Abstract
Background
Alcohol abuse, which impairs antioxidant defenses and promotes acute lung injury, increases Nrf2 nuclear translocation but nevertheless inhibits its activation of the antioxidant response element (ARE). Thioredoxin‐1 (Trx1) is required for optimal Nrf2 binding and activation of the ARE, and we hypothesized that its inhibition contributes to impaired Nrf2‐ARE signaling in the alcoholic lung.
Methods
Lung tissue and primary lung fibroblasts (PLFs) were isolated from C57/BL6 wild‐type (WT) and transgenic mice overexpressing the human Trx1 gene with a nuclear localizing sequence (NLS‐Tg); some mice consumed alcohol in water prior to lung tissue and PLF isolation; in some mice, acute lung injury was induced with intratracheal bleomycin. In other experiments, PLFs were isolated from WT and NLS‐Tg mice and then exposed to alcohol. Finally, PLF isolated from WT mice were transfected with Trx1 expression vector containing either a cytosolic localized sequence (NES) or a nuclear localized sequence (NLS) prior to alcohol exposure.
Results
Alcohol treatment in vivo or in vitro decreased Trx1 expression, and bleomycin‐treated alcohol‐fed mice had fibrotic disrepair in their lungs. In parallel, whereas alcohol exposure in vitro increased TGF β1 expression and decreased Nrf2‐ARE activity in PLF from WT mice, these effects were not observed in PLF from NLS‐Tg mice. Finally, selective overexpression of Trx1 in the nucleus but not in the cytosol preserved Nrf2‐ARE activity during alcohol exposure.
Conclusions
Although alcohol‐induced redox stress actually promotes Nrf2 nuclear translocation, the coincident suppression of Trx1 impairs Nrf2‐ARE activity within the nuclear compartment. Nuclear overexpression of Trx1 restored Nrf2‐ARE activity and attenuated alcohol‐induced TGF β1 expression and alcohol‐induced exaggerate response to bleomycin‐induced acute lung injury.
Keywords: Thioredoxin‐1, Bleomycin, TGFβ1, Lung Fibroblasts, Alcohol
Previously, we identified that alcohol exposure induces oxidative stress in the lung in part through suppression of nuclear factor (erythroid‐derived 2)‐like 2 (Nrf2) and its ability to activate the antioxidant response element (ARE) in alveolar epithelial cells and lung fibroblasts (Jensen et al., 2013; Sueblinvong et al., 2014). These discoveries resolved a long‐standing paradox; specifically, how does alcohol cause such profound depletion of the antioxidant glutathione (GSH) in the lungs of humans and experimental animals (Holguin et al., 1998; Moss et al., 1999) in the absence of any apparent inflammation? In parallel, we determined that chronic alcohol ingestion increases the expression and activation of transforming growth factor‐β1 (TGFβ1) in the lung (Bechara et al., 2004) and that alcohol‐induced TGFβ1 is associated with fibrotic disrepair following bleomycin‐induced acute lung injury in mice that could be prevented by dietary supplementation with the thiol antioxidant S‐adenosylmethionine (SAMe; Sueblinvong et al., 2014). Further, this alcohol‐mediated fibrotic disrepair was associated with discrete phenotypic/functional alterations in lung fibroblasts including evidence of myofibroblast transdifferentiation (Sueblinvong et al., 2014). Interestingly, even though alcohol suppressed total Nrf2 protein expression in lung fibroblasts, it somewhat paradoxically increased Nrf2 nuclear translocation even though Nrf2‐ARE signaling was dampened (Sueblinvong et al., 2014).
Nrf2 is a transcription factor that regulates cellular responses to oxidative stress by binding to the ARE sequence in the promoter regions of hundreds of genes including glutathione‐s‐transferases (GST; Rushmore and Pickett, 1993), thioredoxin‐1 (Trx1; Kim et al., 2003), and glutamate cysteine ligase (GCL; Moinova and Mulcahy, 1999). Within the cytosolic compartment, Nrf2 is maintained in a “ready” but inactive state by its association with Keap1, and this state requires adequate levels of GSH in the cytosol to maintain a reduced redox potential. In the context of an oxidative stress in the cell, oxidation of discrete cysteine residues in Keap1 causes it to dissociate from Nrf2, thereby freeing it up to translocate to the nucleus (Hansen et al., 2004; Nguyen et al., 2003). Within the nuclear compartment, Nrf2 is vulnerable to oxidative attack and subsequent oxidation of a different cysteine residue that results in a conformational change that limits its ability to bind to the ARE and activate the array of ARE‐dependent genes (Hansen et al., 2004). Thioredoxins, which include thioredoxin‐1 (Trx1) and thioredoxin‐2 (Trx2), and GSH are components of the two principal cellular thiol redox regulatory systems, and their synthesis is in fact mediated by ARE‐dependent gene products (Go and Jones, 2010, 2013). There is evidence that the Nrf2 redox state within the nuclear compartment is primarily regulated by Trx1 (Hansen et al., 2004). However, whether or not alcohol‐mediated inhibition of Nrf2‐ARE signaling, despite evidence for its increasing Nrf2 translocation, involves changes in Trx1 expression has not been investigated.
We speculated that chronic alcohol exposure induces chronic oxidative stress state through suppression of Nrf2‐ARE activity, which decreases the synthesis of GSH as well as the expression of Trx1 and thereby establishes a vicious cycle in which Nrf2‐ARE activity is further inhibited as Trx1 becomes progressively less available within the nuclear compartment to preserve the ability of Nrf2 to bind to the ARE. This would also explain how alcohol increases Nrf2 translocation even though Nrf2‐ARE activity is suppressed (Sueblinvong et al., 2014); specifically, the long‐recognized depletion of GSH by alcohol would be expected to increase the dissociation of Nrf2 from Keap1, but if nuclear levels of Trx1 are inadequate, then Nrf2 would be vulnerable to oxidation and inactivation. Therefore, we hypothesized that alcohol inhibits Trx1 expression and that targeted nuclear overexpression of Trx1 would improve Nrf2‐ARE activity and protect the alcoholic lung from fibroproliferative disrepair following bleomycin‐induced injury.
Materials and Methods
Animals and Alcohol Treatment
Three‐month‐old C57/BL6 (from Jackson Laboratories) and transgenic mice overexpressing human thioredoxin‐1 gene (NLS‐Tg) were utilized (Go et al., 2011). The presence of the NLS‐Trx1 gene was confirmed using mouse genomic DNA prepared from ear clip by Transnetyx genotype service (Go et al., 2011). Heterozygous or wild‐type (WT) littermates were utilized. Utilizing a modified version of the Meadows–Cook alcohol feeding model, mice were fed with standard chow ± 20% alcohol (v/v) for 8 weeks (starting at 5% of alcohol, ramping up over 2 weeks by 5% every 3 to 4 days to the final concentration of 20%) in water ad lib (Spitzer and Meadows, 1999; Sueblinvong et al., 2014). We analyzed the lungs of these mice after 8 weeks of alcohol ingestion based on our previous findings that experimental animals display abnormalities in the lung similar to those seen in alcoholic individuals, including glutathione depletion and an increased susceptibility to acute lung injury (Bechara et al., 2004; Holguin et al., 1998; Moss et al., 1999). This 8‐week ingestion period is comparable to experimental studies by other investigators. Further, this model has been validated by multiple investigators, and it has been previously documented that blood alcohol levels in these mice range from 0 to 400 mg/dl (0 to 0.4%) depending on when in a 24‐hour cycle the levels are checked (Cook et al., 2007); therefore, we did not measure blood alcohol levels in this study. Mice were injected intratracheally with bleomycin (2 units/kg in phosphate‐buffered saline (PBS; Sigma‐Aldrich, St Louis, MO; Sueblinvong et al., 2014). At two weeks, the mice were sacrificed and whole lung bronchoalveolar lavage (BAL) was performed and the lungs collected for analyses. All studies were approved by the Institutional Animal Care and Use Committee (IACUC) at Emory University and conformed to institutional standards for the humane treatment of laboratory animals.
Histology
Masson's trichrome staining (for collagen deposition) was performed on 5 μm paraffin‐embedded lung sections. Morphometric analyses for collagen deposition quantification were performed on 3 random lung sections (3 high power fields [40x] per section) using ImageJ 1.42 (http://rsbweb.nih.gov/ij/). In short, three nonadjacent randomly selected sections were imaged in a systematic manner to ensure capture of similar areas of the lungs from all sections. The background staining was subtracted and then the total area of the collagen deposition (blue stain) was measured. The percent of collagen deposition is calculated to the percent of total tissue area. The relative amount of collagen deposition was averaged and normalized to that of lungs from untreated mice.
Hydroxyproline Assay
The frozen right lower lobes of the lungs were analyzed for hydroxyproline content using a commercially available assay kit according to the manufacturer's protocol (BioVision, San Francisco, CA) and analyzed as previously described (Sueblinvong et al., 2014).
Enzyme‐Linked Immunosorbent Assay for TGFβ1
We previously determined that all of the TGFβ1 proteins released into the alveolar space of alcohol‐fed animals during acute inflammation are in the activated form (Bechara et al., 2004); therefore, in this study, the BAL fluid supernatants were not acidified prior to performing the enzyme‐linked immunosorbent assay (ELISA). The concentrations of active TGFβ1 in the BAL fluids were determined with a commercially available ELISA kit (R&D Systems, Minneapolis, MN) using the manufacturer's protocol (Sueblinvong et al., 2014).
Primary Lung Fibroblast Isolation, Culture, and Treatment
Primary lung fibroblasts (PLFs) were isolated from untreated NLS‐Tg or WT mice and expanded in culture (Sueblinvong et al., 2014). Cells were exposed to alcohol in culture (60 mM for 24 hours) and analyzed for mRNA and protein expression. In parallel, fibroblasts from WT mice were used for transfection experiments.
Analysis of TGFβ1 Protein Expression by Flow Cytometry
Flow cytometry was performed to assess for cell‐bound and intracellular TGFβ1 protein expression (Sueblinvong et al., 2014). In short, PLFs from wild‐type and NLS‐Tg mice were treated with alcohol (60 mM). Cells were harvested 24 hours later, permeabilized, and stained at a 1:50 concentration with a rabbit anti‐mouse antibody against TGFβ1 (Abcam, Cambridge, MA) and at a 1:100 concentration with a goat anti‐rabbit‐Alexa 488 secondary antibody (Invitrogen, Carlsbad, CA). Cells were analyzed for TGFβ1 expression using a FACSCalibur (Becton Dickinson, Mountain View, CA). Flow cytometry data were analyzed using the FlowJo vX.0.7 software (Tree Star, San Carlos, CA).
Assessing Nrf2‐ARE Activity
To assess for Nrf2‐ARE activity, we performed a Nrf2‐ARE luciferase activity assay using a Cigna ARE reporter (Sueblinvong et al., 2014). Transfected cells were then exposed to alcohol (60 mM for 24 hours), and a dual‐luciferase reporter assay was performed according to the manufacturer's protocol (Promega Corp., Madison, WI). Nrf2‐ARE promoter activity values were expressed as the ratios of arbitrary units of firefly luciferase/Renilla luciferase activity.
Messenger RNA Expression Analyses
Messenger RNA was isolated from PLFs, and first‐strand cDNA was synthesized using RNeasy kit (Qiagen, Valencia, CA). Quantitative PCR was performed with primers set for 18s, glutathione‐s‐transferase theta 2 (GSTT2), and the glutamate cysteine ligase catalytic subunit (GCLC). Details of the procedures and analyses were previously described (Sueblinvong et al., 2014).
Protein Isolation and Analysis
Total protein from whole lung lysates and PLFs lysates were isolated, and in some groups, the cytosolic and nuclear protein fractions were separated from PLFs. Western blot analyses were then performed as previously described (Sueblinvong et al., 2014). The immunoblots were incubated with a rabbit anti‐mouse thioredoxin‐1 antibody (Trx1; 1:1,000), a rabbit anti‐mouse Nrf2 antibody (1:500), a mouse anti‐mouse β‐actin antibody (1:1,000), or a rabbit anti‐mouse histone antibody (for nuclear protein; 1:1,000) and then a horseradish peroxidase–conjugated secondary antibody (Amersham Biosciences, Pittsburgh, PA). Immunoblots were visualized via enzyme‐linked chemiluminescence using the SuperSignal West Pico kit (Pierce Biotechnology, Rockford, IL).
Overexpression of Human Trx1 in the Nucleus (NLS‐Trx1) and the Cytosol (NES‐Trx1) of Mouse Lung Fibroblasts
Primary lung fibroblasts were transfected with the vectors containing human Trx1 (hTrx1) using the HiPerFect transfection reagent according to the manufacturer's protocol (Qiagen). The hTrx1 was modified to contain a c‐Myc epitope tag at the N‐terminus and a nuclear localization signal (NLS) at the C‐terminus (defined as NLS‐Trx1; Go et al., 2011, 2013) or to contain a hemagglutinin epitope tag at the N‐terminus and a nuclear export signal (NES) at the C‐terminus (defined as NES‐Trx1). Trx1 cDNA was inserted between the cytomegalovirus (CMV) promoter and the simian virus (SV‐40) terminator of a pCMV‐Myc or a pCMV‐HA mammalian expression vector purchased from Clontech Laboratories (Mountain View, CA; Go et al., 2011). The cells were then either treated with alcohol (60 mM for 72 hours) for protein expression analyses or transfected with a Cigna ARE reporter for analyses of Nrf2‐ARE activity as described previously.
Statistical Analyses
Unpaired t‐test or one‐way ANOVA was used for comparisons between or among groups using GraphPad InStat version 5. Post‐test analyses using Bonferroni's method were performed if statistical significance was reached by one‐way ANOVA. Significant differences were accepted at a p level of <0.05.
Results
Chronic Alcohol Ingestion Decreases Thioredoxin‐1 Gene and Protein Expression in the Lung
We previously showed that chronic alcohol ingestion decreased Nrf2 and downstream expression of ARE‐dependent genes in the lung, PLFs isolated from alcohol‐fed mice, and isolated PLFs or 3T3 fibroblasts exposed to alcohol in vitro (Jensen et al., 2013; Sueblinvong et al., 2014). We speculated that chronic alcohol exposure would therefore decrease thioredoxin‐1 (Trx1) expression as it is a Nrf2‐ARE‐dependent gene. As shown in Fig. 1, chronic alcohol ingestion for 8 weeks decreased (p < 0.05) Trx1 gene (Fig. 1, panel A) and protein (Fig. 1, panel B) expression in the whole lung by ~80% (p < 0.05) as compared to the expression in the lungs of control‐fed mice. This inhibition may explain the previously identified glutathione depletion in the airways of alcohol‐fed experimental animals as well as in the lungs of human subjects with alcohol use disorders.
Figure 1.
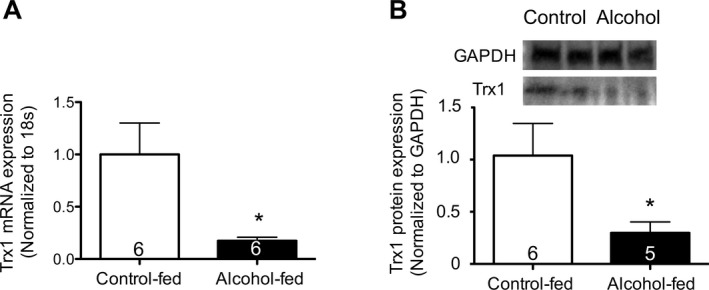
Chronic alcohol ingestion in vivo decreased Trx1 gene and protein expression in the whole lung. Control‐fed and alcohol‐fed mice were sacrificed after 8 weeks of feeding. The lungs were harvested and analyzed for (A) Trx1 mRNA expression by quantitative PCR and (B) Trx1 protein expression by Western immunoblot (representative gels shown above summary data). *p < 0.05 decreased compared to lungs from control‐fed mice. N = 5 to 6.
Alcohol Treatment Decreases Total Cellular as well as Cytosolic and Nuclear Trx1 Protein Expression in Lung Fibroblasts
To assess the direct effects of alcohol on Trx1 expression in PLFs, PLFs were exposed to alcohol (60 mM) for 72 hours and analyzed for Trx1 protein expression. As shown in Fig. 2, panel A, alcohol exposure decreased (p < 0.05) total cellular Trx1 protein expression by 52%. In parallel, we determined that alcohol exposure decreased (p < 0.05) both cytosolic and nuclear Trx1 expression by 80% and 60%, respectively (Fig. 2, panel B).
Figure 2.
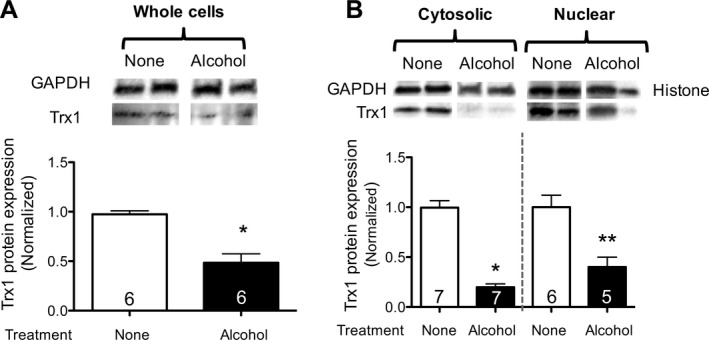
Alcohol exposure in vitro decreased Trx1 protein expression in lung fibroblasts. 3T3 lung fibroblasts were cultured ± alcohol (60 mM), and protein was isolated at 72 hours. (A) Total protein was analyzed for Trx1 expression by Western immunoblot (representative gels shown above summary data). (B) Total protein was separated into nuclear and cytosolic fractions and analyzed for Trx1 protein expression by Western immunoblot analyses (normalized to GAPDH in the cytosolic fraction and to histone in the nuclear fraction; representative gels shown above summary data). *p < 0.05 decreased compared to Trx1 expression in the cytosolic protein from unexposed cells (no alcohol). **p < 0.05 decreased compared to Trx1 expression in the nuclear protein from unexposed cells. N = 5 to 7.
Nuclear Overexpressing Trx1 Mice (NLS‐Tg) Are Protected Against Alcohol‐Mediated Fibrotic Disrepair Following Bleomycin‐Induced Lung Injury
We previously reported that chronic alcohol ingestion primes the lung toward exaggerated fibrotic disrepair following bleomycin‐induced lung injury likely through suppression of Nrf2‐ARE activity (Sueblinvong et al., 2014). We speculated that overexpressing Trx1 in the nucleus could prevent alcohol‐induced oxidative stress by stabilizing Nrf2‐DNA nuclear binding and thereby attenuate fibrotic disrepair following bleomycin‐induced lung injury. Alcohol ingestion for 8 weeks augmented collagen deposition as compared to WT control‐fed animals at 14 days following bleomycin‐induced lung injury; representative histological findings are shown in Fig. 3, panel A. In contrast, although bleomycin‐treated NLS‐Tg mice had increased collagen deposition as compared to untreated mice (Fig. 3, panel A), alcohol‐fed NLS‐Tg mice showed significantly less fibrosis in response to bleomycin when compared to alcohol‐fed WT mice following bleomycin‐induced lung injury (Fig. 3, panel A). These histological findings were confirmed by quantitative morphometric analyses that showed a significant increase (p < 0.05) in collagen deposition in alcohol‐fed, bleomycin‐treated WT mice (~180% increase) as compared to untreated and control‐fed, bleomycin‐treated WT mice (Fig. 3, panel B). All NLS‐Tg mice showed no statistically significant difference in collagen deposition (Fig. 3, panel B). Consistent with our previous study (Sueblinvong et al., 2014), there was a significant increase (~40%; p < 0.05) in hydroxyproline content in alcohol‐fed, bleomycin‐treated WT mice compared to untreated WT mice (Fig. 3, panels C and D). However, there was no significant change in hydroxyproline content in the lungs of control‐fed and alcohol‐fed, bleomycin‐treated NLS‐Tg mice when compared to untreated WT, untreated NLS‐Tg, and control‐fed, bleomycin‐treated, NLS‐Tg mice (Fig. 3, panels C and D).
Figure 3.
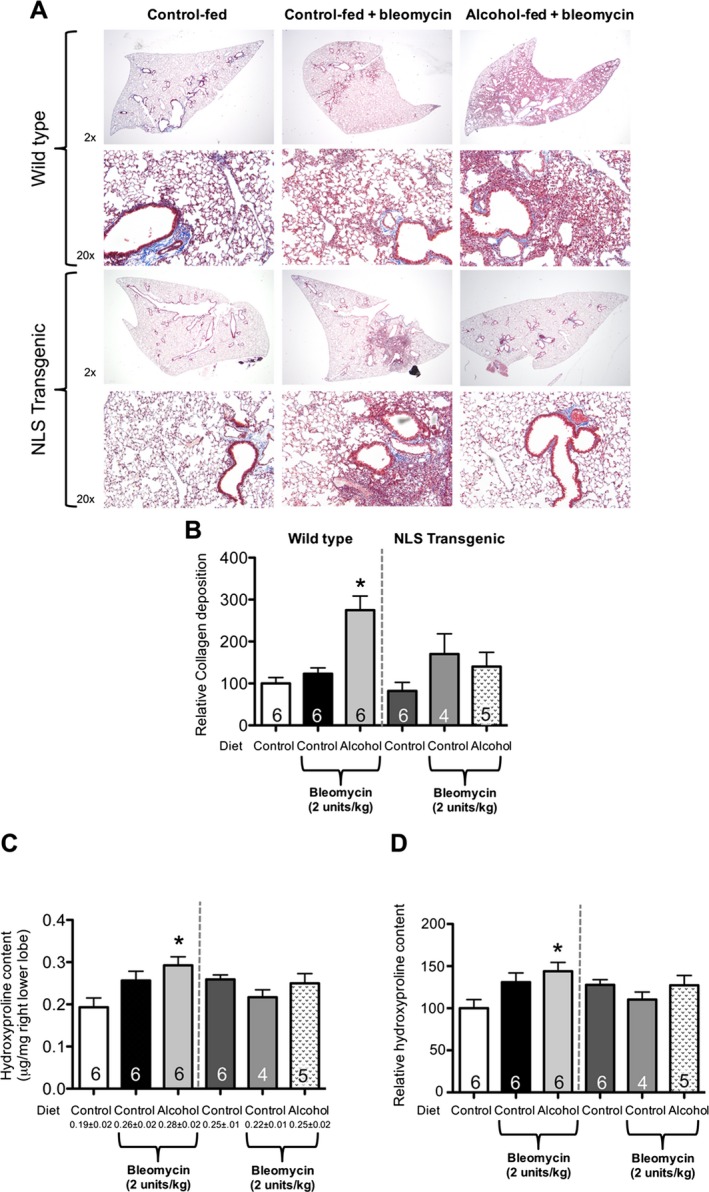
Nuclear overexpressing Trx1 (NLS‐Tg) mice were protected from alcohol‐mediated fibroproliferative disrepair following bleomycin‐induced lung injury. 3‐month‐old WT and NLS‐Tg littermates were treated ± alcohol (20% v/v in water for 8 weeks) then treated with 2 units/kg bleomycin intratracheally. Lungs were harvested at 14 days and analyzed for (A) histological changes by Masson's trichrome staining, (B) morphological analyses for collagen deposition, and (C) actual and (D) relative hydroxyproline content per mg of the right upper lobe of the lung. *p < 0.05 increased compared to untreated WT. N = 4 to 6.
Nuclear Overexpression of Trx1 Mitigates the Exaggerated TGFβ1 Expression Induced by Chronic Alcohol Ingestion Following Bleomycin‐Induced Lung Injury
We previously identified that alcohol increases TGFβ1 expression and accentuates bleomycin‐induced lung injury (Sueblinvong et al., 2014). We speculated that nuclear overexpression of Trx1 would blunt TGFβ1 expression during the response to lung injury. Exposure to bleomycin increased (p < 0.05) TGFβ1 expression in the BAL fluid of control‐fed WT mice, and this was significantly (p < 0.05) exaggerated in alcohol‐fed, bleomycin‐treated WT mice (Fig. 4). In contrast, although there was a trend toward increased TGFβ1 expression in control‐fed NLS‐Tg mice in response to bleomycin treatment, this did not reach statistical significance. More importantly, TGFβ1 expression in alcohol‐fed, bleomycin‐treated NLS‐Tg mice was significantly (p < 0.05) lower than in alcohol‐fed, bleomycin‐treated WT mice (Fig. 4). Taken together, the results argue that nuclear overexpression of Trx1 mitigates the susceptibility to fibrotic disrepair following acute lung injury caused by chronic alcohol ingestion.
Figure 4.
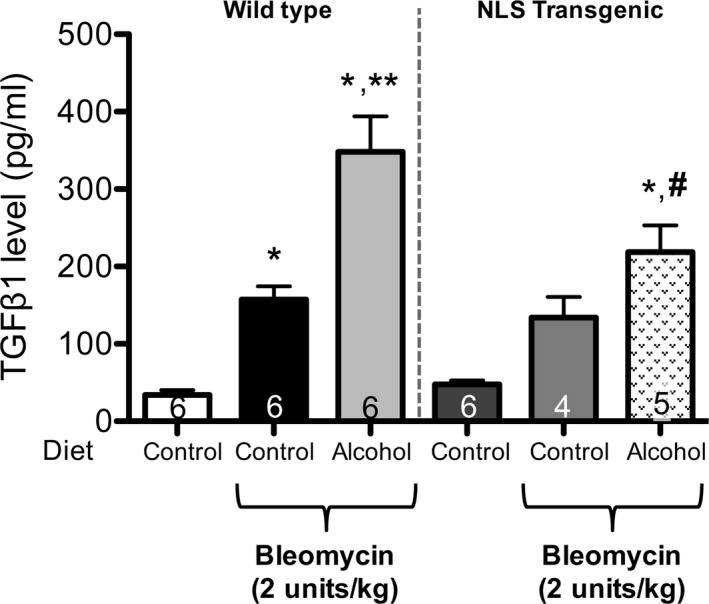
Nuclear overexpression of Trx1 mitigates the exaggerated TGF β1 expression induced by chronic alcohol ingestion following bleomycin‐induced lung injury in NLS‐Tg mice as compared to littermates. 3‐month‐old WT or NLS‐Tg were treated ± alcohol (20% v/v in water for 8 weeks) then treated with 2 units/kg bleomycin intratracheally. Lungs and BAL fluid were harvested at 14 days after and analyzed for activated TGF β1 in BALF by ELISA. *p < 0.05 increased compared to untreated WT. **p < 0.05 increased compared to control‐fed, bleomycin‐treated WT. # p < 0.05 decreased compared to alcohol‐fed, bleomycin‐treated WT. N = 4 to 6.
Alcohol Exposure In Vitro Fails to Induce TGFβ1 Expression in Primary Lung Fibroblasts From NLS‐Tg Mice
Primary lung fibroblasts from WT and NLS‐Tg mice were isolated and expanded in culture. Cells were exposed to alcohol (60 mM for 24 hours), and cell‐bound TGFβ1 expression was assessed by flow cytometry. Alcohol exposure increased (p < 0.05) TGFβ1 expression in WT PLF (Fig. 5; representative histograms are shown above the summary data). There was no change in TGFβ1 expression in alcohol‐treated NLS‐Tg PLF as compared to the expression in PLF from either untreated WT mice or untreated NLS‐Tg mice (Fig. 5).
Figure 5.
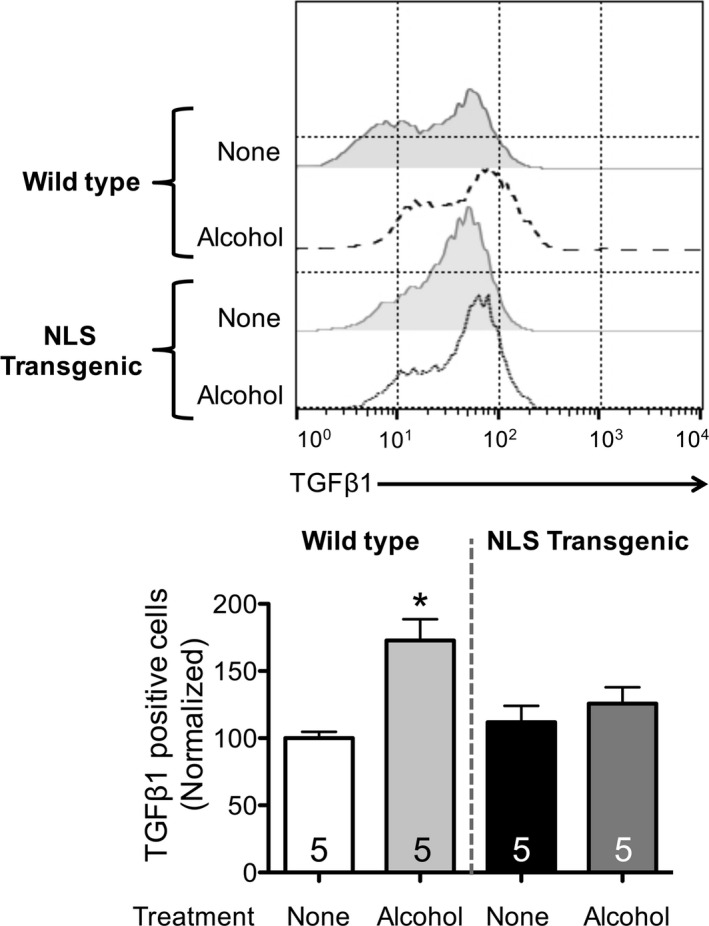
Alcohol exposure failed to induce TGF β1 expression in PLF isolated from NLS‐Tg mice. PLF were isolated from WT and NLS‐Tg mice; cells between passages 3 to 5 were used. Cells were cultured ± alcohol (60 mM) for 24 hours and analyzed for cell‐bound (intracellular and extracellular) TGF β1 expression by flow cytometry. *p < 0.05 increased compared to untreated WT. N = 5.
Nuclear Overexpression of Trx1 Prevents Alcohol‐Induced Nrf2‐ARE Activity Suppression in Lung Fibroblasts
We previously showed that alcohol exposure suppressed Nrf2 expression and Nrf2‐ARE activity in both alveolar epithelial cells and lung fibroblasts (Hansen et al., 2004; Sueblinvong et al., 2014). We speculated that nuclear overexpression of Trx1 could enhance Nrf2‐ARE activity in the presence of alcohol by preserving a relatively healthier redox balance and allowing Nrf2 to bind to the ARE sequence more effectively. PLFs from WT and NLS‐Tg mice were exposed to alcohol, and Nrf2‐ARE activity was measured. Alcohol suppressed Nrf2‐ARE activity by ~40% (p < 0.05) in alcohol‐exposed PLF from WT mice (60 mM for 24 hours, Fig. 6, panel A). There was no significant inhibition of Nrf2‐ARE activity in alcohol‐exposed PLF from NLS‐Tg mice as compared to untreated PLF from WT or NLS‐Tg mice (Fig. 6, panel A). Nuclear overexpression of Trx1 preserved the expression of the ARE‐dependent genes for GSTT2 and GCLC in alcohol‐exposed PLF (Fig. 6, panel B).
Figure 6.
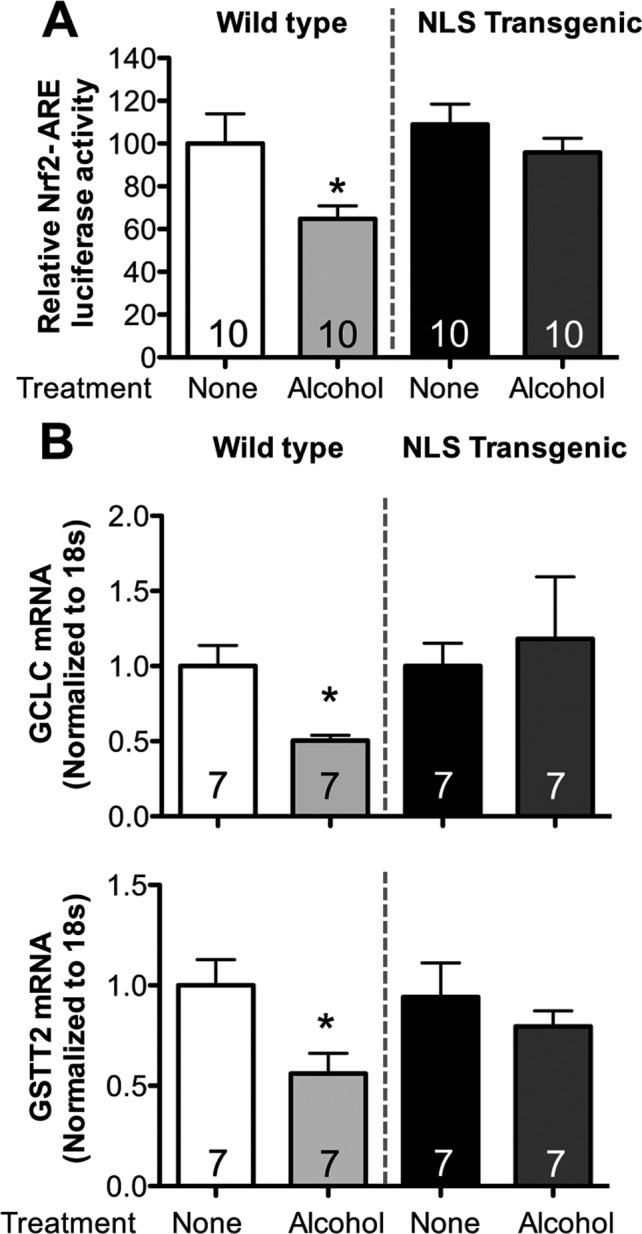
Alcohol failed to suppress Nrf2‐ARE activity in PLF isolated from NLS‐Tg mice. PLF were isolated from WT and NLS‐Tg mice; cells between passages 3 to 5 were used. (A) Cells were transfected with ARE‐luciferase or Renilla luciferase for 24 hours and then cultured ± alcohol (60 mM) for 20 hours at which time relative Nrf2‐ARE activity (Nrf2 luciferase activity normalized to Renilla luciferase activity) was quantified. (B) Cells were cultured ± alcohol (60 mM) for 24 hours and then analyzed for GCLC and GSTT2 gene expression by quantitative PCR of mRNA. *p < 0.05 decreased compared to untreated WT. N = 7 to 10.
Overexpression of Trx1 in Either the Cytosolic or the Nuclear Compartment Maintained Nrf2 Protein Expression in Both Compartments During Alcohol Exposure
We previously showed that alcohol decreases cytosolic Nrf2 protein levels while increasing Nrf2 protein levels in the nucleus, showing that nuclear translocation is actually increased by alcohol (Sueblinvong et al., 2014). We analyzed Nrf2 protein expression in both the nuclear and cytosolic compartments of PLF transfected with either the cytosolic‐targeted (NES) Trx1 or the nuclear‐targeted (NLS) Trx1 vector (Myc‐tagged for NLS or HA‐tagged for NES as detailed in Methods), then exposed to alcohol (60 mM for 72 hours). As shown in Fig. 7, alcohol did not suppress Nrf2 expression in the cytosolic compartments of either NLS‐Trx1‐transfected cells (panel A) or NES‐Trx1‐transfected cells (panel C) compared to cells transfected with the empty vector. In parallel, there were no significant differences in Nrf2 protein expression in untreated and alcohol‐exposed cells which were transfected with either NLS‐Trx1 or NES‐Trx1 (panels B and D).
Figure 7.
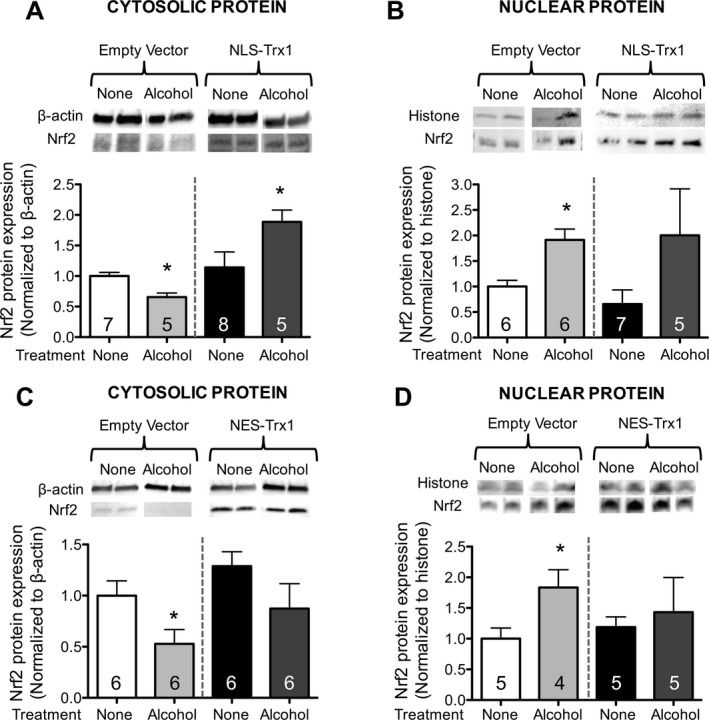
Overexpression of Trx1 in either cytosol or nucleus prevented alcohol‐mediated Nrf2 protein suppression in the cytosol and inhibited Nrf2 nuclear translocation in lung fibroblasts. PLF from WT mice were transfected with nuclear localized sequence Trx1 (NLS; panels A,B) or nuclear export sequence Trx1 (NES; panels C,D), or appropriate empty vector controls, cultured ± alcohol (60 mM) for 72 hours, cytosolic and nuclear protein were separated and analyzed for Nrf2 expression by Western immunoblot. *p < 0.05 changed compared to untreated, empty vector (EV)‐transfected lung fibroblasts. N = 4 to 8.
Whereas Nuclear‐Specific Overexpression of Trx1 Preserved Nrf2‐ARE Activity During Alcohol, Cytosol‐Specific Overexpression of Trx1 Failed to Reverse Alcohol‐Mediated Inhibition of Nrf2‐ARE Signaling
The ability of Nrf2 to bind the ARE depends on it being maintained in a reduced state by Trx1 (Cho et al., 2006; Surh et al., 2008). To assess the role of Trx1 in different cellular compartments on Nrf2‐ARE activity, we cotransfected cells with either NLS‐Trx1 or NES‐Trx1 (or appropriate empty vector) and Nrf2‐ARE luciferase reporter vector, then exposed to alcohol (60 mM for 24 hours). As shown in Fig. 8, cytosolic overexpression of Trx1 did not preserve Nrf2‐ARE activity during alcohol exposure while nuclear overexpression of Trx1 did.
Figure 8.
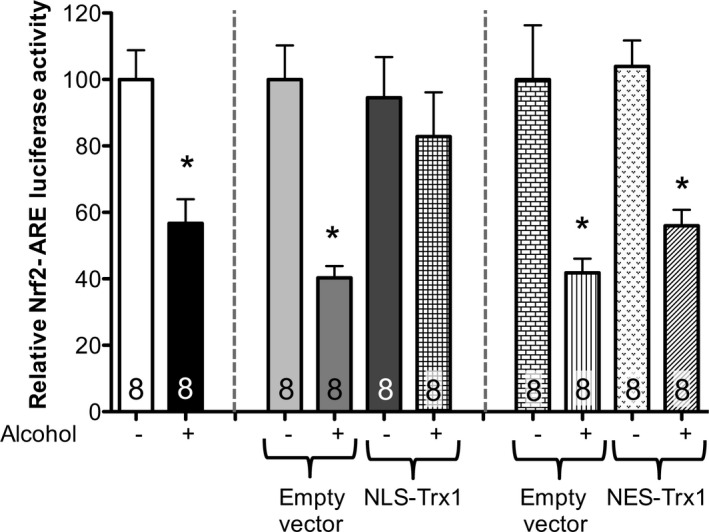
Nuclear localized Trx1 overexpression attenuated alcohol‐mediated Nrf2‐ARE activity suppression but not cytosolic localized Trx1 overexpression in lung fibroblasts. PLF from WT mice were transfected with nuclear localized sequence Trx1 (NLS) or nuclear export sequence Trx1 (NES), incubated overnight, transfected by ARE‐luciferase or Renilla luciferase for 24 hours and then cultured ± alcohol (60 mM) for 20 hours at which time relative Nrf2‐ARE activity (Nrf2 luciferase activity normalized to Renilla luciferase activity) was quantified. *p < 0.05 decreased compared to untreated lung fibroblasts (NT). N = 8.
Discussion
In this study, we determined that chronic alcohol ingestion suppressed Trx1 expression in the experimental mouse lung in vivo. Further, we found that alcohol exposure in vitro decreased overall and compartment‐specific (i.e., cytosolic and nuclear) Trx1 protein expression in PLF. Mice overexpressing Trx1 in the nucleus (NLS‐Tg) were relatively protected from alcohol‐induced fibrotic disrepair in response to bleomycin‐induced lung injury as reflected by decreased collagen deposition, lower tissue levels of hydroxyproline, and histological evidence of less fibroproliferation. In parallel, nuclear overexpression of Trx1 also attenuated alcohol‐induced TGFβ1 expression, and these findings were further confirmed in PLF isolated from NLS‐Tg mice and exposed to alcohol in vitro. Nuclear overexpression of Trx1 in PLF maintained Nrf2‐ARE activity in the presence of alcohol. Further analyses suggested that this is specific only to nuclear overexpression of Trx1, as cytosolic overexpression of Trx1 had no ability to preserve Nrf2‐ARE activity in alcohol‐exposed cells. Taken together, these results build on our previous work and reveal a role for the suppression of Trx1 in the alcoholic lung in which alcohol‐induced TGFβ1 inhibits Nrf2 signaling and Nrf2‐ARE activity which in turn suppresses the synthesis of GSH and Trx1, which further dampens Nrf2‐ARE activity and induces the expression and activation of even more TGFβ1 as the redox potential becomes progressively more oxidized. Finally, our study extends previous work that first revealed compartment‐specific role for GSH and Trx1 in Nrf2‐ARE signaling (Hansen et al., 2004), and the subsequent maintenance of healthy antioxidant systems that are particularly necessary within the highly oxidizing environment of the lung.
In this study, we further explored the intricate relationship between TGFβ1 expression and Nrf2‐ARE signaling during chronic alcohol exposure. Previously, we identified that alcohol suppressed Nrf2‐ARE signaling through activation of TGFβ1 expression, even though the protein expression of Nrf2 within the nuclear compartment was actually increased (Sueblinvong et al., 2014). It appears that alcohol‐induced oxidative stress activates Nrf2 (i.e., dissociates Nrf2 from Keap1) in the cytosol and thereby increases its nuclear translocation (Cho et al., 2006; Surh et al., 2008). However, an optimal redox state is required in the nucleus to maintain a specific cysteine residue (cysteine506) of Nrf2 in the reduced state and thereby enable efficient binding to the consensus ARE sequence. The redox state in the nucleus is largely maintained by one of the Nrf2‐ARE‐dependent genes, Trx1 (Hansen et al., 2004). We speculated that concomitant alcohol‐induced changes in the redox status in the nucleus promote the oxidation of Nrf2 and inhibit its binding to the ARE through suppression of Trx1. We determined that either chronic alcohol ingestion in vivo or alcohol exposure in vitro suppressed Trx1 protein expression by ~80% and ~52%, respectively; this inhibition was evident in both the whole lung and lung fibroblasts. In parallel, alcohol suppressed Trx1 expression in both the cytosolic and nuclear compartments in lung fibroblast by ~80% and ~60%, respectively. This is in contrast to our aforementioned previous finding that alcohol actually increases the translocation of Nrf2 protein into the nuclear compartment (Sueblinvong et al., 2014). Taken together, our previous and current studies reveal that alcohol‐induced oxidative stress appears to be sensed in the cytosol, as reflected by the dissociation of Nrf2 from Keap1 and its translocation into the nucleus, but that Nrf2‐ARE signaling is dampened when it should be amplified. This is likely caused, at least in part, by an inadequate supply of Trx1 in the nuclear compartment to facilitate Nrf2 binding to the ARE. As a result, alcohol inhibits the expression of all of the ARE‐responsive genes including Trx1. The suppression of Trx1 further inhibits Nrf2‐ARE activity and establishes a vicious feed‐forward pathological cycle, consistent with what has been previously reported in HeLa cells in which glutathione depletion was induced by treatment with buthionine sulfoximine (Hansen et al., 2004).
Our findings implicating a central role for Trx1 are consistent with work by other investigators showing that chronic alcohol ingestion decreased Trx1 expression in the liver by ~50% and exogenous supplementation of Trx inhibited alcohol‐induced oxidative stress damage in the liver (Cohen et al., 2009). We speculated that overexpressing Trx1 in the nucleus might prevent alcohol‐mediated fibrotic disrepair following acute lung injury. We analyzed the combined effects of chronic alcohol ingestion and bleomycin in inducing lung injury, and how these effects were modified by nuclear overexpression of Trx1. Alcohol‐fed, bleomycin‐treated WT mice developed overt lung fibrosis 14 days following intratracheal instillation of bleomycin. Alcohol‐fed, bleomycin‐treated NLS‐Tg mice showed significantly less lung fibrosis by histological analyses and quantification of hydroxyproline content. Bleomycin treatment induced TGFβ1 expression in both WT and NLS‐Tg mice, and alcohol ingestion further exacerbated bleomycin‐induced TGFβ1 expression in the BAL fluid of both WT and NLS‐Tg mice. However, TGFβ1 expression was significantly lower in the alcohol‐fed, bleomycin‐treated NLS‐Tg mice. In parallel, alcohol exposure in vitro failed to increase TGFβ1 expression in PLF isolated from NLS‐Tg mice. These findings suggest that nuclear overexpression of Trx1 protected the lung and lung fibroblasts from alcohol‐induced TGFβ1 expression.
We previously reported an antagonistic relationship between TGFβ1 and Nrf2‐ARE signaling in alcohol‐exposed lung fibroblasts, with evidence that TGFβ1 signaling is upstream to the Nrf2‐ARE signaling pathway (Sueblinvong et al., 2014). We assessed Nrf2‐ARE activity in PLF isolated from NLS‐Tg mice and found that alcohol failed to suppress Nrf2‐ARE activity and the expression of the ARE‐dependent genes, GST and GCLC. These results provide evidence that Trx1 influences the redox state within the nucleus and thereby regulates Nrf2 function and Nrf2‐ARE activity. Further, these results complement our previous findings that alcohol induces oxidative stress through TGFβ1 and that enhancing Nrf2‐ARE activity suppressed TGFβ1 expression through a negative feedback loop (Sueblinvong et al., 2014).
Thioredoxin‐1 is a small disulfide‐containing redox regulator protein that is expressed in both the nucleus and the cytosol (Burke‐Gaffney et al., 2005; Go and Jones, 2009; Holmgren and Lu, 2010). Hansen and colleagues elucidated the importance of nuclear Trx1 expression in regulating Nrf2 function (Hansen et al., 2004). To assess the specific effect of Trx1 on Nrf2‐ARE signaling in the nucleus, we transfected lung fibroblasts with hTrx1 containing either a nuclear localized sequence (NLS) or a nuclear export sequence (NES) to overexpress Trx1 in each specific cellular compartment (Hansen et al., 2007). Overexpression of Trx1 in either compartment preserved the relative expression and distribution of Nrf2 in both compartments during alcohol exposure. However, whereas nuclear overexpression of Trx1 also preserved Nrf2‐ARE signaling function suppression during alcohol exposure, cytosolic overexpression of Trx1 did not. These findings suggest that overexpression of Trx1 in the cytosol might restrict (but does not completely eliminate) Nrf2‐Keap1 dissociation and subsequent Nrf2 nuclear translocation through maintaining a relatively reduced redox potential in the cytosol which limits Keap1 oxidization. The Nrf2 that does dissociate and translocate to the nucleus is nevertheless less functional due to the stressful oxidizing environment in the nucleus. In contrast, overexpression of Trx1 in the nucleus preserves Nrf2 in a relatively reduced and functional state that enables it to bind the ARE and turn on the antioxidant and other cellular defenses that provide protection against alcohol exposure (and other stresses).
Overall, this study provides further insight into the mechanisms by which alcohol primes the lung for fibroproliferative disrepair. Despite strong evidence suggesting that oxidative stress and glutathione depletion render the lung susceptible to infection, injury, and fibrosis, treatments aimed at increasing lung glutathione levels in these clinical settings have been disappointing (Adhikari et al., 2004; Homma et al., 2012; Jain and Chandel, 2013). The evidence from this study, together with previous studies, suggests that increasing the intracellular glutathione levels alone would not have a sufficient impact on global antioxidant defenses, and specifically on the Nrf2‐ARE signaling pathway (Hansen et al., 2004, 2007). Targeting normalization of the redox balance in the nucleus may be more important, or at least additive with glutathione supplementation, in preserving cellular defenses during severe stresses. If this is indeed the case, then a challenge is to identify therapeutic strategies that can maintain a healthier redox balance in the nucleus and prove to be effective at limiting the pathological effects of acute lung injury and fibrosis.
In summary, in this study, we have further defined the mechanisms by which alcohol exposure mediates chronic oxidative stress and thereby produces an “alcoholic lung” phenotype that not only renders the lung susceptible to injury but that also promotes fibroproliferative disrepair following lung injury. Specifically, alcohol induces oxidative stress via a vicious cycle in which aberrant TGFβ1 expression inhibits Nrf2 expression and Nrf2‐ARE activity, which in turn dampens the production of glutathione and Trx1 and further inhibits Nrf2‐ARE signaling. The consequent limitation of the available pools of glutathione and Trx1 eventually reaches a critical threshold, and the cell loses its ability to mount adequate antioxidant defenses. The resulting oxidative stress induces even more TGFβ1, and the cycle feeds forward with potentially grave consequences to the host when faced with an acute inflammatory insult such as pneumonia or sepsis. In addition, this study provides further evidence that Nrf2‐ARE activity is regulated by the glutathione and Trx1 thiol redox systems in a compartment‐specific fashion and that nuclear Trx1 is essential to maintain the ability of Nrf2 to bind and activate the ARE. These findings reveal that nuclear overexpression of Trx1 protects the alcoholic lung from fibrotic disrepair through promotion of Nrf2‐ARE signaling and the maintenance of robust antioxidant defenses. Strategies such as treatment with glutathione precursor, that is, SAMe, alone, or perhaps in combination with other agents such as phytochemicals that induce Nrf2 and also increase Nrf2‐ARE signaling, might be effective in individuals at high risk for lung injury and fibroproliferative disrepair because of underlying alcohol use disorders or other chronic conditions that weaken cellular defenses.
Authors' contribution
VS and DMG designed the research project; VS, DCN, and STM performed experiments and analyzed data; VS and DMG interpreted the results of the experiments and then drafted, edited, and revised the manuscript. Y‐MG and DPJ provided NLS‐Tg mice and designed NLS and NES constructs for transfection.
Disclosures
No conflict of interests, financial or otherwise, are declared by the authors.
Acknowledgments
The authors wish to thank Mike Koval, Bashar Staitieh, and Xian Fan for their scientific discussions and helpful suggestions with this manuscript.
Research supports for this work include NIH K08 AA021404‐01 for VS, NIH P50 AA013757 for VS and DMG, NIH R01 AA017627 and a VA Merit Review for DMG, NIH R01 ES023485 for Y‐MG and DPJ.
References
- Adhikari N, Burns KE, Meade MO (2004) Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev CD004477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara RI, Brown LA, Roman J, Joshi PC, Guidot DM (2004) Transforming growth factor beta1 expression and activation is increased in the alcoholic rat lung. Am J Respir Crit Care Med 170:188–194. [DOI] [PubMed] [Google Scholar]
- Burke‐Gaffney A, Callister ME, Nakamura H (2005) Thioredoxin: friend or foe in human disease? Trends Pharmacol Sci 26:398–404. [DOI] [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Kleeberger SR (2006) Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal 8:76–87. [DOI] [PubMed] [Google Scholar]
- Cohen JI, Roychowdhury S, Dibello PM, Jacobsen DW, Nagy LE (2009) Exogenous thioredoxin prevents ethanol‐induced oxidative damage and apoptosis in mouse liver. Hepatology 49:1709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RT, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, Jerrells TR, Nashelsky MB, Ray NB, Haugen TH, Waldschmidt TJ (2007) Thymocytes, pre‐B cells, and organ changes in a mouse model of chronic ethanol ingestion–absence of subset‐specific glucocorticoid‐induced immune cell loss. Alcohol Clin Exp Res 31:1746–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Jones DP (2009) Thioredoxin redox western analysis. Curr Protoc Toxicol, Chapter 17, Unit17, 12. [DOI] [PubMed] [Google Scholar]
- Go YM, Jones DP (2010) Redox control systems in the nucleus: mechanisms and functions. Antioxid Redox Signal 13:489–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Jones DP (2013) The redox proteome. J Biol Chem 288:26512–26520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Kang SM, Roede JR, Orr M, Jones DP (2011) Increased inflammatory signaling and lethality of influenza H1N1 by nuclear thioredoxin‐1. PLoS One 6:e18918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Orr M, Jones DP (2013) Increased nuclear thioredoxin‐1 potentiates cadmium‐induced cytotoxicity. Toxicol Sci 131:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JM, Moriarty‐Craige S, Jones DP (2007) Nuclear and cytoplasmic peroxiredoxin‐1 differentially regulate NF‐kappaB activities. Free Radic Biol Med 43:282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JM, Watson WH, Jones DP (2004) Compartmentation of Nrf‐2 redox control: regulation of cytoplasmic activation by glutathione and DNA binding by thioredoxin‐1. Toxicol Sci 82:308–317. [DOI] [PubMed] [Google Scholar]
- Holguin F, Moss I, Brown LA, Guidot DM (1998) Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin‐mediated acute edematous lung injury in rats. J Clin Invest 101:761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A, Lu J (2010) Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem Biophys Res Commun 396:120–124. [DOI] [PubMed] [Google Scholar]
- Homma S, Azuma A, Taniguchi H, Ogura T, Mochiduki Y, Sugiyama Y, Nakata K, Yoshimura K, Takeuchi M, Kudoh S, Japan NACCSG (2012) Efficacy of inhaled N‐acetylcysteine monotherapy in patients with early stage idiopathic pulmonary fibrosis. Respirology 17:467–477. [DOI] [PubMed] [Google Scholar]
- Jain M, Chandel NS (2013) Rethinking antioxidants in the intensive care unit. Am J Respir Crit Care Med 188:1283–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JS, Fan X, Guidot DM (2013) Alcohol causes alveolar epithelial oxidative stress by inhibiting the nuclear factor (erythroid‐derived 2)‐like 2‐antioxidant response element signaling pathway. Am J Respir Cell Mol Biol 48:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Yamaguchi Y, Kondo N, Masutani H, Yodoi J (2003) Thioredoxin‐dependent redox regulation of the antioxidant responsive element (ARE) in electrophile response. Oncogene 22:1860–1865. [DOI] [PubMed] [Google Scholar]
- Moinova HR, Mulcahy RT (1999) Up‐regulation of the human gamma‐glutamylcysteine synthetase regulatory subunit gene involves binding of Nrf‐2 to an electrophile responsive element. Biochem Biophys Res Commun 261:661–668. [DOI] [PubMed] [Google Scholar]
- Moss M, Steinberg KP, Guidot DM, Duhon GF, Treece P, Wolken R, Hudson LD, Parsons PE (1999) The effect of chronic alcohol abuse on the incidence of ARDS and the severity of the multiple organ dysfunction syndrome in adults with septic shock: an interim and multivariate analysis. Chest 116:97S–98S. [PubMed] [Google Scholar]
- Nguyen T, Sherratt PJ, Pickett CB (2003) Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 43:233–260. [DOI] [PubMed] [Google Scholar]
- Rushmore TH, Pickett CB (1993) Glutathione S‐transferases, structure, regulation, and therapeutic implications. J Biol Chem 268:11475–11478. [PubMed] [Google Scholar]
- Spitzer JH, Meadows GG (1999) Modulation of perforin, granzyme A, and granzyme B in murine natural killer (NK), IL2 stimulated NK, and lymphokine‐activated killer cells by alcohol consumption. Cell Immunol 194:205–212. [DOI] [PubMed] [Google Scholar]
- Sueblinvong V, Tseng V, Smith T, Saghafi R, Mills ST, Neujahr DC, Guidot DM (2014) TGFbeta1 mediates alcohol‐induced Nrf2 suppression in lung fibroblasts. Alcohol Clin Exp Res 38:2731–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh YJ, Kundu JK, Na HK (2008) Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med 74:1526–1539. [DOI] [PubMed] [Google Scholar]


