). Sulfated GAG content was determined with the Blyscan Glycosaminoglycan Assay (Biocolor). Total DNA content was analyzed with a PicoGreen assay (Invitrogen) and converted to total cell number assuming 7.7pg DNA/cell. Total collagen content was quantified with a modified chloramine-T hydroxyproline assay (Woessner, 1961). Sircol collagen standard (Biocolor) was used to generate a standard curve reflecting the collagen amount.
2.8 Statistical analysis
Individual factor main effects, all two-way interactions, and the single three-way interaction were entered into linear regression models of all response parameters. Non-significant factors and interactions were removed in a stepwise manner to obtain a model with all remaining factors and interactions statistically significant (p < 0.05) using a Wald test. When an interaction was found to be statistically significant, the factors are also included, even if they are not significant; these factors are designated as NS. Tables 1B, 2B, and 3B show the result of statistical analysis. These Tables show the main factor effects and interaction terms and list the relevant contributors to the model for each response. In the Results and Discussion sections, references to main factor effects indicate all groups that received that factor (i.e., hyperosmolarity includes hyperosmolarity, hyperosmolarity+4αPDD, hyperosmolarity+PC, and hyperosmolarity+4αPDD+PC). Statistical analyses were performed with JMP 9.0.1 (SAS Institute). An n of 6–8 was employed. Data are represented as mean±standard deviation.
Table 1.
A shows all gross morphological raw data from this study, represented as mean±SD. Control group contains a standard in vitro culture medium calcium level, no Hyp, and no 4αPDD.
B displays models with statistically significant (p < 0.05) main effect factors and interactions predicting gross morphological parameters and their respective p-values; + and − symbols indicate whether the main effect factor had a positive or negative effect on the parameter, respectively.
| A. Gross Morphological Data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Response | Groups | |||||||
| CHG alone |
Hyp +CHG |
4aPDD +CHG |
Hyp+4aPDD +CHG |
PC alone |
Hyp +PC |
4aPDD +PC |
Hyp+4aPDD +PC |
|
| Diameter (mm) | 6.14±0.17 | 6.20±0.08 | 6.05±0.19 | 6.09±0.08 | 6.07±0.13 | 6.00±0.04 | 5.98±0.13 | 6.04±0.06 |
| Thickness (mm) | 0.55±0.05 | 0.55±0.02 | 0.52±0.07 | 0.50±0.05 | 0.57±0.06 | 0.56±0.04 | 0.54±0.05 | 0.52±0.05 |
| Wet Weight (mg) | 18.97±0.90 | 18.51±0.87 | 17.66±1.15 | 17.40±0.41 | 19.09±1.12 | 17.78±0.42 | 16.78±0.97 | 16.52±1.10 |
| Water Content (%) | 88.46±0.33 | 88.59±0.29 | 87.90±0.39 | 88.17±0.29 | 87.88±0.26 | 88.06±0.41 | 86.91±0.26 | 87.14±0.37 |
| B. Summary of Statistics Models for Gross Morbholo | ||||||||
|---|---|---|---|---|---|---|---|---|
| Response | Main Effects | Interactions | Model | |||||
| Hyp | 4αPDD | PC | Hyp*4αPDD | Hyp*PC | 4aPDD*PC | Hyp*4aPDD*PC | ||
| Diameter (mm) | NI | −, p < 0.05* | −, p < 0.005* | NI | NI | NI | NI | p < 0.005* |
| Thickness (mm) | NI | −, p < 0.01* | NI | NI | NI | NI | NI | p < 0.01* |
| Wet Weight (mg) | −, p < 0.05* | −, p < 0.0001* | −, p < 0.05* | NI | NI | NI | NI | p < 0.0001* |
| Water Content (%) | +, p < 0.05* | −, p < 0.0001* | −, p < 0.0001* | NI | NI | p < 0.05* | NI | p < 0.0001* |
NI indicates that the main effect or interaction was not included in the model.
Hyp: hyperosmolarity; PC: physiological calcium.
Statistically significant findings are marked with an asterisk (*).
Table 2.
A shows all biochemical raw data from this study, represented as mean±SD. Control group contains a standard in vitro culture medium calcium level, no Hyp, and no 4αPDD.
B displays models with statistically significant (p < 0.05) main effect factors and interactions predicting biochemical analysis parameters and their respective p-values; + and − symbols indicate whether the main effect factor had a positive or negative effect on the parameter, respectively.
| A. Biochemical Data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Response | Groups | |||||||
| CHG alone |
Hyp +CHG |
4αPDD +CHG |
Hyp+4αPDD +CHG |
PC alone |
Hyp +PC |
4αPDD +PC |
Hyp+4αPDD +PC |
|
| GAG/DW (mg/mg) | 0.701±0.043 | 0.746±0.036 | 0.723±0.049 | 0.689±0.045 | 0.753±0.043 | 0.725±0.033 | 0.713±0.050 | 0.694±0.057 |
| GAG/DNA (ng/ng) | 43.94±5.02 | 44.84±2.67 | 43.51±1.72 | 41.57±5.19 | 50.78±2.89 | 48.44±4.95 | 45.71±5.44 | 44.82±3.91 |
| Col/DW (mg/mg) | 0.118±0.007 | 0.106±0.007 | 0.116±0.011 | 0.117±0.002 | 0.116±0.009 | 0.105±0.011 | 0.114±0.006 | 0.113±0.003 |
| Col/DNA (ng/ng) | 7.42±0.84 | 6.36±0.75 | 7.20±0.86 | 6.72±0.43 | 8.02±0.93 | 7.00±0.81 | 7.32±1.06 | 7.14±0.82 |
| Cell Number (Millions) | 4.64±0.20 | 4.61±0.28 | 4.38±0.18 | 4.39±0.36 | 4.37±0.25 | 4.19±0.34 | 4.41±0.39 | 4.49±0.16 |
| B. Summary of Statistics Models for Biochemical Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Response | Main Effects | Interactions | Model | |||||
| Hyp | 4αPDD | PC | Hyp*4αPDD | Hyp*PC | 4αPDD*PC | Hyp*4αPDD*PC | ||
| GAG/DW (mg/mg) | NI | −, p < 0.05* | NI | NI | NI | NI | NI | p < 0.05* |
| GAG/DNA (ng/ng) | NI | −, p < 0.005* | +, p < 0.005* | NI | NI | NI | NI | p < 0.0005* |
| Col/DW (mg/mg) | −, p < 0.005* | NS | NI | p < 0.005* | NI | NI | NI | p < 0.0005* |
| Col/DNA (ng/ng) | −, p < 0.005* | NI | +, p < 0.05* | NI | NI | NI | NI | p < 0.005* |
| Cell Number (Millions) | NS | NS | NS | NI | NI | p < 0.01* | NI | p < 0.05* |
NI indicates that the main effect or interaction was not included in the model. NS indicates that the factor, while included in the model, was not significant.
Hyp: hyperosmolarity; PC: physiological calcium.
Statistically significant findings are marked with an asterisk (*).
Table 3.
A shows all mechanical raw data from this study, represented as mean±SD. Control group contains a standard in vitro culture medium calcium level, no Hyp, and no 4αPDD.
B displays models with statistically significant (p < 0.05) main effect factors and interactions predicting mechanical analysis parameters and their respective p-values; + and − symbols indicate whether the main effect factor had a positive or negative effect on the parameter, respectively.
| A. Biomechanical Data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Response | Groups | |||||||
| CHG alone |
Hyp +CHG |
4αPDD +CHG |
Hyp+4αPDD +CHG |
PC alone |
Hyp +PC |
4αPDD +PC |
Hyp+4αPDD +PC |
|
| Shear Modulus (MPa) | 0.049±0.013 | 0.056±0.016 | 0.060±0.013 | 0.059±0.017 | 0.043±0.002 | 0.055±0.016 | 0.065±0.027 | 0.069±0.011 |
| Aggregate Modulus (MPa) | 0.106±0.028 | 0.118±0.031 | 0.142±0.036 | 0.132±0.036 | 0.096±0.009 | 0.117±0.034 | 0.157±0.062 | 0.160±0.023 |
| Poisson's Ratio | 0.042±0.038 | 0.092±0.071 | 0.170±0.064 | 0.141±0.064 | 0.113±0.089 | 0.096±0.067 | 0.204±0.017 | 0.177±0.073 |
| Permeability (10^–15) | 12.81±11.39 | 6.46±2.23 | 13.82±5.79 | 9.90±6.59 | 17.08±7.72 | 8.82±4.23 | 17.05±7.47 | 12.62±5.61 |
| Young's Modulus (MPa) | 0.833±0.207 | 0.742±0.148 | 0.809±0.091 | 0.942±0.338 | 0.902±0.160 | 0.783±0.199 | 0.874±0.251 | 1.001±0.094 |
| UTS (MPa) | 0.330±0.072 | 0.353±0.069 | 0.284±0.051 | 0.311±0.095 | 0.315±0.064 | 0.354±0.146 | 0.275±0.068 | 0.329±0.022 |
| B. Summary of Statistics Models for Mechanical Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Response | Main Effects | Interactions | Model | |||||
| Hyp | 4αPDD | PC | Hyp*4αPDD | Hyp*PC | 4αPDD*PC | Hyp*4αPDD*PC | ||
| Shear Modulus (MPa) | NI | +, p < 0.005* | NI | NI | NI | NI | NI | p < .005* |
| Aggregate Modulus (MPa) | NI | +, p < 0.001* | NI | NI | NI | NI | NI | p < 0.001* |
| Poisson's Ratio | NI | +, p < 0.0001* | +, p < 0.05* | NI | NI | NI | NI | p < 0.0001* |
| Permeability (10^–15) | −, p < 0.005* | NI | NI | NI | NI | NI | NI | p < 0.005* |
| Young's Modulus (MPa) | NS | NS | NI | p < 0.05* | NI | NI | NI | p < 0.05* |
| UTS (MPa) | NI | NI | NI | NI | NI | NI | NI | NS |
NI indicates that the main effect or interaction was not included in the model. NS indicates that the factor, while included in the model, does not contribute significantly.
Hyp: hyperosmolarity; PC: physiological calcium.
Statistically significant findings are marked with an asterisk (*).
3. Results
3.1 Gross morphology
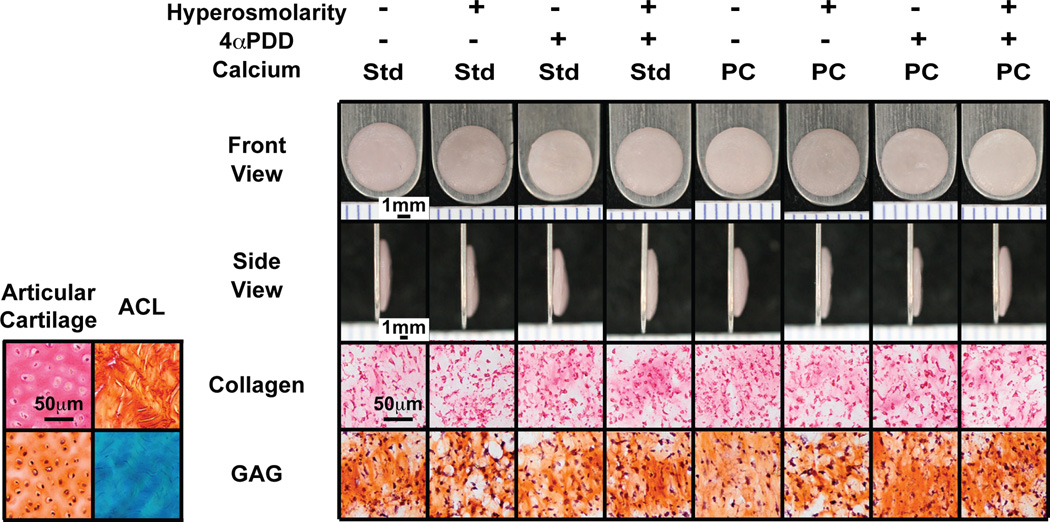
Gross morphologically, constructs appeared hyaline-like with no observed abnormalities (Figure 1). Gross morphological results are shown in Table 1. Across all groups, engineered neocartilage was 6.07±0.13mm in diameter and 0.54±0.05mm thick. All main effect factors significantly (p<0.05) reduced neocartilage wet weight, with 4αPDD and physiological calcium reducing water content as well (Table 1B). The reduced hydration indicates a denser matrix, as compared to non-treated constructs.
Figure 1.
Gross morphology and histology of engineered neocartilage. Across groups, all constructs appeared hyaline-like with no observed abnormalities and are approximately 6mm in diameter. Staining with picrosirius red and Safranin-O/Fast Green dyes revealed the presence of collagen and glycosaminoglycans, respectively. Std: standard in vitro culture medium level of calcium (1.8mM); PC: physiological level of calcium (4mM); ACL: anterior cruciate ligament; GAG: glycosaminoglycans. Ruler ticks are 1mm; scale bar represents 50µm.
3.2 Histology
Histological analysis demonstrated ECM uniformity throughout each construct, with no apparent void or empty spaces (Figure 1). Staining for collagen and glycosaminoglycans (GAGs) revealed even distribution within constructs.
3.3 Biochemical analysis
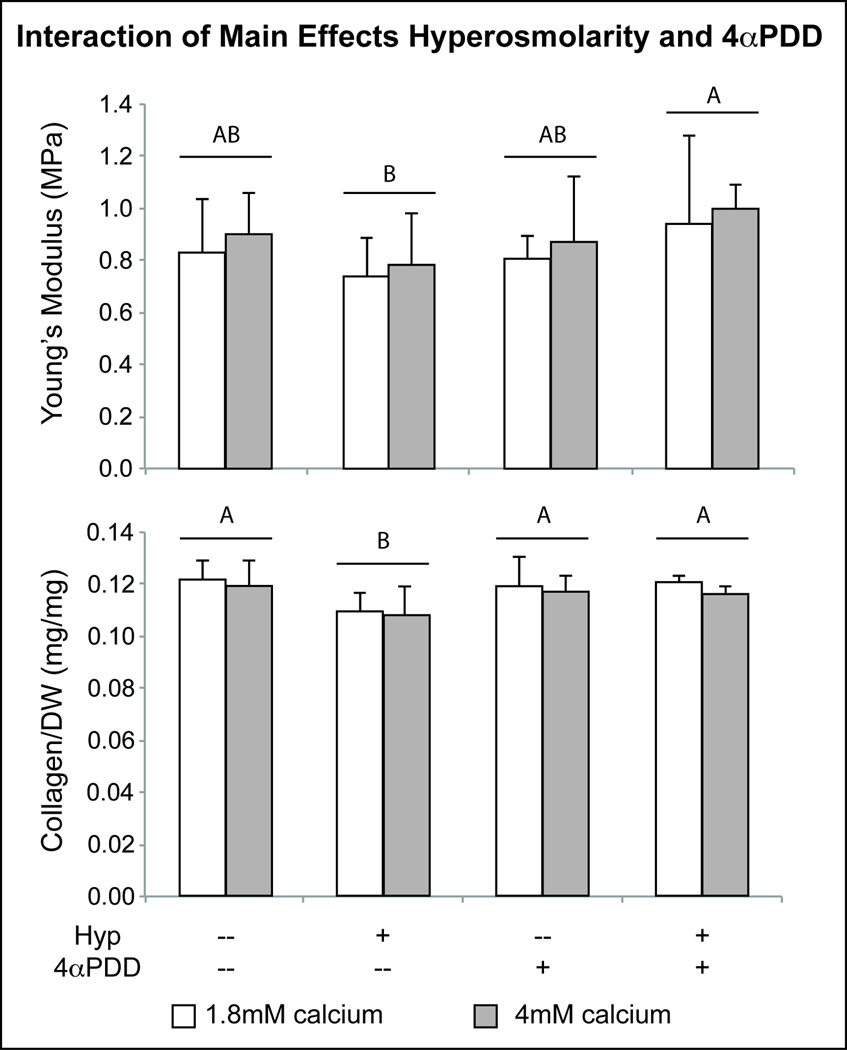
Biochemical analysis evaluated constructs for cell number and collagen and GAG content per dry weight of tissue and per cell (Table 2A). Hyperosmolarity as a factor caused a significant decrease in collagen per dry weight (COL/DW), while 4αPDD, as a factor, did not alter collagen content (Table 2B). The negative effect of hyperosmolarity alone was reversed when combined with 4αPDD—the interaction of factors hyperosmolarity and 4αPDD yielded constructs with significantly increased COL/DW, as compared to hyperosmolarity-treated constructs (Figure 2). On a per cell level, groups receiving hyperosmotic treatment exhibited a 9.32% reduction in collagen synthesis (Table 2). Also, a physiological level of calcium significantly increased collagen content (COL/DNA) by 6.50% over the standard in vitro culture medium level (Table 2).
Figure 2.
Statistical analysis demonstrating interactive effects of hyperosmolarity and 4αPDD on properties of engineered cartilage neotissue. Irrespective of calcium level, in combination with hyperosmolarity, 4αPDD mediates the negative effect of hyperosmolarity on Young’s modulus, increasing tensile stiffness significantly over hyperosmolarity alone. Similarly, the negative effect of hyperosmolarity on collagen content was eliminated with 4αPDD addition. Letters designate results of statistical analysis of hyperosmolarity and 4αPDD interaction; groups not connected by the same letter are statistically significant. Bar heights indicate mean value and error bars represent one standard deviation.
4αPDD as a factor had a significantly negative effect on GAG/DW (3.56% reduction as compared to no 4αPDD treatment; shown in Table 2). Similarly, 4αPDD groups exhibited a significant 7.07% decrease in GAG/DNA. On the contrary, GAG/DNA was increased by 8.05% when a physiological level of calcium was used. Hyperosmolarity as a factor did not affect the GAG content of engineered neocartilage (Table 2B).
In examining the interaction of 4αPDD and a physiological level of calcium, these agents reduced the cellular content of constructs to 4.38±0.28 × 106 and 4.28±0.30 × 106 cells, respectively, while constructs receiving neither 4αPDD nor a physiological level of calcium contained an average of 4.62±0.23 × 106 cells/construct; constructs were initially seeded with 4.48 × 106 cells/construct. The standard in vitro culture medium calcium concentration maintained cell numbers, whereas culturing chondrocytes using a physiological level of calcium caused a decrease in cell number (Table 2B). Interestingly, groups treated with 4αPDD resulted in a marked increase in cells per construct (4.44±0.31 × 106 cells/construct versus 4.28±0.30 × 106 cells/construct) only at a physiological calcium level.
3.4 Mechanical analysis
Aggregate modulus (HA) is reported in Table 3A. Notably, HA significantly increased in groups receiving 4αPDD treatment (Table 3B), resulting in a modulus of 0.148±0.042MPa, 1.33-times greater than non-4αPDD treated constructs. Groups treated with 4αPDD significantly affected Poisson’s ratio (ν) (Table 3B), increasing it 2.00–times non-4αPDD treated constructs. Permeability was 9.45±5.18 × 10−15m4/N*s for all constructs treated with hyperosmolarity, a significant decrease from non-hyperosmolarity treated construct values (15.22±8.31 × 10−15m4/N*s) (Table 3A).
The tensile properties of treated constructs were largely unaffected by any of the treatments alone (Table 3). However, in combination, hyperosmolarity and 4αPDD yielded a significantly higher Young’s modulus (Table 3B). Compared to no treatment, groups treated with hyperosmolarity or 4αPDD had a non-significant negative effect on Young’s modulus (0.761±0.168MPa and 0.844±0.190MPa, respectively, compared to controls of 0.867±0.182MPa) (Figure 2). Interestingly, combined hyperosmolarity and 4αPDD treatment led to a significantly higher modulus (0.972±0.240MPa) than hyperosmolarity-treated constructs, and markedly higher modulus than constructs not treated with either factor (Figure 2). The ultimate tensile strength (UTS) across all groups was similar.
4. Discussion
In this study, we evaluated the effects of hyperosmolarity and 4αPDD on the properties of engineered neocartilage, as applied in medium containing a standard in vitro culture level or a physiological level of calcium, toward establishing these agents as appropriate stimuli for cartilage tissue engineering. This study was motivated by the hypothesis that interaction of these agents would enhance neocartilage properties over application of each agent alone. This hypothesis was shown to be true as 4αPDD positively interacted with both hyperosmolarity and calcium levels in producing neocartilage with increased properties. In contrast, hyperosmolarity in either CHG or PC did not elicit significant effects. This study, for the first time, investigated the interaction of these agents and examined how hyperosmolarity and 4αPDD manifest in functional tissue properties. Specifically, we determined that 4αPDD modulation of the TRPV4 channel, as a proposed osmosensor and mechanosensor, contributes most significantly to osmolarity-mediated changes in engineered neocartilage. Identification of 4αPDD as a key player in regulating the cellular ionic and osmotic microenvironment and, thus, modulating the tissue response may be combined with other non-ionic agents in the future to beneficially affect neocartilage properties.
Based on this study’s results, 4αPDD as a factor had the most significant impact on measured parameters. In particular, 4αPDD increased tissue density and significantly improved compressive stiffness. Additionally, application of combinations, especially those involving 4αPDD, resulted in beneficial interactions, supporting the hypothesis that these treatments interact to produce results different than any treatment alone. Specifically, hyperosmolarity and 4αPDD interacted to beneficially increase tensile stiffness (EY) to 972±24kPa, a significant improvement over hyperosmolarity-treated construct values of 761±168kPa (Figure 2), which is an increase over our previous work using 4αPDD (Eleswarapu and Athanasiou, 2013), likely as a result of the lower seeding density used in the present study. Additionally, 4αPDD and hyperosmolarity interacted to enhance collagen content over hyperosmolarity alone (Figure 2). Previous studies have not examined the interaction of 4αPDD and hyperosmolarity on functional neocartilage properties, though they have investigated these agents independently (O'Conor et al., 2014; Sampat et al., 2013). Overall, the results of this study support 4αPDD as critical in affecting enhanced neocartilage properties, with 4αPDD able to augment the effects of other agents.
In cartilage tissue engineering, hyperosmolarity has resulted in disparate effects on ECM production. At the genetic level, hyperosmolarity has been shown to up-regulate anabolic genes (i.e., collagen I and II), but simultaneously induce catabolic gene expression (i.e., matrix metalloproteinase (MMP)-3 and MMP-13) (Mizuno and Ogawa, 2011). Alternatively, hyperosmotic loading of monolayer chondrocytes reduced aggrecan gene expression (Palmer et al., 2001). Applied to agarose-encapsulated articular chondrocytes, hyperosmolarity (400mOsm) elicited significant increases in tensile stiffness and GAG and DNA content (Sampat et al., 2013). In contrast, when applied to 2D-expanded chondrocytes in hydrogels, hyperosmolarity (400mOsm) decreased Young’s modulus, and GAG, collagen, and DNA content (Oswald et al., 2011). The present study revealed that hyperosmolarity (450mOsm) applied to self-assembled chondrocytes reduced only collagen content. This result is in contrast to the increased collagen content seen in agarose-embedded chondrocytes (Sampat et al., 2013), but in agreement with the decreases seen with expanded chondrocytes (Oswald et al., 2011). The discrepancy in hyperosmolarity’s effects on ECM production suggests that further optimization of hyperosmotic loading levels in neocartilage engineering is needed.
In addition to hyperosmotic loading levels, inconsistencies among hyperosmolarity studies may be due to differences in loading duration, culture system, or osmotic agents (i.e., sucrose versus sodium chloride) (Urban et al., 1993). The present study employed a loading duration optimized for a scaffold-free, self-assembling process (Elder and Athanasiou, 2009), whereas previous work employed continuous hyperosmotic loading of agarose-embedded chondrocytes (O'Conor et al., 2014; Sampat et al., 2013). The differences in collagen production among studies may be attributed not only to the variation in loading level, but also to the scaffold-free system used in this study, as scaffolds may better retain collagen produced by cells. Indeed, loading regimen and culture condition can significantly affect neocartilage properties: continuous hyperosmolarity in scaffold-free self-assembly led to deleterious effects, as compared to non-hyperosmolarity treated controls (Supplemental Figure 1A–I). Finally, while sucrose acts purely as an osmotic agent, sodium chloride contributes to ion channel activation and ionic fluxes. The role of hyperosmotic loading in improving engineered neocartilage requires further elucidation, and may rely on identification of an optimal dosage and timing regimen for a given tissue engineering system, coupled with thorough gene and protein analysis.
Hyperosmolarity and calcium level, while both significant factors, did not interact with one another to produce noteworthy changes (Tables 1B, 2B, 3B). Mechanistically, hyperosmolarity is hypothesized to create an osmotic pressure that induces fluxes of ions, including calcium, in 2D culture (Dascalu et al., 1996; Erickson et al., 2001); this ionic flux requires activation of ion channels that regulate ion flow and concentration. In addition, hyperosmolarity is hypothesized to reduce cell volume to affect changes on the cellular and DNA level—in particular, altered protein and ionic concentrations, and gene expression in 3D culture (Tew et al., 2009). However, these cellular level changes did not manifest in improvements in tissue properties when hyperosmolarity was applied in PC in this study. At the calcium levels examined here, hyperosmolarity may not exert enough osmotic pressure to elicit calcium flux, whereas 4αPDD directly activates ion channels to induce calcium fluxes. Thus, for concentrations examined in this study, the effects of hyperosmolarity (450mOsm) are not as potent as 4αPDD (10uM), though further optimization of the concentrations of these agents will likely alter their potency.
Excitingly, the application of 4αPDD in the presence of hyperosmolarity appears to reverse or ameliorate the potentially negative effects of hyperosmolarity, in particular on collagen content. Recent increased interest in the channelome of chondrocytes has identified several ion channels that mediate chondrocyte response to osmotic stress and volume changes (Barrett-Jolley et al., 2010), and the concomitant ionic fluxes. In particular, the TRPV4 channel, to which 4αPDD is an agonist, has been identified as not only a stretch-activated calcium channel that can regulate chondrogenesis via Sox9 (Muramatsu et al., 2007), but also as an osmosensor that modulates the chondrocyte’s response to osmotic changes (Clark et al., 2010; Guilak et al., 2010; Liedtke and Friedman, 2003). Therefore, in situations of increased osmolarity, such as with dynamic joint loading or simulated in a hyperosmotic loading regimen like in this study, TRPV4 may positively modulate the cellular response and subsequent matrix production.
Increases in calcium influx via a TRPV4 agonist should mimic native conditions and positively affect neocartilage properties. When applied to chondrocytes in culture, both hyperosmotic loading and 4αPDD have been shown to increase the percent of cells responding with changing calcium levels (Phan et al., 2009). By analyzing the functional properties of engineered neocartilage, this study suggests that TRPV4 is not only an osmosensor that positively regulates cell response to changing osmotic conditions, but that this response can be translated into beneficial tissue-level effects when 4αPDD is combined with hyperosmolarity or a physiological level of calcium. These findings are significant as they demonstrate, for the first time, that combinations of these agents may be used to enhance neocartilage properties. Further exploration into the concentration, levels, and application timing of these three factors is needed to optimize neocartilage matrix production.
A limitation of the present study is the focus on tissue properties without monitoring cellular changes. Extensive investigation into the cellular level effects of manipulating the TRPV4 channel have been completed in prior studies (O'Conor et al., 2014). And, as noted above, the genetic-level effects of hyperosmolarity are mixed (Mizuno and Ogawa, 2011). Additional studies involving gene expression analysis, measurements of cell and nuclear volume, imaging of intracellular calcium signaling, or other channel-modulating drugs may help elucidate the specific interactions of 4αPDD-activation of TRPV4, hyperosmolarity, and a physiological level of calcium, and the subsequent effects on matrix production. For instance, changes in intracellular calcium signaling due to these agents may be responsible for increasing neocartilage functional properties. These additional studies may elucidate the cellular response specifically in the self-assembling process, as compared to monolayer or other 3D culture modalities. Since hyperosmolarity and 4αPDD concentrations were previously optimized at a standard in vitro culture medium level of calcium (1.8mM), additional studies identifying an effective concentration range for these agents at varying calcium concentrations are warranted. Similarly, 4αPDD concentration optimization in the presence of hyperosmolarity may further increase the beneficial interaction of these agents. Finally, as calcium entry via TRPV4 may induce fluxes through other ion channels, such as in the case of calcium-induced potassium channels, coupling of TRPV4 activation and potassium channel modulation may further increase matrix synthesis. An increased understanding of the relationship among these ion channels, specific methods to manipulate those relationships, and their applicable concentration ranges, would immensely benefit the field of cartilage tissue engineering.
To our knowledge, this is the first study to systematically investigate hyperosmolarity and 4αPDD in the presence of both a standard in vitro culture and a physiological calcium level, on the functional properties of scaffold-free, engineered neocartilage. Each factor alone contributed significantly to construct properties, while 4αPDD had the greatest effect. Excitingly, combinations including 4αPDD were shown to positively modulate the effects of hyperosmolarity or calcium on properties such as Young’s modulus and collagen content. Constructs stimulated with 4αPDD exhibited functional properties approaching native articular cartilage values, supporting it as a suitable agent to generate tissue engineered neocartilage replacements for cartilage diseases.
Supplementary Material
Acknowledgments
This publication was made possible by NIH R01AR061496 and by Grant Number T32-GM00799 from NIH-NIGMS (JKL). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS or NIH. We thank Dr. Derek Cissell for assistance with statistical analysis and Dr. Grayson DuRaine for technical assistance.
Abbreviations
- TRPV4
transient receptor potential vanilloid 4
- 4αPDD
4α-Phorbol 12,13-didecanoate
- ECM
extracellular matrix
- GAG
glycosaminoglycan
- MMP
matrix metalloproteinase
Definitions of Variables and Constants Used in the Paper
- HA
aggregate modulus
- ν
Poisson’s ratio
- k
permeability
- G
shear modulus
- EY
Young’s modulus
Footnotes
Conflict of Interest Statement
The authors declare no conflicts of interest associated with the work presented in this manuscript.
All authors contributed to this work and the manuscript is not being considered for publication elsewhere.
References
- Barrett-Jolley R, Lewis R, Fallman R, Mobasheri A. The Emerging Chondrocyte Channelome. Front Physiol. 2010;1:1–11. doi: 10.3389/fphys.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SS, Falcovitz YH, Schneiderman R, Maroudas A, Sah RL. Depth-dependent compressive properties of normal aged human femoral head articular cartilage: relationship to fixed charge density. Osteoarthritis Cartilage. 2001;9:561–569. doi: 10.1053/joca.2001.0424. [DOI] [PubMed] [Google Scholar]
- Clark AL, Votta BJ, Kumar S, Liedtke W, Guilak F. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis Rheum. 2010;62:2973–2983. doi: 10.1002/art.27624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascalu A, Korenstein R, Oron Y, Nevo Z. A hyperosmotic stimulus regulates intracellular pH, calcium, and S-100 protein levels in avian chondrocytes. Biochem Biophys Res Commun. 1996;227:368–373. doi: 10.1006/bbrc.1996.1514. [DOI] [PubMed] [Google Scholar]
- Elder BD, Athanasiou KA. Effects of temporal hydrostatic pressure on tissue-engineered bovine articular cartilage constructs. Tissue Eng Part A. 2009;15:1151–1158. doi: 10.1089/ten.tea.2008.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleswarapu SV, Athanasiou KA. TRPV4 channel activation improves the tensile properties of self-assembled articular cartilage constructs. Acta Biomater. 2013;9:5554–5561. doi: 10.1016/j.actbio.2012.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson GR, Alexopoulos LG, Guilak F. Hyper-osmotic stress induces volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways. J Biomech. 2001;34:1527–1535. doi: 10.1016/s0021-9290(01)00156-7. [DOI] [PubMed] [Google Scholar]
- Finan JD, Guilak F. The effects of osmotic stress on the structure and function of the cell nucleus. J Cell Biochem. 2010;109:460–467. doi: 10.1002/jcb.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Leddy HA, Liedtke W. Transient receptor potential vanilloid 4: The sixth sense of the musculoskeletal system? Ann N Y Acad Sci. 2010;1192:404–409. doi: 10.1111/j.1749-6632.2010.05389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen RK, Herzog W. Depth-dependent analysis of the role of collagen fibrils, fixed charges and fluid in the pericellular matrix of articular cartilage on chondrocyte mechanics. J Biomech. 2008;41:480–485. doi: 10.1016/j.jbiomech.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci U S A. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris EA, Hu JC, Athanasiou KA. Hypoxia-induced collagen crosslinking as a mechanism for enhancing mechanical properties of engineered articular cartilage. Osteoarthritis Cartilage. 2013;21:634–641. doi: 10.1016/j.joca.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas A. Physicochemical properties of articular cartilage. Kent, UK: Pitman Medical; 1979. [Google Scholar]
- Maroudas A, Evans H. A Study of Ionic Equilibria in Cartilage. Connective Tissue Research. 1972;1:69–77. [Google Scholar]
- Mizuno S, Ogawa R. Using changes in hydrostatic and osmotic pressure to manipulate metabolic function in chondrocytes. Am J Physiol Cell Physiol. 2011;300:C1234–C1245. doi: 10.1152/ajpcell.00309.2010. [DOI] [PubMed] [Google Scholar]
- Mow VC, Gibbs MC, Lai WM, Zhu WB, Athanasiou KA. Biphasic indentation of articular cartilage--II. A numerical algorithm and an experimental study. J Biomech. 1989;22:853–861. doi: 10.1016/0021-9290(89)90069-9. [DOI] [PubMed] [Google Scholar]
- Muramatsu S, Wakabayashi M, Ohno T, Amano K, Ooishi R, Sugahara T, Shiojiri S, Tashiro K, Suzuki Y, Nishimura R, Kuhara S, Sugano S, Yoneda T, Matsuda A. Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. J Biol Chem. 2007;282:32158–32167. doi: 10.1074/jbc.M706158200. [DOI] [PubMed] [Google Scholar]
- Murphy MK, Huey DJ, Reimer AJ, Hu JC, Athanasiou KA. Enhancing post-expansion chondrogenic potential of costochondral cells in self-assembled neocartilage. PLoS ONE. 2013;8:e56983. doi: 10.1371/journal.pone.0056983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natoli RM, Skaalure S, Bijlani S, Chen KX, Hu J, Athanasiou KA. Intracellular Na(+) and Ca(2+) modulation increases the tensile properties of developing engineered articular cartilage. Arthritis Rheum. 2010;62:1097–1107. doi: 10.1002/art.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci U S A. 2014;111:1316–1321. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek G, Revell CM, Hu JC, Allison DD, Grande-Allen KJ, Athanasiou KA. Matrix development in self-assembly of articular cartilage. PLoS ONE. 2008;3:e2795. doi: 10.1371/journal.pone.0002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald ES, Ahmed HS, Kramer SP, Bulinski JC, Ateshian GA, Hung CT. Effects of Hypertonic (NaCl) Two-Dimensional and Three-Dimensional Culture Conditions on the Properties of Cartilage Tissue Engineered from an Expanded Mature Bovine Chondrocyte Source. Tissue Eng Part C Methods. 2011;17:1041–1049. doi: 10.1089/ten.tec.2011.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald ES, Chao PH, Bulinski JC, Ateshian GA, Hung CT. Dependence of zonal chondrocyte water transport properties on osmotic environment. Cellular and molecular bioengineering. 2008;1:339–348. doi: 10.1007/s12195-008-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer GD, Chao Ph PH, Raia F, Mauck RL, Valhmu WB, Hung CT. Time-dependent aggrecan gene expression of articular chondrocytes in response to hyperosmotic loading. Osteoarthritis Cartilage. 2001;9:761–770. doi: 10.1053/joca.2001.0473. [DOI] [PubMed] [Google Scholar]
- Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, Lee SH, Liedtke W, Guilak F. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60:3028–3037. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampat SR, Dermksian MV, Oungoulian SR, Winchester RJ, Bulinski JC, Ateshian GA, Hung CT. Applied osmotic loading for promoting development of engineered cartilage. J Biomech. 2013;46:2674–2681. doi: 10.1016/j.jbiomech.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez JC, Wilkins RJ. Changes in intracellular calcium concentration in response to hypertonicity in bovine articular chondrocytes. Comparative Biochemistry and Physiology - Part A: Molecular and Integrative Physiology. 2004;137:173–182. doi: 10.1016/j.cbpb.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Tew SR, Peffers MJ, McKay TR, Lowe ET, Khan WS, Hardingham TE, Clegg PD. Hyperosmolarity regulates SOX9 mRNA posttranscriptionally in human articular chondrocytes. Am J Physiol Cell Physiol. 2009;297:C898–C906. doi: 10.1152/ajpcell.00571.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JPG, Hall AC, Gehl KA. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. Journal of Cellular Physiology. 1993;154:262–270. doi: 10.1002/jcp.1041540208. [DOI] [PubMed] [Google Scholar]
- Villanueva I, Gladem SK, Kessler J, Bryant SJ. Dynamic loading stimulates chondrocyte biosynthesis when encapsulated in charged hydrogels prepared from poly(ethylene glycol) and chondroitin sulfate. Matrix Biol. 2010;29:51–62. doi: 10.1016/j.matbio.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.