Introduction
Histopathological examination is currently the gold standard for the diagnosis of melanocytic lesions. However, distinguishing Spitz nevi (SN) from Spitzoid melanomas (SM) using current histopathological criteria may be very challenging and is one the most difficult areas in Dermatopathology.1-4 Some problematic cases may show overlapping features of both SN and SM and are often termed Atypical Spitzoid Neoplasms (ASN). It has been demonstrated in a number of studies that it can be difficult to predict the clinical behavior of ASN from assessment of histopathological features and the interobserver reproducibility of pathologists for the diagnosis of these tumors is generally poor.1-5
Previously, we highlighted proteomic differences between the melanocytic tumor cells of SN and SM and identified a molecular signature of five peptides that could correctly classify the tumors.6 In this study, we sought to determine whether Imaging Mass Spectrometry (IMS) could assist in the diagnosis and risk stratification of ASN by performing IMS in a large series of cases with known clinical follow-up, by comparing in each case the diagnosis rendered by IMS with the histopathological diagnosis and also correlating the diagnoses with clinical outcome.
Materials and Methods
Sample and Clinical Data Collection
We defined ASNs as melanocytic lesions with architectural and cytologic features shared by both Spitz nevi and Spitzoid melanomas, for which a definitive histopathological diagnosis of either SN or SM could not be confidently rendered by an experienced dermatopathologist.4, 7-9 In our opinion, ASN is the most appropriate term for these lesions, since they are truly “neoplasms” and can be either benign or malignant, and “Spitzoid” describes the cytologic and architectural resemblance to Spitz nevus.
To the best of our knowledge, this is the largest study of ASNs performed to date. A total of 252 ASN cases were contributed by centers from 11 countries throughout the world and 11 centers from the US including the Yale Spitzoid Neoplasm Repository. All cases had some degree of ambiguity that prompted an original diagnosis of ASN. Although in some cases either Spitz nevus or Spitzoid melanoma was favored, such diagnoses were considered by no means reasonably certain by the experienced dermatopathologists who reviewed the cases. The entire cohort of ASN cases consisted of difficult ambiguous lesions in the gray/borderline area. All submitted cases were reviewed by a minimum of three experienced dermatopathologists who agreed on the classification of ASN. The cases of ASN were originally diagnosed as such by the dermatopathologist(s) who contributed the case to the study. These cases were reviewed at intradepartmental consensus conference and only cases with diagnostic uncertainty were included in the study. In addition, the first author re-reviewed all cases before they entered the study. Histopathological criteria used in the evaluation and classification of a lesion as an ASN included (but were not limited to) the following: asymmetry, sheet-like growth pattern, expansile nodular growth, lack of maturation, ulceration of the epidermis, presence of mitotic figures, deep or atypical mitotic figures, deep extension into the subcutis, or large size (>1 cm). Initial inclusion criteria were a histopathological diagnosis of ASN and the availability of clinical follow-up information. A total of 252 ASN cases were initially included. Due to lack of follow-up, insufficient melanocytic component to perform the analysis, or technical difficulties 150 ASN cases were subsequently excluded.
A total of 102 cases meeting all of the above criteria were identified. Clinical and histopathological parameters were recorded for all cases including patient age, gender, tumor thickness, primary tumor anatomical location, presence or absence of ulceration, and mitotic rate in accordance with the American Joint Committee on Cancer (AJCC).10 We adopted the clinical classification used by Gerami et al11 in their study of ASNs – the cases were categorized into 4 clinical groups on the basis of their clinical behavior (Table 1).
Table 1.
Clinical groups of Atypical Spitzoid Neoplasms
| Group | Category | SLN | CLDN | Residual disease | Locoregional disease | Distant metastases | Death of disease |
|---|---|---|---|---|---|---|---|
| 1 | 1a | NA | NA | − | − | − | − |
| 1b | − | NA | − | − | − | − | |
| 1c | + | − | − | − | − | − | |
| 2 | + | + | +/− | + | − | − | |
| 3 | +/− | +/− | +/− | +/− | + | − | |
| 4 | +/− | +/− | +/− | +/− | + | + |
Definitions of clinical groupings:
Group 1:
− a – Cases with no evidence of disease after re-excision but without SLN performed
− b – Cases with no evidence of disease after re-excision and negative SLN
− c – Cases with no evidence of disease after re-excision and positive SLN
Group 2: Patients with locoregional disease in other nodes but without distant metastases
Group 3: Patients with distant metastases
Group 4: Patients with distant metastases and death due to disease
Of the total cohort of 102 cases, 94 cases were in Group 1, which was further subdivided into 3 subgroups: 1a (58 cases), 1b (26 cases), and 1c (10 cases). There were 4 cases in Group 2, 1 case in Group 3, and 3 cases in Group 4. Groups 2, 3 and 4 included patients with poor clinical outcome defined as either recurrence of disease, metastasis in non-sentinel lymph node(s) and/or distant metastases and death of disease (Table 2).
Table 2.
Summary of clinical, histopathological, and Imaging Mass Spectrometry data by clinical stage
| Clinical group |
Number of cases |
Average age (years) |
Sex Ratio (M: F) |
Mean tumor thickness (mm) |
Ulceration Y/N |
Average mitotic rate/mm2 |
Histopathological diagnosis |
IMS diagnosis |
Patients with IMS diagnosis of SM |
Average Follow – up (years) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Total: 94 | 31.3 | 44:50 | 1.99 | 9/85 | 1.3 | 27 SN/30 SM/37 ASN | 86 SN/8 SM | (8 cases) 8.5% | 7.7 |
| 1a | 58 | (2 cases) 3.4% | 9.1 | |||||||
| 1b | 26 | (2 cases) 7.7% | 5.4 | |||||||
| 1c | 10 | (4 cases) 40% | 5.3 | |||||||
| 2 | 4 | 39 | 2:2 | 3.6 | 1/3 | 1 | 1 SN/3 SM | 2 SN/2 SM | (2 cases) 50% | 11.8 |
| 3 | 1 | 41 | 1:0 | 2.25 | 0/1 | 1 | 1 SM | 1 SM | 100% | 1* |
| 4 | 3 | 63.3 | 2:1 | 3.4 | 1/2 | 4 | 3 SM | 3 SM | 100% | 2.8 |
Clinical Group 1a: Cases with no evidence of disease after re-excision but without SLN performed
Clinical Group 1b: Cases with no evidence of disease after re-excision and negative SLN
Clinical Group 1c: Cases with no evidence of disease after re-excision and positive SLN
Clinical Group 2: Patients with locoregional disease in other nodes but without distant metastases
Clinical Group 3: Patients with distant metastases
Clinical Group 4: Patients with distant metastases and death due to disease
Lost to follow-up after one year
IMS – Imaging Mass Spectrometry
M – male
F – female
Y – yes
N – no
SLN – sentinel lymph node
ASN – Atypical Spitzoid Neoplasm
SM – Spitzoid melanoma
FU – follow – up
Imaging Mass Spectrometry
IMS was performed on all ASN cases using a previously published methodology in which IMS was employed to discriminate between SN and SM.6 In brief, 5μm-thick formalin-fixed, paraffin-embedded tissue sections were used from each case after de-paraffinization. On average, twenty areas of interest from the melanocytic component, each measuring 300 μm in diameter, were mapped on each slide representing a single case. The mass spectral profile was acquired for each one of these areas of interest. The resultant spectra were compared to a proteomic signature composed of 5 proteins and each area of interest was designated as either consistent with SN or consistent with SM. For each case to be diagnosed as SN or SM, an overwhelming number of the areas of interest (>66%) had to be designated by the instrument as such. A detailed description of this method and the instruments used can be found elsewhere.6, 12 In this study we used the very same algorithm, classification method and criteria that we did in our original study.6 A diagnosis of either SN or SM was rendered by IMS, which was then correlated with various clinicopathologic features and compared with the histopathological diagnosis and clinical outcome.
Statistical analysis
Fisher's exact test was used to compare the frequency of cases diagnosed as SM by IMS between Group 1 versus Groups 2, 3 and 4. The same analysis was also performed comparing Groups 1 and 2 versus Groups 3 and 4 and P-values were calculated. Possible associations were examined by using logistic regression analyses with the AJCC prognostic factors (patient age, gender, primary tumor anatomical location, tumor thickness, ulceration status, and mitotic rate), considered separately as predictors of clinical category (Group 1 versus groups to 2-4). The association of histopathological diagnosis and clinical outcome was also assessed using Fisher's exact test.
Results
A total of 102 ASNs with sufficient clinical follow-up data were included in the study and analyzed by IMS. The patients’ ages ranged from 2 to 83 years (average 32.6). There were 53 women and 49 men. The most common primary tumor anatomical location was the lower extremity (41 cases) followed by the trunk (28 cases), the upper extremity (16 cases), and the head and neck region (16 cases). The anatomical site of one case was unknown. The tumor thickness ranged from 0.4 mm to 5.5 mm (mean 2.1). The follow-up ranged from 0.5 to 25 years (mean 7.7).
The patients were categorized in 4 clinical groups. Group 1 comprised 94 patients and included 3 categories - a, b, and c. Group 1a comprised 58 cases, in which the patients had no evidence of disease after re-excision and in which SLN biopsy was not performed. Of these 2/58 (3.4%) cases were diagnosed as SM by IMS and the rest of the cases, 56/58 (96.6%) were diagnosed as SN. In case #9, diagnosed as SM by IMS, a diagnosis of SM with depth of 3.0 mm was also favored histopathologically. The patient was alive with no evidence of disease (ANED) 15 years after the initial diagnosis.
Group 1b included 26 cases with no evidence of disease after re-excision and negative SLN biopsy. Of those 2/26 (7.7%) cases (#39 and #40) were diagnosed as SM by IMS and 24 (92.3%) cases were diagnosed as SN. In the 2 cases diagnosed as SM by IMS, a diagnosis of SM was also favored histopathologically. The lesions were 1.0 and 4.2 mm thick, respectively, and both patients were ANED at 3 and 2 years, respectively. There were 10 cases in Group 1c, which consisted of patients with positive SLN(s) but no evidence of disease after re-excision. Four cases (40%), (#41, 100, 145 and 146), were diagnosed as SM by IMS and a diagnosis of SM was favored histopathologically. The depth in these cases ranged between 1.65 and 2.45 mm (mean 2.0). All patients were ANED at 3, 1, 3 and 3 years of follow-up, respectively. The other 6/10 cases in Group 1c (#47, 106, 108, 96, 112, 148) were diagnosed as SN by IMS. Three of these patients (#47, 106 and 108) had a histopathological diagnosis favoring SM with thickness ranging between 3.25 and 3.7 mm (mean 3.5). The follow-up interval ranged between 2.5 and 13 years (mean 7.2). Patient #106, who was 12-years-old at the time of the initial histopathological diagnosis favoring SM with thickness of 3.7 mm, was ANED for 6 years until he was killed in a car accident at the age of 18.13 Patient #108 with a histopathological diagnosis favoring SM with thickness of 3.25 mm had 2 positive SLNs and FISH results showing 3 out of 4 genetic abnormalities in favor of melanoma. The patient was ANED 2.5 years after the initial diagnosis. The other 3 cases from clinical Group 1c (#148, 96 and 112), diagnosed as SN by IMS, had a histopathological diagnosis of ASN with tumor thicknesses of 1.75, 4.0 and 5.0 mm, respectively (mean 3.58). The follow-up period ranged between 4 and 10 years (mean 7.2) and the patients were ANED.
Clinical Group 2 included 4 patients with locoregional disease in non-sentinel lymph nodes (#33, 35, 36 and 93). Two of them, cases #33 and #36, were diagnosed as SM by IMS. A diagnosis of SM for these cases was also favored histopathologically. Patient #36, diagnosed as SM by IMS, had a follow-up of 16 years and was ANED after a single recurrence at the same site 8 years following the original diagnosis. The other 2 patients (#35 and 93) were diagnosed as SN by IMS. Patient #35, a 58-year-old man, whose lesion from the ear (depth 4.25 mm) was originally diagnosed favoring SN histopathologically, was also diagnosed as SN by IMS. This lesion was completely excised but 15 years later there was a recurrence at the site of the primary lesion, which was also excised and diagnosed histopathologically as favoring recurrent SN. A subsequent follow-up for another 10 years (25 years total) did not show any further recurrences or metastases.
Clinical Group 3 comprised 1 patient with distant metastases, case #94. A diagnosis of SM was rendered by IMS and favored histopathologically (depth 2.25 mm). The patient was alive with disease but was lost to follow-up 1 year after the initial diagnosis. In clinical Group 4 were 3 patients with distant metastases and death of disease, cases # 12, 38, 131. All cases were diagnosed as SM by IMS, and a diagnosis of SM was also favored histopathologically. The patient characteristics of the 102 cases together with the IMS diagnosis and clinical outcome are summarized in Table 3.
Table 3.
Clinical and histopathological parameters, histopathological and Imaging Mass Spectrometry diagnosis data for all patients
| # | Patient # | Age (years) | Gender | Location | Histopathological Diagnosis |
Imaging Mass Spec |
Depth (mm) |
Ulceration | Mitotic Rate (per mm2) |
# positive | # positive | Clinical | Clinical Group |
FU | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | SLN/Total | CLD/Total | Status | (years) | |||||||||||
| 1 | 12 | 57 | M | Left ear | SM | SM | 3 | N | 4 | NK | NK | DOD | 4 | 5 | |
| 2 | 131 | 83 | F | Right abdomen | SM | SM | 5 | N | 6 | 4/5 | 14/27 | DOD | 4 | 0.5 | |
| 3 | 38 | 50 | M | Thorax | SM | SM | 2.2 | Y | 1 | DOD | 4 | 3 | |||
| 4 | 94 | 41 | M | Left shin | SM | SM | 2.25 | N | 1 | 1/1 | AWD | 3 | 1 | Lett groin mass ot metastatic MM: lost to follow-up after 1 year | |
| 5 | 33 | 6 | F | Left neck | SM | SM | 2.5 | N | 3 | 1/1 | 8/12 | ANED | 2 | 3 | |
| 6 | 36 | 28 | F | Thigh | SM | SM | 2.1 | Y | 0 | ANED | 2 | 16 | Metastasis in a LN 8 years after primary; aCGH | ||
| 7 | 41 | 39 | F | Left leg | SM | SM | 1.65 | N | 1 | 3/3 | 0/11 | ANED | 1c | 3 | |
| 8 | 100 | 14 | M | Right back | SM | SM | 2.45 | Y | 9 | 1/1 | ANED | 1c | 1 | ||
| 9 | 145 | 23 | F | Left calf | SM | SM | 2 | N | 1 | 2/2 | 0/8 | ANED | 1c | 3 | |
| 10 | 146 | 39 | F | Left leg | SM | SM | 1.9 | N | 1 | 3/3 | 0/11 | ANED | 1c | 3 | Patient had prior MM with 1/1 SLN and 0/18 CLD |
| 11 | 39 | 43 | M | Back | SM | SM | 1 | N | 0 | 0/1 | ANED | 1b | 3 | ||
| 12 | 9 | 29 | F | Right back | SM | SM | 3 | N | 0 | ANED | 1a | 15 | |||
| 13 | 40 | 57 | F | NK | SM | SM | 4.2 | Y | 2 | 0/1 | ANED | 1b | 2 | ||
| 14 | 93 | 64 | M | Back | SM | SN | 5.5 | N | 1 | 0/0 | 2/17 | ANED | 2 | 3 | |
| 15 | 47 | 9 | M | Right upper arm | SM | SN | 3.6 | N | 2 | 1/1 | NK | ANED | 1c | 13 | |
| 16 | 106 | 12 | M | Left lower leg | SM | SN | 3.7 | Y | 3 | 1/1 | 0/NK | DOC | 1c | 6 | Patient died in a car accident at age 18 |
| 17 | 108 | 17 | F | Right foot | SM | SN | 3.25 | N | 3 | 2/2 | 0/21 | ANED | 1c | 2.5 | FISH - 3 of 4 genetic abnormalities consistent with MM |
| 18 | 31 | 15 | F | Left neck | SM | SN | 2.5 | N | 3 | 0/4 | 0/5 | ANED | 1b | 4 | |
| 19 | 42 | 55 | M | Right mid back | SM | SN | 0.6 | N | 0 | 0/1 | ANED | 1b | 2.5 | ||
| 20 | 34 | 59 | F | Right arm | SM | SN | 1.8 | X | 1 | 0/2 | ANED | 1b | 13 | ||
| 21 | 46 | 40 | F | Right anterior leg | SM | SN | 0.5 | N | 0 | 0/1 | ANED | 1b | 11.5 | ||
| 22 | 52 | 38 | M | Middle back | SM | SN | 2.15 | Y | 5 | 0/4 | ANED | 1b | 8 | ||
| 23 | 71 | 17 | M | Chest | SM | SN | 1 | N | 1 | 0/2 | ANED | 1b | 1.5 | ||
| 24 | 118 | 54 | F | Right upper arm | SM | SN | 4 | Y | 10 | 0/7 | ANED | 1b | 10 | ||
| 25 | 122 | 44 | F | Right upper arm | SM | SN | 1.2 | N | 1 | 0/4 | ANED | 1b | 10 | ||
| 26 | 124 | 44 | F | Right buttock | SM | SN | 1.2 | N | 1 | 0/7 | ANED | 1b | 10.5 | ||
| 27 | 128 | 50 | F | Left upper back | SM | SN | 1.4 | N | 1 | 0/2 | ANED | 1b | 2.5 | ||
| 28 | 132 | 65 | M | Left temple | SM | SN | 5.1 | Y | 6 | 0/9 | ANED | 1b | 7 | ||
| 29 | 135 | 69 | M | Abdomen | SM | SN | 1.5 | N | 0 | 0/7 | ANED | 1b | 2.5 | ||
| 30 | 136 | 42 | M | Back | SM | SN | 1.4 | N | 1 | 0/5 | ANED | 1b | 14.5 | ||
| 31 | 137 | 16 | M | Back | SM | SN | 1.42 | N | 2 | 0/3 | ANED | 1b | 13 | ||
| 32 | 139 | 25 | F | Back | SM | SN | 2.75 | N | 1 | 0/1 | ANED | 1b | 9 | ||
| 33 | 8 | 30 | F | Right shin | SM | SN | 1.6 | N | 1 | ANED | 1a | 15 | |||
| 34 | 18 | 57 | M | Right thigh | SM | SN | 3.5 | N | 3 | ANED | 1a | 13 | |||
| 35 | 119 | 75 | F | Left upper back | SM | SN | 2.8 | N | 5 | ANED | 1a | 7 | |||
| 36 | 120 | 34 | M | Left foot | SM | SN | 1.8 | N | 0 | ANED | 1a | 24 | |||
| 37 | 121 | 45 | F | Left upper arm | SM | SN | 2.8 | Y | 4 | ANED | 1a | 14 | |||
| 38 | 96 | 9 | M | Right knee | ASN | SN | 1.75 | Y | 1 | 1/1 | 0/NK | ANED | 1c | 7.5 | |
| 39 | 112 | 9 | M | Right knee | ASN | SN | 4 | N | 1 | 2/5 | 0/7 | ANED | 1c | 10 | |
| 40 | 148 | 13 | M | Back | ASN | SN | 5 | N | 0 | 1/1 | ANED | 1c | 4 | ||
| 41 | 1 | 31 | F | Left anterior thigh | ASN | SN | 2.4 | N | 1 | ANED | 1a | 19 | |||
| 42 | 14 | 25 | F | Right shin | ASN | SN | 1.2 | N | 0 | ANED | 1a | 14 | |||
| 43 | 22 | 34 | F | Right forearm | ASN | SN | 2.6 | N | 1 | ANED | 1a | 12 | BRAF negative by PCR | ||
| 44 | 23 | 31 | F | Left upper back | ASN | SN | 0.9 | N | 0 | ANED | 1a | 11 | |||
| 45 | 25 | 37 | F | Rear | ASN | SN | 3.75 | N | 1 | ANED | 1a | 7 | |||
| 46 | 26 | 39 | M | Right posterior arm | ASN | SN | 525 | N | 1 | ANED | 1a | 7 | |||
| 47 | 101 | 6 | M | Left buttock | ASN | SN | 5.5 | N | 0 | 0/NK | NK | ANED | 1b | 1 | |
| 48 | 102 | 17 | F | Left upper breast | ASN | SN | 4 | N | 0 | 0/NK | NK | ANED | 1b | 1.5 | |
| 49 | 103 | 10 | M | Left foot | ASN | SN | 0.9 | N | 1 | 0/NK | NK | ANED | 1b | 1 | |
| 50 | 109 | 45 | M | Right posterior leg | ASN | SN | 0.7 | N | 0 | 0/4 | ANED | 1b | 2.5 | ||
| 51 | 117 | 43 | M | Left posterior axilla | ASN | SN | 1 | N | 1 | 0/6 | ANED | 1b | 2 | ||
| 52 | 68 | 45 | M | Right calf | ASN | SN | 0.7 | N | 0 | 0/3 | ANED | 1b | 1.5 | ||
| 53 | 60 | 49 | F | Left medial thigh | ASN | SN | 0.9 | N | 2 | 0/1 | 0/NK | ANED | 1b | 5.5 | |
| 54 | 66 | 29 | F | Left buttock | ASN | SN | 1.1 | N | 0 | 0/2 | ANED | 1b | 2 | ||
| 55 | 53 | 35 | F | Left thigh | ASN | SN | 0.6 | N | 0 | ANED | 1a | 7 | |||
| 56 | 54 | 34 | F | Right shin | ASN | SN | 0.82 | N | 4 | ANED | 1a | 6 | |||
| 57 | 55 | 13 | F | Right medial knee | ASN | SN | 0.8 | N | 1 | ANED | 1a | 6 | |||
| 58 | 56 | 22 | F | Left anterior thigh | ASN | SN | 0.4 | N | 1 | ANED | 1a | 7 | |||
| 59 | 57 | 39 | F | Right foot | ASN | SN | 0.4 | N | 0 | ANED | 1a | 6 | |||
| 60 | 58 | 72 | F | Left arm | ASN | SN | 0.9 | N | 1 | ANED | 1a | 7 | |||
| 61 | 59 | 38 | M | Right cheek | ASN | SN | 1.1 | N | 1 | ANED | 1a | 6 | |||
| 62 | 61 | 3 | M | Right cheek | ASN | SN | 1.2 | N | 1 | ANED | 1a | 6 | |||
| 63 | 62 | 30 | F | Right thigh | ASN | SN | 1.7 | N | 2 | ANED | 1a | 5.5 | |||
| 64 | 63 | 26 | F | Right thigh | ASN | SN | 0.75 | N | 0 | ANED | 1a | 6 | |||
| 65 | 64 | 6 | F | Right medial thigh | ASN | SN | 0.4 | N | 0 | ANED | 1a | 5 | |||
| 66 | 65 | 8 | M | Right elbow | ASN | SN | 1.9 | X | 2 | ANED | 1a | 5.5 | |||
| 67 | 81 | 42 | F | Left distal thigh | ASN | SN | 0.5 | N | 0 | ANED | 1a | 7.5 | |||
| 68 | 97 | 48 | F | Right forearm | ASN | SN | 2.25 | N | 1 | ANED | 1a | 5.5 | |||
| 69 | 104 | 3 | M | Right ear (helix) | ASN | SN | 5 | N | 6 | ANED | 1a | 7 | |||
| 70 | 113 | 2 | F | Right elbow | ASN | SN | 1.75 | N | 2 | ANED | 1a | 95 | |||
| 71 | 123 | 43 | F | Left upper arm | ASN | SN | 0.7 | X | 0 | ANED | 1a | 9 | |||
| 72 | 125 | 16 | M | Left ear | ASN | SN | 2.5 | N | 1 | ANED | 1a | 10 | |||
| 73 | 129 | 4 | F | Right thigh | ASN | SN | 1.6 | X | 2 | ANED | 1a | 9 | |||
| 74 | 130 | 39 | F | Right ankle | ASN | SN | 2.12 | N | 2 | ANED | 1a | 5.5 | |||
| 75 | 30 | 7 | M | Scalp | ASN | SN | 4.9 | X | 2 | ANED | 1a | 5 | |||
| 76 | 35 | 58 | M | Ear | SN | SN | 4.25 | N | 0 | ANED | 2 | 25 | Recurrence at original site - 15 years after primary; aCGH | ||
| 77 | 5 | 27 | F | Left shin | SN | SN | 2.6 | Y | 2 | ANED | 1a | 16 | |||
| 78 | 11 | 31 | F | Right posterior thigh | SN | SN | 1.4 | N | 1 | ANED | 1a | 8 | |||
| 79 | 15 | 31 | M | Right posterior calf | SN | SN | 2.5 | N | 0 | ANED | 1a | 14 | |||
| 80 | 16 | 48 | M | Left flank | SN | SN | 0.6 | N | 0 | ANED | 1a | 14 | |||
| 81 | 17 | 9 | M | Right elbow | SN | SN | 1.1 | N | 0 | ANED | 1a | 14 | |||
| 82 | 20 | 48 | M | Left back | SN | SN | 0.7 | X | 0 | ANED | 1a | 13 | |||
| 83 | 21 | 36 | M | Mid back | SN | SN | 1.2 | N | 0 | ANED | 1a | 7 | |||
| 84 | 24 | 53 | F | Right cheek | SN | SN | 1.5 | N | 1 | ANED | 1a | 11 | |||
| 85 | 27 | 15 | M | Left shin | SN | SN | 1.8 | N | 1 | ANED | 1a | 14 | |||
| 86 | 28 | 16 | M | Right canthus | SN | SN | 1.55 | N | 1 | ANED | 1a | 6 | |||
| 87 | 32 | 14 | M | Right upper arm | SN | SN | 3.9 | N | 1 | ANED | 1a | 9 | |||
| 88 | 37 | 28 | F | Breast | SN | SN | 4 | N | 0 | ANED | 1a | 12 | aCGH | ||
| 89 | 48 | 55 | F | Left upper back | SN | SN | 1 | N | 0 | ANED | 1a | 8 | |||
| 90 | 49 | 33 | M | Back | SN | SN | 0.7 | X | 0 | ANED | 1a | 8 | |||
| 91 | 45 | 13 | M | Right upper back | SN | SN | 0.75 | N | 1 | ANED | 1a | 7.5 | |||
| 92 | 50 | 14 | M | Left arm | SN | SN | 0.9 | N | 0 | ANED | 1a | 8.5 | |||
| 93 | 51 | 11 | M | Posterior neck | SN | SN | 1.9 | X | 1 | ANED | 1a | 8 | |||
| 94 | 82 | 45 | M | Left posterior shoulder | SN | SN | 1.4 | N | 0 | ANED | 1a | 6.5 | |||
| 95 | 83 | 62 | F | Right upper back | SN | SN | 0.7 | N | 0 | ANED | 1a | 5.5 | |||
| 96 | 87 | 22 | F | Left knee | SN | SN | 0.8 | X | 0 | ANED | 1a | 5.5 | |||
| 97 | 88 | 43 | M | Left scalp | SN | SN | 1.75 | N | 0 | ANED | 1a | 5.5 | |||
| 98 | 89 | 25 | F | Left posterior thigh | SN | SN | 2.5 | N | 0 | ANED | 1a | 5 | |||
| 99 | 90 | 23 | F | Right thigh | SN | SN | 0.4 | N | 0 | ANED | 1a | 5 | |||
| 100 | 91 | 33 | M | Back | SN | SN | 2.1 | N | 1 | ANED | 1a | 5 | |||
| 101 | 110 | 17 | M | Right buttock | SN | SN | 5 | X | 4 | ANED | 1a | 5.5 | |||
| 102 | 10 | 31 | F | Right thigh | SN | SM | 0.7 | N | 0 | ANED | 1a | 15 |
ANED-alive with no evidence of disease; DOD-dead of disease; AWD-alive with disease; DOC-dead of another cause; NK-not known; SN-Spitz nevus; ASN-Atypical Spitzoid Neoplasm; SM-Spitzoid melanoma; SLN-sentinel lymph node; CLD-completion lymphadenectomy; MM-malignant melanoma; PCR-polymerase chain reaction; aCGH-array comparative genomic hybridization; FISH-fluorescent in situ hybridization; BRAF-a human gene that makes a protein called b-RAF
The p-value comparing the frequency of IMS SM diagnosis in Group 1 versus Groups 2-4 by the Fisher's exact test was p<0.0001, indicating a very strong association between the IMS diagnosis of SM and poor clinical outcome based on clinical follow-up. We also compared the cases in Groups 1 and 2 against those in Groups 3 and 4. The Fisher's exact test showed statistically significant difference with p=0.0002. In this comparison there was also a strong association between the IMS diagnosis of SM and clinical category. For comparison, the association of histopathological diagnosis of SM and the clinical category was also statistically significant but not as strong (p=0.001 for the comparison of Group 1 vs. Groups 2-4, p=0.02 for the comparison of Groups 1 and 2 vs. Groups 3 and 4).
In 13 cases, SM was the diagnosis rendered by IMS and favored on histopathological analysis (Table 3). In 10 out of these 13 cases (77%), there were adverse events including SLN metastasis, positive non-sentinel lymph nodes, recurrence, distant metastases and/or death (Figure 1). In 24 cases, while the histopathological examination favored SM, the IMS diagnosis was SN and the patients had no adverse clinical outcome (Figure 2). In 38 cases the histopathological diagnosis was ASN (without favoring either SN or SM) while the IMS diagnosis was SN. The clinical behavior in all these cases was benign (Figure 3). In 26 cases, SN was favored histopathologically and a diagnosis of SN was also rendered by IMS and all of these patients were ANED (Figure 4).
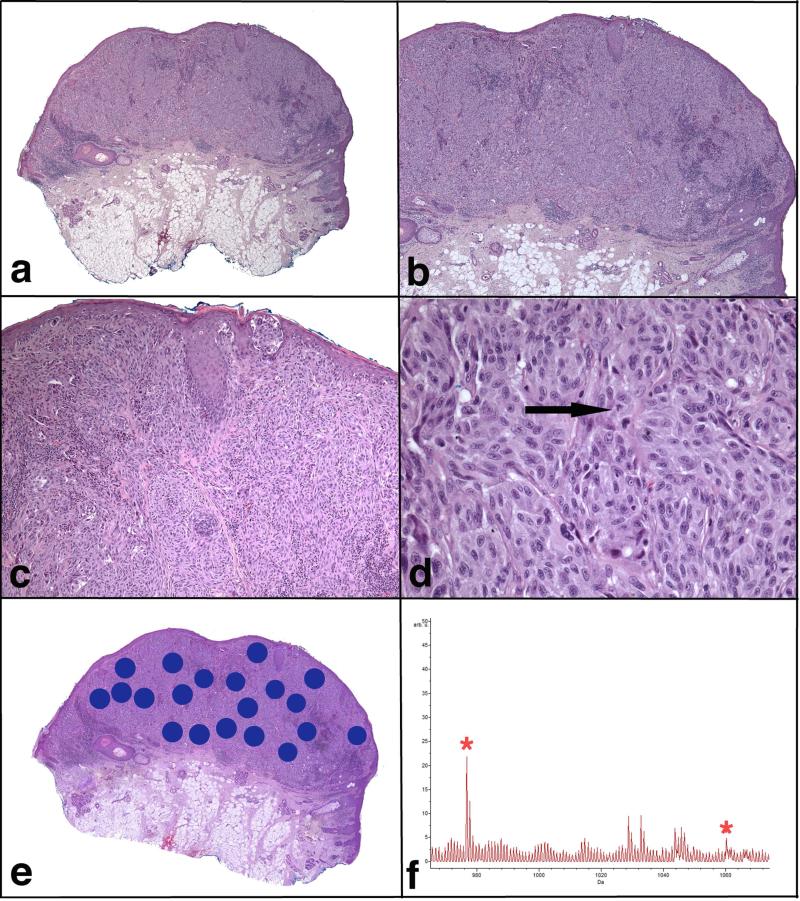
FIGURE 1.
Spitzoid melanoma. Patient #12, a 57-year-old man with a lesion from the left ear, which was diagnosed histopathologically as SM with 3.0 mm tumor thickness, was also classified as SM by IMS. This patient (clinical Group 4) had distant metastases and was dead of disease 5 years after the diagnosis.
a) Low magnification microphotograph showing a large asymmetric lesion. b) There is an expansile proliferation of melanocytes with pushing borders, involving the reticular dermis and extending close to the subcutaneous fat. c) Sheet-like growth pattern of the melanocytes is seen in the dermis. There is focal junctional component. d) The melanocytes are large, pleomorphic and with abundant eosinophilic cytoplasm. Mitotic figures are easily identified (arrow). e) A scanned image of the specimen containing areas marked for Imaging Mass Spectrometry analysis. f) Average spectrum of the selected m/z range 965-1075. Two peaks at m/z 976.5 and m/z 1060.2, which are part of the classifier, are marked by *.
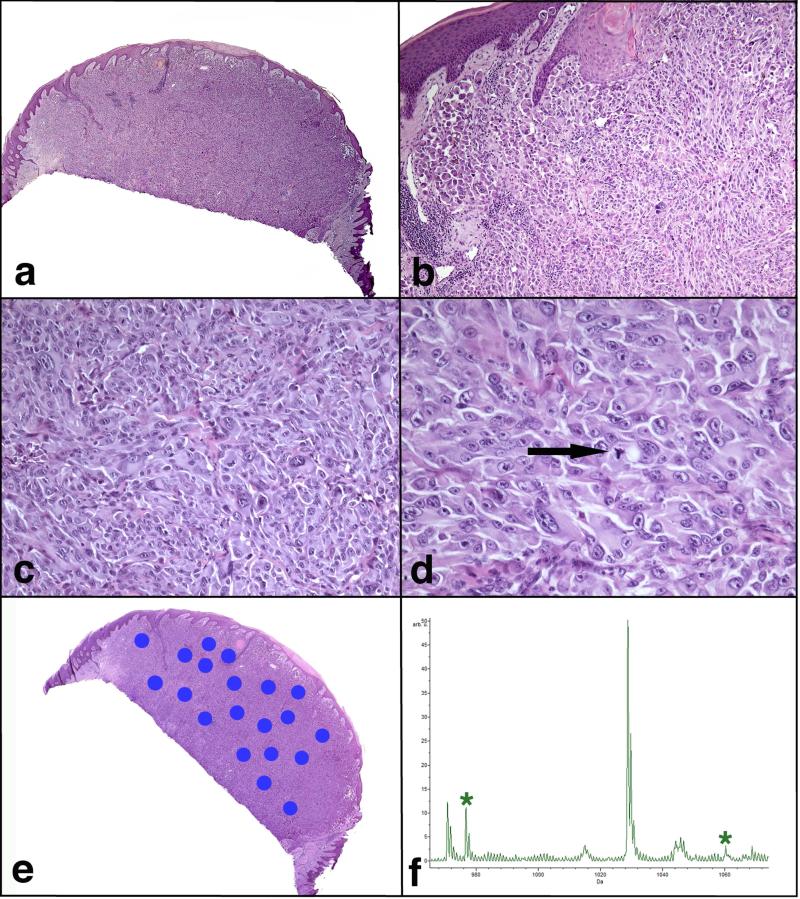
FIGURE 2.
Atypical Spitzoid Neoplasm diagnosed as Spitzoid melanoma by histopathological examination and as Spitz nevus by IMS. Patient #106, a 12-year-old boy with a nodule on the left lower leg, was rendered a histopathological diagnosis of SM with a tumor thickness of 3.7 mm. The lesion was ulcerated and there were 3 mitotic figures per mm2. This lesion was classified as SN by IMS. This patient (clinical Group 1c) had 1/1 SLN positive and no positive lymph nodes on completion lymphadenectomy. The patient was alive and free of disease 6 years after the diagnosis. He died in a car accident at the age of 18. a) Large nodular and poorly defined melanocytic lesion. b) There is mostly intradermal melanocytic proliferation with only focal junctional component. The epidermis is hyperplastic and shows hypergranulosis and hyperkeratosis. c) The proliferation is dense, forming sheets of melanocytes. d) The melanocytes are large, pleomorphic, with vesicular nuclei, prominent purple nucleoli and abundant eosinophilic cytoplasm. Mitotic figures are noted throughout the lesion (arrow). e) A scanned image of the specimen containing areas marked for Imaging Mass Spectrometry analysis. f) Average spectrum of the selected m/z range 965-1075. Two peaks at m/z 976.5 and m/z 1060.2, which are part of the classifier, are marked by *.
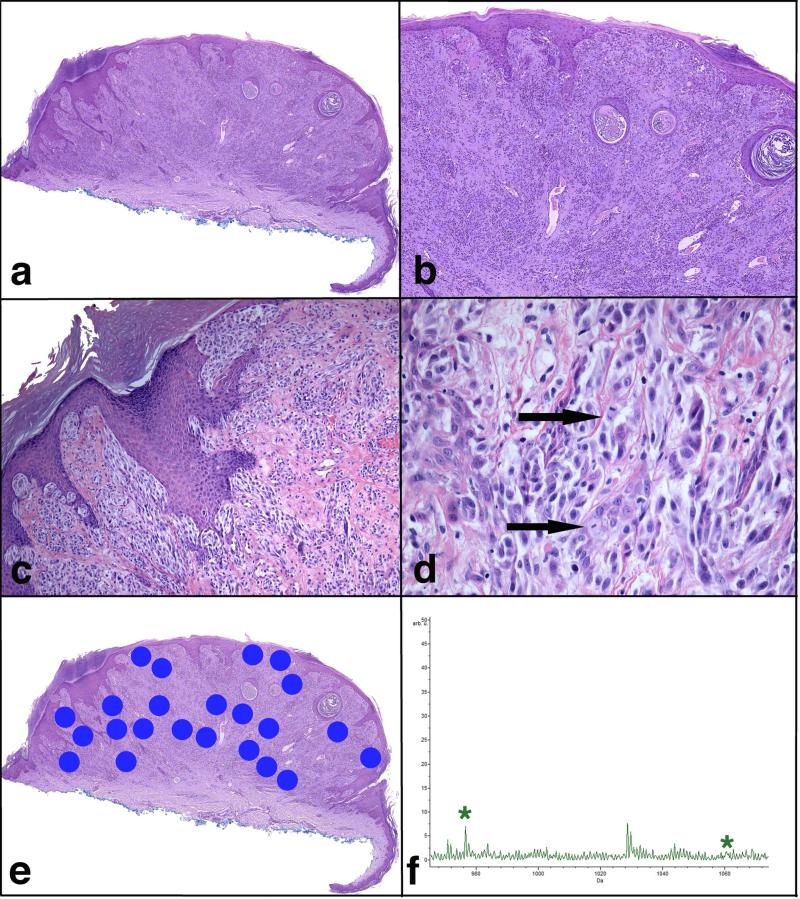
FIGURE 3.
Atypical Spitzoid Neoplasm with a diagnosis of ASN by histopathological examination and of Spitz nevus by IMS. Patient #97 is a 48-year-old woman with a lesion from the right volar forearm diagnosed histopathologically as ASN with tumor thickness of 2.25 mm and 1 mitosis per mm2. The lesion was classified as SN by IMS. This patient (clinical Group 1a) was alive and free of disease 5.5 years after the diagnosis. a) Broad and asymmetric proliferation of melanocytes. b) The epidermis is hyperplastic and shows hypergranulosis and hyperkeratosis. The majority of the lesion is intradermal and shows nests and fascicles of melanocytes. c) There are irregular nests of melanocytes in the epidermis, which vary in size and shape and are not equidistant from one another. Melanocytic nests are also seen down adnexal epithelium and in the dermis. d) The melanocytes are large, pleomorphic, some with hyperchromatic nuclei and abundant pale cytoplasm. Two mitotic figures are designated by arrows. e) A scanned image of the specimen containing areas marked for Imaging Mass Spectrometry analysis. f) Average spectrum of the selected m/z range 965-1075. Two peaks at m/z 976.5 and m/z 1060.2, which are part of the classifier, are marked by *.
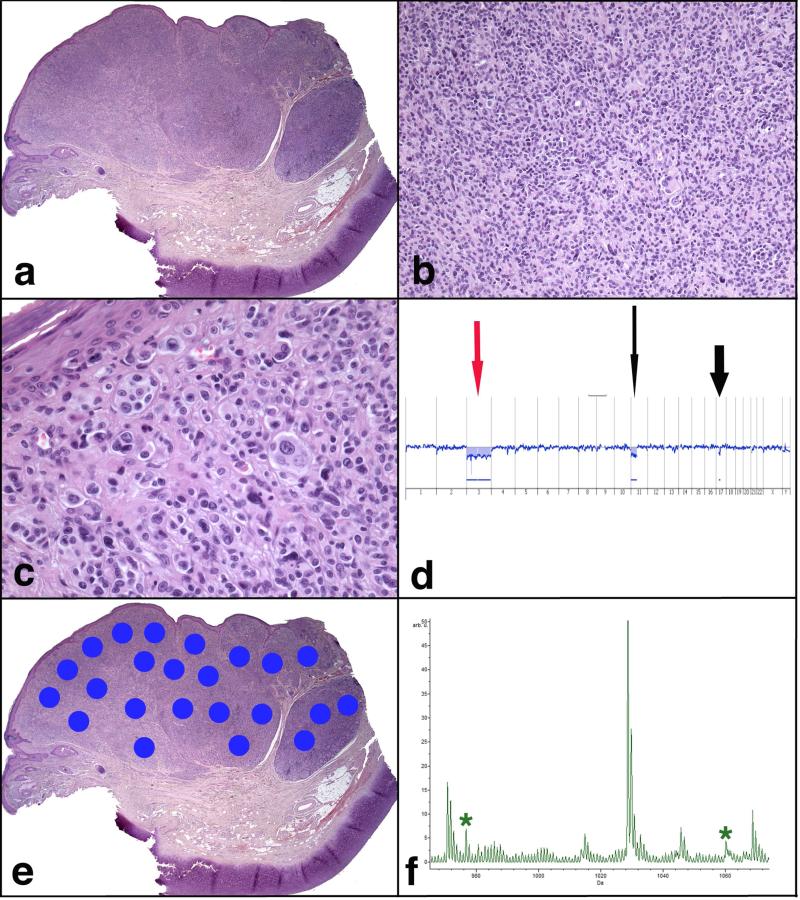
FIGURE 4.
Atypical Spitzoid Neoplasm diagnosed as Spitz nevus by both, histopathological examination and IMS. Patient #35 (clinical Group 2), a 58-year-old man, with a lesion from the ear diagnosed as favoring SN on histopathological assessment, was also diagnosed as SN by IMS. This lesion was completely excised. Fifteen years later there was a recurrence at the site of the primary lesion, which was also excised and diagnosed histopathologically as favoring a recurrent SN. Subsequent follow-up for another 10 years (25 years total) did not show any further recurrences or metastases. a) Large, asymmetric and somewhat multi-lobular melanocytic lesion. b) Very dense, sheet – like proliferation of melanocytes in the dermis. c) Single large melanocytes are seen at the dermal epidermal junction. In the underlying dermis there are large pleomorphic melanocytes with vesicular nuclei and abundant eosinophilic cytoplasm, focally forming small nests. d) An aCGH analysis shows loss of chromosome 3 (red arrow) and partial loss of chromosome 11p (long black arrow) and 17p (short black arrow), findings not entirely sufficient to support the diagnosis of melanoma. e) A scanned image of the specimen containing areas marked for Imaging Mass Spectrometry analysis. f) Average spectrum of the selected m/z range 965-1075. Two peaks at m/z 976.5 and m/z 1060.2, which are part of the classifier, are marked by *.
Discussion
The ‘gray’ category of ASNs is artificial and relatively broad category.2-4,14-17 Currently, based on histopathological examination and using the available histopathological criteria, we cannot categorize these cases with certainty and thus predict their behavior.1,8,11,18-22 IMS is a bio-analytical method for identifying the nature and spatial distribution of metabolites, peptides and proteins, DNA segments and lipids from tissue samples. It has been used to clarify the molecular signatures of various types of cancer and other diseases.23-26 Molecular signatures usually present as a unique combination of 5-20 proteins and enable a specific diagnosis.26 Protein and peptide analysis is superior to gene expression analysis, as it represents the actual functional state of the disease/tumor rather than the potential risk of developing it. Furthermore, post-translational modification is thus also taken into account. In a recent IMS study, we identified differences on proteomic level between SN and SM.6 Five peptides, comprising a specific proteomic signature, were differentially expressed by the melanocytic component of SN and SM in formalin-fixed, paraffin-embedded tissue samples.
In this study we have analyzed 102 ASNs, for some of which the original pathologist(s) favored SM histopathologically, while in others SN was favored. In a third group neither SN nor SM was favored histopathologically and the diagnosis remained as ASN. Clinical Groups 2, 3 and 4 included patients with “adverse” clinical outcome. All 4 patients with advanced locoregional disease, distant metastases, and/or death (in Groups 3 and 4) were correctly diagnosed as SM by IMS. The patients in Group 4 were dead of disease and the patient in Group 3 was alive with disease but lost to follow-up 1 year after diagnosis. In clinical Group 1c, 6/10 cases were diagnosed as SN by IMS. Three of these patients had a histopathological diagnosis favoring SM and the other 3 of ASN. The lesions had tumor thicknesses between 1.75 and 5.0 mm (mean 3.5 mm). The patients were ANED at a follow-up ranging between 2.5 and 13 years (mean 7.2). In comparison, patients with melanoma with similar thicknesses would have had a predicted lesser survival rate and disease free interval. This suggests that there may be some characteristics on molecular level that are detected by mass spectrometry but are “invisible” to standard histopathology, thus a better predictor of clinical behavior.10
In 13 cases SM was favored on histopathological assessment and a diagnosis of SM was rendered by IMS. All of these patients with the exception of 3 patients had either a positive SLN, positive nodes in completion lymphadenectomy, recurrence, distant metastases and/or death. In 24 cases, in which SM was favored histopathologically, IMS rendered a diagnosis of SN, which correlated well with their benign clinical behavior. In additional 38 cases, diagnosed histopathologically as ASN, IMS rendered a diagnosis of SN and the clinical outcome was also benign. In these 62 cases in total, the histopathological diagnosis was either inconclusive or favored SM while IMS classified the lesions as benign SN. All of these cases had benign clinical behavior with a mean follow-up of 7.6 years. IMS appeared to be more accurate in predicting the benign character of ASNs than histopathology and correlated better with their clinical behavior. Histopathology appeared to have a tendency to overdiagnose either atypical features or malignancy. In the final group of 26 patients, in which SN was favored histopathologically, the same diagnosis was rendered by IMS. All patients were ANED with a follow-up between 5 and 16 years.
We found a strong association between the diagnosis of SM by IMS and an adverse clinical outcome when clinical Group 1 was compared to Groups 2, 3 and 4. Of different melanoma prognostic factors that we compared, only older age and greater tumor thickness were strongly associated with adverse clinical behavior and poorer outcome. IMS diagnosis showed stronger association with clinical outcome than did the histopathological diagnosis by Fisher's exact test. In addition, the diagnosis of SM by IMS was statistically strongly associated with adverse clinical behavior. IMS analysis using a proteomic signature may be able to provide reliable diagnosis as well as clinically useful and statistically significant risk assessment of ASNs, beyond the information provided by histology and other ancillary techniques.
Capsule summary.
Spitzoid lesions may be very challenging and represent one of the most difficult areas in Dermatopathology
There is a strong association between the diagnosis of Spitzoid melanoma by Imaging Mass Spectrometry and adverse clinical outcome
Imaging Mass Spectrometry analysis may be helpful in the diagnosis of difficult Atypical Spitzoid Neoplasms
Acknowledgments
Funding: Supported in part by a grant: NIH/NIGMS 5P41 GM103391-05
Abbreviation and acronym list
- IMS
Imaging Mass Spectrometry
- SN
Spitz nevus
- SM
Spitzoid melanoma
- ASN
Atypical Spitzoid Neoplasm
- SLN
sentinel lymph node
- FU
follow – up
- CGH
comparative genomic hybridization
- aCGH
array comparative genomic hybridization
- FISH
fluorescent in situ hybridization
- MALDI
matrix-assisted laser desorption ionization
- ANED
alive with no evidence of disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
The data has not been presented previously.
References
- 1.Miteva M, Lazova R. Spitz nevus and atypical spitzoid neoplasm. Semin Cutan Med Surg. 2010;29:165–73. doi: 10.1016/j.sder.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Farmer ER, Gonin R, Hanna MP. Discordance in the histopathologic diagnosis of melanoma and melanocytic nevi between expert pathologists. Hum Pathol. 1996;27:528–31. doi: 10.1016/s0046-8177(96)90157-4. [DOI] [PubMed] [Google Scholar]
- 3.Ackerman AB. Discordance among expert pathologists in diagnosis of melanocytic neoplasms. Hum Pathol. 1996;27:1115–6. doi: 10.1016/s0046-8177(96)90301-9. [DOI] [PubMed] [Google Scholar]
- 4.Barnhill RL, Argenyi ZB, From L, Glass LF, Maize JC, Mihm MC, Jr., et al. Atypical Spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum Pathol. 1999;30:513–20. doi: 10.1016/s0046-8177(99)90193-4. [DOI] [PubMed] [Google Scholar]
- 5.Cerroni L, Barnhill R, Elder D, Gottlieb G, Heenan P, Kutzner H, et al. Melanocytic tumors of uncertain malignant potential: results of a tutorial held at the XXIX Symposium of the International Society of Dermatopathology in Graz, October 2008. Am J Surg Pathol. 2010;34:314–26. doi: 10.1097/PAS.0b013e3181cf7fa0. [DOI] [PubMed] [Google Scholar]
- 6.Lazova R, Seeley EH, Keenan M, Gueorguieva R, Caprioli RM. Imaging mass spectrometry--a new and promising method to differentiate Spitz nevi from Spitzoid malignant melanomas. Am J Dermatopathol. 2012;34:82–90. doi: 10.1097/DAD.0b013e31823df1e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo S, Sepehr A, Tsao H. Spitz nevi and other Spitzoid lesions part I. Background and diagnoses. J Am Acad Dermatol. 2011;65:1073–84. doi: 10.1016/j.jaad.2011.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo S, Sepehr A, Tsao H. Spitz nevi and other Spitzoid lesions part II. Natural history and management. J Am Acad Dermatol. 2011;65:1087–92. doi: 10.1016/j.jaad.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnhill RL, Flotte TJ, Fleischli M, Perez-Atayde A. Cutaneous melanoma and atypical Spitz tumors in childhood. Cancer. 1995;76:1833–45. doi: 10.1002/1097-0142(19951115)76:10<1833::aid-cncr2820761024>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerami P, Scolyer RA, Xu X, Elder DE, Abraham RM, Fullen D, et al. Risk assessment for atypical spitzoid melanocytic neoplasms using FISH to identify chromosomal copy number aberrations. Am J Surg Pathol. 2013;37:676–84. doi: 10.1097/PAS.0b013e3182753de6. [DOI] [PubMed] [Google Scholar]
- 12.Aerni HR, Cornett DS, Caprioli RM. Automated acoustic matrix deposition for MALDI sample preparation. Analytical chemistry. 2006;78:827–34. doi: 10.1021/ac051534r. [DOI] [PubMed] [Google Scholar]
- 13.Powers JG, Boyd AS. What is your diagnosis? Spitzoid melanoma. Cutis. 2012;90:180, 7–8. [PubMed] [Google Scholar]
- 14.Lallas A, Kyrgidis A, Ferrara G, Kittler H, Apalla Z, Castagnetti F, et al. Atypical Spitz tumours and sentinel lymph node biopsy: a systematic review. Lancet Oncol. 2014;15:e178–83. doi: 10.1016/S1470-2045(13)70608-9. [DOI] [PubMed] [Google Scholar]
- 15.Tom WL, Hsu JW, Eichenfield LF, Friedlander SF. Pediatric “STUMP” lesions: evaluation and management of difficult atypical Spitzoid lesions in children. J Am Acad Dermatol. 2011;64:559–72. doi: 10.1016/j.jaad.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 16.Zedek DC, McCalmont TH. Spitz nevi, atypical spitzoid neoplasms, and spitzoid melanoma. Clin Lab Med. 2011;31:311–20. doi: 10.1016/j.cll.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara G, Argenziano G, Soyer HP, Chimenti S, Di Blasi A, Pellacani G, et al. The spectrum of Spitz nevi: a clinicopathologic study of 83 cases. Arch Dermatol. 2005;141:1381–7. doi: 10.1001/archderm.141.11.1381. [DOI] [PubMed] [Google Scholar]
- 18.Paradela S, Fonseca E, Pita-Fernandez S, Prieto VG. Spitzoid and non-spitzoid melanoma in children: a prognostic comparative study. J Eur Acad Dermatol Venereol. 2013;27:1214–21. doi: 10.1111/j.1468-3083.2012.04686.x. [DOI] [PubMed] [Google Scholar]
- 19.Lott JP, Wititsuwannakul J, Lee JJ, Ariyan S, Narayan D, Kluger HH, et al. Clinical characteristics associated with Spitz nevi and Spitzoid malignant melanomas: the Yale University Spitzoid Neoplasm Repository experience, 1991 to 2008. J Am Acad Dermatol. 2014;71:1077–82. doi: 10.1016/j.jaad.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Requena C, Requena L, Kutzner H, Sanchez Yus E. Spitz nevus: a clinicopathological study of 349 cases. Am J Dermatopathol. 2009;31:107–16. doi: 10.1097/DAD.0b013e3181934218. [DOI] [PubMed] [Google Scholar]
- 21.Hafiji J, Rytina E, Burrows NP. The spectrum of spitzoid tumours: A clinical study. Australas J Dermatol. 2012;53:211–5. doi: 10.1111/j.1440-0960.2012.00902.x. [DOI] [PubMed] [Google Scholar]
- 22.Ferrara G, Gianotti R, Cavicchini S, Salviato T, Zalaudek I, Argenziano G. Spitz nevus, Spitz tumor, and spitzoid melanoma: a comprehensive clinicopathologic overview. Dermatol Clin. 2013;31:589–98, viii. doi: 10.1016/j.det.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Yanagisawa K, Shyr Y, Xu BJ, Massion PP, Larsen PH, White BC, et al. Proteomic patterns of tumour subsets in non-small-cell lung cancer. Lancet. 2003;362:433–9. doi: 10.1016/S0140-6736(03)14068-8. [DOI] [PubMed] [Google Scholar]
- 24.Cornett DS, Mobley JA, Dias EC, Andersson M, Arteaga CL, Sanders ME, et al. A novel histology-directed strategy for MALDI-MS tissue profiling that improves throughput and cellular specificity in human breast cancer. Mol Cell Proteomics. 2006;5:1975–83. doi: 10.1074/mcp.M600119-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Conrad DH, Goyette J, Thomas PS. Proteomics as a method for early detection of cancer: a review of proteomics, exhaled breath condensate, and lung cancer screening. J Gen Intern Med. 2008;23(Suppl 1):78–84. doi: 10.1007/s11606-007-0411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nimesh S, Mohottalage S, Vincent R, Kumarathasan P. Current status and future perspectives of mass spectrometry imaging. Int J Mol Sci. 2013;14:11277–301. doi: 10.3390/ijms140611277. [DOI] [PMC free article] [PubMed] [Google Scholar]