Abstract
A multigene analysis of a combined ITS-LSU-SSU-rpb2-tef1 sequence data matrix was applied to infer the phylogenetic position of the genus Thyridaria in the Pleosporales. The generic type of Thyridaria, T. broussonetiae (syn. T. incrustans), is situated in a clade currently named Roussoellaceae, which becomes a synonym of Thyridariaceae. However, Thyridaria rubronotata does not belong to this clade, but is here recognised as Cyclothyriella rubronotata in its own family Cyclothyriellaceae. The Thyridariaceae contain the genera Thyridaria, Roussoella, Roussoellopsis, Neoroussoella and the new genus Parathyridaria. Roussoella acaciae is combined in Thyridaria and Roussoella percutaenea in Parathyridaria. Ohleria modesta and an additional new thyridaria-like genus, Hobus, are found to represent isolated lineages with unresolved phylogenetic affinites within the Pleosporales. For Ohleria the new family Ohleriaceae is established. Melanomma fuscidulum belongs to Nigrograna, and three new species are described in this genus. A strain named Biatriospora marina clusters with Nigrograna. Based on the newly recognised species in Nigrograna, morphology and ecology do in no way correlate among these genera, therefore we erect the new family Nigrogranaceae for Nigrograna and recommend to discontinue the use of the family name Biatriosporaceae until fresh material of B. marina becomes available for sequencing.
Key words: Ascomycota, Cyclothyrium, Dothideomycetes, Melanomma, Phylogenetic analysis, Pleosporales
Taxonomic novelties: New families: Cyclothyriellaceae Jaklitsch & Voglmayr, Nigrogranaceae Jaklitsch & Voglmayr, Ohleriaceae Jaklitsch & Voglmayr
New genera: Cyclothyriella Jaklitsch & Voglmayr, Hobus Jaklitsch & Voglmayr, Parathyridaria Jaklitsch & Voglmayr
New species: Hobus wogradensis Jaklitsch & Voglmayr; Nigrograna mycophila Jaklitsch, Friebes & Voglmayr; N. norvegica Jaklitsch & Voglmayr; N. obliqua Jaklitsch & Voglmayr; Parathyridaria ramulicola Jaklitsch, Fourn. & Voglmayr
New combinations: Cyclothyriella rubronotata (Berk. & Broome) Jaklitsch & Voglmayr; Nigrograna fuscidula (Sacc.) Jaklitsch & Voglmayr; Parathyridaria percutanea (S.A. Ahmed, D.A. Stevens, W.W.J. van de Sande & G.S. de Hoog) Jaklitsch & Voglmayr; Thyridaria acaciae (Crous & M.J. Wingf.) Jaklitsch & Voglmayr
Epitypifications (basionyms): Cucurbitaria broussonetiae Sacc., Sphaeria fuscidula Sacc., Melogramma rubronotatum Berk.
Introduction
Besides Thyronectria (Jaklitsch and Voglmayr, 2014, Checa et al., 2015), Thyridaria is another genus that Saccardo derived and separated from Thyridium. In order to clarify the concept of the genus, the identity and history of its generic type has to be evaluated: Cucurbitaria broussonetiae was described by Saccardo (1873). In 1875 (Saccardo 1875a) he described Thyridaria incrustans in the schedae of his Mycotheca Veneta and based it on Cucurbitaria broussonetiae, giving Broussonetia as the exclusive host. In the same year (Saccardo 1875b) he established the genus Thyridaria with T. incrustans as its generic type and C. broussonetiae as its synonym. Later, he (Saccardo 1883) listed many different hosts for T. incrustans and thus produced a collective name rather than a well-defined name for a single species (see below). As C. broussonetiae is older than T. incrustans, Traverso (1906, p. 302) noted that Berlese (1894) and Saccardo (1875b) preferred the name Thyridaria incrustans contrary to nomenclatural rules and combined C. broussonetiae in Thyridaria with T. incrustans as a synonym. Traverso's (1906) treatment is nomenclaturally correct.
Several authors studied Thyridaria or selected members of this genus. Chesters (1938) studied type material of T. rubronotata and compared morphology, ascoma ontogeny and a putative asexual morph of fresh material of T. rubronotata collected and isolated from Acer and Ulmus with Melanomma pulvis-pyrius and M. fuscidulum. He also recognised conspecificity of T. delognensis (originally described from Acer pseudoplatanus) and Massaria lateritia Tul. (described from Aesculus) with T. rubronotata (originally described from Ulmus). He reported synchronous development of pycnidia with ascomata and found that the asexual morph characterised by pycnidia forming slimy masses of amerosporous conidia on phialides lacking conidiophores, is like the aposphaeria-like morphs of Melanomma, only that the conidia turn brownish and are thus coniothyrium-like. He accepted the name Cytoplea juglandis, originally described as Phoma ulmicola Berk., for it. Wehmeyer (1941) monographed Thyridaria, accepted fifteen species in the genus and excluded nine species. He examined type material of T. incrustans extant in PAD. He reported on the difficulty to distinguish Thyridaria from Kalmusia, noting that both Thyridaria and Kalmusia differed from Thyridium only in the lack of longitudinal septa in the ascospores. Kalmusia was further differentiated by scattered perithecia in an effused stroma from Thyridaria, which was characterised by aggregated perithecia or valsoid stromata. However, the latter difference was hampered by an extremely wide variation in the aggregation of the perithecia within the genus Thyridaria such as in T. rubronotata, where ascomata may be definitely aggregated in numerous small erumpent pustules, densely crowded in extensive layers or scattered singly. Oddly enough, he accepted T. incrustans instead of T. broussonetiae as the generic type of Thyridaria. Barr (1990) recognised T. broussonetiae as the generic type of Thyridaria and placed the genus in the Platystomaceae. Later (Barr 2003) she referred it to the Didymosphaeriaceae. The concept of Kalmusia, which is additionally characterised by long-stipitate asci, was recently stabilised by neotypification of the type species K. ebuli (Zhang et al. 2014), albeit with a specimen not collected from the type host genus Sambucus but from Populus.
We studied many specimens having thyridaria-like morphology and found that they are distributed among at least nine clades of the Pleosporales. We treat here taxa of four unrelated clades. Below we report that Thyridaria is polyphyletic, that T. broussonetiae, which belongs to a clade encompassing the Roussoellaceae and in effect the Thyridariaceae, is not congeneric with T. rubronotata, erect the new generic name Cyclothyriella for the latter, which forms a family of its own, and describe some other thyridaria-like fungi in different new or known genera. We provide also DNA data and a redescription of Ohleria modesta.
Materials and methods
Isolates and specimens
All newly prepared isolates used in this study originated from ascospores or conidia of fresh specimens. Strain numbers including NCBI GenBank accession numbers of gene sequences used to compute the phylogenetic trees are listed in Table 1. Strain acronyms other than those of official culture collections are used here primarily as strain identifiers throughout the work. Representative isolates have been deposited at the CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands (CBS). Details of the specimens used for morphological investigations are listed in the Taxonomy section under the respective descriptions. Herbarium acronyms are according to Thiers (2016). Specimens have been deposited in the Herbarium of the Institute of Botany, University of Vienna (WU).
Table 1.
Isolates and accession numbers used in the phylogenetic analyses. Isolates/sequences in bold were isolated/sequenced in the present study.
| Taxon | Strain | Voucher | GenBank accession numbers |
||||
|---|---|---|---|---|---|---|---|
| ITS | LSU | SSU | rpb2 | tef1 | |||
| Alternaria alternata | CBS 916.96 | – | DQ678082 | KC584507 | KC584375 | DQ677927 | |
| Amniculicola lignicola | CBS 123094 | – | EF493861 | EF493863 | EF493862 | GU456278 | |
| Anteaglonium parvulum | SMH5223 | – | GQ221909 | – | – | GQ221918 | |
| Arthopyrenia salicis | CBS 368.94 | KF443410 | AY538339 | AY53833 | KF443397 | KF443404 | |
| Cyclothyriella rubronotata | CBS 419.85 | – | GU301875 | – | GU371728 | GU349002 | |
| CBS 121892; TR | WU 36862 | KX650541 | KX650541 | – | KX650571 | KX650516 | |
| TR1 | WU 36863 | KX650542 | KX650542 | – | KX650572 | KX650517 | |
| TR3 | WU 36859 | KX650543 | KX650543 | – | KX650573 | KX650518 | |
| CBS 141486; TR9 | WU 36858 | KX650544 | KX650544 | KX650507 | KX650574 | KX650519 | |
| TR9a | WU 36858 | KX650545 | KX650545 | – | – | KX650520 | |
| Dendryphion europaeum | CPC 22943 | KJ869146 | KJ869203 | – | – | – | |
| Herpotrichia diffusa | CBS 250.62 | – | DQ678071 | GU205239 | DQ677968 | DQ677915 | |
| Hobus wogradensis | CBS 141484; TI | WU 36874 | KX650546 | KX650546 | KX650508 | KX650575 | KX650521 |
| Karstenula rhodostoma | CBS 690.94 | – | GU301821 | GU296154 | GU371788 | – | |
| Leptosphaeria doliolum | CBS 505.75 | – | GU301827 | GU296159 | KT389640 | GU349069 | |
| Lophiostoma macrostomum | JCM 13544 | – | AB619010 | AB618691 | JN993491 | – | |
| Lophiotrema nucula | CBS 627.86 | – | GU301837 | GU296167 | GU371792 | GU349073 | |
| Massaria campestris | M28 | – | HQ599385 | HQ599449 | HQ599459 | HQ599325 | |
| M. inquinans | M19 | – | HQ599402 | HQ599444 | HQ599460 | HQ599342 | |
| Massarina eburnea | CBS 473.64 | – | GU301840 | GU296170 | GU371732 | GU349040 | |
| Massariosphaeria phaeospora | CBS 611.86 | – | GU301843 | GU296173 | GU371794 | – | |
| Mauritiana rhizophorae | BCC 28866 | – | GU371824 | GU371832 | GU371796 | GU371817 | |
| Melanomma pulvis-pyrius | CBS 124080 | – | GU456323 | GU456302 | GU456350 | GU456265 | |
| Neooccultibambusa chiangraiensis | MFLUCC 12-0559 | KU712442 | KU764699 | KU712458 | – | KU872761 | |
| Neoroussoella bambusae | MFLUCC 11-0124 | KJ474827 | KJ474839 | – | KJ474856 | KJ474848 | |
| Nigrograna fuscidula | CBS 141476; MF1 | WU 36881 | KX650547 | KX650547 | KX650509 | KX650576 | KX650522 |
| MF1a | WU 36881 | KX650548 | KX650548 | – | – | KX650523 | |
| MF3 | WU 36880 | KX650549 | KX650549 | – | – | KX650524 | |
| CBS 141556; MF7 | WU 36879 | KX650550 | KX650550 | – | – | KX650525 | |
| MF8 | WU 36883 | KX650551 | KX650551 | – | – | – | |
| MF9 | WU 36884 | KX650552 | KX650552 | – | – | – | |
| N. mackinnonii | CBS 110022 | KF015653 | GQ387614 | GQ387553 | KF015704 | KF407985 | |
| CBS 674.75 | NR_132037 | GQ387613 | GQ387552 | KF015703 | KF407986 | ||
| E9303e | JN545759 | LN626681 | LN626678 | LN626666 | LN626673 | ||
| N. mycophila | CBS 141478; MF5 | WU 36886 | KX650553 | KX650553 | – | – | KX650526 |
| MF6 | WU 36887 | KX650554 | KX650554 | – | – | KX650527 | |
| CBS 141483; TDK | WU 36888 | KX650555 | KX650555 | KX650510 | KX650577 | KX650528 | |
| N. norvegica | CBS 141485; TR8 | WU 36885 | KX650556 | KX650556 | KX650511 | KX650578 | – |
| N. obliqua | BW4 | – | KX650557 | KX650557 | – | – | KX650529 |
| CBS 141475; KE | WU 36876 | KX650558 | KX650558 | KX650512 | KX650579 | KX650530 | |
| MF | WU 36878 | KX650559 | – | – | – | – | |
| CBS 141477; MF2 | WU 36875 | KX650560 | KX650560 | – | KX650580 | KX650531 | |
| MRP | WU 36877 | KX650561 | KX650561 | – | KX650581 | KX650532 | |
| Occultibambusa bambusae | MFLUCC 13-0855 | KU940123 | KU863112 | KU872116 | KU940170 | KU940193 | |
| O. fusispora | MFLUCC 11-0127 | KU940125 | KU863114 | – | KU940172 | KU940195 | |
| O. pustula | MFLUCC 11-0502 | KU940126 | KU863115 | KU872118 | – | – | |
| Ohleria modesta | MGC | WU 36870 | KX650562 | KX650562 | – | KX650582 | KX650533 |
| CBS 141480; OM | WU 36873 | KX650563 | KX650563 | KX650513 | KX650583 | KX650534 | |
| Paradictyoarthrinium diffractum | MFLUCC13-0466 | KP744455 | KP744498 | KP753960 | – | – | |
| P. tectonicola | MFLUCC 13-0465 | KP744456 | KP744500 | KP753961 | – | – | |
| Parathyridaria percutanea | CBS 128203 | KF322117 | KF366448 | KF366450 | KF366453 | KF407988 | |
| CBS 868.95 | KF322118 | KF366449 | KF366451 | KF366452 | KF407987 | ||
| P. ramulicola | MF4 | WU 36868 | KX650564 | KX650564 | – | – | KX650535 |
| CBS 141479; MRR1 | WU 36867 | KX650565 | KX650565 | KX650514 | KX650584 | KX650536 | |
| Pleomassaria siparia | CBS 279.74 | – | DQ678078 | DQ678027 | DQ677976 | DQ677923 | |
| Roussoella angustior | MFLUCC 15-0186 | – | KT281979 | – | – | – | |
| R. chiangraina | MFLUCC 10-0556 | KJ474828 | KJ474840 | – | KJ474857 | KJ474849 | |
| R. hysterioides | CBS 546.94 | KF443405 | KF443381 | AY642528 | KF443392 | KF443399 | |
| R. intermedia | NBRC 106245 | KJ474831 | AB524624 | AB524483 | – | – | |
| R. japanensis | MAFF 239636 | KJ474829 | AB524621 | AB524480 | AB539101 | AB539114 | |
| R. magnatum | MFLUCC 15-0185 | – | KT281980 | – | – | – | |
| R. mexicana | CPC 25355 | KT950848 | KT950862 | – | – | – | |
| R. neopustulans | MFLUCC 11-0609 | KJ474833 | KJ474841 | – | – | KJ474850 | |
| R. nitidula | MFLUCC 11-0182 | KJ474835 | KJ474843 | – | KJ474859 | KJ474852 | |
| MFLUCC 11-0634 | KJ474834 | KJ474842 | – | KJ474858 | KJ474851 | ||
| R. pustulans | MAFF 239637 | KJ474830 | AB524623 | AB524482 | AB539103 | AB539116 | |
| R. scabrispora | MFLUCC 11-0624 | KJ474836 | KJ474844 | – | KJ474860 | KJ474853 | |
| RSC | WU 33540 | KX650566 | KX650566 | – | – | KX650537 | |
| R. siamensis | MFLUCC 11-0149 | KJ474837 | KJ474845 | KJ474861 | KJ474854 | ||
| Roussoella sp. | CBS 170.96 | KF443407 | KF443382 | KF443390 | KF443394 | KF443398 | |
| R. thailandica | MFLUCC 11-0621 | KJ474838 | KJ474846 | – | – | – | |
| R. verrucispora | CBS 125434 | KJ474832 | AB524622 | AB524481 | AB539102 | AB539115 | |
| Roussoellopsis macrospora | MFLUCC 12-0005 | KJ739604 | KJ474847 | KJ739608 | KJ474862 | KJ474855 | |
| Roussoellopsis sp. | NBRC 106246 | – | AB524626 | AB524485 | – | – | |
| R. tosaensis | MAFF 239638 | – | AB524625 | AB524484 | AB539104 | AB539117 | |
| Seriascoma didymospora | MFLUCC 11-0179 | KU940127 | KU863116 | KU872119 | KU940173 | KU940196 | |
| Teichospora trabicola | CBS 140730; C134 | – | KU601591 | – | KU601600 | KU601601 | |
| Tetraplosphaeria sasicola | MAFF 239677 | – | AB524631 | AB524490 | – | – | |
| Thyridaria acaciae | CBS 138873 | KP004469 | KP004497 | – | – | – | |
| T. broussonetiae | CBS 121895; TB | WU 36865 | KX650567 | KX650567 | – | KX650585 | KX650538 |
| CBS 141481; TB1 | WU 36864 | KX650568 | KX650568 | KX650515 | KX650586 | KX650539 | |
| TB1a | WU 36864 | KX650569 | KX650569 | – | – | – | |
| CBS 141482; TB2 | WU 36866 | KX650570 | KX650570 | – | KX650587 | KX650540 | |
| Torula herbarum | CBS 140066 | KR873260 | KR873288 | – | – | – | |
| CBS 111855 | KF443409 | – | KF443391 | KF443396 | KF443403 | ||
| T. hollandica | CBS 220.69 | KF443406 | KF443384 | KF443389 | KF443393 | KF443401 | |
| Trematosphaeria pertusa | CBS 122368 | – | FJ201990 | FJ201991 | FJ795476 | KF015701 | |
| Ulospora bilgramii | CBS 110020 | – | DQ678076 | DQ678025 | DQ677974 | DQ677921 | |
| Versicolorisporium triseptatum | JCM 14775 | AB365596 | AB330081 | AB524501 | – | – | |
| Westerdykella ornata | CBS 379.55 | – | GU301880 | GU296208 | GU371803 | GU349021 | |
Culture preparation, growth rate determination and phenotype analysis
Cultures were prepared and maintained as described previously (Jaklitsch 2009). Microscopic observations were made in tap water except where noted. Morphological analyses of microscopic characters were carried out as described earlier (Jaklitsch 2009). Methods of microscopy included stereomicroscopy using a Nikon SMZ 1500 and Nomarski differential interference contrast (DIC) using the compound microscope Nikon Eclipse E600. Images and data were gathered using a Nikon Coolpix 4500 or a Nikon DS-U2 digital camera and measured with NIS-Elements D v. 3.0. Measurements are reported as maximum and minimum in parentheses and the mean plus and minus the standard deviation of a number of measurements given in parentheses.
DNA extraction and sequencing methods
The extraction of genomic DNA was performed as reported previously (Voglmayr and Jaklitsch, 2011, Jaklitsch et al., 2012) using the DNeasy Plant Mini Kit (QIAgen GmbH, Hilden, Germany). The following loci were amplified and sequenced: the complete internally transcribed spacer region (ITS1-5.8S-ITS2) and a ca. 900 bp fragment of the large subunit nuclear ribosomal DNA (nLSU rDNA) amplified and sequenced as a single fragment with primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990); a ca. 1.1–1.4 kb fragment of the small subunit nuclear ribosomal DNA (nSSU rDNA) with primers SL1 (Landvik et al. 1997) and NSSU1088 (Kauff & Lutzoni 2002), a ca. 1.2 kb fragment of the RNA polymerase II subunit 2 (rpb2) with primers fRPB2-5f and fRPB2-7cr (Liu et al. 1999); and a ca. 1.3–1.5 kb fragment of the translation elongation factor 1-alpha (tef1) containing two introns and a part of the exon with primers EF1-728F (Carbone & Kohn 1999) and TEF1LLErev (Jaklitsch et al. 2005). For a herbarium specimen of Nigrograna obliqua (MF), the ITS was directly amplified from ascomatal contents according to a modified protocol described in Jaklitsch & Voglmayr (2012). Ascomata were cut with a sterile razor blade, the content transferred with a sterile forceps first to 1× TE buffer, and then to a reaction tube containing the PCR master mix with primers V9G and ITS5 (White et al. 1990). PCR products were purified using an enzymatic PCR cleanup (Werle et al. 1994) as described in Voglmayr & Jaklitsch (2008). DNA was cycle-sequenced using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit v. 3.1 (Applied Biosystems Warrington UK) with the same primers as in PCR. In addition, the primers ITS4 (White et al. 1990) and LR3 (Vilgalys & Hester 1990) were used for the ITS-LSU region. Sequencing was performed with an automated DNA sequencer (3730xl Genetic Analyzer Applied Biosystems).
Analysis of sequence data
For phylogenetic analyses, a combined matrix of ITS-LSU, SSU, rpb2 and tef1 sequences was produced. According to results of BLAST searches of the LSU and the tree topology of Hyde et al. (2013), GenBank sequences of selected Pleosporales (Table 1) were included to reveal the phylogenetic relationships of the taxa treated here. In addition, selected members of the families Occultibambusaceae (Dai et al. 2016), Paradictyoarthriniaceae (Liu et al. 2015) and Torulaceae (Crous et al. 2015) were added. Karstenula rhodostoma was included as a member of Didymosphaeriaceae according to Tanaka et al. (2015). Two species of Massaria (Massariaceae) were selected as outgroup according to Voglmayr & Jaklitsch (2011) and Hyde et al. (2013). All alignments were produced with the server version of MAFFT 7 (http://mafft.cbrc.jp/alignment/server/), using the default settings for the SSU rDNA and the rpb2; for the ITS-LSU and the tef1 the E-INS-i and the G-INS-i iterative refinement methods were implemented, respectively, with a gap opening penalty of 1.0. The resulting alignments were checked and refined using BioEdit v. 7.0.4.1 (Hall 1999). For phylogenetic analyses, all sequence alignments were combined. After exclusion of ambiguously aligned regions from the ITS1 (the first 262 characters) and tef1 introns (418 characters) and large insertions from the SSU, the final matrix contained 1 484 nucleotide characters from the ITS-LSU rDNA, 995 from the SSU rDNA, 1082 from rpb2 and 1316 from tef1.
Maximum parsimony (MP) bootstrap analysis was performed with PAUP v. 4.0a149 (Swofford 2002), with 1 000 bootstrap replicates using 5 rounds of heuristic search replicates with random addition of sequences and subsequent TBR branch swapping (MULTREES option in effect, steepest descent option not in effect) during each bootstrap replicate, with each replicate limited to 1 million rearrangements. All molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data; the COLLAPSE command was set to minbrlen.
Maximum likelihood (ML) analyses were performed with RAxML (Stamatakis 2006) as implemented in raxmlGUI 1.3 (Silvestro & Michalak 2012), using the ML + rapid bootstrap setting and the GTRGAMMAI substitution model with 1 000 bootstrap replicates. The matrix was partitioned for the individual gene regions, and substitution model parameters were calculated separately for them. Bootstrap support of <70 % was considered low, between 70–90 % medium, and > 90 % high.
Results
Molecular phylogeny
The final alignment and the tree obtained were deposited in TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S19648). Of the 4 877 nucleotide characters included in the phylogenetic analyses, 1 495 are parsimony informative (435 of ITS-LSU, 112 of SSU, 550 of rpb2, 398 of tef1).
The best ML tree (lnL = −47750.4257) revealed by the RAxML analysis is shown as phylogram in Fig. 1. The Thyridariaceae are highly supported in both MP and ML analyses (100 % ML and 98 % MP). Sister group relationship of Thyridaria to Parathyridaria received low (65 %) and medium (82 %) bootstrap support in ML and MP analyses, respectively, while the subclade containing Arthopyrenia salicis, Neoroussoella, Roussoella and Roussoellopsis received medium support in both analyses (87 % ML and 74 % MP; Fig. 1). Cyclothyriella rubronotata, formerly classified in Thyridaria, is phylogenetically remote from T. broussonetiae, the generic type. The genus Nigrograna received maximum bootstrap support in both analyses, and sister group relationship of N. fuscidula to N. mackinnonii and of N. obliqua to N. mycophila received maximum support as well. Nigrograna is not closely related to Melanomma, within which N. fuscidula was formerly classified, but sister clade to Occultibambusaceae with high (99 % ML) and medium (77 % MP) bootstrap support. Ohleria and Hobus formed an isolated unsupported clade within Pleosporales with uncertain phylogenetic affinities.
Fig. 1.
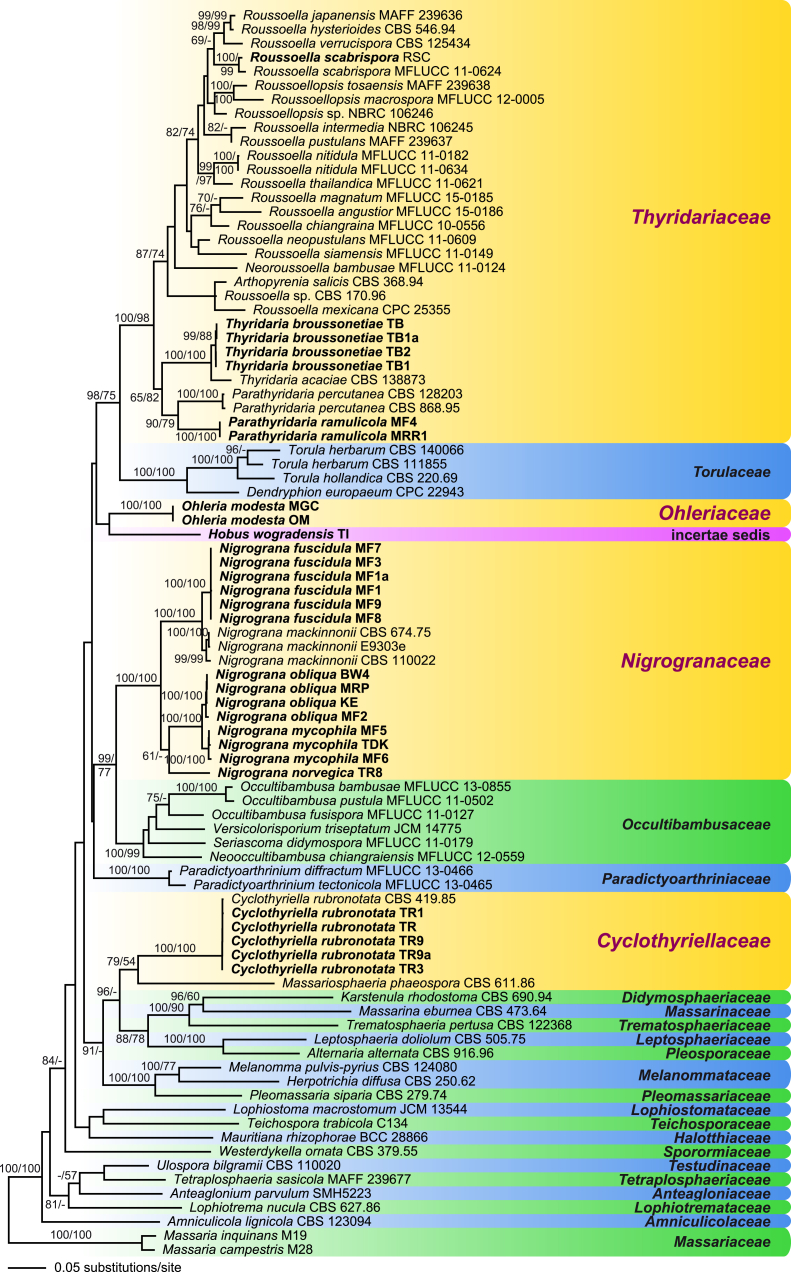
Phylogram of the best maximum likelihood tree (lnL = -47750.4257) revealed by RAxML from an analysis of the combined ITS-LSU-SSU-rpb2-tef1 sequence data matrix of selected Pleosporales to reveal the phylogenetic position of the genera Cyclothyriella, Hobus, Nigrograna, Ohleria, Parathyridaria and Thyridaria. ML and MP bootstrap support above 50 % are given above or below the branches. The tree was rooted with two species of Massaria. Taxa in bold were sequenced in the present study. Familial classification follows Hyde et al. 2013, with updates from Crous et al., 2015, Dai et al., 2016, Liu et al., 2015, Tanaka et al., 2015.
Taxonomy
Contrary to, e.g., Teichospora (Jaklitsch et al. 2016), which forms a highly supported monophyletic lineage but where the partial lack of internal support of the tree backbone and morphological features currently do not support recognition of separate genera, thyridaria-like fungi are much more complex. The taxa studied here are treated below according to the phylogenetic clades (see Fig. 1) as follows:
1) As revealed by the molecular phylogenetic results, Thyridaria rubronotata forms a stable clade of its own, together with only one additional taxon, Massariosphaeria phaeospora. Thyridaria rubronotata is neither congeneric nor confamilial with T. broussonetiae, therefore it needs a different name. We chose Cyclothyriella as new generic name on the basis of the asexual morph name Cyclothyrium, which was originally intended for it (Petrak 1923). However, the latter cannot be used due to nomenclatural and taxonomic reasons (for details, see below).
2) The generic type of Thyridaria, T. broussonetiae, is contained within a highly supported clade until now named Roussoellaceae. Due to priority this clade now becomes Thyridariaceae in a new circumscription, with Roussoellaceae in synonymy. The Thyridariaceae contain two subclades which both receive only low to medium support (Fig. 1), the large Roussoella sensu lato subclade, and a subclade containing Thyridaria sensu stricto and the new genus Parathyridaria. The latter remotely resembles Thyridaria or Melanomma, but differs from the type species of both genera in several features. Roussoella acaciae, which was described from leaves of Acacia tortilis in Tanzania, based on its coniothyrium- or cytoplea-like asexual morph forming clustered pycnidia producing unicellular pale brown conidia (Crous et al. 2014), is part of the first subclade and therefore combined in Thyridaria. Roussoella percutanea, which was isolated as an opportunistic pathogen of humans causing subcutaneous mycosis and is characterised by pycnidia forming minute unicellular hyaline conidia (Ahmed et al. 2014a), is part of the second subclade and therefore combined in Parathyridaria.
3) An unsupported clade with uncertain affinities consists of Ohleria modesta and a thyridaria-like fungus from Juglans, which clusters with O. modesta but differs substantially from this fungus morphologically and ecologically and is therefore described in the new genus Hobus, as H. wogradensis. As Ohleria does not fall into any described family, we describe the new family Ohleriaceae, which does not encompass Hobus due to its unstable affiliation, for it.
4) The fourth and last clade is phylogenetically highly supported, but does not belong to any family recognised in phylogenetic trees of the Pleosporales. It basically consists of four more or less cryptic species difficult to distinguish morphologically. Thus they are representatives of a single genus. One of these species is Melanomma fuscidulum, which thus is not congeneric with the type species of Melanomma, M. pulvis-pyrius, and does not belong to the Melanommataceae. NCBI GenBank sequences of the isolate CY 1228 labelled Biatriospora marina, which forms the basis of the Biatriosporaceae (Hyde et al. 2013), cluster in this clade. All other species in this clade differ substantially in ascospore morphology, ecology and other features from the mangrove-inhabiting Biatriospora (Hyde & Borse 1986). This casts doubt whether the isolate CY 1228 indeed represents Biatriospora marina, therefore we do not include the latter in our tree. On the other hand, Biatriospora mackinnonii, which was combined in this genus due to sequence similarity (Ahmed et al. 2014b), is also part of this clade. For this species Gruyter et al. (2012) described the genus Nigrograna with uncertain familial affinity. Therefore we combine Melanomma fuscidulum in Nigrograna, describe the three additional species in this genus and recognise this highly supported clade as the new family Nigrogranaceae.
Cyclothyriellaceae Jaklitsch & Voglmayr, fam. nov. MycoBank MB817772.
Etymology: Referring to the name of the type genus.
Ascomata and/or pycnidia scattered or more commonly clustered in valsoid configuration within KOH-positive tissue or in purple-coloured plant tissue, immersed-erumpent, more or less globose, black, peridium pseudoparenchymatous. Ostiolar discs brightly coloured or black, ostioles periphysate. Hamathecium of apically free paraphyses and narrow branched and anastomosing, trabeculate pseudoparaphyses. Asci cylindrical to clavate, bitunicate, 8-spored. Ascospores ellipsoid to fusoid, with several eusepta, brown, thick-walled, with or without a sheath. Asexual morph pycnidial, historically called aposphaeria-like. Peridium pseudoparenchymatous, dark. Conidiophores absent or inconspicuous. Conidiogenous cells phialidic. Conidia cylindrical, oblong to ellipsoid, 1-celled, hyaline or brown, smooth.
Type genus: Cyclothyriella Jaklitsch & Voglmayr
Second genus: Massariosphaeria (E. Müll.) Crivelli
Notes: We instate this family name, because the respective clade has proven to be stable (see e.g. Hyde et al. 2013). It has been called Thyridariaceae, but family names are based on their type genus, which itself is defined by its type species. The family Thyridariaceae is therefore to be used for Thyridaria broussonetiae, if no other family name applies in the respective clade, depending on priority.
Cyclothyriella Jaklitsch & Voglmayr, gen. nov. MycoBank MB817773.
Etymology: In analogy to the generic name Cyclothyrium.
Ascomata and pycnidia clustered in valsoid configuration within KOH-positive tissue in bark, more or less globose, black, peridium pseudoparenchymatous. Brightly coloured ostiolar discs present, ostioles periphysate. Hamathecium of apically free paraphyses and narrow branched and anastomosing, trabeculate pseudoparaphyses. Asci cylindrical, bitunicate, fissitunicate but relatively stable in water mounts, with thick endotunica, small ocular chamber, short stipe and furcate base, containing 8 uniseriate to overlapping ascospores. Ascospores ellipsoid, oblong to fusoid, with several thick and dark eusepta, straight or curved, dark brown when mature, verruculose. Asexual morph pycnidial, morphologically similar to ascomata. Conidiophores absent. Conidia cylindrical, oblong to ellipsoid, 1-celled, hyaline when immature, turning dark brown, smooth, produced on variously shaped phialides.
Type species: Cyclothyriella rubronotata.
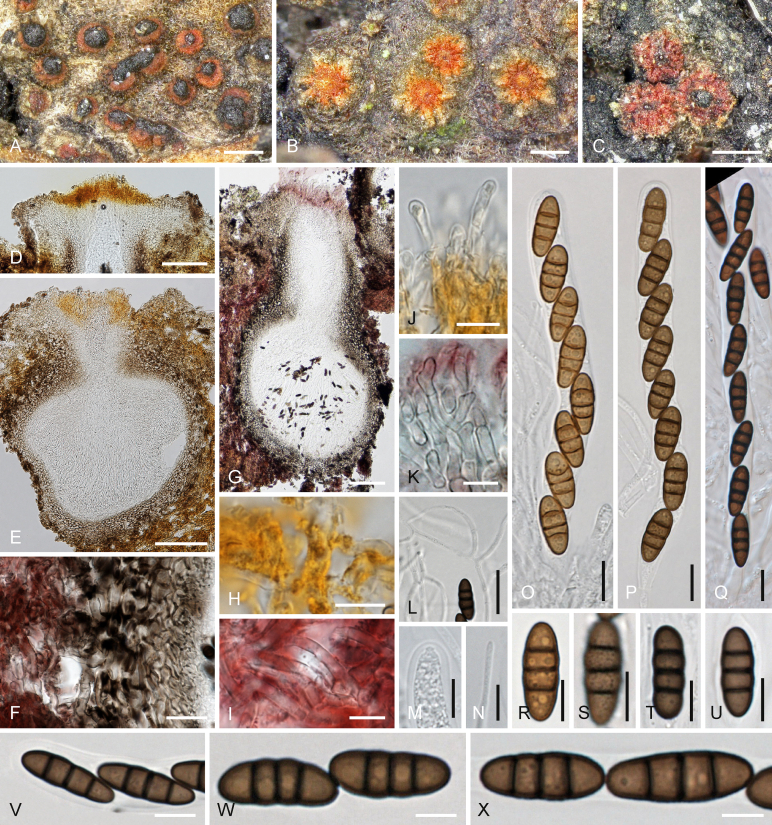
Cyclothyriella rubronotata (Berk. & Broome) Jaklitsch & Voglmayr, comb. nov. MycoBank MB817803. Fig. 2, Fig. 3A–L.
Fig. 2.
Cyclothyriella rubronotata, sexual morph. A–C. Ostiolar discs and stroma surface in face view (A. showing pulvinate spore deposits). D. Ostiolar disc in vertical section. E, G. Ascomata in vertical section. F. Peridium and stroma hyphae in section. H, I. Stroma hyphae. J, K. Apical ostiolar hyphae. L. Trabeculate pseudoparaphyses. M. Tip of immature ascus showing ocular chamber. N. Apically free paraphysis. O–Q. Asci (O. immature). R–X. Ascospores (S. showing verruculose surface). D, E, J. in 40 % glycerol; F, G, I, K, Q, T–X. in 3 % KOH; H. in lactic acid. A, L, X. WU 36859; B–K, M–P, R–T, W. WU 36858; Q. WU 36863. U, V. holotype. Scale bars: A–C = 200 μm; D, E, G = 100 μm; F, L = 20 μm; H–J, M–Q = 10 μm; K, R–V = 7 μm; W, X = 5 μm.
Fig. 3.
A–L.Cyclothyriella rubronotata, asexual morph. A–G. On the natural host. A. Pycnidia in red hyphal stroma in vertical section. B. Part of pycnidium (left side) adjacent to an ascoma (right side). C. Pycnidial wall and stroma hyphae. D, E. Phialides. F, G. Conidia. H–L. In culture. H. Pycnidium. I, J. Phialides. K, L. Conidia. M–P.Phoma (Aposphaeria) ulmicola. M. Pycnidia. N. Phialide. O, P. Conidia. B. in lactic acid; C–G, I–L, N–P. in 3 % KOH. A–G. WU 36858; H–L. culture TR9a; M–P. holotype of Phoma ulmicola. Scale bars: A, H, M = 400 μm; B = 150 μm; C, I = 15 μm; D, E, J, L = 10 μm; F, G, K, N–P = 5 μm.
Basionym: Melogramma rubronotatum Berk. & Broome, Ann. Mag. nat. Hist. Ser. 3, 3: 375. 1859.
Synonyms: Thyridaria rubronotata (Berk. & Broome) Sacc., Syll. fung. 2: 141, pl. III, figs. 1–4. 1883.
Thyridaria delognensis Speg. & Roum., Revue mycol. Toulouse 2: 21. 1880.
Kalmusia delognensis (Speg. & Roum.) Wint. in Rab., Krypt.-Fl. 1(2): 764. 1887.
Massaria lateritia Tul., in sched., vide Tul., sel. fung. carp. 2: 244. 1863.
? Thyridaria minor (Sacc.) Sacc., Syll. fung. 24(2): 769. 1928.
Stromata 0.5–6.5 mm long, 0.6–1.5 mm high, variable, with an inconspicuous or conspicuous ectostroma projecting up to 1 mm from the host surface, consisting of dark red or orange (2.0–)3.0–5.2(–7.0) μm (n = 54) wide, thin-walled hyphae, turning purple in 3 % KOH and bright yellow in lactic acid and 40 % glycerol; containing ascomata and/or pycnidia in variable, mostly valsoid configuration. Ascomata (330–)380–550(–630) μm high, (340–)380–550(–615) μm wide (n = 20), globose or depressed globose, black. Peridium (26–)34–51(–60) μm (n = 20) wide, comprising a t. angularis to t. epidermoidea of thin-walled cells (2.5–)4.5–15.5(–22) × (1.5–)3.0–8.2(–12.5) μm (n = 30), compressed inside, becoming more isodiametric, thicker-walled and darker towards the surface, surrounded by a layer of dark brown hyphae followed by red stromal hyphae. Ostiolar discs (140–)155–343(–415) μm wide, bright to dull red or orange, paler to greenish-yellow with age, not projecting, with a radial to stellate structure in surface view, sometimes with a black pulvinate spore deposit; consisting of small-celled, hyaline to pale brownish t. angularis terminating in short, vertically arranged, thick-walled hyaline hyphae, in the central upper area incrusted by yellow to orange-brown pigment dissolving and diffusing in 3 % KOH and lactic acid, purplish in the former and bright yellow in the latter; often surrounded by yellowish, whitish, orange or pale brownish mycelium. Ostiolar canal (156–)205–355(–420) μm long, interior (62–)759–122(–154) μm wide (n = 20), periphysate. Hamathecium of narrow branched and anastomosing, trabeculate, 1.5–3.5 μm wide pseudoparaphyses and some true paraphyses of similar width with free ends among immature asci. Asci (105–)108–125(–140) × (8.2–)8.7–10.5(–11.5) μm (n = 21), cylindrical, bitunicate, fissitunicate, but relatively stable in water mounts, with thick endotunica, small ocular chamber, short stipe and furcate base, containing 8 uniseriate, partly overlapping ascospores. Ascospores (12.8–)15.0–18.2(–21.2) × (5.0–)5.7–6.7(–7.2) μm, l/w (2.1–)2.4–3(–3.5) (n = 91), narrowly ellipsoid or oblong with narrowly or broadly rounded ends and second cell sometimes slightly widened, 3 thick and dark eusepta, slightly constricted at all septa, straight or slightly curved, yellowish brown when young, turning dark chocolate to blackish brown upon maturation, multiguttulate, with finely verruculose perispore, sheath absent, unchanged in 3 % KOH.
Asexual morph on the natural host: Pycnidia (290–)430–695(–840) μm high, (335–)500–930(–1 210) μm diam (n = 20), immersed in valsoid configuration, often together with ascomata in red or orange mycelium, depressed globose to nearly conical. Ostiolar discs as with ascomata, ca. 0.2–0.4 mm diam outside, ostiolar canal ca. 0.2–0.4 mm long. Pycnidial wall similar to the ascomatal wall, (20–)26–42(–47) μm (n = 20) thick, comprising a dark brown t. angularis of thick-walled, inhomogeneously pigmented cells (3.0–)4.0–9.5(–13.0) × (2.7–)3.0–6.8(–9.6) μm (n = 40); surface turning purple in 3 % KOH. Interior lined by hyaline thin-walled isodiametric cells, giving rise to highly variable, ampulliform to lageniform, cylindrical or oddly shaped hyaline phialides (5.7–)7.3–11.8(–15.0) × (2.7–)3.3–4.7(–5.3) μm (n = 30), producing cylindrical, oblong to ellipsoid, 1-celled conidia (5.3–)5.8–6.7(–7.2) × (2.8–)3.0–3.2(–3.5) μm l/w (1.7–)1.9–2.2(–2.4) (n = 30), first hyaline, turning dark brown, smooth.
Cultures and asexual morph in culture: On CMD at 22 °C colony radius 13–18 mm after 1 mo, colony with irregular outline, purple, vinaceous to violaceous, margin hyaline, thin, odour indistinct to slightly fruity. Hyphae submerged in agar partly orange or dark red; pigment encrusted, turning purple to violaceous and dissolving in 3 % KOH leaving hyphae smooth and colourless. Depending on the isolate, pycnidia absent or forming after 1–2 mo, 0.3–0.8 mm diam, globose with a prominent papilla releasing olivaceous or brown conidial drops, black, densely surrounded by radial orange hairs. Peridium dark brown, pseudoparenchymatous, inhomogeneously pigmented, containing dark brown granules. Phialides originating on more or less globose hyaline cells, (8–)9–14.5(–16) × (2.8–)3.0–4.0(–4.3) μm (n = 13), mostly lageniform to cylindrical, often with a swollen base, sometimes on an intercalary, more or less cylindrical cell. Conidia (2.0–)4.5–6.0(–6.5) × (2.0–)2.7–3.5(–4.0) μm, l/w (1.0–)1.4–2.1(–2.5) (n = 41), first hyaline, turning medium brown, oblong, ellipsoid, subglobose or rhomboid with one end often truncate, smooth, with 1–3 guttules. On MEA at 22 °C colony radius up to ca. 25 mm within 1 mo, colony vinaceous and covered with a whitish mat of aerial hyphae; odour mushroomy; no pycnidia produced after 1–2 mo.
Habitat: in bark of moderately decayed twigs, particularly of Acer spp., Aesculus hippocastanum and Ulmus spp., often on and in association with other fungi, frequently parasitised by Nitschkia parasitans.
Distribution: Europe, possibly also North America.
Holotype: UK, Northamptonshire, Peterborough, King′s Cliffe, in bark of Ulmus, 24 Dec. 1852, M. J. Berkeley (K(M) 202878!, as Melogramma rubro-notatum).
Epitype, here designated due to possible confusion with other similar fungi: Austria, Niederösterreich, Gießhübl, on branches of Ulmus glabra on the ground, soc. Nitschkia parasitans, Nectria nigrescens, Cosmospora sp., 1 Nov. 2014, W. Jaklitsch & H. Voglmayr (WU 36858; ex-epitype culture CBS 141486 = TR9 from ascospores; culture from conidia: TR9a; MBT372419).
Other material examined: Austria, Niederösterreich, Gießhübl, on Acer campestre, partly overgrown by Nitschkia parasitans, soc. Diplodia sp., Thyronectria rhodochlora, 18 Mar. 2012, H. Voglmayr (WU 36859; culture TR3); Vienna, 19th district, Himmelstraße, grid square 7763/2, on branch of Acer pseudoplatanus on the ground, 14 Oct. 1995, W. Jaklitsch W.J. 746 (WU 36860); near parking place at Schloß Cobenzl, grid square 7 763/2, on branch of Acer campestre lying on the ground, 27 Jun. 1999, W. Jaklitsch W.J. 1332 (WU 36861); at the base of the Kahlenberg, grid square 7 763/2, on branches of Acer pseudoplatanus on the ground, 25 May 2006, W. Jaklitsch W.J. 2916 (WU 36862, culture CBS 121892 = TR); Vienna, 21st district, at the eastern base of the Bisamberg, on branches of Acer pseudoplatanus on the ground, 25 Feb. 2012 W. Jaklitsch (WU 36863, culture TR1).
Nomenclatural background and additional notes: The fungus redescribed above was originally described from Ulmus and has been known under the name Thyridaria rubronotata. However, it is not congeneric with T. broussonetiae, the generic type of Thyridaria, and therefore requires a different generic name.
The conidial stage of the fungus was referred to Phoma ulmicola Berk. by Saccardo (1883) and was redescribed and erroneously referred to as P. ulmigenum Berk. by Tulasne & Tulasne (1863, p. 243). This wrongly spelled name was taken up by von Höhnel (1917), who combined it as Melanconiopsis ulmigena (Berk.) Höhn. As already pointed out by Petrak (1923), it is very unlikely that Melanconiopsis fits for these fungi, as its generic type, Melanconiopsis inquinans Ellis & Everh., was described as a “Melanconium with a Cytospora stroma” for asexual morphs of diaporthalean fungi such as Melanconis or Massariovalsa. Its conidia are quite different and very large, given as 20–30 × 12–15 μm in the protologue. Petrak (1923) erected Cyclothyrium for the asexual morphs of Thyridaria, viz. C. ulmigenum (generic type) and C. incrustans, those species, which, according to Petrak, 1923, Von Höhnel, 1917 had erroneously treated under Melanconiopsis. In a later publication, Petrak & Sydow (1927) treated Cyclothyrium preliminarily as a subgenus of Cytoplea Bizz. & Sacc., a genus apparently similar to Cyclothyrium, the generic type of which, Cytoplea arundinicola, has not yet been sequenced. Petrak & Sydow (1927) explicitly used Cyclothyrium for the asexual morph of Thyridaria rubronotata but stated that the latter is most common on Juglans, and therefore the type species must be called C. juglandis, based on Naemospora juglandis Schum. Thus they synonymised several names including Cyclothyrium ulmigenum with Cytoplea juglandis, although they only studied own material from Juglans. They did not study Naemospora juglandis nor Phoma ulmicola, and they ignored any possibility of host specificity and the fact that Thyridaria rubronotata was described from Ulmus. This was already criticised by Chesters (1938) and Wehmeyer (1941).
We examined the holotype of Phoma ulmicola and give a short description here:
Phoma ulmicola Berk., Hook. J. Bot. 5: 40. 1853. Fig. 3M–P.
Synonyms: Aposphaeria ulmicola (Berk.) Sacc., Syll. fung. 3: 175. 1884.
Coniothyrium ulmicola (Berk.) Kuntze, Revis. gen. pl. (Leipzig) 3(2): 459. 1898.
Pycnidia 100–250 μm diam, erumpent from decorticated wood, scattered to aggregated in small numbers, black, globose, flattened above (by pressure); peridium pseudoparenchymatous, bearing some rounded, dark unicellular protruding cells, surrounded by some dark brown hyphae not reacting in 3 % KOH. Phialides lageniform to cylindrical, (6.8–)7.0–9.2(–10.5) × (1.8–)2.0–3.0(–3.5) μm (n = 17). Conidia (2.2–)3.0–4.0(–4.8) × (1.1–)1.5–2.0(–2.3) μm, l/w (1.6–)1.7–2.4(–3.8) (n = 46), narrowly ellipsoid to fusoid or drop-like, 1-celled, hyaline, often truncate at one end, smooth.
Holotype: UK, England, King's Cliffe, in wood of Ulmus, 25 Nov. 1851 (Herb. Berk. in Kew, K(M) 201530!, as Aposphaeria ulmicola).
This makes clear that Phoma ulmicola cannot be the asexual morph of Thyridaria rubronotata, as it differs from the latter by smaller pycnidia, smaller and hyaline conidia, by lack of flattened orange to red ostiolar discs and colour reactions in KOH, and by a different ecology, i.e. growth in weathered decorticated wood vs. bark. For this reason, earlier applications of Berkeley's name Phoma ulmicola for the asexual morph of Thyridaria rubronotata are erroneous and thus Cyclothyrium cannot be used for T. rubronotata, because it is nomenclaturally based on Phoma ulmicola. On Juglans, the host given as typical for Thyridaria rubronotata by Petrak & Sydow (1927), we have collected three different but morphologically similar fungi in Austria referable to Thyridaria, but none of them is conspecific neither with T. rubronotata nor with T. broussonetiae. One of them has reddish hyphae and reddish pulvinate ostioles and might be the same fungus that Petrak studied. However, the latter is not Thyridaria rubronotata, but belongs to a different clade of the Pleosporales to be published later on.
We erect Cyclothyriella as a holomorphic name for Thyridaria rubronotata. Cyclothyriella rubronotata differs from Thyridiaria broussonetiae, the generic type of Thyridaria, in several respects: in the latter the secondary septa are distosepta and appear incomplete when young, the subiculum is KOH-negative and only the tissue in the ostiolar region reacts to KOH, and there are no elongate hyphal elements present in the ostiolum. Below we redescribe a fungus, which may be related to Cyclothyriella rubronotata:
Thyridaria sambuci (P. Karst.) Sacc., Syll. fung. 2: 141. 1883. Fig. 4.
Fig. 4.
Thyridaria sambuci. A, B, D. Stroma, ascomata and ostiolar apices in face view. C. Stroma hyphae in 3 % KOH. E. Trabeculate pseudoparaphysis. F. Ascospore and part of hymenium in 3 % KOH. G–I. Asci (G. young, I. in 3 % KOH). J–M. Ascospores. C, F, H, I, M. in 3% KOH. A, C, E–G, I, L. H 1180; B, D, H, J, K, M. lectotype H 1179. Scale bars: A, B = 0.5 mm; C, E–H, K = 10 μm; D = 0.2 mm; I, J = 7 μm; L, M = 5 μm.
Basionym: Kalmusia sambuci P. Karst., Meddeland Soc. Fauna Flora fenn. 6: 54. 1880.
On bark roundish to longish stromata of 1.3–5 mm length or diam, erumpent to 1.3 mm, compact, irregularly tuberculate, with distinctly projecting cylindrical ostiolar necks; stroma substance between necks partly orange to reddish. On wood ascomata scattered to densely aggregated, with only ostiolar necks visible between wood fibres or erumpent to superficial, usually with the base immersed, covered by a yellow-brown to rust tomentum. Ostiolar necks papillate, conical or cylindrical; apices (50–)52–112(–187) μm (n = 24) wide, often flat, yellow, pale orange to black or black with orange centre, contents at upper levels yellow to orange. Ascomata (224–)325–527(–583) μm (n = 14) diam, (245–)300–440(–493) μm (n = 12) high, subglobose. Peridium brown, inhomogeneously pigmented, comprising a rather thin-walled t. angularis of 3–10 μm wide cells. Stromatic tissue, part of the peridium, subhymenium and even immature asci turning purple to violaceous in 3 % KOH. Hamathecium comprising 1–2.5 μm wide branched trabeculate pseudoparaphyses. Asci (66–)68–91(–108) × (9.0–)9.5–12.0(–13.0) μm (n = 12), clavate, bitunicate, fissitunicate, with endotunica swelling in 3 % KOH, containing 8 ascospores biseriately arranged in the upper part, with short stipe and simple base. Ascospores (12.0–)13.5–16.0(–18.8) × (3.7–)4.3–5.2(–5.6) μm, l/w (2.6–)2.9–3.5(–4.1) (n = 51), fusoid, symmetrical or inequilateral, straight to slightly curved, (1–)3-euseptate, slightly to distinctly constricted at the median septum, second cell slightly wider than others, pale to medium brown, not darkening in 3 % KOH, smooth, collapsing upon access of air.
Habitat: on branches of Sambucus racemosa.
Distribution: Finland, only known from type material.
Material examined: Finland, Kanta-Häme (“Travastia australis”), Tammela, Mustiala, on Sambucus racemosa, 16 Apr. 1872, P.A. Karsten (H 1180); ibid., 17 Apr. 1872 (H 1179), syntypes of Kalmusia sambuci. Lectotype, here designated: H 1179 (MBT372889).
Notes: This species may be related to Cyclothyriella rubronotata, but for the determination of phylogenetic affinities fresh material is necessary. This material was examined also to compare with similar fungi on Sambucus racemosa such as Nigrograna obliqua (see below). Thyridaria sambuci is a typical member of Thyridaria in the sense of Wehmeyer (1941).
Thyridariaceae Q. Tian & K.D. Hyde, Fungal Diversity 63: 254. 2013, emend.
Synonym: Roussoellaceae J.K. Liu et al., Phytotaxa 181: 7. 2014.
Ascomata immersed-erumpent to superficial, scattered or aggregated under a clypeus or in a subiculum, sometimes with yellowish or reddish pigments around the ostiolar neck forming a disc, black, more or less globose, usually with well-developed ostiolar neck and periphysate ostiole. Peridium brown, pseudoparenchymatous. Hamathecium consisting of apically free paraphyses and/or trabeculate pseudoparaphyses. Asci cylindrical or clavate, bitunicate. Ascospores yellowish- to dark brown, ellipsoid, oblong or fusoid, with transversal eusepta or eu- and distosepta, variously ornamented, sometimes with a sheath. Asexual morphs coelomycetous. Saprobic on leaves and branches of woody plants including monocotyledons such as bamboos and palms, sometimes human pathogenic.
Type genus: Thyridaria Sacc.
Thyridaria Sacc., Grevillea 4(no. 29): 21. 1875.
Ascomata immersed-erumpent, separate or gregarious in valsoid groups in brown prosenchymatous tissue, sometimes with yellowish or reddish pigments around the ostiolar neck forming a disc, black, more or less globose, with well-developed ostiolar neck; ostiole periphysate. Peridium brown, pseudoparenchymatous. Hamathecium consisting of apically free paraphyses and trabeculate pseudoparaphyses. Asci cylindrical, bitunicate. Ascospores yellowish- to dark brown, ellipsoid or fusoid, symmetric, with transversal eu- and distosepta, verruculose. Asexual morphs coelomycetous, forming simple or compound pycnidia. Saprobic on branches of woody plants, asexual morph also known from leaves.
Type species: Thyridaria broussonetiae (Sacc.) Traverso
Thyridaria broussonetiae (Sacc.) Traverso, Fl. ital. crypt., Pyrenomycetae (Florence) 1(1): 301. 1906. Fig. 5.
Fig. 5.
Thyridaria broussonetiae. A–C. Ostiolar discs and cushions in face view (B, C. showing surrounding hyphae). D. Stroma in culture at plug side (2 % MEA, 22 °C, 97 d). E–G. Ascomata in vertical section (E. Valsoid group of ascomata in lactic acid, F. in 3% KOH, G. in 50% glycerol). H. Stroma hyphae. I. Ostiolar tissue in 3 % KOH. J. Peridium in section. K. Trabeculate pseudoparaphyses. L–O. Asci (L. immature). P. Periphyses. Q, R. Ascus apices (Q. immature). S–V. Ascospores (S, T. immature). W. Conidia. A, E–L, N–P, R, S, U. WU 36864; B, M. PAD 1876; C, Q, T, V. WU 36866; D, W. culture TB2. Scale bars: A = 300 μm; B, C, E = 200 μm; D = 2 mm; F, G = 100 μm; H, K–P = 20 μm; I, J, U, V = 10 μm; Q–T = 7 μm; W = 5 μm.
Basionym: Cucurbitaria broussonetiae Sacc., Mycologiae Venetae specimen. Atti della Società Veneto-Trentina di Scienze Naturali 2: 166, pl. XII, f. 12–17. 1873.
Synonym: Thyridaria incrustans Sacc., Mycotheca Veneta II, no. 170. 1875, in sched.
Stromata ca. 0.6–1.5 mm diam, more or less conical to pulvinate, scattered or aggregated in variable numbers, consisting of loosely or densely intertwined brown, (2.5–)3.0–4.5(–5.3) μm (n = 31) wide hyphae, one or several, usually less than ten ascomata in valsoid configuration, standing on the wood, and a pruinose layer of yellow to reddish or orange-brown material forming a circular to elongate flat to convex disc or pulvillus 0.2–0.7 mm diam around the apices of the ostiolar necks; discs sometimes confluent to ca. 2 mm. Ascomata (280–)330–450(–495) μm high, (400–)440–580(–665) μm diam (n = 21), depressed globose to globose, usually width exceeding height, dark brown to black; peridium (20–)22–30(–40) μm wide (n = 21), but usually appearing thicker due to the often tightly adhering subiculum, pseudoparenchymatous, of thin-walled compressed basally more isodiametric, pale to medium brown cells (4–)6–16(–21) × (2–)3–5(–7.5) μm (n = 34). Ostiolar necks (335–)390–510(–580) μm long, (107–)133–250(–312) μm wide outside including encasing tissue, interior (46–)75–148(–178) μm wide (n = 20), containing 0.5–2 μm wide periphyses, cylindrical, upright or oblique and converging in a common disc, straight or curved, consisting of thin-walled light coloured cells (3.8–)5.0–7.3(–8.5) μm (n = 25); tissue encasing the ostiolar necks consisting of an amorphous substance, reddish- to orange-brown in 50 % glycerol, yellow and releasing yellow pigment in lactic acid (after 1 d nearly colourless) and turning pink and releasing pinkish to purple pigment in 3 % KOH, ostiolar cells in KOH hyaline but those situated close to the venter remaining dark; ostiolar apices inconspicuous in the disc or appearing as dark spots or slightly projecting dots. Hamathecium of branched, trabeculate, 1–2.5 wide pseudoparaphyses and apically free paraphyses. Asci (109–)124–160(–183) × (12–)13–16(–19) μm (n = 33), bitunicate, stable in microscopic mounts, cylindrical with a short stalk to ca. 20 μm, a simple or furcate base and 8 uniseriate (or partly biseriate by pressure in mounts) ascospores; wall thin, endotunica thick at the apex, with a distinct ocular chamber. Ascospores (18.3–)22.5–27.8(–33.3) × (6.6–)8.0–9.7(–11.0) μm, l/w (2.0–)2.5–3.2(–3.8) (n = 122), oblong, narrowly ellipsoid to fusoid, ends narrowly rounded, straight or curved, pale or yellowish brown when young, dark to blackish brown at maturity, 3-septate with one median euseptum and 2 additional incomplete septa, the latter developing to distosepta when mature, not constricted at the septa, containing several guttules, end cells often slightly longer, sheath absent, surface finely punctate to verruculose. No asexual morph seen in nature.
Cultures and asexual morph: Colony radius on CMD at 22 °C ca. 50 mm after 1 mo; colony first hyaline or whitish, turning dull yellowish green to olivaceous from the centre, centre either dull brown or yellow with yellow crystals forming on the surface; aerial hyphae sometimes amassing at the margin, sometimes pycnidia ca. 0.2–0.5 mm diam with pseudoparenchymatous wall formed, but remaining sterile; odour indistinct. Colony radius on MEA at 22 °C after 1 mo ca. 35 mm; colony zonate, brown, with narrow prominent whitish rings of aerial hyphae; odour indistinct, sometimes pycnidia with pseudoparenchymatous wall formed, but usually remaining sterile, only once hyaline to pale greyish brown, 1-celled, ellipsoid-oval-oblong, smooth conidia (4.3–)4.8–6(–6.5) × (3–)3.3–3.7(–4) μm. l/w (1.2–)1.3–1.7(–1.9) (n = 42), with few small guttules, found in the periphery of a compound stroma after 97 d in an ill-defined stroma ca. 6.4 × 4.7 mm developing at one side of the inoculation plug. Stroma soft, surface dull olive-brown with yellow dots (as in ostiolar discs of naturally grown stromata), containing several locules either of immature ascomata or pycnidia; no conidiogenous cells seen.
Habitat: in bark of various shrubs; confirmed for Amorpha fruticosa, Broussonetia papyrifera and Hippocrepis emerus.
Distribution: Southern Europe.
Typification: Lectotype, here designated: Italy, Padua, on Broussonetia papyrifera, soc. Diplodia sp., Mar. 1873, P.A. Saccardo, distributed as Mycotheca Veneta 170 (W 2009-01175!; MBT372890).
Notes: Mycotheca Veneta 170, where Saccardo (1875a) based T. incrustans on Cucurbitaria broussonetiae, was wrongly labelled by Saccardo himself for the latter name as Mycol. ven. Spec, pag. 118 (see also Saccardo 1883). The correct page is 166. Isotypes of Mycotheca Veneta 170 (as Thyridaria incrustans) in FH (FH 00313543!), K (K(M) 202716!), PAD, UPS (F-736475!; material overmature). All these materials were examined and apparently represent parts of the original collection of Thyridaria (Cucurbitaria) broussonetiae. In the lectotype material one twig contains mature material, all others contain immature stromata with the characteristic yellow to reddish brown ostiolar tops. Diplodia is also tightly associated in this specimen. For the FH material the identity is deduced from the received slide, which shows typical ascospores and asci. At PAD there are two specimens from Broussonetia, the isotype from March 1873 (a small packet on the second sheet of the T. incrustans folder) and an authentic specimen from May 1876 (larger packet on the first sheet); the latter contains better material and is therefore also illustrated in Fig. 5B, M.
Epitype here designated: Hungary, south of Eger, 47°51'16“ N 20°22'00” E, elev. 235 m, on twigs of Amorpha fruticosa, soc. Valsaria robiniae, 30 May 2014, W. Jaklitsch & H. Voglmayr (WU 36864; ex-epitype culture CBS 141481 = TB1; MBT372416).
Other material examined: Croatia, Istrija, Opatija, Mošcenička Draga, in the village heading north, on dead standing branch of Hippocrepis (Coronilla) emerus, 29 Mar. 2007, W. Jaklitsch & H. Voglmayr (WU 36865, culture CBS 121895 = TB). Italy, Veneto, Colli Euganei, Padova, Arqua Petrarca, roadside, on a dead branch of Broussonetia papyrifera, 1 Nov. 2015, W. Jaklitsch (WU 36866, culture CBS 141482 = TB2).
Notes: Apart from the specimens collected from Broussonetia, the Thyridaria incrustans folder at PAD contains specimens, labelled either as Thyridaria incrustans or Cucurbitaria broussonetiae, from Albizzia, Calycanthus, Chimonanthus, Colutea, Fagus sylvatica, Juglans regia, Morus, Prunus padus, Rhus and Robinia, as Saccardo (1875a) and Traverso (1906) had (at least in part) reported. Only few of them show a yellow substance around the ostiolar tops. These specimens contain at least two species, which corroborates that widening of the concept of Cucurbitaria broussonetiae to Thyridaria incrustans by Saccardo produced a collective species name, which is besides priority another argument not to use T. incrustans. In the PAD specimen from Juglans the fungus is overmature with many aberrant ascospores, thus the identity is questionable, and the fungus on Prunus padus is different, with smaller euseptate ascospores, (14.5–)16.7–20.5(–22.5) × (5.8–)6.7–7.8(–8.2) μm, l/w (2.1–)2.3–2.8(–3.1) (n = 30), uniseriately arranged in cylindrical asci. Ascospore sizes given by Saccardo on the label for his Colutea specimen are 15–24 × 4 (and 17–19 × 4.5) μm and on the Fagus specimen 15–18 × 5–6 μm, i.e., they are also different species. Traverso (1906) noticed that the species is very variable, as its ascomata are either arranged in well-defined valsoid groups or in irregular aggregations. Below the cortex and on the wood a black effuse pseudostromatic layer is formed, which was also seen in the sectioned sample. A good character for recognition is the yellow to reddish brown furfuraceous layer which envelops the upper part of the ostiolar necks, and in particular also the secondary distosepta in the ascospores.
Frequent and tight association of ascomata with Diplodia pycnidia led to the view that the latter is an asexual morph of T. broussonetiae, named Diplodia incrustans by Saccardo (1883), who had earlier (Saccardo 1875c) identified another asexual morph as Coniothyrium incrustans Sacc., both by association with the sexual morph on the natural hosts. Petrak (1921) classified the asexual morph in Melanconiopsis and compared its morphology with that of in his opinion very similar asexual morphs of Valsaria (compare Jaklitsch et al. 2015). His conclusions were based on material from Juglans, a host, on which we have seen at least three different thyridaria-like fungi but not T. broussonetiae. Petrak's (1921) description suggests that he had collected Cyclothyriella rubronotata (Thyridaria rubronotata) rather than T. broussonetiae, as he characterised the fungus as a cluster of 2–6 irregular pycnidial chambers imbedded in a loose felty brownish stroma in the surface layers of the bark, containing numerous one-celled, brown, ellipsoid to cylindrical conidia 4–7×2–3 μm. We have not seen a Coniothyrium, but plenty of Diplodia tightly associated with T. broussonetiae particularly between ostiolar necks on Broussonetia (WU 36866) and a Botryosphaeria morph with pale brown unicellular ascospores (19–)23–29(–30) × (6.0–)7.5–10.0 μm (n = 20) in the epitype material on Amorpha (WU 36864). In MEA culture we found conidia only once in a stroma on the plug; unfortunately we could not repeat this result. Fully mature conidia may be brown.
Thyridaria acaciae (Crous & M.J. Wingf.) Jaklitsch & Voglmayr, comb. nov. MycoBank MB817774.
Basionym: Roussoella acaciae Crous & M.J. Wingf., Persoonia 33: 259. 2014.
Notes: For this species only the asexual morph is known, which occurs on leaves of Acacia tortilis collected in Tanzania (Crous et al. 2014). Brown 1-celled conidia formed on phialides in multilocular conidiomata of this species may be typical for asexual morphs of Thyridaria.
Parathyridaria Jaklitsch & Voglmayr, gen. nov. MycoBank MB817775.
Etymology: The generic name is based on the phylogenetic vicinity to Thyridaria broussonetiae, the generic type of Thyridaria.
Ascomata more or less globose, black, immersed in wood and bark, wood surface stromatised, grey to black, subiculum absent or inconspicuous. Peridium pseudoparenchymatous. Ostiolar necks discoid, less commonly papillate or short-cylindrical, apices black or light-coloured, ostioles periphysate. Hamathecium of numerous sparsely branched, trabeculate pseudoparaphyses. Asci bitunicate, fissitunicate, narrowly clavate, with a small but distinct ocular chamber, a short stipe and simple or furcate base, containing 8 ascospores biseriately arranged at upper levels. Ascospores fusoid, with several eusepta and rarely a longitudinal septum, pale to greyish brown, not darkening in 3 % KOH, upper part or second cell often slightly broader than lower part, smooth, guttulate, sheath absent.
Asexual morph (deduced from cultures of P. percutanea): Pycnidia black, globose to subglobose, with thin pseudoparenchymatous wall. Conidiogenous cells hyaline, phialidic. Conidia small, ellipsoid, unicellular, hyaline to pale brown.
Habitat: on plant substrates (e.g., decaying twigs), sometimes human pathogenic.
Type species: Parathyridaria ramulicola Jaklitsch & Voglmayr
Parathyridaria ramulicola Jaklitsch, Fourn. & Voglmayr, sp. nov. MycoBank MB817776. Fig. 6.
Fig. 6.
Parathyridaria ramulicola. A–C. Ostiolar apices and upper part of ascomata in face view (A. with whitish ostioles in bark, B. in bark fissure, C. in stromatised wood). D. Ascoma with short-cylindrical ostiole in vertical section. E. Ascus apices and hamathecium. F, G. Peridium (F. in face view, G. in vertical section). H. Apex of immature ascus. I. Periphysis. J, K. Asci. L–P. Ascospores (O. muriform; P. with 4 septa). A, B, D–L, N. WU 36867; C, M, O. WU 36868; P. JF16012. Scale bars: A, B = 150 μm; C, D = 100 μm; E, G, I, N = 7 μm; F, J, K = 10 μm; H, L, M, O, P = 5 μm.
Etymology: ramulicola = dwelling on branches and twigs.
Ascomata (200–)260–400(–460) μm (n = 24) diam, (200–)210–335(–375) μm (n = 9) high, globose to subglobose, black, immersed in wood and bark, becoming visible in bark fissures, solitary or aggregated into clusters up to ca. 50 individuals, on wood upper half often becoming erumpent. Wood surface typically stromatised in irregular patches, grey to black. Peridium 15–30 μm thick, consisting of a thin dark brown layer and a thin hyaline layer, both of angular thin-walled cells (3.5–)5.5–10.0(–13.5) × (2.0–)3.3–7.2(–9.3) μm (n = 37), sometimes inconspicuous and sparse brown, 1–3 μm wide hyphae present between ascomata. Ostioles (25–)35–100(–110) μm diam (n = 20) outside, typically discoid with a flat or concave surface, less commonly papillate or short-cylindrical, usually projecting to less than 200 μm, with a black, whitish to pale brownish, rarely orange-reddish top, interior periphysate. Hamathecium of numerous sparsely branched, trabeculate, 1–2.5(–3.5) μm wide pseudoparaphyses. Asci (67–)75–89(–96) × (9.2–)10.0–11.7(–12.7) μm (n = 37), bitunicate, fissitunicate, narrowly clavate, with a small but distinct ocular chamber, a short stipe and simple or furcate base, containing 8 ascospores biseriately arranged at upper levels. Ascospores (12.7–)14.0–16.2(–19.5) × (3.8–)4.8–5.6(–6.0) μm, l/w (2.3–)2.6–3.2(–3.9) (n = 120), fusoid, with (2–)3(–4) darker, slightly constricted eusepta at approximately equal distances, rarely with a longitudinal septum in one cell, pale to greyish brown, not darkening in 3 % KOH, straight or slightly curved, upper part or second cell often slightly broader than lower part, ends narrowly rounded, smooth, guttulate, sheath absent.
Cultures: Colony radius on CMD at 22 °C after 1 mo 17–22 mm, colony deep dark or olive-brown, with light aerial hyphae in the centre, sometimes with a radial segmentation; odour indistinct; no asexual morph detected.
Habitat: On decaying twigs, known from Ribes rubrum and Sambucus nigra.
Distribution: France, Germany.
Holotype: Germany, Nordrhein-Westfalen, Velen, Geeste 133, on twigs of Ribes rubrum, soc. pycnidia with hyaline rod-like conidia, 2 Dec. 2013, K. Siepe (WU 36867; ex-type culture CBS 141479 = MRR1).
Other material examined: France, Ariege, Rimont, Las Muros, elev. 480 m, on decorticated twigs of Sambucus nigra, 19 Feb. 2016, J. Fournier JF 16002 (WU 36868; culture MF4); ibid., same host, 12 Apr. 2016, J. Fournier JF 16012 and JF 16015 (WU 36869).
Notes: The typical feature of this fungus is the discoid ostiolar apices, which are always present, even if some in a specimen may be cylindrical and projecting. Also characteristic are the pale to greyish brown ascospores, which do not become darker in 3 % KOH. A subiculum is usually absent or confined to very inconspicuous hyphae.
Parathyridaria percutanea (S.A. Ahmed et al.) Jaklitsch & Voglmayr, comb. nov. MycoBank MB817777.
Basionym: Roussoella percutanea S.A. Ahmed et al., Medical Mycology 52(7): 696. 2014.
This species was based on two clinical isolates obtained from human subcutaneous mycoses (Ahmed et al., 2014a, Ahmed et al., 2014b). No sexual morph is known. ITS sequence JX951180 of an Indian isolate from roots of Tinospora cordifolia (Menispermaceae) also represents this species, indicating that its primary ecology is endophytic or saprobic on plants.
Description (adapted from Ahmed et al., 2014a, Ahmed et al., 2014b): Colonies on oatmeal agar floccose, dark greyish green, with pale grey margin. Hyphae turning dark brown with age. Pycnidia observed after 8 wk, 59–102 × 54–96 μm, black, solitary, globose to subglobose, with thin wall of t. angularis. Conidiogenous cells hyaline, phialidic, obclavate. Conidia 1.2–2.0 × 0.7–0.9 μm, hyaline to pale brown, unicellular, ellipsoid.
Ohleriaceae Jaklitsch & Voglmayr, fam. nov. MycoBank MB817828.
Etymology: Referring to the name of the type genus.
Ascomata scattered or aggregated, erumpent-superficial on wood or black crusts, globose to subconical, ostiolate, black. Peridium pseudoparenchymatous, dark. Hamathecium of narrow pseudoparaphyses. Asci cylindrical, 8-spored, bitunicate, fissitunicate. Ascospores brown, fusoid or ellipsoid, transversely septate, often disarticulating into two parts. Asexual morphs coelomycetous where known; syanamorphs possibly monodictys-like. Saprobic on wood.
Type genus: Ohleria Fuckel.
Notes: We describe this family, because the application of various phylogenetic methods on the sequence dataset of Ohleria modesta always resulted in a position, which has no affinity to any other family. The genus Hobus is here only tentatively included, as clustering with Ohleria does not receive significant support.
Ohleria Fuckel, Fungi rhenani exsic. suppl., fasc. 7, no. 2173. 1868.
Ascomata scattered to aggregated in large numbers, superficial with base or venter embedded in a black crust or wood, globose to subconical, papillate, black. Peridium pseudoparenchymatous, dark. Hamathecium of narrow pseudoparaphyses. Asci cylindrical, 8-spored, bitunicate, fissitunicate. Ascospores brown, fusoid to narrowly ellipsoid, 3-septate, disarticulatng into two parts at the median primary septum. Asexual morph phoma-like, synanamorph presumably monodictys-like. Saprobic on wood.
Type species: Ohleria modesta Fuckel.
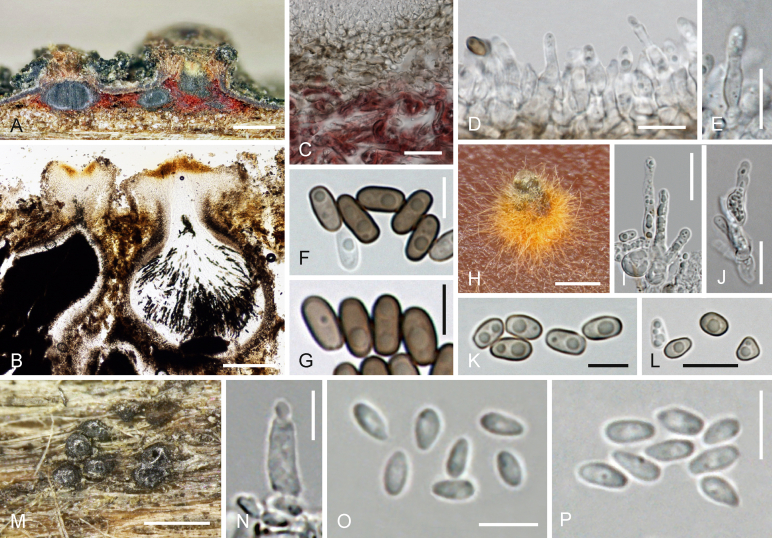
Ohleria modesta Fuckel, Fungi rhenani exsic. suppl., fasc. 7, no. 2173. 1868. Fig. 7.
Fig. 7.
A–U.Ohleria modesta. A–E. Ascomata in face view. F–H. Asci. I. Peridium in section, including hyphae of surrounding crust. J. Trabeculate pseudoparaphyses. K. Ascus apex. L–O. Ascospores (L. young). P–U. Asexual morph. P. Pycnidium with conidial drop. Q. Pycnidial peridium in face view. R. Phialides. S–U. Conidia. A, F, I, J, L, M, O. WU 36873; B. WU 36870; C, E. lectotype G; D, G, H, K. WU 36872; N. isotype W; P, Q, U. culture MGC; R–T. culture OM. V–Z.Ohleria rugulosa (lectotype G). V. Ascomata in face view. W–Z. Ascospores. Scale bars: A–E, V = 300 μm; P = 100 μm; F–J, Q, R = 10 μm; K–O, T, W–Z = 5 μm; S, U = 3 μm.
Ascomata superficial with their bases immersed in a grey to black crust or wood, loosely or densely aggregated, sometimes fusing laterally, often in large numbers, variable in shape, subglobose, semiglobose, pyriform to nearly conical, shiny greyish black, (177–)220–335(–372) μm high, (180–)230–350(–440) μm diam (n = 30); surface smooth or variously warted or wrinkled. Apex variable, usually obtuse or broadly papillate, sometimes conical and pointed or laterally compressed, (45–)68–132(–185) μm (n = 60) diam. Peridium dark brown, (20–)30–65 μm thick, usually thinner at the base, comprising an inner, often degenerated, pale brown amorphous layer, sometimes also containing thin-walled cells, and an outer textura angularis of cells (1.5–)2.5–7(–12) μm (n = 57) tending to be smaller, darker brown and thick-walled towards the outside, at the base or lower levels surrounded by a dense crust of dark brown, thick-walled compacted, up to 6 μm wide hyphae with various inclusions including ejected ascospores. Hamathecium comprising 1–2.5 μm wide, branched and anastomosing trabeculate pseudoparaphyses. Asci (80–)92–115(–120) × (6.2–)6.8–8.3(–9.0) μm (n = 18), cylindrical, bitunicate, fissitunicate, with short stipe and simple or furcate base, containing 8 overlapping uni-, partly biseriate ascospores; endotunica wide, swelling in 3 % KOH, ocular chamber minute. Ascospores (13–)14–16.5(–21) μm long, l/w (2.8–)3.2–3.8(–4.8) (n = 86), fusoid, with three thick and dark eusepta, pale brown when young, turning dark brown, biconical, disarticulating inside the asci at the middle septum to form two conical, 2-celled parts; parts dimorphic, distal part (6.2–)6.7–8.1(–9.8) × (3.8–)4.1–4.7(–5.7) μm, blunt conical, proximal part (6.6–)7.2–8.7(–11.3) × (3.4–)3.7–4.3(–5.5) μm (n = 86), narrowly conical, smooth.
Cultures and asexual morph in culture: Hyphae very thin, moderately growing, entirely covering laterally inoculated plates on CMD at 25 °C after ca. 2 mo. Colony circular, dull or olive-brown, centre black, cottony mat of aerial hyphae forming (particularly thick on PDA), first white, turning greyish- or olive-brown, after ca. 1 month black conical or subglobose ostiolate pycnidia appearing in the mat and immersed in agar, surrounded by 2–4.5 μm wide in part moniliform brown hyphae with drop-like mucous deposits, extruding hyaline mucous mass of conidia through the ostiole. Pycnidia 70–125(–150) μm high and wide (n = 20), up to 200 μm high including conidial mass, ostiole (43–)47–70(–84) μm long, 15–27(–38) μm wide (n = 16); peridium thin, comprising a t. angularis in face view, olive-brown to dark brown, consisting of cells (3.5–)5.0–8.8(–15) × (2.7–)4.0–6.5(–13) μm (n = 35), dark and thick-walled outside, lighter and thinner-walled inside. Inside lined by hyaline cells giving rise to parallel phialides. Phialides (5.5–)6.5–10(–12.5) × (2–)2.5–3.2(–3.5) μm (n = 42), lageniform to cylindrical. Conidia (2.3–)2.8–3.8(–4.7) × (1.5–)1.7–2.2(–2.5) μm, l/w (1.3–)1.5–2.1(–2.4) (n = 70), oblong to ellipsoid, 1-celled, hyaline, smooth, with 1-3 guttules, agglutinated in very pale brownish masses. Pycnidia also numerous on PDA.
Habitat: saprobic on decorticated wood.
Distribution: Europe, possibly also North America
Lectotype, here designated: Germany, Hessen, Oestrich, Oestricher Wald (Aepfelbach), on roots of Fagus sylvatica, in autumn, L. Fuckel (G!, Fungi Rhenani exsicc. 2173; MBT372891; given as holotype by Samuels (1980); isotype in W: W2016-02664!).
Other material examined (all from branches of Chamaecytisus proliferus): Spain, Canarias, La Palma, El Paso, 30 Dec. 2003, P. Karasch W.J. 2805 (WU 36870; culture MGC); San Isidro, 5 Jan. 2005, soc. Patellaria atrata, P. Karasch W.J. 2798 (WU 36871); El Paso, soc. Lophiostoma macrostomoides, 15 Jan. 2005, P. Karasch W.J. 2804 (WU 36872); opposite the old chestnut plantation at the road LP 301 heading north, close to the crossing with LP 3, 2 Dec. 2010, W. Jaklitsch (WU 36873; culture CBS 141480 = OM).
Notes: Although we are convinced about the identity of our material, we do not designate an epitype, because of different host (Chamaecytisus proliferus) and different region (Canary Islands). At first sight, Ohleria modesta looks macroscopically much like Melanomma pulvis-pyrius. In all specimens from La Palma most of the material collected is overmature. The ascomata are mostly densely crowded and more or less superficial. In the lectotype of O. modesta most ascomata are scattered, but on one piece of wood they are very densely crowded and for a large part immersed in a thick dark hyphal crust. The surface of the ascomata is generally rough and often concentrically ridged or variably tuberculate. As ascospores disarticulate early in the ascus, we calculated their length by addition of each part and the l/w ratio by length divided by the width of the broader distal part. This procedure yields a more reliable size range. Ohleria rugulosa was reported by Fuckel (1870) to differ from O. modesta by larger semiglobose ascomata and late disarticulation of the ascospores of similar size. We examined type material of O. rugulosa from G: Lectotype, here designated: Germany, Hessen, Oestrich (Nassau), Oestricher Wald, on decaying wood of Carpinus betulus, in spring (G 00266073, Herb. Barbey Boissier: MBT 373517). Several differences from O. modesta were given on the annotation label of the latter written by Gary Samuels, and by Samuels (1980). We agree with several arguments and summarise the more important ones and add others. While the size of ascomata with O. modesta has never been found to exceed a width of 450 μm, those of O. rugulosa may reach 780 μm; they are in comparison with O. modesta more amorphous, globose to subglobose or semiglobose or grossly tubercular. No asci were found in the type material in G, but ejected ascospores, of which still many are entire, i.e. they disarticulate late. Their end cells are paler than the median cells and the ascospores and its parts are curved, with more rounded sides, in contrast to O. modesta. Species of Ohleria are saprobes on dead wood and thus unlikely to be host-specific. They are rarely collected and this may the reason that every mycologist described his own species in the nineteenth century. No convincing differences can be found in the descriptions of several species. Therefore and based on examination of available types, Samuels (1980) synonymised Ohleria adjecta Pass., O. obducens G. Winter, O. quercicola Fabre and O. ulmi Fabre with O. modesta and accepted the two additional species O. rugulosa and O. brasiliensis in the genus. He determined that O. clematidis Fautrey is a species of Passeriniella. Other species he referred to Sporormia or Preussia based on ascospore features. For O. brasiliensis Starbäck, Samuels (1980) described a hyphomycetous asexual morph in Monodictys. As we have shown that Ohleria forms an aposphaeria- or phoma-like asexual morph and a similar asexual morph was described by Fuckel (1870) for O. rugulosa by association on the natural host, we interpret the Monodictys morph as a synanamorph, as, e.g., described by Grondona et al. (1997) for Pyrenochaeta dolichi (now a species of Coniothyrium; Gruyter et al. 2012). Alternatively, O. brasiliensis may not be congeneric with Ohleria. See also Karasch et al. (2005) and Zhang et al. (2012) for descriptions of Ohleria modesta.
Disarticulation of the brown ascospores is diagnostic for this genus. Other dothideomycetous genera with brown disarticulating ascospores are the representatives of the Sporormiaceae including Ohleriella, the patellariaceous apothecial Colensoniella (Hafellner 1979) and Glyphium (Boehm et al. 2015), also Delitschia (Luck-Allen & Cain 1975) and Hypsostroma (Huhndorf 1992), but most similar although much larger are the ascospores of Hysterodifractum (Carneiro de Almeida et al. 2014), which forms hysterothecia. Phylogenetically, Ohleria is neither closely related to Melanomma nor Trematosphaeria (Samuels 1980), nor to the Sporormiaceae.
Hobus Jaklitsch & Voglmayr, gen. nov. MycoBank MB817778.
Etymology: The generic name is the southern Carinthian word for fungus, with the masculine Latin ending -us.
Sexual morph erumpent from bark through fissures as groups of black, papillate or cylindrical ostiolar necks, free to nearly completely incorporated by stromatic tissue. Stroma compact, forming a t. oblita, at upper levels a t. intricata of densely interwoven, thick-walled, hyaline to brownish hyphae and roundish cells, delimited by a brown subiculum. Ascomata scattered to more commonly aggregated in variable numbers within stromatic tissue. Peridium carbonaceous, brittle, complex, pseudoparenchymatous, not clearly delimited outside, dark reddish brown in glycerol, black in KOH. Ostioles periphysate. Hamathecium of numerous narrow pseudoparaphyses and apically free paraphyses. Asci clavate, fissitunicate, thick-walled, with a small ocular chamber, 8 biseriately arranged ascospores, an up 30 μm long stipe and a furcate base with croziers. Ascospores fusoid with the second cell slightly enlarged, with several slightly constricted eusepta, dark brown, end cells sometimes slightly paler; wall thick, verruculose.
Type species: Hobus wogradensis Jaklitsch & Voglmayr
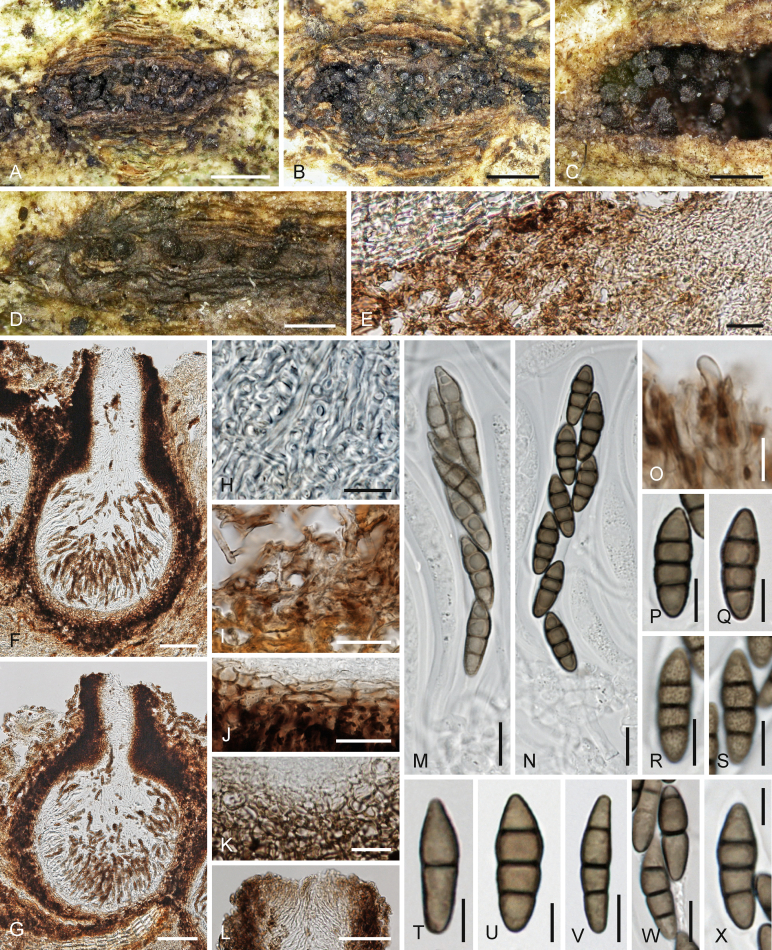
Hobus wogradensis Jaklitsch & Voglmayr, sp. nov. MycoBank MB817779. Fig. 8.
Fig. 8.
Hobus wogradensis (WU 36874). A–D. Ostiolar apices and stroma surface in face view in bark fissures. E. Stroma tissue in vertical section (note epidermis cells at upper left, brown hyphae left and compact stroma right). F, G. Ascomata in vertical section. H. Section through compact hyaline stroma. I. Brown hyphae and amorphous tissue above lower bark tissue at the margin of the compact stroma. J, K. Basal peridium in section (J. median section, K. peripheral section). L. Ostiolar apex in vertical section. M, N. Asci (M. young). O. Apical ostiolar hypha. P–X. Ascospores (R, S. showing verruculose surface; R is the same ascospore as P). Scale bars: A = 1 mm; B = 0.7 mm; C, D = 0.4 mm; E, I–K = 20 μm; F, G = 70 μm; H, M, N = 10 μm; L = 50 μm; O–S, V, W = 7 μm; T, U, X = 5 μm.
Etymology: The epithet reflects the collection site “Wograda”, which is an old southern Carinthian word for pasture, in this case reflecting a defined site.
Sexual morph erumpent from bark through fissures as inconspicuous and roundish or large and conspicuous, narrow and longish, 0.2–8.6 mm long and 0.2–1.8 mm broad groups of black, papillate or cylindrical ostiolar necks, variably surrounded, rarely nearly completely incorporated by stromatic tissue. Stroma compact, forming a t. oblita, at upper levels a t. intricata of densely interwoven, thick-walled refractive, (1.5–)1.8–3.5(–4.5) μm (n = 40) wide hyphae and roundish cells (1.8–)2.8–5.2(–7.5) μm (n = 55) diam, hyaline to brownish, often with some darker inclusions, often thick and present below and around ascomata, outside variably delimited by a layer of (1.8–)2.2–4.2(–6.3) μm (n = 30) wide brown hyphae, sometimes reduced to the latter; stroma surface therefore appearing pale brown to greyish or dark brown to nearly black. Ascomata scattered to mostly closely aggregated in variable numbers (up to 80 per group when well-developed) in stromatic tissue below the host epidermis, standing on the wood; often lifting and disintegrating the epidermis and projecting up to ca. 1.4 mm above the bark surface, globose to subglobose, often slightly higher than broad, (230–)250–315(–353) μm high, (174–)200–300(–332) μm diam (n = 22). Peridium (14.5–)29–56(–82) μm wide (n = 21), carbonaceous, brittle, often thickened and opaque at the base of the ostiolar neck, consisting of an inconspicuous narrow compressed hyaline tissue inside, a layer of brownish angular more or less thin-walled cells (5–)6–14(–20) × (3.5–)4–8.5(–12) μm (n = 40), and at the outside a poorly defined, not clearly delimited, inhomogeneously pigmented, dark to blackish brown amorphous layer of small particles, cells and hyphae partially peeling off, dark reddish brown in 40 % glycerol, dark brown in LA, black in KOH. Ostiolar necks often for a large part free or surrounded by stroma, (143–)176–255(–280) μm long (n = 21), (70–)94–160(–230) μm (n = 67) wide outside, (40–)51–77(–88) μm (n = 21) wide inside, filled with periphyses of the same width as the pseudoparaphyses, apically partially forming brownish, apically rounded, to 3.5 μm wide hyphae originating from the interior, outside cellular; apex flat or rounded, rough or glabrous and shiny. Hamathecium of numerous branched, 1–2.5(–3) μm wide pseudoparaphyses and some apically free paraphyses. Asci (58–)76–99(–106) × (10.2–)10.8–13.2(–14.2) μm (n = 21), clavate, fissitunicate, with thick walls, a small ocular chamber, 8 biseriately arranged ascospores, an up 30 μm long stipe and a furcate base with croziers. Ascospores (13.8–)15.7–19.2(–22.5) × (4.5–)5.2–6.2(–6.5) μm, l/w (2.5–)2.7–3.4(–4.3) (n = 62), fusoid, with the second cell slightly enlarged, with 3 slightly constricted eusepta in ca. equal distances, straight or slightly curved, yellowish to pale brown when young, dark to blackish brown when fully mature, end cells sometimes slightly paler; wall thick, verruculose.
Cultures: On MEA colony radius 26 mm after 7 wk at 22 °C, colony dark brown, mat of aerial hyphae grey to greyish brown, containing numerous droplets, reverse black. On CMD colony radius 14 mm after 1 mo at 22 °C, blackish brown with yellow tint. Dilute reddish pigment diffusing through the agar (particularly on MEA and PDA); odour indistinct. No asexual morph detected.
Habitat: on dead logs and branches of Juglans regia.
Distribution: Central Europe (Austria), only known from the holotype.
Holotype: Austria, Kärnten, St. Margareten im Rosental, Wograda, grid square 9 452/3, on a fallen log of Juglans regia, 14 Apr. 2006, W. Jaklitsch W.J. 2902 (WU 36874, ex-type culture CBS 141484 = TI).
Notes: This species is characterised by a well-developed stroma, which is absent in all other species studied here. Notable is also the formation of a diffusing reddish pigment in fresh cultures, particularly on MEA and PDA. In contrast, the purple pigment in cultures of Cyclothyriella rubronotata is confined to the colony, i.e. it does not diffuse into the agar.
Nigrogranaceae Jaklitsch & Voglmayr, fam. nov. MycoBank MB817780.
Etymology: Referring to the name of the type genus.
Ascomata immersed-erumpent from wood and bark, sometimes superficial, scattered or aggregated, more or less globose, black, usually seated on or surrounded by a subiculum. Ostiolar necks papillate to cylindrical; ostioles periphysate. Peridium pseudoparenchymatous. Hamathecium consisting of apically free paraphyses originating in the subhymenium between developing asci, later becoming elongated, branching and anastomosing and appearing as “trabeculate pseudoparaphyses”. Asci clavate, bitunicate, fissitunicate, with short stipe and knob-like base, containing 8 biseriately arranged ascospores. Ascospores asymmetric, fusoid to narrowly ellipsoid with the second cell slightly wider than others, straight or curved, 1–3-euseptate, pale to chocolate brown, smooth or faintly verruculose.
Pycnidia similar to ascomata. Peridium brown, pseudoparenchymatous. Conidiophores when present filiform, simple to sparsely branched, with pegs and terminal phialides. Phialides ampulliform, lageniform, or subcylindrical. Conidia forming on pegs and phialides, rod-like to ellipsoid, 1-celled, hyaline or subhyaline, sometimes pale brown in mass, smooth.
Type genus: Nigrograna Gruyter et al.
Nigrograna Gruyter et al., Stud. Mycol. 75: 31. 2012, emend.
Sexual morph: Ascomata depressed globose to globose, immersed in wood and bark, erumpent, less commonly superficial, scattered or aggregated in small groups, black, seated on or surrounded by olivaceous or brown, KOH-negative subiculum. Ostiolar necks papillate to cylindrical; ostioles periphysate. Peridium pseudoparenchymatous, cells thin-walled and lighter at the inner side, thick-walled and darker at the outer side, usually covered by hyphae. Hamathecium consisting of apically free paraphyses originating in the subhymenium between developing asci, later becoming elongated, branching and anastomosing and appearing as “trabeculate pseudoparaphyses”. Asci clavate, bitunicate, fissitunicate, with short stipe and knob-like base, containing 8 ascospores biseriately arranged in the upper part. Ascospores asymmetric, fusoid to narrowly ellipsoid with the second cell slightly wider than others, straight or curved, 1–3-euseptate, pale to chocolate brown, smooth or faintly verruculose.
Asexual morph: Pycnidia similar to ascomata and usually co-occurring with them. Peridium brown, pseudoparenchymatous. Conidiophores filiform, simple to sparsely branched, with pegs along one or two sides and solitary terminal phialides; reduced in culture (from clinical isolates). Phialides ampulliform, lageniform, or subcylindrical. Conidia forming on pegs and phialides, oblong, cylindrical or allantoid, sometimes ellipsoid, hyaline or subhyaline, 1-celled, smooth.
Habitat: in bark of moderately decayed twigs of shrubs and trees, often in old fructifications of pyrenomycetes, sometimes human pathogenic.
Type species: Nigrograna mackinnonii (Borelli) Gruyter et al.
Notes: Nigrograna was described by Gruyter et al. (2012) as a monotypic genus. Nigrograna mackinnonii was isolated from human mycetomata in Mexico and Venezuela, and no sexual morph was known. Here we add the sexual morph and asexual morph from natural substrates. For pycnidia of Nigrograna mackinnonii in culture no conidiophores were described. Absence of conidiophores may be an adaptive reduction from the natural situation.
All species of Nigrograna are morphologically very similar and may be interpreted as cryptic species. As Chesters (1938) noted for N. fuscidula (as Melanomma fuscidulum), ascospores first develop their median primary septum and consecutively the two 2 additional septa. In all species of Nigrograna with available sexual morph apically free paraphyses were found among immature asci, just as in other genera of the Thyridariaceae or in Teichospora (Jaklitsch et al. 2016).
Nigrograna fuscidula (Sacc.) Jaklitsch & Voglmayr, comb. nov. MycoBank MB817787. Fig. 9, Fig. 10.
Fig. 9.
Nigrograna fuscidula, sexual morph. A. Specimen label. B–D. Ostiolar necks on the host surface. E, F. Ascomata in vertical section. G. Peridium in section. H. Subicular hyphae. I, J. Free ends of paraphyses among ascus tips. K. Branched (?pseudo)paraphyses above asci. L. Ascus tip. M. Paraphysis in young hymenium (asci at initial stage). N–Q. Asci (N, O. young). R–Y. Ascospores (R, S. young). G, H, J–L, O, R, S, U, V, X, Y. in 3 % KOH. A. isotype (PAD); B, K, O, P, S, T. lectotype K(M) 202882; C–F, R, U, V. WU 36879; G–J, L, N. WU 36881; M, Q, Y. WU 36880; W. WU 36884; X. WU 36883. Scale bars: B, E, F = 100 μm; C, D = 200 μm; G–K, M, O = 10 μm; L, T–Y = 3 μm; N, P–S = 5 μm.
Fig. 10.
Nigrograna fuscidula, asexual morph on the natural host. A, B. Pycnidia in vertical section. C. Pycnidial wall, subicular hyphae and old conidiophores. D–G. Conidiophores with pegs and phialides. H–J. Conidia. A. WU 36880; B, D–I. WU 36882; C, J. WU 36881. Scale bars: A, B = 100 μm; C, D = 10 μm; E–G = 7 μm; H–J = 3 μm.
Basionym: Sphaeria fuscidula Sacc., Myc. Ven. II, No. 159. 1875, in sched.
Synonyms: Melanomma fuscidulum (Sacc.) Sacc. [as “Melanoma fuscidula”], Hedwigia 14: 73. 1875.
Aposphaeria fuscidula (Sacc.) Sacc., Syll. fung. (Abellini) 3: 173. 1884.
Ascomata (180–)215–405(–570) μm (n = 45) diam, (160–)175–260(–340) μm (n = 33) high, depressed globose to globose, grey to black, variable in size and appearance depending on the specimen; immersed in bark and wood with only ostiolar necks visible on the host surface or immersed-erumpent, only rarely becoming superficial, scattered to aggregated in numbers of up to 10, often associated with pycnidia when young, and both surrounded by brown to olivaceous, branched, cylindrical, 2–4 μm wide subicular hyphae. Subiculum often concentrated below the epidermis on the host or occurring as mats or extending in patches on decorticated wood. Peridium to 35 μm thick, pseudoparenchymatous, consisting of a subhyaline to pale brown inner layer of small thin-walled angular cells and an outer dark brown t. angularis of thick-walled cells 3–11 μm diam. Ostiolar necks (31–)52–86(–125) μm (n = 53) diam, ca 90–480 μm long, black, protruding up to 0.4 mm from the host surface, short-papillate to long-cylindrical, rounded or angular in section, erect or oblique, central or eccentric, apex rounded or flattened, black. True and distinct hymenium present, apically free, 1–3.5 μm wide unbranched paraphyses present among initial stages of asci, later branching and also anastomosing above asci. Asci (48–)52–72(–82) × (8.0–)8.3–9.8(–11) μm (n = 38), clavate to oblong, bitunicate, fissitunicate, with short stipe and simple or furcate base; endotunica thick, swelling in 3 % KOH, containing 8 biseriate ascospores. Ascospores (10.2–)12–14.5(–18) × (3.8–)4.2–5.0(–5.4) μm, l/w (2.2–)2.6–3.3(–4.5) (n = 103), asymmetrically fusoid with upper part or second cell slightly wider, often slightly curved, thick-walled, 1-septate in young asci, becoming 3-euseptate, slightly constricted at the primary median septum, first pale greyish brown, turning medium to dark grey-brown, not or slightly and slowly darkening in 3 % KOH, smooth.
Asexual morph on the natural host: Pycnidia ca. 140–340 μm diam, ca. 100–195 μm high, co-occurring with ascomata and very similar to them, but contents grey or greyish brown, interior filled with conidia and a whitish conidiophore layer between the peridium and the conidial mass. Peridium 20–35 μm thick, forming a t. angularis of dark brown, thick-walled 4–10(–11) μm wide cells. Conidiophores arising from the pycnidial wall, to 55 μm long and mainly 2–3.7 μm wide, simple, only branched once near the base, with pegs along one or two sides and solitary phialides terminally. Phialides (5.0–)6.5–8.7(–10.5) × (2.0–)2.5–3.0(–3.5) μm (n = 34), variable in shape, ampulliform-lageniform-subcylindrical. Conidia (3.0–)3.2–4.3(–6.0) × (1.1–)1.3–1.6(–1.8) μm, l/w (2–)2.2–3(–3.8) (n = 72), rod-like, oblong to cylindrical or allantoid, brownish in mass, individually subhyaline to hyaline, 1-celled, containing 2 guttules, smooth.
Cultures: On CMD at 22 °C colony radius 13–17 mm after 1 mo, colony circular, pale to dull brown, darkening with time, distinctly or indistinctly zonate due to aerial hyphae, centre often darker, white aerial hyphae spreading from the centre, containing numerous minute drops, odour often unpleasant, reminiscent of dental clinics; no asexual morph detected.
Habitat: in bark and wood of branches and logs of Sambucus nigra.
Distribution: Europe.
Lectotype, here designated: Italy, Treviso, Vittorio, on branches of Sambucus nigra, Oct. 1873, P.A. Saccardo Mycotheca Veneta No. 159 (K(M) 202882!; MBT372892. PAD: isotype). Epitype, here designated: Austria, Vienna, 22nd district, Lobau, Panozzalacke, on dead branches and twigs of Sambucus nigra lying on the ground, ascomata in wood, 27 Mar. 2016, W. Jaklitsch (WU 36879; ex-epitype culture CBS 141556 = MF7; MBT372418). Designation of an epitype is necessary due to easy mix-up with other species. The Austrian material is selected, because the specimens collected in Italy are for the most part overmature.
Other material examined: Austria, Niederösterreich, close to the highway exit Bad Vöslau, on dead branches of Sambucus nigra attached to the shrub, 22 Feb. 2016, W. Jaklitsch & H. Voglmayr (WU 36880; culture MF3); Vienna 22nd district, Lobau, Panozzalacke, on dead branches of Sambucus nigra attached to the shrub, holomorph, ascomata mainly in bark, often immature, 21 Mar. 2015, W. Jaklitsch (WU 36881; culture from ascospores CBS 141476 = MF1; culture from conidia MF1a); ibidem, same substrate, same collector, 20 Feb. 2016, W. Jaklitsch (WU 36882). France, Ariege, Rimont, Las Muros, elev. 480 m, on decorticated twigs of Sambucus nigra, 12 Apr. 2016, J. Fournier JF 16013 (WU 36890). Italy, Veneto, Padua, Colli Euganei, Arqua Petrarca, on branches of Sambucus nigra on the ground, ascomata mostly overmature, mostly in wood, soc. old Diaporthe sp., 6 Apr. 2016, W. Jaklitsch & H. Voglmayr (WU 36883; culture MF8). Veneto, Treviso, colle Montello, on branches of Sambucus nigra on the ground, ascomata in bark and wood, mostly overmature, 8 Apr. 2016, W. Jaklitsch & H. Voglmayr (WU 36884; culture MF9).
Notes: Melanomma fuscidulum is not congeneric with M. pulvis-pyrius, the generic type of Melanomma, the type genus of the Melanommataceae. All species with sexual morphs here recognised in Nigrograna would earlier have been identified as Melanomma fuscidulum. Most seem to be fungicolous. Chesters (1938) studied Melanomma fuscidulum in detail. However, his descriptions and conclusions regarding the asexual morph were from material collected on Ulmus and thus, in light of the molecular diversification of species having similar morphology, he most probably studied a different species. We have detected N. fuscidula only on its original host Sambucus nigra. Ascomata may be found on attached branches. In this case they are usually immersed in bark, asci are often immature and contain 2-celled, sometimes even 1-celled spores; also associated pycnidia are more common in such specimens. On twigs on the ground the fungus usually occurs in wood and bark. Interestingly, in wood ascomata sometimes tend to be larger and ascospores smaller and darker. Culture preparation from both forms yielded the same DNA data. Nigrograna fuscidula may be mistaken for Parathyridaria ramulicola, which may occur on the same host, but differs by short discoid ostioles, distinctly stromatised wood around ascomata, absence of a subiculum and by pale greyish brown ascospores, which do not darken in 3 % KOH. Darkening around ostioles of N. fuscidula may be due to the upper part of erumpent ascomata or subiculum, or by other fungi. A differentiation from N. obliqua, which may also occur on Sambucus nigra is difficult, but the latter has slightly larger, esp. wider and very dark ascospores when mature. The dark brown ascospores including the ascus shown from the slide of the M. fuscidulum lectotype have not been effectively reconstituted and may therefore appear too dark.
Nigrograna mycophila Jaklitsch, Friebes & Voglmayr, sp. nov. MycoBank MB817781. Fig. 11.
Fig. 11.
Nigrograna mycophila. A, B, D. Ostiolar necks in face view. C. Laterally cut ascoma above an effete ascoma of a Diaporthe sp. (note the grey stromatic zone of the Diaporthe in the wood). E. Horizontally cut pycnidium surrounded by subicular hyphae in an ascoma of Calosporella innesii. F. Young ascoma in vertical section, surrounded by subicular hyphae. G. Peridium in section (subicular hyphae in the lower part). H. Subicular hyphae. I–K. Asci (I. immature). L–N. Ascospores. O. Conidiophores with phialides and conidia. P. Phialide. Q, R. Conidia. I, J, O, Q, R. in 3 % KOH. A, L. WU 36887; B, C, J, K. WU 36886; D–I, M–R. WU 36888. Scale bars: A, D, E = 150 μm; B, F = 100 μm; C = 250 μm; G–K, O = 10 μm; L–N, P–R = 5 μm.
Etymology: mycophila for its occurrence on other fungi, particularly Diaporthales.
Ascomata immersed in bark, in or above effete ascomata of Diaporthales, singly or arranged in valsoid groups, globose to subglobose, (172–)213–336(–380) μm wide and high (n = 16), variably surrounded by olivaceous to brown subiculum, the latter also ascending ostiolar necks as stiff seta-like hairs. Ostiolar neck (57–)65–124(–159) μm (n = 20) diam, cylindrical, black, straight or obliquely emerging, apex rounded or flat, concolorous or more frequently lighter in colour, whitish, yellowish, olivaceous or brownish, not or only slightly projecting beyond the bark level. Peridium pseudoparenchymatous, consisting of (3–)3.5–7(–11) μm (n = 30) wide cells, thin-walled and pale brown at the interior, becoming darker and thicker-walled to the outside; there wall densely covered by 2–4 μm wide smooth subicular hyphae making width determination difficult. Hamathecium consisting of numerous 1–3.5 μm wide paraphyses with free ends visible among immature asci and apparently trabeculate pseudoparaphyses widest at their bases. Asci (65–)72–85(–89) × (10.5–)11–13(–14.3) μm (n = 26), clavate, bitunicate, fissitunicate but stable in mounts, with a small ocular chamber, thick endotunica, short stipe and knob-like to furcate base, containing 8 ascospores biseriately arranged, particularly at upper levels. Ascospores (12.7–)14.3–17(–19) × (5.0–)5.5–6.3(–6.9) μm, l/w (2.3–)2.4–2.9(–3.2) (n = 80), fusoid to narrowly ellipsoid, with the second cell or the upper part slightly enlarged, straight or curved, 2-celled and pale brown when young, 3-euseptate and dark brown at maturity, slightly constricted at the median septum, thick-walled, smooth, not or only slightly darker in 3 % KOH.
Asexual morph on the natural host: Pycnidia similar to ascomata but smaller, ca. 100–220 μm diam, immersed in perithecia of the host fungus, with a dark brown pseudoparenchymatous wall, surrounded by olivaceous to brown subiculum, containing simple, sparsely branched, up to 50 μm long conidiophores. Solitary minute pegs and lageniform to subcylindrical phialides (4.2–)7.0–10(–10.8) × (1.8–)2.0–2.5(–2.7) μm (n = 16) inserted along and terminally on the conidiophores producing oblong to cylindrical, 1-celled, hyaline, smooth conidia (3.0–)3.5–4.2(–5.7) × (1.3–)1.5–1.9(–2.1) μm, l/w (1.6–)1.9–2.5(–3) (n = 51).
Cultures: On CMD growth radius at 22 °C ca. 10 mm after 18 d and 15–17 mm after 37 d; colony circular, dark and deep brown or with an olivaceous tinge, with radial texture, indistinctly zonate, with light aerial hyphae in the centre, odour indistinct. On MEA growth radius 18 mm after 41 d at 22 °C (TDK), light grey, turning dark brown with time, with a thick pale (greyish) brown mat of aerial hyphae, reverse dark brown to black, odour indistinct. No asexual morph detected.
Habitat: in ascomata and pseudostromata of Diaporthales on corticated twigs of various shrubs and trees (known from Acer campestre, Acer pseudoplatanus and Corylus avellana).
Distribution: Europe (Austria, Denmark)
Holotype: Austria, Burgenland, Oberwart, Althodis, near parking place of the Baumwipfelweg, in pseudostromata of Diaporthe sp. on twigs of Acer campestre attached to the tree, 27 Feb. 2016, G. Friebes (WU 36886, ex-type culture CBS 141478 = MF5).
Other material examined: Austria, Steiermark, Bruck an der Mur, Kaltenbachergraben, in pseudostromata of ?Diaporthe decedens on twigs of Corylus avellana on the ground, 5 Mar. 2016, G. Friebes (WU 36887, culture MF6). Denmark, Kristiansminde, Sorö Sönderskov, close to the field centre, in ascomata of Calosporella innesii on twigs Acer pseudoplatanus, 27 May 2007, W. Jaklitsch W.J. 3095 (WU 36888, culture CBS 141483 = TDK).
Nigrograna norvegica Jaklitsch & Voglmayr, sp. nov. MycoBank MB817782. Fig. 12.
Fig. 12.
Nigrograna norvegica (WU 36885). A. Ostiolar apices in face view (red: Cosmospora sp.). B. Horizontally cut ascomata in a Diaporthe pseudostroma, surrounded by subicular hyphae. C. Ascoma in vertical section (red: Cosmospora sp.). D. Ascoma and pycnidium in vertical section. E. Ascomatal peridium in section. F. Ascus apex. G. Paraphysis tip. H–J. Asci (H. with apically free paraphyses). K–N. Ascospores. O. Section through pycnidium showing peridium, subicular hyphae and conidiophores. P, Q. Conidiophores and phialides. R, S. Conidia. G, K–M. in 3 % KOH. Scale bars: A, C, D = 150 μm; B = 400 μm; E, H, J–N, R, S = 5 μm; F, G = 3 μm; I, P, Q = 10 μm; O = 15 μm.
Etymology: norvegica, due to its occurrence in Norway.
Ascomata immersed in ascomata and pseudostromata of Diaporthe sp. singly or in valsoid groups, (177–)200–285(–320) μm (n = 14) diam, with slightly smaller height, globose to depressed globose, black, surrounded by a subiculum of brown to olivaceous, 2–5 μm wide hyphae; subiculum also concentrated between wood and bark. Ostiolar necks (50–)51–75(–88) μm (n = 21) diam, black, cylindrical or papillate-conical with rounded top, central or eccentric, vertical or oblique and convergent. Peridium 10–30 μm thick, pseudoparenchymatous, consisting of (2.5–)3.5–7.5(–11.5) μm (n = 64) wide cells, tending to be larger, paler and more thin-walled inside, and smaller, darker reddish brown and thick-walled outside. Hamathecium consisting of apically free paraphyses and possibly trabeculate pseudoparaphyses, branched, anastomosing, 1–3 μm wide. Asci (50–)61–77(–85) × (7.2–)7.5–9(–10) μm (n = 23), oblong to clavate, fissitunicate but with high stability in mounts, thick-walled, with small but distinct ocular chamber, a stipe to ca. 35 μm long and knob-like base, containing 8 ascospores biseriately arranged at upper levels. Ascospores (8.6–)10.8–13.5(–16.5) × (3.3–)3.8–4.4(–4.8) μm, l/w (2.3–)2.7–3.3(–3.8) (n = 80), fusoid to clavate, with 3 eusepta, apical cell often acute, second cell usually slightly widened, constricted at the medium, only slightly at other septa, straight or curved, terminal cells often slightly longer than mid cells, pale to medium brown, slowly turning dark brown in 3 % KOH, smooth.
Asexual morph on the natural host: Pycnidia associated with and of similar size and shape as ascomata, white inside; peridium 20–30 μm thick, pseudoparenchymatous, of brown cells 2–7 μm diam. Conidiophores arising from the wall in palisadic arrangement, filiform, septate, 1- to several-celled, hyaline, to ca. 60 μm long and 3 μm wide, not or sparsely branched, with pegs along one side and a terminal phialide. Phialides (8.2–)8.4–10.5(–11.6) × (2.0–)2.2–2.7(–3.0) μm (n = 11), lageniform to cylindrical. Conidia formed on phialides and pegs, (2.9–)3.2–3.7(–4.1) × (1.5–)1.6–1.8(–2.0) μm, l/w (1.6–)1.8–2.2(–2.6) (n = 50), oblong to cylindrical, unicellular, with 1–2 small guttules, smooth.
Growth on CMD at 22 °C slow, growth radius reaching up to 33 mm after 70 d; colony dark brown with a paler ring around the centre, internally lobed, aerial hyphae forming a loose reticulum; odour indistinct. No asexual morph detected.
Habitat: in pseudostromata of Diaporthales.
Distribution: Norway, only known from the holotype location.
Holotype: Norway, Aust-Agder, Arendal kommune, Nedenes, Langevoll, in pseudostromata of a Diaporthe sp. soc. Cosmospora (s. lato) sp. on a twig of Tilia platyphyllos lying on the ground, 4 Oct. 2014, W. Jaklitsch & H. Voglmayr (WU 36885; ex-type culture CBS 141485 = TR8).
Note: This species is known from a single collection, therefore no prediction about variation of morphological features is possible.
Nigrograna obliqua Jaklitsch & Voglmayr, sp. nov. MycoBank MB817783. Fig. 13.
Fig. 13.
Nigrograna obliqua. A–C. Ascomata and ostiolar apices in face view. D. Ascoma in section, with eccentric, obliquely ascending ostiolar neck. E, F. Peridium in section (F. subicular hyphae in upper part). G. Subicular hyphae. H. Young hymenium with basal part of paraphyses. I–K. Asci (I. immature). L–Q. Ascospores (L. young, in face view showing warts). F, M, N, P, Q. in 3 % KOH. A, B, K, O, P. WU 36875; C. WU 36878; N. WU 36877; D–J, L, M, Q. WU 36876. Scale bars: A, B = 200 μm; C = 400 μm; D = 100 μm; E–J = 10 μm; K, Q = 7 μm; L–P = 5 μm.
Etymology: obliqua owing to the oblique position of its ascomata with respect to the host surface.
Ascomata (210–)275–435(–495) μm (n = 22) diam, ca. 240–400 μm high, globose to subglobose, immersed in bark, sometimes erumpent, becoming visible in bark fissures, standing on wood or inner bark, scattered or in small groups to 5 forming 0.5–2 mm wide irregular pustules, often lying singly parallel or oblique to the bark or wood surface and then with a cylindrical ostiolar neck to 0.5 mm long; often surrounded by an olivaceous to dark brown subiculum of 1.5–5 μm wide brown hyphae, sometimes inflated up to 8.5 μm, unchanged in 3 % KOH. Apex blunt or papillate, black, sometimes apically flattened, (45–)67–120(–150) μm (n = 62) wide; ostioles periphysate. Peridium ca. 20–40 μm wide, often appearing thicker due to densely adhering hyphae, dark brown, turning olivaceous to black in 3 % KOH, pseudoparenchymatous, comprising (4–)5.5–11(–15.5) μm (n = 43) wide cells, thin-walled and lighter at the inner side, thick-walled and darker at the outer side, usually covered by hyphae; the latter remaining dark brown in 3 % KOH. Hamathecium comprising 1–3.5 μm wide trabeculate “pseudoparaphyses” clearly originating in the subhymenium between asci, branched near the bases, also free apices present between immature asci. Asci (55–)65–83(–96) × (8.2–)9.5–12.2(–14.5) μm (n = 56), clavate, bitunicate, fissitunicate, with short stipe and knob-like base, with 8 ascospores biseriately arranged in the upper part. Ascospores (11.2–)13.0–17.0(–19.8) × (3.8–)4.5–5.8(–7) μm, l/w (2.3–)2.6–3.2(–4.3) (n = 162), fusoid to narrowly ellipsoid with the second cell slightly wider than others, straight to slightly curved, (1–)3-euseptate, slightly constricted at the median septum, pale brownish to yellow-brown when young, turning dark to chocolate brown upon maturation (in water and 3 % KOH), faintly verruculose, with irregularly arranged verrucae (best seen in young ascospores) and one or few guttules in each cell.
Cultures: Growth on CMD at room temperature slow, colony radius 20–25 mm after 2 mo, brown, with a radial texture, margin lighter and wavy, odour indistinct; no asexual morph detected. Colonies on MEA thick, dense, light grey-brown, with a thick mat of aerial hyphae.
Habitat: in bark of moderately decayed twigs of various shrubs and trees (known from Ribes uva-crispa, Salix caprea, Sambucus nigra, S. racemosa)
Distribution: Europe (Austria, France, UK).
Holotype: Austria, Kärnten, Millstatt am See, Lammersdorf, at the junction to Pesenthein, on corticated twigs of Salix caprea, soc. Lophiostoma compressum, 2 Nov. 2015, W. Jaklitsch & H. Voglmayr (WU 36875; ex-type culture CBS 141477 = MF2).
Other material examined: Austria, Niederösterreich, Altmelon, Altmeloner Au, on Sambucus racemosa, soc. Diaporthe sp. in excess, 11 Jul. 2015, W. Jaklitsch, H. Voglmayr & I. Greilhuber (WU 36876; culture CBS 141475 = KE); Osttirol, Prägraten am Großvenediger, Umbalfälle, on Ribes uva-crispa, soc. Arthopyrenia sp., Capronia sp., 17 Jun. 2015, W. Jaklitsch & H. Voglmayr (WU 36877; culture MRP). Steiermark, Weiz, Ratten, Kirchenriegel: Bretterhoferwald, 47.482928, 15.711746, 850 m, on a twig of Sambucus racemosa, 21 Nov. 2015, B. Wergen (specimen lost, culture BW4). France, Ariege, Rimont, Las Muros, elev. 480 m, on decorticated twig of Sambucus nigra, 12 Apr. 2016, J. Fournier JF 16011 (WU 36889; culture MF10). UK, England, Devon, Exeter, Killerton Park, on branch of Sambucus nigra, 8 Sep. 2004, W. Jaklitsch, H. Voglmayr & J. Webster W.J. 2689 (WU 36878 = MF; identity confirmed by ITS amplified directly from the specimen).
Notes: Nigrograna obliqua has the darkest ascospores of all Nigrograna species treated here. In contrast to other species, ascomata frequently become superficial and are situated obliquely to the host surface, often with eccentric ostiolar necks. This fungus differs from Thyridaria sambuci, which also occurs on Sambucus racemosa, by a number of features: it is inconspicuous, has no orange ostiolar apices, no reaction in 3 % KOH, and mature ascospores are dark brown in water and verruculose. Another similar but unrelated fungus occurring on the same host has slightly larger, yellow-brown ascospores, which may become up to 5-septate and are formed in long cylindrical asci.
Discussion
Thyridaria-like fungi are characterised by somehow crowded or clustered perithecioid ascomata with bitunicate asci containing brown phragmospores, often surrounded by subicular hyphae, and they often have a bright-coloured ostiolar area. As indicated in the introduction, already Wehmeyer (1941) pointed out that there is a large number of species of thyridaria-like fungi “with many-celled brown ascospores and numerous filiform paraphyses which are now scattered through such genera as Thyridaria, Kalmusia, Melanomma, Leptosphaeria, Cucurbitaria etc., which must undergo a careful comparative study before any usable generic lines can be drawn”. Based on our collections, such fungi occur in at least nine lineages within Pleosporales. Some belonging to Teichospora were treated by Jaklitsch et al. (2016). As a main objective of this work we clarify phylogenetic position and morphology of the generic type of Thyridaria, which has to be called T. broussonetiae, not T. incrustans, contrary to previous practice.
The “probably best known species of Thyridaria”, T. rubronotata, does not belong to the genus. It has taken a stable place in phylogenetic trees of the Pleosporales. This clade is now named Cyclothyriellaceae with Cyclothyriella rubronotata as its type.
Wehmeyer (1941) noted that Thyridaria rubronotata is “extremely variable in its stromatic configuration, as even the type collection of Melogramma rubronotata shows all transitions from free scattered or crowded perithecia of the Melanomma type through widely effuse crust-like masses or smaller erumpent clusters of crowded perithecia of the Cucurbitaria type to definitely stromatic pustules with erumpent discs of the Thyridaria incrustans type. It illustrates the difficulty of delimiting this genus and seems to represent a species complex which includes a variety of forms on different hosts which will be difficult to separate”. He also discussed putative synonyms of this species, but in light of the remarkable phylogenetic divergence of similar fungi, this has little significance from current view.
The generic type of Thyridaria, T. broussonetiae, is part of a highly supported clade that includes Roussoella. This clade becomes the family Thyridariaceae with Roussoellaceae as a younger synonym. All species of this clade could be interpreted as species of Thyridaria, supported on one hand by medium statistical support of its largest subclade (Roussoella sensu lato), which contains species of Roussoella, Roussoellopsis, Neoroussoella (see Ju et al., 1996, Liu et al., 2014) and Arthopyrenia salicis, and on the other hand there is morphological support by the fact that in Roussoella sensu lato ascomata are scattered or clustered in or beneath some stromatic tissue and that the asci are usually very stable in microscopic mounts, just as in Thyridaria, and that asexual morphs are similar in all genera. There are, however, arguments against lumping to such an extent, we therefore treat the subclades taxonomically separately at the generic level. Particularly Roussoellopsis differs from Roussoella in ascospore features and especially in different asexual morphs. A reason why its species are currently contained within a clade of Roussoella spp. may be incompleteness of the DNA data matrix; e.g., ITS of two species labelled Roussoellopsis is lacking. However, it may also turn out that these morphological differences are unsuitable for generic delimitation. Additional detailed taxon and sequence sampling is necessary to clarify the phylogenetic relationships within the Roussoella subclade.
Arthopyrenia salicis appears in the Roussoella subclade. The original source of DNA data for this species is the strain CBS 368.94. This was collected and isolated from Salix bark in the Netherlands in 1994. LSU (AY607730) and SSU (AY607742) sequences from a second accession which stem from DNA extracts from an apparently correctly identified herbarium specimen collected in Scotland (1994 Coppins) are identical to those of CBS 368.94. Two additional strains were only labelled with this name due to sequence similarity. As documented in GenBank, one (strain NRRL 62788) came from crop field soil plating in Illinois, USA and another (strain CBMAI1330) was isolated from the marine sponge Dragmacidon reticulatum in Brazil. Remarkably, also the ITS sequence (KT270306) of a root endophyte isolated from Microthlaspi perfoliatum (Brassicaceae) is identical to that of Arthopyrenia salicis strain CBS 368.94, as well as the ITS sequence of another strain, CBS 170.96, which was isolated from bamboo collected in Papua New Guinea and initially identified as Roussoella intermedia (included as Roussoella sp. in our analyses; see Table 1 and Fig. 1). Arthopyrenia salicis is apparently not congeneric with Arthopyrenia sensu stricto, as it differs from other species of the genus by a different hamathecium, which is confined to periphysoids (Coppins 1988). Absence of interascal filaments does not support an affiliation to the Thyridariaceae. In addition, ascospores of Arthopyrenia spp. do not germinate on artificial media in our experience. In light of this evidence, we suspect that the isolates and correspondent sequences labelled Arthopyrenia salicis rather represent a species of Roussoella. As the generic type of Arthopyrenia has not been sequenced, its phylogenetic position remains obscure.
Ariyawansa et al. (2015) added the monotypic Elongatopedicellata, a wood saprobe with fusiform, 1-septate hyaline ascospores in long-stipitate asci, to the Roussoellaceae, but no DNA data are available for this fungus.
At present a revision of the genus Thyridaria is impossible or at least extremely difficult. Index Fungorum lists 57 taxa in Thyridaria. After exclusion of forms, varieties and those referred to other groups, 37 names still remain for further study. Berlese (1894) identified Thyridaria lateritia (Ellis) Sacc. and T. myriangioides (Berk. & Ravenel) Sacc. as Melogramma campylosporum (as “vagans”). Based on examination of an authentic specimen in PAD, Thyridaria minor seems to be a synonym of Cyclothyriella rubronotata as already pointed out by Wehmeyer (1941) and Barr (1990). The latter referred Thyridaria to the Platystomaceae and combined several species of Lophiostoma in Thyridaria. Probably none of those will remain in this genus. Later she (Barr 2003) changed her concept and referred Thyridaria to the Didymosphaeriaceae. It seems that most species of Thyridaria will find a different generic home. Particularly Melanomma epithets need also to be considered, as we have seen that M. fuscidulum neither belongs to Melanomma nor to the Melanommataceae.
Species of Nigrograna may be interpreted as a result of cryptic speciation, as morphologically they show only subtle differences. All of them would have morphologically been identified as Melanomma fuscidulum, due to its broad definition (see Chesters 1938). We expect many more species in Nigrograna yet to be detected, which will not be possible to be identified without gene sequences.
Important to note is the finding already reported for Teichospora (Jaklitsch et al. 2016) that nearly all species treated here in detail form a true hymenium, i.e. they differ from the “pleosporalean centrum” substantially. This finding can only be observed in immature or young material, when there are only apically free paraphyses present and asci start to develop or form their spores, but also among mature asci apically free paraphyses can be observed. We think that the so-called narrowly cellular or “trabeculate” pseudoparaphyses are true paraphyses, which become considerably elongated, branch and anastomose after ascus maturation. A meaningful study of the hamathecium in fully mature ascomata is however difficult. Liew et al. (2000) reported that Pleosporales (having “cellular” pseudoparaphyses) and Melanommatales (having “trabeculate” pseudoparaphyses) are not separable as monophyla using SSU sequences. However, they did not study ascoma ontogeny and hamathecial features in detail, but rather reviewed findings of earlier workers, who had apparently studied only a small number of genera not being representative for the whole order Pleosporales.
Another feature reported for the genus Roussoella is high stability of the ascal exotunica, particularly in 3 % KOH. This is quite common for nearly all fungi treated here, only in Nigrograna fissitunicate ascus dehiscence can be seen rather frequently.
Other morphological features are shared among genera of the Thyridariaceae, as far as it appears currently: sexual morphs generally have brown ascospores with transverse septa. Asexual morphs are pycnidial to stromatic and form hyaline or brown unicellular conidia on phialides, sometimes annellides (Roussoellopsis). Three-septate brown ascospores are a story of success in evolution, as they are widely distributed in pyrenomycetes. Ascospore features alone seem therefore not to be very useful for classification.
Ecology: Most species of this clade grow on plants, especially wood and bark, sometimes, leaves, some also on other fungi, and few have been isolated as opportunistic pathogens from human tissue, which appears rather an exception from the natural habitat of these fungi.
Acknowledgements
We thank the fungarium curators of BPI, FH, G, K, M, NY, UPS, and W. Till at WU for sending and managing collections, J. Fournier, G. Friebes, K. Siepe and B. Wergen for fresh material or/and data, T. Læssøe and B. Norden for organising field trips in Denmark and Norway, which yielded two species of Nigrograna. The financial support by the Austrian Science Fund (FWF; project P25870-B16) is gratefully acknowledged.
Footnotes
Peer review under responsibility of CBS-KNAW Fungal Biodiversity Centre.
References
- Ahmed S.A., Stevens D.A., van de Sande W.W.J. Roussoella percutanea, a novel opportunistic pathogen causing subcutaneous mycoses. Medical Mycology. 2014;52:689–698. doi: 10.1093/mmy/myu035. [DOI] [PubMed] [Google Scholar]
- Ahmed S.A., van de Sande W.W.J., Stevens D.A. Revision of agents of black-grain eumycetoma in the order Pleosporales. Persoonia. 2014;33:141–154. doi: 10.3767/003158514X684744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyawansa H.A., Hyde K.D., Jayasiri S.C. Fungal diversity notes 111–252—taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity. 2015;75:27–274. [Google Scholar]
- Barr M.E. Melanommatales (Loculoascomycetes) North American Flora Series II. 1990;13:1–129. [Google Scholar]
- Barr M.E. The affinities of Thyridaria. Mycotaxon. 2003;88:271–278. [Google Scholar]
- Berlese A.N. Icones Fungorum omnium hucusque cognitorum ad usum Sylloges Saccardianae adcommodatae. 1894;1:1–243. 162 pl. Avellino; E. Pergola; repr. 1968: Bibliotheca Mycologica 16. [Google Scholar]
- Boehm E.W.A., Marson G., Mathiassen G.H. An overview of the genus Glyphium and its phylogenetic placement in Patellariales. Mycologia. 2015;107:607–618. doi: 10.3852/14-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. [Google Scholar]
- Carneiro de Almeida D.A., Pascholati Gusmão L.F., Miller A.N. A new genus and three new species of hysteriaceous ascomycetes from the semiarid region of Brazil. Phytotaxa. 2014;176:298–308. [Google Scholar]
- Checa J., Jaklitsch W.M., Blanco M.N. Two new species of Thyronectria from Mediterranean Europe. Mycologia. 2015;107:1314–1322. doi: 10.3852/15-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesters C.G.C. Studies of British Pyrenomycetes II. A comparative study of Melanomma pulvis-pyrius, M. fuscidulum and Thyridaria rubronotata. Transactions of the British Mycological Society. 1938;22:116–150. [Google Scholar]
- Coppins B.J. Notes on the genus Arthopyrenia in the British Isles. Lichenologist. 1988;20:305–325. [Google Scholar]
- Crous P.W., Wingfield M.J., Schumacher R.K. Fungal Planet description sheets: 281–319. Persoonia. 2014;33:212–289. doi: 10.3767/003158514X685680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Carris L.M., Giraldo A. The Genera of Fungi – fixing the application of the type species of generic names – G 2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia. IMA Fungus. 2015;6:163–198. doi: 10.5598/imafungus.2015.06.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D.Q., Phookamsak R., Wijayawardene N.N. Bambusicolous fungi. Fungal Diversity. 2016 http://dx.doi.org/10.1007/s13225-016-0367-8 [Google Scholar]
- de Hoog G.S., Gerrits van den Ende A.H.G. Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses. 1998;41:183–189. doi: 10.1111/j.1439-0507.1998.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Fuckel L. [1869–70]. Symbolae Mycologicae. Beiträge zur Kenntnis der Rheinischen Pilze. Jahrbücher des Nassauischen Vereins für Naturkunde. 1870;23–24:1–459. [Google Scholar]
- Grondona I., Monte E., Garcia-Acha I. Pyrenochaeta dolichi: an example of a confusing species. Mycological Research. 1997;101:1404–1408. [Google Scholar]
- Gruyter J. de, Woudenberg J.H.C., Aveskamp M.M. Redisposition of phoma-like anamorphs in Pleosporales. Studies in Mycology. 2012;75:1–36. doi: 10.3114/sim0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafellner J. Karschia. Revision einer Sammelgattung an der Grenze von lichenisierten und nichtlichenisierten Ascomyceten. Beihefte zur Nova Hedwigia. 1979;62:1–248. [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Huhndorf S.M. Neotropical Ascomycetes 2. Hypsostroma, a new genus from the Dominican Republic and Venezuela. Mycologia. 1992;84:750–758. [Google Scholar]
- Hyde K.D., Borse B.D. Marine fungi from Seychelles V. Biatriospora marina gen. et sp. nov. from mangrove wood. Mycotaxon. 1986;26:263–270. [Google Scholar]
- Hyde K.D., Jones E.B.G., Liu J.-K. Families of Dothideomycetes. Fungal Diversity. 2013;63:1–313. [Google Scholar]
- Jaklitsch W.M. European species of Hypocrea – part I. Studies in Mycology. 2009;63:1–91. doi: 10.3114/sim.2009.63.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Fournier J., Dai D.Q. Valsaria and the Valsariales. Fungal Diversity. 2015;73:159–202. doi: 10.1007/s13225-015-0330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Komon M., Kubicek C.P. Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia. 2005;97:1365–1378. doi: 10.3852/mycologia.97.6.1365. [DOI] [PubMed] [Google Scholar]
- Jaklitsch W.M., Olariaga I., Voglmayr H. Teichospora and the Teichosporaceae. Mycological Progress. 2016;15:31. doi: 10.1007/s11557-016-1171-2. (1–20). http://dx.doi.org/10.1007/s11557-016-1171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Stadler M., Voglmayr H. Blue pigment in Hypocrea caerulescens sp. nov. and two additional new species in sect. Trichoderma. Mycologia. 2012;104:925–941. doi: 10.3852/11-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Voglmayr H. Phylogenetic relationships of five genera of Xylariales and Rosasphaeria gen. nov. (Hypocreales) Fungal Diversity. 2012;52:75–98. [Google Scholar]
- Jaklitsch W.M., Voglmayr H. Persistent hamathecial threads in the Nectriaceae, Hypocreales: Thyronectria revisited and re-instated. Persoonia. 2014;33:182–211. doi: 10.3767/003158514X685211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y.-M., Rogers J.D., Huhndorf S.M. Valsaria and notes on Endoxylina, Pseudothyridaria, Pseudovalsaria, and Roussoella. Mycotaxon. 1996;58:419–481. [Google Scholar]
- Karasch P., Dämon W., Jaklitsch W. Beiträge zur Pilzflora der Kanaren-Insel La Palma 2. Weitere bemerkenswerte Pilzfunde auf Chamaecytisus proliferus. Österreichische Zeitschrift für Pilzkunde. 2005;14:275–289. [Google Scholar]
- Kauff F., Lutzoni F. Phylogeny of Gyalectales and Ostropales (Ascomycota, Fungi): among and within order relationships based on nuclear ribosomal RNA small and large subunits. Molecular Phylogenetics and Evolution. 2002;25:138–156. doi: 10.1016/s1055-7903(02)00214-2. [DOI] [PubMed] [Google Scholar]
- Landvik S., Egger K., Schumacher T. Towards a subordinal classification of the Pezizales (Ascomycota): phylogenetic analyses of SSU rDNA sequences. Nordic Journal of Botany. 1997;17:403–418. [Google Scholar]
- Liew E.C.Y., Aptroot A., Hyde K.D. Phylogenetic Significance of the Pseudoparaphyses in Loculoascomycete Taxonomy. Molecular Phylogenetics and Evolution. 2000;16:392–402. doi: 10.1006/mpev.2000.0801. [DOI] [PubMed] [Google Scholar]
- Liu J.K., Phookamsak R., Dai D.-Q. Roussoellaceae, a new pleosporalean family to accommodate the genera Neoroussoella gen. nov., Roussoella and Roussoellopsis. Phytotaxa. 2014;181:1–33. [Google Scholar]
- Liu J.K., Hyde K.D., Jones E.B.G. Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Diversity. 2015;72:1–197. [Google Scholar]
- Liu Y.L., Whelen S., Hall B.D. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- Luck-Allen E.R., Cain R.F. Additions to the genus Delitschia. Canadian Journal of Botany. 1975;53:1827–1887. [Google Scholar]
- Petrak F. Mykologische Beiträge I. Hedwigia. 1921;62:282–319. [Google Scholar]
- Petrak F. Mykologische Notizen. Annales Mycologici. 1923;21:1–69. [Google Scholar]
- Petrak F., Sydow H. Die Gattungen der Pyrenomyzeten, Sphaeropsideen und Melanconieen. 1. Die phaeosporen Sphaeropsideen und die Gattung Macrophoma. Feddes Repertorium Specierum Novarum Regni Vegetabilis, Beiheft. 1927;42:1–551. [Google Scholar]
- Saccardo P.A. Mycologiae Venetae Specimen. Atti della Società Veneto-Trentina di Scienze Naturali. 1873;2:53–264. 14 pl. [Google Scholar]
- Saccardo PA (1875a). 170. Thyridaria incrustans Sacc. (nov. gen.). Mycotheca Veneta, sistens fungos venetos exsiccatos. 1–1600. CenturiaII: 101–200. Patavii.
- Saccardo P.A. Nova ascomycetum genera. Grevillea. 1875;4:21–22. [Google Scholar]
- Saccardo P.A. Fungi Veneti novi vel critici. Series IV. Atti della Società Veneto-Trentina di Scienze Naturali. 1875;4:101–141. [Google Scholar]
- Saccardo P.A. Sphaeriaceae, Phaeophragmiae. Sylloge Fungorum omnium hucusque cognitorum. 1883;2:1–815. Italy, Padua. [Google Scholar]
- Samuels G.J. Ascomycetes of New Zealand 1. Ohleria brasiliensis and its Monodictys anamorph, with notes on taxonomy and systematics of Ohleria and Monodictys. New Zealand Journal of Botany. 1980;18:515–523. [Google Scholar]
- Silvestro D., Michalak I. raxmlGUI: a graphical front-end for RAxML. Organisms Diversity & Evolution. 2012;12:335–337. [Google Scholar]
- Stamatakis E. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, Massachusetts: 2002. PAUP* 4.0b10: phylogenetic analysis using parsimony (*and other methods) [Google Scholar]
- Tanaka K., Hirayama K., Yonezawa H. Revision of the Massarineae (Pleosporales, Dothideomycetes) Studies in Mycology. 2015;82:75–136. doi: 10.1016/j.simyco.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiers B. New York Botanical Garden's Virtual Herbarium; 2016. Index Herbariorum: A global directory of public herbaria and associated staff.http://sweetgum.nybg.org/ih/ [Google Scholar]
- Traverso G.B. Flora Italica Cryptogama. Pars 1: Fungi. Pyrenomycetae. Xylariaceae, Valsaceae, Ceratostomataceae. 1906;2:1–352. Rocca S. Casciano, Italy; Società Botanica Italiana. [Google Scholar]
- Tulasne LR, Tulasne C (1863). Selecta Fungorum Carpologia2. i–xix, 319 pp., 34 tabs. Paris.
- Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H., Jaklitsch W.M. Prosthecium species with Stegonsporium anamorphs on Acer. Mycological Research. 2008;112:885–905. doi: 10.1016/j.mycres.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Voglmayr H., Jaklitsch W.M. Molecular data reveal high host specificity in the phylogenetically isolated genus Massaria (Ascomycota, Massariaceae) Fungal Diversity. 2011;46:133–170. [Google Scholar]
- Von Höhnel F. Fungi imperfecti. Beiträge zur Kenntnis derselben. Hedwigia. 1917;59:236–284. [Google Scholar]
- Wehmeyer L.E. The genus Thyridaria (Pyrenomycetes) Lloydia. 1941;4:241–261. [Google Scholar]
- Werle E., Schneider C., Renner M. Convenient single-step one tube purification of PCR products for direct sequencing. Nucleic Acids Research. 1994;22:4354–4355. doi: 10.1093/nar/22.20.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T.J., Bruns T., Lee S. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenies. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR protocols: a guide to methods and applications. Academic Press; San Diego: 1990. pp. 315–322. [Google Scholar]
- Zhang Y., Crous P.W., Schoch C.L., Hyde K.D. Pleosporales. Fungal Diversity. 2012;53:1–221. doi: 10.1007/s13225-011-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang J., Wang Z. Neotypification and phylogeny of Kalmusia. Phytotaxa. 2014;176:164–173. [Google Scholar]