Abstract
Developmental origins of cardiometabolic diseases have been related to maternal nutritional conditions. In this context, the rising incidence of arterial hypertension, diabetes type II, and dyslipidemia has been attributed to genetic programming. Besides, environmental conditions during perinatal development such as maternal undernutrition or overnutrition can program changes in the integration among physiological systems leading to cardiometabolic diseases. This phenomenon can be understood in the context of the phenotypic plasticity and refers to the adjustment of a phenotype in response to environmental input without genetic change, following a novel, or unusual input during development. Experimental studies indicate that fetal exposure to an adverse maternal environment may alter the morphology and physiology that contribute to the development of cardiometabolic diseases. It has been shown that both maternal protein restriction and overnutrition alter the central and peripheral control of arterial pressure and metabolism. This review will address the new concepts on the maternal diet induced-cardiometabolic diseases that include the potential role of the perinatal malnutrition.
Keywords: developmental plasticity, perinatal nutrition, cardiometabolic control, protein restriction
Introduction
Cardiovascular and metabolic diseases, such as hypertension, type II diabetes, and dyslipidemia are highly prevalent in the world and have important effects on the public health, increasing risk factors for the development of other diseases, including coronary heart disease, stroke, and heart failure (Landsberg et al., 2013). The etiology of these cardiometabolic diseases includes a complex phenotype that arises from numerous genetic, environmental, nutritional, behavioral, and ethnic origins (Landsberg et al., 2013; Ng et al., 2014). In this regard, it has been observed that the eating habits and behaviors and nutritional condition in early phases of life may play a key role on the etiology of these diseases by inducing physiological dysfunctions (Lucas, 1998; Victora et al., 2008; Wells, 2012). This phenomenon can be understood in the context of phenotypic plasticity and it refers to the ability of an organism to react to both an internal and external environmental inputs with a change in the form, state, physiology, or rate of activity without genetic changes (West-Eberhard, 2005b). Indeed the nutritional factors rise as important element in this theme and it has been highlighted since Barker (Barker, 1990, 1994, 1995, 1998, 1999a,b, 2000; Barker and Martyn, 1992; Fall and Barker, 1997; Osmond and Barker, 2000). In this context, new evidence from epidemiological and clinical studies have showed the association of the maternal under- and overnutrition with development of cardiometabolic dysfuntions (Ashton, 2000; Hemachandra et al., 2006; Antony and Laxmaiah, 2008; Conde and Monteiro, 2014; Costa-Silva et al., 2015; Parra et al., 2015). Thus, this review will address the new concepts about the involvement of the maternal protein malnutrition and overnutrition on the development of the cardiometabolic diseases.
Perinatal origin of cardiometabolic diseases: the role of phenotypic plasticity
Biological and medical consequences of perinatal nutritional factors have been extensively studied in the field of the “developmental origins of health and diseases” proposed by Barker and colleagues since 1986 (Barker and Osmond, 1986; Barker et al., 1989, 1993; Barker, 2007). This field of research proposes that cardiometabolic diseases can be “programmed” by the “adaptative” effects of both under- and overnutrition during early phases of growth and development on the cell physiology (Barker and Osmond, 1986; Hales and Barker, 1992; Alfaradhi and Ozanne, 2011; Chavatte-Palmer et al., 2016). As stated before, it aims to study how an organism reacts to a different environmental input, such as malnutrition, and induces changes in the phenotype, but without altering the genotype (Barker et al., 2005; West-Eberhard, 2005a; Labayen et al., 2006; Andersen et al., 2009; Biosca et al., 2011). In this context, epigenetic alterations, such as DNA methylation, histone acetylation, and microRNA expression are considered the molecular basis of the phenotypic plasticity (Wells, 2011). These modifications termed as “epigenetic” were firstly described by Conrad Waddigton in 1940 and it studies the relationship between cause and effect in the genes to produce a phenotype (Jablonka and Lamb, 2002). Nowadays, this concept is employed to describe the process of the gene expression and its linking to modifications in the cromatin structure without altering DNA sequence (Chong and Whitelaw, 2004; Egger et al., 2004). Among all epigenetic modifications, the DNA methylation is one that has been best studied and is related to addition of methyl groups on DNA cytosine residues, normally on the cytosine followed by guanine residue (CpG dinucleotides), which can produce inhibition of the gene expression by impairing transcriptional factor binding (Waterland and Michels, 2007; Mansego et al., 2013; Chango and Pogribny, 2015; Mitchell et al., 2016). In this context, it has been investigated how nutritional aspect may induce these epigenetic modifications.
Macro- and micro-nutrient compositions have been identified as important nutritional factors inducing epigenetic processes, such as DNA methylation (Mazzio and Soliman, 2014; Szarc vel Szic et al., 2015). It is considered at least three ways by which nutrients can induce DNA methylation, alter gene expression, and modify cellular phenotype: (i) by providing methyl group supply for inducing S- adenosyl-L-methionine formation (genomic DNA methylation), modifying the methyltransferase activity, or impairing DNA demethylation process; (ii) by modifying chromatin remodeling, or lysine and arginine residues in the N-terminal histone tails; and (iii) by altering microRNA expression (Chong and Whitelaw, 2004; Egger et al., 2004; Hardy and Tollefsbol, 2011; Stone et al., 2011). In this context, altered contents of amino acids, such as methionine and cysteine, as well as reduced choline and folate diet amount can modify the process of the DNA methylation leading to both DNA hyper- and hypomethylation (Fiorito et al., 2014). For example, deficiency of choline can precipitate DNA hypermethylation associated with organ dysfunction, mainly in liver metabolism (Karlic and Varga, 2011; Wei, 2013).
High fat diet (HFD) during perinatal period has been identified as risk factor to predispose and induce epigenetic processes in the parents and their offspring (Mazzio and Soliman, 2014; Szarc vel Szic et al., 2015). Both hypo- and hypermethylation processes participate in this dysregulation attributed to HFD consumption (Ng et al., 2010; Milagro et al., 2013). In adipose tissue, for example, it was observed that gene promoter of the fatty acid synthase enzyme suffered methylation (Lomba et al., 2010) and that important obesity-related genes such as leptin have disruption on their methylation status (Milagro et al., 2009).
Maternal protein undernutrition: early- and long-term outcomes
Maternal malnutrition is associated with the risk of developing cardiovascular disease and co-morbidities in offspring's later life including hypertension, metabolic syndrome, and type-II diabetes (Barker et al., 2007; Nuyt, 2008; Nuyt and Alexander, 2009). In humans, studies have provided support for the positive association between low birth weight and increased incidence of hypertension (Ravelli et al., 1976; Hales et al., 1991; Sawaya and Roberts, 2003; Sawaya et al., 2004).
Maternal low-protein diet model during both gestation and lactation is one of the most extensively studied animal models of phenotypic plasticity (Ozanne and Hales, 2004; Costa-Silva et al., 2009; Falcão-Tebas et al., 2012; Fidalgo et al., 2013; de Brito Alves et al., 2014; Barros et al., 2015). Feeding a low-protein diet (8% protein) during gestation and lactation is associated with growth restriction, asymmetric reduction in organ growth, elevated systolic blood pressure, dyslipidemia, and increased fasting plasma insulin concentrations in the most of studies in rodents (Ozanne and Hales, 2004; Costa-Silva et al., 2009; Falcão-Tebas et al., 2012; Fidalgo et al., 2013; Leandro et al., 2012; de Brito Alves et al., 2014, 2016; Ferreira et al., 2015; Paulino-Silva and Costa-Silva, 2016). However, it is known that the magnitude of the cardiovascular and metabolic outcomes are dependent on the both time exposure to protein restricted-diet (Zohdi et al., 2012, 2015) and growth trajectory throughout the postnatal period (Wells, 2007, 2011). A rapid and increased catch-up growth and childhood weight gain appear to augment metabolic disruption in end organs, for example liver (Tarry-Adkins et al., 2016; Wang et al., 2016).
Although, the relationship between maternal protein restriction, sympathetic overactivity and hypertension have been suggested (Johansson et al., 2007; Franco et al., 2008; Barros et al., 2015), few studies have described the physiological dysfunctions responsible for producing these effects. Nowadays, it is well accepted that perinatal protein malnutrition raise risks of hypertension by mechanisms that include abnormal vascular function (Franco Mdo et al., 2002; Brawley et al., 2003; Franco et al., 2008), altered nephron morphology and function, and stimulation of the renin-angiotensin system (RAS) (Nuyt and Alexander, 2009; Siddique et al., 2014). Recently, studies have highlighted contribution of the sympathetic overactivity associated to enhanced respiratory rhythm and O2/CO2 sensitivity on the development of the maternal low-protein diet-induced hypertension by mechanisms independent of the baroreflex function (Chen et al., 2010; Barros et al., 2015; Costa-Silva et al., 2015; de Brito Alves et al., 2015; Paulino-Silva and Costa-Silva, 2016). Offspring from dams subjected to perinatal protein restriction had relevant short-term effects on the carotid body (CB) sensitivity and respiratory control. With enhanced baseline sympathetic activity and amplified ventilatory and sympathetic responses to peripheral chemoreflex activation, prior to the establishment of hypertension (de Brito Alves et al., 2014, 2015). The underlying mechanism involved in these effects seems to be linked with up-regulation of hypoxic inducible factor (HIF-1α) in CB peripheral chemoreceptors (Ito et al., 2011, 2012; de Brito Alves et al., 2015). However, the epigenetic mechanisms in these effects are still unclear. It is hypothesized that epigenetic mechanism produced by DNA methylation could be involved (Altobelli et al., 2013; Prabhakar, 2013; Nanduri and Prabhakar, 2015).
The central nervous system (CNS) compared to other organ systems has increased vulnerability to reactive oxygen species (ROS). ROS are known to modulate the sympathetic activity and their increased production in key brainstem sites is involved in the etiology of several cardiovascular diseases, for example, diseases caused by sympathetic overexcitation, such as neurogenic hypertension (Chan et al., 2006; Essick and Sam, 2010). Ferreira and colleagues showed that perinatal protein undernutrition increased lipid peroxidation and decreased the activity of several antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activities) as well as elements of the GSH system, in adult brainstem. Dysfunction in the brainstem oxidative metabolism, using the same experimental model, were observed in rats immediately after weaning associated to the increase in ROS production, with a decrease in antioxidant defense and redox status (Ferreira et al., 2015, 2016). Related to the metabolic effects on the heart, it was observed that these animals showed decreased mitochondrial oxidative phosphorylation capacity and increased ROS in the myocardium. In addition, maternal low-protein diet induced a significant decrease in enzymatic antioxidant capacity (superoxide dismutase, catalase, glutathione-S-transferase, and glutathione reductase activities) and glutathione level when compared with normoprotein group (Nascimento et al., 2014).
Regarding hepatic metabolism, studies showed that protein restricted rats had suppressed gluconeogenesis by a mechanism primarily mediated by decrease on the mRNA level of hepatic phosphoenolpyruvate carboxykinase, a key gluconeogenic enzyme, and enhancement of the insulin signals through the insulin receptor (IR)/IR substrate (IRS)/phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin complex 1 (mTOR) pathway in the liver (Toyoshima et al., 2010). In relation to lipid metabolism, there was decreased liver triglyceride content in adult rats exposed to protein restriction during gestation and lactation. It was suggested that this effect could be due to increased fatty-acid transport into the mitochondrial matrix or alterations in triglyceride biosynthesis (Qasem et al., 2015). A maternal protein restriction was shown to reduce the lean and increase the fat contents of 6-month old offspring with a tendency for reduced number of muscle myofibers associated with reduced expression of mRNA of Insulin-like growth factor 2 gene (IGF2 mRNA) in pigs (Chavatte-Palmer et al., 2016).
Maternal overnutrition and risk factor for the cardiometabolic dysfuntions
Nutritional transition is a phenomenon well documented in developing countries in the twentieth and twenty-first centuries, and has induced high incidence of the chronic diseases and high prevalence of the obesity (Batista Filho and Rissin, 2003; Batista Filho and Batista, 2010; Ribeiro et al., 2015). It is evident that protein malnutrition was an health problem in the first half of the twentieth century. Now, it was replaced by a diet enriched in saturated fat or other HFDs, predisposing to overweight, and obesity (Batista et al., 2013). Nowadays, it suggested that two billion people in the world are overweight and obese individuals, with major prevalence is related to diet induced-obesity, which have been associated to cardiovascular and endocrine dysfunctions (Hotamisligil, 2006; Aubin et al., 2008; Zhang et al., 2012; Ng et al., 2014; Wensveen et al., 2015).
Recently, the obesity has been considered a physiological state of chronic inflammation, characterized by elevated levels of inflammatory markers including C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) (Wensveen et al., 2015; Erikci Ertunc and Hotamisligil, 2016; Lyons et al., 2016). Maternal HFD chronic consumption enhances the circulating free fatty acids and induce the activation of inflammatory pathways, enhancing chronic inflammation in offspring (Gruber et al., 2015). Studies of Roberts et al. (2015) found that cardiometabolic dysfunction was associated with changes such as elevated serum triglycerides, elevated oxidative stress levels, insulin resistance, vascular disorders, and development of hypertension (Roberts et al., 2015).
In animals on a HFD the hormone leptin has been considered one of the most important physiological mediators of the cardiometabolic dysfunction (Correia and Rahmouni, 2006; Harlan et al., 2013; Harlan and Rahmouni, 2013). Since hyperleptinemia, common in overweight and obesity conditions, produce a misbalance in autonomic system, with sympathetic overactivation (Machleidt et al., 2013; Kurajoh et al., 2015; Manna and Jain, 2015), and reduced sensitivity of vagal afferent neurons (de Lartigue, 2016). This disorder of vagal afferent signaling can activate orexigenic pathways in the CNS and drive hyperphagia, obesity, and cardiometabolic diseases at long-term (de Lartigue, 2016). Some authors have described that, at least in part, cardiovascular dysfuntion elicited by HFD or obesity may be due to changes in the neural control of respiratory and autonomic systems (Bassi et al., 2012, 2015; Hall et al., 2015; Chaar et al., 2016). Part of these effects were suggested to be influenced by atrial natriuretric peptide and renin-angiotensin pathways (Bassi et al., 2012; Gusmão, 2012).
Interestingly, it has been shown that offspring from mothers fed HFD have high risk to develop pathologic cardiac hypertrophy. This condition would be linked to re-expression of cardiac fetal genes, systolic, and diastolic dysfunction and sympathetic overactivity on the heart. These effects lead to reduced cardioprotective signaling that would predispose them to cardiac dysfunctions in adulthood (Taylor et al., 2005; Wang et al., 2010; Fernandez-Twinn et al., 2012; Blackmore et al., 2014). Regarding arterial blood pressure control, it has been described that maternal HFD induces early and persistent alterations in offspring renal and adipose RAS components (Armitage et al., 2005). These changes seem to be dependent upon the period of exposure to the maternal HFD, and contribute to increased adiposity and hypertension in offspring (Samuelsson et al., 2008; Elahi et al., 2009; Guberman et al., 2013; Mazzio and Soliman, 2014; Tan et al., 2015). Studies in baboons subjected to HFD showed that microRNA expression and putative gene targets involved in developmental disorders and cardiovascular diseases were up-regulated and others were down-regulated. The authors suggested that the epigenetic modifications caused by HFD may be involved in the developmental origins of cardiometabolic diseases (Maloyan et al., 2013).
Other metabolic outcomes induced by HFD have been pointed out in the last years and it has demonstrated that HFD displayed a drastic modification on metabolic control of the glucose metabolism and lead to increased insulin level in serum (Fan et al., 2013) and enhanced insulin action through AKT/PKB (protein kinase B) and ERK (extracellular signal-regulated kinase), and activation of mammalian target of rapamycin (mTOR) pathways in cardiac tissue (Fernandez-Twinn et al., 2012; Fan et al., 2013). Offspring from HFD mothers showed alterations in blood glucose and insulin levels, with high predisposition to insulin resistance and cardiac dysfunction (Taylor et al., 2005; Wang et al., 2010). Part of these effects are associated with enhanced production of ROS and reduction in the levels of the anti-oxidant enzymes, such as superoxide dismutase, suggesting a misbalance in the control of the oxidative stress (Fernandez-Twinn et al., 2012).
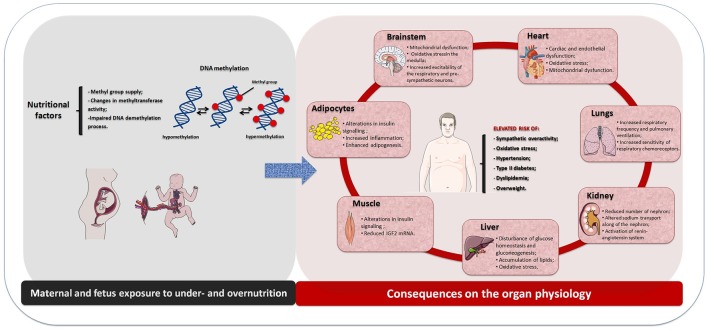
Altogether, this review addressed the new concept on the maternal diet induced-cardiometabolic diseases that include the potential role of the perinatal malnutrition. It showed that the etiology of these diseases is multifactorial involving genetic and environmental influences and their physiological integration. It is well recognized that both perinatal undernutrition and overnutrition are related with the risk of developing metabolic syndrome and hypertension in adult life (Figure 1). The underlying mechanism can be explained in the context of phenotypic plasticity during development that includes adaptive change on the CNS, heart, kidney, liver, muscle, and adipose tissue metabolisms with consequent physiology dysfunction and with subsequent cardiometabolic diseases. Moreover, maternal undernutrition or overnutrition may predispose epigenetic modifications in dams and their offspring, with predominance of DNA methylation, leading to altered gene expression during development and growth. Further, it can provide a different physiological condition which may contribute to the developmental origins of the cardiometabolic diseases. These physiological dysfunctions seem to be linked to the impaired central and peripheral control of both metabolic and cardiovascular functions by mechanisms that include enhanced sympathetic-respiratory activities and disruption in metabolism of end organs at early life. It is suggested that those effects could be associated to inflammatory conditions and impaired oxidative balance, which may contribute to adult cardiometabolic diseases.
Figure 1.
Schematic drawing showing the physiological effects induced by maternal and fetus exposure to under- or overnutrition through DNA methylation and their consequences on the organ physiology and increased risk of the cardiometabolic diseases in the offspring.
Author contributions
JC, AS, and MF drafted and revised critically the work for important intellectual content and final review of the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- AKT/PKB
Protein kinase B
- CB
Carotid body
- CNS
Central nervous system
- CRP
C-reactive protein
- ERK
Extracellular signal-regulated kinase
- GSH
Glutathione reduced
- HFD
High fat diet
- HIF-1α
Hypoxic inducible factor 1 alpha
- IGF2
Insulin-like growth factor 2
- IL-6
Interleukin-6
- IR
Insulin receptor
- IRS
Insulin receptor substrate
- mTOR
Mammalian target of rapamycin
- PI3K
Phosphatidylinositol 3-kinase
- RAS
Renin-angiotensin system
- ROS
Reactive oxygen species
- TNF-α
Tumor necrosis factor alpha.
References
- Alfaradhi M. Z., Ozanne S. E. (2011). Developmental programming in response to maternal overnutrition. Front. Genet. 2:27. 10.3389/fgene.2011.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altobelli G., Bogdarina I. G., Stupka E., Clark A. J., Langley-Evans S. (2013). Genome-wide methylation and gene expression changes in newborn rats following maternal protein restriction and reversal by folic acid. PLoS ONE 8:e82989. 10.1371/journal.pone.0082989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen L. G., Angquist L., Gamborg M., Byberg L., Bengtsson C., Canoy D., et al. (2009). Birth weight in relation to leisure time physical activity in adolescence and adulthood: meta-analysis of results from 13 nordic cohorts. PLoS ONE 4:e8192. 10.1371/journal.pone.0008192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony G. M., Laxmaiah A. (2008). Human development, poverty, health & nutrition situation in India. Indian J. Med. Res. 128, 198–205. [PubMed] [Google Scholar]
- Armitage J. A., Lakasing L., Taylor P. D., Balachandran A. A., Jensen R. I., Dekou V., et al. (2005). Developmental programming of aortic and renal structure in offspring of rats fed fat-rich diets in pregnancy. J. Physiol. 565(Pt 1), 171–184. 10.1113/jphysiol.2005.084947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton N. (2000). Perinatal development and adult blood pressure. Braz. J. Med. Biol. Res. 33, 731–740. 10.1590/S0100-879X2000000700002 [DOI] [PubMed] [Google Scholar]
- Aubin M. C., Lajoie C., Clément R., Gosselin H., Calderone A., Perrault L. P. (2008). Female rats fed a high-fat diet were associated with vascular dysfunction and cardiac fibrosis in the absence of overt obesity and hyperlipidemia: therapeutic potential of resveratrol. J. Pharmacol. Exp. Ther. 325, 961–968. 10.1124/jpet.107.135061 [DOI] [PubMed] [Google Scholar]
- Barker D. J. (1990). The fetal and infant origins of adult disease. BMJ 301:1111. 10.1136/bmj.301.6761.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J. (1994). Maternal and fetal origins of coronary heart disease. J. R. Coll. Physicians Lond. 28, 544–551. [PMC free article] [PubMed] [Google Scholar]
- Barker D. J. (1995). The wellcome foundation lecture, 1994. The fetal origins of adult disease. Proc. Biol. Sci. 262, 37–43. 10.1098/rspb.1995.0173 [DOI] [PubMed] [Google Scholar]
- Barker D. J. (1998). In utero programming of chronic disease. Clin. Sci. 95, 115–128. 10.1042/cs0950115 [DOI] [PubMed] [Google Scholar]
- Barker D. J. (1999a). Fetal origins of cardiovascular disease. Ann. Med. 31(Suppl. 1), 3–6. [PubMed] [Google Scholar]
- Barker D. J. (1999b). The long-term outcome of retarded fetal growth. Schweiz. Med. Wochenschr. 129, 189–196. [PubMed] [Google Scholar]
- Barker D. J. (2000). In utero programming of cardiovascular disease. Theriogenology 53, 555–574. 10.1016/S0093-691X(99)00258-7 [DOI] [PubMed] [Google Scholar]
- Barker D. J. (2007). The origins of the developmental origins theory. J. Intern. Med. 261, 412–417. 10.1111/j.1365-2796.2007.01809.x [DOI] [PubMed] [Google Scholar]
- Barker D. J., Gluckman P. D., Godfrey K. M., Harding J. E., Owens J. A., Robinson J. S. (1993). Fetal nutrition and cardiovascular disease in adult life. Lancet 341, 938–941. 10.1016/0140-6736(93)91224-A [DOI] [PubMed] [Google Scholar]
- Barker D. J., Martyn C. N. (1992). The maternal and fetal origins of cardiovascular disease. J. Epidemiol. Commun. Health 46, 8–11. 10.1136/jech.46.1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J., Osmond C. (1986). Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1, 1077–1081. 10.1016/S0140-6736(86)91340-1 [DOI] [PubMed] [Google Scholar]
- Barker D. J., Osmond C., Forsén T. J., Kajantie E., Eriksson J. G. (2005). Trajectories of growth among children who have coronary events as adults. N. Engl. J. Med. 353, 1802–1809. 10.1056/NEJMoa044160 [DOI] [PubMed] [Google Scholar]
- Barker D. J., Osmond C., Forsen T. J., Kajantie E., Eriksson J. G. (2007). Maternal and social origins of hypertension. Hypertension. 50, 565–571. 10.1161/HYPERTENSIONAHA.107.091512 [DOI] [PubMed] [Google Scholar]
- Barker D. J., Winter P. D., Osmond C., Margetts B., Simmonds S. J. (1989). Weight in infancy and death from ischaemic heart disease. Lancet 2, 577–580. 10.1016/S0140-6736(89)90710-1 [DOI] [PubMed] [Google Scholar]
- Barros M. A., De Brito Alves J. L., Nogueira V. O., Wanderley A. G., Costa-Silva J. H. (2015). Maternal low-protein diet induces changes in the cardiovascular autonomic modulation in male rat offspring. Nutr. Metab. Cardiovasc. Dis. 25, 123–130. 10.1016/j.numecd.2014.07.011 [DOI] [PubMed] [Google Scholar]
- Bassi M., Giusti H., Leite C. M., Anselmo-Franci J. A., do Carmo J. M., da Silva A. A., et al. (2012). Central leptin replacement enhances chemorespiratory responses in leptin-deficient mice independent of changes in body weight. Pflugers Arch. 464, 145–153. 10.1007/s00424-012-1111-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi M., Nakamura N. B., Furuya W. I., Colombari D. S., Menani J. V., do Carmo J. M., et al. (2015). Activation of the brain melanocortin system is required for leptin-induced modulation of chemorespiratory function. Acta Physiol. (Oxf). 213, 893–901. 10.1111/apha.12394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista T. M., Ribeiro R. A., da Silva P. M., Camargo R. L., Lollo P. C., Boschero A. C., et al. (2013). Taurine supplementation improves liver glucose control in normal protein and malnourished mice fed a high-fat diet. Mol. Nutr. Food Res. 57, 423–434. 10.1002/mnfr.201200345 [DOI] [PubMed] [Google Scholar]
- Batista Filho M., Batista L. V. (2010). Transição alimentar/nutricional ou mutação antropológica? Ciênc. Cult. 62, 26–30. [Google Scholar]
- Batista Filho M., Rissin A. (2003). A transição nutricional no Brasil: tendências regionais e temporais. Cad. Saúde Pública 19, S181–S191. 10.1590/S0102-311X2003000700019 [DOI] [PubMed] [Google Scholar]
- Biosca M., Rodríguez G., Ventura P., Samper M. P., Labayen I., Collado M. P., et al. (2011). Central adiposity in children born small and large for gestational age. Nutr. Hosp. 26, 971–976. 10.1590/S0212-16112011000500008 [DOI] [PubMed] [Google Scholar]
- Blackmore H. L., Niu Y., Fernandez-Twinn D. S., Tarry-Adkins J. L., Giussani D. A., Ozanne S. E. (2014). Maternal diet-induced obesity programs cardiovascular dysfunction in adult male mouse offspring independent of current body weight. Endocrinology 155, 3970–3980. 10.1210/en.2014-1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley L., Itoh S., Torrens C., Barker A., Bertram C., Poston L., et al. (2003). Dietary protein restriction in pregnancy induces hypertension and vascular defects in rat male offspring. Pediatr. Res. 54, 83–90. 10.1203/01.PDR.0000065731.00639.02 [DOI] [PubMed] [Google Scholar]
- Chaar L. J., Coelho A., Silva N. M., Festuccia W. L., Antunes V. R. (2016). High-fat diet-induced hypertension and autonomic imbalance are associated with an upregulation of CART in the dorsomedial hypothalamus of mice. Physiol. Rep. 4:e12811. 10.14814/phy2.12811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. H., Tai M. H., Li C. Y., Chan J. Y. (2006). Reduction in molecular synthesis or enzyme activity of superoxide dismutases and catalase contributes to oxidative stress and neurogenic hypertension in spontaneously hypertensive rats. Free Radic. Biol. Med. 40, 2028–2039. 10.1016/j.freeradbiomed.2006.01.032 [DOI] [PubMed] [Google Scholar]
- Chango A., Pogribny I. P. (2015). Considering maternal dietary modulators for epigenetic regulation and programming of the fetal epigenome. Nutrients 7, 2748–2770. 10.3390/nu7042748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavatte-Palmer P., Tarrade A., Rousseau-Ralliard D. (2016). Diet before and during pregnancy and offspring health: the importance of animal models and what can be learned from them. Int. J. Environ. Res. Public Health 13:E586. 10.3390/ijerph13060586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H., Tarry-Adkins J. L., Matharu K., Yeo G. S., Ozanne S. E. (2010). Maternal protein restriction affects gene expression profiles in the kidney at weaning with implications for the regulation of renal function and lifespan. Clin. Sci. 119, 373–384. 10.1042/CS20100230 [DOI] [PubMed] [Google Scholar]
- Chong S., Whitelaw E. (2004). Epigenetic germline inheritance. Curr. Opin. Genet. Dev. 14, 692–696. 10.1016/j.gde.2004.09.001 [DOI] [PubMed] [Google Scholar]
- Conde W. L., Monteiro C. A. (2014). Nutrition transition and double burden of undernutrition and excess of weight in Brazil. Am. J. Clin. Nutr. 100, 1617S–1622S. 10.3945/ajcn.114.084764 [DOI] [PubMed] [Google Scholar]
- Correia M. L., Rahmouni K. (2006). Role of leptin in the cardiovascular and endocrine complications of metabolic syndrome. Diabetes Obes. Metab. 8, 603–610. 10.1111/j.1463-1326.2005.00562.x [DOI] [PubMed] [Google Scholar]
- Costa-Silva J. H., Silva P. A., Pedi N., Luzardo R., Einicker-Lamas M., Lara L. S., et al. (2009). Chronic undernutrition alters renal active Na+ transport in young rats: potential hidden basis for pathophysiological alterations in adulthood? Eur. J. Nutr. 48, 437–445. 10.1007/s00394-009-0032-z [DOI] [PubMed] [Google Scholar]
- Costa-Silva J. H., de Brito-Alves J. L., Barros M. A., Nogueira V. O., Paulino-Silva K. M., de Oliveira-Lira A., et al. (2015). New insights on the maternal diet induced-hypertension: potential role of the phenotypic plasticity and sympathetic-respiratory overactivity. Front. Physiol. 6:345. 10.3389/fphys.2015.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito Alves J. L., de Oliveira J. M., Ferreira D. J., de Barros M. A., Nogueira V. O., Alves D. S., et al. (2016). Maternal protein restriction induced-hypertension is associated to oxidative disruption at transcriptional and functional levels in the medulla oblongata. Clin. Exp. Pharmacol. Physiol. [Epub ahead of print]. 10.1111/1440-1681.12667. [DOI] [PubMed] [Google Scholar]
- de Brito Alves J. L., Nogueira V. O., Cavalcanti Neto M. P., Leopoldino A. M., Curti C., Colombari D. S., et al. (2015). Maternal protein restriction increases respiratory and sympathetic activities and sensitizes peripheral chemoreflex in male rat offspring. J. Nutr. 145, 907–914. 10.3945/jn.114.202804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito Alves J. L., Nogueira V. O., de Oliveira G. B., da Silva G. S., Wanderley A. G., Leandro C. G., et al. (2014). Short- and long-term effects of a maternal low-protein diet on ventilation, O2/CO2 chemoreception and arterial blood pressure in male rat offspring. Br. J. Nutr. 111, 606–615. 10.1017/S0007114513002833 [DOI] [PubMed] [Google Scholar]
- de Lartigue G. (2016). Role of the vagus nerve in the development and treatment of diet-induced obesity. J. Physiol. 594, 5791–5815. 10.1113/JP271538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger G., Liang G., Aparicio A., Jones P. A. (2004). Epigenetics in human disease and prospects for epigenetic therapy. Nature 429, 457–463. 10.1038/nature02625 [DOI] [PubMed] [Google Scholar]
- Elahi M. M., Cagampang F. R., Mukhtar D., Anthony F. W., Ohri S. K., Hanson M. A. (2009). Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br. J. Nutr. 102, 514–519. 10.1017/S000711450820749X [DOI] [PubMed] [Google Scholar]
- Erikci Ertunc M., Hotamisligil G. S. (2016). Lipid signaling and lipotoxicity in metabolic inflammation: indications for metabolic disease pathogenesis and treatment. J. Lipid Res. [Epub ahead of print]. 10.1194/jlr.r066514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essick E. E., Sam F. (2010). Oxidative stress and autophagy in cardiac disease, neurological disorders, aging and cancer. Oxid. Med. Cell Longev. 3, 168–177. 10.4161/oxim.3.3.12106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcão-Tebas F., Bento-Santos A., Fidalgo M. A., de Almeida M. B., dos Santos J. A., Lopes de Souza S., et al. (2012). Maternal low-protein diet-induced delayed reflex ontogeny is attenuated by moderate physical training during gestation in rats. Br. J. Nutr. 107, 372–377. 10.1017/S0007114511002947 [DOI] [PubMed] [Google Scholar]
- Fall C. H., Barker D. J. (1997). The fetal origins of coronary heart disease and non-insulin dependent diabetes in India. Indian Pediatr. 34, 5–8. [PubMed] [Google Scholar]
- Fan L., Lindsley S. R., Comstock S. M., Takahashi D. L., Evans A. E., He G. W., et al. (2013). Maternal high-fat diet impacts endothelial function in nonhuman primate offspring. Int. J. Obes. (Lond). 37, 254–262. 10.1038/ijo.2012.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Twinn D. S., Blackmore H. L., Siggens L., Giussani D. A., Cross C. M., Foo R., et al. (2012). The programming of cardiac hypertrophy in the offspring by maternal obesity is associated with hyperinsulinemia, AKT, ERK, and mTOR activation. Endocrinology 153, 5961–5971. 10.1210/en.2012-1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira D. J., da Silva Pedroza A. A., Braz G. R., da Silva-Filho R. C., Lima T. A., Fernandes M. P., et al. (2016). Mitochondrial bioenergetics and oxidative status disruption in brainstem of weaned rats: immediate response to maternal protein restriction. Brain Res. 1642, 553–561. 10.1016/j.brainres.2016.04.049 [DOI] [PubMed] [Google Scholar]
- Ferreira D. S., Liu Y., Fernandes M. P., Lagranha C. J. (2015). Perinatal low-protein diet alters brainstem antioxidant metabolism in adult offspring. Nutr. Neurosci. 19, 369–375. 10.1179/1476830515Y.0000000030 [DOI] [PubMed] [Google Scholar]
- Fidalgo M., Falcão-Tebas F., Bento-Santos A., de Oliveira E., Nogueira-Neto J. F., de Moura E. G., et al. (2013). Programmed changes in the adult rat offspring caused by maternal protein restriction during gestation and lactation are attenuated by maternal moderate-low physical training. Br. J. Nutr. 109, 449–456. 10.1017/S0007114512001316 [DOI] [PubMed] [Google Scholar]
- Fiorito G., Guarrera S., Valle C., Ricceri F., Russo A., Grioni S., et al. (2014). B-vitamins intake, DNA-methylation of One Carbon Metabolism and homocysteine pathway genes and myocardial infarction risk: the EPICOR study. Nutr. Metab. Cardiovasc. Dis. 24, 483–488. 10.1016/j.numecd.2013.10.026 [DOI] [PubMed] [Google Scholar]
- Franco M. C., Casarini D. E., Carneiro-Ramos M. S., Sawaya A. L., Barreto-Chaves M. L., Sesso R. (2008). Circulating renin-angiotensin system and catecholamines in childhood: is there a role for birthweight? Clin. Sci. 114, 375–380. 10.1042/CS20070284 [DOI] [PubMed] [Google Scholar]
- Franco Mdo C., Arruda R. M., Dantas A. P., Kawamoto E. M., Fortes Z. B., Scavone C., et al. (2002). Intrauterine undernutrition: expression and activity of the endothelial nitric oxide synthase in male and female adult offspring. Cardiovasc. Res. 56, 145–153. 10.1016/S0008-6363(02)00508-4 [DOI] [PubMed] [Google Scholar]
- Gruber L., Hemmerling J., Schüppel V., Müller M., Boekschoten M. V., Haller D. (2015). Maternal high-fat diet accelerates development of crohn's disease-like ileitis in TNFDeltaARE/WT offspring. Inflamm. Bowel Dis. 21, 2016–2025. 10.1097/MIB.0000000000000465 [DOI] [PubMed] [Google Scholar]
- Guberman C., Jellyman J. K., Han G., Ross M. G., Desai M. (2013). Maternal high-fat diet programs rat offspring hypertension and activates the adipose renin-angiotensin system. Am. J. Obstet. Gynecol. 209, 262.e1–262.e18. 10.1016/j.ajog.2013.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusmão D. O. (2012). Relação entre Leptina, Peptídeo Natriurético Atrial e Estrógeno em um Modelo Animal de Hipertensão Associada a Obesidade. Universidade Federal da Bahia. [Google Scholar]
- Hales C. N., Barker D. J. (1992). Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35, 595–601. 10.1007/BF00400248 [DOI] [PubMed] [Google Scholar]
- Hales C. N., Barker D. J., Clark P. M., Cox L. J., Fall C., Osmond C., et al. (1991). Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303, 1019–1022. 10.1136/bmj.303.6809.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. E., do Carmo J. M., da Silva A. A., Wang Z., Hall M. E. (2015). Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ. Res. 116, 991–1006. 10.1161/CIRCRESAHA.116.305697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy T. M., Tollefsbol T. O. (2011). Epigenetic diet: impact on the epigenome and cancer. Epigenomics 3, 503–518. 10.2217/epi.11.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan S. M., Guo D. F., Morgan D. A., Fernandes-Santos C., Rahmouni K. (2013). Hypothalamic mTORC1 signaling controls sympathetic nerve activity and arterial pressure and mediates leptin effects. Cell Metab. 17, 599–606. 10.1016/j.cmet.2013.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan S. M., Rahmouni K. (2013). Neuroanatomical determinants of the sympathetic nerve responses evoked by leptin. Clin. Auton. Res. 23, 1–7. 10.1007/s10286-012-0168-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemachandra A. H., Klebanoff M. A., Duggan A. K., Hardy J. B., Furth S. L. (2006). The association between intrauterine growth restriction in the full-term infant and high blood pressure at age 7 years: results from the Collaborative Perinatal Project. Int. J. Epidemiol. 35, 871–877. 10.1093/ije/dyl080 [DOI] [PubMed] [Google Scholar]
- Hotamisligil G. S. (2006). Inflammation and metabolic disorders. Nature 444, 860–867. 10.1038/nature05485 [DOI] [PubMed] [Google Scholar]
- Ito T., Funamoto K., Sato N., Nakamura A., Tanabe K., Hoshiai T., et al. (2012). Maternal undernutrition induces the expression of hypoxia-related genes in the fetal brain. Tohoku J. Exp. Med. 226, 37–44. 10.1620/tjem.226.37 [DOI] [PubMed] [Google Scholar]
- Ito T., Tanabe K., Nakamura A., Funamoto K., Aoyagi A., Sato K., et al. (2011). Aberrant expression of hypoxia-inducible factor 1alpha in the fetal heart is associated with maternal undernutrition. Tohoku J. Exp. Med. 224, 163–171. 10.1620/tjem.224.163 [DOI] [PubMed] [Google Scholar]
- Jablonka E., Lamb M. J. (2002). The changing concept of epigenetics. Ann. N.Y. Acad. Sci. 981, 82–96. 10.1111/j.1749-6632.2002.tb04913.x [DOI] [PubMed] [Google Scholar]
- Johansson S., Norman M., Legnevall L., Dalmaz Y., Lagercrantz H., Vanpée M. (2007). Increased catecholamines and heart rate in children with low birth weight: perinatal contributions to sympathoadrenal overactivity. J. Intern. Med. 261, 480–487. 10.1111/j.1365-2796.2007.01776.x [DOI] [PubMed] [Google Scholar]
- Karlic H., Varga F. (2011). Impact of vitamin D metabolism on clinical epigenetics. Clin. Epigenet. 2, 55–61. 10.1007/s13148-011-0021-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurajoh M., Koyama H., Kadoya M., Naka M., Miyoshi A., Kanzaki A., et al. (2015). Plasma leptin level is associated with cardiac autonomic dysfunction in patients with type 2 diabetes: HSCAA study. Cardiovasc. Diabetol. 14, 117. 10.1186/s12933-015-0280-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labayen I., Moreno L. A., Blay M. G., Blay V. A., Mesana M. I., González-Gross M., et al. (2006). Early programming of body composition and fat distribution in adolescents. J. Nutr. 136, 147–152. [DOI] [PubMed] [Google Scholar]
- Landsberg L., Aronne L. J., Beilin L. J., Burke V., Igel L. I., Lloyd-Jones D., et al. (2013). Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment–a position paper of the obesity society and The American Society of Hypertension. Obesity (Silver Spring) 21, 8–24. 10.1002/oby.20181 [DOI] [PubMed] [Google Scholar]
- Leandro C. G., da Silva Ribeiro W., Dos Santos J. A., Bento-Santos A., Lima-Coelho C. H., Falcão-Tebas F., et al. (2012). Moderate physical training attenuates muscle-specific effects on fibre type composition in adult rats submitted to a perinatal maternal low-protein diet. Eur. J. Nutr. 51, 807–815. 10.1007/s00394-011-0259-3 [DOI] [PubMed] [Google Scholar]
- Lomba A., Martínez J. A., García-Díaz D. F., Paternain L., Marti A., Campión J., et al. (2010). Weight gain induced by an isocaloric pair-fed high fat diet: a nutriepigenetic study on FASN and NDUFB6 gene promoters. Mol. Genet. Metab. 101, 273–278. 10.1016/j.ymgme.2010.07.017 [DOI] [PubMed] [Google Scholar]
- Lucas A. (1998). Programming by early nutrition: an experimental approach. J. Nutr. 128(2 Suppl.), 401S–406S. [DOI] [PubMed] [Google Scholar]
- Lyons C. L., Kennedy E. B., Roche H. M. (2016). Metabolic inflammation-differential modulation by dietary constituents. Nutrients 8:E247. 10.3390/nu8050247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machleidt F., Simon P., Krapalis A. F., Hallschmid M., Lehnert H., Sayk F. (2013). Experimental hyperleptinemia acutely increases vasoconstrictory sympathetic nerve activity in healthy humans. J. Clin. Endocrinol. Metab. 98, E491–E496. 10.1210/jc.2012-3009 [DOI] [PubMed] [Google Scholar]
- Maloyan A., Muralimanoharan S., Huffman S., Cox L. A., Nathanielsz P. W., Myatt L., et al. (2013). Identification and comparative analyses of myocardial miRNAs involved in the fetal response to maternal obesity. Physiol. Genomics 45, 889–900. 10.1152/physiolgenomics.00050.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna P., Jain S. K. (2015). Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 13, 423–444. 10.1089/met.2015.0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansego M. L., Milagro F. I., Campión J., Martínez J. A. (2013). Techniques of DNA methylation analysis with nutritional applications. J. Nutrigenet. Nutrigenomics 6, 83–96. 10.1159/000350749 [DOI] [PubMed] [Google Scholar]
- Mazzio E. A., Soliman K. F. (2014). Epigenetics and nutritional environmental signals. Integr. Comp. Biol. 54, 21–30. 10.1093/icb/icu049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milagro F. I., Campión J., García-Díaz D. F., Goyenechea E., Paternain L., Martinez J. A. (2009). High fat diet-induced obesity modifies the methylation pattern of leptin promoter in rats. J. Physiol. Biochem. 65, 1–9. 10.1007/BF03165964 [DOI] [PubMed] [Google Scholar]
- Milagro F. I., Mansego M. L., De Miguel C., Martínez J. A. (2013). Dietary factors, epigenetic modifications and obesity outcomes: progresses and perspectives. Mol. Aspects Med. 34, 782–812. 10.1016/j.mam.2012.06.010 [DOI] [PubMed] [Google Scholar]
- Mitchell C., Schneper L. M., Notterman D. A. (2016). DNA methylation, early life environment, and health outcomes. Pediatr. Res. 79, 212–219. 10.1038/pr.2015.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri J., Prabhakar N. R. (2015). Epigenetic regulation of carotid body oxygen sensing: clinical implications. Adv. Exp. Med. Biol. 860, 1–8. 10.1007/978-3-319-18440-1_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento L., Freitas C. M., Silva-Filho R., Leite A. C., Silva A. B., da Silva A. I., et al. (2014). The effect of maternal low-protein diet on the heart of adult offspring: role of mitochondria and oxidative stress. Appl. Physiol. Nutr. Metab. 39, 880–887. 10.1139/apnm-2013-0452 [DOI] [PubMed] [Google Scholar]
- Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., et al. (2014). Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781. 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S. F., Lin R. C., Laybutt D. R., Barres R., Owens J. A., Morris M. J. (2010). Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature 467, 963–966. 10.1038/nature09491 [DOI] [PubMed] [Google Scholar]
- Nuyt A. M. (2008). Mechanisms underlying developmental programming of elevated blood pressure and vascular dysfunction: evidence from human studies and experimental animal models. Clin. Sci. 114, 1–17. 10.1042/CS20070113 [DOI] [PubMed] [Google Scholar]
- Nuyt A. M., Alexander B. T. (2009). Developmental programming and hypertension. Curr. Opin. Nephrol. Hypertens. 18, 144–152. 10.1097/MNH.0b013e328326092c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond C., Barker D. J. (2000). Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ. Health Perspect. 108(Suppl. 3), 545–553. 10.1289/ehp.00108s3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne S. E., Hales C. N. (2004). Lifespan: catch-up growth and obesity in male mice. Nature 427, 411–412. 10.1038/427411b [DOI] [PubMed] [Google Scholar]
- Parra D. C., Iannotti L., Gomez L. F., Pachón H., Haire-Joshu D., Sarmiento O. L., et al. (2015). The nutrition transition in Colombia over a decade: a novel household classification system of anthropometric measures. Arch. Public Health 73, 12. 10.1186/s13690-014-0057-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulino-Silva K. M., Costa-Silva J. H. (2016). Hypertension in rat offspring subjected to perinatal protein malnutrition is not related to the baroreflex dysfunction. Clin. Exp. Pharmacol. Physiol. 43, 1046–1053. 10.1111/1440-1681.12628 [DOI] [PubMed] [Google Scholar]
- Prabhakar N. R. (2013). Sensing hypoxia: physiology, genetics and epigenetics. J. Physiol. 591(Pt 9), 2245–2257. 10.1113/jphysiol.2012.247759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasem R. J., Li J., Tang H. M., Browne V., Mendez-Garcia C., Yablonski E., et al. (2015). Decreased liver triglyceride content in adult rats exposed to protein restriction during gestation and lactation: role of hepatic triglyceride utilization. Clin. Exp. Pharmacol. Physiol. 42, 380–388. 10.1111/1440-1681.12359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravelli G. P., Stein Z. A., Susser M. W. (1976). Obesity in young men after famine exposure in utero and early infancy. N. Engl. J. Med. 295, 349–353. 10.1056/NEJM197608122950701 [DOI] [PubMed] [Google Scholar]
- Ribeiro A. M., Lima Mde C., de Lira P. I., da Silva G. A. (2015). [Low birth weight and obesity: causal or casual association?]. Rev. Paul. Pediatr. 33, 341–349. 10.1016/j.rpped.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts V. H., Frias A. E., Grove K. L. (2015). Impact of maternal obesity on fetal programming of cardiovascular disease. Physiology (Bethesda) 30, 224–231. 10.1152/physiol.00021.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson A. M., Matthews P. A., Argenton M., Christie M. R., McConnell J. M., Jansen E. H., et al. (2008). Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51, 383–392. 10.1161/HYPERTENSIONAHA.107.101477 [DOI] [PubMed] [Google Scholar]
- Sawaya A. L., Martins P. A., Grillo L. P., Florêncio T. T. (2004). Long-term effects of early malnutrition on body weight regulation. Nutr. Rev. 62(7 Pt 2), S127–S133. 10.1111/j.1753-4887.2004.tb00082.x [DOI] [PubMed] [Google Scholar]
- Sawaya A. L., Roberts S. (2003). Stunting and future risk of obesity: principal physiological mechanisms. Cad. Saude Publica 19(Suppl. 1), S21–S28. 10.1590/S0102-311X2003000700003 [DOI] [PubMed] [Google Scholar]
- Siddique K., Guzman G. L., Gattineni J., Baum M. (2014). Effect of postnatal maternal protein intake on prenatal programming of hypertension. Reprod. Sci. 21, 1499–1507. 10.1177/1933719114530186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone N., Pangilinan F., Molloy A. M., Shane B., Scott J. M., Ueland P. M., et al. (2011). Bioinformatic and genetic association analysis of microRNA target sites in one-carbon metabolism genes. PLoS ONE 6:e21851. 10.1371/journal.pone.0021851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarc vel Szic K., Declerck K., Vidakovic M., Vanden Berghe W. (2015). From inflammaging to healthy aging by dietary lifestyle choices: is epigenetics the key to personalized nutrition? Clin. Epigenetics 7, 33. 10.1186/s13148-015-0068-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H. C., Roberts J., Catov J., Krishnamurthy R., Shypailo R., Bacha F. (2015). Mother's pre-pregnancy BMI is an important determinant of adverse cardiometabolic risk in childhood. Pediatr. Diabetes 16, 419–426. 10.1111/pedi.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarry-Adkins J. L., Fernandez-Twinn D. S., Hargreaves I. P., Neergheen V., Aiken C. E., Martin-Gronert M. S., et al. (2016). Coenzyme Q10 prevents hepatic fibrosis, inflammation, and oxidative stress in a male rat model of poor maternal nutrition and accelerated postnatal growth. Am. J. Clin. Nutr. 103, 579–588. 10.3945/ajcn.115.119834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. D., McConnell J., Khan I. Y., Holemans K., Lawrence K. M., Asare-Anane H., et al. (2005). Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, 23. 10.1152/ajpregu.00355.2004 [DOI] [PubMed] [Google Scholar]
- Toyoshima Y., Tokita R., Ohne Y., Hakuno F., Noguchi T., Minami S., et al. (2010). Dietary protein deprivation upregulates insulin signaling and inhibits gluconeogenesis in rat liver. J. Mol. Endocrinol. 45, 329–340. 10.1677/JME-10-0102 [DOI] [PubMed] [Google Scholar]
- Victora C. G., Adair L., Fall C., Hallal P. C., Martorell R., Richter L., et al. (2008). Maternal and child undernutrition: consequences for adult health and human capital. Lancet 371, 340–357. 10.1016/S0140-6736(07)61692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Cao M., Zhuo Y., Che L., Fang Z., Xu S., et al. (2016). Catch-up growth following food restriction exacerbates adulthood glucose intolerance in pigs exposed to intrauterine undernutrition. Nutrition 32, 1275–1284. 10.1016/j.nut.2016.03.010 [DOI] [PubMed] [Google Scholar]
- Wang J., Ma H., Tong C., Zhang H., Lawlis G. B., Li Y., et al. (2010). Overnutrition and maternal obesity in sheep pregnancy alter the JNK-IRS-1 signaling cascades and cardiac function in the fetal heart. FASEB J. 24, 2066–2076. 10.1096/fj.09-142315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterland R. A., Michels K. B. (2007). Epigenetic epidemiology of the developmental origins hypothesis. Annu. Rev. Nutr. 27, 363–388. 10.1146/annurev.nutr.27.061406.093705 [DOI] [PubMed] [Google Scholar]
- Wei L. N. (2013). Non-canonical activity of retinoic acid in epigenetic control of embryonic stem cell. Transcription 4, 158–161. 10.4161/trns.25395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. C. (2007). The programming effects of early growth. Early Hum. Dev. 83, 743–748. 10.1016/j.earlhumdev.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Wells J. C. (2011). The thrifty phenotype: an adaptation in growth or metabolism? Am. J. Hum. Biol. 23, 65–75. 10.1002/ajhb.21100 [DOI] [PubMed] [Google Scholar]
- Wells J. C. (2012). Obesity as malnutrition: the role of capitalism in the obesity global epidemic. Am. J. Hum. Biol. 24, 261–276. 10.1002/ajhb.22253 [DOI] [PubMed] [Google Scholar]
- Wensveen F. M., Jelencic V., Valentic S., Ŝestan M., Wensveen T. T., Theurich S., et al. (2015). NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat. Immunol. 16, 376–385. 10.1038/ni.3120 [DOI] [PubMed] [Google Scholar]
- West-Eberhard M. J. (2005a). Phenotypic accommodation: adaptive innovation due to developmental plasticity. J. Exp. Zool. B Mol. Dev. Evol. 304, 610–618. 10.1002/jez.b.21071 [DOI] [PubMed] [Google Scholar]
- West-Eberhard M. J. (2005b). Developmental plasticity and the origin of species differences. Proc. Natl. Acad. Sci. U.S.A. 102(Suppl 1), 6543–6549. 10.1073/pnas.0501844102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yuan M., Bradley K. M., Dong F., Anversa P., Ren J. (2012). Insulin-like growth factor 1 alleviates high-fat diet-induced myocardial contractile dysfunction: role of insulin signaling and mitochondrial function. Hypertension 59, 680–693. 10.1161/HYPERTENSIONAHA.111.181867 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zohdi V., Lim K., Pearson J. T., Black M. J. (2015). Developmental programming of cardiovascular disease following intrauterine growth restriction: findings utilising a rat model of maternal protein restriction. Nutrients 7, 119–152. 10.3390/nu7010119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohdi V., Sutherland M. R., Lim K., Gubhaju L., Zimanyi M. A., Black M. J. (2012). Low birth weight due to intrauterine growth restriction and/or preterm birth: effects on nephron number and long-term renal health. Int. J. Nephrol. 2012:136942. 10.1155/2012/136942 [DOI] [PMC free article] [PubMed] [Google Scholar]