Significance
New antifungal drugs that act via novel mechanisms are urgently needed to combat the high mortality of invasive fungal disease and the emergence of resistance to existing therapies. We describe the discovery, structure, activity, and mechanism of action of F901318, a new antifungal agent. A member of a novel class of antifungals, the orotomides, F901318 acts via inhibition of dihydroorotate dehydrogenase, an enzyme of de novo pyrimidine biosynthesis. F901318 is currently in clinical development for the treatment of invasive aspergillosis.
Keywords: antifungal drug, Aspergillus fumigatus, mechanism of action, dihydroorotate dehydrogenase
Abstract
There is an important medical need for new antifungal agents with novel mechanisms of action to treat the increasing number of patients with life-threatening systemic fungal disease and to overcome the growing problem of resistance to current therapies. F901318, the leading representative of a novel class of drug, the orotomides, is an antifungal drug in clinical development that demonstrates excellent potency against a broad range of dimorphic and filamentous fungi. In vitro susceptibility testing of F901318 against more than 100 strains from the four main pathogenic Aspergillus spp. revealed minimal inhibitory concentrations of ≤0.06 µg/mL—greater potency than the leading antifungal classes. An investigation into the mechanism of action of F901318 found that it acts via inhibition of the pyrimidine biosynthesis enzyme dihydroorotate dehydrogenase (DHODH) in a fungal-specific manner. Homology modeling of Aspergillus fumigatus DHODH has identified a predicted binding mode of the inhibitor and important interacting amino acid residues. In a murine pulmonary model of aspergillosis, F901318 displays in vivo efficacy against a strain of A. fumigatus sensitive to the azole class of antifungals and a strain displaying an azole-resistant phenotype. F901318 is currently in late Phase 1 clinical trials, offering hope that the antifungal armamentarium can be expanded to include a class of agent with a mechanism of action distinct from currently marketed antifungals.
A recent estimate puts the annual death toll from serious fungal infections at 1.5 million (1). As one of the four greatest killers, Aspergillus species are opportunistic human pathogens, particularly affecting immunocompromised persons, such as transplant recipients and those with hematologic malignancies. Invasive aspergillosis has a high mortality (30–90%) and is estimated to affect more than 200,000 people annually. Other diseases caused by Aspergillus species, including allergic bronchopulmonary aspergillosis (2) and chronic pulmonary aspergillosis (3), have a significant global impact, affecting millions of patients.
There has been a dearth of new drug classes developed to treat systemic fungal infections arriving in the clinic, with the most recent being the echinocandins, introduced in 2001. Only three other classes of antifungal drugs are currently available for the treatment of invasive fungal disease: polyenes (amphotericin B), azoles (e.g., voriconazole, posaconazole, and the recently licensed isavuconazole), and flucytosine (4). These agents work via a limited range of cellular targets. Echinocandins, such as caspofungin, inhibit β-(1,3)-glucan synthase, exploiting the most striking difference between the fungal cell and its human counterpart—the cell wall. Two antifungal drug classes target the cell membrane: azoles inhibit ergosterol biosynthesis, and polyenes disrupt fungal membranes via ergosterol binding. Flucytosine is a pyrimidine analog, converted to 5-fluorouracil within fungal cells, that disrupts DNA and RNA synthesis; however, owing to rapid development of resistance, it is used primarily in combination therapy.
Issues exist with current therapies, including overt toxicity, drug–drug interactions, variable pharmacokinetics, and increasing levels of drug resistance (5, 6). In particular, the development of resistance to the azole class of antifungals is concerning, because they are currently the sole orally available antifungal agent for the treatment of aspergillosis (7). Azole-resistant clinical isolates of Aspergillus fumigatus have been observed and isolated from patients worldwide, including Europe, the United States, Asia, Africa, Australia, and the Middle East (8, 9). Apparently exacerbated by the environmental use of azole fungicides in agriculture (10), reported rates of azole resistance have approached 30% at certain sites in Europe, with rates outside Europe varying between 0.6% and 11.2% (9).
Results
Discovery of F901318.
With the aim of identifying new antifungal chemistries, a library of 340,292 small molecules was screened in vitro against A. fumigatus, and multiple chemical series with antifungal activity were identified. The initial hits in one such series, originally termed the “F3 series,” were developed by a medicinal chemistry program that was driven by classical structure–activity relationships based on in vitro activity. This series was characterized by excellent in vitro potency against Aspergillus spp., but was devoid of activity against Candida spp. This unusual pattern perhaps explains why similar chemicals have not been found previously. Typically, antifungal screens have depended on first finding activity against Candida. Modifications to improve physicochemical properties, antifungal potency, pharmacokinetics, ADMET (absorption, distribution, metabolism, excretion, and toxicity) properties, in vivo efficacy in infection models, and toxicology have led to the development of F901318 (Fig. 1). Antifungal susceptibility testing of F901318 using standardized techniques demonstrated its potent activities against clinical isolates of aspergilli, with minimum inhibitory concentrations (MICs) of <0.1 µg/mL against multiple strains of A. fumigatus, Aspergillus terreus, Aspergillus niger, and Aspergillus flavus, including isolates resistant to other antifungal agents (Table 1).
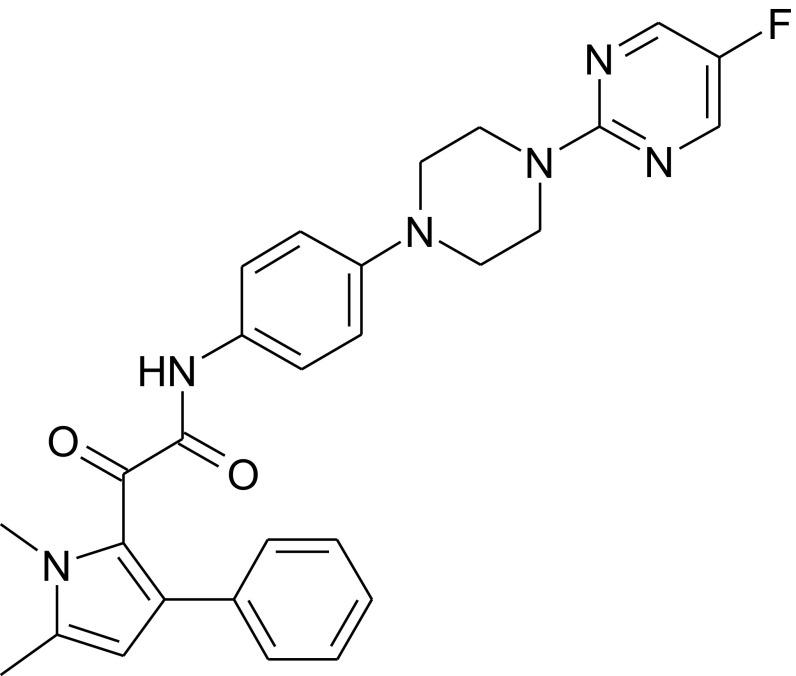
Fig. 1.
Structure of F901318.
Table 1.
Antifungal potency of F901318 and other antifungal drugs against the major Aspergillus species
| Drug | Parameter | A. fumigatus (n = 55) | A. terreus (n = 21) | A. flavus (n = 19) | A. niger (n = 19) |
| F901318 | MIC mean, µg/mL | 0.029 | 0.014 | 0.021 | 0.031 |
| MIC range, µg/mL | 0.008–0.06 | 0.004–0.03 | 0.015–0.06 | 0.015–0.06 | |
| Amphotericin B | MIC mean, µg/mL | 1.55 | 2.07 | 1.39 | 0.46 |
| MIC range, µg/mL | 0.5–2 | 1–4 | 1–2 | 0.25–1 | |
| Caspofungin | MEC* mean, µg/mL | 0.096 | 0.112 | 0.06 | 0.062 |
| MEC* range, µg/mL | 0.06–0.12 | 0.06–0.12 | 0.06 | 0.06–0.12 | |
| Voriconazole | MIC mean, µg/mL | 0.69 | 0.59 | 0.96 | 0.77 |
| MIC range, µg/mL | 0.25–16 | 0.25–1 | 0.5–1 | 0.5–16 |
The MICs (in µg/mL) of F901318, amphotericin B, and voriconazole were determined for the Aspergillus spp. indicated (n, number of different strains tested). *For caspofungin, the MEC is shown, because growth is not completely inhibited with this drug. Data are displayed as the geometric mean of the MICs and the range of MIC from lowest to highest for the strains of a particular species.
Mechanism of Action Screen.
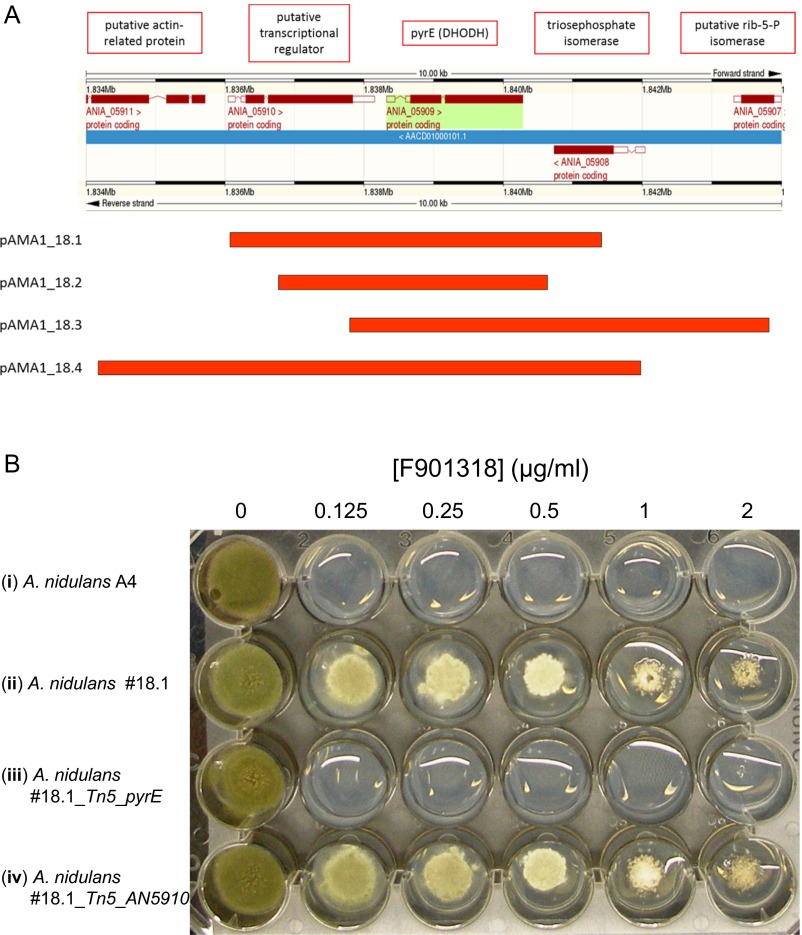
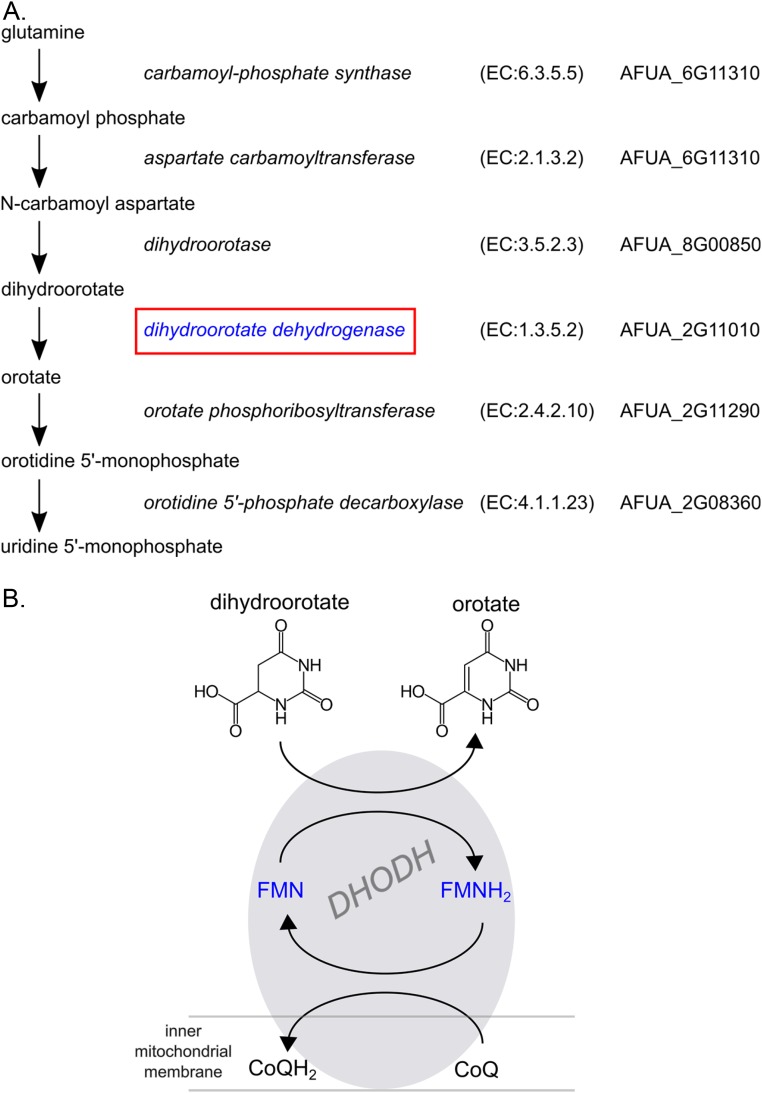
Initially, owing to the method of discovery, the mechanism of action of this series was unknown. A combination of microbiological, genetic, and biochemical approaches were used to identify the target of this drug series. A genetic screen, similar to a multicopy suppressor screen, was performed to identify genes that, when present in multiple copies, provided resistance to F901318. This approach was previously validated for the antifungal drugs itraconazole and terbinafine, by demonstrating that the presence of additional copies of cytochrome P450 C-14 lanosterol α-demethylase and squalene epoxidase, respectively, led to resistance to those agents (11, 12). In the present study, Aspergillus nidulans spores that had been transformed with an A. nidulans genomic library carried by the autonomously replicating plasmid pAMA1 were exposed to F901318. Four independent resistant clones were obtained, pAMA1 DNA was isolated, and the genomic DNA insert was sequenced. All resistant clones contained inserts that mapped to the same region of chromosome I (Fig. S1A). Although sequence data from five genes was retrieved, only one gene—ANIA_05909—was intact in all four genomic fragments. This gene, named pyrE in Aspergillus spp., encodes the pyrimidine biosynthesis enzyme dihydroorotate dehydrogenase (DHODH; EC 1.3.5.2).
Fig. S1.
(A) Map of AMA inserts recovered from the mechanism of action screen. An A. nidulans genomic screen was performed for genes that when present in a high copy number provided resistance to F901318 (Materials and Methods). pAMA1 clones 18.1–18.4 were recovered from four independent colonies that were resistant to F901318. DNA was extracted from the resistant A. nidulans and transformed into E. coli. Plasmid DNA was isolated and sequenced, and the genomic inserts (red bars) found were mapped to the A. nidulans genome using EnsemblFungi. (B) A. nidulans carrying extra copies of pyrE is resistant to F901318. The pAMA1_18.1 plasmid was transformed into A. nidulans A767 before (#18.1; ii) and after mutagenesis with the EZ-Tn5 bacterial transposon system. The transposon disrupted the pyrE gene (#18.1_Tn5_pyrE; iii) or the neighboring ANIA_05910 gene (#18.1_Tn5_AN5910; iv). Conidia from the resulting transformants and from the WT A. nidulans A4 (i) were inoculated onto media in the presence and absence of varying concentrations of F901318 and incubated at 37 °C for 4 d.
To confirm that extra copies of pyrE led to F901318 resistance, the recovered plasmid pAMA1_18.1 was treated with a bacterial transposon (Tn5) to disrupt either pyrE or a neighboring gene, ANIA_05910, and the resulting plasmids were transformed into A. nidulans. Strains carrying the intact pAMA1_18.1 or the ANIA_05910 disruptant exhibited resistance to F901318; however, on disruption of pyrE, the strain returned to wild-type (WT) levels of susceptibility to F901318 (Fig. S1B). This finding confirmed that extra copies of the gene encoding DHODH were responsible for the resistance to F901318, implicating DHODH as the target of the drug.
DHODH Is the Target of F901318.
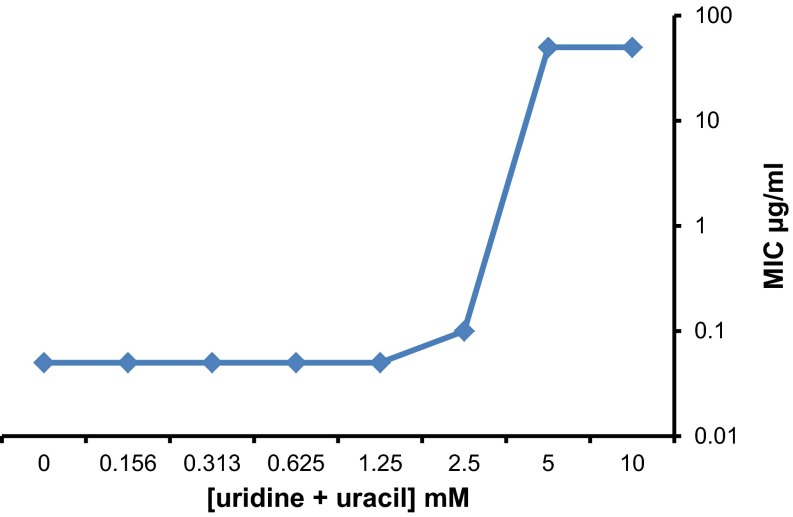
DHODH is an oxidoreductase that catalyzes the fourth step of the pyrimidine biosynthesis pathway (Fig. S2), the conversion of dihydroorotate to orotate. Confirmation that the drug disrupts pyrimidine biosynthesis was obtained after the addition of exogenous pyrimidines (uridine and uracil) to the media during susceptibility testing. A reversal of the antifungal effect of F901318 on A. fumigatus was observed, but only at millimolar concentrations of pyrimidines (5 mM and above; Fig. S3). Interestingly, human serum contains low levels of pyrimidines, estimated to be ∼15 µM (13), which are insufficient to reverse the effect of F901318 on A. fumigatus in vivo. Indeed, mutants of A. fumigatus (14), Candida albicans (15), Histoplasma capsulatum (16), and Cryptococcus neoformans (17), which are disrupted in pyrimidine biosynthesis, have exhibited attenuated virulence in animal models of infection, indicating that targeting pyrimidine synthesis is a valid antifungal strategy.
Fig. S2.
DHODH is an enzyme of pyrimidine biosynthesis. (A) Pathway of de novo pyrimidine biosynthesis in A. fumigatus. Enzyme names, classification (according to the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology; www.chem.qmul.ac.uk/iubmb/enzyme/), and predicted A. fumigatus genes (from KEGG; www.genome.jp/kegg/ and EnsemblFungi; www.fungi.ensembl.org/index.html/) are listed next to each step of the pathway. (B) Reaction catalyzed by DHODH. In mammals and many fungi, DHODH is located at the mitochondria, where a transmembrane domain anchors the enzyme to the outside of the inner mitochondrial membrane. In the intermembrane space, DHODH catalyzes the oxidation of dihydroorotate to orotate. FMN and ubiquinone from the mitochondrial membrane (coenzyme Q) act as electron acceptors in the reaction.
Fig. S3.
Reversal of the antifungal effect of F901318 at high pyrimidine concentrations. Antifungal susceptibility testing of F901318 against A. fumigatus AF293 was carried out according to CLSI protocol M38-A2 in the presence of different concentrations of uridine and uracil. The MIC of F901318 was obtained for each pyrimidine concentration.
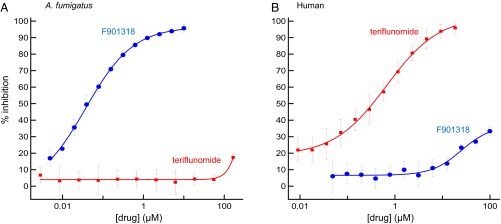
Biochemical evidence confirming that the target of F901318 was DHODH was obtained from in vitro enzyme assays that were set up with recombinant A. fumigatus DHODH using 2,6-dichloroindophenol as a redox indicator. F901318 inhibited A. fumigatus DHODH in a dose-dependent manner, with an IC50 of 44 ± 10 nM (n = 11; Fig. 2). DHODH is also present in mammals, although there is a low overall identity to Aspergillus DHODH (∼30%; Fig. S4). A known inhibitor of human DHODH, teriflunomide (18), which is used to treat multiple sclerosis in man, did not inhibit A. fumigatus DHODH in vitro. Species selectivity of F901318 was confirmed in an assay in which little inhibition of human DHODH was observed, whereas teriflunomide inhibited human DHODH, as expected. In fact, the IC50 value for F901318 against human DHODH was not reached at 100 µM, the highest concentration used in these experiments, indicating that F901318 was >2,200-fold more potent against the A. fumigatus enzyme. Thus, fungal DHODH was confirmed as the target of F901318, and despite the presence of a mammalian version of the enzyme, no target-based toxicity was predicted. On elucidation of the mechanism of action, the F3 series was renamed the orotomides, combining the mechanism (dihydroorotate) with the chemistry (α-ketoamide).
Fig. 2.
F901318 inhibits A. fumigatus DHODH in vitro. Recombinant A. fumigatus DHODH (A) and human DHODH (B) were incubated in the presence and absence of varying concentrations of F901318 and teriflunomide. The activity of the enzymes was measured for each drug concentration, and the percent inhibition compared with no drug controls was calculated.
Fig. S4.
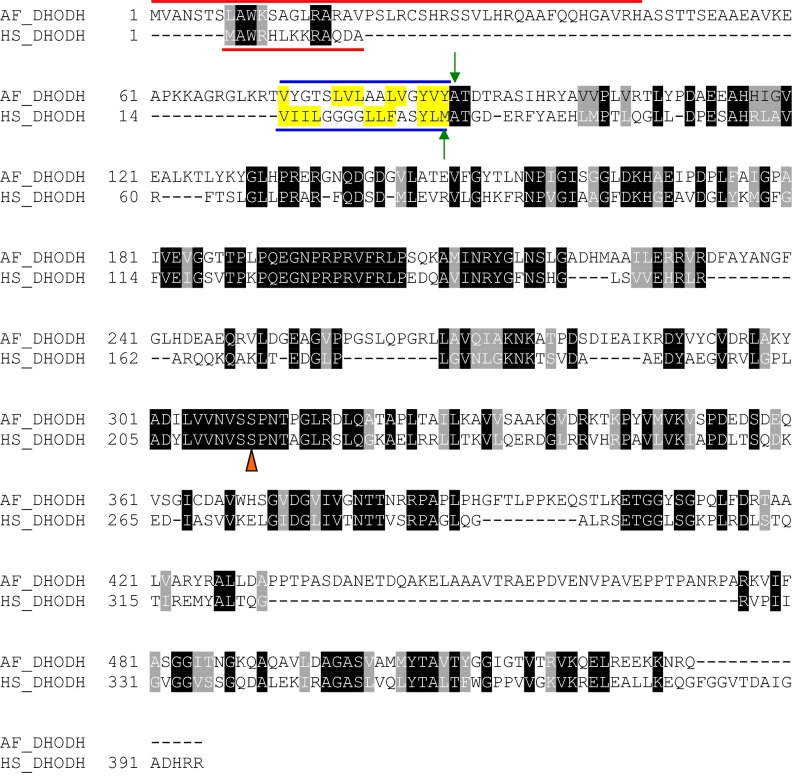
Alignment of A. fumigatus (AF_DHODH) and human (HS_DHODH) DHODH amino acid sequences. The DHODH protein sequences for A. fumigatus and human were aligned using CLUSTALW, followed by minor realignment of the mitochondrial targeting sequences and transmembrane domains, defined previously for the human sequence (35). The conserved residues are highlighted in black, and similar residues are highlighted in gray (using BOXSHADE), except in the transmembrane domain, where hydrophobic residues are highlighted in yellow. Mitochondrial targeting sequences are denoted by a red line; transmembrane sequences, by a blue line. Green arrows denote the first residue of the expressed recombinant proteins. The conserved catalytic serine is labeled with an orange triangle.
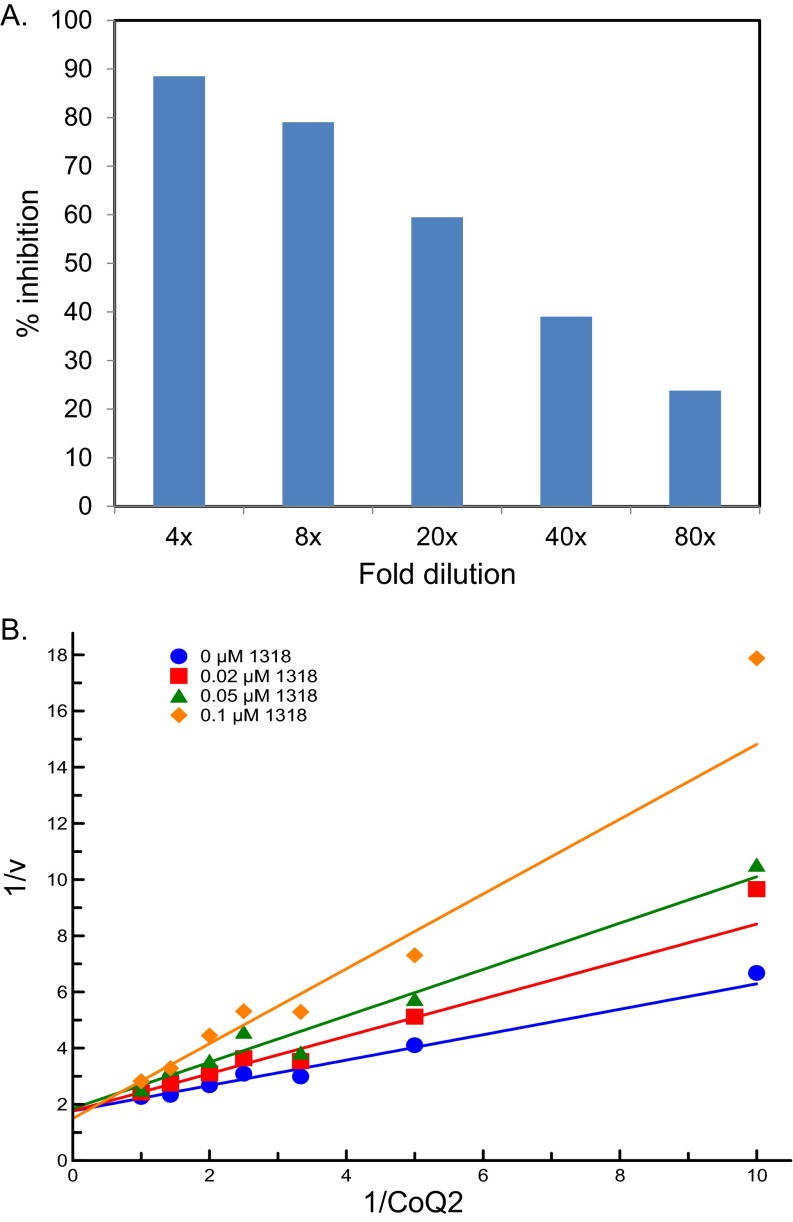
Further enzyme kinetic experiments revealed that F901318 is a reversible inhibitor of A. fumigatus DHODH (Fig. S5A) and is a competitive inhibitor with respect to the ubiquinone (coenzyme Q) cofactor, which functions as an electron acceptor in the reaction (Fig. S5B). This latter property is perhaps not unexpected, given that structural studies have shown that known inhibitors of human DHODH (teriflunomide and brequinar) (19) and the Plasmodium falciparum enzyme (DSM265) (20) bind in a region of the protein predicted to be a channel through which the ubiquinone enters the molecule from the inner mitochondrial membrane.
Fig. S5.
Kinetics of F901318 inhibition of A. fumigatus DHODH. (A) F901318 is a reversible inhibitor of A. fumigatus DHODH. A. fumigatus DHODH was incubated in the presence or absence of 4 µM F901318 for 30 min at room temperature. Aliquots were then diluted in assay buffer [50 mM Tris⋅HCl, 150 mM KCl, 10% (wt/vol) glycerol and 0.1% (wt/vol) Triton X-100, pH 8] and then further diluted by addition of the mixture that starts the reaction (1 mM l-dihydroorotic acid, 0.05 mM coenzyme Q2, and 0.1 mM 2,6-dichlorindophenol in assay buffer). The final dilution was 4- to 80-fold, giving a final drug concentration of 1–0.05 µM. DHODH activity was calculated for each condition in duplicate and expressed as percent inhibition compared with no drug controls. Dilution of the inhibitor-enzyme mixture releases drug from the enzyme, increasing the activity (and hence decreasing the inhibition) with greater dilution. Thus, F901318 is a reversible inhibitor of A. fumigatus DHODH. (B) F901318 is a competitive inhibitor of A. fumigatus DHODH. Lineweaver Burk analysis of F901318 inhibition of A. fumigatus DHODH. The reciprocal of the coenzyme Q2 concentration (in mM) is plotted vs. the reciprocal of the reaction velocity (v, nmol min−1). For a competitive inhibitor, the lines should cross the y-axis at the same place.
Structural Insights of F901318 Binding to A. fumigatus DHODH.
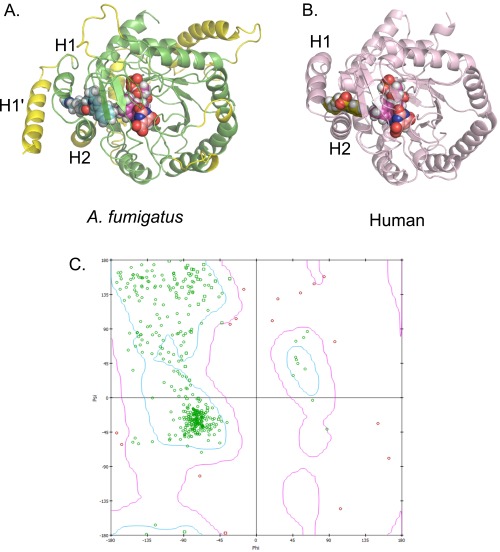
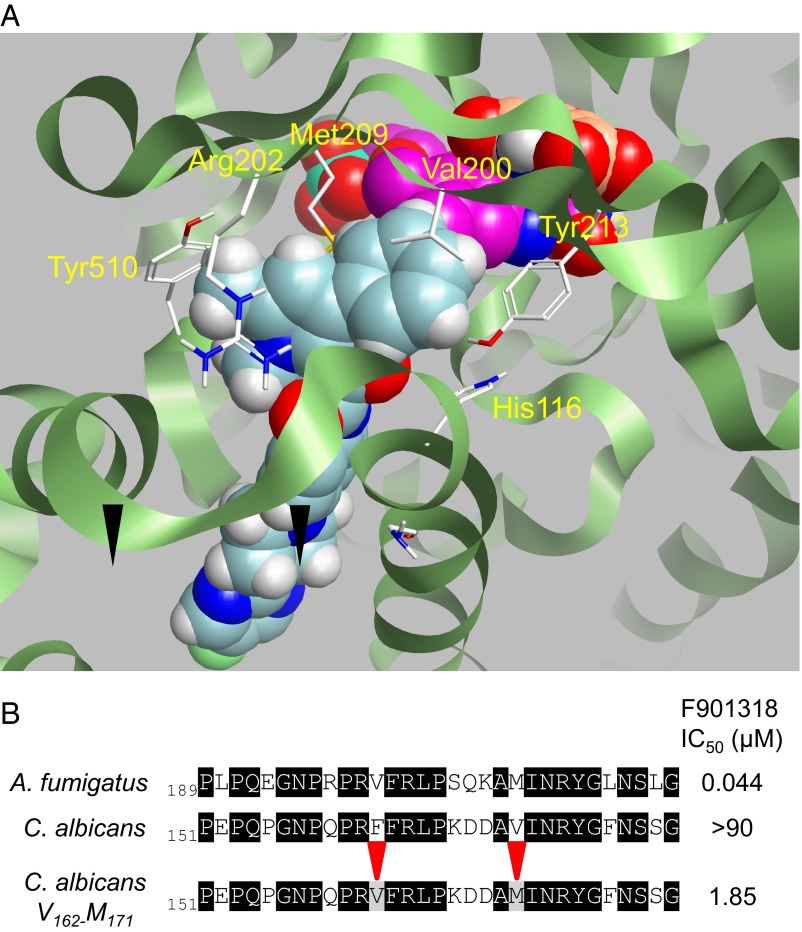
In the absence of a crystal structure, we investigated the binding of F901318 to A. fumigatus DHODH by creating a homology model of A. fumigatus DHODH (Fig. S6) using the structural information provided by other class 2 DHODH enzymes, including the structure of human DHODH (19). F901318 and other members of the series were used to identify a likely binding mode. Key residues for binding were identified (Fig. 3A). Validation of the importance of two of these residues was obtained by mutagenesis of C. albicans DHODH. The WT C. albicans DHODH was not inhibited by F901318, but mutation of two residues, Phe162 and Val171, to the residues predicted to occupy the same positions in the A. fumigatus enzyme, Val200 and Met209, respectively, created a mutant C. albicans DHODH that was inhibited by F901318 (Fig. 3B). The IC50 of the mutant C. albicans_V162_M171 was still ∼40-fold higher than the IC50 of F901318 against the A. fumigatus enzyme, indicating the existence of other important differences in DHODH between the two species; however, both of these residues are clearly important for inhibition. These data, along with the observed competition between F901318 and coenzyme Q in the in vitro assay, suggest that the orotomides bind in the “quinone channel,” where ubiquinone enters the enzyme from the inner mitochondrial membrane, preventing reoxidation of the dihydroflavin mononucleotide (FMNH2) cofactor essential for the reaction to proceed (Fig. S2).
Fig. S6.
Inhibitor binding to DHODH. (A) Homology model of A. fumigatus DHODH with the estimated binding mode of F901318. The A. fumigatus DHODH was modeled using information from known class 2 DHODH structures, with the human structure (PDB ID code 1D3G) forming the protein template. Manually modeled loop/helix insertions are shown in yellow and include an extra N-terminal alpha-helix (H1′) compared with the human protein. Helices H1 and H2 are common to these structures and are predicted to be the entry point for ubiquinone from the mitochondrial membrane (19). F901318 is shown as cyan spheres, FMN is in magenta, and orotate is in orange. (B) Brequinar bound to human DHODH (PDB ID code 1D3G). Adapted from Liu et al. (19). Brequinar is in green; FMN, in magenta; orotate, in orange. (C) Ramachandran plot of an A. fumigatus DHODH homology model. The model was assessed by calculating the ϕ and ψ torsional angles of the amino acid residues in the model. The bulk of the model appears to be energetically allowable (green residues). All residues with ϕ–ψ angle irregularities are exclusively in regions with lower homology to known structures and thus are more speculative. These discrepancies (pink residues) occur in residues distant from the predicted inhibitor-binding region and should not affect the derived ligand-binding hypothesis.
Fig. 3.
(A) Binding of F901318 to A. fumigatus DHODH. A homology model of A. fumigatus DHODH was created, and the binding mode of F901318 (cyan) was estimated. The product orotate (orange) and the cofactor flavin mononucleotide (FMN; magenta) are also shown. Residues predicted to be close to the molecule are highlighted. (B) F901318 inhibits a mutant version, but not the WT version, of C. albicans DHODH. Recombinant C. albicans DHODH residues Phe162 and Val171 were mutated to Val and Met, respectively (their predicted equivalents in A. fumigatus DHODH). The IC50 of F901318 inhibition of the WT and mutant DHODH proteins is displayed in the right-hand column (A. fumigatus IC50, n = 11, SD = 0.01 μM; WT C. albicans IC50, n = 7, all seven replicates had an IC50 >90 μM; C. albicans_V162_M171 IC50, n = 7, SD = 0.91 μM).
Spectrum, Resistance, and in Vivo Efficacy of F901318.
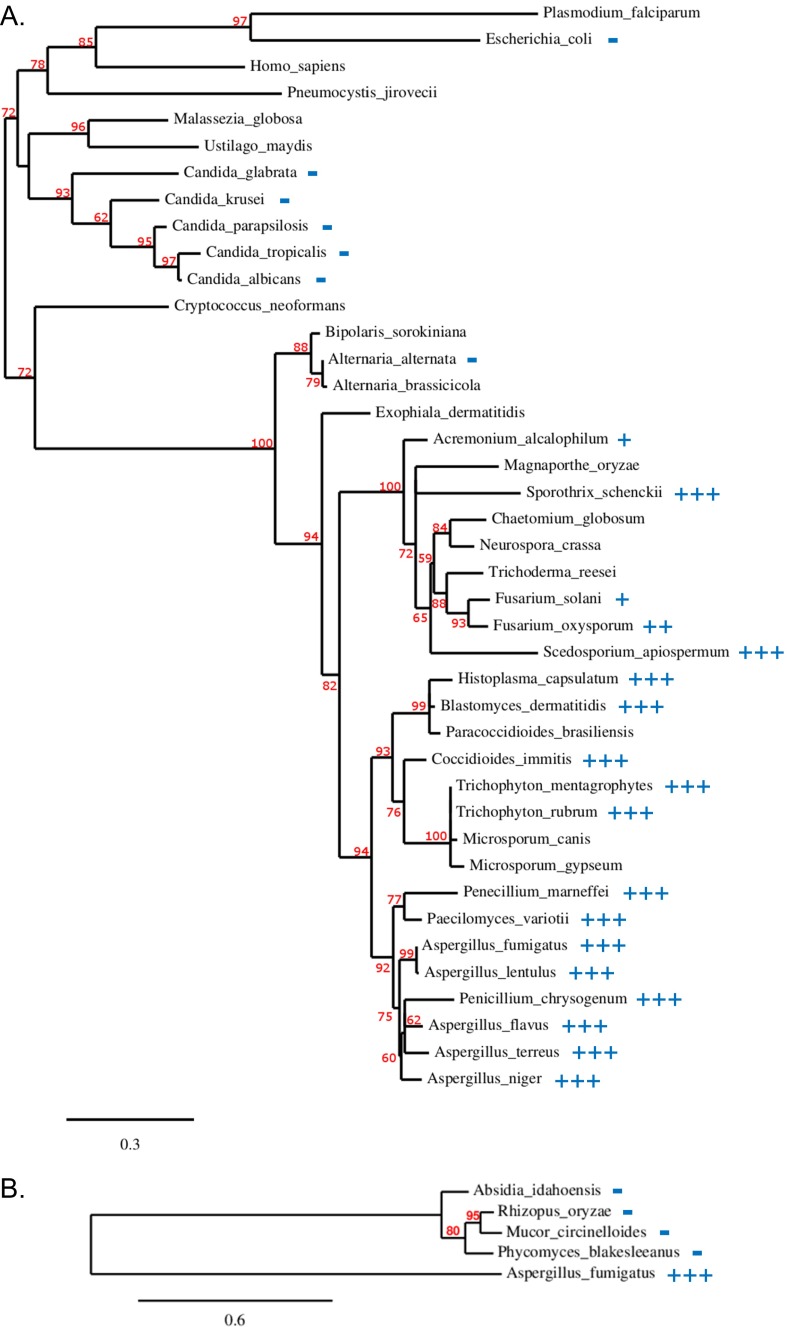
Although we observed no activity against Candida spp. and the zygomycetes in antifungal susceptibility testing, F901318 displayed excellent potency against a broad range of pathogenic filamentous and dimorphic fungi, including Penicillium spp., Coccidiodes immitis, H. capsulatum, Blastomyces dermatitidis, Fusarium spp., and the difficult-to-treat Scedosporium spp. This spectrum appears to be sequence-driven, given that the sensitive organisms were found to be grouped together in a phylogenetic analysis of DHODH (Fig. S7). This finding is consistent with observations that other DHODH inhibitors exhibit specificity of action owing to interspecies variations in the architecture of the hydrophobic channel in which the inhibitors are predicted to bind (19–21). Thus, the unique spectrum of F901318 is a reflection of the structure of the target, DHODH, and the binding mode of the orotomides.
Fig. S7.
Phylogeny of DHODH and F901318 susceptibility. (A) Class 2 DHODH sequences from fungi and selected other organisms. (B) Zygomycete DHODH phylogeny. Branch support values expressed as a percentage are indicated in red. The scale bar corresponds to the branch length for an expected number of 0.3 and 0.6 substitution per site in A and B, respectively. Antifungal susceptibility testing of F901318 against the species indicated was carried out according to CLSI protocol M38-A2. For the fungi with a score, at least five different strains were tested, except for the following organisms: A. lentulus, n = 4; F. solani, n = 3; F. oxysporum, n = 2; S. apiospermum, n = 2; and P. variotti, n = 3. For S. prolificans, three different strains were tested, all giving an MIC of ≤0.06 µg/mL, but no DHODH sequence could be obtained for phylogenetic analysis. MIC scoring: +++, MIC ≤0.06 µg/mL; ++, MIC = 0.125–0.5 µg/mL; +, MIC = 1–2 µg/mL; −, MIC >32 µg/mL.
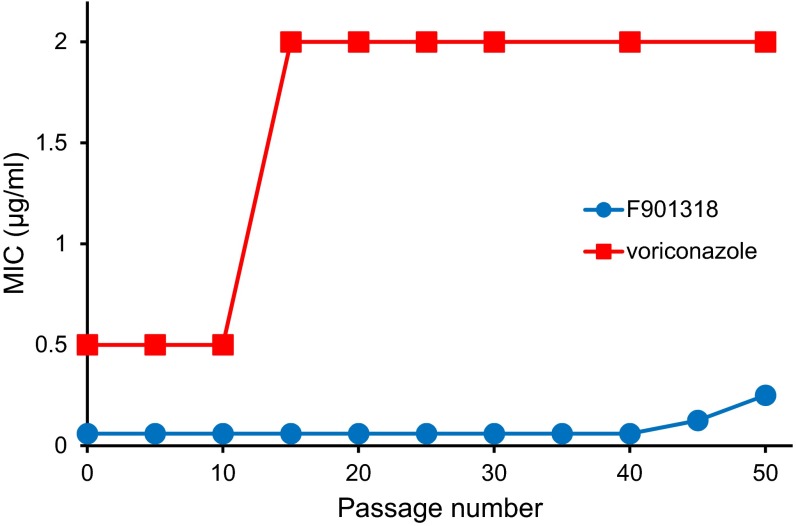
We investigated the resistance to F901318 in A. fumigatus by repeated exposure to a concentration gradient of the drug on an agar plate and selection from the margins of growth. This was carried out for 50 passages, with no change in the MIC observed for the first 40 passages and only a modest increase thereafter (Fig. S8). In contrast, voriconazole exhibited an increase in the MIC between 10 and 15 passages. Based on these results, it appears that F901318 resistance is not easily induced in A. fumigatus.
Fig. S8.
Effect on MIC of repeated exposure of A. fumigatus to F901318 or voriconazole. A. fumigatus 210 conidia were inoculated on Sabouraud agar. Then 100 µL of 500 µg/mL F901318 (blue circles) or voriconazole (red squares) was dispensed into a well in the center of the plate and allowed to diffuse. After incubation at 35 °C for 4 d, conidia were collected from the margins of growth and used to inoculate the next plate. After every five passages, the MIC of the conidia was determined.
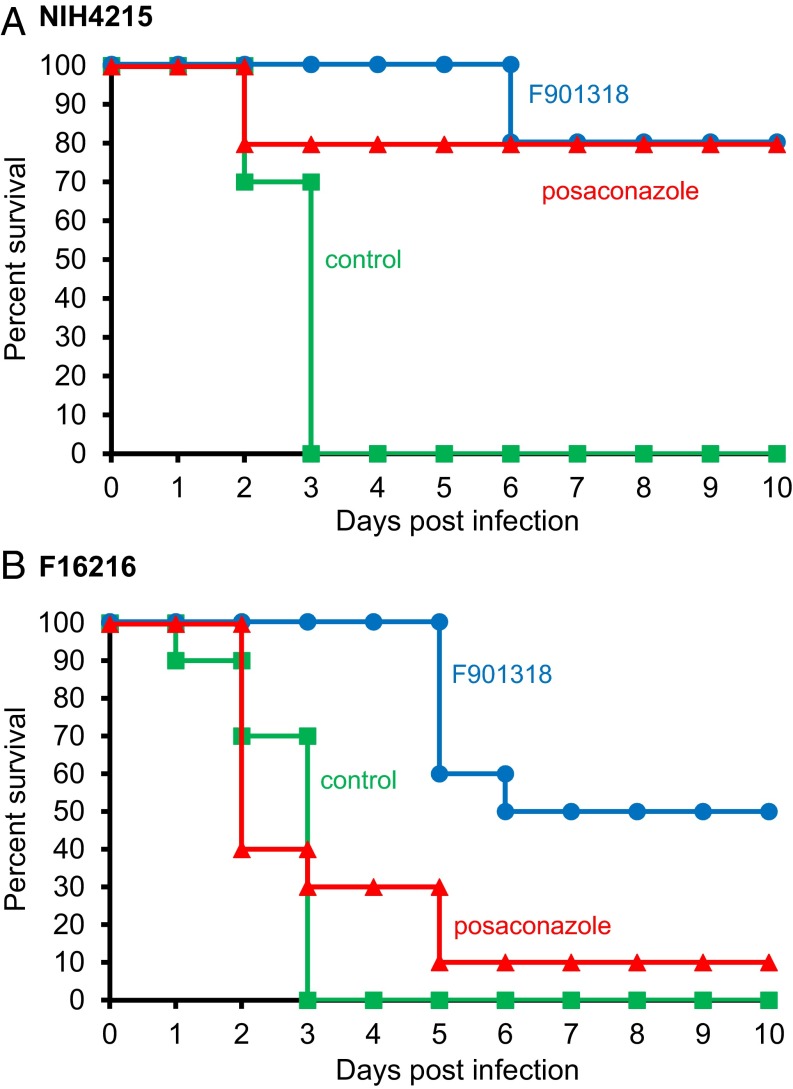
Pharmacokinetic studies in mice have identified good distribution of F901318 to tissues including the kidney, liver, and lung, with detection in the brain, albeit at lower levels, suggesting that the drug reaches key sites of infection. The efficacy of F901318 was demonstrated in a neutropenic murine model of invasive pulmonary aspergillosis. After infection with a well-characterized A. fumigatus strain (NIH 4215), survival was significantly improved by F901318 treatment (Fig. 4A). Treatment with the triazole drug posaconazole also increased survival after infection with this strain.
Fig. 4.
In vivo efficacy of F901318 in a murine model of A. fumigatus infection. Groups of 10 immunosuppressed mice were infected intranasally with A. fumigatus NIH 4215 (A) or the azole-resistant A. fumigatus F16216 (B) conidia on day 0. Treatment with F901318 (15 mg/kg i.v., three times daily; blue circles), posaconazole (7.5 mg/kg orally, once daily; red triangles), or control (green squares) was initiated at 6 h postinfection. Kaplan–Meier curves of surviving mice in each group were plotted. After infection with NIH 4215, F901318 treatment significantly improved survival compared with controls (P < 0.001, Mantel–Haenszel test). After infection with F16216, F901318 treatment significantly improved survival compared with controls (P < 0.001) and compared with posaconazole treatment (P < 0.005).
Mutations in the gene encoding the target molecule of the azole class of antifungal drugs, Cyp51A, that cause resistance have been identified. Several azole-resistant strains of A. fumigatus carry a combination of a tandem repeat in the promoter and a point mutation in the coding sequence. One such strain, A. fumigatus F16216, carrying the TR34/L98H mutation of Cyp51A, has proven to be resistant to multiple azole drugs, including itraconazole, voriconazole, and posaconazole (22). In vitro, A. fumigatus F16216 displayed no resistance to F901318, with an MIC of 0.03 µg/mL, which is comparable to the data presented in Table 1. In vivo, in the pulmonary aspergillosis model, A. fumigatus F16216 caused an infection that cannot be treated with posaconazole (Fig. 4B); however, F901318 therapy led to a significant increase in survival in this severe model, demonstrating that the different mechanism of action of the orotomides enables F901318 to overcome azole resistance caused by Cyp51A mutations.
Preclinical safety pharmacology and toxicology studies of F901318 have supported the progression and evaluation of this antifungal in Phase 1 oral and i.v. single and repeated dose trials.
Discussion
As highlighted by Denning and Bromley (23), the antifungal pipeline has failed to produce new antifungal drugs with mechanisms of action different from those of existing classes in the years since caspofungin was licensed in 2001. Many potential antifungal targets have been investigated, but translating these early stage projects into clinical candidates has proven elusive. This difficulty has mirrored the issues with target-based screening encountered in the antibacterial arena (24). In fact, a review of new mechanism, first-in-class medicines approved by the US Food and Drug Administration between 1999 and 2008 revealed that target-based screens were responsible for the discovery of only 3 of 10 drugs for infectious disease, with the majority discovered by phenotypic screening (i.e., “whole-cell screens” for antibiotics/antifungals) (25). The orotomides were discovered via a whole-cell screening approach, providing hits that were known to have antifungal activity from the start, but with no knowledge of mechanism of action. This classical approach was coupled with a genetic screen to identify the target of the drug, DHODH. A recent review of antifungal drug discovery suggested that similar approaches, taking advantage of genetic tools such as haploinsufficiency strain collections and new technologies such as next-generation sequencing, may accelerate the translation of antifungal chemistries toward the clinic (26).
Pyrimidines are essential to the cell, not just for the synthesis of DNA and RNA, but also for the formation of precursors for lipid and carbohydrate metabolism. For example, synthesis of the cell wall requires UDP-activated sugars at multiple stages, including UDP-glucose for β-(1,3)-glucan synthesis. Pyrimidines are synthesized in the de novo pyrimidine biosynthesis pathway (Fig. S2), in which DHODH is a key enzyme, but they also can be scavenged by fungi from the environment via the salvage pathway. The pyrimidine salvage pathway appears to be inefficient for A. fumigatus, however (Fig. S3). In animal models of infection, pyrimidine biosynthesis mutants from several pathogenic fungi are highly attenuated for virulence, including studies on A. fumigatus (14), C. albicans (15), H. capsulatum (16), and C. neoformans (17). In Saccharomyces cerevisiae, a ura3 deletion strain lacking the orotidine-5′-phosphate (OMP) decarboxylase enzyme of pyrimidine biosynthesis was unable to survive in vivo, exhibiting a decrease in the competitive index versus a WT or reconstituted strain observed after just 4 h (27). In C. albicans, URA3 has been commonly used as a selectable marker, but concerns have been raised that in some virulence studies, the ectopic expression of URA3, leading to reduced OMP decarboxylase activity, had a greater effect on virulence compared with the disruption of the target gene of interest (28). Thus, the evidence in the literature supports the targeting of pyrimidine biosynthesis as a valid antifungal strategy.
Identifying DHODH as the target of the orotomides has helped explain the spectrum of antifungal activity that we observed. F901318 has activity against numerous pathogenic filamentous and dimorphic fungi, including Aspergillus spp., H. capsulatum, Blastomyces dermatitides, and C. immitis, along with the difficult-to-treat Scedosporium prolificans. These F901318-susceptible organisms group together on the phylogenetic tree of DHODH (Fig. S7), whereas DHODH from Candida spp., C. neoformans, and the human and Plasmodium enzymes are more distantly related while still classified as class 2 DHODH enzymes. DHODH from the zygomycota, such as Rhizopus and Mucor, align more closely with class 1A DHODHs, cytosolic enzymes that occur in Gram-positive bacteria and the trypanosmatids that use alternative cofactors, such as fumarate.
DHODH has been suggested as a target for therapy in multiple diverse disease areas, including oncology, rheumatoid arthritis, multiple sclerosis, and infectious diseases caused by such agents as Plasmodium, bacteria, and viruses (21, 29). There are currently two marketed agents that have activity against human DHODH: leflunomide for rheumatoid arthritis and teriflunomide for multiple sclerosis. DSM265 is an antimalarial drug targeting plasmodial DHODH that is currently in Phase 2 clinical trials (20); however, to our knowledge, no other human antifungal therapies have progressed with DHODH as a target.
Although at first consideration, the breadth of therapeutic areas for which DHODH has been proposed as a drug target is surprising, in each case limiting the pool of pyrimidines prevents proliferation of a population of cells. In some cases, the host cells are targeted, such as lymphocytes in autoimmune diseases and proliferating cancerous cells in oncology. Alternatively, the DHODH of invading pathogens is targeted to selectively limit the pyrimidine pools of the infective agent. Between these two effects, antiviral action has been reported for human DHODH inhibitors, given that viruses require host pyrimidines for replication (29).
In conclusion, to combat the increasing problem of resistance to existing antifungal therapies, the discovery of new cellular targets for antifungals, along with viable chemistry against these new targets (23), is vitally important. F901318 is an antifungal drug, currently in both intravenous and oral Phase 1 clinical trials (ClinicalTrials.gov identifiers: NCT02142153, NCT02342574, NCT02394483, and NCT02737371), that acts via inhibition of the pyrimidine biosynthesis enzyme DHODH, validating an alternative target for antifungal drug discovery.
Materials and Methods
Primers.
The sequences of the primers used in this work are listed in Table S1. Primers were supplied by Eurofins MWG.
Table S1.
Sequences of the primers used in this study
| Primer | Sequence 5′–3′ |
| AF_DHODH_F1 | GACGACGACAAGATGGCGACGGATACCAGGGCAAG |
| AF_DHODH_R1 | GAGGAGAAGCCCGGTCTATTGACGGTTTTTCTTTTCC |
| HS_DHODH_F1 | GACGACGACAAGATGGCCACGGGAGATGAGCG |
| HS_DHODH_R1 | GAGGAGAAGCCCGGTTCACCTCCGATGATCTGCTC |
| CA_DHODH_fusF1 | ATGTTTCGTCCAAGTATCAAATTC |
| CA_DHODH_fusR1 | TCACTTATCATCAGAGCCAA |
| CA_DHODH_mutF1 | ATGTTTCGTCCAAGTATCAAATTCAAACAGTCGACTTTGTCC |
| CA_DHODH_mutR1 | CCAACTTATGTCCCGACTCTGCATCTGTGAAAGT |
| CA_DHODH_mutF2 | CACAGATGCAGAGTCGGGACATAAGT |
| CA_DHODH_mutR2 | TCACTTATCATCAGAGCCAATTATTTGCTC |
| CA_DHODH_F1 | GACGACGACAAGATGTCAAGATCAGCAATCCATGA |
| CA_DHODH_R1 | GAGGAGAAGCCCGGTTCACTTATCATCAGAGCCAA |
| CA_DHODH_VM_F | CCAAAAGATGATGCTATGATTAACAGAT |
| CA_DHODH_FV_R | CAATCTGAAAACTCTTGGCTGA |
Synthesis of F901318.
The 2-(1,5-dimethyl-3-phenyl-1H-pyrro-2-yl)-N-(4-[4-(5-fluoro-pyrimidin-2-yl-piperazin-1yl]-phenyl)-2-oxo-acetamide (F901318) was prepared as described in SI Materials and Methods.
In Vitro Antifungal Susceptibility Testing.
MICs of antifungal drugs were determined according to Clinical and Laboratory Standards Institute (CLSI) protocol M38-A2 in RPMI 1640 medium buffered to pH 7.0 with MOPS buffer at 35 °C. For caspofungin, the minimum effective concentration (MEC) was defined as the lowest drug concentration causing abnormal growth (i.e., short-branching hyphae).
Mechanism of Action Screen.
An A. nidulans genomic library carried on the pRG3-AMA1-NotI vector was obtained from the Fungal Genetic Stock Center. Protoplasts from pyrG- strains of A. nidulans (A767) were transformed with the genomic library by PEG-mediated transformation. Transformants were exposed to lethal concentrations of F901318 on Vogel’s minimal agar (30). Plasmid DNA was extracted from resistant colonies and sequenced.
DHODH Assays.
These assays were carried out using recombinant DHODH prepared from A. fumigatus cDNA, C. albicans gDNA, or, for the human protein, IMAGE clone 6064723 (Geneservice Ltd.) cloned into the vector pET44 (Novagen) minus the N-terminal 88 (A. fumigatus), 56 (C. albicans), or 28 (human) amino acids. For C. albicans, CTG-encoded serines were mutated to TCG. Further mutations altered Phe162 and Val171 to become Val and Met to create the mutant protein C. albicans_V162_M171. Primers are listed in Table S1, and further methodological details are provided in SI Materials and Methods. The assay was conducted as described elsewhere (31).
Homology Modeling of A. fumigatus DHODH.
Human DHODH [Protein Data Bank (PDB) ID code 1D3G] served as the protein template for construction of the A. fumigatus DHODH model. Other DHODH structures from human (PDB ID codes 1PRH, 2PRL, 2PRM, 3G0X, 3KVM, 2WV8, and 2FQI), rat (PDB ID codes 1UUM and 1UUO), Trypanosoma cruzi (PDB ID code 2E68), P. falciparum (PDB ID codes 3I68, 1ITV, and 3O8A) Leishmania major (PDB ID code 3MJY), and Escherichia coli (PDB ID code 1F76) also informed the process. Coarse refinement of the structure with Discovery Studio 4.1 (Accelerys) was followed by fine refinement with XEDraw (Cresset). More details of the homology modeling process and ligand binding are provided in SI Materials and Methods.
Resistance Testing.
A. fumigatus 210 conidia were inoculated onto Sabouraud agar (Oxoid) in a 9-cm Petri dish. An 8-mm-diameter circle of agar was removed from the center of the plate to create a well. 100 µL of 500 µg/mL drug was loaded into the well and allowed to diffuse into the agar, creating a concentration gradient. After 4 d of incubation at 35 °C, a zone of inhibition was observed, and conidia were collected from the margins of growth and then used to create the next plate. After every fifth passage, the MIC was determined as described above.
In Vivo Efficacy Testing.
All experiments were conducted under U.K. Home Office project license 40/3630 and approved by the University of Liverpool’s Animal Welfare Committee. Groups of 10 CD-1 mice were immunosuppressed with 200 mg/kg cyclophosphamide i.p. at 4 d before infection and with cyclophosphamide and 250 mg/kg cortisone acetate s.c. at 1 d before infection. A. fumigatus F16216 carries an L98H mutation of cyp51A and a 34-bp tandem repeat in the cyp51A promoter, leading to resistance to azole drugs (22). Conidia from this strain and from the WT A. fumigatus NIH 4215 were administered intranasally on day 0. Treatment with F901318 (15 mg/kg i.v. three times daily) or posaconazole (7.5 mg/kg/day orally) was initiated at 6 h postinfection.
SI Materials and Methods
Fungal Strains.
For susceptibility testing, a range of clinical isolates were tested for each species plus standard strains: A. fumigatus AF210, A. niger AN1, A. terreus AT4, A. flavus AFl1, C. albicans CA6862, E. coli NCTC 10418, and Staphylococcus aureus Oxford strain NCTC 6571. A. nidulans A4 (WT) and A. nidulans A767 (pyrG89 nicA) were used for the mechanism of action screen. A. fumigatus NIH4215 and A. fumigatus F16216 were used for efficacy testing.
Synthesis of F901318.
The synthesis of 1,2-dimethyl-4-phenyl-1H-pyrrole was done as described previously (32).
(1,5-Dimethyl-3-phenyl-1H-pyrrol-2-yl)-oxo-acetyl chloride.
Oxalyl chloride (78.42 mL, 0.91 mol) was slowly added to a cooled solution of 1,2-dimethyl-4-phenyl-1H-pyrrole (105.5 g, 0.61 mol) in dry dichloromethane (3 × 200 mL) at 0 °C. The reaction mixture was then warmed to room temperature and stirred for 1 h. The solvent was evaporated to dryness in vacuo to afford 124 g (78% yield) of (1,5-dimethyl-3-phenyl-1H-pyrrol-2-yl)-oxo-acetyl chloride as a brown oily liquid, which was used directly in the next step (TLC system: EtOAc:pet ether, 3:7; Rf value, 0.65).
5-Fluoro-2-[4-(4-nitro-phenyl)-piperazin-1-yl]-pyrimidine.
2-Chloro-5-fluoropyrimidine (78.76 mL, 0.64 mol) was added to suspension of 1-(4-nitro-phenyl)-piperazine (110 g, 0.53 mol) and potassium carbonate (367.2 g, 2.65 mol) in acetonitrile (3 L). The reaction mixture was refluxed for 48 h. On completion of reflux, the mixture was cooled to 0 °C and then filtered, and the residue was taken in water (3 L) and stirred for 30 min. The suspension was filtered, the solid cake was washed with water (1 L) and pet ether (1 L) and then dried under vacuum to afford 148 g (92%) of 5-fluoro-2-[4-(4-nitro-phenyl)-piperazin-1-yl]-pyrimidine as a yellow-colored solid. (TLC system: EtOAc:pet ether, 3:7; Rf value, 0.70). 1H NMR (300 MHz, CDCl3): δ 8.24 (s, 2 H), 8.17–8.13 (d, J = 9.6 Hz, 2 H), 6.87–6.84 (d, J = 9.6 Hz, 2 H), 3.97–3.94 (m, 4 H), 3.55–3.52 (m, 4 H). m.p. 205–206 °C. (m/z, ES, positive scan): 304.21 (100%; M+H).
4-[4-(5-Fluoro-pyrimidin-2-yl)-piperazin-1-yl]-phenyl amine.
A solution of 5-fluoro-2-[4-(4-nitro-phenyl)-piperazin-1-yl]-pyrimidine (80 g, 0.26 mol) in methanol (1 L) was hydrogenated over Raney Nickel (30 g) under 70-psi pressure at room temperature for 6 h. The reaction mixture was filtered over celite, and the filtrate was concentrated in vacuo to yield a crude product, which was washed with hexane (500 mL) to afford 52 g (72%) of 4-[4-(5-fluoro-pyrimidin-2-yl)-piperazin-1-yl]-phenyl amine as a brown-colored solid (TLC system: methanol:chloroform, 1:9; Rf value, 0.50). 1H NMR (300 MHz, CDCl3): δ 8.21 (s, 2 H), 6.86–6.84 (d, J = 8.7 Hz, 2 H), 6.68–6.65 (d, J = 9.0 Hz, 2 H), 3.92–3.89 (m, 4 H), 3.45 (broad s, 2 H, NH2), 3.09–3.06 (m, 4 H). m.p. 138–139 °C. (m/z, ES, positive scan): 288.14 (100%; M+H).
2-(1,5-Dimethyl-3-phenyl-1H-pyrro-2-yl)-N-{4-[4-(5-fluoro-pyrimidin-2-yl)-piperazin-1-yl]-phenyl}-2-oxo-acetamide F901318.
A solution of 1,5-dimethyl-3-phenyl-1H-pyrrol-2-yl)-oxo-acetyl chloride (64.65 g, 0.24 mol) in dichloromethane (500 mL) was added to a stirred solution of 4-[4-(5-fluoro-pyrimidin-2-yl)-piperazin-1-yl]-phenyl amine (45 g, 0.16 mol) and triethylamine (46 mL, 0.32 mol) in dichloromethane (150 mL) at 0 °C. The reaction mixture was warmed to room temperature and then stirred for 30 min. The reaction mixture was quenched with water and extracted with dichloromethane (6 × 500 mL). The combined organic layers were washed successively with saturated sodium bicarbonate solution (1.5 L), water (1 L), and brine (1.5 L), and finally dried over anhydrous sodium sulfate. The organic layer was stirred with neutral alumina (500 g) at room temperature for 30 min and then filtered. The filtrate was concentrated in vacuo to yield the crude product, which was washed successively with diethyl ether (300 mL) and hexane (200 mL) to afford 60 g (73%) of 2-(1,5-dimethyl-3-phenyl-1H-pyrro-2-yl)-N-{4-[4-(5-fluoro-pyrimidin-2-yl-piperazin-1yl]-phenyl}-2-oxo-acetamide F901318 as a yellow-colored solid (TLC system: EtOAc:pet ether, 1:1; Rf value, 0.65). 1H NMR (400 MHz, CDCl3): δ 8.21 (s, 2 H), 8.19 (broad s, 1 H), 7.32–7.22 (m, 5 H), 7.10 (d, J = 8.7 Hz, 2 H), 6.83 (d, J = 8.7 Hz, 2 H), 6.13 (s, 1 H), 3.92–3.88 (m, 4 H), 3.81 (s, 3 H), 3.19–3.15 (m, 4 H), 2.33 (s, 3 H). m.p. 194–197 °C. (m/z, APCI, positive scan): 499.41 (100%; M+H).
Mechanism of Action Screen.
A. nidulans A767 protoplasts were prepared from fungal biomass by digestion with 5% (wt/vol) glucanex (Novozymes) in 0.6 M KCl and 50 mM CaCl2 for 60–120 min at 30 °C with shaking at 100 rpm. The protoplasts were washed in 0.6 M KCl and 50 mM CaCl2, isolated by centrifugation at 820 × g for 9 min at 4 °C, and then washed and resuspended in STC buffer (1.2 M sorbitol, 50 mM CaCl2, and 10 mM Tris⋅HCl pH 7.5). Then 2–5 μg of an A. nidulans genomic library carried on the pRG3-AMA1-NotI vector (Fungal Genetic Stock Center) was transformed into protoplasts in the presence of 40% (wt/vol) PEG 6000 in STC buffer for 5 min, followed by vortexing and inoculation onto Vogel’s minimal medium, 1.2 M sorbitol, and 5 µg/mL nicotinic acid. The plates were incubated at 37 °C for 5 d, after which the spores were collected in PBS-0.1% Tween 80 and filtered through glass wool. A total of 105 spores were inoculated onto Vogel’s minimal medium (30) plus 0.25 µg/mL F901318, and the plates were incubated at 37 °C for 7 d. DNA was isolated from resistant colonies using the FastDNA Spin Kit (MP Biomedicals), and plasmid DNA was transformed into E. coli (MegaX DH10B T1R Electrocomp cells; Invitrogen).
DNA was mutagenized using the EZ-Tn5 bacterial transposon system (Epicentre Biotechnologies). A DNA fragment comprising A. fumigatus pyrG (GenBank accession no. Y11303.1), followed by the EM7 promoter upstream of the She ble ORF (codes for resistance to zeocin: 1,924–2,426 bp of pEM7/zeo; Invitrogen) with Tn5 mosaic ends at both ends, was included in the transposase reaction. After transformation into E. coli, insertion of the Tn5 transposon was detected by growth in the presence of 50 μg/mL zeocin, and PCR established the insertion site.
Cloning, Expression, and Purification of DHODH.
To overcome initial problems with solubility of A. fumigatus DHODH expressed in E. coli, the N-terminal 88 amino acids consisting of the mitochondrial targeting sequence and transmembrane domain were omitted, and the truncated DHODH (amino acids 89–531) was cloned into pET44 (Novagen) that codes for an N-terminal NusA fusion protein to aid solubility. A. fumigatus cDNA was prepared from A. fumigatus RNA using AMV reverse transcriptase (Promega). PCR of N-truncated A. fumigatus DHODH coding cDNA was carried out using primers AF_DHODH_F1 and AF_DHODH_R1 (primer sequences given in Table S1) and KOD DNA polymerase (Novagen). This product was cloned into pET44 by ligation-independent cloning (Novagen). N-truncated (amino acids 29–395) human DHODH cDNA was cloned from IMAGE clone 6064723 (MGC70636; Geneservice) into pET44 after PCR with primers HS_DHODH_F1 and HS_DHODH_R1.
C. albicans DHODH contains two CTG-encoded serine residues at codons 11 and 78 that were mutated to TCG codons by incorporating the new codons in two overlapping fragments, which were subsequently joined by fusion PCR. The fragments were created by PCR from gDNA with primer pairs CA_DHODH_mutF1 with CA_DHODH_mutR1 and CA_DHODH_mutF2 with CA_DHODH_mutR2. The fusion PCR used primers CA_DHODH_fusF1 with CA_DHODH_fusR1, and the product was cloned into pGEMTeasy (Promega) and sequenced. N-truncated (amino acids 57–444) C. albicans DHODH cDNA was cloned into pET44 for protein expression. Previous studies on recombinant DHODH from a variety of organisms, including human (33) and C. albicans (31), have found that removal of the N terminus, including the mitochondrial targeting sequence and transmembrane regions, improves expression, simplifies purification, and has little effect on kinetics.
C. albicans DHODH was further mutated at two residues. Phe162 and Val171 were altered to become Val and Met, respectively, using the Phusion Site-Directed Mutagenesis Kit (Thermo Fisher Scientific). PCR was carried out with phosphorylated primers CA_DHODH_VM_F and CA_DHODH_FV_R and template pET44_CA_DHODH. The product was religated into a plasmid using T4 DNA ligase to create pET44_CA_DHODH_V162_M171.
Protein expression plasmids were transformed into E. coli BL21 (DE3) cells, and expression was stimulated with 0.5 mM isopropyl β-D-1-thiogalactopyranoside, followed by incubation in LB broth in the presence of 100 μg/mL ampicillin and 100 μM flavin mononucleotide at 18–20 °C for 16–18 h. Bacterial pellets were lysed with BugBuster (Novagen), and His-tagged DHODH protein was isolated by immobilized metal affinity chromatography using His-Bind resin (Novagen) according to the manufacturer’s instructions.
DHODH Assays.
These assays were carried out broadly as described elsewhere (31). Approximately 2 μg of recombinant A. fumigatus DHODH or 0.5 μg of recombinant human DHODH was incubated in the presence of 1 mM l-dihydroorotic acid, 0.05 mM coenzyme Q2, and 0.1 mM 2,6-dichlorindophenol in 50 mM Tris⋅HCl, 150 mM KCl, 10% (wt/vol) glycerol, and 0.1% (wt/vol) Triton X-100, pH 8 at 25 °C. DHODH activity was determined using the redox indicator 2,6-dichloroindophenol by measuring the decrease in absorbance at 600 nm. Initial reaction velocities were determined, and the percent inhibition in the presence of the drug was calculated compared with controls in the absence of drug. The inhibition curve data were fitted to a four-parameter sigmoidal plot (r2 >0.998 for IC50 plots) using XLFit (IDBS), and the IC50 value was calculated as the concentration of drug causing 50% inhibition.
Homology Modeling of A. fumigatus DHODH.
A range of DHODH structures, including those from human, rat, P. falciparum, and E. coli, were compared with the fungal sequences for Candida and Aspergillus strains. Sequences were aligned manually with reference to the protein superimpositions (PyMOL). RCSB PDB ID codes for the structures used were as follows: 1D3G, 1PRH, 2PRL, 2PRM, 3G0X, 3KVM, 2WV8, 2FQI (human); 1UUM, 1UUO (rat); 2E68 (Trypanosoma cruzi); 3I68, 1ITV, 3O8A (P. falciparum); 3MJY (Leishmania major); and 1F76 (E. coli) (www.rcsb.org).
Human DHODH (PDB ID code 1D3G) (19) served as the protein template for construction of the A. fumigatus DHODH model. Residue replacement, bump checking, and coarse refinement were done in Discovery studio 4.1 (Accelrys). Fine residue refinement and new secondary structure or loop insertions were built in XEDraw (Cresset’s internal research software, results of which can be visualized in Torch; www.cresset-group.com/products/torch/). When no information was known for a given loop, the loop was constructed manually based on the local environment and the nature of the primary sequence (i.e., using the predicted beta sheet or helical secondary structural preference) to yield appropriate packing that minimized the exposure of lipophilic residues and maximized the exposure of polar and charged residues to solvent. The final protein was coarse minimized (0.1 rmsd) using the XED force field.
In parallel to the initial protein analysis, the fungal inhibitor data were compared with known DHODH activities for which X-ray data were available. Full multiple ligand alignments were generated, and 3DQSAR was performed for the fungal inhibitors (Forge; Cresset; www.cresset-group.com/products/forge/).
All of the available information for the ligand centric and protein complex data were combined to provide a best estimate of correct placement of the F901318 inhibitor structure in the homology model. The resultant complex was then reminimized in place initially using shell minimization in XEDraw and then using full minimization to 0.01 rmsd (Fig. S6A).
The homology model was assessed using a Ramachandran plot (Fig. S6C). This plot exhibited good geometries for the core, with the only discrepancies being distant from the ligand and confined to more speculative regions of the model, i.e., where no homology existed.
By bringing both the protein sequence and structural data together with ligand SAR and field data, we were able to conclude with reasonable confidence the most likely nature of the interactions of this DHODH inhibitor chemotype. As with known inhibitors, a bidirectional H-bonding arrangement was observed for these fungal ligands, in keeping with the likely interactions within the putative ubiquinone-binding site. These interactions, from observation across multiple examples, involve equivalents of His116, Arg202, Tyr213, and Tyr505 (A. fumigatus residue numbering) as the corresponding H-bonding partners (Fig. 3A). From the sequence, structure analysis, and ligand field analysis, further lipophilic contacts—Val200, Met209, and Tyr510—were defined. This ligand-binding model is supported by the mutagenesis of the C. albicans recombinant protein (Fig. 3B). Phe162 and Val171 of C. albicans align with Val200 and Met209 of A. fumigatus DHODH, respectively. Mutating these residues to their A. fumigatus equivalents introduced inhibition of DHODH activity by F901318 in the mutant, where no inhibition had occurred with the WT enzyme.
Creation of DHODH Phylogenetic Trees.
DHODH sequences were obtained from searching sequence databases held by the National Center for Biotechnology Information, EnsemblFungi, and the Joint Genome Initiative (JGI). The accession codes for the proteins are as follows, with alternative fungal names in parentheses: Absidia idahoensis (Lichtheimia ramosa), CDS13591.1; Acremonium_alcalophilum, jgi|Acral2|2024193; Alternaria alternata, jgi|Altal1|982905; Alternaria brassicicola, jgi|Altbr1|1659; A. flavus, XP_002379410.1; A. fumigatus, XP_755434.1; Aspergillus lentulus, GAQ06647.1; A. niger, XP_001399431.2; A. terreus, protein sequence derived from gene ATEG_01477 (EnsemblFungi); Bipolaris sorokiniana, XP_007699125.1; B. dermatitidis (Ajellomyces dermatitidis), EEQ85598.1; C. albicans, XP_723522.1; Candida glabrata, XP_449903.1; Candida krusei (Pichia kudriavzevii), KGK37337.1; Candida parapsilosis, CCE41941.1; Candida tropicalis, XP_002549089.1; Chaetomium globosum, XP_001223311.1; C. immitis, XP_001240778.2; C. neoformans, XP_569657.1; E. coli, WP_001330055.1; Exophiala dermatitidis, XP_009159400.1; Fusarium oxysporum, EGU79150.1; Fusarium solani (Nectria hematococca), XP_003046434.1; H. capsulatum (Ajellomyces capsulatus), EGC45332.1; Homo sapiens, NP_001352.2; Magnaporthe_oryzae, XP_003719157.1; Malassezia globosa, XP_001729892.1; Microsporum canis (Arthroderma otae), XP_002849178.1; Microsporum gypseum, XP_003175518.1; Mucor circinelloides, EPB81175.1; Neurospora crassa, XP_961804.1; Paecilomyces variotii (Byssochlamys spectabilis), GAD93826.1; Paracoccidioides brasiliensis, XP_010757420.1; Penicillium chrysogenum, jgi|Pench1|33932; Penecillium marneffei (Talaromyces marneffei), KFX48065.1; Phycomyces blakesleeanus, jgi|Phybl2|177367; P. falciparum, XP_966023.1; Pneumocystis jirovecii, CCJ31177.1; Rhizopus delemar, EIE79352.1; Scedosporium apiospermum, JOWA01000121.1; Sporothrix schenckii, ERT03286.1; Trichoderma reesei, XP_006962857.1; Trichophyton mentagrophytes (Arthroderma benhamiae), XP_003012466.1; Trichophyton rubrum, XP_003234264.1; and Ustilago maydis, XP_011389794.1.
Sequences in FASTA format were submitted for phylogenetic analysis at www.phylogeny.fr using the “Advanced” analysis (34). Sequences were aligned using MUSCLE (“Full mode”) and curated with GBlocks (default settings). Phylogeny was established by the maximum likelihood method in PhyML using the approximate likelihood ratio test set to SH-like with the WAG substitution method. The tree was visualized in TreeDyn.
Class 2 DHODH sequences are present in all mammals, many fungi, and some bacteria, including E. coli (29). They are membrane-bound (integral mitochondrial membrane proteins in mammals and some fungi; membrane-associated in some bacteria) monomers that have a catalytic serine and use ubiquinone as a terminal electron acceptor. Class 1A and 1B DHODHs are soluble and multimeric, have a catalytic cysteine, and use fumarate or NAD as a terminal electron acceptor. Class 2 DHODHs (Fig. S7A) were analyzed separately from the zygomycete DHODHs (Fig. S7B), which appear to be from class 1A.
Acknowledgments
We thank John Rex, Richard White, and Markus Heep for their critical reading of the manuscript. S.d.P. is supported by the European Union Grant PITN-GA-2013-607963.
Footnotes
Conflict of interest statement: J.D.O., G.E.M.S., N.B., S.d.P., A.J.K., D.L., and M.B. are employees of F2G Ltd. K.S.D. and M.J.B. are ex-employees of F2G Ltd. J.D.O., G.E.M.S., K.S.D., A.J.K., D.L., and M.B. are shareholders of F2G Ltd. W.W.H. has received research funding from Pfizer, Gilead, Astellas, AiCuris, and F2G, and has acted as a consultant and/or given talks for Pfizer, Basilea, Astellas, F2G, Nordic Pharma, Mayne Pharma, and Pulmocide. G.E.M.S., D.L., J.D.O., and M.B. are listed as inventors on patent WO2009130481. J.D.O., M.J.B., G.E.M.S., and M.B. are listed as inventors on patent WO200913379.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1608304113/-/DCSupplemental.
References
- 1.Brown GD, et al. Hidden killers: Human fungal infections. Sci Transl Med. 2012;4(165):165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication, chronic pulmonary aspergillosis, in adults. Med Mycol. 2013;51(4):361–370. doi: 10.3109/13693786.2012.738312. [DOI] [PubMed] [Google Scholar]
- 3.Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ. 2011;89(12):864–872. doi: 10.2471/BLT.11.089441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pound MW, Townsend ML, Dimondi V, Wilson D, Drew RH. Overview of treatment options for invasive fungal infections. Med Mycol. 2011;49(6):561–580. doi: 10.3109/13693786.2011.560197. [DOI] [PubMed] [Google Scholar]
- 5.Denning DW, Hope WW. Therapy for fungal diseases: Opportunities and priorities. Trends Microbiol. 2010;18(5):195–204. doi: 10.1016/j.tim.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Nett JE, Andes DR. Antifungal agents: Spectrum of activity, pharmacology, and clinical indications. Infect Dis Clin North Am. 2016;30(1):51–83. doi: 10.1016/j.idc.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Verweij PE, Chowdhary A, Melchers WJ, Meis JF. Azole resistance in Aspergillus fumigatus: Can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis. 2016;62(3):362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivero-Menendez O, Alastruey-Izquierdo A, Mellado E, Cuenca-Estrella M. Triazole resistance in Aspergillus spp.: A worldwide problem? J Fungi. 2016;2(3):21. doi: 10.3390/jof2030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonçalves SS, Souza AC, Chowdhary A, Meis JF, Colombo AL. Epidemiology and molecular mechanisms of antifungal resistance in Candida and Aspergillus. Mycoses. 2016;59(4):198–219. doi: 10.1111/myc.12469. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhary A, Kathuria S, Xu J, Meis JF. Emergence of azole-resistant Aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog. 2013;9(10):e1003633. doi: 10.1371/journal.ppat.1003633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osherov N, Kontoyiannis DP, Romans A, May GS. Resistance to itraconazole in Aspergillus nidulans and Aspergillus fumigatus is conferred by extra copies of the A. nidulans P-450 14α-demethylase gene, pdmA. J Antimicrob Chemother. 2001;48(1):75–81. doi: 10.1093/jac/48.1.75. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, May GS, Lionakis MS, Lewis RE, Kontoyiannis DP. Extra copies of the Aspergillus fumigatus squalene epoxidase gene confer resistance to terbinafine: Genetic approach to studying gene dose-dependent resistance to antifungals in A. fumigatus. Antimicrob Agents Chemother. 2004;48(7):2490–2496. doi: 10.1128/AAC.48.7.2490-2496.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parry TE, Blackmore JA. Serum “uracil plus uridine” levels in normal subjects and their possible significance. J Clin Pathol. 1974;27(10):789–793. doi: 10.1136/jcp.27.10.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Enfert C, et al. Attenuated virulence of uridine-uracil auxotrophs of Aspergillus fumigatus. Infect Immun. 1996;64(10):4401–4405. doi: 10.1128/iai.64.10.4401-4405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noble SM, Johnson AD. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot Cell. 2005;4(2):298–309. doi: 10.1128/EC.4.2.298-309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Retallack DM, Heinecke EL, Gibbons R, Deepe GS, Jr, Woods JP. The URA5 gene is necessary for Histoplasma capsulatum growth during infection of mouse and human cells. Infect Immun. 1999;67(2):624–629. doi: 10.1128/iai.67.2.624-629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Gontijo FA, et al. The role of the de novo pyrimidine biosynthetic pathway in Cryptococcus neoformans high-temperature growth and virulence. Fungal Genet Biol. 2014;70:12–23. doi: 10.1016/j.fgb.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bar-Or A, Pachner A, Menguy-Vacheron F, Kaplan J, Wiendl H. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs. 2014;74(6):659–674. doi: 10.1007/s40265-014-0212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S, Neidhardt EA, Grossman TH, Ocain T, Clardy J. Structures of human dihydroorotate dehydrogenase in complex with antiproliferative agents. Structure. 2000;8(1):25–33. doi: 10.1016/s0969-2126(00)00077-0. [DOI] [PubMed] [Google Scholar]
- 20.Phillips MA, et al. A long-duration dihydroorotate dehydrogenase inhibitor (DSM265) for prevention and treatment of malaria. Sci Transl Med. 2015;7(296):296ra111. doi: 10.1126/scitranslmed.aaa6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vyas VK, Ghate M. Recent developments in the medicinal chemistry and therapeutic potential of dihydroorotate dehydrogenase (DHODH) inhibitors. Mini Rev Med Chem. 2011;11(12):1039–1055. doi: 10.2174/138955711797247707. [DOI] [PubMed] [Google Scholar]
- 22.Howard SJ, Pasqualotto AC, Denning DW. Azole resistance in allergic bronchopulmonary aspergillosis and Aspergillus bronchitis. Clin Microbiol Infect. 2010;16(6):683–688. doi: 10.1111/j.1469-0691.2009.02911.x. [DOI] [PubMed] [Google Scholar]
- 23.Denning DW, Bromley MJ. Infectious disease: How to bolster the antifungal pipeline. Science. 2015;347(6229):1414–1416. doi: 10.1126/science.aaa6097. [DOI] [PubMed] [Google Scholar]
- 24.Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat Rev Drug Discov. 2007;6(1):29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- 25.Swinney DC, Anthony J. How were new medicines discovered? Nat Rev Drug Discov. 2011;10(7):507–519. doi: 10.1038/nrd3480. [DOI] [PubMed] [Google Scholar]
- 26.Roemer T, Krysan DJ. Antifungal drug development: Challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med. 2014;4(5):a019703. doi: 10.1101/cshperspect.a019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldstein AL, McCusker JH. Development of Saccharomyces cerevisiae as a model pathogen: A system for the genetic identification of gene products required for survival in the mammalian host environment. Genetics. 2001;159(2):499–513. doi: 10.1093/genetics/159.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brand A, MacCallum DM, Brown AJ, Gow NA, Odds FC. Ectopic expression of URA3 can influence the virulence phenotypes and proteome of Candida albicans but can be overcome by targeted reintegration of URA3 at the RPS10 locus. Eukaryot Cell. 2004;3(4):900–909. doi: 10.1128/EC.3.4.900-909.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munier-Lehmann H, Vidalain PO, Tangy F, Janin YL. On dihydroorotate dehydrogenases and their inhibitors and uses. J Med Chem. 2013;56(8):3148–3167. doi: 10.1021/jm301848w. [DOI] [PubMed] [Google Scholar]
- 30.Vogel HJ. A convenient growth medium for Neurospora (medium N) Microbiol Genet Bull. 1956;13:42–43. [Google Scholar]
- 31.Zameitat E, Gojković Z, Knecht W, Piskur J, Löffler M. Biochemical characterization of recombinant dihydroorotate dehydrogenase from the opportunistic pathogenic yeast Candida albicans. FEBS J. 2006;273(14):3183–3191. doi: 10.1111/j.1742-4658.2006.05327.x. [DOI] [PubMed] [Google Scholar]
- 32.Sibley GEM, et al. 2009. Pyrrole antifungal agents. Patent Cooperation Treaty Appl WO2009130481.
- 33.Copeland RA, et al. Recombinant human dihydroorotate dehydrogenase: Expression, purification, and characterization of a catalytically functional truncated enzyme. Arch Biochem Biophys. 1995;323(1):79–86. doi: 10.1006/abbi.1995.0012. [DOI] [PubMed] [Google Scholar]
- 34.Dereeper A, et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36(Web Server issue):W465–469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rawls J, Knecht W, Diekert K, Lill R, Löffler M. Requirements for the mitochondrial import and localization of dihydroorotate dehydrogenase. Eur J Biochem. 2000;267(7):2079–2087. doi: 10.1046/j.1432-1327.2000.01213.x. [DOI] [PubMed] [Google Scholar]