Abstract
Context
Randomized controlled trials (RCTs) investigating the efficacy of vitamin D (Vit D) in depression provided inconsistent results.
Objective
We aim to summarize the evidence of RCTs to assess the efficacy of oral Vit D supplementation in depression compared to placebo.
Data Sources
We searched electronic databases, two conference proceedings, and gray literature by contacting authors of included studies.
Study Selection
We selected parallel RCTs investigating the effect of oral Vit D supplementation compared with placebo on depression in adults at risk of depression, with depression symptoms or a primary diagnosis of depression.
Data Extraction
Two reviewers independently extracted data from relevant literature.
Data Synthesis
Classical and Bayesian random-effects meta-analyses were used to pool relative risk, odds ratio, and standardized mean difference. The quality of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation tool.
Results
Six RCTs were identified with 1203 participants (72% females) including 71 depressed patients; five of the studies involved adults at risk of depression, and one trial used depressed patients. Results of the classical meta-analysis showed no significant effect of Vit D supplementation on postintervention depression scores (standardized mean difference = −0.14, 95% confidence interval =−0.41 to 0.13, P =.32; odds ratio =0.93, 95% confidence interval =0.54 to 1.59, P = .79). The quality of evidence was low. No significant differences were demonstrated in subgroup or sensitivity analyses. Similar results were found when Bayesian meta-analyses were applied.
Conclusions
There is insufficient evidence to support the efficacy of Vit D supplementation in depression symptoms, and more RCTs using depressed patients are warranted.
Depression is highly prevalent worldwide and is associated with increased morbidity and mortality and decreased quality of life (1–4). Major depressive disorder was the second ranking cause of years lived with disability in the United States in 2010 (5), and it is anticipated that depression will become the leading cause of disease burden and morbidity worldwide by 2030 (6, 7). Nevertheless, it is not uncommon that older adults with depression are underdiagnosed and untreated in primary care settings (8). Furthermore, poor acceptability of treatment (9) and side effects of antidepressants (10, 11) result in suboptimal therapy and treatment discontinuation for depressed patients. Simpler and more acceptable pharmacological interventions are urgently required.
Vitamin D (Vit D) can be produced endogenously in the skin by sun exposure, and humans also obtain Vit D from the diet and from supplements to a minor extent. Vit D is well known for its role in maintaining calcium homeostasis and bone health (12). However, Vit D insufficiency (defined as serum 25-hydroxyvitamin D [25(OH)D] level from 50 to 75 nmol/L approximately) has been reported in many Western countries with astonishingly high prevalence (13), and it is projected that about 1 billion people globally have Vit D deficiency [defined as serum 25(OH)D level < 50 nmol/L] or insufficiency (12).
Because Vit D receptor is found in areas of the brain that are involved in the pathophysiology of depression (14) and cross-talk between Vit D and glucocorticoids in the hippocampus is demonstrated (15), the promising and intriguing role of Vit D as a therapeutic agent in depression is being investigated. Recently, many studies have examined the relationship between Vit D and depression symptoms, especially given the complexity of treating depression and the high prevalence of Vit D deficiency. A systematic review summarizing the evidence from observational studies concluded that Vit D deficiency is positively associated with depression in adults (16). However, based on these observations, it is not possible to conclude that there is a causal relationship between Vit D and depression due to potential confounders including age, dietary intake, time spent outdoors, physical activity, smoking, alcohol use, etc (17). Many randomized controlled trials (RCTs) of Vit D supplementation in depression have been reported, but their findings have been inconsistent. Although some RCTs indicate a promising effect of Vit D supplementation on depression symptoms (18, 19), others show no such effect (20, 21).
In light of these discrepancies, we conducted a systematic review and meta-analysis of RCTs to clarify the efficacy of Vit D supplementation in depression in adults. Specifically, we aimed to evaluate whether Vit D supplementation compared with placebo improves depression symptoms in patients diagnosed with depression or prevents depression in adults who are at risk of depression or have depression symptoms.
Materials and Methods
We conducted the systematic review in accordance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (22). Data were reported following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement recommendations (23). The methods have been described in detail in a published protocol (24).
Search strategy
Briefly, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, PsychINFO, and ClinicalTrials.gov (up to April 2013). An additional search of PubMed (up to July 10, 2013) was conducted to retrieve relevant studies. Unpublished work was identified by searching two major conference proceedings—the International Vitamin Conference (from 2010), and the Anxiety Disorders and Depression Conference (from 2008)—whereas gray literature was acquired by contacting authors of included studies (up to July 2013).
Eligibility criteria
Parallel RCTs investigating the effect of oral Vit D supplementation on depression in adults (18 years of age and older) were included in this review. To be eligible for inclusion, a study’s participants were adults at risk of depression, having depression symptoms, or having a primary diagnosis of depression based on the authors’ definition. Because recognizing that some studies would use different scales to measure depression symptoms and they would choose various cutoff points to dichotomize participants as depressed and nondepressed, we adopted the original authors’ definition of the differentiation between nondepressed and depressed participants in their respective studies (25, 26). To meet our inclusion criteria, at least one of the arms had to include oral Vit D as an intervention arm. Only trials using placebos in their control groups were included. Specifically, the primary comparison was oral Vit D supplementation vs placebo.
Outcomes
The primary outcomes were the postintervention scores of depression symptoms measured by scales (for continuous outcome) and the proportion of patients with symptomatic improvement according to original authors’ definition (for dichotomous outcome), comparing Vit D supplementation with placebo. Secondary outcomes included quality of life, adverse events, and treatment discontinuation.
Data collection
Two authors (G.L. and S.Z.) independently screened and selected studies for possible inclusion in the study. Any disagreements were resolved by discussion and consensus between the two reviewers, and all the other reviewers were available to help if consensus was not reached. Initial agreement was quantified using the κ statistic.
Data extraction was completed by two authors (G.L. and S.Z.) using specially developed data extraction forms that included: 1) participant characteristics (eg, age, sex, number of participants, diagnosis or symptoms of depression, etc); 2) intervention details (eg, number of arms in the trial, sample size for each arm, dose and type of supplementation, dropouts, etc); and 3) outcome measures (eg, results of intervention including scores of depression and interim/final serum 25(OH)D levels, adverse outcomes, etc). If the study authors reported data of depression scores using several different scales corresponding with our definition of outcomes, we gave preference to the Beck Depression Inventory (BDI) for self-rating questionnaires and the Hamilton Depression Rating Scale (HDRS) for rater-administered scales.
Statistical analysis
A random-effects meta-analysis was performed to synthesize the data by pooling the postintervention scores and the proportion of patients with symptomatic improvement in depression. Heterogeneity among included studies was assessed using both the Q test and the I2 statistic (27, 28). In addition, we synthesized the results from the RCTs using a hierarchical Bayesian random-effects model (29–31) combined with observational studies included in a recent systematic review (16).
We analyzed the data using Review Manager (RevMan) version 5.2 for Windows (Nordic Cochrane Center, Cochrane Collaboration) (32). We calculated the pooled relative risk or the odds ratio (OR) for dichotomous data and the standardized mean difference (SMD) for continuous data measured on different scales (22). We used the software WinBUGS 1.4 (MRC Biostatistics Unit) (33) to apply three prior distributions to the Bayesian random-effects model: a “noninformative” prior distribution (34, 35), an “informative” prior distribution (29, 36), and a “skeptical” prior distribution (35), the latter two being based on the pooled observational studies (16). The intervention efficacy was acquired from the posterior distribution of the Bayesian analysis, presented as a SMD, relative risk, or OR, and the relevant 95% credible intervals (CrIs). We fitted the models in WinBUGS using 100 000 Markov Chain Monte Carlo cycles with two chains of simulations, a burn-in of 10 000, and a thin of 10. Convergence was assessed using the Gelman Rubin statistic (37). Convergence was approached if the Gelman Rubin statistic tended to 1. The autocorrelation was assessed based on the autocorrelation function plots. In addition to convergence and autocorrelation, a sensitivity analysis with different prior distributions for between-study variance or SD (ie, γ distribution for between-study variance and uniform distribution for between-study SD) was used to assess the robustness of the results of the Bayesian analyses.
As per our protocol (24), we planned to carry out the following a priori subgroup analyses: 1) different Vit D dosages, ie, less than 4000 IU/d vs more than 4000 IU/d where the cutoff point was chosen according to the tolerable upper intake levels in some guidelines (38, 39); 2) different study settings, ie, high vs low latitude where study was conducted; 3) males vs females; 4) institutional vs community dwellers; and 5) clinical vs general population samples. We also planned some predefined sensitivity analyses by excluding studies with high risk of bias and with short duration (ie, less than 6 mo). In addition, we conducted a fixed-effects model as part of sensitivity analyses.
Publication bias was investigated by a funnel plot and Begg’s rank correlation (40) and Egger’s regression tests (41).
Quality assessment
We assessed the quality of evidence of this systematic review using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool (42). We examined risk of bias for each included study by an adapted Cochrane Collaboration “risk of bias” assessment tool, including sequence generation, allocation concealment, blinding, incomplete outcome data/loss to follow-up, use of intention-to-treat analysis, selective outcome reporting, and other issues (22).
Results
Study identification
We identified 1251 citations. After removing 121 duplicates, 1130 citations remained for title and abstract screening, from which 31 articles were retrieved for full-text screening. Eight additional studies identified from PubMed and reference lists led to a total of 39 full-text papers assessed against the eligibility criteria. There were eight discrepancies resolved by discussion between reviewers (unweighted κ = 0.88; 95% confidence interval [CI], 0.80 to 0.96). No further studies were identified from unpublished or gray literature. Six studies (18–21, 43, 44) met the inclusion criteria and were included in the final meta-analyses (for the flow diagram showing the study selection process, see Supplemental Figure 1, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org).
Characteristics of included studies
Among the six RCTs (Table 1), two were conducted in Norway (18, 20), two in the United States (21, 43), one in Australia (44), and one in Iran (19). A total of 1203 participants (72% females) including 71 depressed patients were randomized in total, with mean/median ages varying from 38.1 years (19) to 75.0 years (44). All studies were published between 2008 and 2013.
Table 1.
Characteristics of Included RCTs
| First Author, Year (Ref.) | Country | Participants
|
Intervention
|
Outcome
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total No. (No. of Females) | Age, y | Eligibility in Each Study | Baseline Serum 25(OH)D Level, nmol/L | Baseline Depression Scores Measured | Vit D Type/Dosage | Intervention Duration | Postintervention Serum 25(OH)D Level, nmol/L | Postintervention Depression Scores Measured | MD (95% CI) | ||
| Jorde, 2008 (18)a | Norway | 441 (282) | 47.0 (21–70) | Obese adults with BMI between 28.0 and 47.0 kg/m2, no use of antidepressant or weight-reducing drugs | DD group, 55.2 (16.8–97.0); placebo group, 52.4 (18.5–99.4); DP group, 52.2 (15.4–111.5) | Total BDI: DD group, 4.5 (0–24.0); placebo group, 4.0 (0–24.5); DP group, 5.0 (0–28.0) | DD group: 2 capsules Vit D/wk (20 000 IU cholecalciferol per capsule); DP group, 1 capsule Vit D and one placebo capsule per week | 12 mo | DD group, 112.1 (46.7–193.4); placebo group, 50.0 (20.3– 99.8); DP group, 87.8 (51.5–162.3) | DD group, 3.0 (0–23.0); placebo group, 3.8 (0–18.0); DP group, 4.0 (0–26.0) | DD group, −0.80 (−1.69, 0.09); DP group, 0.20 (−0.79, 1.19) |
| Arvold, 2009 (43) | United States | 100 (36) | Vit D group, 59.7 (14.0); placebo group, 57.8 (15.8) | Patients with mild to moderate Vit D deficiency identified by Vit D screening | Vit D group, 56.92 (11.13); placebo group, 57.56 (12.72) | FIQ: Vit D group, 2.9 (2.3); placebo group, 2.4 (2.6) | One capsule containing 50 000 IU cholecalciferol weekly | 8 wk | Vit D group, 143.1 (38.16); placebo group, 68.37 (17.49) | Vit D group, 2.8 (2.7); placebo group, 2.1 (2.0) | 0.70 (−0.27, 1.67) |
| Sanders, 2011 (44) | Australia | 137 (137) | Vit D group, 74.5 (72.6– 77.9); placebo group, 75.0 (72.9–80.4) | Females > 70 y old at risk of fracture, and/or at risk of low Vit D and osteoporosis, not taking Vit D supplement > 400 IU/d | Vit D group, pre-dose, 70 (22.2).b Placebo group, pre-dose, 49.6 (14.8)b | WHO Well-Being Index.c Vit D group, pre-dose, 19 (3.33);b placebo group, pre-dose, 18 (4.44)b | 10 tablets containing total of 500 000 IU Vit D3 taken one day annually during autumn/winter | 3 to 5 y | Vit D group: 1 mo, 122 (29.6); 3 mo, 90 (22.2).b Placebo group: 1 mo, 40 (14.8); 3 mo, 40 (18.5)b | Vit D group: 1 mo, 18 (3.33); 3 mo, 18 (4.81).b Placebo group: 1 mo, 18 (5.93); 3 mo, 16.5 (5.56)b | 1.50 (−0.38, 3.38)d |
| Kjærgaard, 2012 (20) | Norway | 237 (129) | Vit D group, 53.4 (10.3); placebo group, 53.3 (10.1) | Participant with low serum 25(OH)D level and without clinical depression, and no use of antidepressant or Vit D supplement | Vit D group, 47.4 (15.8); placebo group, 47.7 (15.5) | Total BDI: Vit D group,4 (0–31); placebo group,4 (0–49) | 2 Vit D3 capsules (20 000 IU cholecalciferol) per week | 6 mo | Vit D group, 147.7 (29.2); placebo group, 52.5 (16.1) | Vit D group, 3 (0–35); placebo group, 2 (0–35) | 1.00 (−0.51, 2.51) |
| Yalamanchili, 2012 (21)e | United States | 246 (246) | Vit D group, 71.8 (3.4); placebo group, 71.1 (3.7) | Older postmenopausal women with normal range of femoral neck density | Vit D group, 97.31 (28.89); placebo group, 100.81 (34.98) | GDS: Vit D group, 4.5 (4.5); placebo group, 4.6 (4.5) | One pill containing calcitriol 0.25 μg, twice a day | 3 y | Not given | Vit D group, 3.9 (3.9); placebo group, 4.0 (4.0) | −0.10 (−1.09, 0.89) |
| Khoraminya, 2013 (19) | Iran | 42 (34) | Vit D group, 38.1 (10.07); placebo group, 39.65 (8.27) | Adults with a diagnosis of major depressive disorder without psychotic features, no use of any antidepressant or dietary supplements during the previous 2 mo | Vit D group, 74.89 (12.82); placebo group, 73.30 (14.06) | HDRS: Vit D group, 29.4 ± 5.23; placebo group, 30.2 ± 5.83 | Daily either 1.5 tablets (1500 IU) of Vit D3 plus one capsule (20 mg) fluoxetine or placebo plus 20 mg fluoxetine | 8 wk | Vit D group, 149.0 (45.0); placebo group, not given | Vit D group: wk 2, 23.94 ± 4.49; wk 4, 18.5 ± 3.76; wk 6, 14.6 ± 4.17; wk 8, 11.7 ± 4.60. Placebo group: wk 2, 25.23 ± 4.60; wk 4, 21.35 ± 3.63; wk 6, 19.0 ± 3.37; wk 8, 17.2 ± 4.16 | −5.50 (−8.22, −2.78)f |
Abbreviations: MD, mean difference (postintervention scores in Vit D group minus that in placebo group); BMI, body mass index; DD group, participants who took two capsules of Vit D; DP group, participants who took one capsule of Vit D and one capsule of placebo. Data are expressed as mean (SD) or median (interquartile range), except for Jorde’s study where medians present the ranges as minimum to maximum range and for Kjœrgaard where ranges denote minimum to maximum range.
Results from per protocol analysis since no sufficient information could be extracted for intension-to-treat analysis.
Results estimated from graphs.
Lower WHO Well-Being Index scores indicating more severe depressive symptoms.
MD calculated from 3-month scores.
Participants extracted for analysis as all subjects randomized because no exact postintervention number of participants in Vit D and placebo groups was reported.
MD calculated from week 8 scores.
The six identified RCTs included adults with a diagnosis of depression (19) or at risk of depression (18, 20, 21, 43, 44). The risk factors for depression in these studies were: obesity for adults (18), female sex for the elderly (21, 44), as well as Vit D deficiency in older adults (20, 43), which had been identified in other systematic reviews as a risk factor for depression (16, 45, 46). Baseline serum 25(OH)D varied from 47 nmol/L (20) to 100 nmol/L (21) approximately. All studies applied Vit D3 (cholecalciferol) with dosages ranging from 1500 IU/d (19) to 7100 IU/d roughly (43), except for one study using calcitriol in the intervention arm (21). The duration of Vit D supplementation varied from 8 weeks (19, 43) to 3–5 years (44).
The extracted scales used to measure depression in the identified studies included the BDI (18, 20), the Fibromyalgia Impact Questionnaire (FIQ) (43), the World Health Organization (WHO) Well-Being Index (44), the Geriatric Depression Scale (GDS) (21), and the HDRS (19). One study used both the BDI and the HDRS to assess depression; however, we only extracted HDRS scores because the HDRS was for the primary outcome measures (19). For postintervention scores of depression symptoms, means and SD values were estimated from graphs in one study (44) and calculated from medians and ranges in two other studies (18, 20). Compared to the postintervention scores in placebo groups, for adults at risk of depression, postintervention measures in the Vit D group did not show significantly lower scores where mean differences were not significant, as presented in Table 1. However, for adults with depression diagnosis, postintervention scores using HDRS in the Vit D group in week 8 were significantly lower than in the placebo group (mean difference, −5.50; 95% CI, −8.22 to −2.78) (19).
Assessment of the risk of bias showed low risk of bias in one RCT (20), moderate risk of bias in four RCTs (18, 19, 43, 44), and high risk of bias in one trial (21). The reasons for moderate risk of bias were mainly due to unclear reporting of allocation concealment (18, 19), unclear selective outcome reporting (18, 44), and intention-to-treat analyses plans (19, 43). A trial was assessed as high risk of bias because of clear reporting of selective outcomes and unclear reporting of dropouts (21).
Efficacy of Vit D supplementation in depression
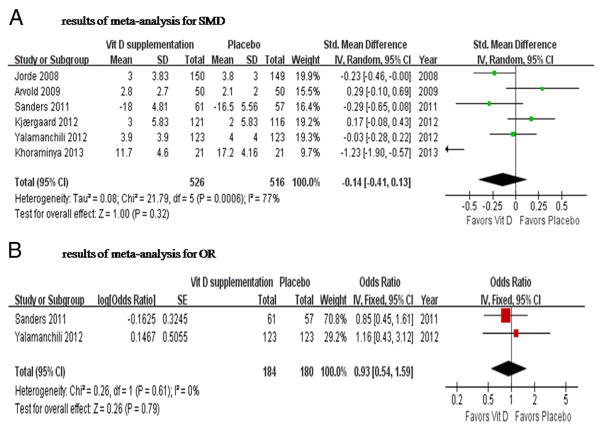
The point estimate of efficacy for each RCT and the total meta-analysis result for the Vit D group vs placebo are shown in Figure 1A. There was no significant effect of Vit D supplementation on depression, with the SMD of −0.14 (95% CI, −0.41 to 0.13; P = .32). The heterogeneity among studies was substantial (I2 = 77%; χ2 = 21.79; P < .001).
Figure 1.
Forest plot of the postintervention SMD of depression scores (A) and the OR of depression (B) for Vit D supplementation vs placebo. The size of the data markers (squares) for the SMD/OR corresponds to the weight of the study in the meta-analysis; the horizontal lines correspond to the 95% CI values.
Data on the proportion of patients with symptomatic improvement were not available in the included studies. However, there were two trials reporting the effect of Vit D supplementation on depression with the use of dichotomized depression scores (cutoff point of 10 on GDS in one trial [21], and cutoff of 13 or any score below 2 for any item on the WHO Well-Being Index in the other trial [44]). Vit D supplementation had no effect on depression in any trial (Figure 1B). There was no overall effect of Vit D supplementation on depression based on the meta-analysis of the two trials using a fixed-effects model (OR=0.93; 95%CI, 0.54 to 1.59; P=.79).
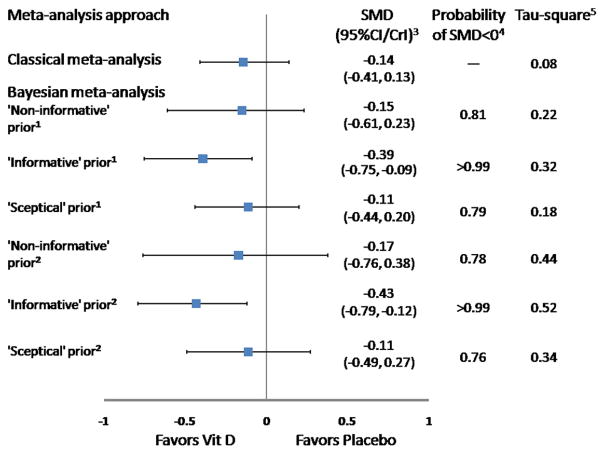
When the Bayesian approach was applied using a non-informative prior distribution (γ distribution for the between-study variance), the SMD was −0.15 (95% CrI, −0.61 to 0.23), with the posterior probability of favoring Vit D supplementation of 0.81 (Figure 2). These findings were similar to classical analysis results (Figure 1A).
Figure 2.
Results of combination of RCTs and observational studies in Bayesian approach for SMD. 1, Analyses using γ distribution for the between-study variance; 2, analyses using uniform distribution for the between-study SD; 3, CrI, credible interval; 4, SMD < 0 means that results favor Vit D supplementation; 5, tau-square means between-study variance.
The informative prior distribution was from one case-control study based on a recent systematic review (16), with SMD of −0.60 (95% CI, −0.97 to −0.23). When data of the six trials were meta-analyzed using the informative prior distribution, there was a significant effect of Vit D supplementation on depression (SMD, −0.39; 95% CrI, −0.75 to −0.09). The posterior probability of symptomatic improvement comparing Vit D supplementation with placebo was very close to 1 (Figure 2).
With the use of skeptical prior distribution, the SMD was −0.11 (95% CrI, −0.44 to 0.20), and the posterior probability of favoring Vit D supplementation was 0.79 (Figure 2).
Bayesian sensitivity analyses using a different prior distribution (uniform distribution for the between-study SD) led to results similar to those based on the γ prior distribution (Figure 2) (see Supplemental Table 1 for codes of Bayesian models and initial values).
Assessment of heterogeneity
Subgroup analyses
We performed subgroup analysis stratified by Vit D dosages, sex, study location, different sampling, and population using both classical and Bayesian random-effects approaches with a noninformative prior (γ distribution for the between-study variance). However, none of the subgroup analyses showed any significant effect of Vit D supplementation on depression (Table 2). When random-effects models were conducted, there was substantial heterogeneity: for studies with low Vit D dosage (I2 = 87%; χ2 = 15.76; P < .001) (19, 20, 44), for studies located in low latitude (I2 =80%; χ2 =15.25; P =.002) (19, 21, 43, 44), for studies with community sampling (I2 =52%; χ2 = 6.29; P = .10) (18, 20, 21, 44), and for studies using the general population as participants (I2 = 55%; χ2 = 8.79; P = .07) (18, 20, 21, 43, 44).
Table 2.
Results of Subgroup Analysis and Sensitivity Analysis for SMD
| Analysis | Classical Analysis
|
Bayesian Approacha
|
||
|---|---|---|---|---|
| SMD (95% CI) | P Value | SMD (95% CrI) | Probability of SMD < 0 | |
| Subgroup analysis | ||||
| Different Vit D dosage | ||||
| Highb | −0.08 (−0.31, 0.14) | .45 | c | |
| Low | −0.38 (−1.02, 0.27) | .25 | −0.38 (−2.03, 1.13) | .80 |
| Sex | ||||
| Malesd | ||||
| Females | −0.11 (−0.32, 0.10) | .30 | c | |
| Study location | ||||
| High latitude | −0.03 (−0.21, 0.15) | .76 | c | |
| Low latitude | −0.24 (−0.68, 0.21) | .29 | −0.25 (−1.15, 0.53) | .80 |
| Sampling | ||||
| Institutional | −0.12 (−0.48, 0.23) | .50 | c | |
| Community | −0.08 (−0.28, 0.12) | .45 | −0.08 (−0.37, 0.18) | .76 |
| Population | ||||
| Clinicale | ||||
| General | −0.03 (−0.22, 0.17) | .79 | −0.03 (−0.26, 0.22) | .61 |
| Baseline Vit D level | ||||
| Sufficientf | −0.16 (−0.32, 0.01) | .06 | −0.17 (−0.50, 0.14) | .91 |
| Deficientf | −0.19 (−0.87, 0.50) | .60 | −0.20 (−2.13, 1.60) | .64 |
| Sensitivity analysis | ||||
| Excluding studies with high risk of bias | −0.18 (−0.53, 0.17) | .31 | −0.19 (−0.83, 0.37) | .80 |
| Excluding studies with short durationg | −0.08 (−0.28, 0.12) | .45 | −0.08 (−0.37, 0.18) | .76 |
| Fixed-effects model | −0.07 (−0.20, 0.06) | .27 | h | |
| Using changed scores from baseline | −0.12 (−0.39, 0.15) | .39 | −0.13 (−0.56, 0.26) | .79 |
Noninformative priors (γ distribution for the between-study variance) were used.
>4000 IU/d.
No Bayesian random-effects model was conducted because only two studies were included.
No meta-analysis was applied because no data could be extracted from included studies.
No meta-analysis was conducted because of only one study included.
Based on original authors’ definition in included studies.
Less than 6 months.
No Bayesian random-effects approach was applied.
Three trials used adults with Vit D deficiency whose baseline serum 25(OH)D levels were approximately 47 nmol/L (20), 57 nmol/L (43), and 74 nmol/L (19), respectively. We conducted a post hoc subgroup analysis stratified by dichotomized baseline 25(OH)D levels (ie, sufficient vs deficient baseline Vit D levels). No significant difference was observed between the deficient Vit D levels and depression (classical analysis—SMD, −0.19; 95% CI, −0.87 to 0.50; Bayesian analysis—SMD, −0.20; 95% CrI, −2.13 to 1.60) (19, 20, 43). There was a marginal but not statistically significant effect of Vit D supplementation on depression symptoms in subjects without Vit D deficiency at baseline: classical analysis—SMD, −0.16; 95% CI, −0.32 to 0.01; P = .06; Bayesian analysis—SMD, −0.17; 95% CrI, −0.50 to 0.14; posterior probability of favoring Vit D supplementation = 0.91 (18, 21, 44) (Table 2).
Sensitivity analyses
Three a priori sensitivity analyses were conducted by excluding studies with a high risk of bias and short duration of intervention and by applying a fixed-effects model. In all three analyses, there was no statistically significant effect of Vit D supplementation on depression (Table 2).
Moreover, because one trial also reported the changed scores of depression from baseline (20), we performed another post hoc sensitivity analysis after imputing SD values of the changed scores for the other trials based on the recommendation of the Cochrane Handbook for Systematic Reviews of Interventions (22). The results did not favor Vit D supplementation (classical analysis—SMD, −0.12;95%CI, −0.39 to 0.15;Bayesian analysis—SMD, −0.13; 95% CrI, −0.56 to 0.26) (Table 2), which was very similar to the pooled results using postintervention scores (classical analysis—SMD, −0.14; 95% CI, −0.41 to 0.13; Bayesian analysis—SMD, −0.15; 95% CrI, −0.61 to 0.23) (Figure 2).
However, the heterogeneity among studies was statistically significant for the analysis, excluding studies with high risk of bias (I2 =81%; χ2 =21.55; P <.001) (18–20, 43, 44), short duration of intervention (I2 =52%; χ2 =6.29; P =.10) (18, 20, 21, 44), and using changed scores from baseline (I2 = 75%; χ2 =20.24; P =.001) (18–21, 43, 44).
Secondary outcomes
Quality of life
Only one trial reported the effect of Vit D on quality of life (44). No significant association was found between Vit D supplementation and quality of life as measured by the General Health Questionnaire (OR =1.06; 95% CI, 0.81 to 1.37) (44).
Adverse events
As reported in the included RCTs, either no participants reported adverse events related to Vit D supplementation (19, 43) or no significant difference in adverse events was found between placebo and Vit D groups (18, 20, 44).
Treatment discontinuation
The rate of withdrawal from the trials was low, except for one trial with a dropout rate of 22.7% (18). The reported withdrawal and discontinuation reasons were: one participant discontinued Vit D supplementation for personal reasons (dropout rate, 0.8%) (20), two were lost to follow-up (4%) (43), 116 withdrew from the study (10.3%) (44), and one was excluded from study because of anxiety (5%) (19).
There were three trials (18, 20, 21) reporting high compliance with the Vit D supplementation, which varied from 93% (21) to 95% (18).
Assessment of quality of evidence across studies
The quality of evidence obtained from the included trials was graded as low, because of consistently unexplained heterogeneity and the risk of selective outcome reporting bias (see Supplemental Table 2 for the summary of findings for efficacy of Vit D supplementation in depression) (42). The Q tests and I2 statistics for assessment of heterogeneity among studies were statistically significant, as found for the overall effect of Vit D supplementation (Figure 1A) and the subgroup and sensitivity analyses when random-effects models were used. Meanwhile, there was unclear risk of selective outcome reporting bias in two trials (18, 44) and clear risk of bias in one RCT (21).
Assessment of publication bias
Publication bias was examined by the construction of a funnel plot showing the relationship between the SMD and the SE of logarithmic SMD, the Begg’s rank correlation, and Egger’s regression tests. The symmetric funnel plot suggested no evidence of publication bias (see Supplemental Figure 2 for the funnel plot to assess publication bias). Egger’s test and Begg’s test yielded similar results to the visual inspection for symmetry of funnel plot: Egger P = .258; Begg P = .546.
Discussion
Main findings
Six RCTs were identified in this systematic review investigating the efficacy of Vit D supplementation in depression. The results of the classical meta-analysis showed no significant effect of Vit D supplementation on depression symptoms (Figure 1A: SMD, −0.14; 95% CI, −0.41 to 0.13; P = .32; Figure 1B: OR = 0.93, 95% CI, 0.54 to 1.59; P =.79). These findings were consistent in subgroup analyses stratified by Vit D dosages, sex, study location, different sampling, and population, and were robust in sensitivity analyses that excluded studies with high risk of bias and short intervention duration, applied a fixed-effects model, and used changed scores from baseline for analysis. When Bayesian meta-analyses were conducted, the results remained nonsignificant with the use of non-informative or skeptical prior distributions.
We also dichotomized Vit D levels into sufficient and deficient levels, based on the definitions used in the selected articles recognizing that there is no consensus on what is the optimal serum 25(OH)D level (12). There was a marginal but not statistically significant effect observed on depression symptoms in participants without Vit D deficiency at baseline (SMD, −0.16; 95% CI, −0.32 to 0.01; P = .06), in which the posterior probability of a beneficial effect of Vit D supplementation was very high (0.91) using a Bayesian analysis with a noninformative prior distribution (Table 2). Compared with those with Vit D deficiency (19, 20, 43), participants with normal serum 25(OH)D levels were elderly women (mean age, 73 years approximately) (21, 44), or obese adults (18). It was possible that these participants consciously or unconsciously consumed other supplementation or food that could help mitigate depression, but they failed to report this to the data collectors, such that the marginal but not significant effect of Vit D was observed. However, taking into account the criteria of evaluating subgroup effect, especially that the analysis (including hypothesis and direction of subgroup effect) was not specified a priori but post hoc (47), we would place uncertainty to this subgroup finding and interpret the result with caution. Also, we conducted the sensitivity analyses by choosing the cutoff points of 25(OH)D levels based on clinical relevance as 50 and 75 nmol/L, respectively; however, no significant effect of Vit D supplementation on depression could be found.
The populations included in the current systematic review were diverse, varying from obese adults (18), elderly females (21, 44), and Vit D-deficient adults (20, 43) to depressed patients (19). The duration of intervention in two studies (19, 43) was very short (ie, 8 wk) (Table 1), which may fail to observe the intervention effect over time because they stopped early (48). Moreover, there was one trial at high risk of bias (21), and four trials were at moderate risk of bias (18, 19, 43, 44). All the aforementioned issues in the quality of the included studies, as well as the quantitative assessment of heterogeneity, resulted in the low quality of evidence for this systematic review.
The scales to measure depression symptoms in the included trials consisted of BDI (18, 20), GDS (21), FIQ (43), HDRS (19), and WHO Well-Being Index (44). Arvold et al (43) used FIQ to measure depression symptoms in older outpatients in which the FIQ was not a specific scale of depression, although it covered the domain of depression (Table 1). However, the participants in the study were not diagnosed with fibromyalgia, but only with Vit D deficiency. According to the authors’ statement, “vitamin D deficiency can cause bone pain, muscle weakness, and a symptom complex that can mimic fibromyalgia, myopathy, or chronic fatigue syndrome”; therefore, “the FIQ was chosen because it captures many of the symptoms reported by some vitamin D-deficient patients” (43). In this systematic review, given that we tried to retrieve all potential eligible evidence and this study met our eligibility criteria accurately, the decision was made to include these participants at risk of depression and extract the data on the domain of depression. Nevertheless, findings of the post hoc sensitivity analysis showed that the pooled SMD not including this study (43) yielded similar results to those from all the six studies (18–21, 43, 44): SMD, −0.22; 95% CI, −0.51 to 0.08, for the classical analysis; and SMD, −0.22; 95% CrI, −0.77 to 0.21, for the Bayesian analysis with a noninformative prior using γ distribution for the between-study variance (see Figure 2 for results of the classical and Bayesian meta-analyses including all of the six studies).
Only one trial included individuals with a depression diagnosis (19). There may be some underlying interaction between the routine antidepressant (ie, fluoxetine) and Vit D on depression symptoms because the intervention was fluoxetine plus Vit D vs fluoxetine plus placebo (Table 1). However, we decided to include this study, given that it was the first and unique RCT using diagnosed patients to evaluate efficacy of Vit D supplementation in depression. The results were robust and insensitive when a subgroup analysis was conducted and stratified by clinical vs general population (Table 2).
Comparison with other reviews
Vit D is essential for the maintenance of calcium homeostasis and for bone health (12). However, the plausibility of association between Vit D and depression has not yet been confirmed. Several narrative reviews suggested an association between Vit D and depression (49–55), whereas a recent systematic review based on observational studies has substantiated the significant association (16). Nevertheless, it is difficult to identify the causal relation given the observational design and the numerous potential confounders, especially when there was reverse causality between serum Vit D level and depression (eg, less outdoor activity/nutrient intake, and thus low Vit D) in observational studies (17, 54–58).
In this systematic review of RCTs, no effect of Vit D supplementation was found on depression, which was supported by the pooled SMD and OR. Furthermore, as shown in Table 1 for each specific study, despite the higher levels of Vit D observed post hoc in the intervention groups (18, 20, 43, 44), no significant mean differences of postintervention scores could be obtained, which meant that the depression scores were not significantly different in Vit D and placebo groups after intervention.
Bayesian meta-analysis can synthesize the evidence of RCTs in conjunction with observational studies (35, 59). Using a noninformative prior distribution, the posterior probability of favoring Vit D supplementation was 0.81 with the SMD of −0.15, which was very similar to the results of the classical meta-analysis. When we used results from observational studies as the informative prior distribution, there was a significant effect of Vit D supplementation on depression with the posterior probability of almost 1. However, if we placed uncertainty on the results from observational studies, again the posterior results of the skeptical prior distribution were not significant, and the posterior probability was only 0.79. Similar results could be found when another prior distribution for the between-study SD (uniform distribution) was performed, which presented the robustness of Bayesian analyses (Figure 2). Hence, there was convincing evidence that exaggerated results from observational studies failed to unveil the true association between Vit D and depression, and no efficacy of Vit D supplementation in depression could be clarified, based on the findings of RCTs in conjunction with observational research.
Limitations and strengths
There are certain limitations to this systematic review. Initially, the heterogeneity persisted significantly in the overall analysis, subgroup, and sensitivity analyses. The unexplained heterogeneity may be, at least in part, related to the different scales used and the diverse populations at risk of depression. Moreover, there was only one trial with a low risk of bias (20). Thus, the underlying risk of bias may influence the estimate of effect of Vit D supplementation. In this systematic review, most included studies were conducted in developed countries (18, 20, 21, 43, 44), whereas only one trial was performed in a developing country (19). Lack of studies in developing countries may limit the generalizability and weaken the findings. Furthermore, most included trials examined a nonclinical sample, which may have decreased the likelihood of success because participants without a diagnosis of depression would have a high placebo response rate and less likelihood of response to Vit D supplementation than patients with depression (60). Significant symptomatic improvement was reported in the study with a clinical sample from week 2 to week 8 compared to placebo (Table 1) (19). However, only one trial using depressed patients could be retrieved and analyzed in this review (19), whereas data of another trial could not be extracted due to insufficient information, although it included 12 and 17 patients with depression in Vit D and placebo groups, respectively (21). Therefore, given all the analyses, there is insufficient evidence to corroborate efficacy of Vit D supplementation in depression at present, and more evidence for the effect of Vit D as an adjunct to antidepressants in depressed patients is urgently needed.
To our knowledge, this is the first systematic review and meta-analysis to evaluate the efficacy of Vit D supplementation in depression in RCTs. We performed a comprehensive and exhaustive search to retrieve all relevant studies. We extracted and managed data in duplicate with a good level of consensus. A priori and post hoc subgroup analyses and sensitivity analyses were carried out to better synthesize the available evidence. The particular strength of the review was use of the Bayesian approach, which allowed us to incorporate external information from observational studies in our synthesis while exploring the robustness of the results under different assumptions (ie, with different prior distributions) and to calculate the posterior probability of Vit D efficacy.
Implications of the study
The existing body of evidence does not support the efficacy of Vit D supplementation in depression. More RCTs using mildly, moderately, or severely depressed patients are needed to identify efficacy of Vit D supplementation in depression.
This systematic review does not provide enough information to update the current guidelines on the use of Vit D, given that there is no attested evidence of Vit D for prevention effect on depression symptoms or enough studies investigating treatment effect on depressed patients. Depressed patients and participants at risk of depression with Vit D deficiency should consume Vit D supplementation (12, 61). However, for those participants without Vit D deficiency, Vit D supplementation is not recommended for the purpose of prevention or treatment of depression.
Conclusion
In conclusion, in our systematic review there is insufficient evidence to support the efficacy of Vit D supplementation in depression symptoms, and more RCTs using depressed patients are imperative and warranted.
Acknowledgments
The authors thank Miss Zhixiong Cheng for her help with writing, editing, and proofreading of this manuscript.
Abbreviations
- BDI
Beck Depression Inventory
- CI
confidence interval
- CrI
credible interval
- FIQ
Fibromyalgia Impact Questionnaire
- GDS
Geriatric Depression Scale
- HDRS
Hamilton Depression Rating Scale
- 25(OH)D
25-hydroxyvitamin D
- OR
odds ratio
- RCT
randomized controlled trials
- SMD
standardized mean difference
- Vit D
vitamin D
Footnotes
Study registration: PROSPERO Identifier, CRD42013003849.
Disclosure Summary: The authors have nothing to disclose.
The authors received no funding for this study.
References
- 1.Penninx BW, Beekman AT, Honig A, et al. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry. 2001;58:221–227. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- 2.Wells KB, Stewart A, Hays RD, et al. The functioning and well-being of depressed patients. Results from the Medical Outcomes Study. JAMA. 1989;262:914–919. [PubMed] [Google Scholar]
- 3.Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 4.Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22:613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 5.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 6.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. The Global Burden of Disease: 2004 update. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 8.Klap R, Unroe KT, Unützer J. Caring for mental illness in the United States: a focus on older adults. Am J Geriatr Psychiatry. 2003;11:517–524. [PubMed] [Google Scholar]
- 9.Jorm AF. Mental health literacy. Public knowledge and beliefs about mental disorders. Br J Psychiatry. 2000;177:396–401. doi: 10.1192/bjp.177.5.396. [DOI] [PubMed] [Google Scholar]
- 10.Anderson HD, Pace WD, Libby AM, West DR, Valuck RJ. Rates of 5 common antidepressant side effects among new adult and adolescent cases of depression: a retrospective US claims study. Clin Ther. 2012;34:113–123. doi: 10.1016/j.clinthera.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 11.Goethe JW, Woolley SB, Cardoni AA, Woznicki BA, Piez DA. Selective serotonin reuptake inhibitor discontinuation: side effects and other factors that influence medication adherence. J Clin Psychopharmacol. 2007;27:451–458. doi: 10.1097/jcp.0b013e31815152a5. [DOI] [PubMed] [Google Scholar]
- 12.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 13.Mithal A, Wahl DA, Bonjour JP, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:1807–1820. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 14.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 α-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Obradovic D, Gronemeyer H, Lutz B, Rein T. Cross-talk of vitamin D and glucocorticoids in hippocampal cells. J Neurochem. 2006;96:500–509. doi: 10.1111/j.1471-4159.2005.03579.x. [DOI] [PubMed] [Google Scholar]
- 16.Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100–107. doi: 10.1192/bjp.bp.111.106666. [DOI] [PubMed] [Google Scholar]
- 17.Bertone-Johnson ER. Vitamin D and the occurrence of depression: causal association or circumstantial evidence? Nutr Rev. 2009;67:481–492. doi: 10.1111/j.1753-4887.2009.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008;264:599–609. doi: 10.1111/j.1365-2796.2008.02008.x. [DOI] [PubMed] [Google Scholar]
- 19.Khoraminya N, Tehrani-Doost M, Jazayeri S, Hosseini A, Djazayery A. Therapeutic effects of vitamin D as adjunctive therapy to fluoxetine in patients with major depressive disorder. Aust N Z J Psychiatry. 2013;47:271–275. doi: 10.1177/0004867412465022. [DOI] [PubMed] [Google Scholar]
- 20.Kjærgaard M, Waterloo K, Wang CE, et al. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case-control study and randomised clinical trial. Br J Psychiatry. 2012;201:360–368. doi: 10.1192/bjp.bp.111.104349. [DOI] [PubMed] [Google Scholar]
- 21.Yalamanchili V, Gallagher JC. Treatment with hormone therapy and calcitriol did not affect depression in older postmenopausal women: no interaction with estrogen and vitamin D receptor genotype polymorphisms. Menopause. 2012;19:697–703. doi: 10.1097/gme.0b013e31823bcec5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0. http://handbook.cochrane.org. Updated March 2011.
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review protocol. Syst Rev. 2013;2:64. doi: 10.1186/2046-4053-2-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omori IM, Watanabe N, Nakagawa A, et al. Fluvoxamine versus other anti-depressive agents for depression. Cochrane Database Syst Rev. 2010;3:CD006114. doi: 10.1002/14651858.CD006114.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cipriani A, Koesters M, Furukawa TA, et al. Duloxetine versus other anti-depressive agents for depression. Cochrane Database Syst Rev. 2012;10:CD006533. doi: 10.1002/14651858.CD006533.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res. 2001;10:277–303. doi: 10.1177/096228020101000404. [DOI] [PubMed] [Google Scholar]
- 30.Smith TC, Spiegelhalter DJ, Thomas A. Bayesian approaches to random-effects meta-analysis: a comparative study. Stat Med. 1995;14:2685–2699. doi: 10.1002/sim.4780142408. [DOI] [PubMed] [Google Scholar]
- 31.Sung L, Hayden J, Greenberg ML, Koren G, Feldman BM, Tomlinson GA. Seven items were identified for inclusion when reporting a Bayesian analysis of a clinical study. J Clin Epidemiol. 2005;58:261–268. doi: 10.1016/j.jclinepi.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Review Manager (RevMan) [computer program] Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration; 2012. Version 5.2. [Google Scholar]
- 33.WinBUGS. The BUGS project. MRC Biostatistics Unit; Cambridge, UK: 1996–2012. [Accessed July 1, 2013]. http://www.mrc-bsu.cam.ac.uk/bugs/winbugs/contents.shtml. Updated August 2007. [Google Scholar]
- 34.Lambert PC, Sutton AJ, Burton PR, Abrams KR, Jones DR. How vague is vague? A simulation study of the impact of the use of vague prior distributions in MCMC using WinBUGS. Stat Med. 2005;24:2401–2428. doi: 10.1002/sim.2112. [DOI] [PubMed] [Google Scholar]
- 35.Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian Approaches to Clinical Trials and Health-Care Evaluation (Statistics in Practice) Hoboken, NJ: John Wiley & Sons Ltd; 2004. [Google Scholar]
- 36.Higgins JP, Whitehead A. Borrowing strength from external trials in a meta-analysis. Stat Med. 1996;15:2733–2749. doi: 10.1002/(SICI)1097-0258(19961230)15:24<2733::AID-SIM562>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 37.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comp Graph Stat. 1998;7:434–455. [Google Scholar]
- 38.Health Canada. [Accessed September 1, 2013];Vitamin D and calcium: updated dietary reference intakes. http://www.hc-sc.gc.ca/fn-an/nutrition/vitamin/vita-d-eng.php.
- 39.European Food Safety Authority Panel on Dietetic Products, Nutrition, and Allergies. Scientific opinion on the tolerable upper intake level of vitamin D. EFSA J. 2012;10:2813. [Google Scholar]
- 40.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 41.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arvold DS, Odean MJ, Dornfeld MP, et al. Correlation of symptoms with vitamin D deficiency and symptom response to cholecalciferol treatment: a randomized controlled trial. Endocr Pract. 2009;15:203–212. doi: 10.4158/EP.15.3.203. [DOI] [PubMed] [Google Scholar]
- 44.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose vitamin D3 and mental well-being: randomised controlled trial. Br J Psychiatry. 2011;198:357–364. doi: 10.1192/bjp.bp.110.087544. [DOI] [PubMed] [Google Scholar]
- 45.Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 46.Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160:1147–1156. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- 47.Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ. 2010;340:c117. doi: 10.1136/bmj.c117. [DOI] [PubMed] [Google Scholar]
- 48.Haynes R, Sackett D, Guyatt G, Tugwell P. Clinical Epidemiology: How to Do Clinical Practice Research. 3. Philadelphia, PA: Lippincott Williams, Wilkins; 2005. [Google Scholar]
- 49.Murphy PK, Wagner CL. Vitamin D and mood disorders among women: an integrative review. J Midwifery Womens Health. 2008;53:440–446. doi: 10.1016/j.jmwh.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 50.Barnard K, Colón-Emeric C. Extraskeletal effects of vitamin D in older adults: cardiovascular disease, mortality, mood, and cognition. Am J Geriatr Pharmacother. 2010;8:4–33. doi: 10.1016/j.amjopharm.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Penckofer S, Kouba J, Byrn M, Estwing Ferrans C. Vitamin D and depression: where is all the sunshine? Issues Ment Health Nurs. 2010;31:385–393. doi: 10.3109/01612840903437657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howland RH. Vitamin D and depression. J Psychosoc Nurs Ment Health Serv. 2011;49:15–18. doi: 10.3928/02793695-20110111-02. [DOI] [PubMed] [Google Scholar]
- 53.Humble MB. Vitamin D, light and mental health. J Photochem Photobiol B. 2010;101:142–149. doi: 10.1016/j.jphotobiol.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34:47–64. doi: 10.1016/j.yfrne.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Parker G, Brotchie H. ‘D’ for depression: any role for vitamin D? ‘Food for Thought’ II. Acta Psychiatr Scand. 2011;124:243–249. doi: 10.1111/j.1600-0447.2011.01705.x. [DOI] [PubMed] [Google Scholar]
- 56.Truesdell DD. The efficacy of nutrition and lifestyle approaches in the treatment of depression. Top Clin Nutr. 2009;24:55–66. [Google Scholar]
- 57.Berk M, Sanders KM, Pasco JA, et al. Vitamin D deficiency may play a role in depression. Med Hypotheses. 2007;69:1316–1319. doi: 10.1016/j.mehy.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Cherniack EP, Troen BR, Florez HJ, Roos BA, Levis S. Some new food for thought: the role of vitamin D in the mental health of older adults. Curr Psychiatry Rep. 2009;11:12–19. doi: 10.1007/s11920-009-0003-3. [DOI] [PubMed] [Google Scholar]
- 59.Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE. Bayesian meta-analysis of hormone therapy and mortality in younger post-menopausal women. Am J Med. 2009;122:1016–1022. e1. doi: 10.1016/j.amjmed.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 60.Berk M, Jacka F. Preventive strategies in depression: gathering evidence for risk factors and potential interventions. Br J Psychiatry. 2012;201:339–341. doi: 10.1192/bjp.bp.111.107797. [DOI] [PubMed] [Google Scholar]
- 61.Pearce S, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ. 2010;340:b5664. doi: 10.1136/bmj.b5664. [DOI] [PubMed] [Google Scholar]