Abstract
Over half of the nearly 2 million healthcare-associated infections can be attributed to indwelling medical devices. In this review we highlight the difficulty in diagnosing implantable device related infection and how this leads to a likely underestimate of the prevalence. We then provide a length-scale conceptualization of device related infection pathogenesis. Within this conceptualization we focus specifically on biofilm formation and the role of host immune and coagulation systems. Using this framework, we describe how current and developing preventative strategies target specific processes along the entire length-scale. In light of the significant time horizon for the development and translation of new preventative technologies, we also emphasize the need for parallel development of in situ treatment strategies. Specific examples of both preventative and treatment strategies and how they align with the length-scale conceptualization are described.
Keywords: Biofilm, CLABSI, CAUTI, nosocomial infection
Over the past half-century, rapid improvements in electronics and materials have produced a breathtaking array of implantable devices for the treatment of many illnesses. Diseases of every organ system have benefitted from these technologies, and it is unlikely that anyone reading this report does not personally know someone whose life has been improved by devices such as heart valves, endovascular stents, joint prostheses, implantable meshes, artificial lenses or cochlear implants. Ventricular assist devices, artificial hearts, and deep brain stimulators are becoming an increasingly common aspect of daily life. While these innovations have prolonged and improved the quality of life for countless patients, introducing foreign material into a patient unavoidably sets the stage for potential microbial colonization and infection. Of the nearly 2 million healthcare-associated infections reported by the Centers for Disease Control, 50–70% can be attributed to indwelling medical devices(1, 2). Attributable mortality is highly device dependent but can range from <5% for devices such as dental implants and foley catheters to >25% for mechanical heart valves(3). Barring revolutionary advances in material sciences and despite process improvements at the time of implantation, this number is likely to increase over time. Reasons include increasing rates and types of device utilization, the aging of the population, and the increasing frequency of comorbidities leading to immunocompromised states(4, 5).
Despite the detailed understanding of the epidemiology of the problem, and a growing appreciation for the underlying mechanisms of device related infections, relatively little is known of the inflammatory and immunological aspects of this common clinical challenge. In this review we address several relevant aspects of device infection. After briefly highlighting clinical features that inform the pathophysiology of the process, we will discuss the current understanding of two central pathophysiological events – bacterial adhesion to artificial surfaces and the development of complex bacterial communities known as biofilms. Lastly, to the extent possible given current understanding, we will outline what is known about the host response to infected devices and propose areas where more intense research focus may improve patient care.
THE CLINICAL PROBLEM
A major obstacle in diagnosing and treating device related infections is the lack of clear diagnostic definitions. A potential definition of implanted device related sepsis is: a patient that meets criteria for systemic inflammatory response syndrome (SIRS) where the only attributable source (i.e., no evidence for infection in the skin, urine, lung, spinal fluid, abdomen, etc.) is the implanted device. However, with this definition there will be a high rate of false positives leading to unnecessary and potential harmful interventions. Also, many presentations of device related infection are subtle or associated with immunocompromised patients that do not mount a full SIRS response. Medical device complications not previously thought to be infection are secondarily related to bacterial contamination. For instance, a devastating complication of breast reconstruction surgery is capsular calcification of the implant. Classically thought to be related to a noninfectious inflammatory response, it may actually be triggered by microbial contamination(6, 7). Likewise, cases of prosthetic joint failure may be related to undiagnosed infection or contamination(8). This population of subtle cases represents a “hidden” prevalence of implantable device related infection. A more specific definition might include clinical signs and symptoms with intraoperative evidence for infection and culture positive explanted specimens. However, if a goal of future treatment strategies is to preserve devices with limited or no surgical intervention, this definition would not be useful. Clearly novel algorithms/technologies for early diagnosis are needed.
At this time, there are no biomarkers that specifically indicate the presence of biofilm or device related infection. Nonspecific biomarkers such as erythrocyte sedimentation rate and C-reactive protein are often considered but are too nonspecific to be of significant diagnostic utility. Bacteremia is the hallmark of intravascular device infection(1), although many components of intravascular devices such as pacemakers and ventricular assist devices (VADs) have large surface areas that are not in direct contact with the blood. Furthermore, there is a significant time lag in getting blood culture results and biofilms can be present without detectable bacteremia. Likewise, culture of aspirated synovial fluid from around a prosthetic joint is only 50% sensitive(1). In addition, microbes isolated from surrounding tissue/fluid may only represent a subset of the larger diverse population of bacteria in a biofilm. On the other hand, the presence of bacteria on a device does not equate to infection and can merely indicate a benign colonization. Furthermore, there are complex interactions between different bacterial species such that benign colonization of a device surface by one species confers protection against a potential pathogen(9).
Novel diagnostics are currently being developed and evaluated to increase the sensitivity of detecting microorganisms in the blood, other body fluids, and wounds(10, 11). More specifically, detection of microbial virulence genes are being considered to diagnose medical device related biofilms(12–14). For instance, detection of the ica gene, which codes for a primary extracellular component of biofilms, by polymerase chain reaction (PCR) has been used to quickly diagnose staphylococcal device related infections(12). However, this approach is flawed by the fact that there are ica-negative biofilm producing strains(15). The fundamental problem with the current use of high-tech molecular diagnostics (e.g., PCR, and mass spectroscopy) of microorganisms is in trading specificity for sensitivity. By increasing our capacity to detect bacteria we are likely increasing the number of false-positive events, particularly in the setting of benign colonization. Since the treatment for these infections can involve high-risk surgery and broad spectrum antibiotics with significant potential for toxicity, differentiating between colonization and infection is a true diagnostic dilemma with profound consequences.
The lack of a clear definition for diagnosis has major implications for the practice modern medicine. In the current reimbursement environment, infection of specific implantable devices (e.g., central venous catheters, foley catheters) and surgical site infections are considered medical errors. As reimbursement rates become more closely tied with outcomes, the rates of device related infection, repeat surgery, and readmission will be more closely monitored. Indeed, device manufacturers are targeting infection prevention strategies to a 30-day window in response to potential lost reimbursement by the Center for Medicare and Medicaid Services.
Device infection rates
Keeping in mind the uncertainty in diagnosis and potential underestimate of prevalence, we have tried to summarize the rates of infection for some of the most common implantable medical devices (Table 1). There are many ways to classify implanted medical devices. For instance, devices can be divided by those that are fully implanted versus those that cross the skin or mucosal barriers. They can be classified by their materials (i.e., metal, polymers, ceramics, and devitalized human/animal tissue). They can also be classified by the implantation site and intended lifespan. The large difference in infection rates across the different devices is likely a function of these classifications which provides clues on the pathogenesis of device infection as well as application specific strategies to mitigate infection.
Table 1.
Infection rates for implanted devices with associated references
| Vascular access devices | *BSI per 1000 †IVD days | References |
|---|---|---|
| Peripheral intravenous catheter | <1 | (140) |
| Arterial catheter | 1–3.4 | (141, 142) |
| Central venous catheter (non-dialysis) | 2.5–4 | (19, 20) |
| Peripherally inserted central catheter | 2–3 | (143, 144) |
| Dialysis catheters | 4.2–5.3 | (20, 21) |
| Venous access ports | 0.11–0.76 | (145, 146) |
| Cardiac devices | Incidence | References |
| Intra-aortic balloon pump | 0.08–0.13% | (147, 148) |
| Left ventricular assist device | 16–36% | (28, 29, 149) |
| Heart valve | 7–15% | (24, 25) |
| Cardiac implantable electronic devices | 5–20% | (150) |
| Prosthetic joints | Incidence | References |
| Shoulder | 1.2% | (31) |
| Hip | 1–2% | (31, 32, 35) |
| Knee | 1–4% | (31, 32, 35) |
| Elbow | 5% | (34) |
| Urinary devices | ‡UTI per 1000 catheter days | References |
| Foley catheter | 3.1–7.5 | (37) |
| Other devices | Incidence | References |
| Mesh for ventral hernia repair | 1–10% | (38, 39) |
| Ventriculoperitoneal shunt | 0.25–1% | (43) |
| Peritoneal dialysis catheter | 20% | (41) |
BSI – Bloodstream infection
IVD – Intravascular device
UTI – Urinary tract infection
Intravascular devices are those that are in contact with the blood in the intravascular space. Examples include: peripheral IVs, central lines, dialysis catheters, heart valves, vascular grafts, stents, and ventricular assist devices. A comparison of the infection rates for various types of intravascular devices is summarized in detail by Maki et al(16). Although peripheral IV infection is rare and typically localized, those infections can lead to infection of other devices such as pacemaker/defibrillators(17). In the United States there are over 15 million days of exposure to central venous catheters annually for intensive care units (ICU) alone(18). This does not include chronic dialysis catheters and outpatient peripherally inserted central lines. The incidence of non-dialysis catheter related bloodstream infection ranges from 2.5 to 4 per 1000 catheter days(19, 20). Dialysis catheters have higher infection rates (3.8–5.5 per 1000 catheter days)(20, 21). In the ICU these infections can equate to financial costs as high as $30,000 per infection with the potential for increased duration of mechanical ventilation, 1 week increased ICU stay, and 2–3 weeks of additional hospital stay(22, 23).
Prosthetic heart valves and VADs are unique intravascular devices due to their prolonged periods of implantation and the associated high risk surgery required for removal and replacement. Over 20 years there is a 7–15% risk of endocarditis with either mechanical or bioprosthetic heart valves(24, 25). First generation VADs had infection rates as high as 80%(26, 27) which has improved to less than 40%(28, 29) for the second generation continuous flow pumps. Of note, VAD infections can be localized to the device (i.e., drive line or pocket infection) or related to the device (i.e., endocarditis or bloodstream infection). Infection of arterial stents (e.g., coronary stents) is rare(30).
Orthopedic devices include primarily prosthetic joints but also fixation hardware. Prosthetic joint infections range from 0.5–5% depending on the location(31–35). Hip and shoulder prostheses have relatively low incidence of infection (~1%)(31, 35) while elbows have significantly higher (5%)(34). The reason for this difference remains unclear. Although less likely to be life-threatening, these infections are associated with significant morbidity and disability. The cost for a surgical site infection of a total knee replacement exceeds $150,000(36).
Urologic devices include the ubiquitous foley catheter as well as ureteral stents, bladder slings/meshes, and urostomy/nephrostomy tubes. Catheter associated urinary infections (CAUTI) are the most common nosocomial infection. Data from National Health Safety Network in 2006 showed a range of pooled mean CAUTI rates of 3.1–7.5 infections per 1000 catheter-days(37). While improved selection of patients requiring urinary catheters can improve this infection rate, there will always be patients that require indwelling foley catheters and therefore new technologies to prevent, appropriately diagnose, and treat these infections is needed.
Other common devices include ventral hernia repair mesh, peritoneal dialysis catheters and ventriculoperitoneal shunts. Ventral hernia repair mesh infection rates range from 1–10% depending on the surgical procedure and type of mesh used(38, 39) but as high as 60% in contaminated cases with bioprosthetic mesh(40). Infections associated with peritoneal dialysis catheters can manifest in exit site infections, tunnel infections, or peritonitis. Annual rates of peritoneal dialysis associated infection range from 0.24–1.66 episodes per patient per year(41). Specifically peritoneal dialysis associated peritonitis, which carries nearly a 4% mortality(41), occurs once every 9 to 53 catheter months(42). Ventriculoperitoneal shunt infection rates are typically less than 1%(43).
Temporal trends in infection rate
From the late 1970’s through 2007 nosocomial bloodstream infections have been increasing(44–48). This increase is multifactorial, arising from increased device utilization, increasing prevalence of antibiotic resistance, increasing patient medical co-morbidities, and increased reporting. However, starting around 2006 this trend leveled off and we are now beginning to see decreases in these device related infections(49). Although potentially informative, it is difficult to determine what factors have led to this change. Increasing awareness is likely a key component. Hospital initiatives to improve protocols and compliance with sterile technique, perioperative antibiotics, and patient selection for implanted devices have also been implicated(50, 51). However, these interventions are not always sustained and their affect can wane. A Cochrane review was also not able to demonstrate consistent benefit of these types of interventions to reduce infection rates(52). Although, this is likely a result of the current quality of studies as much as a true lack of efficacy. In 2008, the Centers for Medicare and Medicaid Services implemented a policy to reduce payment for perceived preventable infections related to central venous catheters and urinary tract infections. However, a systemic evaluation of the trends in these infections was not able to attribute the decreasing incidence to the nonpayment policy(49). Regardless, despite current trends toward decreasing incidence, there continues to be large numbers of patients that suffer from significant medical morbidity and mortality and the associated economic burdens of device related infection. This prompts the need to better understand how implanted devices interact with host and pathogen to develop more infection resistant materials, improved diagnostic protocols, and devise noninvasive or device preserving treatment strategies.
PATHOGENESIS OF DEVICE INFECTION
Every infection at its heart is a spatial phenomenon; pathogens and host immune cells alike are in motion, creating and responding to chemical gradients that may constitute an attack or a signal. The sense of ‘locality’ is particularly acute in device infection, and we have found it informative to use a length-scale framework for understanding the pathogenesis of device infection. Host-pathogen interactions as well as current and future interventions to treat and prevent implanted device related infection all can be described with this approach. A pictorial representation of this length-scale conceptualization is shown in Figure 1.
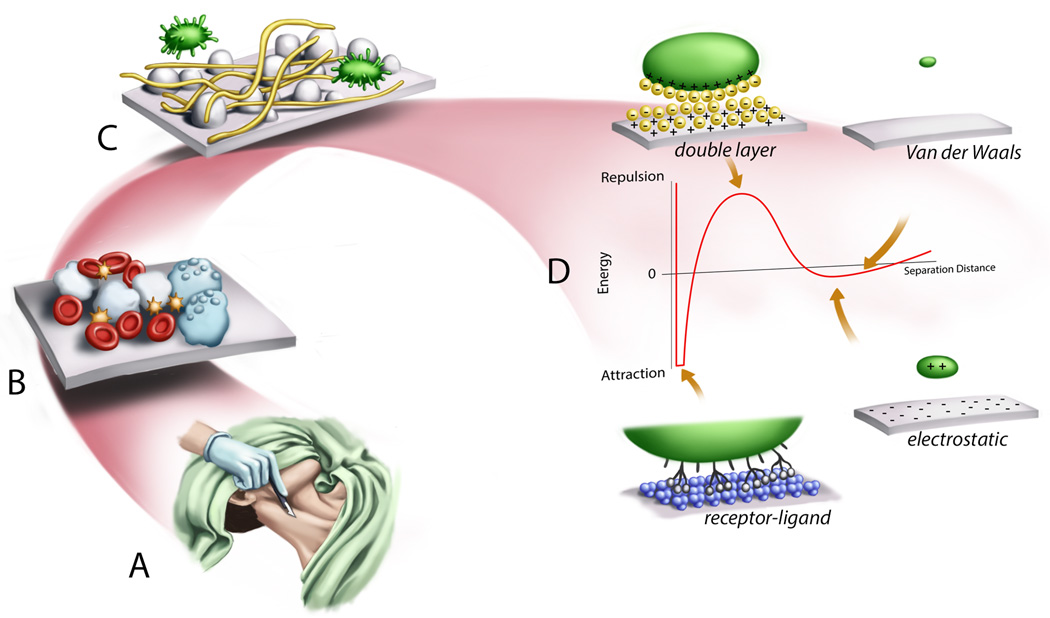
Figure 1.
Illustration of the length-scale conceptualization of the pathogenesis of implantable device related infection. (A) Surgical incision and wound creation leads to continuity between sterile and non-sterile compartments of the body as well as inflammatory reaction. (B) Extracellular fluids and blood cells in the wound come into contact with the abiotic implanted material initiating both inflammatory and coagulation cascades. (C) Host proteins (e.g., fibrin, thrombin, albumin) also interact with the surface as well as bacteria leading to the development of a biofilm. (D) At the nano and sub-nanometer scale the interaction of a cell or molecule with a surface is governed by basic physiochemical properties. A particle approaching the surface by bulk transport may have an opposite or similar charge as the surface leading to electrostatic attraction or repulsion. In an aqueous environment a double layer of water molecules will develop on the charge surface, further contributing to the energy of interaction. Van der Waals forces will provide additional attractive forces. Finally, biological particles will have receptor-ligand interactions which provide strong and sometimes irreversible attractive interaction.
On the scale of several centimeters, surgical insertion of a device violates an anatomic barrier to infection, such as the skin or a mucous membrane. This brings an otherwise sterile compartment in continuity with one that is unavoidably colonized with bacteria, such as the skin, upper airway, or urinary bladder. In many instances (e.g., central venous catheters, peritoneal dialysis catheters, VAD drive lines) the disruption in the barrier persists and is an ongoing opportunity for colonizing bacteria to become pathogenic. Of note, most clinical practice guideline-based infection control methods are employed on this gross scale (Figure 1A).
At millimeter distances, the surgical wound itself permits contact between the abiotic material, extracellular fluids and to a greater or lesser extent, blood. At the micron scale, many well-recognized features of host immunity and bacterial growth are active (Figure 1B). Bacteria have specific surface receptors for various host proteins (e.g., fibringogen) or abiotic surfaces(53, 54) (Figure 1C). Contact between plasma proteins, peripheral blood cells and the device surface leads to activation of inflammatory and coagulation cascade mediators. While immune cell activation in a wound can be protective against infection(55), imbalanced or persistent inflammation can lead to poor wound healing and local tissue damage(56, 57). This perpetuates epithelial barrier dysfunction and increases the potential for bacterial contamination.
At nanometer distances physiochemical forces govern how a particle (i.e., cell, protein, molecule) will interact with other particles or surfaces (Figure 1D). Small fluctuations in the electron clouds of these particles or molecules lead to Van der Waals forces that provide a weak attraction. Electrostatic interactions can be attractive or repulsive depending on the respective charges of the interacting substances. In most biological cases, a double layer of water molecules sets up on charged surfaces and provide a repulsive barrier to binding. This energy barrier however is traversed or lowered by the presence of certain specific receptor-ligand binding (e.g., fibrinogen receptor on bacterial cell wall) that provide more permanent attachment. The exclusion of water from between two hydrophobic materials (known as the hydrophobic effect) is a driving force for protein folding and binding of two hydrophobic substances. A complete description of the intermolecular interactions governing the adsorption of biological materials to abiotic surfaces is beyond the scope of this paper and described in detail in multiple texts(58). However, it is important to understand that these basic physiochemical forces are the underpinnings for the design of all biomaterials. Going forward we will focus on two critical aspects along this length-scale, namely the adhesion of bacteria to a surface leading to biofilm formation, and the interaction with the coagulation and immune systems. These processes provide numerous targets for potential clinical intervention.
BIOFILMS
The specific biofilm-forming organisms that cause device related infection depend on the location of the device. In general, the most common organism isolated from infected medical devices is coagulase negative staphylococci, typically Staphylococcus epidermidis (1, 48). Staphylococcus aureus is slightly less common but certainly more virulent. Other important but less common pathogens include Enterococcus species, Candida, Escherichia coli, and Klebsiella species. This trend is true for intravascular devices and prosthetic joints. However in the urinary tract, E. coli, Candida, and Enterococcus are the most common and Gram-positive organisms are less common(59).
Bacteria are found in one of two different “lifestyles”. They can be found free floating in solution (i.e., planktonic) or associated into sessile communities encapsulated in an extracellular matrix (i.e., biofilm). Table 2 highlights some of the key features distinguishing bacteria in planktonic growth from biofilms. It is important to note that as part of a biofilm, bacteria have slower metabolism, are resistant to host immune response, and are tolerant to high doses of antibiotics (which should be distinguished from true resistance as the same bacteria will become susceptible once dispersed from the biofilm)(60, 61). Specifically, both E. coli and S. epidermidis in biofilms have decreased growth rate and a resultant decreased antibiotic susceptibility when compared to the same strains in planktonic solution (62, 63). This presents the central challenge with implanted device related infections which are cause by biofilms rather than planktonic cells.
Table 2.
Comparison of planktonic and biofilm lifestyles
| Planktonic | Biofilm |
|---|---|
| Single Cells | Cell aggregates |
| Free-floating in solution | Usually associated with a surface |
| Antibiotic responsive | Antibiotic tolerant (not true resistance) |
| Susceptible to host immune response | Resistant to host immune response |
| Rapid metabolism and cell growth | Slow metabolism and cell growth |
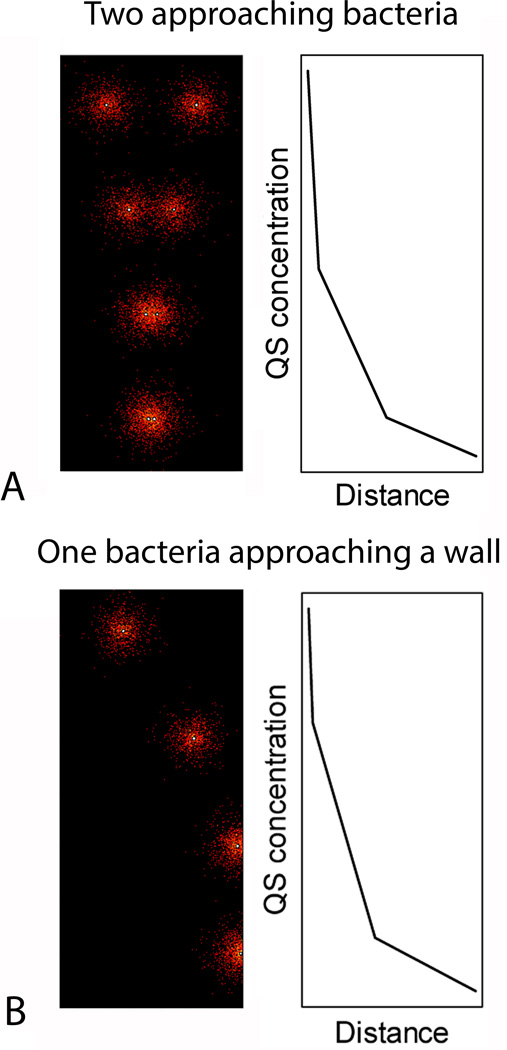
Biofilm producing organisms readily transition between planktonic and biofilm “lifestyles”. The transition represents a phenotypic switch that involves significant changes in both gene and protein expression(64, 65). For instance, in pseudomonas over 50% of the proteome has a six fold or greater change in expression level when comparing planktonic cells with mature biofilm cells(64). The method by which cells probe their environment and respond with the appropriate gene and protein expression changes is known as quorum sensing (QS). Although each microbial species has its own set of QS systems(66), they all share specific characteristics. In all cases, bacteria synthesize and release small probe molecules. The bacterium also has a receptor that detects the presence of that probe molecule. The surrounding concentration of the probe molecule will be dependent on the environment. For example, the probe molecule will quickly diffuse away and have a low concentration in a planktonic environment but will increase in concentration as the cell approaches a surface (Figure 2B). Also, a cell can sense the presence of other cells by the resultant increasing probe concentration from the neighboring cell (Figure 2A). QS systems have been identified and characterized in detail for many of the most common pathogens causing device related and infection(66). These pathways are a target-rich environment for the prevention and treatment of infections involving biofilms.
Figure 2.
Computational simulation of quorum sensing (QS). Bacteria were modeled as point sources of QS molecules. Those molecules were free to diffuse by simple Brownian motion. In the top we demonstrate the concentration of QS molecules surround two bacteria approaching each other. The plot shows the concentration of the QS molecules on the bacterial surface as a function of the separation distance. Note, that this is a computation simulation using dimensionless parameters, therefore there are no units. This demonstrates how a bacterium can sense the presence of a neighboring bacterium in close proximity. In the bottom a similar simulation demonstrates the concentration and distribution QS molecules as a bacterium approaches a wall. Again the QS concentration rapidly increases providing a signal to the bacterium.
It is generally accepted that the lifecycle of a biofilm is a four step process: (1) Adhesion, (2) Accumulation, (3) Maturation, and (4) Detachment (67–69). In this section, we will review these processes and how they contribute to treatment resistance. Our goal is to highlight potential therapeutic targets.
Adhesion
Microbial adhesion to a medical device surface occurs in two phases(68, 69). The first phase involves transport of the bacterial particle to the surface and reversible binding via physicochemical interactions (recall Figure 1D). The second phase is the formation of an irreversible bond through specific cellular/molecular interactions. For the reversible phase, bacteria are transported to the surface of interest via bulk flow of the surrounding fluid (i.e., convection) as well as diffusion through the fluid via Brownian motion(70). As it approaches the surface, the bacterium is then acted upon by physicochemical forces (described above in Figure 1D) to form a reversible bond(70). At this point, depending on the strength of those interactions, the bacterium can be carried away by the bulk flow or the bond can “mature” into an irreversible adhesion. This is accomplished by cell surface proteins that can bind abiotic surfaces directly or bind to host proteins (e.g., serum proteins) that have already conditioned the surface(69). In S. epidermidis the autolysin surface protein AtlE has been shown to bind directly to abiotic surfaces(71). S. aureus has a homologue with similar function(72). AtlE also has the role of causing host cell lysis and the release of extracellular DNA which has been shown to be a major component of the extracellular matrix of biofilms(73). Staphylococcal species also produce a family of surface proteins known as microbial surface component recognizing adhesive matrix molecules, known as MSCRAMMS(74). These molecules bind to host serum proteins such as fibrinogen, collagen, and fibronectin.
Accumulation
The next step in biofilm formation is the accumulation of cells. This occurs either by proliferation of already adhered cells with the progeny remaining adhered to the surface or by recruitment of additional cells from the surrounding environment(70, 75). Although cellular metabolism and growth is slowed in biofilms, cells will continue to divide, particularly early in the formation of the biofilm as evidenced by the development of Streptococcus pneumoniae diplococci and small clusters in the few hours after initial adhesion to a surface(76). In addition, adhered bacteria begin to change their phenotype from planktonic to sessile. As part of that change they begin to produce extracellular matrix (ECM). The prototypical ECM substance known as polysaccharide intercellular adhesion is produced by staphylococcal species(77). Other ECM substances include proteinaceous factors, teichoic acids, and extracellular DNA(73, 78). This ECM provides targets for receptor specific recruitment and adhesion of bacteria from the surrounding environment(79, 80).
Maturation
As the cells begin to accumulate and generate the gelatinous ECM they become encased in this glycocalyx(81). This results in a protective, physical barrier for restricted diffusion which conveys significant antimicrobial tolerance without true resistance(75, 82, 83). The restricted diffusion reduces the local concentration of antibiotic as well as altering the pH, oxygen tension, and concentration of certain ions which further compromises the activity of the antimicrobials(67, 82, 83). As the oxygen tension decreases, the cells slow their metabolism and growth rate which reduces uptake of and susceptibility to antimicrobials that target the cell cycle and protein translation(67, 83). The biofilm will now have clusters of cells connected by fluid filled channels that deliver oxygen/nutrients and remove waste(70, 84, 85). They also provide an efficient medium for lateral gene transfer between cells which can facilitate the development of true antibiotic resistance(86, 87). At this point there has been a dramatic change in the phenotype of most of the cells as described above. They have switched their priority from cell division to ECM production. They have started to express genes related to antimicrobial resistance(88). Furthermore, there are significant local variations in the expression patterns of cells related to the local nutrient microenvironment within the biofilm(89). Specifically, a small population of cells within the biofilm known as persister cells will develop(90, 91). These cells are inactive and highly protected which allows them to repopulate the biofilm after antibiotic treatment. Due to their persistence they acquire antibiotic resistance genes making the repopulated biofilm even more resistant to repeat treatment.
Detachment
The final stage in biofilm formation is detachment. Eventually individual cells or small parts of the biofilm will be released where they can transition back to a planktonic phenotype which can then disseminate into systemic infection(67–69). This is not a passive release of loosely bound bacteria but rather a distinct, active process in the maturation of the biofilm that is control by QS systems. In staphylococcal bacteria the agr QS system controls the release of phenol soluble modulins (e.g., delta toxin) that inhibit the hydrophobic interaction between bacterial cell surfaces thereby reducing their surface tension causing them to detach(92, 93).
The process of biofilm development is an excellent demonstration of the length-scale conceptualization. It provides a mechanism by which a bulk inoculum of bacteria is transported from their commensal environment (e.g., skin) to a device surface, adhere, colonize and become a pathogenic biofilm. Treatment and prevention strategies at all points along that length-scale include improvements to surgical/sterile technique that reduce the initial inoculum; materials with specific physiochemical properties that resist initial adhesion of bacteria or host proteins; drugs to target induction of sessile phenotype; activation and antibiotic targeting of persister cells; and biofilm stabilization to prevent dissemination. We will describe examples of specific treatment and prevention strategies and how they related to this length-scale framework later in the article.
LINK TO HOST IMMUNITY AND THOMBOSIS
As mentioned previously, surgical implantation of a device can lead to contact between the device’s surface and blood. This contact leads to activation of host immune and coagulation systems. In this section we will describe how thrombus formation can contribute to device infection. Likewise we will describe how host immune cell activation and/or dysfunction contribute to the pathogenesis of device infection.
Thrombosis
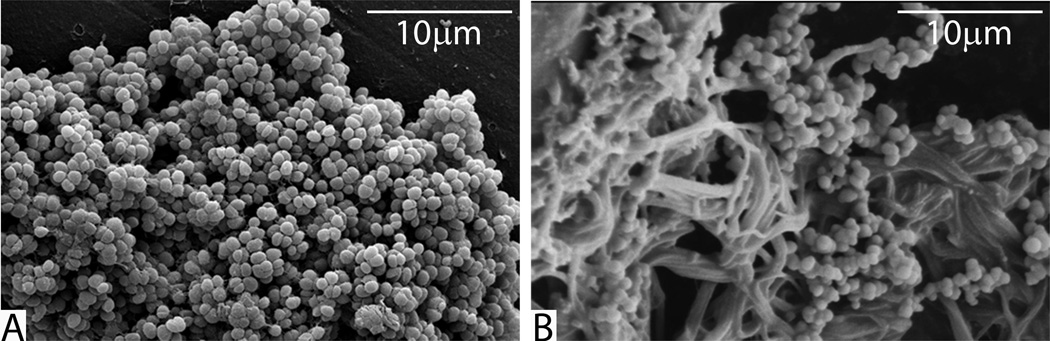
There is an intimate bi-directional relationship between biofilm infection and thrombosis(94–97). Lordick et al found that 12 of 14 patients with central venous catheter (CVC) related infection had a preceding CVC related thombus(98). In patients with CVC related infection, 44% had a clinically manifest thrombosis whereas only 3% without an infection had a thrombosis(99). This is equivalent to a 17.6 relative risk of thrombosis in the presence of infection. In addition, ensuring excellent hemostasis in the subcutaneous pocket of pacemaker/defibrillators has been shown to reduce post-operative generator infections(100). The mechanism of this phenomenon is based on the idea that the presence of a clot increases the likelihood of bacterial adherence and conversely biofilms are able to activate the coagulation cascade to increase thrombus formation. Specifically, microbial receptor-specific binding to fibrin and fibronectin within the fibrin sheath coating central venous catheters has been demonstrated(101, 102). Many of these organisms also produce a coagulase enzyme which enhances the development of a thrombus(96). The presence of infection leads to inflammatory activation with subsequent activation of the coagulation cascade via tissue factor and concomitant inhibition of anticoagulation factors and thrombolysis(97, 103). Recently, Kwiecinski et al. demonstrated that host derived fibrin was a major component of S. aureus biofilm matrix(104). This intimate relationship between host proteins and microbes is visually evident in explanted infected implants (Figure 3). Kwiecinski et al. also demonstrated that dissolution of the matrix by host or bacterial activation of plasminogen increased antibiotic susceptibility and neutrophil phagocytosis(104). This is an excellent example of a potential therapy derived from understanding the interactions between host coagulation and microbiology.
Figure 3.
Scanning electron micrographs of staphylococcal biofilms (A) grown in vitro on polystyrene culture pegs and (B) harvested from an infected ventriculoperitoneal shunt. Note the scant ECM on the in vitro biofilm in (A) and the bacteria adhered to dense fibrin clot matrix in (B).
Local Infection
The presence of a foreign material can have profound effects on the surrounding tissue via host inflammatory response(105, 106). This response can then modulate the degree of bacterial colonization and potential for local infection as well as the development of a systemic inflammatory response and disseminated infection. With respect to the local reaction to a foreign material, the activation of complement has been implicated as a key feature of implant associated infections. For instance, silicone has been shown to increase complement activation and has a higher infection rate when compared to polyurethane or polyvinylchloride(107, 108). More specifically, S. epidermidis biofilms induced more C3a than planktonic growth(109). However, phagocytic killing by polymorphonuclear neutrophils (PMNs) was reduced secondary to decreased deposition of IgG and C3b(109). This has also been demonstrated in vivo where PMNs harvested from peri-implant lavage in patients with post-traumatic osteomyelitis had upregulation of the high affinity receptor for antibodies but down regulation of the adherence protein CD62L necessary for migration from the vascular compartment(110). Furthermore, they demonstrated enhanced production of reactive oxygen species but diminished chemotaxis(111). However, Zimmerli et al. demonstrated that PMNs in contact with the non-phagocytosable materials had decreased respiratory burst(105). The end result is persistent PMN infiltration due to complement activation but decreased opsonization and phagocytic killing. This leads to spilling of the bactericidal and cytotoxic PMN contents resulting in persistent local tissue destruction without eradication of the infection(57).
Biomaterial associated neutrophil inhibition described above appears to be independent of the type of material(112). However, macrophage phagocytosis is modulated in a material specific manner. Specifically, bacterial clearance by macrophages was significant improved on cross-linked polyethylene glycol over fluorinated ethylene polypropylene, silicone, and glass(113). This affect is thought to be related to weak adhesion between the bacteria and the surface as well as weak adhesion of the macrophages allowing for increased mobility and chemotaxis(113). On the other hand, Brodeck et al. demonstrated that anionic and hydrophilic materials had decreased macrophage adherence, increased apoptosis, and decreased fusion/giant cell formation(114). Based on these findings, novel biomaterials will require carefully titrated surface properties that balance bacterial adhesion with sufficient macrophage adhesion without hindering chemotaxis. In general however, biofilms have a similar effect on macrophages as PMNs. That is the immune cells are able to migrate to the site of infection but are unable to clear the pathogen.
Disseminated Infection
Infections of devices implanted in the vascular space certainly have a high risk of dissemination. As described above, the natural life cycle of a biofilm involves dissemination, which in the bloodstream can rapidly lead to septic shock and death. Devices implanted into other anatomic compartments however have a slower progression. They will likely have significant local tissue destruction and inflammation like that seen in periprosthetic osteomyelitis(57). Disseminated infection is a very late manifestation for these types of devices. However, even local inflammatory activation described above can cause systemic inflammatory response and sepsis without dissemination of the infection. Indeed, in the case of implant associated osteomyelitis there was concomitant activation of immune cells in the peripheral blood, elevated C-reactive protein, fever, and evidence of SIRS in at least 30% of the patients(57).
TREATMENT AND PREVENTATIVE STRATEGIES
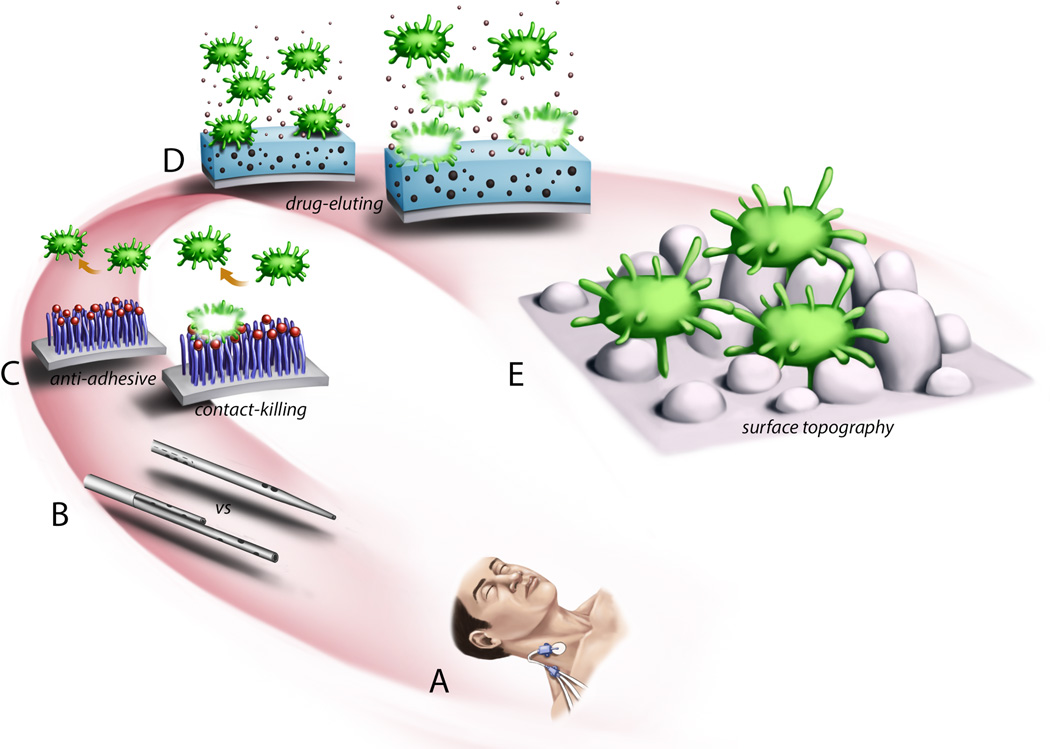
The length-scale description of device infection pathogenesis (described in Figure 1) provides a framework for understanding various potential treatment and prevention strategies. That is, specific interventions to control or eradicate infection can be “mapped” on to that conceptualization. Figure 4 provides an illustration of this. At the centimeter length-scale are procedural modifications to reduce the inoculate load when a sterile anatomic compartment is breached. These interventions include perioperative antibiotics, sterile techniques, procedure checklists, chlorhexedeine sponge dressings (e.g., biopatch shown in Figure 4A), and careful hemostasis. In general they are aimed at prevention and when implemented, have demonstrated reduced infection rates(100, 115, 116). However, they target one point in the pathogenesis of infection and therefore, despite widespread adoption, implanted devices continue to be the number one cause of nosocomial infection(1, 52). At the millimeter length-scale, specific device geometry can alter the probability of infection. Geometry at this length-scale is particularly important for intravascular devices (Figure 4B). As describe above, cells (host or bacterial) and proteins are transported to the device surface from the bloodstream via convection of the blood flow and Brownian diffusion. Particle transport and deposition is a function of the device geometry (117, 118). Device geometry is extensively studied and controlled for dialysis catheters, heart valves, ventricular assist devices and oxygenators to reduce platelet deposition and thrombosis (119–122). At micron length-scales there has been extensive research developing polymer coatings to prevent infection. As shown in Figure 4C they are designed for two purposes. First, they attempt to change the physiochemical properties of the surface to prevent bacteria adhesion. Second they can have bacteriocidal properties that can kill on contact. While these strategies show promising results in the laboratory, their efficacy can wane in vivo due to binding of host proteins which pacify the surface. At sub-micron distances, devices can be impregnated with small molecule antimicrobial agents such as rifampin, minocycline, silver, or chlorohexedeine. These substances are then eluted from the surface to kill bacteria that are in proximity or contact with the surface. Antibiotic impregnated catheters are now common in clinical practice, although a Cochrane review(123) could not conclusively recommend widespread use as the data seemed most useful in populations where the infection rate remains high despite the implementation of other less expensive interventions. In addition, these catheters did not seem to affect sepsis rates or mortality. Again these surfaces have a limited life span due to depletion of the molecule from continuous elution or pacification by host proteins. Finally, at the nanometer length-scale are fabrication techniques that alter the nano-scale topography of the surface (Figure 4E). It has been well demonstrated that cell adhesion and proliferation can be controlled by the surface topography(124–126). A more realistic example of bacteria interacting with a surface with specific nano-engineered topography is shown in Figure 5.
Figure 4.
Demonstration of specific preventative anti-infective strategies mapped onto the length-scale conceptualization of implantable device related infection. (A) Chlorhexidine sponge at the insertion site of a triple lumen central venous catheter to reduce transport of skin bacteria along the catheter to the bloodstream. (B) Dialysis catheters with different geometries can altered blood flow patterns which results in altered transport of cells or other materials to the catheter surface. (C) Polymer coatings with specific chemical end groups that prevent adhesion or have antibacterial properties upon contact. (D) Devices can be impregnated with antimicrobial substances that are eluted to kill bacteria in proximity to the device surface. (E) Nano-scale patterning of a surface can alter bacterial adhesion and proliferation.
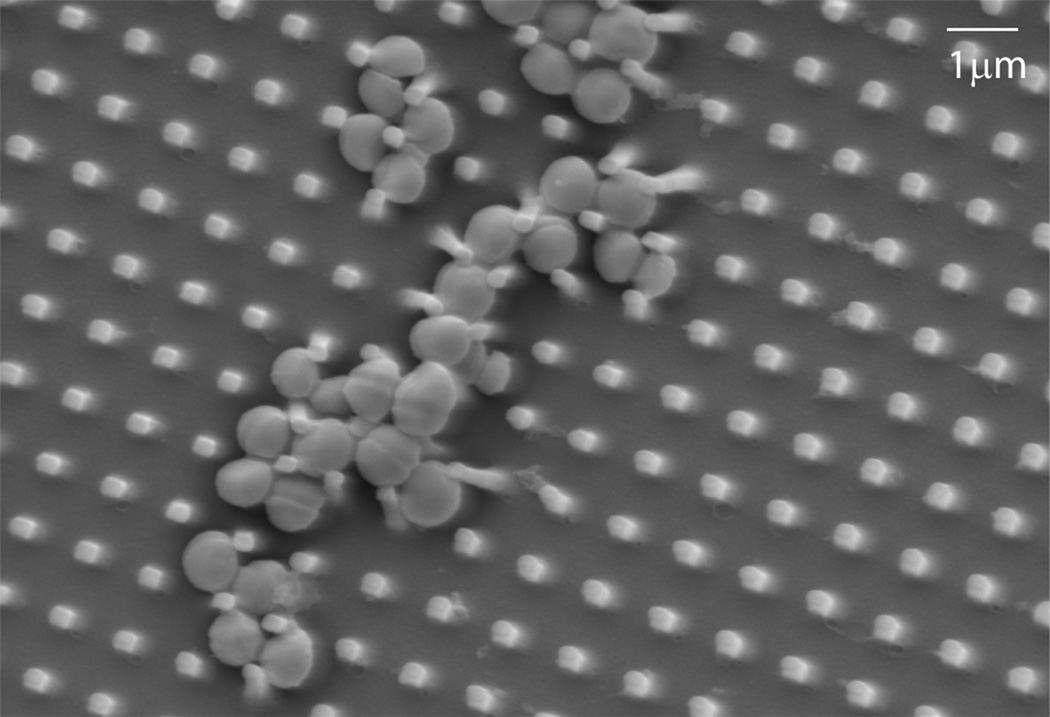
Figure 5.
Scanning electron micrograph of Staphylococcus aureus interacting with a nano-textured polyurethane surface.
Keeping the length-scale and specific pathologic features of infection in mind is critically important to developing new prevention strategies. Let us look at an example of a potential technology and how understanding the mechanism of action of that technology, and mapping it to the appropriate length-scale conceptualization will guide device design. Zinc oxide (ZnO) particularly in the form of nanoparticles (NP) has recently been studied as a novel antimicrobial(127, 128). However, the specific mechanism by which ZnO inhibits and/or kills bacteria remains controversial. There are two commonly postulated mechanisms. The first is the generation of reactive oxygen species (ROS). There are multiple groups who have shown ROS generation by ZnO-NP suspensions(129–131). However, this finding is not universal and may require the presence of light (not present in vivo) or be dependent on the NP synthesis technique. Regardless, this mechanism is similar to an antibiotic impregnated surface. That is, a ZnO coated surface provides a catalytic site for the generation of ROS that can diffuse away and work at a distance. It has the capacity to kill bacteria in proximity but not necessarily in contact. One specific benefit is that since the ZnO is not “consumed” but rather a catalyst; it may have a significantly longer lifespan than traditional antimicrobial eluting surfaces which are eventually exhausted. The second mechanism is that the ZnO-NPs make contact with the bacterial surface causing cell membrane disruption(132, 133). Assuming this mechanism to be true, the length-scale might be similar to a “contact killing” polymer surface. Or, the coating would have to be engineered to release the ZnO-NPs into the surrounding fluid. Clearly, as novel antibacterial materials (e.g., metal oxides, polymers, peptides, etc.) are being developed, it is crucial that we have a well-developed understanding of the mechanism. This provides critical information required to design the delivery system and application. Indeed, many silver coated devices have failed to live up to their promised efficacy due to a lack of understanding of the mechanism of action and appropriate translation to a device coating(134, 135).
It should be noted that the vast majority of strategies engineered to combat this problem are preventative. That is, they aim to reduce bacterial inoculum (e.g., antibiotics and sterile/surgical technique), prevent adhesion (e.g., repulsive polymer surfaces), kill / inhibit bacterial growth on contact (e.g., antimicrobial eluting or contact killing surfaces). While these technologies show promise and we advocate for continued pursuit in this field, we also acknowledge that clinical realization of these new technologies has a significant time horizon. Furthermore, even after their clinical adoption, there will continue to be a significant proportion of the population that have already received early generation devices that will continued to get infected for many years to come. As such, it is vitally important that parallel research be focused on strategies that treat device infection in situ. Examples of treatment strategies under development include persister cell targeting(136), quorum sensing inhibitors(137), and mechanical debridement of biofilms(138, 139). Detailed descriptions of these strategies are the subject another review article in and of itself. Regardless, the length-scale pathogenesis framework described here provides numerous potential targets for future research endeavors to treat medical device associated biofilms in situ in parallel with efforts to develop anti-infective materials. Both strategies will reduce the number of devices that have to be removed and replaced, thereby reducing the significant morbidity and mortality.
CONCLUSIONS
Implantable device related infections are the most common cause of healthcare associated infections. Given the uncertainty in diagnosis they may be responsible for more device related complications and morbidity than previous thought. The process by which bacteria adhere to, colonize, and eventually infect a device is a spatially oriented problem that spans distances from centimeters to nanometers. Understanding the processes at each of these length-scales provides targets for the development of new procedures, processes, and materials that resist contamination and infection. Understanding the mechanism of action of novel antimicrobial substances and how they are mapped on to the pathogenic process is crucial to the design and success of new technologies. At the same time, current technology devices (with significant risk for infection) will continue to be implanted while these new materials are being developed. Therefore we also encourage parallel research efforts to develop novel strategies to treat infections in situ.
Acknowledgments
We would like to thank Nicholas A. Kotov, PhD from the University of Michigan for providing the nano-engineered surfaces used for bacteria adhesion testing shown in Figure 5.
Funding Support:
NIGMS R01 GM081702 Biomechanics of Bloodstream Infection (JGY,JSV))
SAEM Research Fellowship Award (JSV)
REFERENCES
- 1.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 2.Bryers JD. Medical biofilms. Biotechnology and Bioengineering. 2008;100(1):1–18. doi: 10.1002/bit.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstein RA, Darouiche RO. Device-Associated Infections: A Macroproblem that Starts with Microadherence. Clinical Infectious Diseases. 2001;33(9):1567–1572. doi: 10.1086/323130. [DOI] [PubMed] [Google Scholar]
- 4.Uslan DZ, Tleyjeh IM, Baddour LM, Friedman PA, Jenkins SM, St Sauver JL, Hayes DL. Temporal trends in permanent pacemaker implantation: a population-based study. Am Heart J. 2008;155(5):896–903. doi: 10.1016/j.ahj.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol. 2010;33(4):414–419. doi: 10.1111/j.1540-8159.2009.02569.x. [DOI] [PubMed] [Google Scholar]
- 6.Gundeslioglu O, Altundag O, Altundag K. Nanobacteria and breast implant capsule contracture and calcification: a hypothesis. Aesthetic Plast Surg. 2005;29(6):582. doi: 10.1007/s00266-005-0094-0. [DOI] [PubMed] [Google Scholar]
- 7.Pajkos A, Deva AK, Vickery K, Cope C, Chang L, Cossart YE. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg. 2003;111(5):1605–1611. doi: 10.1097/01.PRS.0000054768.14922.44. [DOI] [PubMed] [Google Scholar]
- 8.Portillo ME, Salvado M, Alier A, Sorli L, Martinez S, Horcajada JP, Puig L. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res. 2013;471(11):3672–3678. doi: 10.1007/s11999-013-3200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters BM, Jabra-Rizk MA, O’May GA, Costerton JW, Shirtliff ME. Polymicrobial Interactions: Impact on Pathogenesis and Human Disease. Clinical Microbiology Reviews. 2012;25(1):193–213. doi: 10.1128/CMR.00013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincent JL, Brealey D, Libert N, Abidi NE, O’Dwyer M, Zacharowski K, Mikaszewska-Sokolewicz M, Schrenzel J, Simon F, Wilks M, Picard-Maureau M, Chalfin DB, Ecker DJ, Sampath R, Singer M. Rapid Diagnosis of Infection in the Critically Ill, a Multicenter Study of Molecular Detection in Bloodstream Infections, Pneumonia, and Sterile Site Infections. Crit Care Med. 2015;43(11):2283–2291. doi: 10.1097/CCM.0000000000001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fournier P-E, Drancourt M, Colson P, Rolain J-M, Scola BL, Raoult D. Modern clinical microbiology: new challenges and solutions. Nat Rev Micro. 2013;11(8):574–585. doi: 10.1038/nrmicro3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arciola CR, Gamberini S, Campoccia D, Visai L, Speziale P, Baldassarri L, Montanaro L. A multiplex PCR method for the detection of all five individual genes of ica locus in Staphylococcus epidermidis. A survey on 400 clinical isolates from prosthesis-associated infections. J Biomed Mater Res A. 2005;75(2):408–413. doi: 10.1002/jbm.a.30445. [DOI] [PubMed] [Google Scholar]
- 13.Arciola CR, Baldassarri L, Montanaro L. Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. J Clin Microbiol. 2001;39(6):2151–2156. doi: 10.1128/JCM.39.6.2151-2156.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arciola CR, Campoccia D, Gamberini S, Baldassarri L, Montanaro L. Prevalence of cna, fnbA and fnbB adhesin genes among Staphylococcus aureus isolates from orthopedic infections associated to different types of implant. FEMS Microbiol Lett. 2005;246(1):81–86. doi: 10.1016/j.femsle.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Schaeffer CR, Woods KM, Longo GM, Kiedrowski MR, Paharik AE, Büttner H, Christner M, Boissy RJ, Horswill AR, Rohde H, Fey PD. Accumulation-Associated Protein Enhances Staphylococcus epidermidis Biofilm Formation under Dynamic Conditions and Is Required for Infection in a Rat Catheter Model. Infection and Immunity. 2015;83(1):214–226. doi: 10.1128/IAI.02177-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171. doi: 10.4065/81.9.1159. [DOI] [PubMed] [Google Scholar]
- 17.Othman H, Fishbain JT, Khatib R. The role of intravenous catheters in cardiovascular implantable electronic device infections: Identifying potential targets for prevention. American Journal of Infection Control. 2013;41(4):376–377. doi: 10.1016/j.ajic.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 18.O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S and the Healthcare Infection Control Practices Advisory, C. Summary of Recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2011;52(9):1087–1099. doi: 10.1093/cid/cir138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marik PE, Flemmer M, Harrison W. The risk of catheter-related bloodstream infection with femoral venous catheters as compared to subclavian and internal jugular venous catheters: a systematic review of the literature and meta-analysis. Crit Care Med. 2012;40(8):2479–2485. doi: 10.1097/CCM.0b013e318255d9bc. [DOI] [PubMed] [Google Scholar]
- 20.Napalkov P, Felici DM, Chu LK, Jacobs JR, Begelman SM. Incidence of catheter-related complications in patients with central venous or hemodialysis catheters: a health care claims database analysis. BMC Cardiovascular Disorders. 2013;13:86–86. doi: 10.1186/1471-2261-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fysaraki M, Samonis G, Valachis A, Daphnis E, Karageorgopoulos DE, Falagas ME, Stylianou K, Kofteridis DP. Incidence, clinical, microbiological features and outcome of bloodstream infections in patients undergoing hemodialysis. Int J Med Sci. 2013;10(12):1632–1638. doi: 10.7150/ijms.6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blot SI, Depuydt P, Annemans L, Benoit D, Hoste E, De Waele JJ, Decruyenaere J, Vogelaers D, Colardyn F, Vandewoude KH. Clinical and Economic Outcomes in Critically Ill Patients with Nosocomial Catheter-Related Bloodstream Infections. Clinical Infectious Diseases. 2005;41(11):1591–1598. doi: 10.1086/497833. [DOI] [PubMed] [Google Scholar]
- 23.Frasca D, Dahyot-Fizelier C, Mimoz O. Prevention of central venous catheter-related infection in the intensive care unit. Crit Care. 2010;14(2):212. doi: 10.1186/cc8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. Journal of the American College of Cardiology. 2000;36(4):1152–1158. doi: 10.1016/s0735-1097(00)00834-2. [DOI] [PubMed] [Google Scholar]
- 25.Oxenham H, Bloomfield P, Wheatley DJ, Lee RJ, Cunningham J, Prescott RJ, Miller HC. Twenty year comparison of a Bjork-Shiley mechanical heart valve with porcine bioprostheses. Heart. 2003;89(7):715–721. doi: 10.1136/heart.89.7.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baddour LM, Bettmann MA, Bolger AF, Epstein AE, Ferrieri P, Gerber MA, Gewitz MH, Jacobs AK, Levison ME, Newburger JW, Pallasch TJ, Wilson WR, Baltimore RS, Falace DA, Shulman ST, Tani LY, Taubert KA. Nonvalvular Cardiovascular Device-Related Infections. Circulation. 2003;108(16):2015–2031. doi: 10.1161/01.CIR.0000093201.57771.47. [DOI] [PubMed] [Google Scholar]
- 27.Grossi P, Dalla Gasperina D, Pagani F, Marone P, Viganò M, Minoli L. Infectious complications in patients with the novacor left ventricular assist system. Transplantation Proceedings. 2001;33(1–2):1969–1971. doi: 10.1016/s0041-1345(00)02757-3. [DOI] [PubMed] [Google Scholar]
- 28.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Ulisney KL, Baldwin JT, Young JB. Second INTERMACS annual report: More than 1,000 primary left ventricular assist device implants. Journal of Heart and Lung Transplantation. 2010;29(1):1–10. doi: 10.1016/j.healun.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaffer JM, Allen JG, Weiss ES, Arnaoutakis GJ, Patel ND, Russell SD, Shah AS, Conte JV. Infectious complications after pulsatile-flow and continuous-flow left ventricular assist device implantation. Journal of Heart and Lung Transplantation. 2011;30(2):164–174. doi: 10.1016/j.healun.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Antonios VS, Baddour LM. Intra-arterial Device Infections. Curr Infect Dis Rep. 2004;6(4):263–269. doi: 10.1007/s11908-004-0046-x. [DOI] [PubMed] [Google Scholar]
- 31.Kunutsor SK, Whitehouse MR, Blom AW, Beswick AD. Patient-Related Risk Factors for Periprosthetic Joint Infection after Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. PLoS One. 2016;11(3):e0150866. doi: 10.1371/journal.pone.0150866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips JE, Crane TP, Noy M, Elliott TS, Grimer RJ. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br. 2006;88(7):943–948. doi: 10.1302/0301-620X.88B7.17150. [DOI] [PubMed] [Google Scholar]
- 33.Parvizi J, Walinchus L, Adeli B. Molecular diagnostics in periprosthetic joint infection. Int J Artif Organs. 2011;34(9):847–855. doi: 10.5301/ijao.5000054. [DOI] [PubMed] [Google Scholar]
- 34.Krenek L, Farng E, Zingmond D, SooHoo NF. Complication and Revision Rates Following Total Elbow Arthroplasty. The Journal of Hand Surgery. 2011;36(1):68–73. doi: 10.1016/j.jhsa.2010.09.036. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-Joint Infections. New England Journal of Medicine. 2004;351(16):1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 36.Daniels AH, Kawaguchi S, Contag AG, Rastegar F, Waagmeester G, Anderson PA, Arthur M, Hart RA. Hospital charges associated with “never events”: comparison of anterior cervical discectomy and fusion, posterior lumbar interbody fusion, and lumbar laminectomy to total joint arthroplasty. J Neurosurg Spine. 2016:1–5. doi: 10.3171/2015.11.SPINE15776. [DOI] [PubMed] [Google Scholar]
- 37.Edwards JR, Peterson KD, Andrus ML, Tolson JS, Goulding JS, Dudeck MA, Mincey RB, Pollock DA, Horan TC. National Healthcare Safety Network (NHSN) Report, data summary for 2006, issued June 2007. American Journal of Infection Control. 2007;35(5):290–301. doi: 10.1016/j.ajic.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Cevasco M, Itani KM. Ventral hernia repair with synthetic, composite, and biologic mesh: characteristics, indications, and infection profile. Surg Infect (Larchmt) 2012;13(4):209–215. doi: 10.1089/sur.2012.123. [DOI] [PubMed] [Google Scholar]
- 39.Falagas ME, Kasiakou SK. Mesh-related infections after hernia repair surgery. Clinical Microbiology and Infection. 2005;11(1):3–8. doi: 10.1111/j.1469-0691.2004.01014.x. [DOI] [PubMed] [Google Scholar]
- 40.Primus FE, Harris HW. A critical review of biologic mesh use in ventral hernia repairs under contaminated conditions. Hernia. 2013;17(1):21–30. doi: 10.1007/s10029-012-1037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akoh JA. Peritoneal dialysis associated infections: An update on diagnosis and management. World Journal of Nephrology. 2012;1(4):106–122. doi: 10.5527/wjn.v1.i4.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiggins KJ, Johnson DW, Craig JC, Strippoli GF. Treatment of peritoneal dialysis-associated peritonitis: a systematic review of randomized controlled trials. Am J Kidney Dis. 2007;50(6):967–988. doi: 10.1053/j.ajkd.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 43.Kanev PM, Sheehan JM. Reflections on Shunt Infection. Pediatric Neurosurgery. 2003;39(6):285–290. doi: 10.1159/000075255. [DOI] [PubMed] [Google Scholar]
- 44.Allan JM, Jr, Freer CV, Searcy MA, Landry SM, Wenzel RP. Nosocomial Bloodstream Infections: Secular Trends in a Statewide Surveillance Program in Virginia. Infection Control. 1986;7(11):550–553. doi: 10.1017/s0195941700065309. [DOI] [PubMed] [Google Scholar]
- 45.Allen JR, Hightower AW, Martin SM, Dixon RE. Secular trends in nosocomial infections: 1970–1979. The American Journal of Medicine. 1981;70(2):389–392. doi: 10.1016/0002-9343(81)90777-4. [DOI] [PubMed] [Google Scholar]
- 46.Ammerlaan HSM, Harbarth S, Buiting AGM, Crook DW, Fitzpatrick F, Hanberger H, Herwaldt LA, van Keulen PHJ, Kluytmans JAJW, Kola A, Kuchenbecker RS, Lingaas E, Meessen N, Morris-Downes MM, Pottinger JM, Rohner P, dos Santos RP, Seifert H, Wisplinghoff H, Ziesing S, Walker AS, Bonten MJM. Secular trends in nosocomial bloodstream infections: antibiotic-resistant bacteria increase the total burden of infection. Clinical Infectious Diseases. 2012 doi: 10.1093/cid/cis1006. [DOI] [PubMed] [Google Scholar]
- 47.Banerjee SN, Emori TG, Culver DH, Gaynes RP, Jarvis WR, Horan T, Edwards JR, Tolson J, Henderson T, Martone WJ and National Nosocomial Infections Surveillance, S. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. The American Journal of Medicine. 1991;91(3, Supplement 2):S86–S89. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- 48.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial Bloodstream Infections in US Hospitals: Analysis of 24,179 Cases from a Prospective Nationwide Surveillance Study. Clinical Infectious Diseases. 2004;39(3):309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 49.Lee GM, Kleinman K, Soumerai SB, Tse A, Cole D, Fridkin SK, Horan T, Platt R, Gay C, Kassler W, Goldmann DA, Jernigan J, Jha AK. Effect of nonpayment for preventable infections in U.S. hospitals. N Engl J Med. 2012;367(15):1428–1437. doi: 10.1056/NEJMsa1202419. [DOI] [PubMed] [Google Scholar]
- 50.Willson M, Wilde M, Webb M-L, Thompson D, Parker D, Harwood J, Callan L, Gray M. Nursing Interventions to Reduce the Risk of Catheter-Associated Urinary Tract Infection: Part 2: Staff Education, Monitoring, and Care Techniques. Journal of Wound Ostomy & Continence Nursing. 2009;36(2):137–154. doi: 10.1097/01.WON.0000347655.56851.04. 10.1097/01.WON.0000347655.56851.04. [DOI] [PubMed] [Google Scholar]
- 51.Ramritu P, Halton K, Cook D, Whitby M, Graves N. Catheter-related bloodstream infections in intensive care units: a systematic review with meta-analysis. Journal of Advanced Nursing. 2008;62(1):3–21. doi: 10.1111/j.1365-2648.2007.04564.x. [DOI] [PubMed] [Google Scholar]
- 52.Flodgren G, Conterno LO, Mayhew A, Omar O, Pereira CR, Shepperd S. Interventions to improve professional adherence to guidelines for prevention of device-related infections. Cochrane Database Syst Rev. 2013;3 doi: 10.1002/14651858.CD006559.pub2. CD006559. [DOI] [PubMed] [Google Scholar]
- 53.Campoccia D, Cangini I, Selan L, Vercellino M, Montanaro L, Visai L, Arciola CR. An overview of the methodological approach to the in vitro study of anti-infective biomaterials. Int J Artif Organs. 2012;35(10):800–816. doi: 10.5301/ijao.5000140. [DOI] [PubMed] [Google Scholar]
- 54.Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials. 2012;33(26):5967–5982. doi: 10.1016/j.biomaterials.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 55.Nathan C. Points of control in inflammation. Nature. 2002;420(6917):846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 56.Cheung K, Taylor K, Jameson J. Immunomodulation at epithelial sites by obesity and metabolic disease. Immunologic Research. 2012;52(3):182–199. doi: 10.1007/s12026-011-8261-7. [DOI] [PubMed] [Google Scholar]
- 57.Wagner C, Obst U, Hansch GM. Implant-associated posttraumatic osteomyelitis: collateral damage by local host defense? Int J Artif Organs. 2005;28(11):1172–1180. doi: 10.1177/039139880502801115. [DOI] [PubMed] [Google Scholar]
- 58.Israelachvili JN. Intermolecular and surface forces. London: Academic; 1992. [Google Scholar]
- 59.Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3:23. doi: 10.1186/2047-2994-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bjarnsholt T, Jensen PØ, Moser C, Høiby N. Biofilm Infections. Springer; 2010. [Google Scholar]
- 61.Padera RF. Infection in ventricular assist devices: the role of biofilm. Cardiovascular Pathology. 2006;15(5):264–270. doi: 10.1016/j.carpath.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Evans DJ, Allison DG, Brown MRW, Gilbert P. Effect of growth-rate on resistance of Gram-negative biofilms to cetrimide. Journal of Antimicrobial Chemotherapy. 1990;26(4):473–478. doi: 10.1093/jac/26.4.473. [DOI] [PubMed] [Google Scholar]
- 63.Duguid IG, Evans E, Brown MRW, Gilbert P. Growth-rate-independent killing by ciprofloxacin of biofilm-derived Staphylococcus epidermidis evidence for cell-cycle dependency. Journal of Antimicrobial Chemotherapy. 1992;30(6):791–802. doi: 10.1093/jac/30.6.791. [DOI] [PubMed] [Google Scholar]
- 64.Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. Journal of Bacteriology. 2002;184(4):1140–1154. doi: 10.1128/jb.184.4.1140-1154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yao Y, Sturdevant DE, Otto M. Genomewide analysis of gene expression in Staphylococcus epidermidis biofilms: insights into the pathophysiology of S. epidermidis biofilms and the role of phenol-soluble modulins in formation of biofilms. J Infect Dis. 2005;191(2):289–298. doi: 10.1086/426945. [DOI] [PubMed] [Google Scholar]
- 66.Waters CM, Bassler BL. QUORUM SENSING: Cell-to-Cell Communication in Bacteria. Annual Review of Cell and Developmental Biology. 2005;21(1):319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 67.McCann MT, Gilmore BF, Gorman SP. Staphylococcus epidermidis device-related infections: pathogenesis and clinical management. J Pharm Pharmacol. 2008;60(12):1551–1571. doi: 10.1211/jpp/60.12.0001. [DOI] [PubMed] [Google Scholar]
- 68.Katsikogianni M, Missirlis YF. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cell Mater. 2004;8:37–57. doi: 10.22203/ecm.v008a05. [DOI] [PubMed] [Google Scholar]
- 69.Vuong C, Otto M. Staphylococcus epidermidis infections. Microbes Infect. 2002;4(4):481–489. doi: 10.1016/s1286-4579(02)01563-0. [DOI] [PubMed] [Google Scholar]
- 70.Pavithra D, Doble M. Biofilm formation, bacterial adhesion and host response on polymeric implants--issues and prevention. Biomed Mater. 2008;3(3):034003. doi: 10.1088/1748-6041/3/3/034003. [DOI] [PubMed] [Google Scholar]
- 71.Heilmann C, Hussain M, Peters G, Gotz F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997;24(5):1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 72.Costerton JW, Montanaro L, Arciola CR. Biofilm in implant infections: its production and regulation. Int J Artif Organs. 2005;28(11):1062–1068. doi: 10.1177/039139880502801103. [DOI] [PubMed] [Google Scholar]
- 73.Qin Z, Ou Y, Yang L, Zhu Y, Tolker-Nielsen T, Molin S, Qu D. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology. 2007;153(Pt 7):2083–2092. doi: 10.1099/mic.0.2007/006031-0. [DOI] [PubMed] [Google Scholar]
- 74.Clarke SR, Foster SJ. Surface adhesins of Staphylococcus aureus. Adv Microb Physiol. 2006;51:187–224. doi: 10.1016/S0065-2911(06)51004-5. [DOI] [PubMed] [Google Scholar]
- 75.Patel JD, Ebert M, Ward R, Anderson JM. S. epidermidis biofilm formation: effects of biomaterial surface chemistry and serum proteins. J Biomed Mater Res A. 2007;80(3):742–751. doi: 10.1002/jbm.a.31103. [DOI] [PubMed] [Google Scholar]
- 76.Allegrucci M, Hu FZ, Shen K, Hayes J, Ehrlich GD, Post JC, Sauer K. Phenotypic characterization of Streptococcus pneumoniae biofilm development. J Bacteriol. 2006;188(7):2325–2335. doi: 10.1128/JB.188.7.2325-2335.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ziebuhr W, Hennig S, Eckart M, Kranzler H, Batzilla C, Kozitskaya S. Nosocomial infections by Staphylococcus epidermidis: how a commensal bacterium turns into a pathogen. Int J Antimicrob Agents. 2006;28(Suppl 1):S14–S20. doi: 10.1016/j.ijantimicag.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 78.Hussain M, Heilmann C, Peters G, Herrmann M. Teichoic acid enhances adhesion of Staphylococcus epidermidis to immobilized fibronectin. Microb Pathog. 2001;31(6):261–270. doi: 10.1006/mpat.2001.0469. [DOI] [PubMed] [Google Scholar]
- 79.Mack D, Siemssen N, Laufs R. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect Immun. 1992;60(5):2048–2057. doi: 10.1128/iai.60.5.2048-2057.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mack D, Nedelmann M, Krokotsch A, Schwarzkopf A, Heesemann J, Laufs R. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect Immun. 1994;62(8):3244–3253. doi: 10.1128/iai.62.8.3244-3253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dunne WM., Jr Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev. 2002;15(2):155–166. doi: 10.1128/CMR.15.2.155-166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harrison JJ, Ceri H, Stremick C, Turner RJ. Differences in biofilm and planktonic cell mediated reduction of metalloid oxyanions. FEMS Microbiol Lett. 2004;235(2):357–362. doi: 10.1016/j.femsle.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 83.Fey PD, Olson ME. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol. 2010;5(6):917–933. doi: 10.2217/fmb.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Habash M, Reid G. Microbial biofilms: their development and significance for medical device-related infections. J Clin Pharmacol. 1999;39(9):887–898. doi: 10.1177/00912709922008506. [DOI] [PubMed] [Google Scholar]
- 85.Freitas RA. Nanomedicine: Biocompatibility. Volume IIA. Landes Bioscience; 2002. [Google Scholar]
- 86.de Araujo GL, Coelho LR, de Carvalho CB, Maciel RM, Coronado AZ, Rozenbaum R, Ferreira-Carvalho BT, SáFigueiredo AM, Teixeira LA. Commensal isolates of methicillin-resistant Staphylococcus epidermidis are also well equipped to produce biofilm on polystyrene surfaces. Journal of Antimicrobial Chemotherapy. 2006;57(5):855–864. doi: 10.1093/jac/dkl071. [DOI] [PubMed] [Google Scholar]
- 87.Saginur R, Stdenis M, Ferris W, Aaron SD, Chan F, Lee C, Ramotar K. Multiple combination bactericidal testing of staphylococcal biofilms from implant-associated infections. Antimicrob Agents Chemother. 2006;50(1):55–61. doi: 10.1128/AAC.50.1.55-61.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maira-Litran T, Allison DG, Gilbert P. An evaluation of the potential of the multiple antibiotic resistance operon (mar) and the multidrug efflux pump acrAB to moderate resistance towards ciprofloxacin in Escherichia coli biofilms. J Antimicrob Chemother. 2000;45(6):789–795. doi: 10.1093/jac/45.6.789. [DOI] [PubMed] [Google Scholar]
- 89.Stewart PS, Franklin MJ. Physiological heterogeneity in biofilms. Nat Rev Microbiol. 2008;6(3):199–210. doi: 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- 90.Lewis K. Persister cells and the riddle of biofilm survival. Biochemistry (Mosc) 2005;70(2):267–274. doi: 10.1007/s10541-005-0111-6. [DOI] [PubMed] [Google Scholar]
- 91.Roberts ME, Stewart PS. Modelling protection from antimicrobial agents in biofilms through the formation of persister cells. Microbiology. 2005;151(Pt 1):75–80. doi: 10.1099/mic.0.27385-0. [DOI] [PubMed] [Google Scholar]
- 92.Vuong C, Kocianova S, Yao Y, Carmody AB, Otto M. Increased colonization of indwelling medical devices by quorum-sensing mutants of Staphylococcus epidermidis in vivo. J Infect Dis. 2004;190(8):1498–1505. doi: 10.1086/424487. [DOI] [PubMed] [Google Scholar]
- 93.Yarwood JM, Bartels DJ, Volper EM, Greenberg EP. Quorum Sensing in Staphylococcus aureus Biofilms. Journal of Bacteriology. 2004;186(6):1838–1850. doi: 10.1128/JB.186.6.1838-1850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raad II, Luna M, Khalil SA, Costerton JW, Lam C, Bodey GP. The relationship between the thrombotic and infectious complications of central venous catheters. JAMA. 1994;271(13):1014–1046. [PubMed] [Google Scholar]
- 95.Smith RS, Zhang Z, Bouchard M, Li J, Lapp HS, Brotske GR, Lucchino DL, Weaver D, Roth LA, Coury A, Biggerstaff J, Sukavaneshvar S, Langer R, Loose C. Vascular catheters with a nonleaching poly-sulfobetaine surface modification reduce thrombus formation and microbial attachment. Sci Transl Med. 2012;4(153) doi: 10.1126/scitranslmed.3004120. 153ra132. [DOI] [PubMed] [Google Scholar]
- 96.Boersma RS, Jie KS, Verbon A, van Pampus EC, Schouten HC. Thrombotic and infectious complications of central venous catheters in patients with hematological malignancies. Ann Oncol. 2008;19(3):433–442. doi: 10.1093/annonc/mdm350. [DOI] [PubMed] [Google Scholar]
- 97.Levi M, van der Poll T, Büller HR. Bidirectional Relation Between Inflammation and Coagulation. Circulation. 2004;109(22):2698–2704. doi: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- 98.Lordick F, Hentrich M, Decker T, Hennig M, Pohlmann H, Hartenstein R, Peschel C. Ultrasound screening for internal jugular vein thrombosis aids the detection of central venous catheter-related infections in patients with haemato-oncological diseases: a prospective observational study. Br J Haematol. 2003;120(6):1073–1078. doi: 10.1046/j.1365-2141.2003.04199.x. [DOI] [PubMed] [Google Scholar]
- 99.van Rooden CJ, Schippers EF, Barge RM, Rosendaal FR, Guiot HF, van der Meer FJ, Meinders AE, Huisman MV. Infectious complications of central venous catheters increase the risk of catheter-related thrombosis in hematology patients: a prospective study. J Clin Oncol. 2005;23(12):2655–2660. doi: 10.1200/JCO.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 100.Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, Lockhart PB, Masoudi FA, Okum EJ, Wilson WR, Beerman LB, Bolger AF, Estes NAM, Gewitz M, Newburger JW, Schron EB, Taubert KA on behalf of the American Heart Association Rheumatic Fever, E., Young, K. D. C. o. t. C. o. C. D. i. t., Surgery, C. o. C., Anesthesia, Nursing, C. o. C., Cardiology, C. o. C., Care, t. I. C. o. Q. o. and Research, O. Update on Cardiovascular Implantable Electronic Device Infections and Their Management: A Scientific Statement From the American Heart Association. Circulation. 2010;121(3):458–477. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- 101.Herrmann M, Vaudaux PE, Pittet D, Auckenthaler R, Lew PD, Schumacher-Perdreau F, Peters G, Waldvogel FA. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988;158(4):693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- 102.Vaudaux P, Pittet D, Haeberli A, Huggler E, Nydegger UE, Lew DP, Waldvogel FA. Host factors selectively increase staphylococcal adherence on inserted catheters: a role for fibronectin and fibrinogen or fibrin. J Infect Dis. 1989;160(5):865–875. doi: 10.1093/infdis/160.5.865. [DOI] [PubMed] [Google Scholar]
- 103.Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131(4):417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 104.Kwiecinski J, Peetermans M, Liesenborghs L, Na M, Bjornsdottir H, Zhu X, Jacobsson G, Johansson BR, Geoghegan JA, Foster TJ, Josefsson E, Bylund J, Verhamme P, Jin T. Staphylokinase Control of Staphylococcus aureus Biofilm Formation and Detachment Through Host Plasminogen Activation. J Infect Dis. 2016;213(1):139–148. doi: 10.1093/infdis/jiv360. [DOI] [PubMed] [Google Scholar]
- 105.Zimmerli W, Waldvogel FA, Vaudaux P, Nydegger UE. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982;146(4):487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]
- 106.Zimmerli W, Lew PD, Waldvogel FA. Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. J Clin Invest. 1984;73(4):1191–1200. doi: 10.1172/JCI111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sherertz RJ, Carruth WA, Marosok RD, Espeland MA, Johnson RA, Solomon DD. Contribution of vascular catheter material to the pathogenesis of infection: the enhanced risk of silicone in vivo. J Biomed Mater Res. 1995;29(5):635–645. doi: 10.1002/jbm.820290511. [DOI] [PubMed] [Google Scholar]
- 108.Marosok R, Washburn R, Indorf A, Solomon D, Sherertz R. Contribution of vascular catheter material to the pathogenesis of infection: depletion of complement by silicone elastomer in vitro. J Biomed Mater Res. 1996;30(2):245–250. doi: 10.1002/(SICI)1097-4636(199602)30:2<245::AID-JBM15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 109.Kristian SA, Birkenstock TA, Sauder U, Mack D, Gotz F, Landmann R. Biofilm formation induces C3a release and protects Staphylococcus epidermidis from IgG and complement deposition and from neutrophil-dependent killing. J Infect Dis. 2008;197(7):1028–1035. doi: 10.1086/528992. [DOI] [PubMed] [Google Scholar]
- 110.Wagner C, Kondella K, Bernschneider T, Heppert V, Wentzensen A, Hansch GM. Post-traumatic osteomyelitis: analysis of inflammatory cells recruited into the site of infection. Shock. 2003;20(6):503–510. doi: 10.1097/01.shk.0000093542.78705.e3. [DOI] [PubMed] [Google Scholar]
- 111.Wagner C, Kaksa A, Muller W, Denefleh B, Heppert V, Wentzensen A, Hansch GM. Polymorphonuclear neutrophils in posttraumatic osteomyelitis: cells recovered from the inflamed site lack chemotactic activity but generate superoxides. Shock. 2004;22(2):108–115. doi: 10.1097/01.shk.0000132488.71875.15. [DOI] [PubMed] [Google Scholar]
- 112.Shanbhag A, Yang J, Lilien J, Black J. Decreased neutrophil respiratory burst on exposure to cobalt-chrome alloy and polystyrene in vitro. Journal of Biomedical Materials Research. 1992;26(2):185–195. doi: 10.1002/jbm.820260205. [DOI] [PubMed] [Google Scholar]
- 113.Saldarriaga Fernandez IC, Da Silva Domingues JF, van Kooten TG, Metzger S, Grainger DW, Busscher HJ, van der Mei HC. Macrophage response to staphylococcal biofilms on crosslinked poly(ethylene) glycol polymer coatings and common biomaterials in vitro. Eur Cell Mater. 2011;21:73–79. doi: 10.22203/ecm.v021a06. discussion 79. [DOI] [PubMed] [Google Scholar]
- 114.Brodbeck WG, Patel J, Voskerician G, Christenson E, Shive MS, Nakayama Y, Matsuda T, Ziats NP, Anderson JM. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. Proceedings of the National Academy of Sciences. 2002;99(16):10287–10292. doi: 10.1073/pnas.162124199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Safdar N, O’Horo JC, Ghufran A, Bearden A, Didier ME, Chateau D, Maki DG. Chlorhexidine-impregnated dressing for prevention of catheter-related bloodstream infection: a meta-analysis*. Crit Care Med. 2014;42(7):1703–1713. doi: 10.1097/CCM.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tang HJ, Lin HL, Lin YH, Leung PO, Chuang YC, Lai CC. The impact of central line insertion bundle on central line-associated bloodstream infection. BMC Infect Dis. 2014;14:356. doi: 10.1186/1471-2334-14-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tan J, Shah S, Thomas A, Ou-Yang HD, Liu Y. The influence of size, shape and vessel geometry on nanoparticle distribution. Microfluidics and nanofluidics. 2013;14(1–2):77–87. doi: 10.1007/s10404-012-1024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schoephoerster RT, Oynes F, Nunez G, Kapadvanjwala M, Dewanjee MK. Effects of local geometry and fluid dynamics on regional platelet deposition on artificial surfaces. Arterioscler Thromb. 1993;13(12):1806–1813. doi: 10.1161/01.atv.13.12.1806. [DOI] [PubMed] [Google Scholar]
- 119.Clark TW, Isu G, Gallo D, Verdonck P, Morbiducci U. Comparison of symmetric hemodialysis catheters using computational fluid dynamics. J Vasc Interv Radiol. 2015;26(2):252–259. doi: 10.1016/j.jvir.2014.11.004. e2. [DOI] [PubMed] [Google Scholar]
- 120.Dasi LP, Simon HA, Sucosky P, Yoganathan AP. FLUID MECHANICS OF ARTIFICIAL HEART VALVES. Clinical and experimental pharmacology & physiology. 2009;36(2):225–237. doi: 10.1111/j.1440-1681.2008.05099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Graefe R, Borchardt R, Arens J, Schlanstein P, Schmitz-Rode T, Steinseifer U. Improving oxygenator performance using computational simulation and flow field-based parameters. Artif Organs. 2010;34(11):930–936. doi: 10.1111/j.1525-1594.2010.01157.x. [DOI] [PubMed] [Google Scholar]
- 122.Bluestein D, Einav S, Slepian MJ. DEVICE THROMBOGENICITY EMULATION: A NOVEL METHODOLOGY FOR OPTIMIZING THE THROMBORESISTANCE OF CARDIOVASCULAR DEVICES. Journal of biomechanics. 2013;46(2):338–344. doi: 10.1016/j.jbiomech.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lai NM, Chaiyakunapruk N, Lai NA, O’Riordan E, Pau WS, Saint S. Catheter impregnation, coating or bonding for reducing central venous catheter-related infections in adults. Cochrane Database Syst Rev. 2013;6 doi: 10.1002/14651858.CD007878.pub2. CD007878. [DOI] [PubMed] [Google Scholar]
- 124.Decuzzi P, Ferrari M. Modulating cellular adhesion through nanotopography. Biomaterials. 2010;31(1):173–179. doi: 10.1016/j.biomaterials.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 125.Epstein AK, Hochbaum AI, Kim P, Aizenberg J. Control of bacterial biofilm growth on surfaces by nanostructural mechanics and geometry. Nanotechnology. 2011;22(49):494007. doi: 10.1088/0957-4484/22/49/494007. [DOI] [PubMed] [Google Scholar]
- 126.Hochbaum AI, Aizenberg J. Bacteria pattern spontaneously on periodic nanostructure arrays. Nano Lett. 2010;10(9):3717–3721. doi: 10.1021/nl102290k. [DOI] [PubMed] [Google Scholar]
- 127.McGuffie MJ, Hong J, Bahng JH, Glynos E, Green PF, Kotov NA, Younger JG, VanEpps JS. Zinc oxide nanoparticle suspensions and layer-by-layer coatings inhibit staphylococcal growth. Nanomedicine. 2016;12(1):33–42. doi: 10.1016/j.nano.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cha S-H, Hong J, McGuffie M, Yeom B, VanEpps JS, Kotov NA. Shape-Dependent Biomimetic Inhibition of Enzyme by Nanoparticles and Their Antibacterial Activity. ACS Nano. 2015;9(9):9097–9105. doi: 10.1021/acsnano.5b03247. [DOI] [PubMed] [Google Scholar]
- 129.Leung YH, Chan CM, Ng AM, Chan HT, Chiang MW, Djurisic AB, Ng YH, Jim WY, Guo MY, Leung FC, Chan WK, Au DT. Antibacterial activity of ZnO nanoparticles with a modified surface under ambient illumination. Nanotechnology. 2012;23(47):475703. doi: 10.1088/0957-4484/23/47/475703. [DOI] [PubMed] [Google Scholar]
- 130.Lipovsky A, Tzitrinovich Z, Friedmann H, Applerot G, Gedanken A, Lubart R. EPR Study of Visible Light-Induced ROS Generation by Nanoparticles of ZnO. The Journal of Physical Chemistry C. 2009;113(36):15997–16001. [Google Scholar]
- 131.Xia T, Kovochich M, Liong M, Madler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2(10):2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti MF, Fievet F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006;6(4):866–870. doi: 10.1021/nl052326h. [DOI] [PubMed] [Google Scholar]
- 133.Zhang L, Jiang Y, Ding Y, Povey M, York D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids) Journal of Nanoparticle Research. 2007;9(3):479–489. [Google Scholar]
- 134.Darouiche RO. Anti-infective efficacy of silver-coated medical prostheses. Clin Infect Dis. 1999;29(6):1371–1377. doi: 10.1086/313561. quiz 1378. [DOI] [PubMed] [Google Scholar]
- 135.Politano AD, Campbell KT, Rosenberger LH, Sawyer RG. Use of silver in the prevention and treatment of infections: silver review. Surg Infect (Larchmt) 2013;14(1):8–20. doi: 10.1089/sur.2011.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lu TK, Collins JJ. Engineered bacteriophage targeting gene networks as adjuvants for antibiotic therapy. Proceedings of the National Academy of Sciences. 2009;106(12):4629–4634. doi: 10.1073/pnas.0800442106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brackman G, Coenye T. Quorum sensing inhibitors as anti-biofilm agents. Curr Pharm Des. 2015;21(1):5–11. doi: 10.2174/1381612820666140905114627. [DOI] [PubMed] [Google Scholar]
- 138.Sahm N, Becker J, Santel T, Schwarz F. Non-surgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine: a prospective, randomized, controlled clinical study. Journal of Clinical Periodontology. 2011;38(9):872–878. doi: 10.1111/j.1600-051X.2011.01762.x. [DOI] [PubMed] [Google Scholar]
- 139.Orizondo RA, Babcock CI, Fabiilli ML, Pavlovsky L, Fowlkes JB, Younger JG, Cook KE. Characterization of a reverse-phase perfluorocarbon emulsion for the pulmonary delivery of tobramycin. J Aerosol Med Pulm Drug Deliv. 2014;27(5):392–399. doi: 10.1089/jamp.2013.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rickard CM, Webster J, Wallis MC, Marsh N, McGrail MR, French V, Foster L, Gallagher P, Gowardman JR, Zhang L, McClymont A, Whitby M. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012;380(9847):1066–1074. doi: 10.1016/S0140-6736(12)61082-4. [DOI] [PubMed] [Google Scholar]
- 141.Lucet JC, Bouadma L, Zahar JR, Schwebel C, Geffroy A, Pease S, Herault MC, Haouache H, Adrie C, Thuong M, Francais A, Garrouste-Orgeas M, Timsit JF. Infectious risk associated with arterial catheters compared with central venous catheters. Crit Care Med. 2010;38(4):1030–1035. doi: 10.1097/CCM.0b013e3181d4502e. [DOI] [PubMed] [Google Scholar]
- 142.Safdar N, O’Horo JC, Maki DG. Arterial catheter-related bloodstream infection: incidence, pathogenesis, risk factors and prevention. Journal of Hospital Infection. 85(3):189–195. doi: 10.1016/j.jhin.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 143.Advani S, Reich NG, Sengupta A, Gosey L, Milstone AM. Central Line-Associated Bloodstream Infection in Hospitalized Children with Peripherally Inserted Central Venous Catheters: Extending Risk Analyses Outside the Intensive Care Unit. Clinical Infectious Diseases. 2011;52(9):1108–1115. doi: 10.1093/cid/cir145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Safdar N, Maki DG. Risk of catheter-related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495. doi: 10.1378/chest.128.2.489. [DOI] [PubMed] [Google Scholar]
- 145.Lebeaux D, Fernandez-Hidalgo N, Chauhan A, Lee S, Ghigo JM, Almirante B, Beloin C. Management of infections related to totally implantable venous-access ports: challenges and perspectives. Lancet Infect Dis. 2014;14(2):146–159. doi: 10.1016/S1473-3099(13)70266-4. [DOI] [PubMed] [Google Scholar]
- 146.Toure A, Vanhems P, Lombard-Bohas C, Cassier P, Pere-Verge D, Souquet JC, Ecochard R, Chambrier C. Totally implantable central venous access port infections in patients with digestive cancer: incidence and risk factors. Am J Infect Control. 2012;40(10):935–939. doi: 10.1016/j.ajic.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 147.Stone GW, Ohman EM, Miller MF, Joseph DL, Christenson JT, Cohen M, Urban PM, Reddy RC, Freedman RJ, Staman KL, Ferguson JJ., Iii Contemporary utilization and outcomes of intra-aortic balloon counterpulsation in acute myocardial infarction: The benchmark registry. Journal of the American College of Cardiology. 2003;41(11):1940–1945. doi: 10.1016/s0735-1097(03)00400-5. [DOI] [PubMed] [Google Scholar]
- 148.Azeem T, Stephens-Lloyd A, Spyt T, Hartshorne R, Gershlick AH. Intra-aortic balloon counterpulsation: variations in use and complications. International Journal of Cardiology. 2004;94(2–3):255–259. doi: 10.1016/j.ijcard.2003.04.007. [DOI] [PubMed] [Google Scholar]
- 149.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, Iii, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH. Advanced heart failure treated with continuous-flow left ventricular assist device. New England Journal of Medicine. 2009;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 150.Uslan DZ, Sohail MR, St. Sauver JL, et al. Permanent pacemaker and implantable cardioverter defibrillator infection: A population-based study. Archives of Internal Medicine. 2007;167(7):669–675. doi: 10.1001/archinte.167.7.669. [DOI] [PubMed] [Google Scholar]