Abstract
Epithelial Na+ channels mediate the transport of Na across epithelia in the kidney, gut, and lungs and are required for blood pressure regulation. They are inhibited by ubiquitin protein ligases, such as Nedd4 and Nedd4-2, with loss of this inhibition leading to hypertension. Here, we report that these channels are maintained in the active state by the G protein-coupled receptor kinase, Grk2, which has been previously implicated in the development of essential hypertension. We also show that Grk2 phosphorylates the C terminus of the channel β subunit and renders the channels insensitive to inhibition by Nedd4-2. This mechanism has not been previously reported to regulate epithelial Na+ channels and provides a potential explanation for the observed association of Grk2 overactivity with hypertension. Here, we report a G protein-coupled receptor kinase regulating a membrane protein other than a receptor and provide a paradigm for understanding how the interaction between membrane proteins and ubiquitin protein ligases is controlled.
Keywords: amiloride, ubiquitin protein ligases
Epithelial Na+ channels (ENaC) are expressed in a wide variety of salt-scavenging epithelia, including the distal nephron, the colon, the lungs, and the excretory ducts of salivary and sweat glands (1). They play a critical role in the maintenance of normal extracellular fluid volume (2), blood pressure (2), and fluid volume in the respiratory passages (3). Increases in their activity are associated with hypertension, as in the autosomal dominant form of hypertension Liddle's syndrome (4). Increased Na channel activity has also been proposed as the cause of the dehydration of the respiratory surfaces that occurs in cystic fibrosis (3, 5), although alternative explanations have been proposed (6). Conversely, reductions in their activity, as occurs in pseudohypoaldosteronism type I, lead to hypotension and depletion of extracellular fluid volume (7, 8) as well as the accumulation of an excessive volume in the respiratory passages (9).
In epithelia, the channels are composed of the three following homologous subunits: α-ENaC, β-ENaC, and γ-ENaC (10). Each of the subunits of the channel has two transmembrane domains as well as N and C termini, which are cytosolic. The C-terminal domain of each subunit contains a so-called PY (PPxY) motif, which can be bound by WW domains in the ubiquitin protein ligases Nedd4 (11, 12) and Nedd4-2 (13–16). These ubiquitin protein ligases bind the channels in response to increases in intracellular Na+ (12, 17) and ubiquitinate them (18), causing their endocytosis and destruction (18, 19). Interruption of this regulatory system, as occurs in the autosomal dominant condition Liddle's syndrome, which is due to mutation or deletion of the PY motifs in the β or γ subunits of the channel, leads to increased channel activity and hypertension (4, 11, 20).
In addition to this regulation by ubiquitin protein ligases, ENaC are also regulated by kinases. Studies in stably transfected Madin–Darby canine kidney cells, for example, have shown that aldosterone and insulin, both of which activate the channels, result in the phosphorylation of the C termini of β-ENaC and γ-ENaC (21). In particular, the C-terminal 20 residues of the β subunit showed increased levels of phosphorylation in response to these hormones. Which of the three serines in this region was phosphorylated, however, has not been determined. Furthermore, inhibition of protein phosphatase type I with low concentrations of okadaic acid (OA) has been found to increase the activity of the channels (22), consistent with phosphorylation playing an important role in their regulation.
The present studies were stimulated by the finding that the activity of the ENaC in salivary duct cells depended on the presence of ATP in the cytosol. We found that the requirement for ATP was actually due to the activity of the channels depending on a kinase. We identified this kinase to be the G protein-coupled receptor kinase 2 (Grk2), and we showed that it acts on S633 in the C terminus of β-ENaC. The kinase increases the activity of the channels and renders them insensitive to the ubiquitin protein ligase Nedd4-2. The results demonstrate the existence of a previously unsuspected regulatory mechanism in which Na+ channel activity and the operation of the Na+ feedback regulatory system are regulated by Grk2. They further suggest that increased Grk2 activity, which is known to be associated with hypertension in humans and animal models (23), may act by interrupting Na+ feedback regulation of the Na+ channels in the distal nephron, leading to inappropriate renal Na+ absorption.
Methods
Materials. Adenosine 5′-(β,γ-imido)triphosphate (AMP-PNP), adenosine 5′-(β,γ-methylene)triphosphate (AMP-PCP), ATP, oligomycin, 2-deoxy-d-glucose, and heparin were obtained from Sigma. OA, protein phosphatase inhibitor 2 (PPI2), and LY294002 were obtained from Calbiochem. L. A. Pinna (University of Padua, Padua, Italy) generously provided 4,5,6,7-tetrabromobenzotriazole. Anti-Grk2 and anti-Grk3 antibodies were obtained from Santa Cruz Biotechnology, and the anti-Grk2 and 3 antibody was obtained from Upstate Biotechnology (Lake Placid, NY). βmC10 (MESDSEVEAI) and βmC15 (CQPLDTMESDSEVEAI) were synthesized by Mimotopes (Clayton, Victoria, Australia). The anti-Nedd4/Nedd4-2 antibody is described in ref. 24. The GST-Δ(WW1,WW2)Nedd4-2 construct (Δ1–362mNedd4-2) was generated by PCR amplification, followed by cloning into the EcoRI site of pGEX-2TK. GST-K48R ubiquitin (12) and GST-Δ(WW1,WW2)Nedd4-2 were produced in Escherichia coli. Bovine Grk2 was produced in Sf9 cells (25, 26).
Cell Isolation. Isolated salivary duct cells were prepared by collagenase digestion of mandibular glands from male mice (12).
Patch-Clamp Methods. The standard bath solution contained 145 mM NaCl, 5.5 mM KCl, 1.0 mM CaCl2, 1.2 mM MgCl2, 1.2 mM NaH2PO4, 7.5 mM Na·Hepes, 7.5 mM H·Hepes, and 10 mM glucose (pH 7.4). After establishing the whole-cell configuration in an isolated cell, we replaced the bath solution with a solution containing 145 mM Na-glutamate, 5.0 mM NaCl, 1.0 mM MgCl2, 10 mM H·Hepes, 10 mM glucose, and 1.0 mM EGTA; the pH was adjusted to 7.4 with NaOH. The pipettes were filled with solutions containing N-methyl-d-glucamine-glutamate and Naglutamate (together, total of 150 mM), 1.0 mM MgCl2, 10 mM H·Hepes, 10 mM glucose, and 5.0 mM EGTA (pH 7.2). Glucose was omitted from solutions containing 2-deoxy-d-glucose. ATP was omitted from solutions containing AMP-PCP or AMP-PNP.
Current–voltage relations were obtained by applying 800-ms voltage pulses from a resting potential of 0 mV and were initially measured 4 min after attaining the whole-cell configuration. Steady-state currents are the average current between 700 and 800 ms after the start of the pulse. Amiloride-sensitive current was calculated by subtracting the current after the addition to the bath of 100 μM amiloride from the current before the addition of amiloride. Chord conductances were calculated between -80 mV and the reversal potential of the current (compare with ref. 12).
Phosphorylation Assays. Individual peptides (50 μM) or nonpeptide control were incubated in triplicate at 30°C for 45 min with 8 ng/μl Grk2/1 μl of [γ-32P]ATP (10 mCi/ml; 1 Ci = 37 GBq) in 20 mM Tris·HCl, pH 7.5/2 mM EDTA/10 mM MgCl2/1 mM DTT/100 μM ATP. The final reaction volume was 25 μl. The mixture was then transferred onto 2 × 2-cm P-81 paper squares. Free [γ-32P]ATP was removed by washing the squares five times in 0.75% phosphoric acid (10 ml per square per wash) and one time in acetone before drying and counting.
Statistical Methods. Data are presented as mean ± SEM. Student's unpaired t test was used to assess statistical significance.
Results and Discussion
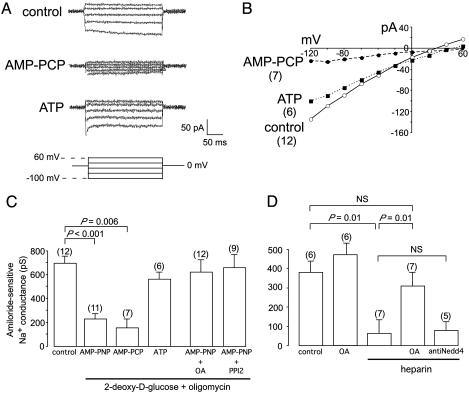
The present experiments stemmed from whole-cell patch-clamp studies in freshly isolated salivary duct cells. These showed that the activity of ENaC was reduced when the cytosol was dialyzed with a solution designed to inhibit phosphorylation reactions by blocking endogenous ATP production and providing an excess of the nonhydrolyzable ATP analogue, AMP-PNP (Fig. 1A). The reduction in channel activity was not due to a nonspecific toxic effect of the inhibitors of ATP production, oligomycin and 2-deoxy-d-glucose, because it was not observed when we replaced AMP-PNP by ATP (Fig. 1 A–C). Furthermore, it could be completely reversed by inhibition of protein phosphatase activity with either OA, which inhibits protein phosphatases type 1, 2A, and 2B (PP1, PP2A, and PP2B), or PPI2, a selective inhibitor of PP1 (Fig. 1 B and C). This reversal was not due to a generalized stimulatory effect on the activity of the channels because the inclusion of OA in the 0-Na+ pipette solution did not increase the magnitude of the amiloride-sensitive Na+ current above that observed with 0-Na+ pipette solution alone (Fig. 1D). Finally, we found that heparin, an inhibitor of kinases such as casein kinase 2 (CK2) that are directed against acidic motifs (27), could reproduce the effects on channel activity of nonspecific blockade of phosphorylation (Fig. 1D). It could, however, be overcome by OA (Fig. 1D), indicating that heparin acts by inhibiting a kinase. The inhibitory action of heparin was not mediated by Nedd4/Nedd4-2 ubiquitin protein ligases because it was not prevented by the inclusion in the pipette solution of an antibody directed against Nedd4 and Nedd4-2 (Fig. 1D).
Fig. 1.
ENaC are regulated by phosphorylation. (A) Amiloride-sensitive currents during dialysis with 0-Na solution or 0-Na+ solution plus 10 mM 2-deoxy-d-glucose/5 μM oligomycin plus either 5 mM AMP-PCP or 5 mM ATP. (B) Mean steady-state current-voltage relations of the amiloride-sensitive current under the conditions in A. (C) Amiloride-sensitive conductance during dialysis with 0-Na solution or 0-Na+ solution plus 2-deoxy-d-glucose/oligomycin plus 5 mM AMP-PNP, AMP-PCP, ATP, AMP-PNP plus 10 μM OA, or AMP-PNP plus 1,000 units/ml PPI2. (D) Amiloride-sensitive conductance during dialysis with 0-Na+ solution plus 200 μg/ml heparin ± OA or 2 μg/ml anti-Nedd4 antibody. Amiloride-sensitive current was calculated by subtracting the current after addition to the bath of 100 μM amiloride from that measured before the addition of amiloride. Data are given as mean ± SEM. Statistical significance was assessed by using Student's unpaired t test.
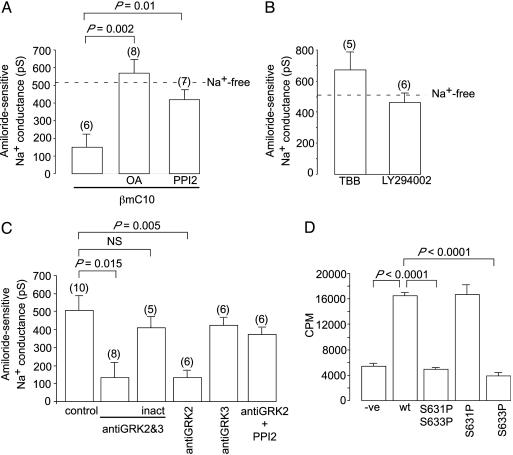
We then attempted to identify the site of the phosphorylation reaction that regulates the activity of the channels. The C-terminal 10 aa of the β subunit of the ENaC (MESDSEVEAI*; βmC10) contain a conserved acidic sequence that is phosphorylated in vivo (21) and is a target for CK2 (27). The C-terminal 10 aa of β-ENaC have also been proposed to be the target for an unknown kinase that maintains ENaC in the active state (28). This proposal was based on the observations that dialysis of the βmC10 peptide into the cytosol of salivary duct cells markedly reduces the activity of the channels and that this inhibitory effect is lost if the serines in the peptide are mutated to glycine (28). Our finding in the present studies that the Na+ channels are maintained in an active state by a kinase led us to examine whether the inhibitory effect of βmC10 can be overcome by inhibiting protein phosphatase activity with OA or PPI2. We found that it could (Fig. 2A). Hence, the inhibitory action of the inclusion in the pipette solution of βmC10 is due to the peptide inhibiting the kinase that maintains the channels in an open state.
Fig. 2.
Grk2 regulates ENaC. (A) Amiloride-sensitive conductance during dialysis with 0-Na+ solution plus βC10 peptide ± the phosphatase inhibitors OA (10 μM) or PPI2 (1,000 units/ml). (B) Amiloride-sensitive conductance during dialysis with 0-Na+ solution plus the CK2 inhibitors, 4,5,6,7-tetrabromobenzotriazole (100 μM) or LY294002 (50 μM). (C) Amiloride-sensitive conductance during dialysis with 0-Na+ solution ± antibodies (2 μg/ml) against Grk2 or Grk3. Antibody was inactivated (inact) at 60°C for 45 min. (D) In vitro incorporation of radioactive phosphate by Grk2 into various phosphopeptides derived from the terminal pentadecapeptide of mβ-ENaC (CQPLDTMESDSEVEAI). WT is unphosphorylated CQPLDTMESDSEVEAI, S631P phosphorylated at S631 [CQPLDTMES(P)DSEVEAI], S633P at S633 [CQPLDTMESDS(P)EVEAI], and S631PS633P at both serines [CQPLDTMES(P)DS(P)EVEAI].
Given that the C-terminal of β-ENaC is known to be phosphorylated by CK2 (27), we investigated whether the kinase could be CK2. We found that, although heparin inhibits the channels (Fig. 1D), other inhibitors of CK2, including 4,5,6,7-tetrabromobenzotriazole (29) and LY294002 (30), failed to do so (Fig. 2B). Having ruled out CK2, we then used predikin, a program that uses the amino acid sequence of a kinase to predict its target sequence (31), to screen the kinome for other candidate kinases. This program revealed the G protein-coupled kinases, Grk2 and Grk3, to have the appropriate target sequence. Furthermore, these kinases are inhibited by heparin (32), and increased activity of Grk2 has been implicated in the development of essential hypertension (23). We then found that inclusion in the pipette solution of an antibody directed against both Grk2 and Grk3 or of an antibody directed specifically against Grk2 inhibited the channels, whereas heat-inactivated anti-Grk2 and Grk3 antibody, as well as an antibody selective for Grk3, were without effect (Fig. 2C). The effect of the anti-Grk2 antibody was completely reversed by inhibition of PP1 (Fig. 2C). Finally, we used recombinant Grk2 (25, 26) to show that Grk2 phosphorylates S633 in the C terminus of β-ENaC (Fig. 2D).
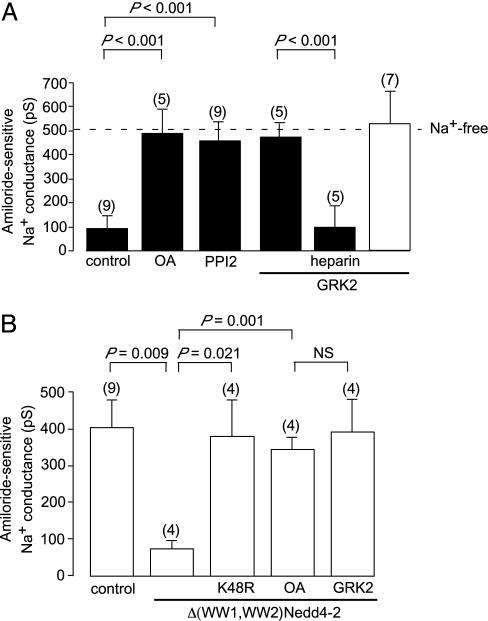
Binding of Nedd4 or Nedd4-2 to ENaC is mediated by the WW domains in these ubiquitin protein ligases, which bind to PPxY motifs (PY motifs) in the C termini of the channel subunits (11, 15, 20, 33). Loss of this interaction due to the deletion or mutation of the PY motif of even a single subunit (for example, the β subunit) leads to increased activity of the channels (20) and causes Liddle's syndrome in humans (4). Thus, we investigated whether phosphorylation of the channel by Grk2 alters its sensitivity to ubiquitin protein ligases. We found that the inactivation of the channels by increased cytosolic Na+ can be prevented by maintaining the channel in a phosphorylated state by inhibition of PP1 (Fig. 3A), indicating that Nedd4 and Nedd4-2 only interact with the dephosphorylated form of the channel. Consistent with this possibility is the finding that the inclusion of recombinant Grk2 in the pipette solution prevented the inhibition of the channels by increased intracellular Na+ (Fig. 3A) but had no impact on the activity of the channels when we used 0-Na+ pipette solution (Fig. 3A). Finally, we found that inactivation of the channels by the inclusion in the pipette solution of recombinant Δ(WW1,WW2)Nedd4-2, which like native Nedd4 and Nedd4-2 causes ubiquitin-dependent inactivation of the channels (Fig. 3B), is prevented by the protein phosphatase inhibitor OA and by the inclusion of recombinant Grk2 in the pipette solution (Fig. 3B). Thus, the Na+ feedback system and the ubiquitin protein ligases that mediate it act only on the dephosphorylated form of the channel. The mechanism by which phosphorylation of S633 of the β subunit of the ENaC prevents the binding of Nedd4 or Nedd4-2 is unclear. It may induce a conformational change in the C-terminal of the subunit that precludes binding of the ubiquitin protein ligase. Alternately, it may recruit another protein that prevents binding of the ligase to the channel.
Fig. 3.
Phosphorylation of ENaC regulates their sensitivity to ubiquitin protein ligases. (A) The effect of OA (10 μM), PPI2 (1,000 units/ml), or recombinant Grk2 (1 μg/ml) on the inhibition of the amiloride-sensitive conductance produced by dialyzing the cells with 70 mM Na+ solution. (B) The effect on the amiloride-sensitive conductance of dialyzing the cells with 0-Na+ solution containing recombinant Δ1–362mNedd4-2 (Δ(WW1,WW2)Nedd4-2, 100 μg/ml) ± the 100 μg/ml K48R mutant of ubiquitin, 10 μM OA, or 1 μg/ml recombinant Grk2.
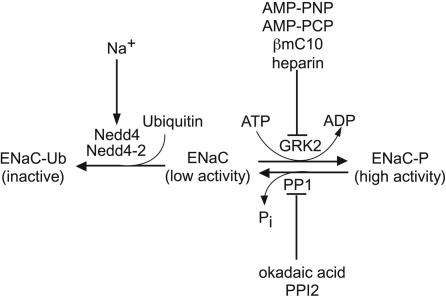
In this article, we have shown that Grk2, which is expressed in renal and other epithelia (34, 35), activates ENaC and renders them insensitive to inhibition by ubiquitin protein ligases (Fig. 4). The ubiquitin protein ligases Nedd4 and Nedd4-2 mediate the inhibition and endocytosis of the channels in response to increases in intracellular Na+ (12, 16, 17), and the present findings suggest that the known association between increased Grk2 activity and the development of essential hypertension in humans (23, 36) and in spontaneously hypertensive and Dahl salt-sensitive rats (36) may not simply be due to defective vascular reactivity (23, 37). Increased Grk2 activity would interrupt the Na+ feedback inhibitory system, leading to increased activity and surface expression of the Na+ channels in the distal nephron, which in turn would lead to hypertension. The consequences of Grk2 activation are, thus, similar to Liddle's syndrome in which mutations of channel β and γ subunits that prevent interaction of Nedd4-2 with the channel lead to interruption of Na+ feedback and hypertension (17). Consistent with this proposal are the findings that hypertensive Dahl salt-sensitive rats have increased expression of ENaC in the kidney (38) and low circulating levels of aldosterone (38).
Fig. 4.
Model summarizing the present findings. These findings indicate that the ENaC exists in the following two states: a phosphorylated state that has high activity and a dephosphorylated state that has low activity. The equilibrium between the phosphorylated and the dephosphorylated states is maintained by the kinase Grk2 and PP1. Inhibition of the kinase by the inclusion in the pipette solution of nonhydrolyzable analogues of ATP, such as AMP-PNP and AMP-PCP, or the Grk2 inhibitor, heparin, or of an excess of its substrate the terminal decapeptide of the β subunit of the channel (βC10), leads to decreased channel activity. Conversely, inhibition of the phosphatase with OA or PPI2, leads to an increase in the activity of the channel. Only the dephosphorylated form of the channel is sensitive to inhibition by Nedd4/Nedd4-2 ubiquitin protein ligases. Hence, the inhibition of the channel by increased intracellular Na+, which is mediated by these ubiquitin protein ligases, can be overcome by maintaining the channel in the phosphorylated state.
An increasing number of membrane transporters have been reported to be regulated by the Nedd4 family of ubiquitin protein ligases, including the ENaC (11), voltage-gated Na+ channels (39), and the Cl- channel, ClC-5 (40). Recent studies (14, 41, 42) have also suggested that hormones that activate ENaC and voltage-gated Na+ channels may do so by phosphorylating Nedd4-2, thus rendering it unable to interact with the channels. Here, we report a paradigm in which phosphorylation of the channel inactivates the regulatory system. Furthermore, our findings indicate that G protein receptor kinases, which have been thought to target only receptors (43), may play a major role in regulating the activity and trafficking of membrane transport proteins.
Acknowledgments
We thank Darrell Capel in R.J.L.'s laboratory for preparing the Grk2. This project was supported by the National Health and Medical Research Council of Australia and the Australian Kidney Foundation. R.J.L. is an Investigator of the Howard Hughes Medical Institute.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AMP-PNP, adenosine 5′-(β,γ-imido)triphosphate; AMP-PCP, adenosine 5′-(β,γ-methylene)triphosphate; OA, okadaic acid; PPI2, protein phosphatase inhibitor 2; CK2, casein kinase 2; ENaC, epithelial Na+ channels.
References
- 1.Duc, C., Farman, N., Canessa, C. M., Bonvalet, J. P. & Rossier, B. C. (1994) J. Cell Biol. 127, 1907-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossier, B. C., Pradervand, S., Schild, L. & Hummler, E. (2002) Annu. Rev. Physiol. 64, 877-897. [DOI] [PubMed] [Google Scholar]
- 3.Matsui, H., Grubb, B. R., Tarran, R., Randell, S. H., Gatzy, J. T., Davis, C. W. & Boucher, R. C. (1998) Cell 95, 1005-1015. [DOI] [PubMed] [Google Scholar]
- 4.Snyder, P. M., Price, M. P., McDonald, F. J., Adams, C. M., Volk, K. A., Zeiher, B. G., Stokes, J. B. & Welsh, M. J. (1995) Cell 83, 969-978. [DOI] [PubMed] [Google Scholar]
- 5.Mall, M., Grubb, B. R., Harkema, J. R., OíNeal, W. K. & Boucher, R. C. (2004) Nat. Med. 10, 487-493. [DOI] [PubMed] [Google Scholar]
- 6.Zabner, J., Smith, J. J., Karp, P. H., Widdicombe, J. H. & Welsh, M. J. (1998) Mol. Cell 2, 397-403. [DOI] [PubMed] [Google Scholar]
- 7.Pradervand, S., Barker, P. M., Wang, Q., Ernst, S. A., Beermann, F., Grubb, B. R., Burnier, M., Schmidt, A., Bindels, R. J., Gatzy, J. T., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 1732-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hummler, E., Barker, P., Talbot, C., Wang, Q., Verdumo, C., Grubb, B., Gatzy, J., Burnier, M., Horisberger, J. D., Beermann, F., et al. (1997) Proc. Natl. Acad. Sci. USA 94, 11710-11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerem, E., Bistritzer, T., Hanukoglu, A., Hofmann, T., Zhou, Z., Bennett, W., MacLaughlin, E., Barker, P., Nash, M., Quittell, L., et al. (1999) N. Engl. J. Med. 341, 156-162. [DOI] [PubMed] [Google Scholar]
- 10.Canessa, C. M., Schild, L., Buell, G., Thorens, B., Gautschi, I., Horisberger, J. D. & Rossier, B. C. (1994) Nature 367, 463-467. [DOI] [PubMed] [Google Scholar]
- 11.Staub, O., Dho, S., Henry, P. C., Correa, J., Ishikawa, T., McGlade, J. & Rotin, D. (1996) EMBO J. 15, 2371-2380. [PMC free article] [PubMed] [Google Scholar]
- 12.Dinudom, A., Harvey, K. F., Komwatana, P., Young, J. A., Kumar, S. & Cook, D. I. (1998) Proc. Natl. Acad. Sci. USA 95, 7169-7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamynina, E., Debonneville, C., Bens, M., Vandewalle, A. & Staub, O. (2001) FASEB J. 15, 204-214. [DOI] [PubMed] [Google Scholar]
- 14.Snyder, P. M., Olson, D. R. & Thomas, B. C. (2002) J. Biol. Chem. 277, 5-8. [DOI] [PubMed] [Google Scholar]
- 15.Fotia, A. B., Dinudom, A., Shearwin, K. E., Koch, J. P., Korbmacher, C., Cook, D. I. & Kumar, S. (2003) FASEB J. 17, 70-72. [DOI] [PubMed] [Google Scholar]
- 16.Harvey, K. F., Dinudom, A., Cook, D. I. & Kumar, S. (2001) J. Biol. Chem. 276, 8597-8601. [DOI] [PubMed] [Google Scholar]
- 17.Kellenberger, S., Gautschi, I., Rossier, B. C. & Schild, L. (1998) J. Clin. Invest. 101, 2741-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staub, O., Gautschi, I., Ishikawa, T., Breitschopf, K., Ciecanover, A., Schild, L. & Rotin, D. (1997) EMBO J. 16, 6325-6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimkets, R. A., Lifton, R. P. & Canessa, C. M. (1997) J. Biol. Chem. 272, 25537-25541. [DOI] [PubMed] [Google Scholar]
- 20.Schild, L., Lu, Y., Gautschi, I., Schneeberger, E., Lifton, R. P. & Rossier, B. C. (1996) EMBO J. 15, 2381-2387. [PMC free article] [PubMed] [Google Scholar]
- 21.Shimkets, R. A., Lifton, R. & Canessa, C. M. (1998) Proc. Natl. Acad. Sci. USA 95, 3301-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becchetti, A., Malik, B., Yue, G., Duchatelle, P., Al-Khalili, O., Kleyman, T. R. & Eaton, D. C. (2002) Am. J. Physiol. 283, F1030-F1034. [DOI] [PubMed] [Google Scholar]
- 23.Feldman, R. D. (2002) Mol. Pharmacol. 61, 707-709. [DOI] [PubMed] [Google Scholar]
- 24.Kumar, S., Harvey, K. F., Kinoshita, M., Copeland, N. G., Noda, M. & Jenkins, N. A. (1997) Genomics 40, 435-443. [DOI] [PubMed] [Google Scholar]
- 25.Lodowski, D. T., Pitcher, J. A., Capel, W. D., Lefkowitz, R. J. & Tesmer, J. J. G. (2003) Science 300, 1256-1262. [DOI] [PubMed] [Google Scholar]
- 26.Pitcher, J. A., Tesmer, J. J. G., Freeman, J. L. R., Capel, W. D., Stone, W. C. & Lefkowitz, R. J. (1999) J. Biol. Chem. 274, 34531-34534. [DOI] [PubMed] [Google Scholar]
- 27.Shi, H., Asher, C., Yung, Y., Kligman, L., Reuveny, E., Seger, R. & Garty, H. (2002) Eur. J. Biochem. 269, 4551-4558. [DOI] [PubMed] [Google Scholar]
- 28.Dinudom, A., Harvey, K. F., Komwatana, P., Joliffe, C. N., Young, J. A., Kumar, S. & Cook, D. I. (2001) J. Biol. Chem. 276, 13744-13749. [DOI] [PubMed] [Google Scholar]
- 29.Sarno, S., Reddy, H., Meggio, F., Ruzzene, M., Davies, S. P., Donella-Deana, A., Shugar, D. & Pinna, L. A. (2001) FEBS Lett. 496, 44-48. [DOI] [PubMed] [Google Scholar]
- 30.Davies, S. P., Reddy, H., Caivano, M. & Cohen, P. (2000) Biochem. J. 351, 95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brinkworth, R. I., Breinl, R. A. & Kobe, B. (2003) Proc. Natl. Acad. Sci. USA 100, 74-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benovic, J. L., Stone, W. C., Caron, M. G. & Lefkowitz, R. J. (1989) J. Biol. Chem. 264, 6707-6710. [PubMed] [Google Scholar]
- 33.Harvey, K. F., Dinudom, A., Komwatana, P., Jolliffe, C. N., Day, M. L., Parasivam, G., Cook, D. I. & Kumar, S. (1999) J. Biol. Chem. 274, 12525-12530. [DOI] [PubMed] [Google Scholar]
- 34.Gainetdinov, R. R., Premont, R. T., Caron, M. G. & Lefkowitz, R. J. (2000) Trends Pharmacol. Sci. 21, 366-367. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe, H., Xu, J., Bengra, C., Jose, P. A. & Felder, R. A. (2002) Kidney Int. 62, 790-798. [DOI] [PubMed] [Google Scholar]
- 36.Gros, R., Chorazyczewski, J., Meek, M. D., Benovic, J. L., Ferguson, S. S. & Feldman, R. D. (2000) Hypertension 35, 38-42. [DOI] [PubMed] [Google Scholar]
- 37.Eckhart, A. D., Ozaki, T., Tevaearai, H., Rockman, H. A. & Koch, W. J. (2002) Mol. Pharmacol. 61, 749-758. [DOI] [PubMed] [Google Scholar]
- 38.Wataru, A., Niisato, N., Miyazaki, H. & Marunaka, Y. (2004) Biochem. Biophys. Res. Commun. 315, 892-896. [DOI] [PubMed] [Google Scholar]
- 39.Abriel, H., Kamynina, E., Horisberger, J. D. & Staub, O. (2000) FEBS Lett. 466, 377-380. [DOI] [PubMed] [Google Scholar]
- 40.Schwake, M., Friedrich, T. & Jentsch, T. J. (2001) J. Biol. Chem. 276, 12049-12054. [DOI] [PubMed] [Google Scholar]
- 41.Debonneville, C., Flores, S. Y., Kamynina, E., Plant, P. J., Tauxe, C., Thomas, M. A., Munster, C., Chraibi, A., Pratt, J. H., Horisberger, J. D., et al. (2001) EMBO J. 20, 7052-7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boehmer, C., Wilhelm, V., Palmada, M., Wallisch, S., Henke, G., Brinkmeier, H., Cohen, P., Pieske, B. & Lang, F. (2003) Cardiovasc. Res. 57, 1079-1084. [DOI] [PubMed] [Google Scholar]
- 43.Pitcher, J. A., Freedman, N. J. & Lefkowitz, R. J. (1998) Annu. Rev. Biochem. 67, 653-692. [DOI] [PubMed] [Google Scholar]