Abstract
Because Δ-9-tetrahydrocannabinol (THC) inhibited luteinizing hormone-releasing hormone (LHRH) in male rats, we hypothesized that the endocannabinoid, anandamide (AEA), would act similarly. AEA microinjected intracerebroventricularly (i.c.v.) decreased plasma luteinizing hormone (LH) at 30 min in comparison to values in controls (P < 0.001). The cannabinoid receptor 1 (CB1-r)-specific antagonist, [N-(piperidin-1-yl)-1-(2,4-dichlorophenyl)-5-(4-chlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide] (AM251), produced a significant elevation in plasma LH (P < 0.01). AEA (10-9 M) decreased LHRH release from medial basal hypothalami incubated in vitro. These results support the concept that endogenous AEA inhibits LHRH followed by decreased LH release in male rats. In ovariectomized (OVX) female rats, AEA i.c.v. also inhibited LH release, but in this case AM251 had an even greater inhibitory effect than AEA. In vitro, AEA had no effect on LHRH in OVX rats. It seems that endogenous AEA inhibits LHRH followed by decreased LH release in OVX rats but that AM251 has an inhibitory action in this case. In striking contrast, in OVX, estrogen-primed (OVX-E) rats, AEA i.c.v. instead of decreasing LH, increased its release. This effect was completely blocked by previous injection of AM251. When medial basal hypothalami of OVX-E rats were incubated, AEA increased LHRH release. The synthesized AEA was higher in OVX-E rats than in OVX and males, indicating that estrogen modifies endocannabinoid levels and effects. The results are interpreted to mean that sex steroids have profound effects to modify the response to AEA. It inhibits LHRH and consequently diminishes LH release in males and OVX females, but stimulates LHRH followed by increased LH release in OVX-E-primed rats.
Keywords: AM251, CB1 receptor, medial basal hypothalamus
It is well known that Δ-9-tetrahydrocannabinol (THC), the active principle isolated from Cannabis sativa, alters many reproductive parameters in both male and female laboratory animals and humans (1). It has been reported that THC decreases secretion of hormones that control reproduction in male rats (2) but on the other hand stimulates sexual behavior in female rats (3). Also, a marked depression within 1 h in luteinizing hormone (LH) secretion after THC administration has been reported in male rats (2, 4). The molecular target for the action of this plant-derived cannabinol is the endocannabinoid system. This endogenous system consists of two types of GTP-binding protein-coupled receptors, cannabinoid receptors type 1 (CB1-r) and cannabinoid receptors type 2, and their endogenous ligands named anandamide (AEA) and 2-arachidonyl glycerol (5). This endocannabinoid system plays modulatory roles in many processes of the brain, such as the regulation of appetite, temperature, memory, and sexual and motor behavior (6). The activation of the endocannabinoid system acts in series with changes in the activity of several neurotransmitters including γ-aminobutyric acid (GABA), dopamine, and glutamate (7).
We had previously demonstrated that AEA injected intracerebroventricularly (i.c.v.) suppressed prolactin release in male rats through the activation of the tuberoinfundibular dopaminergic system. In contrast there was no effect on plasma prolactin levels in ovariectomized (OVX) rats. However, in estrogen-primed OVX (OVX-E) rats, the administration of AEA i.c.v. increased plasma prolactin concentrations. This effect was abrogated when the CB1-r antagonist, [N-(piperidin-1-yl)-1-(2,4-dichlorophenyl)-5-(4-chlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide] (AM251), was administered previous to i.c.v. AEA injection. Therefore, we concluded that the endogenous release of AEA acting through CB1-r on dopaminergic neurons that control prolactin secretion is under sex steroid-dependent regulation. We also reported that CB1-r colocalize in the cell bodies of hypothalamic periventricular nucleus tyrosine hydroxylase-containing neurons that may be a site of action of AEA (8).
The aim of the present study was to examine the effect of AEA on luteinizing hormone releasing hormone (LHRH), another important hormone that controls the reproductive system, in intact male, OVX, and OVX-E rats. Because LHRH release is under the control of stimulatory and inhibitory neurotransmitters such as norepinephrine and GABA (9), our aim was also to determine the effect of AEA on norepinephrine content and GABA release from the medial basal hypothalamus (MBH) in vitro.
Furthermore, we examined whether sex differences, ovariectomy, and sex steroid replacement would result in changes in CB1-r protein levels in the MBH by Western blot immunoassay and in the synthesized AEA measured in the same tissue.
Materials and Methods
Animals. Male and female adult Sprague–Dawley rats (220- to 250-g body weight) were kept in group cages in an animal room having a photoperiod of 14 h of light (0700–2100 hours) and room temperature of 22–24°C. Animals had free access to laboratory chow and tap water. The experimental procedures reported here were approved by the Animal Care Committee of the Center of Pharmacological and Botanical Studies of the National Council of Research of Argentina and carried out in accord with the Declaration of Helsinki.
In Vivo Studies. Female rats. The rats were bilaterally OVX while anaesthetized with ether. Two weeks later, a cannula was implanted into the lateral cerebral ventricle, under tribromoethanol (3.5%, 1 ml/100 g of animal body weight, i.p.) anesthesia, by using a stereotaxic instrument and coordinates from the atlas of Pellegrino et al. (10). The location of the cannula in the ventricle was confirmed at the end of the experiment. The experiment was performed 1 wk after the implantation of the cannula. Before the experiment (24 h), an indwelling catheter was placed into the right external jugular vein and advanced to the right atrium for collection of blood samples according to the technique of Harms and Ojeda (11). In the experiments performed with OVX-E rats, 48 h before the experiment the rats were injected with 17-β estradiol (10 μg/100 μl of corn oil, s.c.).
On the day of experiment, conscious, freely moving rats were divided into four groups. After the collection of the first blood sample (0.5 ml), the rats were microinjected i.c.v. during 1 min with: control group: 2 μl of 0.9% NaCl (saline); AEA group: 20 ng of AEA/2 μl saline; AM251 group: a CB1-r-specific antagonist (12), 200 ng/2 μl saline; and AEA plus AM251 group: AM251 was injected 30 min before AEA injection to allow for its diffusion and binding to CB1-r. Every 30 min thereafter, blood samples (0.5 ml) were obtained until 150 min after the i.c.v. injections for the determination of LH in plasma. Each blood sample was immediately replaced with 0.5 ml of saline containing 50 units of heparin per ml. After centrifugation, the plasma was stored frozen at -20°C until the determination of plasma LH by radio-immunoassay.
Male rats. These rats received the implantation of a cannula into the lateral cerebral ventricle as described previously. They were divided also into four groups and treated as described above for female rats. After the i.c.v. injections, blood samples (0.5 ml) were obtained every 30 min until 90 min, and plasma LH was determined.
In Vitro Studies. Untreated normal male, OVX, and OVX-E rats were killed by decapitation. The brains were immediately removed, and MBH were dissected by making frontal cuts just behind the optic chiasm, extending dorsally 1 mm. A horizontal cut extended from this point caudally to just behind the pituitary stalk, where another frontal cut was made. Longitudinally, cuts were made 1-mm lateral to the midline bilaterally.
MBH explants (one per tube) were incubated in Krebs–Ringer bicarbonate-buffered medium (pH 7.4) containing 0.1% glucose in a Dubnoff metabolic shaker (50 cycles per min, 95% O2/5% CO2). After a preliminary incubation of 15 min, the media were discarded and the tissues were incubated for 30 min in fresh medium containing AEA (10-5 to 10-11 M). To maintain the estrogen milieu, estradiol (10-2 M) was added to the incubation medium only of the MBH from OVX-E rats. At the end of the experiment, the media and tissues were processed and stored at -20°C for determination of LHRH and/or GABA.
Radio-Immunoassays. Plasma LH levels were measured by radio-immunoassay by using a kit purchased from the National Institutes of Health, National Hormone and Peptide Program. The results were expressed in terms of the NIH-LH-RP-2 reference preparation. The interassay variation for this assay was 6.6%, and the intraassay variation was 3.6%.
LHRH was measured by radio-immunoassay by using a highly specific LHRH antiserum kindly provided by Ayala Barnea (University of Texas Southwestern Medical Center, Dallas). The sensitivity of the assay was 0.2 pg per tube, and the curve was linear up to 100 pg of LHRH. The coefficient of variation of the LHRH intraassay ranged from 4% to 7.3%, and the coefficient of variation of the interassay was 8.9%. All samples were measured in duplicate.
GABA Assay. For the determination of GABA concentrations, the media were heated for 10 min at 100°C and centrifuged at 10,000 × g for 10 min. The supernatants were stored at -20°C, and GABA was measured by the [H3]muscimol radioreceptor assay as described by Bernasconi et al. (13) (sensitivity range, 12.5–200 pmol). This method measures the concentration of endogenous GABA receptor ligands metabolically related to GABA (14).
Norepinephrine Determination. Norepinephrine was extracted from MBH and determined by HPLC with electrochemical detection as described (15). The final concentration of norepinephrine in tissues was expressed as nanograms per milligram of protein.
Anandamide Synthase Activity. Synthase activity in MBH was assayed as described by Fernández-Solari et al. (16). In brief, the MBH were homogenized in 200 μl of buffer (20 mM Tris·HCl/1 mM EDTA, pH 7.6) and centrifuged 2,000 × g for 15 min. Supernatant protein (150–100 μg) was incubated in a total volume of 200 μl of 5 × 10-2 M Tris·HCl (pH 9.0) with 40 μM (0.1 μCi; 1 Ci = 37 GBq) of [1-14C]arachidonic acid (40–60 mCi/mmol) and 2 × 10-2 M ethanolamine during 5 min at 37°C. After evaporation, organic phases were redissolved and applied on silica gel 60 plates with concentration zone (Merck). The synthesized [14C]AEA was resolved by using the organic layer of an ethyl acetate/hexane/acetic acid/water (100:50:20:100) mixture. After autoradiography, distribution of radioactivity on the plate was counted in a scintillation counter. The Rf values of AEA and arachidonic acid were 0.33 and 0.78, respectively.
Western Immunoblotting. For detection of CB1-r protein, MBH explants of OVX, OVX-E, and male rats were homogenized in lysis buffer containing protease inhibitor mixture and centrifuged, and supernatants were stored at -80°C until used. In brief, the anti CB1-r affinity-purified rabbit Ab (Cayman Chemical, Ann Arbor, MI) was diluted 1:500 in blocker albumin and incubated for 3 h. After washes, the membrane was incubated for 1 h with anti-rabbit IgG-alkaline phosphatase (1:2,000) as secondary Ab. The immunoreactivity was revealed with BCPI/NBT Sigma FAST (5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium).
Chemicals. All materials were purchased from Sigma, except AM251 from Tocris Cookson (Ellisville, MO), LHRH was purchased from Peninsula Laboratories, Division of Bachem (San Carlos, CA), iodine125 for iodination and [3H]muscimol (specific activity: 706.7 GBq/mmol) were purchased from New England Nuclear.
Statistics. Results are expressed as means plus SEM. The n for each group refers to the number of MBH used for in vitro experiments and/or the number of rats used for in vivo experiments. Data were evaluated by t test when two groups were compared and by ANOVA when more groups were compared. Multiple comparisons were made with the Newman–Keuls' test. P values of <0.05 were considered significant.
Results
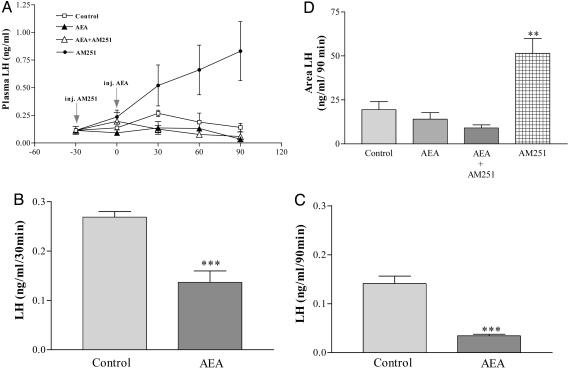
Male Rats. Effect of AEA on plasma LH levels in male rats. AEA (20 ng/2 μl saline) significantly decreased plasma LH levels at 30 and 90 min after the injection in comparison with those of the control group (Fig. 1 A–C). Interestingly enough the CB1-r-specific antagonist (AM251 200 ng/2 μl saline) alone produced a highly significant increase (P < 0.01) of plasma LH levels. Plasma LH levels in AEA and AEA plus AM251 groups were very similar. AEA counteracted the increase of LH induced by AM251. The areas under the curves of LH secretion were evaluated statistically and confirmed the conclusions drawn from observing the individual values (Fig. 1D).
Fig. 1.
Endocannabinoid inhibits LH release in male rats. (A) Effect on plasma LH levels of AEA (20 μg/2 μl), AM251 (200 ng/2 μl), AEA plus AM251, or 2 μlof saline, as a control group, injected i.c.v. in male rats. n = 7–9 animals per group. (B) Effect on plasma LH levels 30 min after i.c.v. injection of AEA. ***, P < 0.001 vs. control group. (C) Effect on plasma LH levels 90 min after the i.c.v. injection of AEA. ***, P < 0.001 vs. control group. (D) Comparison of areas under the curve of plasma LH during 90 min, after the injection of AEA. **, P < 0.01 vs. control group.
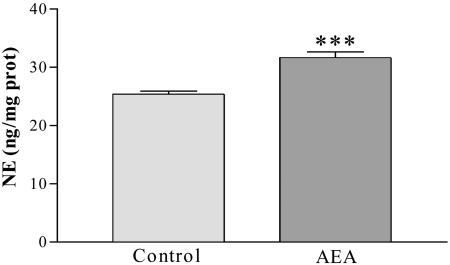
Effect of AEA on norepinephrine concentration in MBH. Norepinephrine concentration was determined in MBH of male rats 90 min after the i.c.v. injection of AEA. AEA significantly increased (P < 0.001) the norepinephrine concentration on comparison with values in the control group (Fig. 2).
Fig. 2.
NE content in MBH 90 min after the i.c.v. injection of AEA (20 ng/2 μl) or saline in male rats. ***, P < 0.01 vs. control group. The number of MBH per group was six.
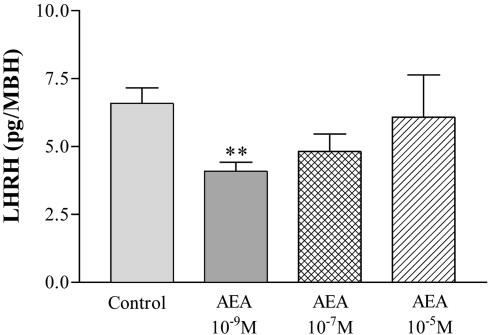
Effect of AEA on LHRH release from MBH in vitro. The dose-response curve of LHRH release showed that AEA only at 10-9 M inhibited significantly (P < 0.01) the basal release of LHRH in male rats (Fig. 3). AEA at a concentration of 10-7 M had a slight, but not significant inhibitory effect on LHRH release, whereas 10-5 M AEA failed to modify this release.
Fig. 3.
Effect of AEA (10-9,10-7, and 10-5 M) on the basal LHRH release from MBH of male rats incubated in vitro. **, P < 0.01 vs. control group. The number of MBH per group was six.
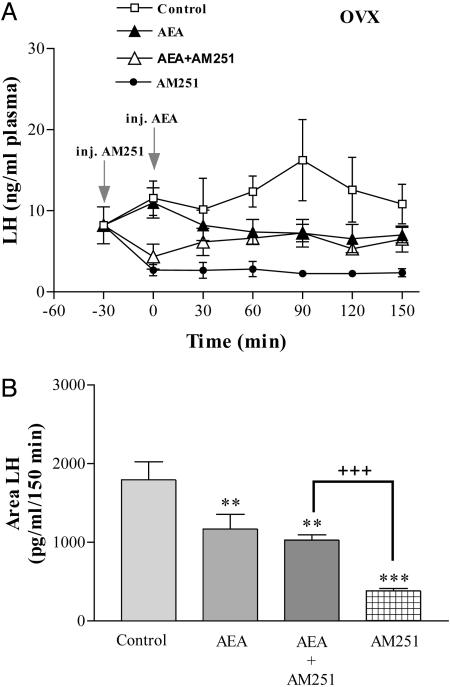
Female Rats. Effect of AEA on plasma LH levels in OVX rats. AEA (20 ng/2 μl saline, i.c.v.) in OVX rats significantly decreased plasma LH levels in comparison with those of the control group (Fig. 4A). The effect was already present at 30 min after injection and persisted for the duration of the experiment (150 min). Interestingly enough, AM251 (200 ng/2 μl) alone produced a much greater inhibition than AEA itself. Plasma LH levels in AEA and AEA plus AM251 groups were very similar at 30 min after AEA injection and for the remainder of the experiment. The areas under the curve of LH secretion showed that all treatments decreased plasma LH significantly (P < 0.01) (Fig. 4B). The greatest inhibitory effect was obtained with AM251 (P < 0.001), and it was significantly greater than that obtained with AEA alone (P < 0.01). The area of plasma LH in the group injected with AEA plus AM251 was significantly greater (P < 0.001) than in the AM251 group and not different from AEA alone.
Fig. 4.
Endocannabinoid inhibits LH release in OVX rats. (A) Effect on plasma LH levels of AEA (20 ng/2 μl), AM251 (200 ng/2 μl), AEA plus AM251, or 2 μl of saline for control group, injected i.c.v. in OVX rats. n = 7–9 animals per group. (B) Comparison of areas under the curve of LH secretion during 150 min after the injection of AEA. ***, P < 0.001 and **, P < 0.01 vs. control group; +++, P < 0.001 vs. AM251.
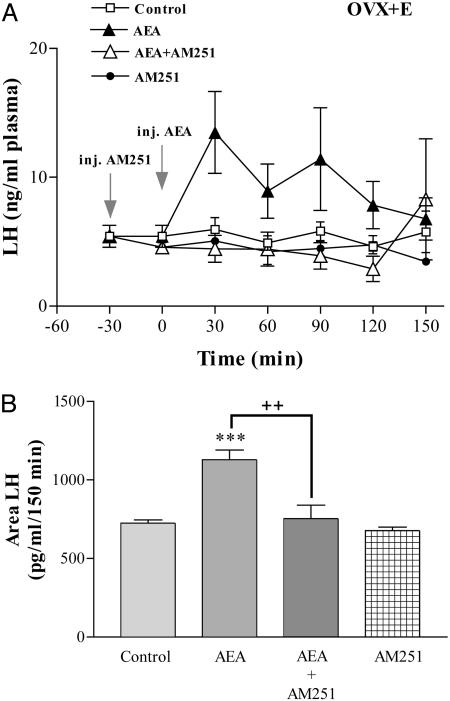
Effect of AEA on plasma LH levels in OVX-E rats. Basal plasma LH values in these rats were significantly lower than those of OVX rats and unaffected by i.c.v. injection of saline (Fig. 5A). In stark contrast to data in OVX rats, microinjection of AEA into OVX-E rats increased significantly (P < 0.001) plasma LH levels reaching a maximum at 30 min, over twice the initial values, and then there was a slow return to values similar to initial levels at 150 min. This increase was completely abolished when AM251 was injected before AEA. The injection of AM251 alone did not significantly modify plasma LH levels in OVX-E rats. Confirming these observations, the area under the curve of plasma LH was significantly increased after AEA injection when compared to that of the control group (P < 0.001), an effect that was significantly blocked (P < 0.01) by the AEA antagonist, which by itself had no effect (Fig. 5B).
Fig. 5.
Stimulation of LH release by endocannabinoid in OVX estrogen-primed rats. (A) Effect on plasma LH levels of AEA (20 ng/2 μl), AM251 (200 ng/2 μl), AEA plus AM251, or 2 μl of saline for control group, injected i.c.v. in OVX-E rats. n = 7–9 animals per group. (B) Comparison of areas under the curve of LH secretion during 150 min, after the injection of AEA. ***, P < 0.001 vs. control group; ++, P < 0.01 vs. AEA plus AM251.
Effect of AEA on norepinephrine concentration in MBH. At the end of the experiment, 150 min after the i.c.v. injection of AEA, the norepinephrine concentrations (54.9 ± 8.6 ng/mg protein) in MBH of OVX rats were not significantly different from those of controls injected only with saline (58.3 ± 18.3 ng/mg protein).
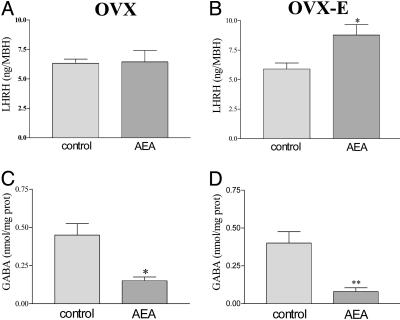
Effect of AEA on LHRH and GABA release from incubated MBH. AEA (10-9 M) had no effect on basal LHRH release from MBH of OVX rats (Fig. 6A). However, AEA significantly increased (P < 0.05) the LHRH release from MBH fragments of OVX-E rats (Fig. 6B). AEA (10-9 M) highly significantly inhibited basal GABA release from MBH fragments of both OVX and OVX-E rats (P < 0.01) (Fig. 6 C and D).
Fig. 6.
Effect of AEA (10-9 M) in vitro on the basal LHRH and GABA release from MBH of OVX (A and C) and OVX-E (B and D) rats. *, P < 0.05 and **, P < 0.01 vs. control group. The number of MBH per group was six.
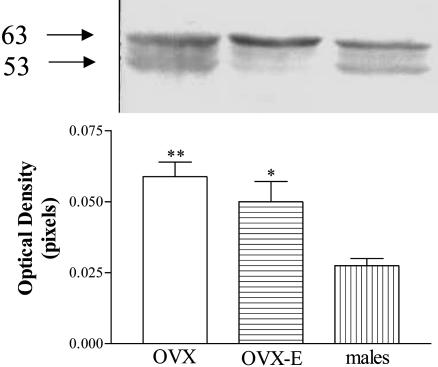
CB1-r Protein Expression. Western immunoblotting of whole MBH by SDS/PAGE revealed the presence of two immunoreactive species ≈53 and 63 kDa. The predicted molecular mass for the unmodified unglycosylated rat CB1-r protein, after appropriate extrapolation of its coding sequence from cDNA is 53 kDa. The band of 63 kDa corresponds with one of the glycosylated species. The results demonstrated a significant increase of CB1-r protein expression in MBH from OVX and OVX-E rats when compared to that of normal male rats (Fig. 7).
Fig. 7.
CB1 receptor protein is reduced in male rats. (Upper) Western blot showing the expression of CB1 receptor protein in MBH from OVX, OVX-E, and normal male rats. (Lower) Optical density of CB1 receptor expressed in MBH of OVX, OVX-E, and male rats (each column represents the mean of four separate determinations). **, P < 0.01 and *, P < 0.05 vs. male rats.
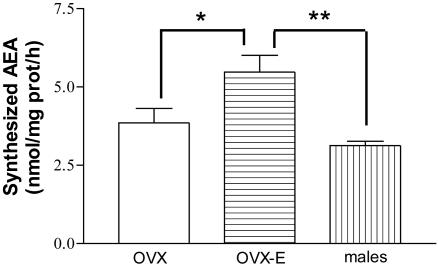
Anandamide Synthesis. The synthesized AEA was not significantly different between MBH of male and OVX rats, but was significantly higher in MBH of OVX-E rats as compared to males or OVX rats (Fig. 8).
Fig. 8.
Synthesized AEA in MBH from OVX, OVX-E, and male rats. There were six rats per group *, P < 0.05 vs. OVX; **, P < 0.01 vs. males.
Discussion
It is well known that THC alters LHRH release in rats (17). Wenger et al. (18) demonstrated that serum LH and testosterone levels were significantly decreased in mutant (CB1-r-/-) mice, indicating that the CB1-r activation is needed for the effects of endocannabinoids on the regulation of reproductive function. Moreover, they found that AEA suppressed LH and testosterone secretion in WT (CB1+/+) mice but had no effect in receptor-inactivated animals.
Furthermore, we have recently demonstrated that AEA inhibits the forskolin-stimulated LHRH release in male rats (16). In the present work we observed that AEA inhibited LHRH release from MBH of males but had no effect on the hypothalamic LHRH release in OVX rats. Additionally, in our in vivo experiments, we observed that microinjections of AEA i.c.v. significantly decreased plasma LH levels in male and in OVX rats. These results are in agreement with the experiments performed by Wenger and Moldrich (1) that demonstrated that both THC and AEA significantly decreased serum LH levels in OVX rats. Furthermore, it has been shown that THC significantly decreased serum LH levels in male rats (2).
In contrast to these inhibitory effects of AEA, we observed an increase in plasma LH levels after injections of AEA in OVX-E rats. The increase in plasma LH levels seen in vivo in OVX-E-primed rats is probably due, at least in part, to increased LHRH release, because AEA evoked LHRH release in vitro from MBH of OVX-E rats. The results clearly indicate that estrogen reverses the inhibitory action of AEA on the hypothalamus–pituitary axis of female rats, which now becomes stimulatory. Indeed, it was reported (3, 19) that THC facilitated the sexual behavior in female rats.
We have recently demonstrated in male rats that AEA increased the release of GABA, a neurotransmitter involved in the inhibition of LHRH release (16). Thus, a stimulation of GABA release could be involved in the inhibition of basal LHRH release by AEA in males. However, an inhibitory effect of AEA on GABA release was observed both in OVX and OVX-E rats, although AEA stimulated LHRH release only in OVX-E rats. Therefore, depressed GABA release may not be the sole cause of the AEA-stimulated LHRH release induced by estrogen.
Tallying with previous reports (20), we observed an increase of hypothalamic norepinephrine content after the i.c.v. injection of AEA in male rats. Although it is well known that norepinephrine stimulates LHRH release from the MBH (21), another mechanism was shown in which norepinephrine increases the inhibitory tone of GABA on LHRH release by increasing nitric oxide production (22).
Additionally, Fernández-Ruiz and coworkers (23) demonstrated that endocannabinoids inhibit the uptake of GABA in GABAergic neurons. Recently, others (24) also found that endocannabinoids play a facilitating role on GABAergic transmission by blocking its uptake. Moreover, endocannabinoids decrease cAMP production that inhibits a cAMP-dependent protein kinase that inactivates the GABA transporters (25). Probably, AEA acts at least through one of these mechanisms increasing GABA inhibitory tone and decreasing LHRH release.
In OVX rats, AEA had no effect on MBH norepinephrine content and that could be related with the lack of effect of AEA on LHRH release in these rats. Because AEA had no effect on LHRH release of OVX rats, but decreased plasma LH levels, we could argue that AEA acts on the anterior pituitary to inhibit LH release in OVX female rats with a lack of estrogen. A possible additional effect that regulates LH release from the pituitary could be involved, and indeed CB1-r have been found in the anterior pituitary gland (26).
The ability of AM251 to increase plasma LH in males may be interpreted as follows: A tonic inhibitory effect of endogenous AEA on LHRH release that can be increased slightly by exogenous AEA resulting in a decrease in plasma LH. This tonic inhibitory effect of AEA is blocked by the CB1-r blocker, AM251, resulting in an increase in plasma LH above levels in the saline-injected rats; or AM251 per se increases plasma LH acting through CB1-r as an inverse agonist probably by reducing the activity of CB1-r. It has been reported that SR141716A, a structural analog of AM251, may produce inverse cannabimimetic effects by itself acting through CB1-r as an inverse agonist (27–29). Furthermore, by using SR141716A, an increase in plasma LH levels in male rats was shown (30). This action of AM251 was blocked by exogenous AEA resulting in a lowering in plasma LH.
In OVX rats the greatest effect was obtained with AM251 but in contrast to males the effect was inhibitory. AEA had a smaller inhibitory effect and vitiated the effect of AM251. The greater decrease of plasma LH levels produced by AM251 alone suggests that this substance can elicit responses on CB1-r by itself acting as an agonist.
Because acute administration of estrogen to OVX rats changes the response of LH to progesterone from inhibitory to stimulatory (31), we hypothesized that estrogen would convert the inhibitory effect of AEA to a stimulatory one and that this effect would be antagonized by AM251 and indeed it did. The mechanism is unclear because there was no effect of estrogen on the CB1-r protein expression and AEA inhibited GABA release in both OVX and OVX-E rats. AM251 behaved as expected in OVX-E rats, having no effect by itself and blocking the stimulatory action of AEA on plasma LH levels. This lack of effect of the antagonist by itself suggests that there was no endogenous AEA effect in OVX-E rats.
The CB1-r has been reported to undergo glycosylation at three potential sites on the N terminus (32), which could account for size variations. The band of 53 kDa found in this study is analogous to the unglycosylated rat CB1-r protein, and the band of 63 kDa corresponds with one of the immunoreactive glycosylated species (33). Estrogen did not modify the expression of CB1-r between OVX and OVX-E rats, but the male rats showed less CB1-r expression, probably due to testosterone. The synthesized AEA in the hypothalamus of OVX-E rats was higher than in OVX and males. These results indicate that estrogen could modify endocannabinoid levels and effects because opposite effects of AEA on LHRH and LH release were found, being inhibitory in OVX and male rats and stimulatory in OVX-E rats.
Because AEA increased plasma prolactin (8) as well as plasma LH in OVX-E, it is clear that estrogen alters the action of endocannabinoids on pituitary hormone secretion. Moreover, estrogen increases LHRH release and LH plasma levels in OVX-E rats but inhibits LHRH release and LH plasma levels in male rats and plasma LH levels in OVX rats, animals that have very low estrogen levels. Therefore, we are in the position to conclude that estrogen alters the action of endocannabinoids on the hypothalamic–pituitary axis.
Acknowledgments
We thank Ana Ines Casella for her administrative help. This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica, Argentina (PICT 99#5-6117) and Consejo Nacional de Investigaciones Científicas y Técnicas.
Abbreviations: THC, δ-9-tetrahydrocannabinol; LH, luteinizing hormone; CB1-r, cannabinoid receptors type 1; AEA, anandamide; GABA, γ-aminobutyric acid; i.c.v., intracerebroventricularly; OVX, ovariectomized; OVX-E, estrogen-primed OVX; LHRH, LH releasing hormone; MBH, medial basal hypothalamus; AM251, [N-(piperidin-1-yl)-1-(2,4-dichlorophenyl)-5-(4-chlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide].
References
- 1.Wenger, T. & Moldrich, G. (2002) Prostaglandins Leukotrienes Essent. Fatty Acids 66, 301-307. [DOI] [PubMed] [Google Scholar]
- 2.Wenger, T., Rettori, V., Snyder, G., Dalterio, S. & McCann S. M. (1987) Neuroendocrinology 46, 488-493. [DOI] [PubMed] [Google Scholar]
- 3.Mani, S., Mitchell, A. & O'Malley, B. (2001) Proc. Natl. Acad. Sci. USA 98, 1249-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marks, P. (1973) Prog. Brain Res. 39, 331-334. [DOI] [PubMed] [Google Scholar]
- 5.Felder, C. C., Briley, E. M., Axelford, J., Simpson, J. T., Mackie, K. & Devane, W. A. (1993) Proc. Natl. Acad. Sci. USA 90, 7656-7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mechoulam, R., Fride, E. & Di Marzo, V. (1998) Eur. J. Pharmacol. 359, 1-18. [DOI] [PubMed] [Google Scholar]
- 7.Pistis, M., Ferraro, L., Pira, L., Flore, G., Tanganelli, S., Gessa, G. L. & Devoto, P. (2002) Brain. Res. 948, 155-158. [DOI] [PubMed] [Google Scholar]
- 8.Scorticati, C., Mohn, C., De Laurentiis, A., Vissio, P., Fernández-Solari, J., Seilicovich, A., McCann, S. M. & Rettori, V. (2003) Proc. Natl. Acad. Sci. USA 100, 2134-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lomniczi, A., Mastronardi, C. A., Faletti, A., Seilicovich, A., De Laurentiis, A., McCann, S. M. & Rettori, V. (2000) Proc. Natl. Acad. Sci. USA 97, 2337-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pellegrino, L. J., Pellegrino, A. S. & Ashman, A. J. (1979) in A Stereotaxic Atlas of the Rat Brain (Plenumn, New York), 2nd Ed.
- 11.Harms, P. & Ojeda, S. R. (1974) J. Appl. Physiol. 36, 391-392. [DOI] [PubMed] [Google Scholar]
- 12.Gatley, S., Gifford, A., Volkow, R., Ruoxi, L. & Makriyannis, A. (1996) Eur. J. Pharmacol. 307, 331-338. [DOI] [PubMed] [Google Scholar]
- 13.Bernasconi, R., Bittiger, H. & Heid, J. (1980) J. Neurochem. 34, 614-618. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell, R., Grieve, G., Dow, R. & Fink, G. (1983) Neuroendocrinology 37, 169-176. [DOI] [PubMed] [Google Scholar]
- 15.De Laurentiis, A., Pisera, D., Caruso, C., Candolfi, M., Mohn, C., Rettori, V. & Seilicovich, A. (2002) Neuroimmunomodulation 10, 30-39. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Solari, J., Scorticati, C., Mohn, C., De Laurentiis, A., Billi, S., Franchi, A., McCann, S. M. & Rettori, V. (2004) Proc. Natl. Acad. Sci. USA 101, 3264-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rettori, V., Lomniczi, A., Mohn, C., Scorticati, C., Vizzio, P., Lasaga, M., Franchi, A. & McCann, S. M. (2002) Prog. Brain Res. 141, 175-181. [DOI] [PubMed] [Google Scholar]
- 18.Wenger, T., Ledent, C., Csemus, V. & Gerendai, I. (2001) Biochem. Biophys. Res. Commun. 284, 363-368. [DOI] [PubMed] [Google Scholar]
- 19.Mani, S. K., Allen, J. M., Rettori, V., McCann, S. M., O'Malley, B. W. & Clark, J. H. (1994) Proc. Natl. Acad. Sci. USA 91, 6468-6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao, S., Avraham, Y., Mechoulam, R. & Berry, E. M. (2000) Eur. J. Pharmacol. 392, 147-156. [DOI] [PubMed] [Google Scholar]
- 21.Negro Vilar, A., Ojeda, S. R. & McCann, S. M. (1979) Endocrinology 104, 1749-1757. [DOI] [PubMed] [Google Scholar]
- 22.Seilicovich, A., Duvilanski, B., Pisera, D., Theas, S., Gimeno, M., Rettori, V. & McCann, S. M. (1995) Proc. Natl. Acad. Sci. USA 92, 3421-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero, J., de Miguel, R., Ramos, J. & Fernández-Ruiz, J. J. (1998) Life Sci. 62, 351-363. [DOI] [PubMed] [Google Scholar]
- 24.Wilson, R. I. & Nicoll, R. A. (2001) Nature 410, 588-592. [DOI] [PubMed] [Google Scholar]
- 25.Tian, Y., Kapatos, G., Granneman, J. G. & Bannon, M. J. (1996) Eur. J. Pharmacol. 308, 161-164.8840127 [Google Scholar]
- 26.Wenger, T., Fernández-Ruiz, J. J. & Ramos, J. A. (1999) J. Neuroendocrinol. 11, 873-878. [DOI] [PubMed] [Google Scholar]
- 27.Pertwee, R. G. & Ross, R. A. (2002) Prostaglandins Leukotrienes Essent. Fatty Acids 66, 101-121. [DOI] [PubMed] [Google Scholar]
- 28.Sim-Selley, L. J., Brunk, L. K. & Selley, D. E. (2001) Eur. J. Pharmacol. 414, 135-143. [DOI] [PubMed] [Google Scholar]
- 29.Bouaboula, M., Perrachon, S., Milligan, L., Canat, X., Rinaldi-Carmona, M., Portier, M., Barth, F., Calandra, B., Pecceu, F., Lupker, J., et al. (1997) J. Biol. Chem. 272, 22330-22339. [DOI] [PubMed] [Google Scholar]
- 30.de Miguel, R., Romero, J., Muñoz, R., García-Gil, L., Gonzalez, S., Villanua, M., Makriyannis, A., Ramos, J. & Fernández-Ruiz, J. (1998) Biochem. Pharmacol. 56, 1331-1338. [DOI] [PubMed] [Google Scholar]
- 31.Crowley, W. R., O'Donohue, T. L., Wachslicht, H. & Jacobowitz, D. M. (1978) Brain. Res. 154, 345-357. [DOI] [PubMed] [Google Scholar]
- 32.Matusda, L. A., Lolait, S. J., Browstein, M. J., Yound, A. C. & Bonner, T. I. (1990) Nature 346, 561-564. [DOI] [PubMed] [Google Scholar]
- 33.Waksman, Y., Olson, J. M., Carlisle, S. J. & Cabral, G. A. (1999) J. Pharmacol. Exp. Ther. 288, 1357-1366. [PubMed] [Google Scholar]