Abstract
Combined inhaled therapy in chronic obstructive pulmonary disease (COPD) is commonly used, but its benefits remain controversial. We assessed the effect of tiotropium in reducing COPD exacerbations when combined with long‐acting β2 agonists (LABA) and/or inhaled corticosteroids (ICS). This new‐user cohort study is based on administrative data from 3 Italian regions. We identified adults hospitalized for COPD from 2006 to 2009 who were newly prescribed a fixed LABA/ICS combination (double therapy). We classified patients according to whether tiotropium was also prescribed (triple therapy), using both intention‐to‐treat and as‐treated approaches, and followed them for 1 year. COPD exacerbations were measured as outcomes. Multivariate and propensity score‐adjusted hazard ratios (HRs, 95%CI) were calculated with Cox regression models. We identified 5717 new users of LABA/ICS of which 31.9% initiated triple therapy. In the intention‐to‐treat analysis, the multivariate adjusted HR for moderate, severe, and any exacerbations were 1.02 (95%CI 0.89‐1.16), 0.92 (95%CI 0.76‐1.12), and 1.08 (95%CI 0.91‐1.28), respectively. The propensity score adjustment produced similar results. In the subcohort of patients with previous exacerbations, triple therapy was significantly associated with reduced risk of moderate exacerbations, compared to double therapy (HR 0.68, 95%CI 0.48‐0.98 in intention‐to‐treat approach). In conclusion, the addition of tiotropium to LABA/ICS did not reduce COPD exacerbations compared to LABA/ICS alone. A protective role for moderate exacerbations was found in patients at risk of frequent exacerbations. Given the impact of exacerbations on health status and prognosis, it is crucial to target COPD patients for optimal treatment.
Keywords: chronic obstructive pulmonary disease, exacerbation, tiotropium, comparative effectiveness, inhaled therapy
For patients with chronic obstructive pulmonary disease (COPD), guidelines suggest a stepwise treatment approach using a combination of long‐acting β2 agonists (LABA) and inhaled corticosteroids (ICS). This approach is shown to improve lung function and quality of life and to reduce the risk of exacerbations.1, 2, 3, 4, 5 For patients with severe COPD, guidelines recommend the use of an additional long‐acting bronchodilator with the LABA/ICS treatment. Tiotropium, a long‐acting muscarinic receptor antagonist, was shown to reduce exacerbations and improve quality of life and lung function compared to a placebo.6
Triple therapy (tiotropium plus LABA/ICS) is widely used by physicians to manage COPD, but this practice has limited scientific support. Although the benefits of tiotropium, LABA, and ICS have been extensively studied independently, the short‐ and long‐term effects of the triple combination therapy on disease outcomes are not well established. Clinical trials assessing the efficacy of triple therapy compared
to control groups show controversial results.7, 8, 9 Recently, systematic reviews have indicated the need for longer‐term studies to provide stronger evidence of the benefits of triple therapy.10, 11, 12 Although this aim can be readily addressed with observational studies, methodological issues (including patient selection, adjustment methods, and study design) must be considered to avoid potential biases that can affect internal validity.13 In particular, time‐related biases may amplify the observed benefits of a drug.14
Recently, patients with frequent exacerbations have been recognized as a distinct clinical subgroup, the “frequent exacerbator” phenotype, that is associated with poorer health outcomes.15 Due to the complexity of the pathophysiology underlying the frequent exacerbator phenotype, this subgroup is a priority for research and treatment.16
To our knowledge, only 1 observational study has assessed the effectiveness of tiotropium added to LABA/ICS in patients with COPD. Triple therapy was found to reduce all‐cause mortality and exacerbations; however, as stated by the authors, this finding should be treated with caution.17 The aims of the present study are (1) to evaluate the role of tiotropium in reducing exacerbations in a large cohort of patients with COPD treated with LABA/ICS and (2) to test the hypothesis of an effect modification of the history of previous COPD exacerbations. To strengthen the validity of results, multiple analytical strategies and several sensitivity analyses were applied.
Methods
Sources of Data
Regional health information systems from 3 large regions in Italy, Emilia Romagna, Lombardia, and Lazio (about 19 million residents), were used for this study: Hospital Information Systems (HIS) (ICD‐9‐CM codes of the International Classification of Diseases), Drug Registers (PHARM), and Mortality Registers. For Lazio region, information from the Emergency Information System (EIS) was also available. Details on these data sets have been described elsewhere.18 Drugs dispensed to patients, which are reimbursable by the National Health Service, the Italian universal coverage healthcare system, are identified by the national drug register code, accordant with the international Anatomical Therapeutic Chemical (ATC) classification. The following information was available for each prescription: patient identification number, ATC code, number of packs, number of units per pack, dosage, unit cost per pack, and prescription date. In compliance with the national law on privacy, records were linked using an anonymous key.
Population
A standardized algorithm was used to identify patients aged ≥45 years who were discharged with a diagnosis of acute exacerbation of COPD between 2006 and 2009 from the HIS of the 3 regions in Italy.19, 20 We selected hospital discharges with COPD as the main diagnosis (ICD 9 CM codes 490, 491, 492, 494, 496) or secondary diagnosis with a main diagnosis of acute respiratory failure (ICD 9 CM codes 518.81‐518.84), dyspnea (786.0), cough (786.2), or abnormal sputum (786.4). In cases with multiple COPD admissions, the first admission was used. Patients were enrolled who received at least 1 prescription of a fixed LABA/ICS combination (ATC code R03AK06, R03AK07) within 6 months of discharge.
Only new users, those patients without a previous prescription for LABA, ICS, or tiotropium (ATC code R03BB04), were included, with a washout period of 6 months. The definition of “new users” and ATC codes can be found in Supplementary Table S1.
Study Design and Exposure Definition
Two analytical approaches were used to assess the role of tiotropium in reducing COPD‐exacerbations (Figure 1). In the intention‐to‐treat (ITT) approach, patients were classified as receiving triple or double therapy (ie, LABA/ICS with or without tiotropium, respectively), based on index date exposure (ie, date of the first fixed LABA/ICS prescription). In the as‐treated approach, the exposure was classified at the index date and tracked throughout the follow‐up period. Drug exposure was measured in terms of defined daily doses (DDD). The number of DDD was converted to the number of days the patient was treated, counting 1 DDD per day and distributing all available DDDs to days of follow‐up (including the days covered by the last prescription). At the end of the exposure period (ie, when all available DDDs were expired), a renewal time of 30 days (ie, grace time) was applied during which the patient was considered “exposed” without being censored for discontinuation.20, 21 In the case of switching, a 7‐day grace time was used to limit the potential misclassification of exposure accounting for the 5‐ to 6‐day half‐life of tiotropium terminal elimination.22
Figure 1.
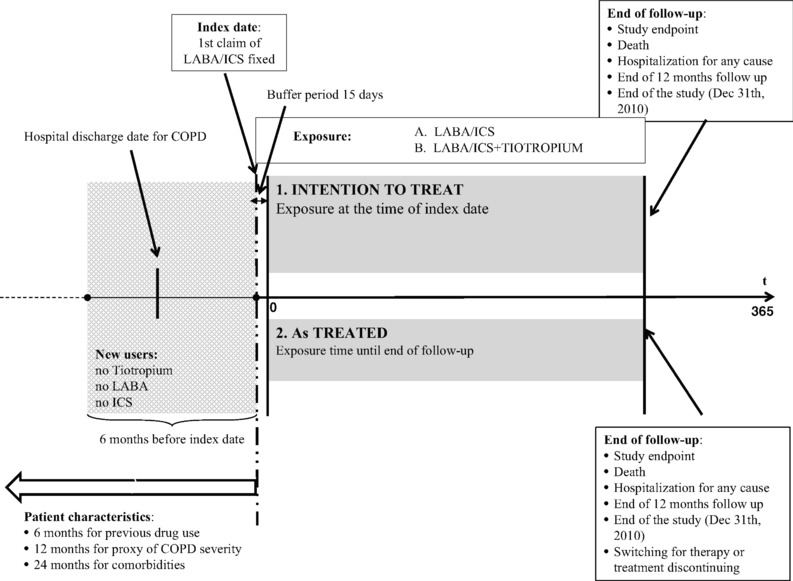
Study designs: (1) intention‐to‐treat and (2) as‐treated approaches. LABA, Long acting beta 2 agonists; ICS, Inhaled corticosteroids.
Demographic and Clinical Characteristics
The following patient characteristics were assessed: (1) proxy of COPD severity based on 12 months of information from HIS and PHARM registries, (2) concomitant respiratory disease based on 24 months of HIS data, (3) comorbidities based on 24 months of HIS information, and (4) prescriptions of respiratory and nonrespiratory drugs based on 6 months of PHARM information. Details on codes are reported in Supplementary Tables S2‐S5.
Endpoints and Follow‐Up
COPD‐related acute exacerbations were defined as events that require acute pharmacological treatment (ie, oral corticosteroid and antibiotics) or a hospital admission.7, 23, 24, 25, 26 The study endpoint, the first observed COPD‐related acute event, was recorded and classified as follows:
“Moderate COPD exacerbation” refers to an event that requires both oral corticosteroids and antibiotics. (ATC codes H02AB and J01), prescribed within 15 days of the event.
“Severe COPD exacerbation” refers to a COPD hospitalization assessed with the criteria used for case identification.
“Any exacerbation” means moderate or severe exacerbation.
We started counting the person‐years on the 15th day of follow‐up (buffer period) to allow for the biologic action of the medication to occur (Figure 1). In the ITT approach, each patient was followed for 12 months after the index date until a study endpoint was observed, death or the end of the study (December 31, 2010), whichever came first. In the as‐treated approach, censoring was applied if there was a switch or discontinuation of the index medication. In both analytical approaches, a lack of information on drug exposure during the first hospitalization (for any reason) resulted in the interruption of follow‐up. Details on the as‐treated approach are reported in Supplementary Figure S1.
Statistical Analysis
The incidence rate for the first episode of moderate, severe, or any exacerbation was calculated as the number of patients who experienced an event divided by the person‐years at risk. To develop the predictive model, each variable measured at baseline in a backward stepwise procedure was included, discarding variables not associated with the outcome.
To balance measured risk factors for the outcome between drug user groups, the propensity scores of initiating triple vs double therapy were estimated with a logistic regression model, including all characteristics potentially associated with the exposure: sociodemographic factors, proxies of COPD severity, concomitant respiratory diseases, comorbidities, and previous use of respiratory and nonrespiratory drugs.27, 28
To test the role of tiotropium in reducing exacerbations, multivariate analysis with Cox proportional hazard models was performed to determine the hazard ratio (HR) and 95% confidence interval (95%CI). The models were run separately, adjusting for (1) characteristics resulting from the predictive model and (2) indicators of propensity score quintiles following standardized techniques.13, 28, 29
After testing a potential interaction between drug exposure and proxies of COPD severity, an analysis was conducted among the subcohort of COPD patients with previous 12‐month exacerbations (both moderate or severe). Statistical significance was set at P < .05 for main effects and P < .10 for interactions.
Sensitivity Analysis
Several sensitivity analyses were performed. First, the ITT analyses were rerun with an exposure definition based on the information collected on the index day and the 4 subsequent days to account for drugs that are not readily available in pharmacies. Second, 2 different intervals, 7 and 15 days, were considered as grace time for treatment discontinuation in the as‐treated approach.30, 31 Third, the analysis for the subcohort of patients in the Lazio region was rerun using (1) a broader definition of the outcome “any exacerbation” by including information (COPD related ICD‐9‐CM codes at emergency visits) registered in the EIS (available only in the Lazio region), and (2) a broader definition of COPD severity by including the variable “use of liquid oxygen” (available only in the Lazio region).
Statistical analyses were performed using SAS 9.2.
Results
From January 1, 2006 to December 31, 2009, we selected 68,795 patients with COPD hospital discharges. Of these, 26,197 (38.1%) had at least 1 prescription of fixed LABA/ICS combinations, and 5717 (21.8%) were classified as new users.
The demographic characteristics of the study population, stratified by treatment regimen, are shown in Table 1. Most patients were male (56.5%) and residents of the Lombardia region (46.8%). The mean age of the population was 73.8 (SD = 10.7) years, and 31.9% (n = 1821) had received triple therapy.
Table 1.
Characteristics of the Study Population According to Therapy (Double and Triple): OUTPUL Study 2006‐2009
| Long‐Acting β2 Agonists and Inhaled Corticosteroidsa (n = 3896) | Long‐Acting β2 Agonists and Inhaled Corticosteroidsa Plus Tiotropium (n = 1821) | P‐Value | Adjusted P‐Valueb | |
|---|---|---|---|---|
| Residence (region) | ||||
| Lazio | 26.3 | 29.2 | 0.077 | 0.930 |
| Emilia Romagna | 29.1 | 19.2 | ||
| Lombardia | 44.6 | 51.6 | ||
| Age (years) | ||||
| 45‐54 | 5.1 | 5.5 | <.001 | .748 |
| 55‐64 | 13.2 | 17.5 | ||
| 65‐74 | 26.3 | 31.1 | ||
| 75‐84 | 37.6 | 33.8 | ||
| 85+ | 17.8 | 12.0 | ||
| Sex | ||||
| Male | 54.3 | 61.1 | <.001 | .725 |
| Female | 45.7 | 38.9 | ||
| Proxy of COPD severity (previous 12 months) | ||||
| Previous COPD hospitalization | 7.8 | 7.7 | .880 | .588 |
| Concomitant use of oral corticosteroids and antibiotics | 10.5 | 8.5 | .018 | .100 |
| Diagnosis of respiratory failure | 42.1 | 51.9 | <.001 | .506 |
| Invasive respiratory procedures | 2.9 | 2.9 | .925 | .340 |
| Staying in intensive care unit during a COPD hospitalization | 3.0 | 3.6 | .235 | .737 |
| Oxygen therapy (gas) | 5.3 | 4.3 | .135 | .151 |
| Concomitant respiratory diseases (previous 24 months) | ||||
| Asthma | 1.8 | 1.3 | .166 | .153 |
| Chronic respiratory disease other than COPD | 3.4 | 3.3 | .901 | .588 |
| Pulmonary infections | 11.3 | 12.3 | .281 | .446 |
| Acute pulmonary symptoms | 3.4 | 3.2 | .654 | .614 |
| Apnea | 2.1 | 2.9 | .061 | .545 |
| Comorbidities (previous 24 months) | ||||
| Diabetes | 17.4 | 18.9 | .143 | .113 |
| Hypertension | 39.8 | 39.2 | .637 | .150 |
| Ischemic heart disease | 17.0 | 14.8 | .035 | .321 |
| Heart failure/pulmonary heart disease | 18.2 | 18.2 | .994 | .701 |
| Other chronic heart diseases | 10.0 | 8.4 | .057 | .090 |
| Arrythmia | 15.6 | 13.8 | .081 | .927 |
| Cerebrovascular diseases | 11.3 | 9.2 | .015 | .288 |
| Peripheral vascular diseases | 5.7 | 5.4 | .574 | .650 |
| Obesity, dyslipidemia | 9.4 | 11.8 | .005 | .714 |
| Liver disease | 4.3 | 3.7 | .349 | .950 |
| Other chronic digestive disease | 1.3 | 1.5 | .598 | .678 |
| Chronic renal diseases | 7.9 | 7.4 | .471 | .934 |
| Neurological and muscle disease | 2.8 | 2.5 | .556 | .874 |
| Anemia and coagulation disorders | 4.7 | 3.4 | .028 | .161 |
| Thyroid disease | 5.3 | 4.8 | .421 | .748 |
| Depression | 3.2 | 2.1 | .032 | .149 |
| Psychiatric disease | 4.4 | 2.3 | <.001 | .457 |
| Peptic ulcer/esophageal reflux | 1.4 | 1.2 | .638 | .410 |
| Rheumatologic/diffuse disease of connective tissue | 1.2 | 0.5 | .023 | .078 |
| Prescriptions of respiratory drugs (previous 6 months) | ||||
| Short‐acting β2 agonists | 7.7 | 6.3 | .065 | .118 |
| Short‐acting anticholinergics | 3.3 | 2.6 | .125 | .236 |
| Xanthines | 13.6 | 10.9 | .004 | .889 |
| Prescriptions of nonrespiratory drugs (previous 6 months) | ||||
| Cardiac therapies | 27.5 | 22.6 | <.001 | .247 |
| Antidiabetic drugs | 16.6 | 16.6 | .995 | .802 |
| Antiplatelets | 37.4 | 32.2 | <.001 | .856 |
| Antihypertensives | 71.1 | 64.6 | <.001 | .784 |
| Statins | 15.6 | 16.9 | .218 | .128 |
Fixed combination.
Adjusted for the quintiles of the propensity score.
Univariate analyses showed that the double‐ and triple‐therapy groups were similar in the proxy of COPD severity variables except for the diagnosis of respiratory failure and the concomitant use of oral corticosteroids and antibiotics. In general, patients with double therapy were more likely than those with triple therapy to have comorbidities, particularly ischemic heart disease, cerebrovascular disease, and psychiatric diseases. Use of respiratory drugs was comparable between the 2 groups, except for xanthines. Patients with triple therapy appeared to receive fewer treatments than those with double therapy for nonrespiratory diseases such as cardiac therapies, antiplatelets, and antihypertensives. For each patient, we calculated the expected probability of being treated with triple therapy, adjusting for all characteristics measured at baseline. There was a reasonably large overlap in the distribution of propensity scores between treatment groups. Box plots displaying the expected probability of being treated with triple therapy can be found in Supplementary Figure S2. The adjusted P‐values are reported in Table 1.
The predictive model for any exacerbation, calculated according to the ITT approach, providing the HR and relative 95%CI for each condition, is shown in Figure 2. As expected, variables defined as a proxy of COPD severity were most highly associated with the outcome. The only variable with a protective effect was the use of antihypertensive drugs in the previous 6 months. When we considered severe and moderate exacerbations separately, other variables were identified in the predictive model. In particular, severe exacerbations were positively associated with the use of cardiac drugs and statins, previous hospitalization for heart failure, and other chronic heart diseases. For moderate exacerbations, treatment with antidiabetic drugs had a protective effect (data not shown).
Figure 2.
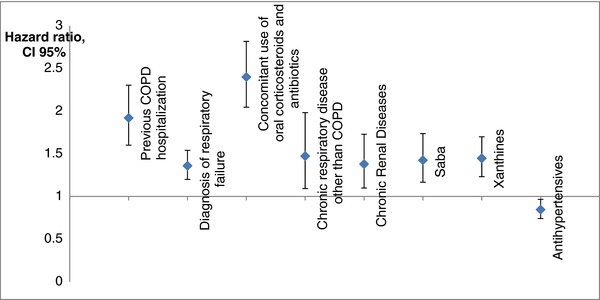
Predictive model for any exacerbation calculated with the intention‐to‐treat approach.
Table 2 shows each study outcome rate and HR for patients who received triple therapy vs double therapy. The overall rates of moderate, severe, and any exacerbations were 13.7, 13.4, and 26.3, per 100 person‐years, respectively. The rates did not differ considerably between the 2 treatment regimens. In the ITT analysis the adjusted HRs for moderate, severe, and any exacerbations were: 0.92 (95%CI 0.76‐1.12), 1.08 (95%CI 0.91‐1.28), and 1.02 (95%CI 0.89‐1.16), respectively. The as‐treated analysis produced consistent results. In particular, for moderate exacerbations HR 0.93 (95%CI 0.98‐1.29), severe exacerbations HR 1.13 (95%CI 0.82‐1.55), and any exacerbation HR 0.95 (95%CI 0.74‐1.22). For both the ITT and as‐treated approaches, the adjustments based on propensity scores led to similar results.
Table 2.
Rates and Hazard Ratios (HRs, CI95%) for Moderate, Severe, and Any Exacerbations in COPD Patients with Triple Therapy vs Those With Double Therapy, Using 2 Different Study Designsa
| Intention to Treat | As Treated | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted With Predictive Model | Adjusted With Propensity Score Index | Adjusted With Predictive Model | Adjusted With Propensity Score Index | |||||||||||||||
| N | Person Years | Rate per 100 PYb | HR | 95%CI | HR | 95%CI | N | Person Years | Rate per 100 PYb | HR | 95%CI | HR | 95%CI | |||||
| Moderate exacerbation | 533 | 3878 | 13.7 | 185 | 1154 | 16.0 | ||||||||||||
| Fixed LABA/ICSc | 385 | 2668 | 14.4 | 1.00 | ‐ | ‐ | 1.00 | ‐ | ‐ | 146 | 857 | 17.0 | 1.00 | ‐ | ‐ | 1.00 | ‐ | ‐ |
| Fixed LABA/ICS plus tiotropium | 148 | 1209 | 12.2 | 0.92 | 0.76 | 1.12 | 0.92 | 0.76 | 1.12 | 39 | 297 | 13.1 | 0.93 | 0.98 | 1.29 | 0.98 | 0.71 | 1.36 |
| Severe exacerbation | 598 | 4476 | 13.4 | 190 | 1296 | 14.7 | ||||||||||||
| Fixed LABA/ICSc | 388 | 3054 | 12.7 | 1.00 | ‐ | ‐ | 1.00 | ‐ | ‐ | 135 | 957 | 14.1 | 1.00 | ‐ | ‐ | 1.00 | ‐ | ‐ |
| Fixed LABA/ICS plus tiotropium | 210 | 1421 | 14.8 | 1.08 | 0.91 | 1.28 | 1.11 | 0.93 | 1.31 | 55 | 338 | 16.3 | 1.13 | 0.82 | 1.55 | 1.11 | 0.80 | 1.53 |
| Any exacerbation | 1020 | 3878 | 26.3 | 342 | 1154 | 29.6 | ||||||||||||
| Fixed LABA/ICSc | 696 | 2668 | 26.1 | 1.00 | ‐ | ‐ | 1.00 | ‐ | ‐ | 257 | 857 | 30.0 | 1.00 | ‐ | ‐ | 1.00 | ‐ | ‐ |
| Fixed LABA/ICS plus tiotropium | 324 | 1209 | 26.8 | 1.02 | 0.89 | 1.16 | 1.04 | 0.91 | 1.19 | 85 | 297 | 28.6 | 0.95 | 0.74 | 1.22 | 0.99 | 0.77 | 1.27 |
OUTPUL study 2006‐2009 (n = 5717).
PY, person years.
LABA, long‐acting β2 agonists; ICS, inhaled corticosteroids.
Table 3 shows each study outcome rate and HR for patients who received triple therapy vs those who received double therapy in the subcohort of patients with a history of 12 months previous exacerbations (n = 945). The overall rates of moderate, severe, and any exacerbation were 35.4, 23.8, and 58.0 per 100 person‐years, respectively. Triple therapy was significantly associated with reduced risk of moderate exacerbations compared to double therapy (HR 0.68, 95%CI 0.48‐0.983 for ITT approach; HR 0.46, 95%CI 0.23‐0.93 for as‐treated approach).
Table 3.
Rates and Hazard Ratios (HRs, 95%CI) for Moderate, Severe, and Any Exacerbations in COPD Patients With Triple Treatment, Those With Double Therapy, Using 2 Different Study Designsa
| Intention to Treat | As Treated | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted With Predictive Model | Adjusted With Propensity Score Index | Adjusted With Predictive Model | Adjusted With Propensity Score Index | |||||||||||||||
| N | Person Years | Rate per 100 PYb | HR | 95%CI | HR | 95%CI | N | Person Years | Rate per 100 PYb | HR | 95%CI | HR | 95%CI | |||||
| Moderate exacerbation | 185 | 522 | 35.4 | 73 | 183 | 39.9 | ||||||||||||
| Fixed LABA/ICSc | 144 | 361 | 39.9 | 1.00 | ‐ | ‐ | 1.00 | ‐ | ‐ | 63 | 135 | 46.7 | 1.00 | ‐ | ‐ | 1.00 | ‐ | ‐ |
| Fixed LABA/ICS plus tiotropium | 41 | 161 | 22.5 | 0.68 | 0.48 | 0.98 | 0.65 | 0.46 | 0.93 | 10 | 48 | 20.8 | 0.46 | 0.23 | 0.93 | 0.47 | 0.24 | 0.93 |
| Severe exacerbation | 158 | 664 | 23.8 | 58 | 217 | 26.7 | ||||||||||||
| Fixed LABA/ICSc | 107 | 461 | 23.2 | 1.00 | ‐ | ‐ | 1.00 | ‐ | ‐ | 42 | 161 | 26.1 | 1.00 | ‐ | ‐ | 1.00 | ‐ | ‐ |
| Fixed LABA/ICS plus tiotropium | 51 | 203 | 25.1 | 0.99 | 0.70 | 1.40 | 0.97 | 0.69 | 1.37 | 16 | 56 | 28.6 | 1.14 | 0.63 | 2.09 | 1.14 | 0.63 | 2.06 |
| Any exacerbation | 303 | 522 | 58.0 | 118 | 183 | 64.5 | ||||||||||||
| Fixed LABA/ICSc | 223 | 361 | 61.8 | 1.00 | ‐ | ‐ | 1.00 | ‐ | ‐ | 96 | 135 | 71.1 | 1.00 | ‐ | ‐ | 1.00 | ‐ | ‐ |
| Fixed LABA/ICS plus tiotropium | 80 | 161 | 49.7 | 0.79 | 0.60 | 1.03 | 0.78 | 0.60 | 1.02 | 22 | 48 | 45.8 | 0.63 | 0.39 | 1.02 | 0.67 | 0.50 | 1.09 |
Subcohort of patients with previous COPD exacerbations (n = 945).
PY, person years.
LABA, long‐acting β2 agonists; ICS, inhaled corticosteroids.
In the first sensitivity analysis, we used a 4‐day time window (instead of only the index day) to define drug exposure in the ITT approach. This analysis produced results consistent with those mentioned above. The second analysis, applying 7‐ and 15‐day grace time for drug discontinuation in the as‐treated approach, showed similar association patterns. However, the numbers of person years (drug exposure) and of outcome events were smaller than in the main analysis, and no statistically significant results were found (Supplementary Tables S6 and S7). Third, an analysis of the subcohort of the Lazio region produced a higher overall rate of any exacerbation (33.4%), but it did not modify the HR (1.09, 95%CI 0.86‐1.37). For this analysis, emergency department visits were added to the definition of exacerbation events. In the last sensitivity analysis, the effect of liquid oxygen as a predictive variable for exacerbation was tested. This variable was found to be highly associated with any exacerbation. Its inclusion in the predictive model produced a reduction of the effects of the other variables considered to be proxies of COPD severity without modifying the HR of triple therapy vs double therapy for any exacerbation (1.06, 95%CI 0.81‐1.38).
Discussion
This study included a large cohort of individuals discharged with a diagnosis of COPD exacerbation to represent patients with moderate to severe COPD and evaluated the effect of tiotropium on COPD exacerbations over the following 12 months. Consistently, no evidence was found of increased benefit associated with its addition to the fixed combination of LABA/ICS. Analyses suggest a protective role for moderate exacerbation in the subgroup of COPD patients with a history of 12 months of previous exacerbations.
COPD exacerbations are episodes in the natural course of the disease characterized by worsening of respiratory symptoms.1, 32 These events are indicative of the health status in COPD patients and are independent predictors of disease progression and mortality.32, 33 In most cases, exacerbations are triggered by viral infections, especially rhinovirus, the cause of the common cold. Therefore, preventing COPD exacerbations is crucial for disease management.1, 32 Anti‐inflammatory drugs, including inhaled corticosteroids, have proven effective in reducing the frequency and severity of exacerbations, while bronchodilators reduce dynamic hyperinflation.34 Tiotropium, a long‐acting, specific, muscarinic receptor antagonist or anticholinergic drug was approved by the Food and Drug Administration in 2004 as an anticholinergic bronchodilator. It has been widely used in clinical practice over the last decade and is considered a first‐line agent for COPD patients.1 In the large UPLIFT trial, tiotropium was found to be associated with a reduction in exacerbation risk.8 In the POET‐COPD trial, tiotropium was more effective than salmeterol in preventing exacerbation.35 The lack of evidence on the impact of tiotropium when added to LABA/ICS, a treatment previously proven to be effective, highlights the need to assess its role in real practice.10, 11, 12 LABA and tiotropium are known to have distinct bronchodilator mechanisms and have exhibited a synergistic action in reducing bronchial tone.36, 37 The beneficial bronchodilator effect of this combination compared to tiotropium alone or LABA/ICS alone has also been demonstrated in recent large clinical trials.38, 39 However, these studies only assessed pulmonary function (ie, forced expiratory flow in the first second, FEV1) and did not measure any patient‐centered outcomes.
In our study, the addition of tiotropium to LABA/ICS did not affect the risk of new exacerbations in comparison to fixed LABA/ICS among patients with moderate or severe COPD in the 12 months following discharge. This finding supports the hypothesis of the importance of anti‐inflammatory treatments, such as ICS combined with LABA, and a lack of an increased effect of the double therapy with the addition of the antimuscarinic or anticholinergic action of tiotropium. A distinct group of COPD patients has been recognized as more susceptible to exacerbations (“frequent exacerbator phenotype”). However, the complex pathophysiology is not fully understood.15 Our study suggests a major effect of LABA/ICS with tiotropium, in comparison to double therapy, for preventing moderate exacerbations among patients with a history of frequent exacerbations. This finding contributes to the complex ongoing debate on this COPD subgroup. The lack of evidence of a higher effect of triple therapy in comparison to LABA/ICS on severe exacerbations (ie, those requiring hospitalization) can be attributed to the large heterogeneity of COPD exacerbations involving multiple interacting factors. For example, cardiovascular comorbidity and environmental pollution may play a role in worsening COPD symptoms and subsequent hospitalizations through mechanisms that remain unclear.40, 41
To the best of our knowledge, only one retrospective study measured patient‐centered outcomes with electronic healthcare data bases, demonstrating a beneficial effect of tiotropium in conjunction with ICS plus LABA on the COPD exacerbation rate.17 However, Short et al highlight a number of limitations that may have affected their results. Most importantly, the absence of an accurate definition of exposure, essential for the research topic, can produce time‐related biases that can lead to an overestimation of the drug's beneficial effect.14, 17
In the present study the risk influence of previous treatments on the outcome was minimized by restricting the cohort to new users.42 Moreover, 2 adjustment techniques were applied to control for confounding; each provided information about the association between the treatment and a COPD exacerbation. In the predictive model, adjustments were performed for the factors most strongly associated with the outcome. The propensity score was used to balance factors that could influence the treatment choice.
Patients taking other respiratory drugs, such as short‐acting β agonists and xanthines, were used as a proxy for increased disease severity and were found to have the highest risk of COPD exacerbation. In accordance with results of previous studies, the use of antihypertensive drugs was protective, but their role remains unclear.23, 43 For moderate exacerbations, antidiabetics showed a protective effect. In this case the effect could be explained by an underestimation of the outcome due to the contraindicated use of oral corticosteroids in patients with diabetes.44 To apply the propensity score adjustment, we calculated the distribution of the probability of receiving a given treatment. Unexpectedly, this analysis did not identify any factors, even for variables related to COPD severity, which are strong predictors of treatment choice. This result may reflect the absence of an indication bias. Alternatively, despite our testing of variables used in previous studies, some variables associated with treatment choice that correlated with patient characteristics and physician preferences may have been omitted. Nevertheless, when information on the use of liquid oxygen for the subcohort of Lazio region was included as a proxy for COPD severity, the results were consistent with those obtained in our main analysis.
The comparability between the 2 treatment groups in terms of COPD severity is critical in this type of study. In theory, triple therapy should be given only when double therapy is no longer effective. Guidelines for COPD management suggest a stepwise treatment approach, which accounts for disease progression.1 Patients in a stable clinical condition with no previous use of respiratory drugs are not expected to benefit from the addition of tiotropium to LABA/ICS compared to LABA/ICS alone. In the present study the 2 treatment groups were not substantially different in terms of COPD severity. Therefore, a possible interpretation of our results could be that the choice of triple or double therapy might have been random, based on the physician's preferences. Alternatively, it may have been based on clinical criteria that we are unable to measure. Testing the added value of a drug in a cohort of patients who are currently in treatment remains a challenge in pharmacoepidemiology due to the difficulty of measuring a possible carryover effect.
To avoid misclassification of exposure, a typical issue in observational studies, we tested 2 different approaches. The number of days in which the patient was treated was measured on the basis of DDDs with potential misclassification of exposure because the pharmaceutical data base used in this study does not include information on prescribed daily doses.45 The ITT approach measures the exposure at the index date; thus, all outcomes that occur in the follow‐up period are associated with the index date. The as‐treated approach accounts for switching therapy and therapy discontinuation during the follow‐up period. However, both switching and discontinuation might be predictors of adverse health outcomes due to drug intolerance or treatment failure, which may have led to informative censoring.20, 21 To limit this potential bias, a 30‐day grace period for drug discontinuation was applied, according to previous studies.21, 28 Sensitivity analyses for varying lengths of grace periods (7 or 15 days) were also performed; it is worthy of note that lower grace time intervals lead to smaller exposure time and lower number of outcome events and may limit the statistical power in the detection of an effect.
Another limitation of this study was the difficulty in measuring the outcome. Exacerbations may require hospitalizations, but in less severe cases, they may not require any use of health resources, which may have underestimated the number of moderate exacerbations. However, a previously used event‐based definition of COPD exacerbation was used for this study.7, 23, 24, 25, 26 When drug prescriptions were used to define the outcome, we attempted to define the prescriptions as specifically as possible, including only combined prescriptions of antibiotics and oral corticosteroids filled within 15 days of the event. Nevertheless, this definition did not account for patients who might have stored drugs at home as a stand‐by therapy, in which case there would be no need to fill the prescription in case of an acute exacerbation. Furthermore, for the subcohort in the Lazio region, emergency department visits for COPD were used to assess the “any exacerbation” outcome. The sensitivity analysis showed that this inclusion did not change the results compared to the main analysis.
In conclusion, our results suggested that tiotropium added to fixed a LABA/ICS combination did not reduce COPD exacerbations within a 1‐year follow‐up period compared to LABA/ICS alone among patients discharged after a COPD exacerbation. Among those with a history of frequent exacerbations, LABA/ICS in conjunction with tiotropium shows a greater effect in preventing moderate exacerbations compared to double therapy. Findings from this type of observational study could have an impact on the management of patients with COPD in current clinical practice. Given the complex pathophysiology of exacerbations and their role on prognosis, targeting COPD patients for appropriate therapy is crucial.
Declaration of Conflicting Interests
The authors report no conflicting interests.
Author Contributions
N.A. and R.P. conceived the idea for the study and designed the study in collaboration with E.F. and V.B. V.B., M.D.M., G.F., and S.C. were responsible for acquiring the data. V.B. was responsible for the data analysis, tables, and graphs with input from N.A., S.C., U.K., M.D.M., and D.F. R.P., E.P., M.D., G.F., and C.A.P. contributed to the interpretation of the results. The initial draft of the manuscript was prepared by E.F., V.B., and N.A. and then circulated repeatedly among all authors for critical revision.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Acknowledgments
We are very grateful to Natalie Terens for her editorial assistance and to Roberta Macci and Sandra Magliolo for their support in finding the cited articles. The project was partially funded by the Ministry of Health‐Agenzia Italiana del Farmaco (AIFA); Prot. FARM8ZBT93.
References
- 1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD ; 2016. http://www.goldcopd.org/.
- 2. Mapel DW, Hurley JS, Dalal AA, et al. The role of combination inhaled corticosteroid/long‐acting beta‐agonist therapy in COPD management. Prim Care Respir J. 2010;19:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cazzola M, Hanania NA. The role of combination therapy with corticosteroids and long‐acting beta2‐agonists in the prevention of exacerbations in COPD. Int J COPD. 2006;1:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanania NA. The impact of inhaled corticosteroid and long‐acting beta‐agonist combination therapy on outcomes in COPD. Pulm Pharmacol Ther. 2008;21:540–550. [DOI] [PubMed] [Google Scholar]
- 5. Mensing M, Aalbers R. Comparison and optimal use of fixed combinations in the management of COPD. Int J Chron Obstruct Pulmon Dis. 2007;2:107–116. [PMC free article] [PubMed] [Google Scholar]
- 6. Tashkin DP, Celli B, Senn S, et al. A 4‐year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. [DOI] [PubMed] [Google Scholar]
- 7. Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone‐salmeterol for treatment of chronic obstructive pulmonary disease (a randomized trial). Ann Intern Med. 2007;146:545–555. [DOI] [PubMed] [Google Scholar]
- 8. Tashkin DP. Impact of tiotropium on the course of moderate‐to‐very severe chronic obstructive pulmonary disease: the UPLIFT trial. Expert Rev Respir Med. 2010;4:279–289. [DOI] [PubMed] [Google Scholar]
- 9. Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:741–750. [DOI] [PubMed] [Google Scholar]
- 10. Welsh EJ, Cates CJ, Poole P. Combination inhaled steroid and long‐acting beta2‐agonist versus tiotropium for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;31;5:CD007891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karner C, Cates CJ. The effect of adding inhaled corticosteroids to tiotropium and long‐acting beta2‐agonists for chronic obstructive pulmonary disease (Review). Cochrane Database Syst Rev. 2011;9:CD009039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kew KM, Dias S, Cates CJ. Long‐acting inhaled therapy (beta‐agonists, anticholinergics and steroids) for COPD: a network meta‐analysis. Cochrane Database Syst Rev. 2014;26;3:CD010844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hlatky MA, Winkelmayer WC, Setoguchi S. Epidemiologic and statistical methods for comparative effectiveness research. Heart Fail Clin. 2013;9:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suissa S. Immortal time bias in observational studies of drug effect. Pharmacoepidemiol Drug Saf. 2007;16:241–249. [DOI] [PubMed] [Google Scholar]
- 15. Wedzicha JA, Brill SE, Allinson JP, et al. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGarvey L, Lee AJ, Roberts J, et al. Characterization of the frequent exacerbator phenotype in COPD patients in a large UK primary care population. Respir Med. 2015;109:228–237. [DOI] [PubMed] [Google Scholar]
- 17. Short PM, Williamson PA, Elder DH, et al. The impact of tiotropium on mortality and exacerbations when added to inhaled corticosteroids and long‐acting β‐agonist therapy in COPD. Chest. 2012;141:81–86. [DOI] [PubMed] [Google Scholar]
- 18. Kirchmayer U, Agabiti N, Belleudi V, et al. Socio‐demographic differences in adherence to evidence‐based drug therapy after hospital discharge from acute myocardial infarction: a population‐based cohort study in Rome, Italy. J Clin Pharmacol Ther. 2012;37:37–44 [DOI] [PubMed] [Google Scholar]
- 19. Agabiti N, Belleudi V, Davoli M, et al. Profiling hospital performance to monitor the quality of care: the case of COPD. Eur Respir J. 2010;35:1031–1038. [DOI] [PubMed] [Google Scholar]
- 20. Di Martino M, Agabiti N, Cascini S, et al. The effect on total Mortality of adding inhaled corticosteroids to long‐acting bronchodilators for COPD: a real practice analysis in Italy. COPD. 2016;13:293–302. [DOI] [PubMed] [Google Scholar]
- 21. Huybrechtd KF, Schneeweiss S, Gerhard T, et al. Comparative safety of antipsychotic medications in nursing home residents. J Am Geriatr Soc. 2012:60:420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barnes PJ. The pharmacological properties of tiotropium. Chest. 2000;117(2 Suppl):63S–66S. [DOI] [PubMed] [Google Scholar]
- 23. Suh DC, Lau H, La Ho, et al. Association between incidence of acute exacerbation and medication therapy in patients with COPD. Curr Med Res Opin. 2010;26:297–306. [DOI] [PubMed] [Google Scholar]
- 24. Calverly PMA, Anderson JA, Celli B. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. [DOI] [PubMed] [Google Scholar]
- 25. Aaron SD, Fergusson D, Marks GB, et al. Counting, analysing and reporting exacerbations of COPD in randomised controlled trials. Thorax. 2008;63:122–128. [DOI] [PubMed] [Google Scholar]
- 26. Akazawa M, Biddle AK, Stearns SC. Economic assessment of early initiation of inhaled corticosteroids in chronic obstructive pulmonary disease using propensity score matching. Clin Ther. 2008;30:1003–1016. [DOI] [PubMed] [Google Scholar]
- 27. Patorno E, Glynn RJ, Hernández‐Díaz S, et al. Studies with many covariates and few outcomes: selecting covariates and implementing propensity‐score‐based confounding adjustments. Epidemiology. 2014;25:268–278. [DOI] [PubMed] [Google Scholar]
- 28. Kirchmayer U, Cascini S, Agabiti N, et al. One‐year mortality associated with COPD treatment: a comparison of tiotropium and long‐acting beta2‐agonists in three Italian regions: results from the OUTPUL study. Pharmacoepidemiol Drug Saf. 2016;25:578–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: analytic strategies using propensity scores. Ann Intern Med. 2002;137:693–695. [DOI] [PubMed] [Google Scholar]
- 30. Caetano PA, Lam JM, Morgan SG. Toward a standard definition and measurement of persistence with drug therapy: examples from research on statin and antihypertensive utilization. Clin Ther. 2006;28:1411–1424; discussion 1410. [DOI] [PubMed] [Google Scholar]
- 31. Monfared AA, Lelorier J. Accuracy and validity of using medical claims data to identify episodes of hospitalizations in patients with COPD. Pharmacoepidemiol Drug Saf. 2006;15:19–29. [DOI] [PubMed] [Google Scholar]
- 32. Barnes PJ. Chronic obstructive pulmonary disease. Clin Chest Med. 2014;35(1):71–86. [DOI] [PubMed] [Google Scholar]
- 33. Donaldson GC, Seemungal TA, Bhowmik A, et al. The relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wedzicha JA1, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of COPD exacerbations. N Engl J Med. 2011;364:1093–1103. [DOI] [PubMed] [Google Scholar]
- 36. Cazzola M, Molimard M. The scientific rationale for combining long‐acting beta2‐agonists and muscarinic antagonists in COPD. Pulm Pharmacol Ther. 2010;23:257–267. [DOI] [PubMed] [Google Scholar]
- 37. Tashkin DP, Ferguson GT. Combination bronchodilator therapy in the management of chronic obstructive pulmonary disease. Respir Res. 2013;14:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mahler DA1, D'Urzo A, Bateman ED, et al. Concurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised, double‐blind comparison. Thorax. 2012;67:781–788. [DOI] [PubMed] [Google Scholar]
- 39. Vogelmeier CF1, Bateman ED, Pallante J, et al. Efficacy and safety of once‐daily QVA149 compared with twice‐daily salmeterol‐fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double‐blind, parallel group study. Lancet Respir Med. 2013;1:51–60. [DOI] [PubMed] [Google Scholar]
- 40. Müllerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147:999–1007. [DOI] [PubMed] [Google Scholar]
- 41. Song Q, Christiani DC, Xiaorong W, Ren J. The global contribution of outdoor air pollution to the incidence, prevalence, mortality and hospital admission for chronic obstructive pulmonary disease: a systematic review and meta‐analysis. Int J Environ Res Public Health. 2014;11:11822–11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ray WA. Evaluating medication effects outside of clinical trials: new‐user designs. Am J Epidemiol. 2003;158:915–920. [DOI] [PubMed] [Google Scholar]
- 43. McGhan R, Radcliff T, Fish R, et al. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132:1748–1755. [DOI] [PubMed] [Google Scholar]
- 44. Wami WM, Buntinx F, Bartholomeeusen S, et al. Influence of chronic comorbidity and medication on the efficacy of treatment in patients with diabetes in general practice. Br J Gen Pract. 2013;63:267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. World Health Organization Collaborating Center for Drug Statistics Methodology . Guidelines for ATC Classification and DDD Assignment 2013 (16th edition). Oslo, Norway: WHO; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article at the publisher's web‐site.


