Abstract
AIM
To investigate the potential of implanting pseudoislets formed from human insulin-releasing β-cell lines as an alternative to islet transplantation.
METHODS
In this study, the anti-diabetic potential of novel human insulin releasing 1.1B4 β-cells was evaluated by implanting the cells, either as free cell suspensions, or as three-dimensional pseudoislets, into the subscapular region of severe combined immune deficient mice rendered diabetic by single high-dose administration of streptozotocin. Metabolic parameters including food and fluid intake, bodyweight and blood glucose were monitored throughout the study. At the end of the study animals were given an intraperitoneal glucose tolerance test. Animals were then culled and blood and tissues were collected for analysis. Insulin and glucagon contents of plasma and tissues were measured by insulin radioimmunoassay and chemiluminescent enzyme-linked immunosorbance assay respectively. Histological analyses of pancreatic islets were carried out by quantitative fluorescence immunohistochemistry staining.
RESULTS
Both pseudoislet and cell suspension implants yielded well vascularised β-cell masses of similar insulin content. This was associated with progressive amelioration of hyperphagia (P < 0.05), polydipsia (P < 0.05), body weight loss (P < 0.05), hypoinsulinaemia (P < 0.05), hyperglycaemia (P < 0.05 - P < 0.001) and glucose tolerance (P < 0.01). Islet morphology was also significantly improved in both groups of transplanted mice, with increased β-cell (P < 0.05 - P < 0.001) and decreased alpha cell (P < 0.05 - P < 0.001) areas. Whereas mice receiving 1.1B4 cell suspensions eventually exhibited hypoglycaemic complications, pseudoislet recipients displayed a more gradual amelioration of diabetes, and achieved stable blood glucose control similar to non-diabetic mice at the end of the study.
CONCLUSION
Although further work is needed to address safety issues, these results provide proof of concept for possible therapeutic applicability of human β-cell line pseudoislets in diabetes.
Keywords: Human β-cell line, 1.1B4, Cell therapy, Insulin, Pseudoislets
Core tip: Human insulin-releasing 1.1B4 β-cell suspensions and psuedoislets were implanted in streptozotocin-diabetic severe combined immune deficient mice to assess their antidiabetic potential. Both cell configurations yielded vascularised, insulin positive β-cell masses. These were associated with beneficial effects on hyperphagia, polydipsia, body weight, hypoinsulinaemia, hyperglycaemia and glucose tolerance. Both treatments were also associated with significant improvements in islet morphology and increased β:α-cell ratio. Pseudoislet recipients displayed gradual glucose normalization, while cell suspension recipients ultimately presented with hypoglycaemic complications. These results provide proof of concept for possible clinical artificial human β-cell psuedoislets, although further work is needed to address the tumourigenicity of clonal cell-lines.
INTRODUCTION
Type 1 diabetes mellitus (T1DM) is caused by autoimmune mediated destruction of insulin producing β-cells in the pancreatic islets[1]. Uncontrolled hyperglycaemia leads to debilitating and in some cases life-limiting complications including retinopathy, nephropathy, neuropathy and metabolic ketoacidosis[2-5]. Protection against these ailments by insulin injections requires frequent monitoring of blood glucose to prevent over - or under-dosage. Hypoglycaemic episodes are not uncommon especially in brittle diabetes where patients often exhibit hypoglycaemia unawareness resulting in dangerous iatrogenic hypoglycaemia[6]. Cellular delivery of insulin achieved by replacement of pancreatic β-cells can help manage diabetes and in some cases eliminate the need for exogenous insulin therapy[7].
At present, the two methods employed to replace lost β-cells in T1DM are pancreatic transplantation (PTx) and islet transplantation (ITx)[8]. PTx involves an invasive procedure performed in combination with kidney transplantation and necessitates chronic immunosuppression to prevent graft rejection[9,10]. In contrast, ITx represents a less invasive alternative to PTx where islets are isolated by enzymatic digestion of donor pancreata and then administered to the recipient by percutaneous infusion into the liver via the portal vein[8]. While less risky than whole organ transplantation, ITx is limited by the requirement for immunosuppression to prevent rejection and promote long-term islet graft functionality but the majority of patients still revert to insulin use within five years of treatment[11,12]. Nevertheless, ITx can provide temporary insulin independence and even partial graft function can prevent dangerous hypoglycaemic events[8,13,14]. Unfortunately, pancreatic donors are scarce and current practices often require use of islets from two or more separate donors. This practice is not practical on a large scale and so there is a great impetus to find alternative solutions especially given that implant function also frequently fails with time[8].
One approach to providing a sustainable supply of insulin releasing tissue for transplantation is to generate insulin-producing cells from stem cells or to engineer cell-lines which mimic the functional response of normal human pancreatic β-cells[15-18]. Over the years, many rodent β-cell lines have been created by methods such as exposure of primary rodent β-cells to radiation or transfection with oncogenic viral vectors such as SV40[19-24]. While such cell-lines have proven invaluable in basic islet research their xenogeneic properties limit their therapeutic utility. Consequently, more recent endeavours have been focused on the creation of insulin-releasing cell-lines from human β-cells[25,26]. Unfortunately, this has proven to be extremely difficult as human β-cells tend to proliferate poorly and undergo rapid dedifferentiation when cultured in vitro. The majority of attempts to develop stable human β-cell lines have yielded cells with limited glucose sensitivity or insufficient insulin content[27-32].
Extensive functional studies using the novel human β-cell line 1.1B4 created by the electrofusion of freshly isolated human β-cells with immortal PANC1 epithelial partner cells have demonstrated that 1.1B4 cells possess intact cellular mechanisms for insulin production and secretion, and that they are responsive to glucose and other modulators of insulin secretion[25]. The cells also appear to possess similar cytoprotective mechanisms to primary β-cells[33-35].
Like many β-cell-lines, 1.1B4 cells spontaneously form three dimensional pseudoislets after 5 to 7 d when grown in suspension culture. These pseudoislets are morphologically similar to isolated primary islets and show increased expression of cell-cell communication genes together with remarkable potentiation of insulin secretory responses to glucose and other secretagogues in vitro[25,36]. Moreover, 1.1B4 cells showed significantly enhanced resistance to cytotoxicity when configured as pseudoislets compared to monolayers[37]. Transplantation of cells configured as pseudoislets may represent an attractive model to improve graft survival, function and resistance to hyperglycaemia. In the present study the ability of human insulin secreting 1.1B4 cells, administered as single cell suspensions or pseudoislets, to rescue diabetes and restore blood glucose control was studied using severe combined immunodeficient (SCID) mice rendered diabetic by administration of streptozotocin (STZ). These immunodeficient mice were used to prevent rejection of human 1.1B4 cell implants.
MATERIALS AND METHODS
Cell culture and pseudoislet formation
The generation and characterisation of the human 1.1B4 β-cell line has been described previously[25]. The cells were maintained at 37 °C with 5% CO2 in RPMI-1640 media (Gibco® Invitrogen, Paisley, United Kingdom) containing 11.1 mol/L glucose and 2.0 mol/L L-glutamine supplemented with 10% (v/v) foetal calf serum (Gibco® Invitrogen, Paisley, United Kingdom) and antibiotics (100 U/mL penicillin and 0.1 g/L streptomycin) (Gibco® Invitrogen, Paisley, United Kingdom). Cells were given fresh media every 2-3 d as necessary and were routinely used from passage 25-35. The cell line is available to purchase from Sigma-Aldrich (Dorset, United Kingdom). To form pseudoislets, 1.1B4 cells were seeded at a density of 1 × 105 cells/well into ultra-low-attachment, six-well, flat-bottomed plates (Corning Inc., NY, United States) with 5-mL/well culture medium. Cells typically formed three-dimensional pseudoislet clusters, each comprising 5000-6000 cells, within 5-7 d of seeding[37].
Animal and surgical procedures
Adult female SCID mice (15-20 wk) were bred and maintained under specific pathogen-free conditions in the Biomedical and Behavioral Research Unit (BBRU) at Ulster University, Coleraine. Food and water were provided ad libitum unless specified otherwise. Diabetes was induced by intraperitoneal administration of streptozotocin (165 mg/kg) after an 8 h fast. Hyperglycaemia was controlled with intensive insulin therapy (15 mg/kg body weight intraperitoneal bovine insulin every 8 h) prior to and during the early engraftment period as indicated in the Figures. Suspensions of 1.1B4 cells (1 × 107 cells/mL) were administered in 500 μL serum-free Roswell park memorial institute (RPMI) medium subscapularly into adipose tissue deposit at back of the neck using a 25-G needle. For pseudoislet implantation, harvested pseudoislets were resuspended at a density of 2000 pseudoislets per ml and 500 μL was injected to the same location using an 18-G needle. Control mice received vehicle only. Food intake, water intake and body weight were monitored daily while blood glucose was measured once every 3 d using Ascensia contour glucose strips (Bayar, Uxbridge, United Kingdom). At the end of the study, glucose tolerance was determined by measuring blood glucose and plasma insulin levels after glucose administration (18 mmol/kg bw i.p.) at 0 and 15, 30, 60, 90 and 120 min. Finally, terminal blood samples were collected and implants and pancreata were collected for both histology and hormone content assessment. Timeline of the procedures is depicted in Figure 1. All animal procedures were performed in adherence to the United Kingdom home office regulations (United Kingdom Animal Scientific Procedures Act 1986) and “Principles of laboratory animal care” (NIH Publication no 86-23, revised 1985).
Figure 1.
Timeline of experiment.
Biochemical assays
Lysates of excised cell masses and pancreata were prepared by overnight extraction at 4 °C with acid ethanol (ethanol 75% v/v, water 23.5% v/v and concentrated HCl 1.5% v/v). Protein contents were determined by Bradford assay. Insulin was determined by radioimmunoassay as described previously[38]. Glucagon was determined using glucagon chemiluminescent assay (EZGLU-30K, Millipore, MA, United States) following manufacturer’s instructions. Glucose in plasma samples was determined using an Analox GM9 glucose analyzer (Analox, London, United Kingdom).
Immunohistochemistry
For peroxidase immunostaining, de-waxed and rehydrated sections were blocked in 0.3% (v/v) H2O2 in 50% (v/v) methanol for 30 min to quench endogenous peroxidase activity, before incubation at 95 °C in citrate buffer (pH 6.0) for antigen retrieval. After cooling, sections were incubated at 4 °C with mouse anti insulin antibody (1:1000, Abcam, United Kingdom) overnight, and then incubated with ImmPRESS HRP anti mouse IgG (peroxidase) reagent (Vector labs, United Kingdom) and developed with 3, 3’-Diaminobenzidine substrate (Vector labs, United Kingdom). Lastly, sections were counterstained with haematoxylin at 60 °C for 5 min, and slides were cleared with Histo-clear II and mounted with Histomount mounting medium. Slides were viewed using Olympus IX51 inverted microscope and photographed using the SPOT RT-Ke camera (Diagnostic Instruments Inc., Sterling Heights, MI, United States).
For fluorescence immunostaining, following dewaxing, rehydration, antigen retrieval with citrate buffer and blocking with BSA solution, sections were incubated at 4 °C overnight with primary antibodies (mouse anti insulin antibody, ab6995, 1:1000, Abcam; guinea pig anti glucagon antibody, PCA2/4, raised in house; rabbit anti Ki67 antibody, ab15580, 1:100, Abcam) prior to incubation at 37 °C for 45 min with secondary antibody (Alexa Fluor 488/594)[35,39]. Finally, slides were mounted with anti-fade mounting medium and viewed under FITC filter (488 nm) or TRITC filter using a fluorescent microscope (Olympus, model BX51) and photographed using a connected DP70 camera adapter system.
Image analysis
Closed polygon tool in Cell-F image analysis software (Olympus Soft Imaging Solutions, GmbH) was used to analyze islet parameters including islet, α cell and β cell areas. Number of islets was counted in a blinded fashion and expressed as number per mm2 of pancreas. For analysis of islet size distribution, islets smaller than 10000 μm2 were considered small, those larger than 10000 μm2 but smaller than 25000 μm2 were considered medium and those larger than 25000 μm2 were considered large. Cells expressing both insulin and either Ki67 or TUNEL were counted and values were expressed as a percentage of the total number of insulin positive cells observed. Approximately 1000 β-cells were analyzed per replicate.
Statistical analysis
Results are expressed as mean ± SEM. Groups of data were compared using Student’s unpaired t-test with two-tailed P-values. Groups were considered significant where P < 0.05.
RESULTS
Effects on food and fluid intake, body weight and blood glucose
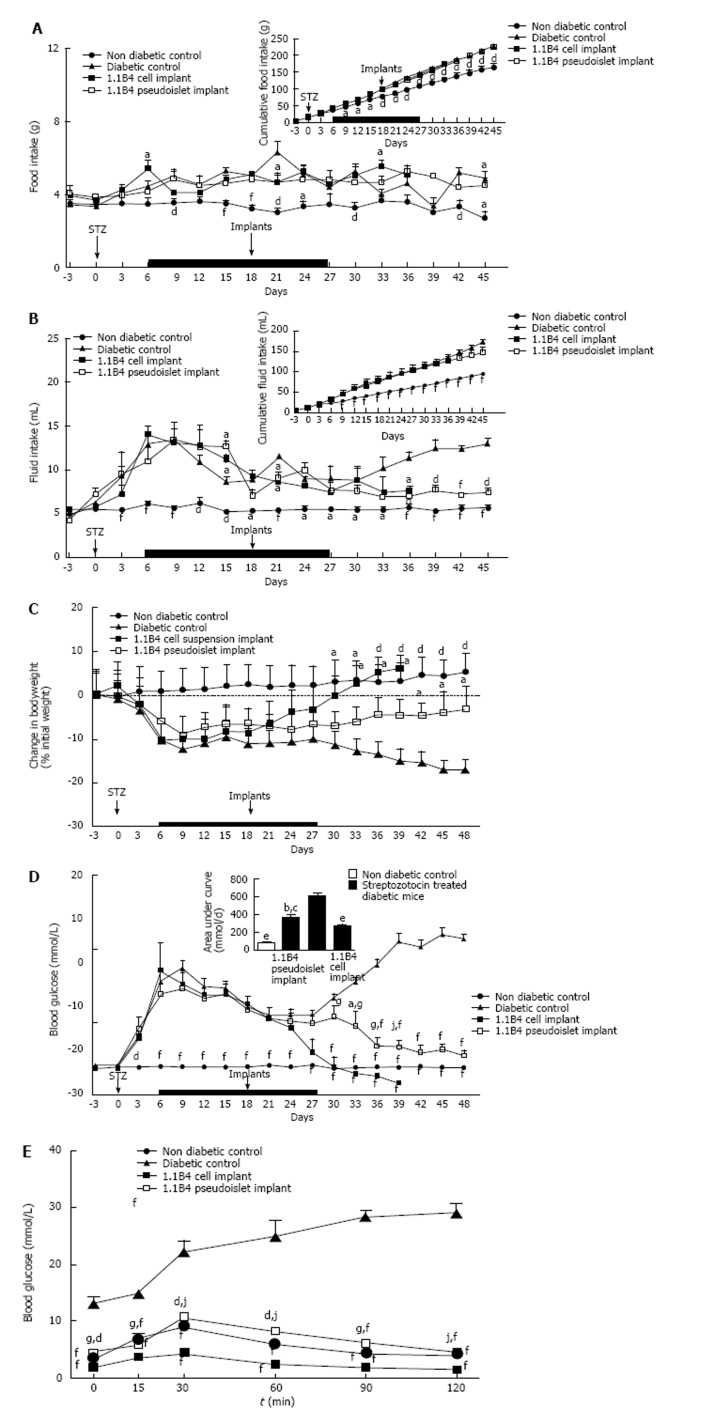
Streptozotocin diabetes caused significant increases in food and fluid intake when compared to non-diabetic controls (P < 0.05, P < 0.01, P < 0.001, Figure 2A and B). Implantation of 1.1B4 cell suspensions or pseudoislets had small inhibitory effects on daily and cumulative food intake (Figure 2A). 1.1B4 pseudoislet transplantation significantly (P < 0.05) decreased fluid intake from day 18 post-implantation compared to the marked polydipsia exhibited by diabetic controls (Figure 2B). Fluid intake of cell suspension recipients did not significantly differ from control diabetic mice, indicating less effective amelioration of blood glucose control.
Figure 2.
Effects on food and fluid intake, body weight and blood glucose of streptozotocin diabetic severe combined immunodeficient mice implanted with 1.1B4 cells/ pseudoislets. A: Food intake; B: Fluid intake; C: Change in body weight; D: Blood glucose. From day 6-27, all diabetic mice were injected with insulin (15 U/kg bw) every 8 h (Indicated by black bar). At the end of the study, glucose tolerance (E) was determined over a time course of 120 min. Values are mean ± SEM (n = 4). aP < 0.05, dP < 0.01 and fP < 0.001 vs diabetic control animals; gP < 0.05, jP < 0.01 vs 1.1B4 cell suspension recipients.
Streptozotocin diabetes resulted in significant and progressive body weight loss compared to non-diabetic controls (P < 0.05, P < 0.01, Figure 2C). Transplantation of 1.1B4 cells resulted in significantly increased body weight compared to diabetic controls 15 d post transplantation (P < 0.05, Figure 2C), while pseudoislets evoked a more gradual increase with values differing significantly from diabetic controls from 24 d post transplantation (P < 0.05, Figure 2C).
Streptozotocin diabetes significantly increased blood glucose levels within 3 d compared to non-diabetic controls (P < 0.001, Figure 2D). The hyperglycaemia was moderated during the period of insulin treatment but rebounded to very high levels thereafter. Blood glucose was significantly decreased at 12 and 15 d after implantation of 1.1B4 cells (P < 0.001, Figure 2D) or pseudoislets (P < 0.05, Figure 2D) respectively. From day 12 onwards, a much more moderate fall of blood glucose was observed in the pseudoislet recipient group (P < 0.05, P < 0.01, Figure 2D). Indeed, whereas mice receiving 1.1B4 cells were culled at 21 d post-transplantation to avoid severe hypoglycaemia, pseudoislet recipients exhibited normoglycaemia when the study was terminated at 30 d.
Effects on glucose tolerance
Following an 8 h fast and intraperitoneal glucose administration, blood glucose levels of both 1.1B4 cell suspension and pseudoislet recipients were significantly lower than diabetic control animals at all time-points observed (P < 0.01, Figure 2E). Furthermore, 1.1B4 cell suspension implants yielded significantly (P < 0.05) lower blood glucose levels than pseudoislet implants or normal control mice (P < 0.05, P < 0.01, Figure 2E). Pseudoislet recipients exhibited normal glucose tolerance.
Effects on plasma and pancreatic hormone content
Insulin content of cell suspension and pseudoislet implant did not differ significantly (Figure 3A). Streptozotocin diabetes significantly decreased plasma insulin compared to non-diabetic mice (P < 0.001). Insulin concentrations were significantly raised in mice receiving 1.1B4 cell suspension and pseudoislet implants (10.8 and 7.9 fold increases respectively, P < 0.05, P < 0.01, Figure 3B). Streptozotocin diabetes also significantly decreased pancreatic insulin content (P < 0.05, Figure 3C) which was not altered by transplantation (Figure 3C). Plasma and pancreatic glucagon levels of diabetic mice were significantly increased compared to non-diabetic controls (P < 0.05, P < 0.01, Figure 3D and E) and this was partly normalized by cell transplantation (P < 0.05, Figure 3D and E).
Figure 3.
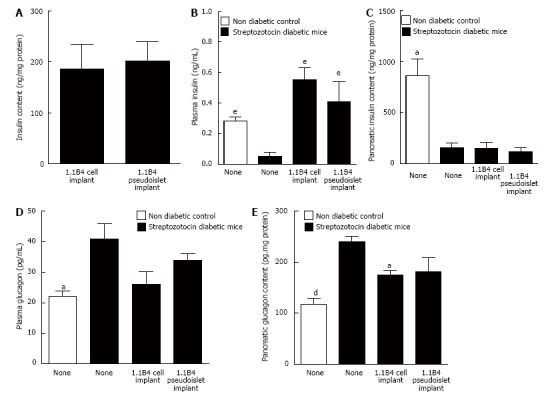
Insulin content (A) of excised 1.1B4 cell/pseudoislet cell masses and the effects of implantation on plasma insulin (B), pancreatic insulin content (C), plasma glucagon (D) and pancreatic glucagon content (E) of normal and streptozotocin diabetic severe combined immunodeficient mice. Values are mean ± SEM (n = 4). aP < 0.05, dP < 0.01, eP < 0.01 vs diabetic control animals.
Effects on pancreatic islets
Representative images showing insulin and glucagon staining in islets of non-diabetic, diabetic and cell/pseudoislet implanted diabetic mice are shown in Figure 4A. Histological analysis of the islets showed that streptozotocin markedly diminished islet area, β cell area, β to α cell ratio and number of islets while increasing alpha cell area (P < 0.05, P < 0.01, P < 0.001, Figure 4B-F). Islet areas of 1.1B4 cell suspension recipients were marginally decreased compared to diabetic controls (P < 0.05, Figure 4B). However, α-cell areas were decreased and both β-cell and β- to α-cell ratios were significantly increased in 1.1B4 cell suspension and pseudoislet recipients (P < 0.05, Figure 4C-E). Percentage of smaller islets increased in diabetic mice which was not normalised by cell or pseudoislet transplantation (Figure 4G).
Figure 4.
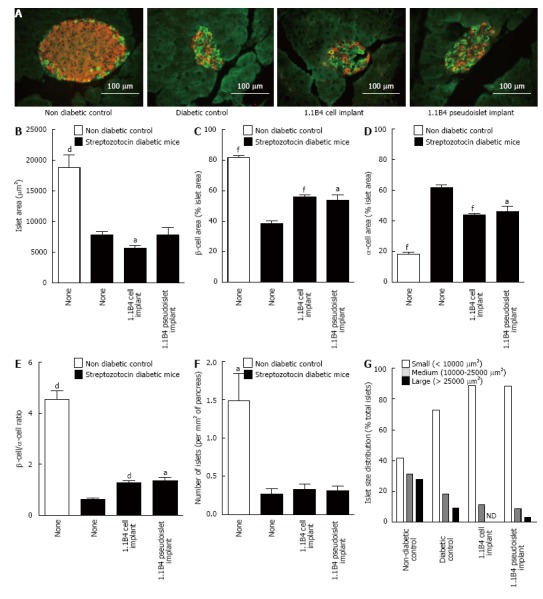
Result of insulin (red) and glucagon (green) staining in islets of non-diabetic and diabetic severe combined immunodeficient mice with or without cell/pseudoislet transplantation. Representative images are shown in A. Islet area (B), β cell area (C), α cell area (D), β to α cell ratio (E), number of islets (F), and islet size distribution (G) were all determined by quantitative histological analysis using cell^F software. Values are mean ± SEM (n = 5). aP < 0.05, dP < 0.01 and fP < 0.001 vs diabetic control.
Representative images showing Ki67/insulin and TUNEL/insulin staining in islets of non-diabetic, diabetic and cell/pseudoislet implanted diabetic mice are shown in Figure 5A. Diabetes induction was associated with significant decreases in β-cell Ki67 to TUNEL ratio indicating an increase in the frequency of β-apoptosis and a decrease in β-cell proliferation (P < 0.05, Figure 5B-D). Implants did not significantly affect β-cell Ki67 or TUNEL expression.
Figure 5.
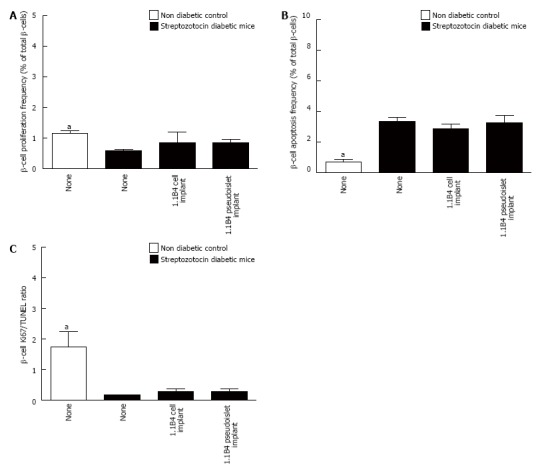
Frequency of β-cell proliferation (A) and apoptosis (B) and ratio of Ki67 to TUNEL positive β-cells (C) were determined by histological analysis. Values are mean ± SEM (n = 4). Approximately 1000 β-cells were counted per replicate. aP < 0.05 vs diabetic controls.
DISCUSSION
The therapeutic potential of novel 1.1B4 human insulin-releasing β-cells configured as cell suspensions or pseudoislets was assessed by implantation into diabetic SCID mice. 1.1B4 cells exhibit marked decreases in secretory function and viability following prolonged exposure to high levels of glucose[33,37]. As a result, mice with chemically-induced diabetes were given insulin therapy for 9-27 d after STZ to moderate blood glucose levels during the engraftment of implanted 1.1B4 cell suspensions and pseudoislets. As expected, control STZ-treated mice characteristically exhibited hyperphagia, polydipsia, weight loss and marked hyperglycaemia which were temporarily moderated during the period of insulin treatment.
Implantation of 1.1B4 cell suspensions or pseudoislets yielded vascularised cell masses (data not included) which restored plasma insulin concentrations and reversed the hyperglycaemic state. We did not have the opportunity to measure human C-peptide for confirmation but we assume that this insulin was derived from extra-pancreatic source because analysis of pancreatic tissue at end of study revealed severe loss of islet beta cells and cellular insulin in both 1.1B4 cell implanted groups similar to untreated diabetic controls. Furthermore, human insulin and C-peptide were readily detectable in 1.1B4 cells[25]. This was associated with significant beneficial effects on glucose tolerance, body weight and both, food and fluid intakes, but plasma glucagon remained elevated. These results have parallels with previous studies where primary islets were implanted into insulin controlled diabetic animals[40-42]. However, recipients of 1.1B4 cell suspensions progressed to low blood glucose levels such that these mice were terminated at 21 d after transplantation. In contrast, the anti-hyperglycaemic effects of pseudoislet implants manifested more slowly, achieving stable normoglycaemia without hypoglycaemic complications. Furthermore, energy and fluid balance, body weight, blood glucose and glucose tolerance improved gradually in these mice. This difference is most likely due to improved insulin secretory function in 1.1B4 pseudoislets compared to single isolated cells as described previously in vitro[25,36,37,43]. This better regulated insulin release is supported by similar insulin contents of the two types of resected β-cell masses. Nevertheless, part of the difference may also reflect the slower cellular proliferation following pseudoislet implantation.
Administration of STZ to SCID mice was associated with significant decreases in islet number, size and β-cell number together with significant α-cell hyperplasia. These observations accompanied by depletion of pancreatic insulin and enhancement of pancreatic glucagon, mirror previous studies of animal models of diabetes induced by STZ[35,39,44-49]. Implantation of 1.1B4 cell suspensions did not affect hormone contents but was associated with decreases in α-cell and islet areas but an increase in β-cell area and the β-cell to α-cell ratio. There were no significant changes in β-cell proliferation or apoptosis, so alterations of these processes in islet α-cells merits further study. However, both pancreatic insulin and glucagon were unchanged in transplanted mice. Given the present interest in changes of α cell populations in diabetes[35,47,49], this observation merits further investigation. The effects on pancreatic hormones and islets were similar in pseudoislet recipients but as with the metabolic effects, they were moderate compared with cell suspension recipients.
Both cell suspensions and, to a lesser extent, 1.1B4 pseudoislets developed into cell masses following transplantation. While no obvious signs of metastasis were apparent in either group following post-mortem examination, the tumorigenic nature of the cells remains an obstacle to therapeutic use. 1.1B4 cells configured as pseudoislets exhibited significantly decreased proliferation rates and are self-limiting in size in vitro[36]. This might be a consequence of cell-cell contacts playing a role in modulation of proliferation and apoptosis rates. However, it seems likely that an additional factor limiting pseudoislet growth in vitro is hypoxia, a common consequence of culturing cell spheroids in static cultures. This view is supported by the ability of MIN6 mouse β-cell pseudoislets cultured in bioreactor with continuous stirring to grow continuously for two wk without exhibiting any signs of hypoxia, reduced functionality, or growth arrest[50]. In vivo 1.1B4 cells pseudoislets were able to quickly muster a blood supply which allowed proliferation of the cells. This contrasts with the limited ability of human islets to establish effective vascularisation which is a major hindrance to clinical islet outcomes[51].
A number of groups have investigated potential ways of getting around the issue of tumorigenicity of engineered β-cells which need to be generated in large numbers in culture. The most popular approach is the use of tailored viral vectors which allows the inactivation or excision of oncogenes from the cell-lines genomes to reverse the immortal status of the cells once enough have been generated for use[17,26,32]. If such an approach could potentially be tailored to reverse the tumorigenic status of 1.1B4 cells, the therapeutic qualities observed in this study could be more usefully exploited for the treatment of T1DM. An additional or alternative approach involves the use of implantation devices that are currently under development[7,52]. These devices, such as TheraCyte™ macroencapsulation system and nanofiber-enabled encapsulation devices support cell function by providing good oxygen tension and protection from autoimmune attack, whilst providing against unwanted growth and spread of implanted cells[7,52,53].
To conclude, implantation of human 1.1B4 cells configured as pseudoislets rescued diabetes and significantly improved glucose tolerance, providing stable blood glucose control. Although the results provide proof-of-concept for possible therapeutic use of genetically engineered human β-cells configured as pseudoislets, further work to circumvent the tumorigenic properties of the cells, by genetic manipulation using viral vectors or implantation devices, will be required before such an approach can be realised in a clinical setting.
COMMENTS
Background
The clinical practicality of anti-diabetic islet transplantation therapy is hampered by poor long-term graft survival and the limited availability of donor pancreata. Implanting bioengineered human insulin releasing β-cell lines could potentially provide unlimited cells for such therapy.
Research frontiers
The electrofusion derived 1.1B4 human β-cell line has previously shown promise as a candidate for such therapy. Furthermore, in vitro studies of these cells have shown marked enhancements in functionality and survival when the cells were configured as psuedoislets rather than isolated cells.
Innovations and breakthroughs
This is the first study to show that the implantation of 1.1B4 pseudoislets can reverse diabetes in an animal model and to demonstrate additional beneficial effects of such treatment on the endocrine pancreas.
Applications
These results provide proof-of-concept for possible therapeutic use of genetically engineered human β-cells configured as psuedoislets as an alternative to the unsustainable practice of implanting primary human islets.
Peer-review
In this study, the authors investigated insulin secreting 1.1B4 cells as an option to rescue diabetes in severe combined immunodeficient mice. The manuscript is interesting, but several concerns need to be addressed before publication.
Footnotes
Supported by University of Ulster Research Strategic funding; and the award of a Northern Ireland Department of Employment and Learning Research Studentship to Alastair D Green.
Institutional review board statement: All the experiments were approved by Animal Welfare and Ethical Review Body at Ulster University.
Institutional animal care and use committee statement: All animal procedures were performed in adherence to the United Kingdom home office regulations (United Kingdom Animal Scientific Procedures Act 1986) and “Principles of laboratory animal care” (NIH Publication No. 86-23, revised 1985). The experiments were approved by the Northern Ireland Department of Health, Social Services and Public Safety and performed under the project license 2691 (Hormonal and Metabolic Studies).
Conflict-of-interest statement: The authors declare no conflicts of interest.
Data sharing statement: All data are included within the manuscript.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: April 12, 2016
First decision: May 19, 2016
Article in press: August 31, 2016
P- Reviewer: Kietzmann T, Liu SH, Malfitano C S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
References
- 1.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiasson JL, Aris-Jilwan N, Bélanger R, Bertrand S, Beauregard H, Ekoé JM, Fournier H, Havrankova J. Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar state. CMAJ. 2003;168:859–866. [PMC free article] [PubMed] [Google Scholar]
- 3.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, Klein R. Retinopathy in diabetes. Diabetes Care. 2004;27 Suppl 1:S84–S87. doi: 10.2337/diacare.27.2007.s84. [DOI] [PubMed] [Google Scholar]
- 4.Aring AM, Jones DE, Falko JM. Evaluation and prevention of diabetic neuropathy. Am Fam Physician. 2005;71:2123–2128. [PubMed] [Google Scholar]
- 5.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 6.Vantyghem MC, Press M. Management strategies for brittle diabetes. Ann Endocrinol (Paris) 2006;67:287–296. doi: 10.1016/s0003-4266(06)72600-2. [DOI] [PubMed] [Google Scholar]
- 7.Fotino N, Fotino C, Pileggi A. Re-engineering islet cell transplantation. Pharmacol Res. 2015;98:76–85. doi: 10.1016/j.phrs.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onaca N, Naziruddin B, Matsumoto S, Noguchi H, Klintmalm GB, Levy MF. Pancreatic islet cell transplantation: update and new developments. Nutr Clin Pract. 2007;22:485–493. doi: 10.1177/0115426507022005485. [DOI] [PubMed] [Google Scholar]
- 9.Lam VW, Pleass HC, Hawthorne W, Allen RD. Evolution of pancreas transplant surgery. ANZ J Surg. 2010;80:411–418. doi: 10.1111/j.1445-2197.2010.05309.x. [DOI] [PubMed] [Google Scholar]
- 10.Gruessner RW, Gruessner AC. The current state of pancreas transplantation. Nat Rev Endocrinol. 2013;9:555–562. doi: 10.1038/nrendo.2013.138. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 12.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 13.McCall M, Shapiro AM. Update on islet transplantation. Cold Spring Harb Perspect Med. 2012;2:a007823. doi: 10.1101/cshperspect.a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chhabra P, Brayman KL. Overcoming barriers in clinical islet transplantation: current limitations and future prospects. Curr Probl Surg. 2014;51:49–86. doi: 10.1067/j.cpsurg.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Hohmeier HE, Newgard CB. Cell lines derived from pancreatic islets. Mol Cell Endocrinol. 2004;228:121–128. doi: 10.1016/j.mce.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Baiu D, Merriam F, Odorico J. Potential pathways to restore β-cell mass: pluripotent stem cells, reprogramming, and endogenous regeneration. Curr Diab Rep. 2011;11:392–401. doi: 10.1007/s11892-011-0218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravassard P, Hazhouz Y, Pechberty S, Bricout-Neveu E, Armanet M, Czernichow P, Scharfmann R. A genetically engineered human pancreatic β cell line exhibiting glucose-inducible insulin secretion. J Clin Invest. 2011;121:3589–3597. doi: 10.1172/JCI58447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godfrey KJ, Mathew B, Bulman JC, Shah O, Clement S, Gallicano GI. Stem cell-based treatments for Type 1 diabetes mellitus: bone marrow, embryonic, hepatic, pancreatic and induced pluripotent stem cells. Diabet Med. 2012;29:14–23. doi: 10.1111/j.1464-5491.2011.03433.x. [DOI] [PubMed] [Google Scholar]
- 19.Santerre RF, Cook RA, Crisel RM, Sharp JD, Schmidt RJ, Williams DC, Wilson CP. Insulin synthesis in a clonal cell line of simian virus 40-transformed hamster pancreatic beta cells. Proc Natl Acad Sci USA. 1981;78:4339–4343. doi: 10.1073/pnas.78.7.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 21.Ashcroft SJ, Hammonds P, Harrison DE. Insulin secretory responses of a clonal cell line of simian virus 40-transformed B cells. Diabetologia. 1986;29:727–733. doi: 10.1007/BF00870283. [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126–132. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- 23.Ishihara H, Asano T, Tsukuda K, Katagiri H, Inukai K, Anai M, Kikuchi M, Yazaki Y, Miyazaki JI, Oka Y. Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia. 1993;36:1139–1145. doi: 10.1007/BF00401058. [DOI] [PubMed] [Google Scholar]
- 24.Radvanyi F, Christgau S, Baekkeskov S, Jolicoeur C, Hanahan D. Pancreatic beta cells cultured from individual preneoplastic foci in a multistage tumorigenesis pathway: a potentially general technique for isolating physiologically representative cell lines. Mol Cell Biol. 1993;13:4223–4232. doi: 10.1128/mcb.13.7.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCluskey JT, Hamid M, Guo-Parke H, McClenaghan NH, Gomis R, Flatt PR. Development and functional characterization of insulin-releasing human pancreatic beta cell lines produced by electrofusion. J Biol Chem. 2011;286:21982–21992. doi: 10.1074/jbc.M111.226795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scharfmann R, Pechberty S, Hazhouz Y, von Bülow M, Bricout-Neveu E, Grenier-Godard M, Guez F, Rachdi L, Lohmann M, Czernichow P, et al. Development of a conditionally immortalized human pancreatic β cell line. J Clin Invest. 2014;124:2087–2098. doi: 10.1172/JCI72674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thivolet C, Chatelain P, Haftek M, Durand A, Pugeat M. [Morphologic and functional study of a human insulin-secreting cell line] C R Acad Sci III. 1986;303:381–386. [PubMed] [Google Scholar]
- 28.Gueli N, Toto A, Palmieri G, Carmenini G, Delpino A. In vitro growth of a cell line originated from a human insulinoma. J Exp Clin Cancer Res. 1987;6:281–285. [Google Scholar]
- 29.Soldevila G, Buscema M, Marini V, Sutton R, James RF, Bloom SR, Robertson RP, Mirakian R, Pujol-Borrell R, Bottazzo GF. Transfection with SV40 gene of human pancreatic endocrine cells. J Autoimmun. 1991;4:381–396. doi: 10.1016/0896-8411(91)90154-5. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Beattie GM, Mally MI, Cirulli V, Itkin-Ansari P, Lopez AD, Hayek A, Levine F. Isolation and characterization of a cell line from the epithelial cells of the human fetal pancreas. Cell Transplant. 1997;6:59–67. doi: 10.1177/096368979700600110. [DOI] [PubMed] [Google Scholar]
- 31.MacFarlane WM, Chapman JC, Shepherd RM, Hashmi MN, Kamimura N, Cosgrove KE, O’Brien RE, Barnes PD, Hart AW, Docherty HM, et al. Engineering a glucose-responsive human insulin-secreting cell line from islets of Langerhans isolated from a patient with persistent hyperinsulinemic hypoglycemia of infancy. J Biol Chem. 1999;274:34059–34066. doi: 10.1074/jbc.274.48.34059. [DOI] [PubMed] [Google Scholar]
- 32.Narushima M, Kobayashi N, Okitsu T, Tanaka Y, Li SA, Chen Y, Miki A, Tanaka K, Nakaji S, Takei K, et al. A human beta-cell line for transplantation therapy to control type 1 diabetes. Nat Biotechnol. 2005;23:1274–1282. doi: 10.1038/nbt1145. [DOI] [PubMed] [Google Scholar]
- 33.Vasu S, McClenaghan NH, McCluskey JT, Flatt PR. Cellular responses of novel human pancreatic β-cell line, 1.1B4 to hyperglycemia. Islets. 2013;5:170–177. doi: 10.4161/isl.26184. [DOI] [PubMed] [Google Scholar]
- 34.Vasu S, McClenaghan NH, McCluskey JT, Flatt PR. Effects of lipotoxicity on a novel insulin-secreting human pancreatic β-cell line, 1.1B4. Biol Chem. 2013;394:909–918. doi: 10.1515/hsz-2013-0115. [DOI] [PubMed] [Google Scholar]
- 35.Vasu S, McClenaghan NH, McCluskey JT, Flatt PR. Mechanisms of toxicity by proinflammatory cytokines in a novel human pancreatic beta cell line, 1.1B4. Biochim Biophys Acta. 2014;1840:136–145. doi: 10.1016/j.bbagen.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 36.Guo-Parke H, McCluskey JT, Kelly C, Hamid M, McClenaghan NH, Flatt PR. Configuration of electrofusion-derived human insulin-secreting cell line as pseudoislets enhances functionality and therapeutic utility. J Endocrinol. 2012;214:257–265. doi: 10.1530/JOE-12-0188. [DOI] [PubMed] [Google Scholar]
- 37.Green AD, Vasu S, McClenaghan NH, Flatt PR. Pseudoislet formation enhances gene expression, insulin secretion and cytoprotective mechanisms of clonal human insulin-secreting 1.1B4 cells. Pflugers Arch. 2015;467:2219–2228. doi: 10.1007/s00424-014-1681-1. [DOI] [PubMed] [Google Scholar]
- 38.Flatt PR, Bailey CJ. Abnormal plasma glucose and insulin responses in heterozygous lean (ob/+) mice. Diabetologia. 1981;20:573–577. doi: 10.1007/BF00252768. [DOI] [PubMed] [Google Scholar]
- 39.Moffett RC, Vasu S, Thorens B, Drucker DJ, Flatt PR. Incretin receptor null mice reveal key role of GLP-1 but not GIP in pancreatic beta cell adaptation to pregnancy. PLoS One. 2014;9:e96863. doi: 10.1371/journal.pone.0096863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merino JF, Nacher V, Raurell M, Aranda O, Soler J, Montanya E. Improved outcome of islet transplantation in insulin-treated diabetic mice: effects on beta-cell mass and function. Diabetologia. 1997;40:1004–1010. doi: 10.1007/s001250050781. [DOI] [PubMed] [Google Scholar]
- 41.Ferrer-Garcia JC, Merino-Torres JF, Pérez Bermejo G, Herrera-Vela C, Ponce-Marco JL, Piñon-Selles F. Insulin-induced normoglycemia reduces islet number needed to achieve normoglycemia after allogeneic islet transplantation in diabetic mice. Cell Transplant. 2003;12:849–857. [PubMed] [Google Scholar]
- 42.Kikawa K, Sakano D, Shiraki N, Tsuyama T, Kume K, Endo F, Kume S. Beneficial effect of insulin treatment on islet transplantation outcomes in Akita mice. PLoS One. 2014;9:e95451. doi: 10.1371/journal.pone.0095451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly C, McClenaghan NH, Flatt PR. Role of islet structure and cellular interactions in the control of insulin secretion. Islets. 2011;3:41–47. doi: 10.4161/isl.3.2.14805. [DOI] [PubMed] [Google Scholar]
- 44.Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24:366–371. doi: 10.1007/BF00251826. [DOI] [PubMed] [Google Scholar]
- 45.Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, Cooper GJ, Holman RR, Turner RC. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9:151–159. [PubMed] [Google Scholar]
- 46.Li Z, Karlsson FA, Sandler S. Islet loss and alpha cell expansion in type 1 diabetes induced by multiple low-dose streptozotocin administration in mice. J Endocrinol. 2000;165:93–99. doi: 10.1677/joe.0.1650093. [DOI] [PubMed] [Google Scholar]
- 47.Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88:2300–2308. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 48.Liu Z, Kim W, Chen Z, Shin YK, Carlson OD, Fiori JL, Xin L, Napora JK, Short R, Odetunde JO, et al. Insulin and glucagon regulate pancreatic α-cell proliferation. PLoS One. 2011;6:e16096. doi: 10.1371/journal.pone.0016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meier JJ, Ueberberg S, Korbas S, Schneider S. Diminished glucagon suppression after β-cell reduction is due to impaired α-cell function rather than an expansion of α-cell mass. Am J Physiol Endocrinol Metab. 2011;300:E717–E723. doi: 10.1152/ajpendo.00315.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lock LT, Laychock SG, Tzanakakis ES. Pseudoislets in stirred-suspension culture exhibit enhanced cell survival, propagation and insulin secretion. J Biotechnol. 2011;151:278–286. doi: 10.1016/j.jbiotec.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 51.Liljebäck H, Grapensparr L, Olerud J, Carlsson PO. Extensive Loss of Islet Mass Beyond the First Day After Intraportal Human Islet Transplantation in a Mouse Model. Cell Transplant. 2016;25:481–489. doi: 10.3727/096368915X688902. [DOI] [PubMed] [Google Scholar]
- 52.Kirk K, Hao E, Lahmy R, Itkin-Ansari P. Human embryonic stem cell derived islet progenitors mature inside an encapsulation device without evidence of increased biomass or cell escape. Stem Cell Res. 2014;12:807–814. doi: 10.1016/j.scr.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 53.An D, Ji Y, Chiu A, Lu YC, Song W, Zhai L, Qi L, Luo D, Ma M. Developing robust, hydrogel-based, nanofiber-enabled encapsulation devices (NEEDs) for cell therapies. Biomaterials. 2015;37:40–48. doi: 10.1016/j.biomaterials.2014.10.032. [DOI] [PubMed] [Google Scholar]