Significance
Sclerotinia sclerotiorum hypovirulence-associated DNA virus 1 (SsHADV-1) was originally isolated from a fungal pathogen; recent research revealed that SsHADV-1–like viruses are widespread in various niches including human tissues. Here, we discovered that SsHADV-1 could infect a mycophagous insect, Lycoriella ingenua, when larvae fed on virus-infected fungus; and viruliferous adults could transmit SsHADV-1 transovarially. We further found that SsHADV-1–infected fungus could suppress the production of repellent volatile substances to attract adults to lay eggs on its colony and that virus infection could stimulate female adults to produce more eggs. Our findings may facilitate the exploration of mycoviruses to control fungal diseases and suggest that insects may play an important role in the transmission and distribution of these newly emerging viruses.
Keywords: mycovirus, biological control, mycophagous insect, mutualism, gemycircularvirus
Abstract
Mycoviruses are usually transmitted horizontally via hyphal anastomosis and vertically via sexual/asexual spores. Previously, we reported that a gemycircularvirus, Sclerotinia sclerotiorum hypovirulence-associated DNA virus 1 (SsHADV-1), could infect its fungal host extracellularly. Here, we discovered that SsHADV-1 could infect a mycophagous insect, Lycoriella ingenua, and use it as a transmission vector. Virus acquired by larvae feeding on colonies of a virus-infected strain of S. sclerotiorum was replicated and retained in larvae, pupae, adults, and eggs. Virus could be transmitted to insect offspring when larvae were injected with virus particles and allowed to feed on a nonhost fungus. Virus replication in insect cells was further confirmed by inoculating Spodoptera frugiperda cells with virus particles and analyzing with RT-PCR, Northern blot, immunofluorescence, and flow cytometry assays. Larvae could transmit virus once they acquired virus by feeding on virus-infected fungal colony. Offspring larvae hatched from viruliferous eggs were virus carriers and could also successfully transmit virus. Virus transmission between insect and fungus also occurred on rapeseed plants. Virus-infected isolates produced less repellent volatile substances to attract adults of L. ingenua. Furthermore, L. ingenua was easily observed on Sclerotinia lesions in rapeseed fields, and viruliferous adults were captured from fields either sprayed with a virus-infected fungal strain or nonsprayed. Our findings may facilitate the exploration of mycoviruses for control of fungal diseases and enhance our understanding of the ecology of SsHADV-1 and other newly emerging SsHADV-1–like viruses, which were recently found to be widespread in various niches including human HIV-infected blood, human and animal feces, insects, plants, and even sewage.
Mycoviruses infect fungi and replicate in fungal cells that are widespread in all major fungal groups (1). As a part of the virus world, mycoviruses may play an ecologically important role in nature (2). Furthermore, hypovirulence-associated mycoviruses that infect fungal plant pathogens may have potential to control plant diseases (1, 3–5). Moreover, mycoviruses could potentially be explored to control human fungal diseases (6). Mycoviruses are also very important toward an understanding of the diversity, evolution, and ecology of viruses; they are often found to be phylogenetically related to plant and animal viruses (7, 8), and some newly discovered mycoviruses showed linkages between dsRNA and ssRNA viruses (9–11). Chiba and Suzuki found that one mycovirus could interfere with the replication of an unrelated mycovirus (12). More recently, Zhang et al. discovered that a capsid protein encoded by a dsRNA mycovirus could package a capsidless single-stranded (+) RNA virus genome (13). Mycoviruses are thought to lack an extracellular phase in their lifecycles, and some mycoviruses are associated with latent infections (1) and are only transmitted horizontally via hyphal anastomosis between vegetatively compatible individuals or transmitted vertically through asexual and/or sexual spores (1). Several studies implied the presence of vector organisms for mycoviruses in nature (14–16); Thyreophagus corticalis (Acari, Acaridae) was found to be involved in the spread of Cryphonectria parasitica hypovirus 1 by carrying undigested virus-infected hyphae (17). However, possible transmission of mycoviruses via vectors still needs to be carefully investigated.
Sclerotinia sclerotiorum hypovirulence-associated DNA virus 1 (SsHADV-1) is a circular single-stranded DNA virus with a genome of 2,166 nt, and its particles are isometric with a diameter of 20–22 nm (18). This virus was originally isolated from a hypovirulent strain of S. sclerotiorum, a fungal plant pathogen. After similar ssDNA viruses were detected in dragonflies, SsHADV-1 and SsHADV-1–like viruses were grouped together in a new genus designated Gemycircularvirus (19). Surprisingly, gemycircularviruses are exceptionally widespread in nature. The DNA of SsHADV-1–like viruses was detected in a microbial community from the Sargasso Sea (18) and was found in fecal samples of unexplained human diarrhea (20) and in various animal feces (21–23), insects (such as mosquitoes, dragonflies, and damselflies) (19, 24–26), and plants (27). SsHADV-1–like viral DNA was found in human brain and serum samples from multiple sclerosis patients (28), in human HIV-positive blood (29), in the cerebrospinal fluids of Sri Lankan patients with unexplained encephalitis (20), in blood samples of experimental rats (30), and in the liver and spleen of a horse with fatal idiopathic hepatopathy (31). These discoveries suggest that gemycircularviruses represent a group of emerging viruses, which are widespread in nature, and some of them may impact human and animal health.
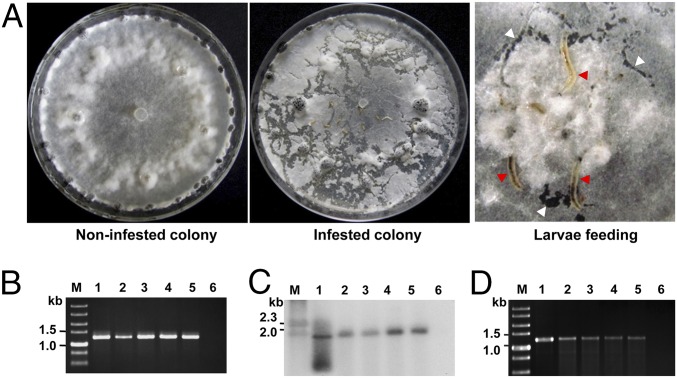
SsHADV-1 has a narrow fungal host range. It could not replicate in Botrytis cinerea, a closely related fungus that shares the same family with S. sclerotiorum (32). However, the fact that viral DNA of SsHADV-1 could be detected in urban river sediments (33) and in damselfly (26) suggests that SsHADV-1 may have hosts belonging to different kingdoms, as S. sclerotiorum usually does not occur in river sewage and does not naturally infect damselfly. Insects in the genus Lycoriella feed on fungi, and some of them, such as Lycoriella ingenua, are notorious pests of cultivated mushrooms (34). We observed that L. ingenua could also feed on mycelia of S. sclerotiorum in the laboratory (Fig. 1A and Fig. S1) and suspected that this mycophagous insect might acquire and transmit fungal viruses when fed on colonies of virus-infected fungi.
Fig. 1.
L. ingenua feeding and virus detection. (A) Larvae feeding on a fungal colony in a PDA plate. Larvae (indicated by red arrowheads) feeding on hyphae and generating galleries (indicated by white arrowheads) on a colony. (B) PCR detection of virus in L. ingenua. A virus-specific DNA product could be amplified from L. ingenua reared on strain DT-8. The diameter of dish was 90 mm, and the width of left photograph was about 13 mm. (C) Southern blot analysis of virus in L. ingenua. (Lanes for B and C) Lane M, DNA size markers; lane 1, DNA from strain DT-8 (positive control); lanes 2–5, DNA samples from eggs, larvae, pupae, and adults reared on strain DT-8, respectively; lane 6, DNA from larvae reared on strain DT-8VF (negative control). A DIG-labeled probe comprising a DNA fragment (814 bp) that corresponds to viral genomic DNA was prepared and used to probe the Southern blots. (D) Virus retention time in the body of larvae, as assayed by PCR. Lane M, DNA marker; lane 1, DNA sample from strain DT-8; lanes 2–5, DNA samples from larvae starved for 1, 3, 5, and 7 d, respectively; lane 6, DNA sample from larvae reared on strain DT-8VF.
Fig. S1.
Morphology of eggs, larva, pupae, and adults of L. ingenua. The various L. ingenua stages were examined under a stereo microscope (Olympus u-Tv0.63xc), and photographs were taken with an Olympus DP72. (Scale bar, 200 μm for eggs and 400 μm for larva, pupa, and adult.)
Results
Acquisition of SsHADV-1 by L. ingenua from a Virus-Infected Fungal Isolate.
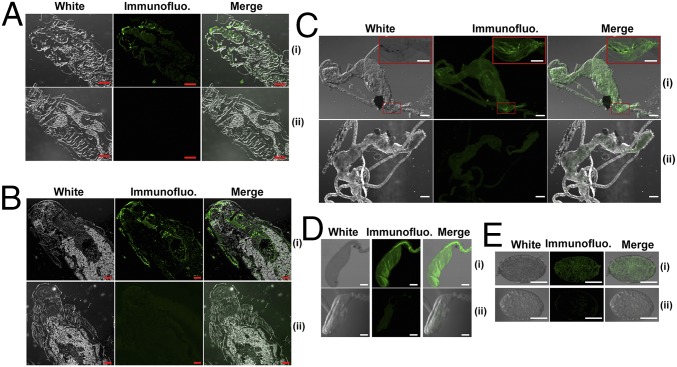
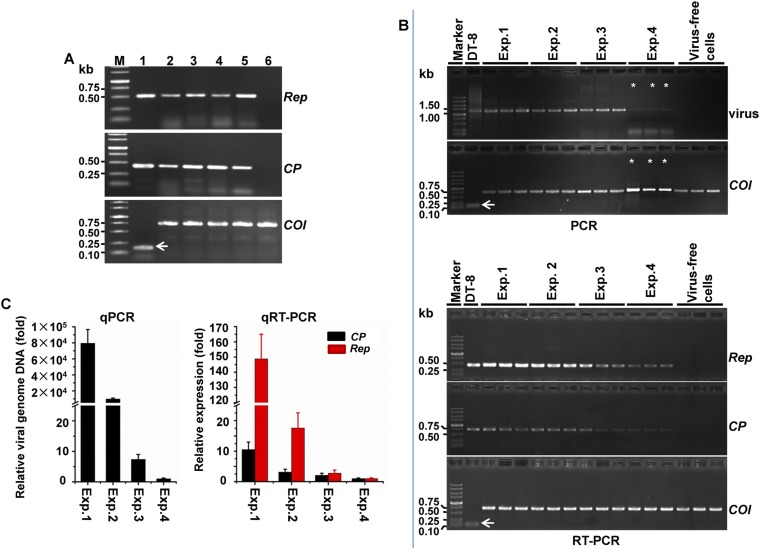
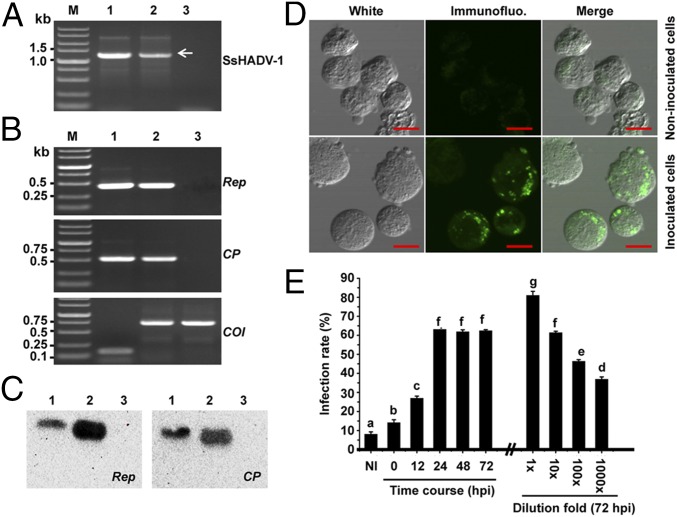
SsHADV-1–free larvae were reared on a colony of virus-free strain DT-8VF, and the emerging pupae were collected and placed on a colony of virus-infected strain DT-8. When adults emerged, the female adults laid eggs, and the newly hatched larvae, which consumed hyphae, were allowed to develop on the virus-infected colony of strain DT-8. Larvae, pupae, adults, and eggs were taken from these colonies and subjected to virus detection assays. Viral genomic DNA was PCR-amplified from larvae, pupae, adults, and eggs (Fig. 1B), and the sequences of the PCR products were identical to that of the SsHADV-1 genomic sequence. Southern blot analysis further confirmed that virus is present on and/or in larvae, pupae, adults, and eggs (Fig. 1C). Immunofluorescence detection with an antibody to viral coat protein showed that virus existed in cells of larvae, pupae, adults, and eggs (Fig. 2). Larvae, when fed on virus-infected colony, could acquire virus in a short period of acquisition feeding, as virus was detected in 5 larvae out of 10 pursuant to feeding on a colony of strain DT-8 for 30 min (Fig. S2A). We could not judge, however, whether the virus had already entered into insect tissue or was still retained in fungal hyphae in the insect digestive tract. Nevertheless, SsHADV-1 viral genomic DNA was detected in larvae that were starved for as long as 7 d (Fig. 1D).
Fig. 2.
Immunofluorescence detection of SsHADV-1 in L. ingenua. Insects developed either on strain DT-8 (i) or DT-8VF (ii) (negative control) were used for detection. (A) Detection in larvae. Longitudinal sections of larvae (head parts) were shown. (B) Detection in pupae. Longitudinal sections of pupae (head parts) were shown. (C) Detection in midgut of female adults. Photographs shown at the upper corner were magnified from a part of the midgut (indicated by red boxes). (D) Detection in ovarian duct of female adults. (E) Detection in eggs dissected from female adults. (Scale bars at the bottom right, 200 μm for A and B, 100 μm for C–E, and 50 μm for the upper corner in C.) Samples were stained with secondary antibody FITC conjugated to viral particle-specific monoclonal antibody prepared with viral CP protein. The immunofluorescence reaction was observed under a confocal microscope (fv1000mp, Olympus).
Fig. S2.
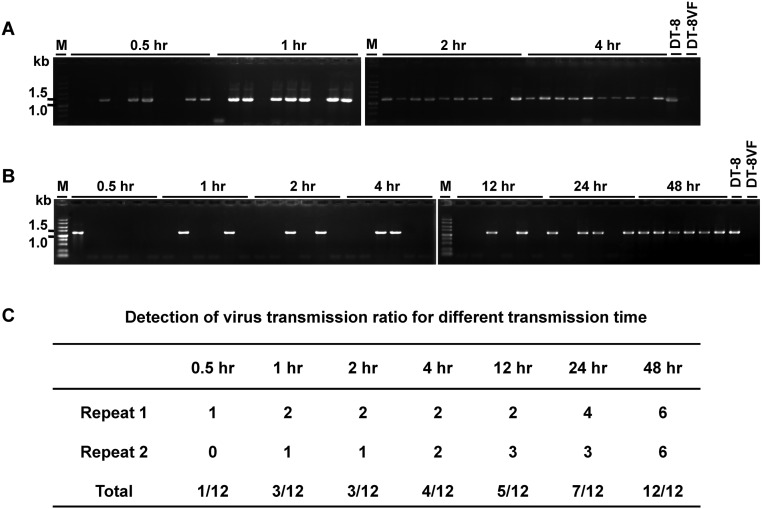
Determination of minimum time required for virus acquisition and transmission by L. ingenua. (A) Minimum time period for acquisition and (B) minimum time period for transmission of SsHADV-1. To determine the minimum time for virus acquisition, larvae reared on a colony of strain DT-8VF were picked up and placed on a colony of strain DT-8 for 0.5, 1, 2, or 4 h and then collected and washed with distilled ddH2O before detection. To find out the minimum time for virus transmission, larvae collected from a colony of strain DT-8 were placed in a small glass beaker starved for 1 d and then were placed on a colony of strain DT-8VF for 0.5, 1, 2, 4, 12, 24, or 48 h. (C) Total virus-infected subcultures of two repeats for the minimum transmission time detection. Six subcultures were tested at each time point. DNA samples of strain DT-8 and DT-8VF were used as controls.
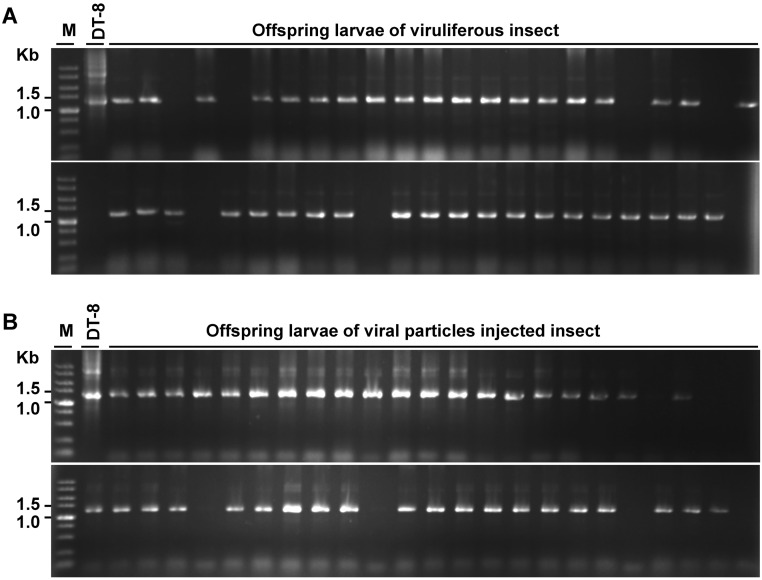
To reduce the probability that virus detected from insect was potentially derived from contaminating virus-infected hyphal debris, either viruliferous pupae developed on a colony of strain DT-8 or virus-free larvae that were injected with virus particles suspension were placed on colonies of the nonhost fungus B. cinerea to generate offspring, and then the newly emerging larvae on B. cinerea colonies were sampled for virus detection individually using PCR amplification. Thirty nine out of 46 and 41 out of 46 of the sampled two groups of larvae (those derived from viruliferous pupae and virus-injected larvae), respectively, were found to be virus-infected (Fig. S3). Thus, L. ingenua could pass SsHADV-1 to its offspring.
Fig. S3.
Detection of SsHADV-1 from progeny larvae. (A) Detection from progeny larvae of viruliferous pupae. Viruliferous pupae developed on strain DT-8 were collected and placed on a colony of B. cinerea to develop progeny larvae for virus detection. (B) Detection from progeny larvae of virus particle-injected larvae. Virus-free larvae reared on strain DT-8VF were injected with virus particles and placed on a colony of B. cinerea to develop progeny larvae for virus detection.
Replication of SsHADV-1 in Insect Cells.
Because SsHADV-1 Rep and CP gene transcripts in insects reared on strain DT-8 were successfully detected with RT-PCR amplification (Fig. S4A), we suspected that SsHADV-1 could replicate in insect cells. To further confirm this finding, purified SsHADV-1 particles (300 ng/µL) either nondiluted or serially diluted were used to inoculate Spodoptera frugiperda cells (Sf9), which were grown in Grace’s medium, and then the cells were subjected to virus detection with RT-PCR amplification, Northern blot, immunofluorescence, and flow cytometry assays. Again, virus-specific DNA could be amplified with virus-specific primers (Fig. 3A). Moreover, RT-PCR amplification (Fig. 3B) and Northern blot analysis (Fig. 3C) established that viral Rep and CP genes were expressed in virus-inoculated Sf9 cells. Furthermore, viral fluorescence signals were observed in virus-inoculated Sf9 cells using immunofluorescence detection assays (Fig. 3D). The infection rates of insect cells were measured with flow cytometry, and the results showed that infection of insect cells inoculated with virus particle suspension (30 ng/μL) could be detected at 12 h postinoculation (hpi); then the infection rate increased up to 60% at 24 hpi, and this infection level was maintained for 72 hpi (Fig. 3E). Virus infection could be still detected when the original virus particle suspension (300 ng/μL) was diluted 1,000-fold and assayed at 72 hpi (Fig. 3E). The results of passage experiments showed that SsHADV-1 replicated in second-passage Sf9 cells, as viral DNA and transcripts of Rep and CP were successfully detected with qRT-PCR (Fig. S4 B and C). Taken together, these results suggest that SsHADV-1 could replicate in cultured cells of an experimental insect host, S. frugiperda, as well as in cells of its presumptive natural host, L. ingenua.
Fig. S4.
Detection of SsHADV-1 from L. ingenua (A) and second-passage Sf9 cells (B and C). (A) RT-PCR for detecting the expression of the SsHADV-1 Rep and CP gene in L. ingenua. Lane M, DNA marker; lanes 1–6, RNA samples of strain DT-8 (positive control); eggs, larvae, pupae, and adults reared on strain DT-8; and larvae reared on strain DT-8VF (negative control), respectively. (B) Detection of DNA and transcripts of Rep and CP of SsHADV-1 from second-passage Sf9 cells with RT-PCR. Four dosages of virus particles (300, 30, 3, and 0.3 ng/μL) were used to inoculate P0 cells and were designated as Exp. 1, Exp. 2, Exp. 3, and Exp. 4, respectively. Three repeats were prepared for each dosage. Noninoculated Sf9 cells (virus-free cells) were used as negative control. In each lane, 3 μL PCR products were loaded, whereas 10 μL PCR products were loaded in the lanes marked with asterisks. (C) qPCR and qRT-PCR detection of viral DNA and transcripts of Rep and CP from second-passage Sf9 cells. Viral DNA and viral transcripts were examined from the second-passage cells at 24 hpi. The expression of the Actin gene for S. sclerotiorum strain DT-8 (shown with a white arrow) and COI gene for L. ingenua and Sf9 cells were amplified as controls.
Fig. 3.
Replication of SsHADV-1 in S. frugiperda cells (Sf9). (A) PCR amplification of SsHADV-1 in virus-inoculated Sf9 cells. Viral DNA was amplified from virus-inoculated Sf9 cells (indicated by white arrow). Lane M, DNA marker; lanes 1–3, DNA from strain DT-8, virus-inoculated Sf9 cells, and noninoculated Sf9 cells. (B) RT-PCR detection of viral Rep and CP gene transcripts in virus-inoculated Sf9 cells. Viral Rep and CP transcripts were amplified in virus-inoculated Sf9 cells. The Actin gene for strain DT-8 (shown with white arrow) and COI gene for Sf9 cells were RT-PCR–amplified as positive controls. Lane M, DNA marker; lanes 1–3, RNA from strain DT-8, virus-inoculated Sf9 cells, and noninoculated Sf9 cells. (C) Northern blot analysis for expression of the viral Rep and CP gene. DIG-labeled DNA probes of 853 bp and 901 bp corresponding to viral DNA for the Rep and CP gene, respectively, were used. Lanes 1–3, RNA from strain DT-8, virus-inoculated Sf9 cells, and noninoculated Sf9 cells. (D) Immunofluorescence detection of SsHADV-1 in Sf9 cells. Cells were inoculated with SsHADV-1 particles, and observations were carried out at 72 hpi, as described in Fig. 2. (Scale bar, 10 μm.) (E) Virus infection rates of Sf9 cells. Sf9 cells were inoculated with 100 μL (30 ng/μL) virus particle suspension and incubated for different time points to detect virus infection rates; Sf9 cells were inoculated with 100 μL serially diluted virus particle suspension (original concentration was 300 ng/μL) and incubated for 72 hpi. NI, noninoculated cells. The lowercase letters on top of each column indicate differences that are significant (P < 0.05).
Transmission of SsHADV-1 to a Virus-Free S. sclerotiorum Isolate by L. ingenua.
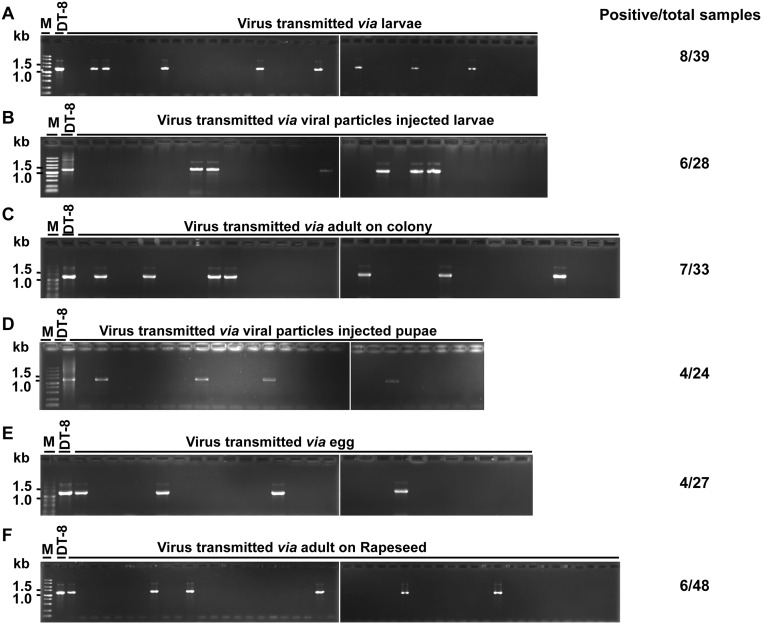
Larvae reared on a virus-infected colony were carefully picked up with sterilized forceps or brush and gently washed in sterilized ddH2O to remove possible hyphal debris attached to the larvae body. To test the possibility of transmission, the water-bathed larvae were further reared on a colony of strain DT-8VF for 1 d, and then hyphal agar plugs were taken from the larvae-feeding galleries (Fig. 1A) and placed on fresh potato dextrose agar (PDA) plates. These new subcultures were subjected to virus detection assays. The viral genomic DNA of SsHADV-1 extracted from the mycelial mass of newly infected subcultures was detected by PCR amplification (Fig. S5A). We tested 39 subcultures, eight of which were found to be infected with SsHADV-1, and virus infection of three subcultures—LT3, LT4, and LT9—was further confirmed by Southern blot analysis (Fig. 4C). Larvae reared on a colony of strain DT-8VF were injected with virus particles and starved for 1 d and then were used to repeat this experiment, and the results showed that 6 subcultures out of 28 tested were infected with SsHADV-1 (Fig. S5B). These results suggest that viruliferous larvae could transmit SsHADV-1 when allowed to feed on a virus-free strain.
Fig. S5.
Verification of SsHADV-1 transmission to fungi via L. ingenua using PCR amplification. (A) Via larvae (8/39 subcultures), viruliferous larvae were fed on strain DT-8VF, and then hyphal agar plugs were taken from the feeding galleries (as indicated in Fig. 1A) to fresh PDA plates to develop new subcultures for virus detection. (B) Via virus particle-injected larvae (6/28 subcultures), virus particle-injected larvae were starved for 1 d to allow virus replication in larvae and then placed on a colony of strain DT-8VF for further feeding. Subcultures isolated from the larvae-feeding galleries were subjected to virus detection. (C) Via adults on a fungal colony (7/33 subcultures), viruliferous pupae were collected and placed in a small glass beaker to physically separate the pupae and fungal colonies. After emergence, the adults laid eggs on colonies of strain DT-8VF, the emerging larvae fed on them, and subcultures were developed from feeding galleries for virus detection. (D) Via virus particle-injected pupae (4/24 subcultures), virus particle-injected pupae were placed in a small glass beaker to physically separate the pupae and fungal colonies. After emergence, the adults laid eggs on colonies of strain DT-8VF and the emerging larvae fed on them. Subcultures were developed from feeding galleries for virus detection. (E) Via eggs (4/27 subcultures), viruliferous adults laid eggs on filter paper, and these eggs were transferred to colonies of strain DT-8VF with a soft brush to allow hatching on these colonies. Subcultures were developed from feeding galleries for virus detection. (F) Via L. ingenua adults (6/48 subcultures) on plants, virus-free larvae were placed on lesions induced by strain DT-8, subcultures of which were recovered from diseased plant residues of virus-free strain 1980-inoculated plants subjected to virus detection. DNA sample of strain DT-8 was used as the control.
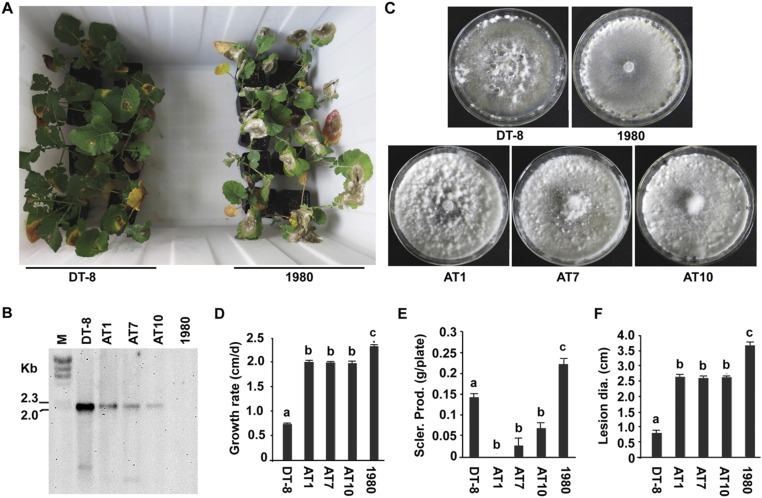
Fig. 4.
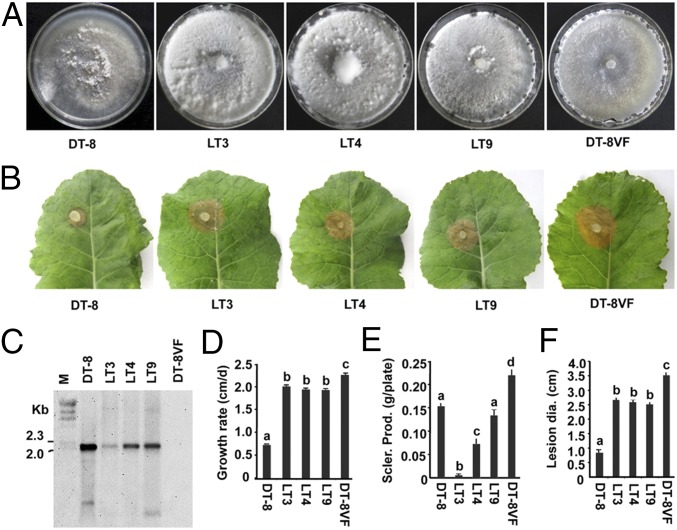
Transmission of SsHADV-1 to virus-free fungal strain via larvae of L. ingenua. (A) Colony morphology of virus-infected isolates (colonies were developed on a PDA plate at 20 °C for 7 d). (B) Virulence analysis of virus-infected isolates on detached rapeseed leaves; lesion diameters were measured at 48 hpi. (C) Confirmation of virus transmission of PCR-positive subcultures (LT3, LT4, and LT9) with Southern blot analysis. See Fig. 1 for probe preparation. Viral DNA was amplified with rolling circle amplification (RCA). DNA samples of strain DT-8 and DT-8VF were used as controls. (D–F) Sclerotial production and growth rate of newly infected subcultures growing on a PDA plate at 20 °C. Sclerotia were counted on the 15th day of cultivation. The lowercase letters on top of each column indicate differences that are significant (P < 0.05).
The acquisition feeding time required for virus transmission by larvae was examined. The results showed that a minimum feeding time of 0.5 h is required, at which time point we checked a total of 12 subcultures and only one subculture was found to be SsHADV-1–infected. The frequency of transmission was enhanced slowly with increasing acquisition feeding time from 1 h to 24 h, eventually following 48 h of acquisition feeding; all examined subcultures were infected with SsHADV-1 (Fig. S2 B and C).
We suspected that L. ingenua adults could transmit virus, as adults also carried SsHADV-1. To test this assumption, adults that emerged from pupae developed on colonies of strain DT-8 were allowed to oviposit on colonies of strain DT-8VF, and the hatched larvae were allowed to feed on the same DT-8VF colonies. Subcultures derived from larvae-feeding galleries were sampled for virus detection, and the results showed that seven subcultures out of 33 tested were infected with SsHADV-1 (Fig. S5C). Alternatively, in experiments using pupae that developed on strain DT-8VF and injected with virus, particles showed that 4 subcultures out of 24 tested were infected with SsHADV-1 (Fig. S5D). Thus, viruliferous adults could transmit SsHADV-1.
The fact that SsHADV-1 was successfully detected in eggs of L. ingenua and that adults of L. ingenua do not feed on fungal colonies suggested that adults transmit virus via eggs. The eggs laid by viruliferous adults were collected and placed on colonies of strain DT-8VF, and 3 d later, the emerged larvae crawled into the colonies and fed on them. Colonies derived from larvae-feeding galleries were sampled for virus detection, and the results showed that 4 out of 27 subcultures were infected with SsHADV-1. These results suggest that virus transmission could occur through viruliferous eggs of L. ingenua (Fig. S5E).
Traits of Virus-Infected Strains Following Insect-Mediated Transmission.
Colony morphology, growth rate, sclerotial production, and virulence on rapeseed of newly virus-infected isolates were examined. The results showed that colonies that developed on PDA plates were rich in aerial hyphae (Fig. 4A) and produced few or no sclerotia at a late stage of colony growth (Fig. 4E). The growth rate of these newly infected isolates was significantly reduced compared with that of strain DT-8VF, but they grew faster than strain DT-8 (Fig. 4D). Although these virus-infected isolates still maintained a certain level of virulence, their virulence was significantly reduced compared with strain DT-8VF (Fig. 4 B and F). These traits were also observed on virus-infected isolates transmitted via adults and eggs.
Virus Transmission from a Fungal Pathogen Residing on Plants via L. ingenua.
Virus-free larvae were placed on lesions induced by strain DT-8 on rapeseed plants at one side of a plastic box, and plants on the other side were inoculated with strain 1980, a virus-free strain that is labeled with a hygromycin resistance gene. Because strain 1980 is vegetatively incompatible with strain DT-8, virus transmission via hyphal anastomosis could be avoided (Fig. S6A). Two weeks later, plants inoculated with strain 1980 were killed, and 48 subcultures of strain 1980 were recovered from diseased plant residues by growing on hygromycin-amended PDA and all subcultures were subjected to virus detection assays. The results showed that viral genomic DNA was detected from six subcultures by PCR amplification (Fig. S5F), and virus infection of subcultures AT1, AT7, and AT10 was confirmed by Southern blot analysis (Fig. S6B). Compared with SsHADV-1–free strain 1980, these virus-infected subcultures often exhibit abnormal colony morphology, such as abundance of aerial hyphae and significant reduction in growth rate, sclerotia production, and virulence (Fig. S6 C–F). These traits are similar to those of virus-infected subcultures via larval transmission.
Fig. S6.
Transmission of SsHADV-1 to a virus-free fungal strain by L. ingenua on rapeseed plants. (A) Arrangement of rapeseed plants inoculated with either strain DT-8 (Left) or SsHADV-1–free strain 1980 (Right) in a plastic box. The distance between plants on the opposite two sides was 30 cm. Virus-free larvae were placed on lesions induced by strain DT-8, subcultures of which were recovered from diseased plant residues of virus-free strain 1980-inoculated plants subjected to virus detection. (B) Confirmation of virus infection of PCR-positive subcultures (AT1, AT7 and AT10) with Southern blot analysis. See Fig. 1 for probe preparation. Viral DNA was amplified with RCA; DNA samples of strain DT-8 and 1980 were used as controls. (C–F) Colony morphology, growth rate, sclerotial production, and virulence of virus-infected subcultures AT1, AT7, and AT10. See Fig. 4 for culturing condition and virulence assay.
Adults Tend to Lay Eggs on Virus-Infected Colonies.
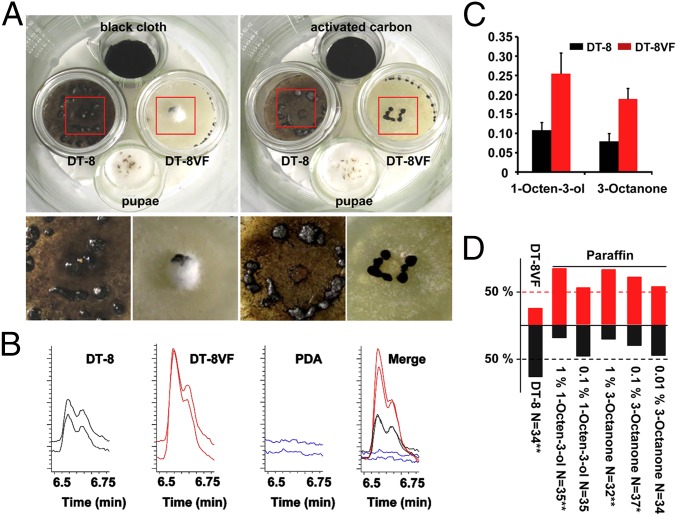
Two glass bottles, one with a colony of strain DT-8VF and another with a colony of strain DT-8, and two small glass beakers with 10 pupae of L. ingenua or a piece of black cloth were placed in a large glass beaker and incubated at 23 °C. When adults emerged from pupae, they chose the colony of strain DT-8 for laying eggs. About 10 d later, a large number of larvae appeared on the colony of strain DT-8 and consumed all hyphae, whereas larvae rarely appeared on the colony of strain DT-8VF. However, when activated carbon was added in the large beaker, both virus-free and SsHADV-1–infected colonies were consumed by larvae (Fig. 5A). The tendency of insects to prefer virus-infected colonies was further confirmed with a “Y”-shaped tube test with volatiles collected from strains DT-8 and DT-8VF. The results showed that 27 out of 35 female adults chose volatiles released by strain DT-8 (Fig. 5D). We collected the volatiles produced by either strain DT-8 or DT-8VF, subjected them to GC–MS analysis, and found that the pattern of volatiles released by strain DT-8 was similar to that by strain DT-8VF (Fig. S7), but the quantity of 1-Octen-3-ol and 3-Octanone produced by strain DT-8 was significantly lower than that by strain DT-8VF (Fig. 5 B and C). Furthermore, Y-shaped tube tests showed that high concentrations of 1-Octen-3-ol and 3-Octanone were likely to repel L. ingenua adults (Fig. 5D).
Fig. 5.
Preference of L. ingenua on virus-infected strain and volatile analysis. (A) Adults tended to lay eggs on the virus-infected strain DT-8 colony, and hyphae of strain DT-8 were consumed by emerging larvae. The large glass beaker containing pupae (p), black cloth (bc), and colonies of strains DT-8 and DT-8VF were maintained at 23 °C for 10 d (Left). (Right) The black cloth was replaced by activated carbon (ac), and others were the same as in Left. The Bottom photographs were magnified from parts of the red boxes. (B) The peak and retention time for 1-Octen-3-ol (6.588 min) and 3-Octanone (6.700 min). The whole profile patterns of volatile chemicals of strains DT-8 and DT-8VF detected by GC–MS were presented in Fig. S7. (C) Quantitative analysis of 1-Octen-3-ol and 3-Octanone volatilized by strain DT-8 and DT-8VF. The number on the y axis was the relative peak area of 1-Octen-3-ol and 3-Octanone compared with nonyl acetate, which was defined as 1. (D) Preference analysis of L. ingenua using a Y-shaped tube test. 1-Octen-3-ol and 3-Octanone resolved in paraffin and volatiles collected from strains DT-8 and DT-8VF were used for the test group and paraffin was used as the control. The number on the y axis represents the ratio of insects choosing the tested chemicals or volatiles. Numbers choosing chemical or volatiles were given “X2” test, *P < 0.05 and **P < 0.01.
Fig. S7.
Comparison of volatile patterns produced by strains DT-8 and DT-8VF and PDA medium. The two strains were allowed to grow on PDA plates for 10 d, and the volatiles were pumped out under room temperature for 12 h. The collected volatiles were then analyzed with GC–MS; the volatile chemicals of the fungal medium, PDA, were analyzed as the control.
L. ingenua Individuals Exist in Rapeseed Fields and Carry SsHADV-1.
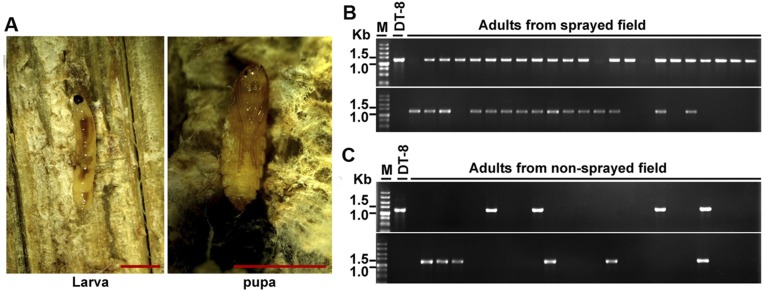
Larvae and pupae were frequently found on diseased residuals of S. sclerotiorum-infected rapeseed, with preference to the inner parts of diseased stems of rapeseed (Fig. S8A). Adults could be found on leaves and petals of rapeseed. Adults of L. ingenua were collected either from a strain DT-8–sprayed rapeseed field or from a nonsprayed rapeseed field and then subjected to virus detection. In the strain DT-8–sprayed field, 43 adults were captured, and viral DNA was successfully detected from 35 adults (Fig. S8B); 49 adults were captured from the nonsprayed field, which is 19.2 km away from the strain DT-8–sprayed field, and 11 of these 49 adults were found to carry the virus (Fig. S8C). The PCR-amplified products from adults in both rapeseed fields were further sequenced and confirmed to originate from SsHADV-1 sequences.
Fig. S8.
Occurrence of L. ingenua in a rapeseed field and virus detection. (A) Larvae and pupae resided in the inner parts of the diseased stem induced by S. sclerotiorum in a rapeseed field. (Scale bars at the bottom right, 400 μm.) Virus detection in adults captured from a strain DT-8–sprayed rapeseed field (B) and adults from a nonsprayed rapeseed field (C), which was about 19.2 km away from the sprayed field.
Discussion
In this study, we discovered that the fungal DNA virus SsHADV-1 could infect larvae of L. ingenua when reared on colonies of a virus-infected strain and that the virus could replicate in larvae, pupae, adults, and eggs of L. ingenua. Furthermore, L. ingenua could transmit SsHADV-1 transovarially. We propose that L. ingenua not only serves as a transmission vector but also is a natural host of SsHADV-1. Additionally, we found that SsHADV-1 could replicate in S. frugiperda cells, suggesting S. frugiperda is a potential host. Previously, the viral DNA genome of SsHADV-1 was detected in nature in damselflies (26), suggesting that damselflies are likely to be a host of SsHADV-1. Thus, SsHADV-1 is most likely to have a broad insect host range and wide distribution in nature, as L. ingenua, S. frugiperda, and damselflies are distantly related phylogenetically and they belong to different orders in Insecta.
Our findings imply that gemycircularviruses have transkingdom hosts and could be transmitted via insects. The gemycircularviruses in animal feces might have originated from foods (such as plants and mushrooms). For example, the ancient caribou feces-associated virus (aCFV), which was discovered in 700-y-old caribou feces, could infect Nicotiana benthamiana (23). Gemycircularviruses, which were detected in samples of human blood, serum, cerebrospinal fluids, and brain (28, 29), as well as horse liver and spleen (31), most likely replicate and are retained in human and animal cells, suggesting that humans and animals are possible hosts of these viruses. The finding that gemycircularviruses were detected in mosquitoes suggests that mosquitoes and other parasitic insects such as fleas as well as parasitic mites and ticks may serve as vectors for transmission of human and animal gemycircularviruses. Our finding may provide a clue to understanding the ecology and evolution of these newly emerging small circular ssDNA viruses and provide an avenue to study multiple ecological interactions among fungi, viruses, and insects.
Insects as virus transmission vectors are very common in nature, as insects transmit many plant and animal viruses. Most plant viruses are transmitted by insects with piercing sucking mouthparts. The virus could enter into the body of an insect and be released into the plant through a circulatory system during the next feeding cycle (35). Transmission of mycoviruses via L. ingenua may not be specific, but the larvae of L. ingenua have chewing mouthparts to uptake hyphae. Virus particles may be released from mechanically broken hyphae during the drawing of hyphae into the mouth, or virus particles may also be released from hyphae digested by enzymes in the intestine of larvae, and then the released virus may infect the insect. This hypothesis is supported by the findings that viral genomic DNA could be detected in starving larvae, pupae, adults, and eggs (Fig. 1) and that SsHADV-1 could replicate in Sf9 cells (Fig. 3). Furthermore, SsHADV-1 has strong extracellular infection ability and is stable (32); SsHADV-1–like viruses are likely to be very stable, as their full-length genomic DNA was detected from 700-y-old animal feces (23). However, details of how SsHADV-1 infects insects and replicates in insect cells need to be studied in the future.
Viruses modulate host secondary metabolism pathways to facilitate their transmission by insect vectors. This is very common in plant/virus/vector systems, such as geminiviruses, which could modulate their hosts to attract whiteflies (36); tomato spotted wilt tospovirus, which could affect its transmission vector Frankliniella fusca by regulating its host plant (37); barley yellow dwarf virus, which could change its host to attract aphids (38); and cucumber mosaic virus, which could change the host’s volatiles to attract pollinators (39). These natural phenomena may be universal, as SsHADV-1–infected fungal strains could release less repellent volatile compounds to attract the adults of L. ingenua than virus-free strains do. Furthermore, we found that viruliferous adults tended to produce more eggs, as 96.4 ± 2.5 eggs were laid per viruliferous female compared with 74.8 ± 1.9 per virus-free female. This phenomenon is of interest and suggests that there is possibly a mutualistic interaction between L. ingenua and SsHADV-1.
Hypovirulence-associated mycoviruses have the potential to control fungal plant diseases. A classic example is the successful use of hypoviruses to control chestnut blight caused by C. parasitica in Europe. However, this approach for controlling plant diseases has some disadvantages to be overcome. Milgroom and Cortesi concluded that the success of using hypoviruses to control chestnut blight at the population level depended on the natural spread of viruses, and they thought that the application of hypovirulence-associated mycoviruses for biological control of other plant-pathogenic fungi was likely to meet the same constraints as those for chestnut blight (40). Our findings and previous studies on potential mycovirus transmission vectors (14–17) suggest that vectors for mycoviruses are likely to exist in nature, and other mycophagous insects may function as mycovirus transmission vectors, as many fungi could release volatiles to attract insects (41). Thus, L. ingenua is not likely to be the only transmission vector for mycoviruses. The existence of other transmission vectors for mycoviruses needs to be explored.
In the rapeseed/S. sclerotiorum system, our previous research showed that spraying the SsHADV-1–infected strain under field conditions could control rapeseed stem rot disease and improve the yield of rapeseed (32). Although in the present study our experiments suggested that SsHADV-1 could occur naturally in L. ingenua, spraying the virus-infected strain in a rapeseed field could enhance the proportion of viruliferous adults, as the virus is transmitted transovarially. However, we also found that the replication of the insect-transmitted virus in fungi was significantly suppressed. With a better understanding of the multiple interactions among S. sclerotiorum, SsHADV-1, L. ingenua, and plants—especially a better understanding of the biological and ecological properties of viruliferous L. ingenua—the possibility to control rapeseed stem rot disease with SsHADV-1 could be markedly enhanced.
Materials and Methods
Fungal Strains and Maintenance.
SsHADV-1–infected strain DT-8 of S. sclerotiorum, SsHADV-1–free strain DT-8VF (18), the virus-free strain 1980 labeled with a hygromycin-resistance gene, and strain B05.10 of B. cinerea (nonhost fungus to SsHADV-1) were used in this study. Details for this and subsequent methods can be found in SI Materials and Methods.
Insect Collection, Identification, and Maintenance.
Mycophagous insects were originally collected from a colony of S. sclerotiorum growing on a PDA plate. Insect identification was made using traditional methods and sequencing analysis of the COI gene (42). Insects were reared and maintained either on a colony of strain DT-8VF or on strain DT-8 in glass bottles.
Virus Particle Preparation.
Purified SsHADV-1 particles were prepared as previously described (18) and used for injecting insects and inoculating Sf9 cells.
Insect Injection with Virus Particles.
Virus-free larvae and pupae developed on colonies of strain DT-8VF were injected with virus particles by a micromanipulator system, NT-88-V3 (Nikon). The injected larvae were starved for 1 d before rearing on either strain DT-8VF or B. cinerea, and the injected pupae were placed in a beaker for emergence.
Virus Replication Assay in Sf9 Cells.
To determine whether SsHADV-1 can replicate in insect cells, SsHADV-1 particles either diluted or nondiluted (300 ng/µL) were used to inoculate S. frugiperda cells (Sf9). The cell-free supernatant of culture liquid of inoculated Sf9 cells was prepared to conduct passage experiments according to a method described by Hadsbjerg et al. (43). The inoculated Sf9 cells were collected to extract DNA and RNA for RT-PCR amplification, Northern blotting, immunofluorescence, and flow cytometry analyses.
DNA and RNA Extraction; RT-, qRT-, and q-PCR; and Southern/Northern Hybridization Blots.
Genomic DNA and RNA from insect, fungi, and Sf9 cells were extracted using a cetyltrimethylammonium bromide (CTAB) method and a TRIzol Kit reagent (Invitrogen), respectively. Specific primer pairs designed based on viral genomic DNA sequence, fungal Actin gene (44), and insect COI gene (42) were used for RT-, qRT-, and q-PCR amplification and for probe preparation. Primer pairs are listed in Table S1. Southern and Northern blot analysis was conducted with a digoxigenin (DIG) High Prime DNA Labeling and CDP-Star Detection Kit.
Table S1.
Primers used for PCR amplification in the study
| Name | Sequence | Use | Expected size, bp |
| SsDVFp | 5'-CGGACGGAAGTTTCGCAGTAGA-3' | To detect viral genomic DNA of SsHADV-1 | 1,272 |
| SsDVRp | 5'-ATTTTGTTTAAGGGGGCAGAGAATG-3' | ||
| RepFp | 5'-GAGCCGATTCCAGACTGTTCCA-3' | To detect the expression of SsHADV-1 Rep gene | 435 |
| RepRp | 5'-GCACTCACCTACACTGCTTTGC-3' | ||
| CPFp | 5'-CTTGGCGGCACATTTATTTGGT-3' | To detect the expression of SsHADV-1 CP gene | 523 |
| CPRp | 5'-TCTCCGTATTCGTCGTCATCGT-3' | ||
| LCOI | 5'-GGTCAACAAATCATAAAGATATTGG-3' | To detect the expression of insect COI gene | 658 |
| HCOI | 5'-TAAACTTCAGGGTGACCAAAAAATCA-3' | ||
| actin-qF2 | 5'-GAGCTGTTTTCCCTTCCATTGTC-3' | To detect the expression of S. sclerotiorum Actin gene | 146 |
| actin-qR4 | 5'-GACGACACCGTGCTCGATTGG-3' | ||
| SsDVsbFp | 5'-TTCGCAGTAGAAAGGCTGATGTATT-3' | To prepare probe for viral genomic DNA of SsHADV-1 detection (Southern blot) | 814 |
| SsDVsbRp | 5'-GCAGCCGATATGGATAATTAATTGA-3' | ||
| RepnbFp | 5'-TTCATCTCCATCCAATCAATGTCCA-3' | To prepare probe for the expression of SsHADV-1 Rep gene detection (Northern blot) | 853 |
| RepnbRp | 5'-TGGATAAACTTTCACTTCTGGGAGC-3' | ||
| CPnbFp | 5'-TTTCGGGCAAGAAGGCCAATC-3' | To prepare probe for the expression of SsHADV-1 CP gene detection (Northern blot) | 901 |
| CPnbRp | 5'-CATGCCAATACATAGTAGAATTAGCGG-3' |
Insect Feeding, Egg Production, and Virus Detection.
Larvae were fed on colonies of either strain DT-8 or strain DT-8VF. Female adults, 3 d postemergence, were dissected under stereomicroscope, and their egg production was counted. To determine whether the insect could acquire SsHADV-1 by feeding on a colony of strain DT-8, larvae reared on such a colony, and subsequently pupae, adults, and eggs, were randomly selected for DNA extraction, PCR detection, and Southern blot analyses.
Immunofluorescence and Flow Cytometry Analyses.
Immunofluorescence detection was performed according to previously described methods (45–47) with larvae, pupae, eggs, ovarian ducts, and midguts of female adults of L. ingenua and Sf9 cells. A monoclonal antibody, prepared with SsHADV-1 CP conjugated to virus particles, was used. Goat anti-mouse IgG/FITC (CWBIO) conjugated to the first monoclonal antibody was used as a secondary antibody. Immunofluorescence reaction was observed under a confocal microscope, fv1000mp (Olympus), and infection rates of cells were counted by NovoCyte flow cytometer (ACEA Biosciences) according to previously described methods (48).
Virus Transmission Assay via L. ingenua.
To determine whether larvae and eggs of L. ingenua could transmit the acquired virus to SsHADV-1–free strains, viruliferous larvae and eggs or virus-injected larvae were placed on a colony of strain DT-8VF, and hyphal-agar discs were taken from larvae-feeding galleries and transferred to fresh PDA plates. The new colonies were then subjected to virus detection using PCR amplification and Southern blot analyses using DNA samples from RCA amplification (49).
To determine whether pupae and adults could transmit virus, viruliferous pupae or virus-injected pupae were placed in a small glass beaker to physically separate the pupae and fungal colonies. After emerging, the adults oviposited eggs on colonies of strain DT-8VF, and offspring larvae fed on the same colonies. Subcultures derived from feeding galleries were then subjected to virus detection.
To determine whether virus transmission could occur on plants, rapeseed plants were inoculated with either strain DT-8 or strain 1980 and were placed at two opposite sides of a plastic box with a distance about 30 cm between the two sides to ensure plants in each side do not contact each other. Two weeks later, strain 1980 was recovered from the diseased stem and subjected to virus detection.
Insect Preference of Virus-Infected Fungal Strains.
Insect preference was determined by direct observation with naked eyes (Fig. 5A) and Y-shaped tube test. The volatiles released by strain DT-8 and strain DT-8VF were collected as previously described (50) and then analyzed with GC–MS (GC/MS-QP 2010 plus) according to previously described methods (51). To test for possible attractants or repellants specifically released by strain DT-8 or DT-8VF, pure compounds were obtained commercially (Sigma), and their ability to attract or repel insects was conducted with a Y-shaped tube test following a method described previously (52).
Biological Properties of Fungal Subcultures.
To assess colony morphology, growth rate, sclerotial production, and virulence on detached rapeseed leaves of insect-mediated virus-infected fungal isolates, the methods described by Yu et al. (18) were followed.
Field Investigation.
To determine whether L. ingenua could survive in a rapeseed field, larvae and pupae were sampled from stem rot lesions on a diseased rapeseed in a field and identified in the laboratory with a stereoscope. Adults collected either from a strain DT-8–sprayed rapeseed field or from a nonsprayed field were subjected to PCR amplification and sequencing analysis.
Statistical Analysis.
Experimental data on fungal growth rate, sclerotial production, and virulence assays of the fungal strains and virus-infection rates of Sf9 cells were subjected to analysis of variance (ANOVA) in SAS (SAS Institute, version 8.1), and treatment means were compared using least significant difference (LSD) test at P = 0.05. The peak areas of the GC–MS results were evaluated by a t test at P = 0.05. The proportion of L. ingenua adults’ choices to strain DT-8 or DT-8VF and pure compounds in the Y-shaped tube test were performed via a χ2 test for an expected proportion of 1:1 for all choice experiments at P = 0.05 or P = 0.01.
SI Materials and Methods
Fungal Strains and Maintenance.
Strain DT-8 of S. sclerotiorum, which is naturally infected with SsHADV-1, was originally isolated from a sclerotium formed on a diseased stem of rapeseed; SsHADV-1–free strain DT-8VF was derived from strain DT-8 via hyphal-tipping isolation (18). The virus-free strain 1980, which is vegetatively incompatible with strain DT-8, was labeled with a hygromycin resistance gene by using Agrobacterium tumefaciens-mediated transformation. Strain B05.10 of B. cinerea was used as the nonhost of SsHADV-1 for rearing insect. These strains and their derivatives were cultured on PDA plates at 20 °C and stored on PDA slants at 4 °C.
Insect Collection, Identification, and Maintenance.
Mycophagous insects were originally collected from a S. sclerotiorum colony growing on a PDA plate. Insect identification was made by both traditional and molecular methods. The morphologies of eggs, larvae, pupae, and adults were observed with a stereo microscope (Olympus u-Tv0.63xc), and photographs were taken with an Olympus DP72. The mitochondrial COI gene (42) was amplified and sequenced. Finally, the insect was identified as L. ingenua, a common pest on cultivated mushrooms. L. ingenua larvae were reared and maintained either on a colony of SsHADV-1–free strain DT-8VF or virus-infected strain DT-8 in glass bottles (ϕ56 mm × 90 mm) covered with a ventilable cap at 23 °C.
Virus Particle Preparation.
SsHADV-1 particles were extracted using the methods of Yu et al. (18). Briefly, mycelia of strain DT-8, which was cultured on PDA plates at 20 °C for 10 d, were harvested and finely ground in liquid nitrogen, and then dissolved in 0.1 M phosphate buffer (pH 7.0) and gently shaken on ice for 1 h. The mixture was centrifuged at 12,000 g for 10 min to remove hyphal debris, and the supernatant was collected with an ultracentrifuge at 125,000 g to extract virus particles. The virus particles were suspended with 0.1 M phosphate buffer (pH 7.0), and virus particle suspension was adjusted to 300 ng/μL before inoculation.
Virus Replication Assay in Sf9 Cells.
To determine whether SsHADV-1 can replicate in insect cells, SsHADV-1 particles passed through a 0.22-μm biofilter were used to inoculate S. frugiperda cells (Sf9). Before inoculation, Sf9 cells were maintained in 10 mL Grace’s insect medium in a flask at 28 °C for 30 min to form monolayer cells and then were inoculated with 100 μL virus particles (300 ng/μL). After incubating at 28 °C for 72 h, the inoculated Sf9 cells were collected and washed three times with distilled ddH2O to extract DNA and RNA for RT-PCR, Northern blotting, and immunofluorescence analyses.
To determine virus infection rates of Sf9 cells, 100 μL virus particles (300 ng/μL), serially diluted 1-, 10-, 100-, and 1,000-fold with Grace’s insect medium, were used to inoculate Sf9 cells and were incubated for 72 h. Additionally, 100 μL virus particles (30 ng/μL) were used to inoculate Sf9 cells and were incubated for 0, 12, 24, 48, and 72 h, respectively, to investigate virus infection rates to Sf9 cells for serial time points. For each dilution fold and infection time point, virus-inoculated Sf9 cells were collected for flow cytometry detection. This experiment was repeated two times.
To determine whether the virus could passage to new Sf9 cells, previously described methods (43) were used with slight modifications. Sf9 cells (P0 cells) were inoculated with virus particles extracted from fungal hyphae and incubated for 24 h, and then the culture liquid was removed and the cells were washed with fresh medium two times. The cells were incubated in fresh medium for 72 h. The supernatant of the culture liquid was collected by centrifuging at 2,400 g for 5 min to remove Sf9 cells, and then the supernatant was used to inoculate new Sf9 cells (first passage), and cells were incubated for 72 h. Again, the supernatant was collected from first-passage Sf9 cells and used to further inoculate Sf9 cells (second passage), and the cells were incubated for 24 h. Finally, the second-passage Sf9 cells were collected for DNA and RNA extraction and for virus detection. This passage experiment was performed with four dosages of virus particles (300, 30, 3, and 0.3 ng/μL) to inoculate P0 cells, and the dosages were designated Exp. 1, Exp. 2, Exp. 3, and Exp. 4, respectively. Three repeats were prepared for each dosage.
DNA and RNA Extraction, RT-PCR and qRT-PCR Amplification, Sequencing, and Southern and Northern Blot Analyses.
Genomic DNA from insect, fungi, and Sf9 cells was extracted using the CTAB method. Specific primer pair design based on viral genomic DNA sequence SsDVFp and SsDVRp (Table S1) was used to amplify viral genomic DNA using extracted genomic DNA as a template. The PCR products were purified with a gel extraction kit and ligated into the pMD19-T simple vector (TaKaRa) for sequencing.
To find out whether SsHADV-1 can replicate in insect tissues, RNA samples were extracted from larvae, pupae, adults, and eggs of L. ingenua and Sf9 cells with a TRIzol Kit reagent (Invitrogen); 10 larvae, pupae, or adults or more than 100 eggs were used for RNA extraction. The RNA samples were treated with DNase I (TaKaRa) to remove all genomic DNA and then were subjected to cDNA synthesis with Moloney murine leukemia virus transcriptase (Promega) and an oligo (dT) primer for RT-PCR amplification. The synthesized cDNA and extracted DNA samples of passaged Sf9 cells were used as temples for qRT-PCR and qPCR analysis with the SYBR Green Supermix on CFX96 Real-Time System (Bio-Rad), respectively. Specific primer pairs based on viral genomic DNA sequence RepFp and RepRp as well as CPFp and CPRp (Table S1) were used for viral Rep and CP expression detection, respectively. At the same time, specific primer pairs LCOI and HCOI as well as actin-qF2 and actin-qR4 (Table S1), according to Hebert et al. (42) and Sexton et al. (44), for the insect COI gene and S. sclerotiorum Actin gene were used as positive controls. This experiment was repeated two times.
Southern blot analysis was conducted with a DIG High Prime DNA Labeling and CDP-Star Detection Kit (GE Healthcare) following the manufacturer’s protocol. An 814-bp fragment corresponding to viral genomic DNA sequence, prepared with primer pair SsDVsbFp and SsDVsbRp (Table S1) and labeled with DIG, was used as a probe. DNA samples of strain DT-8, diluted 10-fold with distilled ddH2O, was used as a positive control, and DNA samples of strain DT-8VF or 1980 were used as the negative control.
Sf9 cells, inoculated with SsHADV-1 particles for 72 h, were collected to extract RNA for Northern blot analysis. Northern blot analysis was conducted following the manufacturer’s protocol with a DIG High Prime DNA Labeling and CDP-Star Detection Kit. Probes of an 853-bp and a 901-bp fragment prepared with primer pair RepnbFp and RepnbRp as well as CPnbFP and CPnbRp (Table S1) corresponding to viral genomic DNA sequence and labeled with DIG were used for viral Rep and CP genes, respectively. Total RNA extracted from strain DT-8 diluted 10-fold with distilled ddH2O was used as a positive control, and RNA extracted from noninoculated Sf9 cells was used as a negative control.
Immunofluorescence Observation and Flow Cytometry Analyses.
Immunofluorescence detection was performed according to previously described methods (45) with eggs, ovarian ducts, and midguts dissected from 3 d-postemergence L. ingenua female adults reared on strain DT-8. Insects reared on strain DT-8VF were used as negative controls. To observe the egg immunofluorescence, eggs were incubated in osmotic buffer [10 mg/mL protease and 0.5% triton X-100 (vol/vol) in 0.1 M PBS buffer] at 20 °C for 4 h to break superficial chorion, allowing antibodies to enter into the egg before being incubated with viral CP-specific antibody. A monoclonal antibody, prepared with SsHADV-1 CP protein conjugated to virus particles, was used. Goat anti-mouse IgG FITC (CWBIO), conjugated to the first monoclonal antibody, was used as a secondary antibody. Immunofluorescence reaction was observed under a confocal microscope (fv1000mp, Olympus), and the excitation and emission wavelengths used were 488 nm and 502 nm, respectively.
Immunofluorescence detection on paraffin sections of L. ingenua reared on strain DT-8 was performed according to previously described methods (45). The paraffin sections were prepared as described by Li et al. (46), and insects reared on strain DT-8VF were used as negative controls. Antibodies used and immunofluorescence observation were as described earlier.
Sf9 cells inoculated with purified SsHADV-1 particles for 72 h were fixed in 4% (wt/vol) paraformaldehyde for immunofluorescence analysis as described previously (47). Noninoculated Sf9 cells were used as a negative control. Antibodies used and immunofluorescence observation were as described earlier.
The flow cytometry detection was performed according to previously described methods (48). The virus-infected Sf9 cells were treated with 0.5% triton X-100 and stained with the SsHADV-1 CP protein-prepared monoclonal antibody and goat anti-mouse IgG FITC (CWBIO) antibody as described earlier. Then the immunofluorescence signal was detected with a NovoCyte flow cytometer (ACEA Biosciences) to detect virus infection rates. Noninoculated and nonantibody-stained Sf9 cells were used as negative controls, and four repeats were prepared for each treatment.
Insect Injection with Virus Particles.
Virus-free larvae and pupae developed on colonies of strain DT-8VF were injected with a virus particle suspension, which was previously passed through a 0.22-μm biofilter. We used 0.4 µL virus particles (300 ng/μL) for injection with a micromanipulator system, NT-88-V3 (Nikon). The injected larvae were starved in a small glass beaker (ϕ50 mm × 60 mm) for 1 d to allow virus replication in the body and then were used for further processing.
Insect Feeding and Virus Acquisition.
To determine whether the insect could acquire SsHADV-1 by feeding on a colony of strain DT-8 of S. sclerotiorum, larvae reared on strain DT-8VF were randomly selected for DNA extraction and confirmed to be SsHADV-1–free by PCR amplification. Ten larvae were picked up with a sterilized soft brush and placed on a colony of strain DT-8. The larvae fed on the colony for 1 d and then were collected and washed three times with sterilized ddH2O to remove possible hyphal debris adhering to the surface of the larval body.
To ensure no hyphal debris still adhered on the surface of larvae after washing three times, 10 washed larvae were placed on a fresh PDA plate and incubated at 20 °C for 1 wk to observe whether the S. sclerotiorum strain could grow out. At the same time, 10 unwashed larvae were placed on a fresh PDA plate as a positive control. At least three plates were used for each treatment, and this experiment was conducted three times. A week later, no S. sclerotiorum grew out, except several bacterial or yeast colonies, on all repeats of washed larvae plates, whereas S. sclerotiorum colonies grew out from unwashed larvae plates. These experimental results demonstrated that larvae could carry hyphal fragments when fed on fungal colonies, but water bathing could clearly remove such fragments. Thus, larvae collected from fungal colonies were carefully washed before any further processing.
Pupae developed on colonies of a virus-infected strain were collected and placed in a small glass beaker (ϕ50 mm × 60 mm) to allow adults to emerge and lay eggs. Pupae, adults, eggs, and the water-bathed larvae were collected and DNA samples were subjected to virus detection via PCR amplification and Southern blot analyses, as described earlier. Virus detection experiments were repeated at least three times.
To determine whether the virus could be detected in subsequent-generation insects, viruliferous pupae developed on strain DT-8 and virus particle-injected larvae were starved for 1 d and both groups were placed on colonies of B. cinerea to develop next-generation larvae for virus detection. To determine the minimum time required for larvae to acquire SsHADV-1, larvae reared on strain DT-8VF were selected at random and placed on a colony of strain DT-8 and allowed to feed for 0.5, 1, 2, or 4 h. The larvae were then collected and washed with distilled ddH2O for virus detection as described earlier. To find out whether SsHADV-1 could be retained in larvae, larvae feeding on a colony of strain DT-8 for 1 d were collected and washed carefully and then placed in a small glass beaker (ϕ50 mm × 60 mm) for starvation periods of different durations (1, 3, 5, or 7 d). At each starvation time point, 10 larvae were collected for virus detection with PCR amplification as described earlier. These experiments were repeated two times.
To assess egg production, female adults, 3 d postemergence, were dissected under stereo microscope and their egg productions were counted. At least 30 female adults were analyzed for adults reared on strain DT-8 or DT-8VF, respectively.
Virus Transmission Assays via L. ingenua.
To determine whether larvae of L. ingenua could transmit acquired SsHADV-1 to virus-free strains, larvae reared on strain DT-8 were collected and carefully washed and then placed on a colony of strain DT-8VF for an additional 1-d feeding. Hyphal-agar discs were taken from the larvae-feeding galleries (Fig. 1A) and transferred to fresh PDA plates to allow new colonies to develop. The new colonies were then subcultured and subjected to virus detection using PCR amplification and Southern blot analyses, as described earlier. These experiments were performed at least four times, with 10 subcultures examined each time. Before carrying out Southern blot analysis, DNA samples from PCR-positive new subcultures were amplified with RCA, as RCA was usually used to enrich circular ssDNA virus according to Kim et al. (49). Briefly, 5 µL of the DNA sample was mixed with 1.5 µL random primers and 8.5 µL ddH2O, heated at 95 °C for 5 min, and then placed in ice for 10 min. Then, 0.5 µL phi29 DNA polymerase (Thermoscientific), 3 µL reaction buffer, 1.5 µL dNTP, and 10 µL ddH2O were added and amplified at 30 °C overnight. After amplification, the RCA products were digested overnight with BamH I, which is a single restriction enzyme site in the genomic sequence of SsHADV-1. After enzyme restriction, the products were precipitated with ethyl-alcohol and dissolved with ddH2O for Southern blot analysis, as described earlier.
Virus particle-injected larvae were starved for 1 d and placed on colonies of strain DT-8VF for further feeding, and subcultures isolated from the larvae-feeding galleries were subjected to virus detection.
To find out the minimum inoculation feeding time required for virus transmission, larvae collected from a colony of strain DT-8 were washed, placed in a small glass beaker (ϕ50 mm × 60 mm), and starved for 1 d; 10 larvae were placed on a colony of strain DT-8VF for inoculation feeding for 0.5, 1, 2, 4, 12, 24, or 48 h; hyphal agar discs were randomly selected from the larvae-feeding galleries and placed on fresh PDA plates; and the subcultures were then subjected to virus detection assays. At least six subcultures were isolated and tested for each acquisition time point; this experiment was performed two times.
To determine whether viruliferous adults could transmit virus, 10 pupae developed on strain DT-8 were collected and placed in a small glass beaker (ϕ50 mm × 60 mm) to physically separate the pupae and fungal colonies, the small beaker and two PDA plates (ϕ60 mm) on which colonies of strain DT-8VF developed were placed together in a large glass beaker (ϕ146 mm × 198 mm), and then the large beaker was sealed with parafilm and placed at 23 °C to await adults emerging and laying eggs on colonies. After larvae-feeding galleries appeared, hyphal agar plugs were picked up and were allowed to develop to new colonies for virus detection. The virus particle-injected pupae were used to replace the pupae developed on strain DT-8 and perform the transmission experiment, as described earlier. To find out whether viruliferous eggs could transmit virus, 10 pupae collected from strain DT-8 were placed in a small glass beaker (ϕ50 mm × 60 mm) to allow the adults to emerge and lay eggs. Then, the eggs were placed on a colony of strain DT-8VF with a sterilized soft brush to allow hatching on this colony. Subcultures isolated from the larvae-feeding galleries were subjected to virus detection. Both transmission experiments via adults or eggs were repeated three times.
To determine whether virus transmission could occur on plants, rapeseed plants (five-leaf stage) were placed at two opposite sides of a plastic box (75 cm × 55 cm × 45 cm) with a distance about 30 cm between the two sides to ensure plants in each side do not contact each other. At one side, plant leaves were wounded and inoculated with strain DT-8, and larvae reared on strain DT8-VF were placed on the resultant small lesions that developed 3 d postinoculation and were allowed to feed there. When pupae appeared on the lesions, plants at the opposite side of the plastic box were inoculated with SsHADV-1–free strain 1980, previously labeled with a hygromycin resistance gene, and then the plastic box was sealed with parafilm and incubated at 20 °C. When adults emerged, they were expected to fly to the lesions induced by strain 1980 and lay eggs there. Two weeks later, plants that were inoculated with strain 1980 were killed, and strain 1980 was recovered from these diseased plant residues using selective medium amended with hygromycin (100 μg/mL). These recovered isolates were subjected to virus detection using PCR amplification and Southern blot analyses as described with larvae transmission isolates. This experiment was repeated two times.
Insect Preference by Virus-Infected Fungal Strain.
A simple setup was designed to test the appeal of strain DT-8 to L. ingenua. Two glass bottles (ϕ56 mm × 90 mm), one containing a colony of strain DT-8VF and the another with strain DT-8, and two small glass beakers (ϕ50 mm × 60 mm), one with 10 pupae and the another with a piece of black cloth, were placed in a large glass beaker (ϕ165 mm × 220 mm); then the large beaker was sealed with parafilm and incubated at 23 °C (Fig. 5A). When adults emerged, they had a choice to lay eggs on the colony of strain DT-8 or DT-8VF. Larvae and feeding galleries, appearing on colonies of both strains, were noted. To ensure the adults’ choice was based on the effect of volatiles produced by S. sclerotiorum, an additional small glass beaker (ϕ50 mm × 60 mm) with 5 g activated carbon instead of the black cloth was placed on the other side of the large glass beaker containing pupae and fungal colonies (Fig. 5A). At least three replications of each treatment were performed, and the experiments were repeated more than three times. To further confirm volatiles’ effect on adults’ tending to strain DT-8, volatiles collected from strain DT-8 and DT-8VF were imported into a Y-shaped tube for testing, respectively, and then adults that chose strain DT-8 or DT-8VF were observed. At least 30 female adults were tested.
Strain DT-8 and DT-8VF grown on PDA plates for 10 d were used for volatile collection as previously described (50) at room temperature for 12 h. Then, 10 µL nonyl acetate was added to the volatiles and analyzed by GS–MS (GC/MS-QP 2010 plus) according to previously described methods (51). A Rxi-5il MS capillary column (Restek) was used, and the temperature of the oven was programmed at 50 °C for 1 min, then increased at a rate of 8 °C/min up to 200 °C and maintained for 3 min, and then increased at a rate of 40 °C/min up to 300 °C and held for 3 min at 300 °C. At the same time, volatiles released by fresh PDA medium were analyzed as a negative control, and at least eight replications for each treatment were analyzed. To examine the different peaks quantitatively, we compared every peak area to nonyl acetate, which was defined as 1, and then performed a t test to find significantly different content peaks. To test possible attractants released by strain DT-8, pure compounds were obtained commercially (Sigma), and their ability to attract or repel insects was conducted with a Y-shape tube test following a method described previously (52). At least 30 female adults were tested for each selected compound in different doses.
Biological Properties of Subcultures Derived from Insect-Mediated Virus-Infected Isolates.
To assess colony morphology, growth rate, sclerotial production, and virulence on detached rapeseed leaves for virus-infected fungal isolates via insect transmission, the methods described by Yu et al. (18) were followed. Virus-infected strain DT-8 was used as a positive control, whereas strains DT-8VF and 1980, a virus-free strain labeled with a hygromycin resistance gene, were used as negative controls. At least three replications for each treatment were performed, and the experiments were repeated three times.
Field Investigation.
To determine whether L. ingenua could survive in a rapeseed field, larvae and pupae were sampled from stem rot lesions on diseased rapeseeds in a field and identified in the laboratory with a stereoscope. To find out whether adults in a rapeseed field could carry SsHADV-1, we sprayed hyphal fragment suspension of strain DT-8 on rapeseed plants at the early flowering stage in a rapeseed field located at XN city. To prepare the hyphal fragment suspension, strain DT-8 was grown in a 500-mL flask containing 200 mL PDB at 20 °C for 7 d on a shaker at 150 rpm, and then the hyphal mass was homogenized in a blender. The hyphal fragment suspension was diluted to 2.0 OD600 units [about 107 colony-forming units (cfu)/mL] with distilled ddH2O and sprayed into the rapeseed field with a hand sprayer. One week before harvest (45 d postspraying), adults were captured with an insect-capture net from the strain DT-8–sprayed field and subjected to virus detection. At the same time, adults were also captured from another rapeseed field, which was about 19.2 km away from the sprayed field located at the same city.
Statistical Analysis.
Experimental data on growth rate, sclerotial production, and virulence assays of the fungal strains and virus infection rates of Sf9 cells were subjected to ANOVA in SAS (SAS Institute, version 8.1), and treatment means were compared using LSD test at P = 0.05. The peak areas of the GC–MS results were obtained using a t test at P = 0.05. The proportion of L. ingenua adults’ preferences of strain DT-8 or DT-8VF and pure compounds in the Y-shaped tube test were performed using χ2 test for an expected proportion of 1:1 for all choice experiments at P = 0.05 or P = 0.01.
Acknowledgments
The authors thank their colleagues Dr. Changying Niu and Mr. Ze Sun for their generous technical assistance, Dr. Jianxiang Wu (Zhejiang University) for viral CP monoclonal antibody preparation, Prof. Said A. Ghabrial (University of Kentucky) for critical reading and editing of the revised manuscript, and the anonymous reviewers for constructive and helpful comments. This research was supported by the National Natural Science Foundation of China (Grants 31430070 and 31201561), the Special Fund for Agro-Scientific Research in the Public Interest of China (Grant 201103016), and China Agriculture Research System (Grant CARS-13).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1608013113/-/DCSupplemental.
References
- 1.Ghabrial SA, Castón JR, Jiang D, Nibert ML, Suzuki N. 50-plus years of fungal viruses. Virology. 2015;479-480:356–368. doi: 10.1016/j.virol.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 2.Márquez LM, Redman RS, Rodriguez RJ, Roossinck MJ. A virus in a fungus in a plant: Three-way symbiosis required for thermal tolerance. Science. 2007;315(5811):513–515. doi: 10.1126/science.1136237. [DOI] [PubMed] [Google Scholar]
- 3.Dawe AL, Nuss DL. Hypovirus molecular biology: From Koch’s postulates to host self-recognition genes that restrict virus transmission. Adv Virus Res. 2013;86:109–147. doi: 10.1016/B978-0-12-394315-6.00005-2. [DOI] [PubMed] [Google Scholar]
- 4.Xie J, Jiang D. New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu Rev Phytopathol. 2014;52:45–68. doi: 10.1146/annurev-phyto-102313-050222. [DOI] [PubMed] [Google Scholar]
- 5.Zhang DX, Nuss DL. Engineering super mycovirus donor strains of chestnut blight fungus by systematic disruption of multilocus vic genes. Proc Natl Acad Sci USA. 2016;113(8):2062–2067. doi: 10.1073/pnas.1522219113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Refos JM, et al. Double-stranded RNA mycovirus infection of Aspergillus fumigatus is not dependent on the genetic make-up of the host. PLoS One. 2013;8(10):e77381. doi: 10.1371/journal.pone.0077381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie J, et al. Characterization of debilitation-associated mycovirus infecting the plant-pathogenic fungus Sclerotinia sclerotiorum. J Gen Virol. 2006;87(Pt 1):241–249. doi: 10.1099/vir.0.81522-0. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, et al. A novel mycovirus that is related to the human pathogen hepatitis E virus and rubi-like viruses. J Virol. 2009;83(4):1981–1991. doi: 10.1128/JVI.01897-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, et al. Evolutionary genomics of mycovirus-related dsRNA viruses reveals cross-family horizontal gene transfer and evolution of diverse viral lineages. BMC Evol Biol. 2012;12(91):91. doi: 10.1186/1471-2148-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, et al. Characterization of a novel Megabirnavirus from Sclerotinia sclerotiorum reveals horizontal gene transfer from single-stranded RNA virus to double-stranded RNA virus. J Virol. 2015;89(16):8567–8579. doi: 10.1128/JVI.00243-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanhayuwa L, Kotta-Loizou I, Özkan S, Gunning AP, Coutts RH. A novel mycovirus from Aspergillus fumigatus contains four unique dsRNAs as its genome and is infectious as dsRNA. Proc Natl Acad Sci USA. 2015;112(29):9100–9105. doi: 10.1073/pnas.1419225112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiba S, Suzuki N. Highly activated RNA silencing via strong induction of dicer by one virus can interfere with the replication of an unrelated virus. Proc Natl Acad Sci USA. 2015;112(35):E4911–E4918. doi: 10.1073/pnas.1509151112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang R, et al. A capsidless ssRNA virus hosted by an unrelated dsRNA virus. New Microbiol. 2016;1:15001. doi: 10.1038/nmicrobiol.2015.1. [DOI] [PubMed] [Google Scholar]
- 14.Hillman BI, Supyani S, Kondo H, Suzuki N. A reovirus of the fungus Cryphonectria parasitica that is infectious as particles and related to the coltivirus genus of animal pathogens. J Virol. 2004;78(2):892–898. doi: 10.1128/JVI.78.2.892-898.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaegashi H, et al. Appearance of mycovirus-like double-stranded RNAs in the white root rot fungus, Rosellinia necatrix, in an apple orchard. FEMS Microbiol Ecol. 2013;83(1):49–62. doi: 10.1111/j.1574-6941.2012.01454.x. [DOI] [PubMed] [Google Scholar]
- 16.Petrzik K, et al. Molecular characterization of a new monopartite dsRNA mycovirus from mycorrhizal Thelephora terrestris (Ehrh.) and its detection in soil oribatid mites (Acari: Oribatida) Virology. 2016;489:12–19. doi: 10.1016/j.virol.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Bouneb M, et al. Occurrence and transmission of mycovirus Cryphonectria hypovirus 1 from dejecta of Thyreophagus corticalis (Acari, Acaridae) Fungal Biol. 2016;120(3):351–357. doi: 10.1016/j.funbio.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Yu X, et al. A geminivirus-related DNA mycovirus that confers hypovirulence to a plant pathogenic fungus. Proc Natl Acad Sci USA. 2010;107(18):8387–8392. doi: 10.1073/pnas.0913535107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosario K, et al. Diverse circular ssDNA viruses discovered in dragonflies (Odonata: Epiprocta) J Gen Virol. 2012;93(Pt 12):2668–2681. doi: 10.1099/vir.0.045948-0. [DOI] [PubMed] [Google Scholar]
- 20.Phan TG, et al. Small circular single stranded DNA viral genomes in unexplained cases of human encephalitis, diarrhea, and in untreated sewage. Virology. 2015;482:98–104. doi: 10.1016/j.virol.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sikorski A, et al. Novel myco-like DNA viruses discovered in the faecal matter of various animals. Virus Res. 2013;177(2):209–216. doi: 10.1016/j.virusres.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Conceição-Neto N, et al. Fecal virome analysis of three carnivores reveals a novel nodavirus and multiple gemycircularviruses. Virol J. 2015;12:79. doi: 10.1186/s12985-015-0305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng TFF, et al. Preservation of viral genomes in 700-y-old caribou feces from a subarctic ice patch. Proc Natl Acad Sci USA. 2014;111(47):16842–16847. doi: 10.1073/pnas.1410429111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng TFF, et al. Broad surveys of DNA viral diversity obtained through viral metagenomics of mosquitoes. PLoS One. 2011;6(6):e20579. doi: 10.1371/journal.pone.0020579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dayaram A, et al. High global diversity of cycloviruses amongst dragonflies. J Gen Virol. 2013;94(Pt 8):1827–1840. doi: 10.1099/vir.0.052654-0. [DOI] [PubMed] [Google Scholar]
- 26.Dayaram A, et al. Identification of diverse circular single-stranded DNA viruses in adult dragonflies and damselflies (Insecta: Odonata) of Arizona and Oklahoma, USA. Infect Genet Evol. 2015;30:278–287. doi: 10.1016/j.meegid.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 27.Dayaram A, et al. Molecular characterisation of a novel cassava associated circular ssDNA virus. Virus Res. 2012;166(1-2):130–135. doi: 10.1016/j.virusres.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Lamberto I, Gunst K, Müller H, Zur Hausen H, de Villiers EM. Mycovirus-like DNA virus sequences from cattle serum and human brain and serum samples from multiple sclerosis patients. Genome Announc. 2014;2(4):e00848–14. doi: 10.1128/genomeA.00848-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uch R, et al. Divergent gemycircularvirus in HIV-positive blood, France. Emerg Infect Dis. 2015;21(11):2096–2098. doi: 10.3201/eid2111.150486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, et al. A novel gemycircularvirus from experimental rats. Virus Genes. 2015;51(2):302–305. doi: 10.1007/s11262-015-1238-1. [DOI] [PubMed] [Google Scholar]
- 31.Li L, et al. Exploring the virome of diseased horses. J Gen Virol. 2015;96(9):2721–2733. doi: 10.1099/vir.0.000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu X, et al. Extracellular transmission of a DNA mycovirus and its use as a natural fungicide. Proc Natl Acad Sci USA. 2013;110(4):1452–1457. doi: 10.1073/pnas.1213755110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraberger S, et al. Discovery of Sclerotinia sclerotiorum hypovirulence-associated virus-1 in urban river sediments of heathcote and Styx rivers in Christchurch City, New Zealand. Genome Announc. 2013;1(4):1–2. doi: 10.1128/genomeA.00559-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewandowski M, Sznyk A, Bednarek A. Biology and morphometry of Lycoriella ingenua (Diptera: Sciaridae) Biol Lett. 2004;1:41–50. [Google Scholar]
- 35.Hogenhout SA, Ammar D, Whitfield AE, Redinbaugh MG. Insect vector interactions with persistently transmitted viruses. Annu Rev Phytopathol. 2008;46:327–359. doi: 10.1146/annurev.phyto.022508.092135. [DOI] [PubMed] [Google Scholar]
- 36.Jiu M, et al. Vector-virus mutualism accelerates population increase of an invasive whitefly. PLoS One. 2007;2(1):e182. doi: 10.1371/journal.pone.0000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stumpf CF, Kennedy GG. Effects of tomato spotted wilt virus (TSWV) isolates, host plants, and temperature on survival, size, and development time of Frankliniella fusca. Entomol Exp Appl. 2005;114:215–225. [Google Scholar]
- 38.Ingwell LL, Eigenbrode SD, Bosque-Pérez NA. Plant viruses alter insect behavior to enhance their spread. Sci Rep. 2012;2:578. doi: 10.1038/srep00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groen SC, et al. Virus infection of plants alters pollinator preference: A payback for susceptible hosts? PLoS Pathog. 2016;12(8):e1005790. doi: 10.1371/journal.ppat.1005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milgroom MG, Cortesi P. Biological control of chestnut blight with hypovirulence: A critical analysis. Annu Rev Phytopathol. 2004;42:311–338. doi: 10.1146/annurev.phyto.42.040803.140325. [DOI] [PubMed] [Google Scholar]
- 41.Hung R, Lee S, Bennett JW. Fungal volatile organic compounds and their role in ecosystems. Appl Microbiol Biotechnol. 2015;99(8):3395–3405. doi: 10.1007/s00253-015-6494-4. [DOI] [PubMed] [Google Scholar]
- 42.Hebert PDN, Cywinska A, Ball SL, deWaard JR. Biological identifications through DNA barcodes. Proc Biol Sci. 2003;270(1512):313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hadsbjerg J, et al. Sequence adaptations during growth of rescued classical swine fever viruses in cell culture and within infected pigs. Vet Microbiol. 2016;192:123–134. doi: 10.1016/j.vetmic.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Sexton AC, Minic Z, Cozijnsen AJ, Pedras MSC, Howlett BJ. Cloning, purification and characterisation of brassinin glucosyltransferase, a phytoalexin-detoxifying enzyme from the plant pathogen Sclerotinia sclerotiorum. Fungal Genet Biol. 2009;46(2):201–209. doi: 10.1016/j.fgb.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Huo Y, et al. Transovarial transmission of a plant virus is mediated by vitellogenin of its insect vector. PLoS Pathog. 2014;10(3):e1003949. doi: 10.1371/journal.ppat.1003949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li JL, et al. Systemic spread and propagation of a plant-pathogenic virus in European honeybees, Apis mellifera. MBio. 2014;5(1):e00898-13. doi: 10.1128/mBio.00898-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei T, et al. Pns12 protein of Rice dwarf virus is essential for formation of viroplasms and nucleation of viral-assembly complexes. J Gen Virol. 2006;87(Pt 2):429–438. doi: 10.1099/vir.0.81425-0. [DOI] [PubMed] [Google Scholar]
- 48.Østergaard E, Frandsen PL, Sandberg E. Determination of freeze damage on HPV vaccines by use of flow cytometry. Biologicals. 2015;43(4):266–273. doi: 10.1016/j.biologicals.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Kim KH, et al. Amplification of uncultured single-stranded DNA viruses from rice paddy soil. Appl Environ Microbiol. 2008;74(19):5975–5985. doi: 10.1128/AEM.01275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Booth E, et al. A rapid column technique for trapping and collecting of volatile fungal hydrocarbons and hydrocarbon derivatives. Biotechnol Lett. 2011;33(10):1963–1972. doi: 10.1007/s10529-011-0660-2. [DOI] [PubMed] [Google Scholar]
- 51.Tenorio-Salgado S, Tinoco R, Vazquez-Duhalt R, Caballero-Mellado J, Perez-Rueda E. Identification of volatile compounds produced by the bacterium Burkholderia tropica that inhibit the growth of fungal pathogens. Bioengineered. 2013;4(4):236–243. doi: 10.4161/bioe.23808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y, Ulyshen MD, Poland TM. Abundance of volatile organic compounds in white ash phloem and emerald ash borer larval frass does not attract Tetrastichus planipennisi in a Y-tube olfactometer. Insect Sci. 2016;23(5):712–719. doi: 10.1111/1744-7917.12227. [DOI] [PubMed] [Google Scholar]