Abstract
To determine the effect of feeding frequency on appetite in normal weight (NW) and obese (OB) prepubertal children, we carried out a prospective, randomized interventional study of 18 NW and 17 OB children ages 6–10. Children received three or five feedings in random order on separate days. Total calories, carbohydrate, protein, and fat composition on each day were equal. Two hours following the last feeding, children were offered ice cream ad lib. The major outcome variable was kilocalories ice cream consumed. A visual analog scale to assess fullness was also administered before consumption of ice cream. We observed that OB children consumed 73.0 ± 37.4 kcal more after five feedings than after three feedings whereas the NW children consumed 47.1 ± 27.8 kcal less. There was significant interaction between meal pattern and weight group indicating that this change in ice cream consumption differed significantly between groups (P = 0.014 by two-factor analysis). Ice cream intake/kg was less in OB compared to NW subjects (P = 0.012). Fullness ratings before ice cream did not differ by meal pattern or weight group. However, pre-ice cream fullness predicted ice cream intake in NW but not OB children. In summary, OB and NW children differed in appetite response to meal frequency. Our data suggest that: (i) satiety in OB children is related more to proximity of calories (larger supper) than to antecedent distribution of calories and; (ii) NW children may be more prone to restrict intake based on subjective fullness.
Introduction
The prevalence of obesity has increased dramatically among children and adolescents in the United States over the past three decades (1, 2). According to the National Health and Nutrition Examination Survey the prevalence of obesity has doubled and overweight tripled for children and adolescents since 1980 (3). Causes appear multifactorial (3–5). Although several studies have addressed the pathophysiology underlying abnormal weight gain in adults, few have been done in children. Obesity and overweight in childhood can lead to obesity in adults. Thus, it is imperative to study the control of food intake in the pediatric population. In particular, prepubertal children merit study since an adiposity rebound occurs between the ages of 5 and 7 when the BMI begins to increase after a steady decline in early childhood (6).
One factor, which may affect appetite, and consequently propensity for obesity, is feeding frequency. Some (7–9) studies of adults suggest that smaller, more frequent meals are associated with reduced body fat. In another report, this effect was gender specific, observed in males but not females (10). In contrast, studies in children (11–17) have not been consistent in demonstrating a relationship between feeding frequency and obesity. Moreover, prior studies in children, and all but a few in adults (8, 9), were epidemiologic rather than interventional and, therefore, limited as to cause and effect. Thus, little is known of the effect of feeding frequency on appetite in children.
Here, we report the results of a randomized, prospective, interventional study in obese (OB) and normal-weight (NW) prepubertal children. We carried out this study to test the hypothesis that more frequent feedings, as compared to equivalent daily caloric intake in the form of three defined meals, would suppress appetite at a subsequent (evening) time point. In addition, to try to gain mechanistic information, we examined the temporal pattern of certain hormonal signals well known to affect appetite (18). These included the adipose hormone, leptin (19), insulin (20), and the gut-derived signals ghrelin (21) and peptide YY (PYY) (22). We report findings somewhat contrary to our hypothesis, challenging existing dogma contending that more frequent feedings favor weight loss in OB individuals.
Methods and Procedures
Statement of ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. The study was approved by the University of Iowa Institutional Review Board and carried out within our Institute for Clinical and Translational Science (former General Clinical Research Center (GCRC)).
Subjects
Eighteen NW and seventeen OB children were recruited. All subjects were between 6 and 10 years of age and had no changes in weight or height (other than consistent with normal growth) for the 3 months before enrollment. All children were prepubertal. In girls, this was defined as absence of breast development and pubic hair. In boys, this was defined as absence of genital development and pubic hair (23, 24).
Inclusion and exclusion criteria
Inclusion criteria required subjects: (i) be otherwise healthy and prepubertal, (ii) age 6–10 years at first study visit. Exclusion criteria included (i) diabetes or any other chronic disease, (ii) any syndrome involving abnormal metabolism or eating, (iii) use of steroids, stimulant medications, or any other medication known to increase or decrease appetite, (iv) signs of puberty on examination.
Because BMI during childhood is age and sex specific, each child's BMI was plotted on growth charts from the Centers for Disease Control. BMI was calculated using an average of three body weight measures divided by an average of three height measures and converted to Z-scores as recommended by the Centers for Disease Control. In accordance with Centers for Disease Control guidelines, a BMI percentile at or above the 95th percentile was considered OB. Demographic information for our subjects is shown in Table 1.
Table 1. Demographic characteristics of the study population.
| Lean | Obese | |
|---|---|---|
| N | 18 | 17 |
| Age (years ± s.e.) | 8.4 ± 0.3 | 8.6 ± 0.3 |
| Age (range) | 6–10 | 6–10 |
| Race (% white) | 83 | 87 |
| Gender (% female) | 61 | 47 |
| Weight (kg ± s.e.) | 28.6 ± 1.2 | 54.9 ± 4.7* |
| BMI (kg/m2 ± s.e.) | 16.65 ± 0.36 | 27.81 ± 1.66* |
| Z-score (±s.e.) | 0.22 ± 0.16 | 2.40 ± 0.09* |
| BMI percentile | 56.67 ± 5.42 | 97.76 ± 0.16* |
P < 0.00001 compared to lean by unpaired two-tailed t-test.
Study protocol
Following screening by telephone or interview, children meeting eligibility criteria were asked to come to the GCRC for study visit 1 (SV1). During SV1 parents signed the informed consent and provided medical history. Children signed an assent form before participating. Children underwent a physical examination and self-reported Tanner staging with parental assistance. Any questions regarding Tanner staging were followed by an examination by a physician. Those subjects meeting inclusion and exclusion criteria continued in the study.
Eligible subjects were admitted to the GCRC at 07:00 h on two separate days, no <2 days or >2 weeks apart (SV2 and SV3). They were asked to eat their last food no later than 20:00 the night before. Children came fasting and were in the GCRC for 13 h. At SV2, the children were randomly assigned one of two meal patterns (five-meal or three-meal pattern) equal in total caloric content served to each subject in a randomized crossover fashion. On return for SV3, subjects were given the alternate meal pattern. The two patterns were as follows: (i) equal caloric (20% total daily energy needs) at 08:00, 10:00, 12:00, 14:00, and 17:00; or (ii) equal caloric (33% of total needs) at 08:00, 12:00, and 17:00. Overall, the same foods were provided to each subject at each study visit differing only in distribution over time (specific food items are listed in Supplementary Data online).
For each of the two meal patterns, energy distribution for each meal, as well as total for the day, were calculated to provide 14% of energy from protein, 32% from fat, and 54% from carbohydrate using the USDA Nutrient Database for Standard Reference and manufacturer's data (25). Total energy expenditure for subjects in the NW group was calculated using the dietary reference intake equation for normal-weight children and weight-maintenance energy expenditure was calculated for subjects in the OB group using the dietary reference intake equation for overweight children (26). Weights of each food on the two meal plans were factored proportionately to provide the estimated energy needs calculated for each subject. To enhance compliance, the research dietitians reviewed the menus with each subject and parent/guardian during the screening visit and, if needed, made adjustments to accommodate food allergies or dislikes. Foods were prepared in the GCRC Metabolic Kitchen and weighed to the nearest gram. Children were asked to consume each meal within 30 min but were allowed 45 min when needed. Food intake was documented after each meal and any food refusals were weighed and recorded. No additional foods or beverages including water were provided between meals.
To control for activity, all subjects were asked to remain in the room or corridor of the GCRC. They watched television, played video games, or read during the day. An intravenous catheter was inserted at 07:30 h following admission to the GCRC at each visit in order to draw blood for insulin, leptin, ghrelin, and PYY. Samples were obtained immediately before breakfast, lunch, and supper as well as 120 min after the last meal. Plasma was obtained from 5 ml of whole blood by centrifugation at 3,000 r.p.m. for 10 min at 4 °C. These samples were immediately stored at −70 °F until assayed.
On SV1, each child was taught to use a visual analogue scale or “Freddy” scale (Supplementary Methods and Procedures online) asking subjects how full they felt according to the parameters established by Keller et al. (27). Each child was introduced to “Freddy” and the slider. They were asked to use “Freddy” to make estimated fullness ratings for five diferent sized portions of French fries and five portions of fruit salads. The children were asked to use the slider to rate how full “Freddy” would feel after consuming the food in the portion displayed. Corresponding to the child's response, a line was drawn across Freddy's stomach and the distance between this line and the bottom of the scale was measured.
At 19:00 on SV2 and SV3 (or 2 h after the last feeding), each child was again presented with the “Freddy” and tested on the proper use. The children were then asked how full their stomach felt. The researcher then made a line across Freddy's stomach on the data sheet corresponding to the child's response and the distance measured as above.
Subjects were then offered ice cream and allowed to select and consume as much as desired, up to a predetermined upper limit of eight scoops. Ice cream was chosen for this study because it is an energy-dense food, palatable to most children and often used as a reward or treat. We opted to offer no more than four scoops at one time (rather than eight), reasoning that this would be simpler and, thus, improve the children's capacity to judge how much they desired. Children were presented with a tray of four premeasured bowls containing one, two, three, or four scoops (corresponding to ¼ cup, ½ cup, ¾ cup, and 1 cup) of chocolate or vanilla ice cream according to their flavor preference. To standardize the effect of any visual cues on ice cream selection, all bowls and spoons were identical in size, shape, and color. Children were asked if they would like some ice cream and instructed to select the bowl they wanted. The selected bowl was given to the child with the instruction to notify the investigator when they were finished eating; the unselected bowls were removed from the child's view. After children finished eating they were asked to rate their level of fullness using the visual analogue scale. Next, they were presented with four more premeasured bowls of ice cream identical to the first tray and were asked if they would like more ice cream. If children selected a second bowl of ice cream, the unselected bowls were again removed and they were allowed to eat until they notified the investigator they were finished. The visual analogue scale was again administered after consumption of the second serving. Children were allowed as much time as they needed to finish their ice cream. For individual subjects, the same flavor was administered at each of the two visits. Ice cream consumption was determined by the difference in weights of the bowls before and after consumption of the amount desired. This procedure allowed us to evaluate the amount of ice cream selected as well as the actual amount consumed.
Hormone and glucose assays
Plasma leptin, insulin, PYY, and acylated ghrelin were measured using commercial radioimmunoassay kits from Linco, St Louis, MO. PYY was assayed as total PYY which measures both PYY1–36 and PYY3–36. HCL (50 mmol/l) and phenylmethylsulfonyl fluoride (100 μg/ml) were included in the samples upon thawing for determination of acylated ghrelin (some loss due to esterase activity on storage or thawing cannot be ruled out). Glucose was measured using a Beckman analyzer.
Statistical analysis
Analyses were performed by two-factor or three-factor linear mixed model analyses for repeated measures, Pearson correlation, stepwise multiple regression, or t-test as indicated in the figure legends, tables, or text. Parameters determined for the same individuals on one or other feeding pattern were analyzed using paired comparisons. A P value <0.05 was considered significant.
Results
All subjects complied with the treatments and followed the protocol.
Consumption of provided meal/snack calories
Total daily calories consumed prior to the ice cream feeding did not differ between the three-meal and five-meal patterns in either the lean or OB children (Table 2).
Table 2. Kilocalories (mean ± s.e.) actually consumed according to group and feeding schedule.
| Kilocalories | ||||
|---|---|---|---|---|
|
| ||||
| Normal weight | Obese | |||
|
|
|
|||
| Three feedings | Five feedings | Three feedings | Five feedings | |
| Breakfast | 530 ± 20 | 324 ± 10 | 770 ± 46 | 465 ± 27 |
| Snack | – | 327 ± 10 | – | 464 ± 27 |
| Lunch | 510 ± 18 | 319 ± 12 | 750 ± 38 | 460 ± 28 |
| Snack | – | 307 ± 12 | – | 466 ± 28 |
| Dinner | 518 ± 18 | 331 ± 9 | 780 ± 41 | 477 ± 28 |
| Total | 1,558 ± 27 | 1,608 ± 28 | 2,300 ± 71 | 2,332 ± 77 |
Ice cream consumption
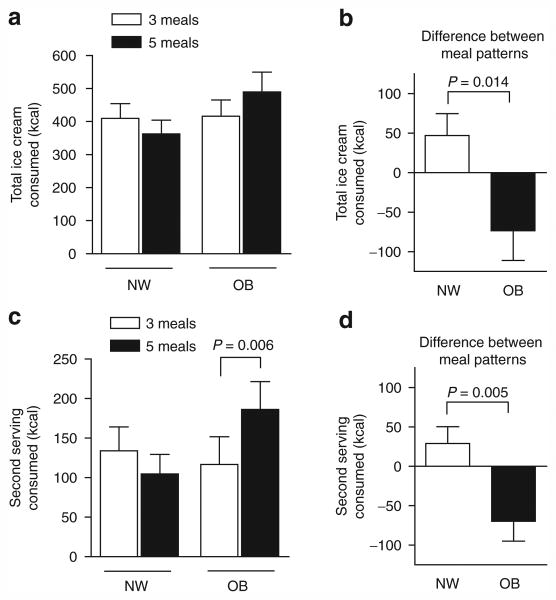
We observed a differential response to meal pattern of the total amount of ice cream consumed between the NW and OB children (Figure 1a,b). There was significant interaction (group × meal pattern, P = 0.014) indicating that the effect of meal pattern was dependent on (differed by) weight group, with OB children consuming more after five meals and NW children consuming more after three meals. Most of the differential in ice cream consumption between the OB and NW children was explained by the amount of ice cream consumed when a second serving was offered (Figure 1c,d). Within the NW or OB groups, Bonferroni post-tests revealed no significant effects of meal pattern on total ice cream consumption. Post-tests did show greater ice cream consumption on the five-meal pattern compared to three by the OB children at the second serving (Figure 1c). However, these post-tests can only be interpreted cautiously in the presence of the significant interaction.
Figure 1.
Ice cream consumption. (a, b) Total ice cream consumed by normal weight (NW, n = 18) and obese (OB, n = 17) children according to meal pattern (a) and as differential values calculated as kcal consumed after three meals minus that consumed after five meals (b). (c, d) Ice cream consumed at the second serving according to meal pattern (c) and as differential values (d). Data were analyzed by two-factor linear mixed model analysis comparing the effect of meal pattern (three or five meals, repeated measures), weight status (NW or OB), and interaction (weight status × meal pattern). The effect of meal pattern differed according to weight group (b, d). Bonferroni post-tests revealed a significant within group increase in ice cream consumption by OB subjects at the second feeding (c).
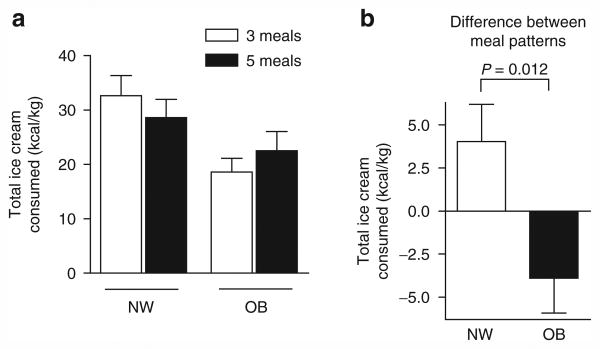
When ice cream consumption was expressed as kcal/kg body mass, similar results were obtained (Figure 2). In this case, there was a significant overall effect of weight group on ice cream consumed per kg body mass (OB < NW, P = 0.012), mostly accounted for by the difference in ice cream consumption following the three meal pattern (Figure 2a).
Figure 2.
Ice cream consumption per kg. (a) Ice cream consumed per kg body weight by normal weight (NW, n = 18) and obese (OB, n = 17) children according to meal pattern. For each child in the OB and NW groups, the differential values were calculated as kcal/kg consumed after three meals minus that consumed after five meals (b). Data were analyzed as in the legend to Figure 1. The effect of meal pattern differed according to weight group (P = 0.012 for interaction). The two-factor analysis also revealed that obese subjects consumed less ice cream per kg body weight than lean (P = 0.031).
We also compared the differential in ice cream consumption (Figures 1b and 2b) between OB and NW children by unpaired t-test (as opposed to the two-factor analyses) and again noted significant differences (P = 0.014 when expressed as kcal and P = 0.004 expressed as kcal/kg).
When the data were examined separately by gender similar results were obtained although the differential effect of meal pattern did not reach significance due to lower numbers of participants.
Using a three-factor linear mixed model analysis with factors consisting of order of study, weight group (NW or OB), and meal pattern there were no significant effects of order (P = 0.85), and no interactive effects for weight group × order (P = 0.50), order × meals (P = 0.95), or weight group × order × meals (P = 0.20) whereas the interaction of weight group × meals remained significant (P = 0.03) consistent with the data in Figure 1. Moreover, we used a backwards stepwise regression model including all 35 children to further assess the impact of factors beyond weight group on the differential ice cream consumption following three or four antecedent feedings. In a model that included age (numerical as 6–10), and categorical variables including weight group, order of study, gender, and flavor of ice cream (chocolate or vanilla), only weight group remained in the model (P = 0.038). Order (P = 0.862), gender (P = 0.418), age (P = 0.379), and flavor (P = 0.382) dropped out.
Amount of ice cream selected
The number of scoops selected at the first and second presentations and total scoops selected are listed in Table 3. Consistent with the data for ice cream consumption OB children selected more scoops after five feedings whereas NW children selected more after three feedings, although significant interaction was observed only upon the second offering.
Table 3. Amount of ice cream selected (number scoops ± s.e.) after each of the two feeding patterns by NW and OB children at the first and second servings and as the total of both selections.
| Group (serving) | Three feedings | Five feedings | Differential |
|---|---|---|---|
| NW (first) | 3.28 ± 0.25 | 2.94 ± 0.29 | 0.33 ± 0.37 |
| OB (first) | 3.29 ± 0.21 | 3.18 ± 0.27 | 0.12 ± 0.21 |
| NW (second) | 1.39 ± 0.36 | 1.28 ± 0.33 | 0.11 ± 0.27 |
| OB (second) | 1.29 ± 0.37 | 2.12 ± 0.39* | −0.82 ± 0.33** |
| NW (total) | 4.67 ± 0.52 | 4.22 ± 0.48 | 0.44 ± 0.47 |
| OB (total) | 4.59 ± 0.52 | 5.29 ± 0.63 | −0.71 ± 0.45 |
NW, normal weight; OB, obese.
Differential amounts were determined by subtracting the selected amount after five feedings from the amount after three feedings. P values were determined by two-factor linear mixed model analysis (weight group and feeding pattern and group × pattern interaction).
P < 0.05 compared to three feeding pattern.
P < 0.05 compared to NW by Bonferroni post-test.
Freddy (visual analogue scale)
Children were considered successfully trained on the proper use of the “Freddy” scale by accurately demonstrating increased fullness of “Freddy” as the portion sizes “consumed by Freddy” increased (P < 0.001). Mean (s.e.) ratings for the smallest and largest portions of French fries were 20.4 (2.8) and 134.9 (4.6) mm for NW and 21.2 (2.9) to 147.4 (4.8) mm for OB; ratings for fruit salad ranged from 25.7 (5.2) to 139.1 (5.2) mm for NW and 20.1 (5.4) to 147.1 (5.4) mm for OB. There was no difference in training scores between NW and OB children and no training effect was observed.
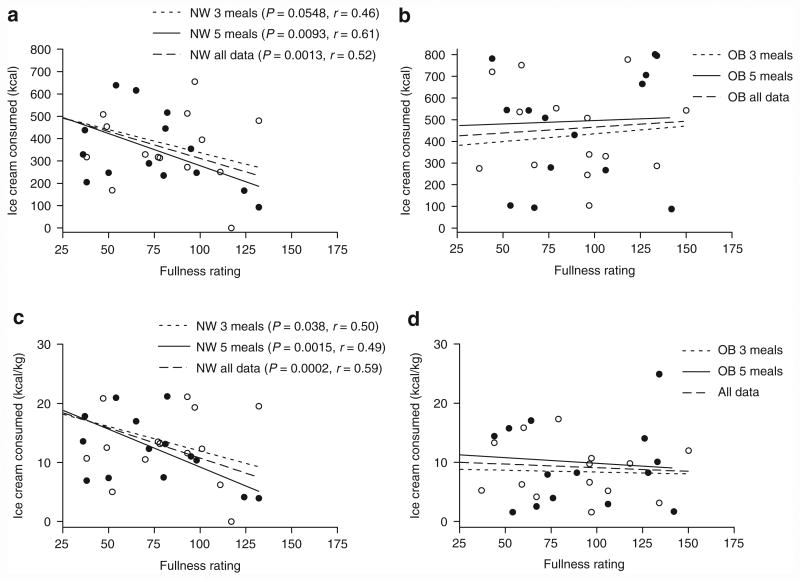
The children's fullness ratings prior to being offered ice cream (either the first or second bowls) or after the second bowl were not different for the two meal patterns or between NW and OB children. However, we found significant correlations between pre-ice cream fullness ratings by the NW children and subsequent total ice cream consumption after both the three- and five-meal patterns (Figure 3). In contrast, no such correlation was evident for the OB children.
Figure 3.
Ice cream consumption as a function of subjective fullness. (a, b) Relationships between pre-supper fullness ratings and total ice cream consumed or (c, d) ice cream consumed per kg for normal we ight (NW) and obese (OB) children. Linear regression analysis was performed for data obtained after the three-meal pattern (open circles) and the five-meal pattern (closed circles) or for the combined data.
Hormone concentrations
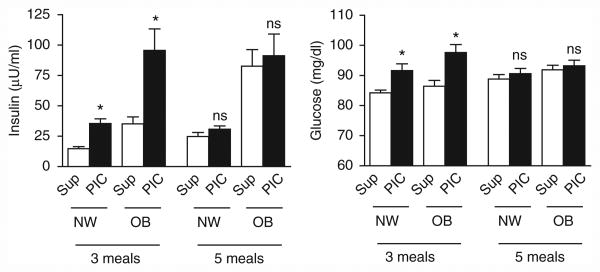
There were no differences in concentrations of leptin or ghrelin on days when the children were fed three meals compared to five (Supplementary Data online, Figure 1). Moreover, immediate pre-ice cream insulin and PYY concentrations did not differ by pattern. Notably, pre-lunch and pre-supper, but not pre-ice cream, insulin concentrations were substantially higher on the five feeding pattern compared to three for both the NW and OB children. The PYY concentrations were also substantially higher pre-lunch and pre-supper on the five feeding pattern compared to three but only for the OB children (Supplementary Data online, Figure 1). For both NW and OB children, plasma glucose concentrations increased significantly between pre-supper and pre-ice cream (2 h post-supper) but only on the three-meal pattern. Pre-supper and pre-ice cream insulin and glucose concentrations are depicted in Figure 4. Complete data for all four hormones and glucose values measured pre-meals and pre-ice cream are included in Supplementary Figure S1 online. The statistical results depicted in Figure 4 consider the complete data sets. However, the results also remain significant if comparisons are limited to the selected data shown in Figure 4.
Figure 4.
Insulin and glucose concentrations determined pre-supper (Sup) and pre-ice cream (PIC) in NW and OB children after the meal pattern indicated. *P < 0.001 compared to pre-supper. Data were analyzed by two-factor linear mixed model analysis comparing the effect of meal pattern (three or five meals) and time (Sup or PIC) with repeated measures for both factors. The analysis considers all the insulin and glucose data (assessed pre-meals and pre-ice cream, and presented in detail in Figure 1 of supplementary data online). NW, normal weight; OB, obese.
It was not our main purpose to compare hormone concentrations between OB and NW children. However, since there is limited data in prepubertal children, we calculated integrated (sum of all) concentrations for each subject combining both study days as well as average fasting concentrations over the two study days. These integrated and fasting values (Table 4) show the insulin and leptin concentrations were far greater in OB than lean subjects. Integrated but not fasting, ghrelin levels were lower in the OB children. There was no significant difference in PYY concentrations.
Table 4. Integrated and average fasting hormone values (mean ± s.e.) over both study days in NW and OB children.
| Normal weight | Obese | P | |
|---|---|---|---|
| Integrateda | |||
| nsulin (μU/ml) | 170 ± 12 | 452 ± 71 | < 0.001 |
| PYY (pg/ml) | 1,176 ± 88 | 1,350 ± 106 | 0.21 |
| Leptin (ng/ml) | 39 ± 7 | 229 ± 41 | < 0.001 |
| Ghrelin (pg/ml) | 264 ± 22 | 191 ± 22 | 0.027 |
| Average fastingb | |||
| Insulin (μU/ml) | 20.2 ± 1.4 | 38.5 ± 4.3 | < 0.001 |
| PYY (pg/ml) | 235 ± 19 | 277 ± 31 | 0.25 |
| Leptin (ng/ml) | 8.9 ± 1.6 | 51.9 ± 8.7 | < 0.001 |
| Ghrelin (pg/ml) | 66.0 ± 5.1 | 55.1 ± 7.9 | 0.26 |
Data were compared by unpaired two-tailed t-test. N = 15–17 for all data indicated and include those subjects for whom all samples on both study days were suitable for analyses (a few samples were not adequate due to insufficient volume).
NW, normal weight; OB, obese.
Summation of pre-meal and pre-bedtime snack values for each child on each day (8 values per child).
Average of two pre-breakfast values.
Discussion
In this study, prepubertal children were offered an ad-lib ice cream dessert following consumption of equal calories and an equal distribution of carbohydrate, fat, and protein on two separate study days. These days differed only in frequency of feeding. We found a differential response to feeding frequency between OB and NW children (Figures 1 and 2) wherein OB children consumed more ice cream after frequent feedings whereas NW children consumed less. Since the last feeding on the three-meal pattern was larger than the last feeding on the five-meal pattern (both administered at 1700 h), our results suggest that appetite in OB children may be influenced more by the number of calories provided at the most recent meal than by the antecedent distribution of the daily caloric supply.
Appetite might be considered to consist of both hunger-based and reward-based eating; the former a necessity for survival, the latter driven more by desire for enjoyable intake (28). We provided full estimated daily energy needs to all children as part of the three- or five-meal pattern before offering ice cream at 1900h. Therefore, although difficult to rigidly document, what we measured likely falls more into the category of reward-based eating or eating in the absence of hunger (29, 30). In this respect, our results, viewed in the context of the clinical obesity epidemic, are all the more meaningful. This is because hunger-based eating would not likely cause obesity, rather representing calories needed for survival and avoidance of malnutrition. On the other hand, by adding calories beyond maintenance needs, eating without hunger would in fact lead to obesity. Not surprisingly, eating in the absence of hunger, has been considered a phenotypic characteristic of overweight or obesity in children (29, 30). Of further note, Fisher et al. (31), in a study of 800 Hispanic children, age 5–18, found that subjects consumed 387 ± 8 kcal in the absence of hunger, very similar to the ice cream energy consumption of our subjects.
Visual clues such as serving size and the size of bowls are known to affect food consumption (32, 33). However, this should not have affected our results because the ice cream was presented in identical fashion after each of the two meal patterns including bowl and spoon size and color and flavor of ice cream.
The meal pattern dependent difference in ice cream consumption between NW and OB children (Figure 1b) may, at first glance, not seem large. However, we point out these differences if repeated on a daily basis over time can add or subtract greatly to body fat stores. For example, a difference of 73.5 cal day (mean difference in meal patterns, Figure 1) over 365 days divided by 3,500 (calories per pound fat) equals 7.7 pounds (3.5 kg) or a fat mass equal to 14% of the mean body weight (Table 1) of our OB children.
The differential response to feeding frequency that we observed (more intake in OB after frequent feedings than lean) may appear discordant with epidemiologic data in adults demon strating an inverse relationship between frequency of eating and obesity (7, 34, 35). However, epidemiologic studies carried out in children are inconsistent, some suggesting a benefit (13, 16, 17) of more frequent feedings whereas others suggesting a trend toward benefit (11, 14) or no significant effect (12, 15). As opposed to other studies of feeding frequency in childhood, our study was not based on associative population data but followed a randomized, interventional design. Our results do not support the concept that more frequent feedings curtail appetite in OB children. However, we acknowledge that our sample size was not large. Moreover, we studied children exposed to only one day of each feeding pattern; whereas, chronic effects of meal pattern on caloric intake may become apparent only after more prolonged study. On the other hand, our results provide impetus to address this issue in a larger, perhaps multicentered, cohort. As suggested in a recent review (36), such studies are clearly needed given the need for preventative approaches to the growing epidemic of childhood obesity.
Another limitation to our work is that it is difficult to precisely match total caloric intake on two different days, especially in prepubertal children. So, some deviation is expected (Table 2). However, this is highly unlikely to have affected our results. First, the differences were small and in significant. Second, most of the difference in the lean subjects was accounted for by two of the 18 subjects. If these are excluded, the intake in the lean subjects becomes 1,560 ± 57 kcal on the three-meal pattern and 1,577 ± 52 on the five-meal pattern, and the results for ice cream intake and selection remain significant for all differences shown in Figures 1 and 2 and Table 3. Third, the OB group actually consumed nonsignificantly more calories during the antecedent five feedings. So, if anything this discrepancy would be expected to have decreased, rather than increased ice cream consumption in the OB group after five meals.
Finally, we acknowledge the limitation that post-tests did not demonstrate that children in either group (OB or NW) consumed significantly greater or significantly less ice cream after the five vs. three feeding patterns (except when expressed as amount consumed at the second feeding). However, this does not detract from the clear differential response between the groups (Figures 1 and 2). Moreover, even if post-tests did reveal differences in ice cream consumption within one or other of the groups, such differences would need to be interpreted with caution given interaction.
Although our major measure of appetite was the quantitative amount of ice cream consumed, we also examined the issue of “fullness” as determined by the “Freddy” scale. We observed no difference in fullness ratings between meal patterns or between NW and OB children. However, in this regard, it may be important to note a limitation to our “Freddy” scale findings based on data by Keller et al. (27). These investigators showed that children were successful at estimating fullness in response to looking at pictures of different sized portions of food but were not able to estimate their own fullness. Thus, it is possible that our subjects may well have been too young for this measure due to limited cognition and lack of ability to quantify their own fullness on a hunger scale.
Interestingly, our data showed that pre-ice cream fullness rating (after both the three- and five-meal patterns) for the NW, but not OB, children correlated negatively with subsequent ice cream consumption (Figure 3). This suggests that either subjective fullness may be a more potent regulator of actual intake in NW than OB children and/or that OB children simply did not recognize fullness as well as the NW group. We were unable to find previous studies wherein fullness had been addressed in predictive fashion in OB compared to lean subjects. Our results appear compatible with the findings of Barkeling et al. (37) who reported that OB compared to NW 11 year old children ate significantly faster and non-significantly more calories. Yet, pre-meal visual analogue data revealed that the OB children demonstrated significantly less pre-meal hunger and desire to eat and nonsignificantly greater fullness. Less sensitivity to fullness might also, at least in part, explain findings in the above mentioned report by Fisher et al. (31) wherein overweight children consumed 14% more energy in the absence of hunger than nonoverweight children.
To seek mechanistic information, we measured pre-meal and pre-ice cream concentrations of certain hormones known to affect appetite (Supplementary Figure S1 online). There were no differences in circulating concentrations of leptin or ghrelin on days when the children were fed three meals compared to five. Moreover, immediate pre-ice cream insulin and PPY concentrations did not differ by pattern, so that differences in these factors do not appear to explain our observed differences in ice cream consumption.
Interestingly, for both NW and OB children, pre-supper insulin concentrations were higher on the five feeding schedule than on the three feeding schedule whereas the pre-ice cream insulin values did not differ by meal pattern (Figure 4). Although the higher pre-supper values may simply reflect proximity to the antecedent mid-afternoon feedings, the equivalent pre-ice cream insulin values were surprising. We had expected lower values following the smaller supper meal (on the five-meal pattern). Although highly speculative, it is possible that the equivalent pre-ice cream insulin on the five-meal pattern (in spite of the smaller supper) was the result of antecedent priming of pancreatic β-cell function (38) due to the multiple feedings. This phenomenon probably deserves further investigation since it is conceivable that children exposed to frequent snacks may experience greater exposure to insulinemia, a concern since hyperinsulinemia is strongly associated with insulin resistance and features of the metabolic syndrome (39). We considered the possibility that persistent elevated glucose on the five-meal pattern might have caused the higher than expected pre-ice cream insulin concentrations. However, this does not appear likely since, for both the lean and OB children, the pre-supper glucose concentrations were similar on the three- and five-meal patterns whereas the pre-supper to pre-ice cream glucose excursion was less on the five-meal pattern. Further investigation of this issue should include more frequent or continuous daylong glucose monitoring and more frequent plasma insulin determinations.
Our major intent was not to compare hormone concentrations between OB and lean children. However, our data do provide information about this issue; for which there is limited data specific to prepubertal children. We compared fasting and integrated pre-feeding concentrations between these groups (Table 4). As expected, insulin and leptin concentrations were far higher in the OB children. Less is known about ghrelin and PYY as affected by obesity. Our data agree with lower preprandial ghrelin levels reported in OB children (40, 41); although subject to the limitation (see Supplementary Methods and Procedures online) that preservatives were added to the plasma specimens only upon thawing. Fasting and integrated PYY levels in OB compared to lean individuals are somewhat controversial, either showing a trend to higher values in OB children (42), lower concentrations in OB adults (43) and children (44), or no correlation (45, 46) with BMI. Here, we observed no difference.
In summary, our findings show that OB and NW children differ in their responses to perturbations in feeding frequency. Our results challenge the contention that more frequent feedings may be beneficial in treating obesity. The biochemical basis underlying our results is not clear from our hormonal studies and needs further investigation. Our study also revealed that subjective fullness prior to an ice cream snack predicted subsequent intake only in the NW children.
Supplementary Material
Acknowledgments
This work was supported by grant M01-RR00059 from the National Institutes of health, National center for Research Resources, General clinical Research centers Program; by funds donated by the Iowa affiliate of the Fraternal Order of the Eagles; by Va Medical affairs medical research funds; and by a 2-year fellowship grant to R.M. from the Genentech Foundation. We thank Judy a. herlein for technical assistance.
Footnotes
Supplementary Material: Supplementary material is linked to the online version of the paper at http://www.nature.com/oby
Disclosure: The authors declared no conflict of interest.
References
- 1.Baskin ML, Ard J, Franklin F, Allison DB. Prevalence of obesity in the United States. Obes Rev. 2005;6:5–7. doi: 10.1111/j.1467-789X.2005.00165.x. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Clément K. Genetics of human obesity. Proc Nutr Soc. 2005;64:133–142. doi: 10.1079/pns2005416. [DOI] [PubMed] [Google Scholar]
- 4.Coll AP, Farooqi IS, O'Rahilly S. The hormonal control of food intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strauss R. Childhood obesity. Curr Probl Pediatr. 1999;29:1–29. doi: 10.1016/s0045-9380(99)80011-5. [DOI] [PubMed] [Google Scholar]
- 6.Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59:955–959. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- 7.Fabry P, Hejl Z, Fodor J, Braun T, Zvolankova K. The frequency of meals. Its relation to overweight, hypercholesterolaemia, and decreased glucose-tolerance. Lancet. 1964;2:614–615. doi: 10.1016/s0140-6736(64)90510-0. [DOI] [PubMed] [Google Scholar]
- 8.Speechly DP, Buffenstein R. Greater appetite control associated with an increased frequency of eating in lean males. Appetite. 1999;33:285–297. doi: 10.1006/appe.1999.0265. [DOI] [PubMed] [Google Scholar]
- 9.Speechly DP, Rogers GG, Buffenstein R. Acute appetite reduction associated with an increased frequency of eating in obese males. Int J Obes Relat Metab Disord. 1999;23:1151–1159. doi: 10.1038/sj.ijo.0801046. [DOI] [PubMed] [Google Scholar]
- 10.Drummond SE, Crombie NE, Cursiter MC, Kirk TR. Evidence that eating frequency is inversely related to body weight status in male, but not female, non-obese adults reporting valid dietary intakes. Int J Obes Relat Metab Disord. 1998;22:105–112. doi: 10.1038/sj.ijo.0800552. [DOI] [PubMed] [Google Scholar]
- 11.Fulkerson JA, Neumark-Sztainer D, Hannan PJ, Story M. Family meal frequency and weight status among adolescents: cross-sectional and 5-year longitudinal associations. Obesity (Silver Spring) 2008;16:2529–2534. doi: 10.1038/oby.2008.388. [DOI] [PubMed] [Google Scholar]
- 12.Huang TT, Howarth NC, Lin BH, Roberts SB, McCrory MA. Energy intake and meal portions: associations with BMI percentile in U.S. children. Obes Res. 2004;12:1875–1885. doi: 10.1038/oby.2004.233. [DOI] [PubMed] [Google Scholar]
- 13.Mota J, Fidalgo F, Silva R, et al. Relationships between physical activity, obesity and meal frequency in adolescents. Ann Hum Biol. 2008;35:1–10. doi: 10.1080/03014460701779617. [DOI] [PubMed] [Google Scholar]
- 14.Nicklas TA, Morales M, Linares A, et al. Children's meal patterns have changed over a 21-year period: the Bogalusa Heart Study. J Am Diet Assoc. 2004;104:753–761. doi: 10.1016/j.jada.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi E, Yoshida K, Sugimori H, et al. Influence factors on the development of obesity in 3-year-old children based on the Toyama study. Prev Med. 1999;28:293–296. doi: 10.1006/pmed.1998.0428. [DOI] [PubMed] [Google Scholar]
- 16.Toschke AM, Küchenhoff H, Koletzko B, von Kries R. Meal frequency and childhood obesity. Obes Res. 2005;13:1932–1938. doi: 10.1038/oby.2005.238. [DOI] [PubMed] [Google Scholar]
- 17.Toschke AM, Thorsteinsdottir KH, von Kries R GME Study Group. Meal frequency, breakfast consumption and childhood obesity. Int J Pediatr Obes. 2009;4:242–248. doi: 10.3109/17477160902763341. [DOI] [PubMed] [Google Scholar]
- 18.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 19.Oswal A, Yeo GS. The leptin melanocortin pathway and the control of body weight: lessons from human and murine genetics. Obes Rev. 2007;8:293–306. doi: 10.1111/j.1467-789X.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- 20.Flint A, Møller BK, Raben A, et al. Glycemic and insulinemic responses as determinants of appetite in humans. Am J Clin Nutr. 2006;84:1365–1373. doi: 10.1093/ajcn/84.6.1365. [DOI] [PubMed] [Google Scholar]
- 21.Kojima M, Hosoda H, Kangawa K. Clinical endocrinology and metabolism. Ghrelin, a novel growth-hormone-releasing and appetite-stimulating peptide from stomach. Best Pract Res Clin Endocrinol Metab. 2004;18:517–530. doi: 10.1016/j.beem.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 22.le Roux CW, Bloom SR. Peptide YY, appetite and food intake. Proc Nutr Soc. 2005;64:213–216. doi: 10.1079/pns2005427. [DOI] [PubMed] [Google Scholar]
- 23.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.U.S. Department of Agriculture ARS. USDA Nutrient Database for Standard Reference. 2004;17 Release. [Google Scholar]
- 26.Medicine Io. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. The National Academies Press; Washington, DC: 2005. [Google Scholar]
- 27.Keller KL, Assur SA, Torres M, et al. Potential of an analog scaling device for measuring fullness in children: development and preliminary testing. Appetite. 2006;47:233–243. doi: 10.1016/j.appet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 29.Faith MS, Berkowitz RI, Stallings VA, et al. Eating in the absence of hunger: a genetic marker for childhood obesity in prepubertal boys? Obesity (Silver Spring) 2006;14:131–138. doi: 10.1038/oby.2006.16. [DOI] [PubMed] [Google Scholar]
- 30.Fisher JO, Birch LL. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. Am J Clin Nutr. 2002;76:226–231. doi: 10.1093/ajcn/76.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher JO, Cai G, Jaramillo SJ, et al. Heritability of hyperphagic eating behavior and appetite-related hormones among Hispanic children. Obesity (Silver Spring) 2007;15:1484–1495. doi: 10.1038/oby.2007.177. [DOI] [PubMed] [Google Scholar]
- 32.Wansink B, van Ittersum K, Painter JE. Ice cream illusions bowls, spoons, and self-served portion sizes. Am J Prev Med. 2006;31:240–243. doi: 10.1016/j.amepre.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Wansink B, Painter JE, North J. Bottomless bowls: why visual cues of portion size may influence intake. Obes Res. 2005;13:93–100. doi: 10.1038/oby.2005.12. [DOI] [PubMed] [Google Scholar]
- 34.Hejda S, Fabry P. Frequency of food intake in relation to some parameters of the nutritional status. Nutr Dieta Eur Rev Nutr Diet. 1964;64:216–228. doi: 10.1159/000175070. [DOI] [PubMed] [Google Scholar]
- 35.Metzner HL, Lamphiear DE, Wheeler NC, Larkin FA. The relationship between frequency of eating and adiposity in adult men and women in the Tecumseh Community Health Study. Am J Clin Nutr. 1977;30:712–715. doi: 10.1093/ajcn/30.5.712. [DOI] [PubMed] [Google Scholar]
- 36.Koletzko B, Toschke AM. Meal patterns and frequencies: do they affect body weight in children and adolescents? Crit Rev Food Sci Nutr. 2010;50:100–105. doi: 10.1080/10408390903467431. [DOI] [PubMed] [Google Scholar]
- 37.Barkeling B, King NA, Näslund E, Blundell JE. Characterization of obese individuals who claim to detect no relationship between their eating pattern and sensations of hunger or fullness. Int J Obes (Lond) 2007;31:435–439. doi: 10.1038/sj.ijo.0803449. [DOI] [PubMed] [Google Scholar]
- 38.Ward WK, Halter JB, Beard JC, Porte D., Jr Adaptation of B and A cell function during prolonged glucose infusion in human subjects. Am J Physiol. 1984;246:E405–E411. doi: 10.1152/ajpendo.1984.246.5.E405. [DOI] [PubMed] [Google Scholar]
- 39.Lann D, LeRoith D. Insulin resistance as the underlying cause for the metabolic syndrome. Med Clin North Am. 2007;91:1063–77. viii. doi: 10.1016/j.mcna.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Reinehr T, de Sousa G, Roth CL. Obestatin and ghrelin levels in obese children and adolescents before and after reduction of overweight. Clin Endocrinol (Oxf) 2008;68:304–310. doi: 10.1111/j.1365-2265.2007.03042.x. [DOI] [PubMed] [Google Scholar]
- 41.Bideci A, Camurdan MO, Cinaz P, Demirel F. Ghrelin, IGF-I and IGFBP-3 levels in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2005;18:1433–1439. doi: 10.1515/jpem.2005.18.12.1433. [DOI] [PubMed] [Google Scholar]
- 42.Lomenick JP, Clasey JL, Anderson JW. Meal-related changes in ghrelin, peptide YY, and appetite in normal weight and overweight children. Obesity (Silver Spring) 2008;16:547–552. doi: 10.1038/oby.2007.129. [DOI] [PubMed] [Google Scholar]
- 43.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 44.Roth CL, Enriori PJ, Harz K, et al. Peptide YY is a regulator of energy homeostasis in obese children before and after weight loss. J Clin Endocrinol Metab. 2005;90:6386–6391. doi: 10.1210/jc.2005-1357. [DOI] [PubMed] [Google Scholar]
- 45.Kim BJ, Carlson OD, Jang HJ, et al. Peptide YY is secreted after oral glucose administration in a gender-specific manner. J Clin Endocrinol Metab. 2005;90:6665–6671. doi: 10.1210/jc.2005-0409. [DOI] [PubMed] [Google Scholar]
- 46.Stock S, Leichner P, Wong AC, et al. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90:2161–2168. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.