Abstract
Purpose
The use of ADHD drugs among adults is controversial and has until recently not been approved for use in adults in most countries. The aim was to investigate use of ADHD drugs (stimulants and atomoxetine) among the entire adult population in the Nordic countries.
Methods
We conducted a multinational population-based prescription register study based on the entire adult population in the five Nordic countries (Denmark, Finland, Iceland, Norway and Sweden). All users of ADHD drugs aged 18–64 years during 2008–2012 were included, which for 2012 comprised 76,896 drug users among 15.8 million adult inhabitants.
Results
Annual prevalence of drug use increased during the study period for both genders and all age groups. The overall prevalence increased from 2.4 to 5.3 per 1000 men and 1.8 to 4.4 per 1000 women. Incidence also increased, but to a lesser extent in the last part of the study period. Methylphenidate was used by 88 % of drug users. Treatment was discontinued within the first year by 21 % of new drug users. Among all users of ADHD drugs, 53 % of men and 64 % of women concurrently used other psychotropic drugs, most frequently antidepressants and hypnotics. Psychotropic co-medication increased with age and was more pronounced among women than men.
Conclusions
Use of ADHD drug among adults more than doubled over a 5-year period, and a majority were concurrently treated with other psychotropics. Adults constitute a substantial proportion of persons treated with ADHD drugs. Thus, evidence for long-term efficacy and safety in adults is urgently needed.
Electronic supplementary material
The online version of this article (doi:10.1007/s00228-016-2125-y) contains supplementary material, which is available to authorized users.
Keywords: ADHD, Psychostimulants, Adults, Pharmacoepidemiology, Nordic countries
Introduction
The diagnosis of attention-deficit hyperactivity disorder (ADHD) among adults is controversial [1–4]. Nevertheless, evidence shows that symptoms of ADHD may persist into adulthood in a substantial proportion of child and adolescent patients [5–11]. The prevalence estimates of ADHD in adults are reported to be between 1 and 8 % [9], with variation by geographic region, diagnostic system used and underlying study population [6, 9, 12–14]. Adults with ADHD have a high degree of psychiatric comorbidity, complicating diagnostics as well as treatment [6, 15]. While physicians have previously had little guidance for diagnosing and treating adult ADHD, adults have been included in recent guidelines [8, 16–19], reflecting the increasing awareness of ADHD symptoms beyond childhood. However, until recently, atomoxetine was the only drug approved for use in adults [8, 9], which reflects that long-term efficacy and safety of these drugs are insufficiently studied in adults [20–22]. Despite these considerations, use of ADHD drugs among adults has increased rapidly throughout the world [14, 23–26]. To ensure rational use of stimulants and other ADHD drugs in the adult population, detailed knowledge on utilization patterns is thus urgently needed. Leveraging high-quality prescription register data available in the five Nordic countries, we aimed to describe the use of stimulants and atomoxetine in the adult population.
Method
Study setting, population and data sources
This population-based study examines the use of ADHD drugs among adults aged 18–64 years in ambulatory care in all five Nordic countries (Denmark, Finland, Iceland, Norway, Sweden), comprising in total 15.8 million inhabitants in this age range. Data on prescription drugs dispensed from pharmacies were retrieved from the nationwide prescription registers in each country [27]. Data on filled prescriptions are sent elec-tronically from all pharmacies to the national registers. Reporting to the registers is mandatory, ensuring high cover-age of prescription drug use in the ambulatory care setting. These registers allow individual-level drug use to be tracked over time by using the unique personal identity number (encrypted) assigned to all citizens [27].
The number of inhabitants in each country by gender and age group was retrieved from national population registers (Social Insurance Institution (Kela) in Finland and the bureau of statistics in the other countries). In 2012, the number of inhabitants aged 18–64 years was as follows: 3.4 million in Denmark, 3.3 million in Finland, 199,000 in Iceland, 3.1 million in Norway and 5.8 million in Sweden (total 15.8 million).
ADHD drugs
Medical products in the Nordic countries are classified according to the Anatomical Therapeutic Chemical (ATC) classification system [28]. For the present study, ADHD drugs were defined as methylphenidate (ATC code N06BA04), atomoxetine (N06BA09), amphetamine (N06BA01) and dexamphetamine (N06BA02). Methylphenidate was further classified in extended release (ER) and immediate release (IR) formulations. Danish data did not include amphetamine, which is not marketed and thus rarely used in Denmark.
Data analysis
Prescription data from each country were uploaded to a server at Statistics Denmark and analysed by a common programme in Stata version 13. Drug utilization was measured during 2008–2012, whereas data from 2006 to 2007 were used in specific analyses as a run-in period to define new users of ADHD drugs. Results are presented by gender and age attained at the start of the year by four age groups (18–24, 25–34, 35–44 and 45–64 years), combined for all Nordic countries (main results) and by country (Online Resource).
ADHD drug use was examined using the following definitions.
Prevalence: Annual prevalence proportion (per 1000) of ADHD drug use was defined as the number of individuals who filled at least one prescription in one calendar year divided by the gender- and age-specific population of the same year.
Incidence: Annual incidence (per 1000) of ADHD drug use was defined as the number of individuals who filled at least one prescription in one calendar year, and with no prescription fills during the previous 2 years (730 days), divided by the gender- and age-specific population of the same year.
Type of ADHD drug used at treatment initiation: The type of ADHD drugs received at treatment initiation (i.e. first prescription fill) was assessed among new users during 2008–2012. New users were defined as individuals that had not previously filled any prescription for ADHD drugs (going back to 2006).
Early discontinuation and switch in drug treatment: Among new users of ADHD drugs (defined as above), early discontinuation of treatment was defined as filling no more than two prescriptions for ADHD drugs during the first year (365 days) after initiating treatment. Prescriptions filled on the same date were counted as on prescription. Early switch in treatment was assumed if an individual who initiated treatment with methylphenidate filled a subsequent prescription for atomoxetine during the first year, or vice versa. In this analysis, users initiating treatment in 2012 were disregarded to ensure 1 year of prescription data follow-up.
Co-medication with other psychotropic drugs: Use of other types of psychotropic drugs was examined among prevalent users of ADHD drugs. For all individuals who filled a prescription for an ADHD drug in one calendar year, the proportion (%) that filled prescriptions for other psychotropic drugs concurrently [29] with an ADHD drug (within 3 months) was calculated. Other psychotropic drugs were classified as antipsychotics (ATC N05A), anxiolytics (N05B), hypnotics and sedatives (N05C), antidepressants (N06A) and antiepileptics (N03A).
Ethical approval
Personal identity numbers were encrypted. In accordance with laws and regulations, no ethical approval is needed for use of the Danish, Finnish and Norwegian prescription register data. The study was approved by the Icelandic Bioethics Committee (VSNb2013010018/03.07) and the Icelandic Data Protection Authority (2013010062TS/--), and in Sweden, it was approved by the the Karolinska Institutet regional ethics committee.
Results
The source population was the Nordic population aged 18–64 years. In 2012, this comprised 15,828,232 inhabitants, with 5.3 per 1000 men (n = 42,450), 4.4 per 1000 women (n = 34,446) and 4.9 per 1000 in total (n = 76,896) filling a prescription for ADHD drugs.
Prevalence
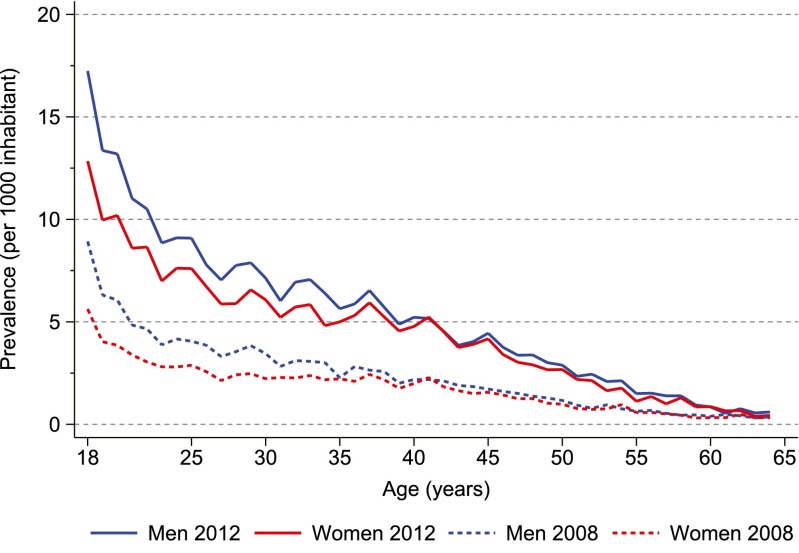
The highest prevalence of ADHD drug use in 2012 was among 18-year olds at 17.2 per 1000 men and 12.8 per 1000 women (Fig. 1). Prevalence decreased with age in both genders. Substantial differences in prevalence were observed between countries (Online Resource Fig. S 1), with the highest prevalence in Iceland and the lowest in Finland (e.g. 25.2 versus 5.3 per 1000 among 18-year-old men).
Fig. 1.
Annual prevalence proportion of ADHD drug use in 2008 and 2012 among persons aged 18–64 years in the Nordic countries, by 1-year age groups
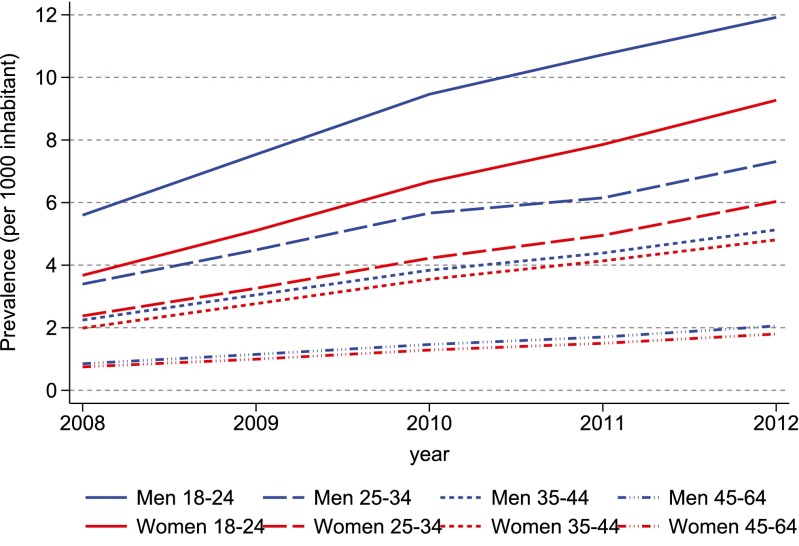
From 2008 to 2012, the prevalence of ADHD drug use more than doubled in all gender and age categories (Fig. 2 and Online Resource Table S1), from 2.4 to 5.3 per 1000 men and from 1.8 to 4.4 per 1000 women aged 18–64 years. Thus, over the study period, the male/female ratio decreased from 1.33 to 1.20. Country-specific analyses showed increasing prevalence for all gender and age categories in all countries except Finland (Online Resource Fig. S2 and Table S1). The prevalence more than doubled in Iceland, Denmark and Sweden, while the increase in Norway was more modest.
Fig. 2.
Annual prevalence proportion of ADHD drug use during 2008–2012 among persons aged 18–64 years in the Nordic countries, by year
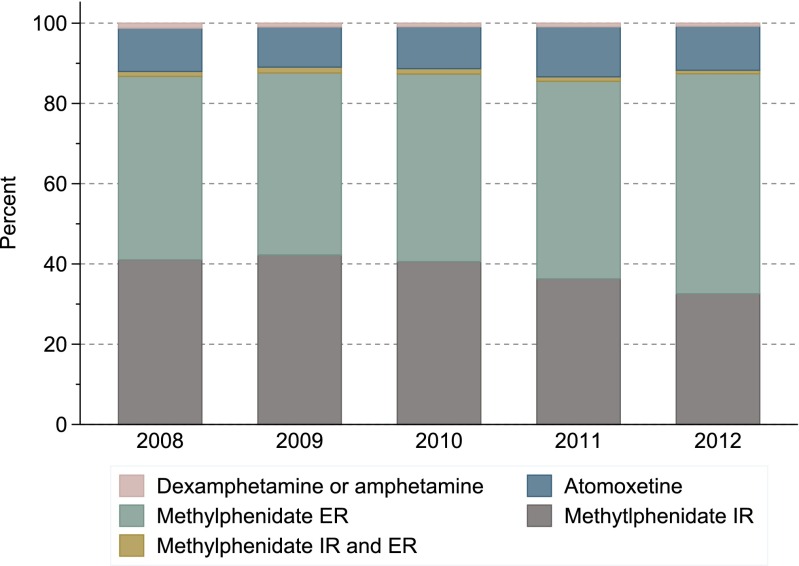
Methylphenidate was the predominant drug dispensed, as it was used by 87–88 % of ADHD drug users in all age groups and both genders throughout the study period. A shift was observed from use of immediate release (IR) to extended release (ER) methylphenidate formulations; 64 vs 73 % of users received ER formulations in 2008 and 2012, respectively, while IR formulations decreased from 41 to 34 % in the same period. The proportion using atomoxetine remained stable at 12–14 %, while less than 5 % used amphetamine and dexamphetamine.
Incidence
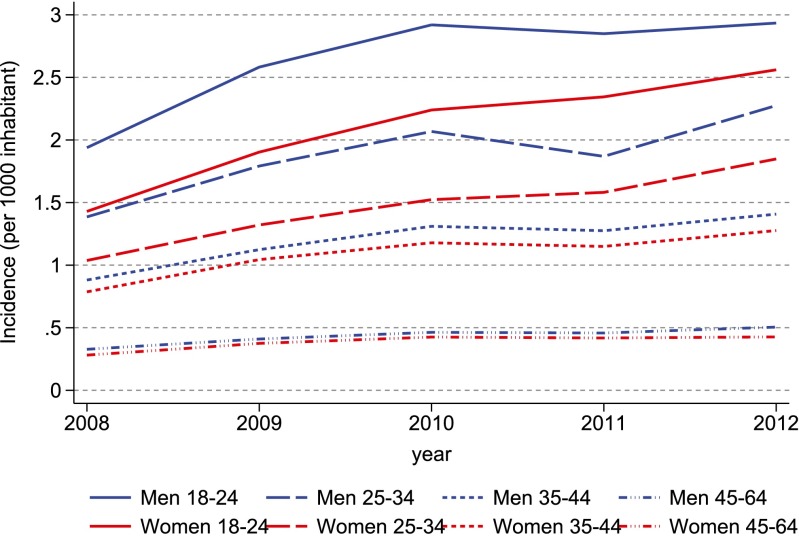
The annual incidence of ADHD drug use increased modestly among all age groups and both genders during the study period, and mainly in 2008–2010 (Fig. 3). The changes in incidence over time varied by country, rising the most in Iceland and to a lesser extent in Denmark and Sweden, while remaining stable in Finland and Norway (Online Resource Fig. S3).
Fig. 3.
Annual incidence of ADHD drug use during 2008–2012 among persons aged 18–64 years in the Nordic countries, by year
Type of ADHD drug used at treatment initiation
Methylphenidate was the preferred drug at treatment initiation throughout the study period; 88 % of new users during 2008–2012 received methylphenidate on the first prescription fill, 11 % received atomoxetine, whereas amphetamine and dexamphetamine were rarely used (Fig. 4). In total, 38 % of the new users received IR methylphenidate formulations and 49 % received ER formulations. There was a shift towards using ER formulations as first-line treatment during the study period. These results were similar for the four age groups (data not shown). Substantial differences were observed between countries, as about 70 % initiated treatment on IR formulations in Denmark and Norway, whereas ER formulations were predominantly used in the other countries (Online Resource Fig. S4).
Fig. 4.
Type of ADHD drug used at treatment initiation during 2008–2012 among persons aged 18–64 years in the Nordic countries, by year. IR immediate release formulation, ER extended release formulation
Early discontinuation and switch in treatment
Among 62,144 new users of ADHD drugs during 2008–2011, 13 % filled only the initial prescription and 9 % filled only one more prescription, while 79 % filled 3 or more prescriptions of any ADHD drug during the first year of treatment. When restricting to users who initiated treatment on methylphenidate, 21 % filled only one or two prescriptions of any ADHD drug during the first year (discontinuation), while 8 % received atomoxetine within the first year (switching). Among users who initiated treatment on atomoxetine, 25 % filled only one or two prescriptions while 32 % received methylphenidate within the first year.
Co-medication with other psychotropic drugs
Co-medication with other psychotropic drugs was common among ADHD drug users in all age groups and both genders (Table 1). Depending on gender and age category, 38–77 % of ADHD drug users received at least one other psychotropic drug concurrently with their ADHD medication. Co-medication was more common among older ADHD drug users and higher among women than men. Antidepressants were the most frequently used co-medication in all gender and age categories, highest among 45–64-year-old women (53 %). The proportion with co-medication was stable over the study period (data not shown).
Table 1.
Proportion (%) of ADHD drug users with co-medication of other psychotropic drugs in 2012 among persons aged 18–64 years in the Nordic countries
| Gender | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age group | 18–24 | 25–34 | 35–44 | 45–64 | 18–64 | 18–24 | 25–34 | 35–44 | 45–64 | 18–64 |
| ADHD users (n) | 14,396 | 11,972 | 9059 | 7023 | 42,450 | 10,693 | 9478 | 8210 | 6065 | 34,446 |
| Any psychotropic | 38 | 56 | 62 | 67 | 53 | 50 | 65 | 71 | 77 | 64 |
| Antidepressants | 20 | 32 | 37 | 40 | 30 | 32 | 44 | 49 | 53 | 43 |
| Antiepileptics | 7 | 15 | 16 | 17 | 13 | 10 | 17 | 19 | 21 | 16 |
| Antipsychotics | 14 | 20 | 20 | 19 | 18 | 13 | 18 | 18 | 19 | 17 |
| Anxiolytics | 7 | 17 | 21 | 25 | 16 | 13 | 23 | 26 | 32 | 22 |
| Hypnotics | 15 | 23 | 27 | 31 | 23 | 22 | 29 | 33 | 40 | 30 |
| Melatonin | 7 | 6 | 5 | 5 | 6 | 8 | 7 | 8 | 7 | 8 |
| Other hypnotics | 10 | 20 | 24 | 29 | 19 | 15 | 25 | 29 | 36 | 25 |
Proportion (%) of ADHD drug users with co-medication, defined as filling a prescription for other psychotropic drugs within 3 months before or after filling a prescription for an ADHD drug (i.e. concomitant use)
Discussion
This multinational study provides a detailed overview of ADHD drug utilization among the entire adult population of the five Nordic countries. Use of ADHD drugs more than doubled during the 5-year study period, with 5.3 per 1000 men and 4.4 per 1000 women filling a prescription in the last year of the study. One in five patients discontinued ADHD drug use within the first year of treatment initiation. Co-medication with other psychotropics among users of ADHD drugs was common.
Strengths and limitations
This study utilises data from high-quality prescription registers with mandatory reporting that cover the entire population of the Nordic countries [27]. Our results may slightly underestimate ADHD drug use, as the prescription registers do not include information on drugs administered in hospitals or institutions. The Finnish prescription register only covers reimbursed prescriptions and includes most prescriptions for ADHD drugs. However, during March 2011–March 2012, methylphenidate was not reimbursed for patients over 30 years. This may explain the slight dip in prevalence and incidence observed for Finland (Online Resource Figs. S2 and S3) but has little impact on the Nordic figures. Further, coverage for hypnotics is incomplete because melatonin is not reimbursable in Finland (Online Resource Table S2). Differences in licencing and reimbursement of psychotropic drugs and melatonin may impact the comparison of co-medication between countries. A limitation is the lack of information on the underlying ADHD diagnosis, which precludes solid conclusions on appropriateness of treatment.
Licencing of ADHD drugs and types of drugs used
Licencing of ADHD drugs for use also in adults may partly explain the substantial increase in use observed in the present study. Methylphenidate has been extensively used in the treatment of ADHD in children and adolescents [14, 23, 25, 26, 30–32]. Over time, the labelling status for prescribing these drugs to both children and adults has varied between the Nordic countries. Continuation of atomoxetine treatment from childhood into adulthood was approved in Nordic countries in 2005, while continuation of methylphenidate treatment was approved from 2011 in some Nordic countries. After the study period of the present study (2008–2012), initiation of drug therapy in treatment-naïve adults has been approved for atomoxetine, methylphenidate and the new substance lisdexamphetamine.
Guidelines that also focus on the management of adult ADHD have only recently been published in all Nordic countries but Finland, stating that methylphenidate is first-line treatment while atomoxetine is second-line treatment [16–19]. Our study finds that methylphenidate was the preferred drug in both prevalent and new users as 9 of 10 patients used it. Use of atomoxetine remained limited, and dexamphetamine and amphetamine were rarely used. This differs markedly from the USA, where amphetamine and dexamphetamine formulations are used by a substantial proportion of adult patients [23, 31, 32]. Our study further revealed that the use of ER formulations of methylphenidate increased while IR formulations decreased. The ER formulation has the advantage of once-daily administration, which may be advantageous given the clinical manifestation of ADHD.
Prevalence and incidence of ADHD drug use
The present study reveals a markedly increased prevalence of ADHD drug use during 2008–2012. However, the prevalences reported in the present study (2.1 per 1000 in 2008, 4.9 per 1000 in 2012) are substantially lower than what has been observed in the USA (12 per 1000 aged 20 years or older in 2005) [23], and higher than in the United Kingdom (1.1 per 1000 aged 18–24 years and 0.07 per 1000 aged 25–45 years in 2008) [24]. Differences in data source, the patient population under study and the definitions of drug use applied in each analysis may explain some of this geographical variation. Nevertheless, we found substantial difference in utilization patterns within the Nordic countries despite leveraging similar and nationwide data in five neighbouring countries, with similar healthcare systems and social structures, and used one programme for data analysis. Similarly, large variations have previously been observed between regions within the individual countries [33, 34]. These geographical variations in use point towards differences in guidelines [35], as well as prescribing practice and tradition being the most important parameters underlying the observed variations.
The prevalence of ADHD drug use increased substantially during the study period and was 4.9 per 1000 in 2012. Nevertheless, it is lower than the reported prevalence of the ADHD diagnosis, ranging from 10 to 80 per 1000 [6, 9, 12–14]. Our results also showed that the incidence of drug use increased, but to a lesser extent in the last part of the study period. Thus, the proportion of adults with ADHD not receiving pharmacological treatment may remain high. We found that drug use was more common in men than women, but the gender ratio was smaller than observed among children in the Nordic countries [30] and internationally [24, 26, 36]. The gender ratio for prescribing of ADHD drugs for adults in the UK is substantially higher than in the present study [24]. A diminishing gender difference from childhood to adulthood is in line with the reported differences by gender for diagnosis of ADHD [8].
Early discontinuation and switch in treatment
Our results indicate that 21 % of persons who initiated treatment had discontinued all ADHD drugs within the first year. Other studies have revealed higher levels of discontinuation in adults [37–40]. Continuity of drug treatment may be compromised when patients transition from the adolescent health and social care systems to the adult system [10, 41], and some physicians may be reluctant to prescribe ADHD drugs to adults [1, 2, 4, 8]. We observed a higher proportion of early discontinuation and drug switching among users who initiated treatment with atomoxetine than those initiating treatment with methylphenidate. As atomoxetine is not considered first-line treatment [16–19], patients initiated directly on atomoxetine may differ from the overall study population, e.g. with respect to liability for substance misuse. Also, stricter regulation of substances classified as narcotics (methylphenidate) may lead to shorter supply per methylphenidate prescription, thus requiring more frequent prescription fills for methylphenidate than atomoxetine. Further studies should elucidate the long-term treatment duration and switch patterns for ADHD drugs.
Co-medication of other psychotropic drugs with ADHD drugs
Studies of epidemiological and clinical populations of adults with ADHD have revealed that comorbid psychiatric illnesses are very common, in particular depression and substance use disorders [6, 8, 9, 15]. In the present study, antidepressants were the most frequently used psychotropic among users of ADHD drugs, with as many as half of the women over 34 years receiving antidepressants. Hypnotics were also commonly used, with the Z-hypnotics, such as zopiclone, preferred over melatonin. The presence of comorbidities poses additional challenges for diagnosis and treatment management of ADHD and may affect both the initiation and continuation of ADHD treatment [6, 15]. Evidence for the efficacy and safety of combined pharmacological treatment of ADHD and other psychiatric illnesses is limited.
Unanswered questions
The present study did not reveal to what extent persons receiving pharmacological treatment with ADHD drugs also had a diagnosis for ADHD or other psychiatric illnesses, or vice versa. There is generally less evidence on the efficacy and safety for ADHD drugs in adults than in children, which is of concern given the substantially higher rates of comorbid psychiatric and somatic disorders in the adult population. For instance, ADHD drugs have been linked to a moderate increase in heart rate and blood pressure although evidence does not point to increased rate of serious cardiovascular events [42–45]. The Nordic countries provide an opportune setting for studies on these and other unanswered questions regarding outcomes of ADHD drug treatment in adults, with several health registers covering the entire population in each country and the possibility to track the exposures and outcomes of all persons over time [27].
Conclusion
The present study revealed that the use of ADHD drug among adults more than doubled over a 5-year period, and a majority were concurrently treated with other psychotropics. Because of these recent developments in ADHD drug use patterns, adults now constitute about half of the persons using ADHD drugs in the Nordic countries. Thus, evidence for long-term treatment efficacy and safety in adults is urgently needed.
Electronic supplementary material
(PDF 556 kb)
Acknowledgments
Contributors
All authors contributed to data collection, writing the protocol and statistical analysis plan, revised the draft manuscript and approved the final version of the manuscript. K.F. initiated the collaborative project. K.F. and Ø.K. obtained the grant from the Norwegian Resource Centre for ADHD, Tourette syndrome and Narcolepsy. A.P. and Ø.K. analysed the data. Ø.K. drafted the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The project was funded by the authors’ affiliations and a grant from the Norwegian Resource Centre for ADHD, Tourette syndrome and Narcolepsy. The funding source had no further role in design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
References
- 1.Asherson P, Adamou M, Bolea B, Muller U, Morua SD, Pitts M, Thome J, Young S. Is ADHD a valid diagnosis in adults? Yes. BMJ. 2010;340(1):c549. doi: 10.1136/bmj.c549. [DOI] [PubMed] [Google Scholar]
- 2.Moncrieff J, Timimi S. Is ADHD a valid diagnosis in adults? No. BMJ. 2010;340(1):c547. doi: 10.1136/bmj.c547. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz A. The Selling of Attention Deficit Disorder. The New York Times [Internet]. 2013 [cited 2015 Sep 10]; Available from: www.nytimes.com/2013/12/15/health/the-selling-of-attention-deficit-disorder
- 4.Shah PJ, Morton MJS. Adults with attention-deficit hyperactivity disorder - diagnosis or normality? Br J Psychiatry. 2013;203(5):317–319. doi: 10.1192/bjp.bp.113.126474. [DOI] [PubMed] [Google Scholar]
- 5.Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC, Adler L, Berkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–723. doi: 10.1176/ajp.2006.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biederman J, Petty CR, Evans M, Small J, Faraone SV. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299–304. doi: 10.1016/j.psychres.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kooij SJ, Bejerot S, Blackwell A, Caci H, Casas-Brugué M, Carpentier PJ, Edvinsson D, Fayyad J, Foeken K, Fitzgerald M, Gaillac V, Ginsberg Y, Henry C, Krause J, Lensing MB, Manor I, Niederhofer H, Nunes-Filipe C, Ohlmeier MD, Oswald P, Pallanti S, Pehlivanidis A, Ramos-Quiroga JA, Rastam M, Ryffel-Rawak D, Stes S, Asherson P. European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD. BMC Psychiatry. 2010;10:67. doi: 10.1186/1471-244X-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramos-Quiroga JA, Montoya A, Kutzelnigg A, Deberdt W, Sobanski E. Attention deficit hyperactivity disorder in the European adult population: prevalence, disease awareness, and treatment guidelines. Curr Med Res Opin. 2013;29(9):1093–1104. doi: 10.1185/03007995.2013.812961. [DOI] [PubMed] [Google Scholar]
- 10.Turgay A, Goodman DW, Asherson P, Lasser RA, Babcock TF, Pucci ML, Barkley R, ADHD Transition Phase Model Working Group Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(2):192–201. doi: 10.4088/JCP.10m06628. [DOI] [PubMed] [Google Scholar]
- 11.Matte B, Rohde LA, Grevet EH. ADHD in adults: a concept in evolution. ADHD Atten Deficit Hyperact Disord. 2012;4(2):53–62. doi: 10.1007/s12402-012-0077-3. [DOI] [PubMed] [Google Scholar]
- 12.Fayyad J, Graaf RD, Kessler R, Alonso J, Angermeyer M, Demyttenaere K, Girolamo GD, Haro JM, Karam EG, Lara C, Lépine J-P, Ormel J, Posada-Villa J, Zaslavsky AM, Jin R. Cross–national prevalence and correlates of adult attention–deficit hyperactivity disorder. Br J Psychiatry. 2007;190(5):402–409. doi: 10.1192/bjp.bp.106.034389. [DOI] [PubMed] [Google Scholar]
- 13.Polanczyk G, Rohde LA. Epidemiology of attention-deficit/hyperactivity disorder across the lifespan. Curr Opin Psychiatry. 2007;20(4):386–392. doi: 10.1097/YCO.0b013e3281568d7a. [DOI] [PubMed] [Google Scholar]
- 14.Hinshaw SP, Scheffler RM, Fulton BD, Aase H, Banaschewski T, Cheng W, Mattos P, Holte A, Levy F, Sadeh A, et al. International variation in treatment procedures for ADHD: social context and recent trends. Psychiatr Serv. 2011;62(5):459–464. doi: 10.1176/ps.62.5.pss6205_0459. [DOI] [PubMed] [Google Scholar]
- 15.Giacobini M, Medin E, Ahnemark E, Russo LJ, Carlqvist P. Prevalence, Patient Characteristics, and Pharmacological Treatment of Children, Adolescents, and Adults Diagnosed With ADHD in Sweden. J Atten Disord. 2014;1087054714554617 [DOI] [PubMed]
- 16.Helsedirektoratet. ADHD/hyperkinetisk forstyrrelse – nasjonal faglig retningslinje for utredning, behandling og oppfølging [In Norwegian] [Internet]. 2014. Report No.: IS-2062. Available from: https://helsedirektoratet.no/retningslinjer/adhd
- 17.Sundhedsstyrelsen. National klinisk retningslinje for udredning og behandling af ADHD hos voksne [In Danish] [Internet]. Sundhedsstyrelsen; 2015. Available from: https://sundhedsstyrelsen.dk/da/udgivelser/2015/nkr-adhd-hos-voksne
- 18.Socialstyrelsen. Läkemedelsbehandling av adhd hos barn och vuxna – Stöd för beslut om behandling [In Swedish] [Internet]. 2015. Available from: http://www.socialstyrelsen.se/publikationer2015/2015-4-14
- 19.Embætti landlæknis. Vinnulag við greiningu og meðferð athyglisbrests með ofvirkni (ADHD) – Stytt útgáfa leiðbeininga [In Icelandic] [Internet]. 2014. Available from: http://www.landlaeknir.is/gaedi-og-eftirlit/heilbrigdisstarfsfolk/klininskar-leidbeiningar/leidbeiningar/item14931/ADHD---vinnulag-vid-greiningu-og-medferd
- 20.Hammerness PG, Karampahtsis C, Babalola R, Alexander ME. Attention-deficit/hyperactivity disorder treatment: what are the long-term cardiovascular risks? Expert Opin Drug Saf. 2015;14(4):543–551. doi: 10.1517/14740338.2015.1011620. [DOI] [PubMed] [Google Scholar]
- 21.Statens beredning för medicinsk utvärdering (SBU). ADHD - diagnostik och behandling, vårdens organisation och patientens delaktighet – En systematisk litteraturöversikt [In Swedish] [Internet]. 2013. Report No.: 217. Available from: http://www.sbu.se/sv/Publicerat/Gul/ADHD---diagnostik-och-behandling-vardens-organisation-och-patientens-delaktighet/
- 22.Volkow ND, Swanson JM. Adult attention deficit–hyperactivity disorder. N Engl J Med. 2013;369(20):1935–1944. doi: 10.1056/NEJMcp1212625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castle L, Aubert RE, Verbrugge RR, Khalid M, Epstein RS. Trends in medication treatment for ADHD. J Atten Disord. 2007;10(4):335–342. doi: 10.1177/1087054707299597. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy S, Wilton L, Murray ML, Hodgkins P, Asherson P, Wong IC. The epidemiology of pharmacologically treated attention deficit hyperactivity disorder (ADHD) in children, adolescents and adults in UK primary care. BMC Pediatr. 2012;12(1):78. doi: 10.1186/1471-2431-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Ban E, Souverein P, Swaab H, van Engeland H, Heerdink R, Egberts T. Trends in incidence and characteristics of children, adolescents, and adults initiating immediate- or extended-release methylphenidate or atomoxetine in the Netherlands during 2001–2006. J Child Adolesc Psychopharmacol. 2010;20(1):55–61. doi: 10.1089/cap.2008.0153. [DOI] [PubMed] [Google Scholar]
- 26.Karanges EA, Stephenson CP, McGregor IS. Longitudinal trends in the dispensing of psychotropic medications in Australia from 2009 to 2012: focus on children, adolescents and prescriber specialty. Aust N Z J Psychiatry. 2014;48(10):917–931. doi: 10.1177/0004867414538675. [DOI] [PubMed] [Google Scholar]
- 27.Furu K, Wettermark B, Andersen M, Martikainen JE, Almarsdottir AB, Sørensen HT. The Nordic countries as a cohort for Pharmacoepidemiological research. Basic Clin Pharmacol Toxicol. 2010;106(2):86–94. doi: 10.1111/j.1742-7843.2009.00494.x. [DOI] [PubMed] [Google Scholar]
- 28.WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment 2016 [Internet]. Oslo, Norway; 2015. Available from: http://www.whocc.no/
- 29.Tobi H, Faber A, van den Berg PB, Drane JW, de Jong-van den Berg LT. Studying co-medication patterns: the impact of definitions. Pharmacoepidemiol Drug Saf. 2007;16(4):405–411. doi: 10.1002/pds.1304. [DOI] [PubMed] [Google Scholar]
- 30.Zoega H, Furu K, Halldórsson M, Thomsen PH, Sourander A, Martikainen JE. Use of ADHD drugs in the Nordic countries: a population-based comparison study. Acta Psychiatr Scand. 2011;123(5):360–367. doi: 10.1111/j.1600-0447.2010.01607.x. [DOI] [PubMed] [Google Scholar]
- 31.Lawson KA, Johnsrud M, Hodgkins P, Sasané R, Crismon ML. Utilization patterns of stimulants in ADHD in the Medicaid population: a retrospective analysis of data from the Texas Medicaid program. Clin Ther. 2012;34(4):944–56.e4. doi: 10.1016/j.clinthera.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 32.Christensen L, Sasané R, Hodgkins P, Harley C, Tetali S. Pharmacological treatment patterns among patients with attention-deficit/hyperactivity disorder: retrospective claims-based analysis of a managed care population. Curr Med Res Opin. 2010;26(4):977–989. doi: 10.1185/03007991003673617. [DOI] [PubMed] [Google Scholar]
- 33.Lillemoen PKS, Kjosavik SR, Hunskår S, Ruths S. Prescriptions for ADHD medication, 2004 – 08. Tidsskr Nor Laegeforen. 2012;132(16):1856–1860. doi: 10.4045/tidsskr.11.1270. [DOI] [PubMed] [Google Scholar]
- 34.Pottegård A, Bjerregaard BK, Glintborg D, Hallas J, Moreno SI. The use of medication against attention deficit hyperactivity disorder in Denmark: a drug use study from a national perspective. Eur J Clin Pharmacol. 2012;68(10):1443–1450. doi: 10.1007/s00228-012-1265-y. [DOI] [PubMed] [Google Scholar]
- 35.Bilenberg N, Gillberg C, Houmann T, Kadesjø B, Lensing MB, Plessen KJ, Strand G, Thomsen PH, Worning A. Prescription rates of ADHD medication in the Scandinavian countries and their national guidelines. Nord J Psychiatry. 2012;66(1):70–71. doi: 10.3109/08039488.2011.593102. [DOI] [PubMed] [Google Scholar]
- 36.Zuvekas SH, Vitiello B. Stimulant medication use in children: a 12-year perspective. Am J Psychiatry. 2012;169(2):160–166. doi: 10.1176/appi.ajp.2011.11030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geirs DP, Pottegård A, Halldórsson M, Zoëga H. A Nationwide study of attention-deficit/hyperactivity disorder drug use among adults in Iceland 2003–2012. Basic Clin Pharmacol Toxicol. 2014;115(5):417–422. doi: 10.1111/bcpt.12243. [DOI] [PubMed] [Google Scholar]
- 38.van den Ban E, Souverein PC, Swaab H, van Engeland H, Egberts TCG, Heerdink ER. Less discontinuation of ADHD drug use since the availability of long-acting ADHD medication in children, adolescents and adults under the age of 45 years in the Netherlands. Atten Deficit Hyperact Disord. 2010;2(4):213–220. doi: 10.1007/s12402-010-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pottegård A, Bjerregaard BK, Kortegaard LS, Zoëga H. Early discontinuation of attention-deficit/hyperactivity disorder drug treatment: a Danish Nationwide drug utilization study. Basic Clin Pharmacol Toxicol. 2015;116(4):349–353. doi: 10.1111/bcpt.12325. [DOI] [PubMed] [Google Scholar]
- 40.Aanonsen N, Lensing MB, Prietz R, Gørvell P, Sandven I, Ljøner L. Utprøvende behandling med sentralstimulerende legemidler til voksne med hyperkinetisk forstyrrelse/ADHD [In Norwegian]. Ullevål universitetssykehus; 2004.
- 41.Swift KD, Sayal K, Hollis C. ADHD and transitions to adult mental health services: a scoping review. Child Care Health Dev. 2014;40(6):775–786. doi: 10.1111/cch.12107. [DOI] [PubMed] [Google Scholar]
- 42.Winterstein AG, Gerhard T, Kubilis P, Saidi A, Linden S, Crystal S, Zito J, Shuster JJ, Olfson M. Cardiovascular safety of central nervous system stimulants in children and adolescents: population based cohort study. BMJ. 2012;345:e4627. doi: 10.1136/bmj.e4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Habel LA, Cooper WO, Sox CM, Chan KA, Fireman BH, Arbogast PG, Cheetham TC, Quinn VP, Dublin S, Boudreau DM, Andrade SE, Pawloski PA, Raebel MA, Smith DH, Achacoso N, Uratsu C, Go AS, Sidney S, Nguyen-Huynh MN, Ray WA, Selby JV. ADHD Medications and Risk of Serious Cardiovascular Events In Young and Middle-Aged Adults. JAMA. 2011;306(24):2673–2683. doi: 10.1001/jama.2011.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westover AN, Halm EA. Do prescription stimulants increase the risk of adverse cardiovascular events?: a systematic review. BMC Cardiovasc Disord. 2012;12:41. doi: 10.1186/1471-2261-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, Murray KT, Quinn VP, Stein CM, Callahan ST, Fireman BH, Fish FA, Kirshner HS, O’Duffy A, Connell FA, Ray WA. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896–1904. doi: 10.1056/NEJMoa1110212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 556 kb)