Abstract
The extrafollicular (EF) plasmablast response to self-antigens that contain Toll-like receptor (TLR) ligands is prominent in murine lupus models and some bacterial infections, but the inhibitors and activators involved have not been fully delineated. Here, we used two conventional dendritic cell (cDC) depletion systems to investigate the role of cDCs on a classical TLR-dependent autoreactive EF response elicited in rheumatoid-factor B cells by DNA-containing immune complexes. Contrary to our hypothesis, cDC depletion amplified rather than dampened the EF response in Fas-intact but not Fas-deficient mice. Further, we demonstrated that cDC-dependent regulation requires Fas and Fas ligand (FasL) expression by T cells, but not Fas expression by B cells. Thus, cDCs activate FasL-expressing T cells that regulate Fas-expressing extrafollicular helper T cells (Tefh). These studies reveal a regulatory role for cDCs in B cell plasmablast responses and provide a mechanistic explanation for the excess autoantibody production observed in Fas-deficiency.
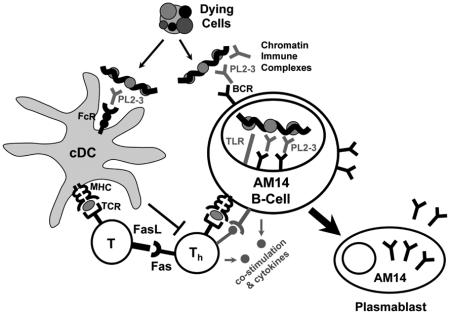
Graphical abstract
INTRODUCTION
B cells are pivotal in the development of systemic autoimmune diseases, which are characterized by the selective loss of tolerance to self-Ags such as DNA, RNA, and IgG (Shlomchik, 2009). In addition to autoantibody-mediated damage, B cells promote disease by stimulating T cell activation and expansion, presumably via antigen (Ag) presentation, and possibly through cytokine secretion (Shlomchik, 2009). Autoreactive B cells are often activated in extrafollicular (EF) foci, via co-ligation of B cell receptors (BCRs) and toll-like receptors (TLRs) by antigens (Ags) containing DNA and RNA. These autoreactive, EF-localized, TLR-driven B cell responses consist of short-lived plasmablasts, as found in MRL/MpJ-Faslpr/J (MRL.Faslpr), and other autoimmune mouse models, such as NZB/W, as well as in human disease (Daridon et al., 2012; Hoyer et al., 2004; Tipton et al., 2015). The EF response is not restricted to autoimmunity, and perhaps evolved to provide pathogen-specific protection. Indeed, EF B cell foci develop in response to various types of T-dependent (TD) and T-independent (TI) Ags, including viruses and bacteria (Cunningham et al., 2007; Di Niro et al., 2015; Maclennan et al., 2003). Such pathogen-induced EF responses are normally transient, indicating that they are tightly regulated. However, in the case of autoimmunity, EF responses persist for the life of the animal, becoming sites of extensive and prolonged clonal expansion, hypermutation, and affinity maturation (William et al., 2002; William et al., 2005b). How normal EF responses become dysregulated in autoimmunity remains unclear.
To study how autoreactive EF B cell responses are regulated, we have used mice transgenic (Tg)—either ectopically inserted or site-directed (sd)—for an immunoglobulin (Ig) heavy chain encoding the anti-IgG2aa rheumatoid factor (RF), AM14 (Sweet et al., 2010). In autoimmune prone MRL.Faslpr and MRL/MpJ mice, AM14 B cells spontaneously expand, class switch, hypermutate and develop into antibody forming cells (AFCs) within EF foci in the spleen (Sweet et al., 2010; William et al., 2002; William et al., 2005b). This spontaneous, TLR-dependent autoactivation occurs in vivo by AM14 BCR binding to DNA- and RNA-containing immune complexes (ICs) (Herlands et al., 2008). We can also induce EF AM14 activation on the BALB/c background by administration of a model physiologic ligand, the IgG2aa anti-chromatin antibody, PL2-3 (Herlands et al., 2007), which results in a plasmablast response that is phenotypically and histologically indistinguishable from the spontaneous model. Like the spontaneous RF and anti-nuclear response, the PL2-3-induced response is TLR7, TLR9, and MyD88 dependent, as well as autoantigen-specific (Herlands et al., 2008). In contrast to the unpredictable onset and variable magnitude of the spontaneous system, the PL2-3 induced response enables precise timing and a directed approach for determination of proximal factors required for autoreactive EF B cell activation. Thus, the PL2-3 induced response has been used by a number of labs to study how a classical autoreactive B cell response is induced (Giltiay et al., 2013; Nundel et al., 2015; Sang et al., 2014).
We initially hypothesized that cDCs are critical supporting cells for the EF response, in part because cDCs are prominent components of such EF foci (Maclennan et al., 2003; William et al., 2002). An activating role for cDCs in the EF response has been inferred from experiments in which anti-CD40 was used to induce cDC proliferation, and appeared to thereby increase EF plasmablast survival in response to a TI-2 Ag (Maclennan et al., 2003). Although cDCs are best known for their interactions with T cells, a small body of literature indicates that cDCs can promote B cell activation. cDCs secrete factors, such as B cell activating factor (BAFF), a proliferation inducing ligand (APRIL), and interleukin-6 (IL-6), which support B cell development, activation and differentiation (MacLennan and Vinuesa, 2002; Mohr et al., 2009). cDCs have also been shown to deliver Ag to, and directly interact with, B cells in lymph nodes (Gonzalez et al., 2010; Qi et al., 2006), and in the spleen (Balázs et al., 2002), thereby promoting B cell activation and the development of humoral immunity. In particular, via a non-degradative Ag uptake and processing pathway, cDCs can present whole Ag on their cell surface to B cells (Bergtold et al., 2005; Zelenay et al., 2012). Although these data suggest that cDCs might aid B cell responses, a more direct cDC ablation study has shown that cDCs are not required for the generation of an EF TI-2 response to 4-hydroxy-3-nitrophenylacetyl (NP)-Ficoll (Hebel et al., 2006), calling into question earlier conclusions that DCs played nonredundant roles.
The autoreactive response to nucleic acids and to the ICs containing them has characteristics of TI-1, TI-2 (Herlands et al., 2008), and TD responses (Sweet et al., 2011). Such nucleic acid-driven responses are therefore likely to be regulated differently from TI-2 responses. In addition, chromatin-containing immune complexes (ICs) have a distinctive capacity to stimulate myeloid cells through both the Fc gamma receptor (FcγR) and TLRs (Boulé et al., 2004). To ask whether cDCs are required for the activation of this type of autoreactive B cell response, we employed both acute (Jung et al., 2002) and constitutive (Birnberg et al., 2008; Teichmann et al., 2010) cDC ablation systems. Both cDC-depletion systems yielded the finding that cDCs negatively regulate, rather than activate, this autoreactive B cell response. Furthermore, we found that this cDC regulatory effect was dependent on Fas and was not observed in Fas-deficiency. We further determined that cDC-dependent regulation of the EF response required Fas and FasL expression by T cells; whereas, Fas expression on B cells was not relevant for this effect. We thus propose that cDCs activate FasL-expressing T cells to regulate Fas-expressing T cells that, in turn, help B cells in EF sites. In addition to revealing a clear regulatory role for cDCs, these studies elucidate an additional mechanism whereby Fas controls autoreactive EF plasmablast responses, helping explain how Fas-deficiency promotes hypergammaglobulinemia, high autoantibody titers, and autoimmunity.
RESULTS
The EF AM14 Rheumatoid Factor Response Does Not Acutely Require, but is Regulated by, cDCs
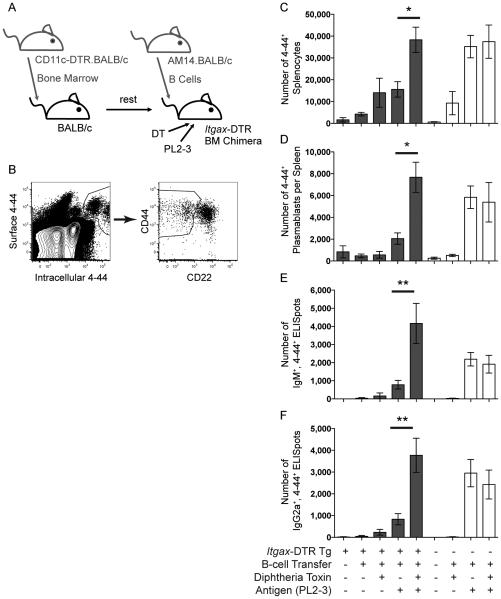
To determine whether cDCs are required for the generation of the EF AM14 rheumatoid factor B cell response, we made radiation chimeras with bone marrow (BM) from mice expressing the diphtheria toxin (DT) receptor (DTR) and green fluorescent protein (GFP) under a Itgax promoter (Itgax-DTR) (Jung et al., 2002), administered DT to deplete cDCs, transferred in sd-Tg AM14 B cells, and injected the activating Ag, PL2-3 (schema in Figure S1). Repeated DT treatment is well tolerated in BM chimeras (Zammit et al., 2005). Because the transferred AM14 B cells do not carry the Itgax-DTR locus, CD11clo AM14 plasmablasts are not deleted by DT in this system (Hebel et al., 2006). The AM14 cells with rheumatoid factor specificity were tracked by ELISpot analysis and flow cytometry using the anti-idiotype marker, 4-44.
Notably, we observed 3, 5, 4 and 7-fold increases in the numbers of 4-44+ AM14 cells, CD22lo CD44hi plasmablasts, IgM and IgG2a antibody forming cells (AFCs), respectively, in cDC-depleted hosts as compared to controls (Figure 1). The location of the response in the cDC-depleted animals was EF (Figure S2), similar to that observed in untreated controls, and as reported (Herlands et al., 2008; Herlands et al., 2007; Sweet et al., 2011; William et al., 2002). Few, if any 4-44+ peanut agglutinin (PNA)+ germinal centers (GCs) could be detected by histology at day 6 (not depicted). These data indicate that cDCs are not required for, and—given the several-fold enhancement in their absence—may regulate, the EF AM14 response.
Figure 1. The EF AM14 Rheumatoid Factor Response does not Acutely Require, but is Regulated by cDCs.
(A) Experiment design. Itgax-DTR.BALB/c or Tg-negative littermates were used to make BM chimeras. cDCs were depleted with DT on days -1, -2, 1, 3 and 5. 9-10 × 106 AM14.BALB/c B cells were transferred into the mice on day 0, and activated with PL2-3 on days 0, 2 and 4. Spleens were harvested on day 6.
(B) Representative flow cytometry gating of live AM14 B cells by surface and intracellular 4-44 (anti-idiotype) staining, and further subgating for CD22lo, CD44hi plasmablasts.
(C) Number of AM14 B cells (surface and intracellular 4-44+) per spleen.
(D) Number of AM14 plasmablasts (4-44+, CD22lo, CD44hi) per spleen.
(E & F) ELISpot assays for the number of 4-44+, IgM+ or IgG2a+ AFCs per spleen. Data were combined from 2 experiments to obtain 4-8 mice per group. Bars represent mean and SEM. *p < 0.05; **p < 0.01 by ANOVA. See also Figures S1 and S2.
cDCs are not Required Constitutively for the AM14 Response
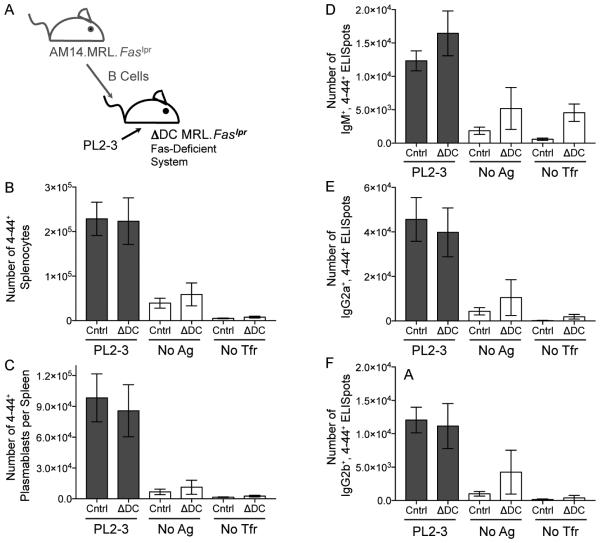
Although acute depletion of cDCs suggested that they are not required for the activation of AM14 B cells, it remained possible that essential cytokines produced by cDCs are retained in the extracellular matrix (Mohr et al., 2009), in which case acute cDC depletion would not reveal an effect of cDCs on B cell activation. We were also concerned that acute local DT-induced cDC death could provide an additional source of chromatin Ag, that could account for the increased AM14 response observed in the acute cDC ablation system. To address these issues, we generated recipient mice constitutively lacking cDCs by crossing mice expressing the Rosa26-flox-stop-DTA locus (Ivanova et al., 2005) to mice expressing Itgax-Cre (Caton et al., 2007), which we call ΔDC mice. We had both strains of these mice fully backcrossed onto the MRL.Faslpr background (Teichmann et al., 2010), which were suitable for our studies, because we used young, pre-diseased animals, as previously reported (Herlands et al., 2007). As had been shown for ΔDC mice, there was extensive depletion of cDCs and a substantial loss of pDCs as well as increased CD11b+ cells (Birnberg et al., 2008; Ohnmacht et al., 2009; Teichmann et al., 2010) (Figure S3). We transferred sd-Tg AM14.MRL. Faslpr B cells into these recipients on day 0, administered the activating Ag, PL2-3 on days 0, 2 and 4, and assessed the response on day 6. In contrast to our acute ablation experiments in BALB/c mice, EF AM14 B cell responses in ΔDC MRL.Faslpr mice were similar to those of control recipients (Figure 2). These results confirm that cDCs are not required either acutely or constitutively for the activation of the autoreactive AM14 B cell response. That cDCs were not required for the EF response was somewhat unexpected, because MacLennan and colleagues have concluded that cDCs are a limiting factor in plasmablast generation and survival in response to TI-2 Ags (Maclennan et al., 2003). Additionally, since we did not observe enhanced EF responses in ΔDC mice, it remained possible that acute DT-induced cDC death within the spleen of Itgax-DTR mice enhanced the AM14 B cell response.
Figure 2. cDCs are not Required Constitutively for the AM14 Response.
(A) Experiment design. 4 × 106 AM14.MRL.Faslpr B cells were transferred into MRL.Faslpr recipients that were either double positive for both the CD11c-cre and Rosa26-flox-stop-DTA Tgs, and thus lacked cDCs (ΔDC mice), or into single Tg positive littermates (Cntrl) on day 0. The AM14 B cell response was activated with PL2-3 on days 0, 2 and 4, or left untreated (no Ag). As additional controls, some mice did not receive cell transfer or Ag (no Tfr). Spleens were harvested on day 6.
(B) Number of AM14 B cells (4-44+) per spleen.
(C) Number of AM14 plasmablasts (4-44+, CD22lo, CD44hi) per spleen.
(D, E, & F) ELISpot assays for the number of 4-44+, IgM+, IgG2a+, or IgG2b+ AFCs per spleen. Data were combined from 3 experiments to obtain 12-24 mice per group receiving PL2-3 and 2-12 mice in the untreated groups. Bars represent mean and SEM. See also Figure S3.
Regulation of the AM14 Response by cDCs Requires Fas
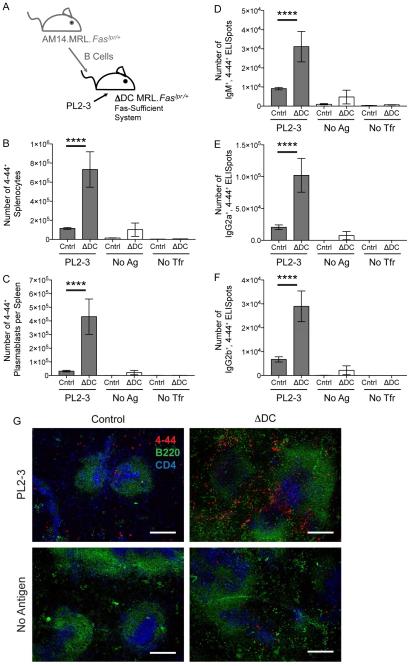
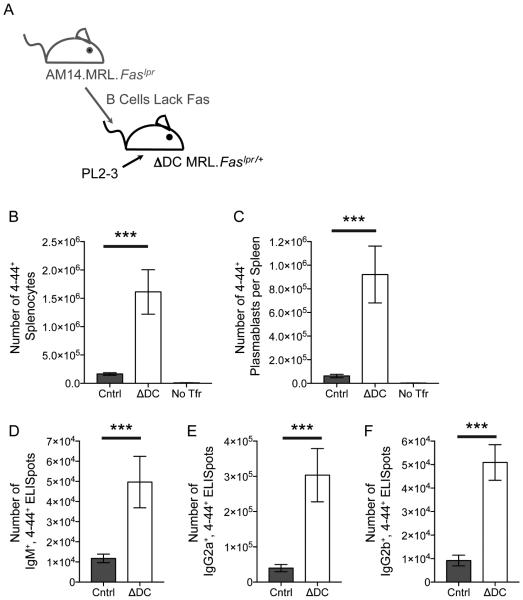
However, another important difference between the acute and the constitutive cDC ablation systems was the genetic background of the mice: BALB/c in the former, and MRL.Faslpr, in the latter. Because Fas-deficient mice have prominent spontaneous autoreactive EF responses (Shlomchik, 2009; William et al., 2002), it was possible that Fas could limit this EF response in normal mice. If this role of Fas were cDC-dependent, then depletion of cDCs in Fas-intact BALB/c mice would result in an increased response, as we observed (Figure 1). However, in Fas-deficient mice, cDC elimination would have no effect on the response, as we also observed (Figure 2). This logic is shown schematically in Figure S4. To test this idea, we compared constitutive cDC depletion on the same MRL genetic background, but in the presence or absence of functional Fas. Notably, in Fas-sufficient ΔDC MRL.Faslpr/+ mice, there was a large enhancement (3, 9, 2, 5, and 4 fold, respectively) of splenic 4-44+ AM14 B cell and plasmablast numbers, as well as, IgM, IgG2a, and IgG2b AFCs, compared to control recipients (Figure 3). Again, this AM14 response was EF (Figure 3G and S5). Hence, enhancement of the EF AM14 response upon cDC depletion was seen in both acute and constitutive cDC depletion, but only when Fas was intact. Therefore, we conclude that cDCs regulate the EF AM14 plasmablast response via a Fas-dependent mechanism.
Figure 3. Regulation of the AM14 Response by cDCs Requires Fas.
(A) Experiment design. 8-9 × 106 Fas-sufficient AM14.MRL.Faslpr/+ B cells were transferred into Fas-sufficient MRL.Faslpr/+ recipients that either lacked cDCs (ΔDC) or into littermate controls (Cntrl) on day 0. The AM14 B cell response was activated with PL2-3 on days 0, 2 and 4, or left untreated (no Ag). Some mice did not receive cell transfer or Ag (no Tfr). Spleens were harvested on day 6.
(B) Number of AM14 B cells (4-44+) per spleen.
(C) Number of AM14 plasmablasts (4-44+, CD22lo, CD44hi) per spleen.
(D, E, & F) ELISpot assays for the number of 4-44+, IgM+, IgG2a+, or IgG2b+ AFCs per spleen. Data were combined from 5 experiments to obtain 15-29 mice per group receiving PL2-3 and 2-7 mice in the other groups. Bars represent mean and SEM. ****p < 0.0001 by Mann-Whitney U test.
(G) Immunofluorescence histology was performed on spleens from experimental mice. Images were taken at a magnification of 100x. Red, AM14 B cells (4-44+); green, B cell follicles (B220+); blue, T cell zones (CD4+). White scale bars are 200 μm. See also Figures S4 and S5.
Fas-Dependent cDC Regulation of the AM14 Response is AM14 B Cell Extrinsic
Next, we investigated which cell type was required to express Fas for proper regulation of the response. Because EF helper T cells (Odegard et al., 2008) enhance AM14 B cell expansion (Sweet et al., 2011), it was possible that FasL-killing of Fas-expressing helper T cells could limit EF B cell responses. Alternatively, because B cells are sensitive to FasL-mediated killing (Rothstein et al., 1995), Fas-expressing AM14 B cells could be directly targeted. To distinguish these possibilities, we transferred Fas-deficient sd-Tg AM14.MRL.Faslpr B cells into Fas-sufficient ΔDC.MRL.Faslpr/+ mice or littermate controls, and induced the AM14 response with PL2-3 (Figure 4). Although caution is required in comparing across experiments, as expected, the response size of these Fas-deficient B cells appeared larger than what we had observed in prior experiments. However, if Fas expression on B cells were responsible for cDC-dependent regulation of AM14 B cells, we would have expected to see, upon transfer of Fas-deficient B cells, similar responses within the ΔDC and cDC-intact mice. Rather, we still saw a large enhancement of the AM14 response within ΔDC recipient mice as compared to controls, even when the B cells could not have been influenced by FasL. In fact, the splenic 4-44+ AM14 B cell, plasmablast, and IgM, IgG2a, and IgG2b AFC numbers were 10, 20, 4, 9, and 9 fold higher, respectively, in recipients lacking cDCs than in controls. Thus, we conclude that cDCs regulate EF autoimmune responses in a Fas-dependent, B cell-extrinsic manner.
Figure 4. Fas-Dependent cDC Regulation of the AM14 Response is AM14 B Cell Extrinsic.
(A) Experiment design. 8 × 106 Fas-deficient AM14.MRL.Faslpr B cells were transferred into either Fas-sufficient, cDC-deficient (ΔDC) MRL.Faslpr/+ recipients or into littermate controls (Cntrl) on day 0. The AM14 B cell response was activated with PL2-3 on days 0, 2 and 4. Some mice did not receive cell transfer or Ag (no Tfr). Spleens were harvested on day 6.
(B) Number of AM14 B cells (surface and intracellular 4-44+) per spleen.
(C) Number of AM14 plasmablasts (4-44+, CD22lo, CD44hi) per spleen.
(D, E, & F) ELISpot assays for the number of 4-44+, IgM+, IgG2a+, or IgG2b+ AFCs per spleen. Data were combined from 2 experiments to obtain 8-15 mice per group receiving PL2-3 and 2 no Tfr controls. Bars represent mean and SEM. ***p < 0.001 by Mann-Whitney U test.
See also Figure S7.
Recipient Mice Lacking Fas, cDCs, or Both Have Higher Numbers of Activated CD4+ T Cells than Control Mice
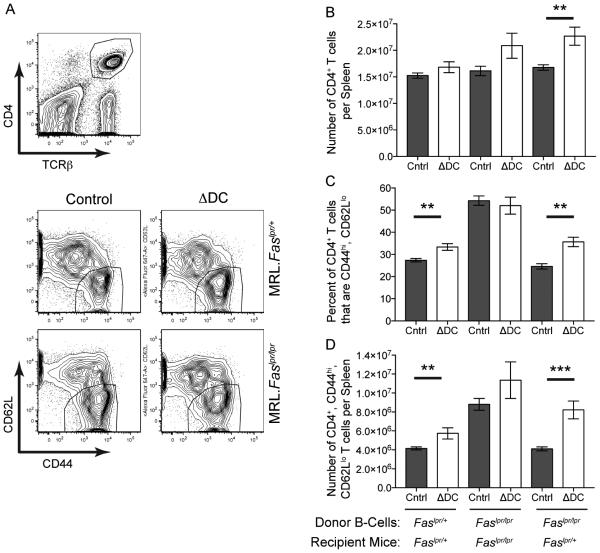
Since Fas-dependent killing of B cells was not the mechanism for Fas-dependent regulation of the AM14 B cell response by cDCs, regulation must depend on a different cDC-dependent, Fas-expressing cell. T cells that help the EF response (Lee et al., 2011; Odegard et al., 2008) would be a leading candidate for such a Fas-expressing cell. Reciprocally, FasL expression could be required either on other T cells or on cDCs (Lu et al., 1997; Süss and Shortman, 1996; Van Parijs and Abbas, 1996) in order to kill the Fas-expressing EF helper T cell (Tefh). The notion that cDCs could activate both Fas-sensitive Tefh and FasL-expressing killer T cells is reasonable, because T cells are regulated by Fas-FasL interactions (Van Parijs and Abbas, 1996), and cDCs can both activate and regulate T cell responses (Reis e Sousa, 2006). Furthermore, T cells can promote AM14 activation and differentiation via CD40L and IL-21 (Sweet et al., 2011). With these possible cDC-T and T-B interactions in mind, we first assessed our three systems of AM14 B cell transfer into ΔDC mice for cDC and Fas-dependent alterations in T cell activation (Figure 5). Fas-intact ΔDC MRL mice had a higher proportion of CD4+ T cells that were CD44hi, CD62Llo than the cDC-sufficient littermate controls. Absolute numbers of this T cell subset were also elevated. These cDC-dependent differences in the T cell compartment were Fas-dependent, because the increases were only seen in Fas-sufficient and not Fas-deficient MRL recipients. Of note, similar differences were present even in mice that had not received Ag or B cell transfer, although Ag injection with B cell transfer also enhanced T cell activation above controls (Figure S6). Further analysis of DT-treated Itgax-DTR.BALB/c bone marrow chimeras revealed that the increase in CD4+ T cells observed in DC-depleted mice included an increase in the PD-1+. Bcl-6+ Tefh cell subset (Figure S6). These data imply that cDC-dependent regulation of AM14 B cells involves the elimination of Fas-expressing Tefh cells.
Figure 5. Recipient Mice Lacking Fas and/or Dendritic Cells Have Higher Numbers of Activated CD4+ T Cells than Control Mice.
Splenic T cells from experimental mice shown in Figures 2-4 were analyzed by flow cytometry.
(A) Representative sample showing the gating of live splenocytes for CD4+, TCRβ+ cells (top) that were further sub-gated (bottom) by their activation markers (CD44hi, CD62Llo).
(B) Number of CD4+, TCRβ+ cells per spleen.
(C) Percent of CD4+, TCRβ+ cells that are activated (CD44hi, CD62Llo).
(D) Number of CD4+, TCRβ+, CD44hi, CD62Llo cells per spleen. ΔDC, mice that lack cDCs; Cntrl, littermate controls. Bars represent mean and SEM. **p < 0.01; ***p < 0.001 by Mann-Whitney U test. See also Figure S6.
Regulation of AM14 Plasmablasts by cDCs Requires T Cells Expressing Fas and FasL
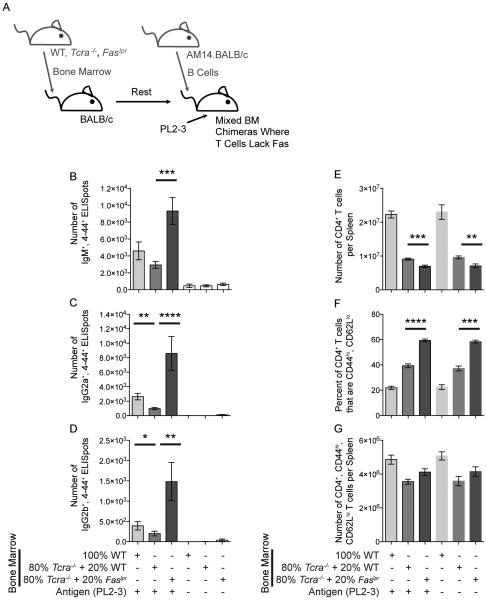
To more definitively determine cell-specific roles for Fas and FasL in limiting autoreactive EF plasmablast responses, we performed a series of mixed bone marrow (BM) chimera experiments. First, we made mice in which irradiated BALB/c recipients received a mix of donor BM that was 80% from BALB/c.Tcra−/− mice and 20% from BALB/c.Faslpr mice. Thus, all T cells within the chimeric animals lacked Fas, while other cell types had normal Fas expression on the great majority of their cells (Figure 6). Control animals received BM that was either 100% BALB/c or a mix of 80% BALB/c.Tcra−/− and 20% BALB/c. In these experiments, when T cells lacked Fas, the AM14 B cell response was enhanced by 3, 7, and 10 fold for IgM, IgG2a, and IgG2b AFCs, respectively. Finally, we made mice in which irradiated BALB/c recipients received a mix of donor BM that was 80% from BALB/c.Tcra−/− mice and 20% from BALB/c.FasLmut mice (Gregory et al., 2011). In these chimeras, no T cells had active FasL, while other cell types were normal (Figure 7A-F). Control mice received BM that was either 100% BALB/c or a mix of 80% BALB/c.Tcra−/− and 20% BALB/c. Here, when T cells could not signal via FasL, AM14 IgM, IgG2a, and IgG2b AFC numbers were 2, 3, and 8 fold higher, respectively. As expected, when T cells lacked either Fas or FasL, an activated T cell subset was elevated in frequency (Figures 6F, G & 7F, G). These experiments confirm that cDC-dependent regulation of autoreactive plasmablasts requires FasL-expressing T cells to regulate Fas-expressing Tefh cells.
Figure 6. Regulation of AM14 Plasmablasts by cDCs Requires T Cells Expressing Fas.
(A) Experiment design. BALB/c mice were used to make chimeras with BM mixed from WT, Tcra−/− or Faslpr mice. 3 × 106 AM14.BALB/c B cells were transferred into the mice on day 0, and activated with PL2-3 on days 0, 2 and 4. Spleens were harvested on day 6.
(B, C, & D) ELISpot assays for the number of 4-44+, IgM+, IgG2a+, or IgG2b+ AFCs per spleen.
(E) Number of CD4+, TCRβ+ cells per spleen.
(F) Percent of CD4+, TCRβ+ cells that are activated (CD44hi, CD62Llo).
(G) Number of CD4+, TCRβ+, CD44hi, CD62Llo cells per spleen.
Data were combined from 2 experiments to obtain 6-12 mice per group receiving PL2-3 and 4-8 controls. Bars represent mean and SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 by Mann-Whitney U test.
See also Figure S7.
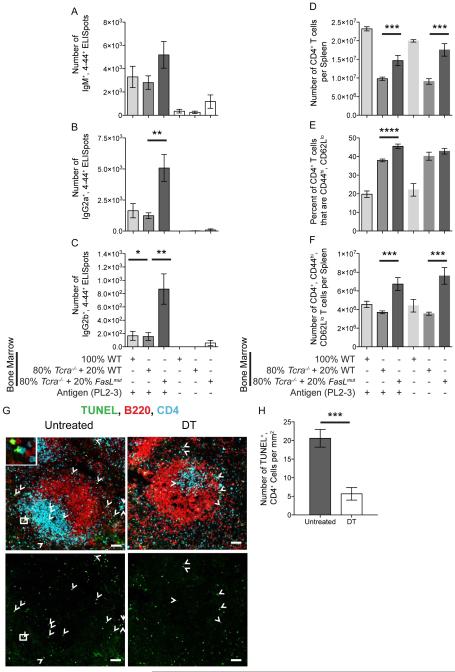
Figure 7. Regulation of AM14 Plasmablasts by cDCs Requires T Cells Expressing FasL and Increases the Death of CD4 T Cells.
(A-F) Experiments performed as described and arranged in same order as in Figure 6, except BM was mixed from WT, Tcra−/− or FasLmut mice. Data were combined from 2 experiments to obtain 6-12 mice per group receiving PL2-3 and 3-8 controls.
(G & H) As described in Figure 1, CD11c-DTR.BALB/c BM chimeras were generated and depleted of cDCs using 16 ng per g weight doses of DT. 8 × 106 AM14.BALB/c B cells were transferred and activated with PL2-3. Spleens were harvested on day 7. Data for untreated versus DT-treated mice are shown. (G) Representative histology of the spleen, taken at a magnification of 200x. Arrowheads indicate TUNEL+, CD4+ cells. Inset upper left shows a digital magnification of a TUNEL+ CD4+ cell in the white box of the larger image. Scale bars are 50 μm. (H) Quantification of TUNEL+, CD4+ cells from a total of 8 fields combined from 2 representative mice per group. Bars represent mean and SEM. *p < 0.05; **p < 0.01; ***p < 0.001 by Mann-Whitney U test.
See also Figures S6 and S7.
Loss of cDCs Reduces Cell Death of Splenic CD4+ T Cells During the AM14 B Cell Response
To determine if cDCs increase the ability of FasL-expressing T cells to kill Fas-expressing helper T cells during the AM14 B cell response, we used the TUNEL assay to detect apoptotic cells in tissue sections of DT-treated Itgax-DTR.BALB/c bone marrow chimeras. Indeed, less TUNEL+ CD4+ cells were detected in cDC-depleted than control mice (Figure 7G,H). These data further support the idea that cDC-dependent regulation of AM14 B cells involves the killing of Fas-expressing helper T cells by FasL-expressing T cells.
DISCUSSION
In normal responses to non-replicating TI and TD Ags, the EF response is transient (Maclennan et al., 2003), though the mechanisms that constrain it are not yet defined. A limiting component is likely the inherent propensity of plasmablasts to die, which does not depend on Fas (Do et al., 2000; Ursini-Siegel et al., 2002; William et al., 2005a). Although regulatory T (Treg) cells can restrain humoral immunity, they have been implicated in GC, rather than EF responses (Sage and Sharpe, 2015), and Treg cell neutralization with the anti-CD25 Ab PC61 had no effect in our system (not depicted). Determining the identity and function of the negative regulators of EF plasmablasts is important for understanding both normal (Di Niro et al., 2015) and pathogenic, autoimmune responses. That the EF plasmablast response was greatly enhanced in the absence of cDCs was an important and unexpected finding, as it revealed a regulatory rather than stimulatory role for cDCs in the humoral immune response.
That cDCs were not even required for the EF response was also somewhat unexpected based on prior reports, albeit in different contexts. MacLennan and colleagues have concluded that cDCs are a limiting factor in plasmablast generation and survival in response to TI-2 Ags (Maclennan et al., 2003). CD11c+ cells are also prominently seen in juxtaposition with dividing and differentiating EF AM14 B cells (William et al., 2002), supporting the notion that cDCs are critical in promoting the response. However, an essential role of cDCs in the EF response has been called into question by Jung, et. al., who found that cDC depletion does not alter the extent of a TI-2 response elicited by NP-Ficoll (Jung et al., 2002). Of note, the response we tested was not a TI-2 response, but one with qualities of TI-1 (given the TLR requirement), TI-2 (giventhe polymeric nature of the Ag) and TD (given the ability of T cells, when present, to enhance it). Because EF responses are typical of autoreactive and probably bacterial (Di Niro et al., 2015) and viral pathogen responses, the EF response we studied may be more physiologically relevant than idealized TI or TD protein antigens given in artificial adjuvant.
There have been few reports of negative regulation of B cells by cDCs. Three groups have observed inhibition of B cell responses by cDCs in vitro via either an IL-6 and soluble CD40L-dependent mechanism (Gilbert et al., 2007), or a mechanism requiring CD22 expression by the B cells (Santos et al., 2008; Sindhava et al., 2012). None of these systems was translated in vivo. Moreover, it is unlikely that they relate directly to our in vivo observations. In the former case only anergic B cells are affected, but AM14 B cells are not anergic (Hannum et al., 1996). In the latter cases CD22 is required, but CD22 is rapidly downregulated early in plasmablast differentiation (William et al., 2005b).
Use of multiple systems for cDC depletion allowed us to rule out some alternate explanations for the enhanced EF response observed in the absence of cDC. Because we saw an elevated AM14 response in both the transient and constitutive cDC depletion systems the acute release of Ag (ie. chromatin) from dying CD11c+ cells that might occur upon DT treatment in the Itgax-DTR system was not the primary cause of the enhanced AM14 responses seen in the absence of cDCs. Macrophage hyperplasia, which we observed to some degree in both cDC depletion systems and as noted by others (Birnberg et al., 2008), was also likely not the cause of enhanced EF responses, because this macrophage expansion was also present within cDC-deficient MRL.Faslpr mice, which did not have an altered frequency of AM14 AFCs. Finally, a role for pDCs is unlikely, because pDCs were not depleted in the CD11c-DTR system, in which acute cDC depletion substantially increased the AM14 AFC response.
We found that the mechanism by which cDCs regulate the EF autoimmune response in vivo was dependent on Fas: cDCs did not regulate the AM14 response in Fas-deficient animals. In this case, the regulatory pathway was short-circuited because the Fas-signaling was already “off.” Depleting cDCs in addition to Fas, therefore, had no additional effect. Fas has previously been implicated in controlling autoimmunity via a number of mechanisms, but the pathway revealed by our studies appears different. For example, FasL and Fas are required for elimination of anergic B cells by CD4+ T cells in vivo (Rathmell et al., 1995). However, AM14 B cells are not anergic (but remain ignorant), and do not need to escape anergy to break tolerance (Hannum et al., 1996; Wang and Shlomchik, 1999). GC B cells upregulate Fas, which regulates the duration and extent of the GC reaction and the generation of long-lived plasma cells (Butt et al., 2015; Hao et al., 2008; Takahashi et al., 2001). In contrast, the response we are studying is EF and generates short-lived plasmablasts.
cDC regulation did not depend on Fas expression by the B cell itself, even though activated B cells can be sensitive to FasL-mediated killing (Rothstein et al., 1995). Rather, we demonstrated that the FasL targets were Fas-expressing T cells that help amplify the EF B cell response, namely Tefh cells (Lee et al., 2011; Odegard et al., 2008; Sweet et al., 2011). Because Tfh cells express Fas (Rasheed et al., 2006), we would expect that the Tefh cells in our system would also be sensitive to elimination by a FasL-expressing cell: either another T cell, the activation of which is cDC-dependent, or the cDC itself (Lu et al., 1997; Süss and Shortman, 1996). We also showed that the FasL-expressing cells required in this system were indeed T cells. There is precedent for such a mechanism, because regulation of T cells by Fas-FasL interactions is well documented (Van Parijs and Abbas, 1996), and T cell restricted Fas- or FasL-deficiency leads to autoantibody production (Mabrouk et al., 2008; Stranges et al., 2007). Seo et al also noted that B cell-specific Fas expression is not required to regulate autoreactive B cell responses to strong T cell help (Seo et al., 2002). Thus, our observations are consistent with prior studies, but extend our knowledge by demonstrating that cDCs can regulate an EF plasmablast response via a mechanism that requires Fas ligation on T cells—most likely Tefh cells—rather than on B cells directly.
We previously showed, using constitutive cDC-depletion, that cDCs can amplify disease in aged MRL.Faslpr mice, although they are not required for disease initiation (Teichmann et al., 2010). These studies did not reveal a regulatory role for cDCs, and in fact, certain types of autoantibodies were reduced in aged cDC-deficient MRL.Faslpr mice. Similarly, Fas expression on cDCs suppresses autoimmunity in older mice (Stranges et al., 2007). This presents the paradox that, in the short term, cDCs negatively regulate the initiation of the EF response, whereas in the long-term cDCs promote specific, chronic aspects of disease. We suggest two ways to understand this paradox. First, as in many complex cellular networks in vivo, cDCs likely have both positive and negative effects. When integrated over the life of an autoimmune-prone animal, these effects are seen as mainly promoting disease, in particular, by aiding the growth of tissue cellular infiltrates (Teichmann et al., 2015; Teichmann et al., 2010). Over time cDC-driven positive feedback cycles of T cell activation could override any short-term negative effects. Second, and most relevant to the present context, our previous study of constitutively cDC-deficient mice was on the Fas-deficient MRL.Faslpr background. In that model, the regulatory effects of cDCs would not have been observed, because the Fas-dependent mechanism was already short-circuited.
These insights into the Fas-dependent regulatory role of cDCs in the generation of autoantibodies could apply to other EF plasmablast responses. The EF response can be driven by simultaneous TLR and BCR signals. In the AM14 system, chromatin-containing ICs promote the EF response in a TLR7- and TLR9-dependent manner (Herlands et al., 2008; Leadbetter et al., 2002). A more general effect of combined TLR and BCR stimulation in directing the EF response has been demonstrated in elegant studies in which a BCR signal was engineered to have a TLR9 signal associated with it on the same synthetic bead (Eckl-Dorna and Batista, 2009). Similarly, many bacteria and viruses contain Ags that can directly stimulate B cell-expressed TLR4, 7, and 9 as well as ligate relevant BCRs. This dual recognition promotes vigorous EF plasmablast responses that can undergo isotype switch, and is probably critical for the early control of pathogens like Salmonella (Cunningham et al., 2007; Di Niro et al., 2015; Neves et al., 2010). Thus, the cDC-regulated pathway we have observed could also be important during the primary response to pathogens.
Initial EF responses are typically supplanted by the GC response, which provides definitive B cell memory and long-lived AFCs. GC and T-dependent responses engage multiple mechanisms to enforce self-tolerance. In contrast, the primary EF response is less selective and partially T-independent, thus making it more liable to be subverted into autoimmunity. Here we have demonstrated a Fas- and cDC-dependent pathway that reduces the magnitude of the EF response by 3-6 fold over the course of a week. cDCs are, therefore, potent regulators of the autoreactive EF response, and possibly of EF responses in general.
EXPERIMENTAL PROCEDURES
Mice
BALB/c, BALB/c.Rag1−/−, MRL/MpJ- Faslpr/J (MRL. Faslpr), MRL/MpJ and BALB/c FVB-Tg(Itgax-DTR/EGFP)57Lan/J (Itgax-DTR.BALB/c) (Jung et al., 2002) mice were purchased from Jackson Laboratories (Bar Harbor, Maine). BALB/c sd-Tg AM14 mice were previously described (Sweet et al., 2011), and were also backcrossed greater than 10 times onto MRL.Faslpr. AM14.BALB/c mice were used at 7-9 weeks of age. AM14.MRL.Faslpr mice were used at 5-7 weeks of age. AM14.MRL.Faslpr/+ mice were used at 5-10 weeks of age. C57BL/6 CD11c-Cre BAC transgenic (Caton et al., 2007) and Rosa26-eGFP-flox-stop-DTA transgenic (Ivanova et al., 2005) mice, gifts from Boris Reizis (Columbia University) and Juan Martinez-Barbera (University College London), respectively, were backcrossed greater than 10 times onto MRL.Faslpr (Teichmann et al., 2010). These two strains were then intercrossed to obtain cDC-deficient (ΔDC MRL.Faslpr), CD11c-Cre+ Rosa26-eGFP-DTA+ mice. Single positive and WT littermates were used as controls. Mice were used at 6-7, 5-9.5 and 7-9 weeks of age in three experiments with equivalent results. To obtain Fas-sufficient cDC-deficient mice (ΔDC.MRL.Faslpr/+) mice and controls, double positive, ΔDC.MRL.Faslpr females were outcrossed to MRL/MpJ males for one generation. Mice were used at 6-7 weeks of age in three experiments and 6-10 weeks of age in two experiments, with equivalent results. BALB/c.Faslpr mice were obtained from Thomas Ferguson (Washington University) and used at 4-9 weeks of age. BALB/c.Tcra−/− mice (Sweet et al., 2011) were obtained from Kim Bottomly (Yale University) and used at 5-7 weeks of age. BALB/c.FasLmut mice, gifted from Ann Marshak-Rothstein (Gregory et al., 2011) were used at 7-9 weeks of age. All animals were housed under SPF conditions and handled according to IACUC approved protocols.
B cell Purification and Adoptive Cell Transfer
Splenocyte suspensions were made in 2% fetal calf serum (FCS), 1mM EDTA, phosphate buffered saline (PBS). B cells were purified using an EasySep Mouse B cell Enrichment Kit (Stemcell Technologies). 3-12 × 106 cells suspended in PBS per mouse (as indicated) were injected intravenously (IV) on day 0.
Anti-Chromatin Preparation and Immunization
PL2-3, an IgG2aa anti-chromatin hybridoma (Losman et al., 1992) was prepared as ascites in Rag−/− mice as described (Herlands et al., 2008; Sweet et al., 2011). PL2-3 protein concentration was determined by IgG2a ELISA. Mice were immunized intraperitoneally (IP) with 0.5 mg equivalent of PL2-3 on days 0, 2, and 4. AM14 B cell activation was assayed on day 6.
Bone Marrow Chimeras and Diphtheria Toxin Treatment
BM from CD11c-DTR donors or Tg-negative littermates was processed in 0.5% bovine serum albumin (BSA), 5mM EDTA, penicillin, streptomycin in PBS. After red blood cell lysis with ACK solution, BM cells were washed with BSA, PBS and resuspended in injection buffer (2.5% v/v ACD-A, 10mM HEPES, penicillin, streptomycin, PBS). Eight week old BALB/c recipients received 450 Rad of irradiation twice from a 137Cs source with a 3-4 h rest period between doses. 1-2 h after the second dose, 8-10 × 106 BM cells were injected IV. Animals were allowed to recover for 6 weeks prior to cDC depletion or B cell transfer. Diphtheria toxin (Sigma) was administered IP at 4-8 ng/g weight on days -2 and -1 prior to and on days 1, 3 and 5 after B cell transfer. Effectiveness of chimerism and depletion was assessed by flow cytometry of splenocytes (Figure S1).
ELISpot Assay
ELISpot assays were performed as described (Hannum et al., 1996). Spots were counted using a dissecting microsope.
Flow Cytometry
Splenocytes were suspended in staining media (SM: 3% FCS, 5 mM EDTA, 0.05% sodium azide, PBS) with the FcR blocking antibody, 2.4G2 and ethidium monoazide (EMA) for live/dead discrimination. Reagents used for staining were either prepared in house: 4-44-biotin, PDCA1 (927)-Alexa 647, CD62L (MEL-14)-Alexa 647; or purchased from Biolegend: CD44 (1M7)-APC/Cy7, CD11c (N418)-PE/Cy7, CD4 (GK1.5)-PE/Cy7, Gr1 (RB6.8C5)-biotin; from BD: CD22.2 (Cy34.1)-PE, TCRβ (H57-597)-PE, I-A/I-E (M5/114.15.2)-PE; from eBioscience: CD11b (MAC-1)-PE, streptavidin-PE/Cy7; or from Invitrogen: streptavidin-PacBlue. Surface stained cells were fixed with 1% PFA, washed and resuspended in SM. For intracellular staining, cells were fixed in 4% PFA in BD Perm/Wash Buffer, washed, blocked (with 5% rat serum), and stained with 4-44-Alexa 647 or GFP-FITC (Rockland) in the BD Perm/Wash Buffer, with a final wash in SM. Cytometry was performed on a LSRII (BD) and data analyzed with FlowJo software.
Immunofluorescence Histology
CD11c-DTR and control spleens were fixed in paraformaldehyde-lysine-periodate (PLP) solution (1% paraformaldehyde, 95 mM L-lysine, 10 mM sodium M-periodate, 0.1 M phosphate buffer (PB), pH 7.2) for 1-7 days at 4°C. They were then washed three times with PB, dehydrated stepwise in 10%, 20%, and 30% sucrose/PB and frozen in OCT (TissueTek). Alternatively, for Bcl-6 and TUNEL staining, spleens were fixed with 4% paraformaldehyde for 2-4h at 4°C and washed for 2-4h in cold PBS and frozen in OCT medium. Spleens from CD11c-cre × Rosa26-eGFP-flox-stop-DTA mice were first frozen in OCT and fixed after cryostat sectioning with 4°C acetone for 10 min. 7-10 μm cryostat sections were cut, rehydrated with PBS, blocked for 20 min with 10% rat serum, 1% BSA, 0.1% Tween-20 in PBS, stained for 1 h with respective antibodies in BSA, Tween-20, PBS, washed three times with BSA, Tween-20, PBS and once with PBS. Staining reagents were either prepared in house: 4-44-biotin, B220 (RA3-6B2)-488, B220 (RA3-6B2)-Dylight 405, B220 (RA3-6B2)-PE, CD4 (GK1.5)-Alexa 647, F4/80 (BM8)-Alexa 647, PNA-Alexa 647, CD19 (1D3.2)-Alexa 647; or purchased from Invitrogen: streptavidin-Alexa 555; from Vector Laboratories: PNA-FITC; from BD: anti-Bcl-6 (K112-91)-Alexa 647; from BioLegend: anti-CD4 (GK1.5)-PE. For the visualization of apoptotic cells by the TUNEL method, ApoAlert DNA Fragmentation Assay Kit (Clontech) was used as per manufacturer’s instructions. After air-drying, slides were mounted with Prolong Antifade (Molecular Probes). Images were captured either with a 10 × lens on a Nikon Exlipse Ti automated wide field microscope with a QImaging Retiga 200R CCD camera (Figures 3, S2, & S5) using NIS Elements software or with a 20 × lens on an Olympus IX83 microscope (Figures 7 & 6S) with an ORCA-flash 4.0 camera (Hamamatsu) using Olympus CellSens Dimension 1.15 software. Images were further processed with Adobe Photoshop software, and CellSens software assisted in TUNEL+ cell quantitation.
Statistical Analysis
P-values were calculated by one-way ANOVA with Tukey’s multiple comparisons test, two-tailed Mann-Whitney U-test, or unpaired T-test, as indicated, using Prism software (Graphpad).
Supplementary Material
HIGHLIGHTS.
- cDCs are not required for extrafollicular (EF) autoreactive B cell activation
- Loss of cDCs markedly enhances the EF B cell response
- cDC regulation of the EF response requires B cell extrinsic Fas
- Loss of Fas or FasL on T cells enhances autoantibody production
IN BRIEF.
cDCs negatively regulate extrafollicular B activation and autoantibody production. Using two cDC ablation systems, Ols and colleagues observe elevated autoantibody production in the absence of cDCs. They further show that the regulatory effect of cDCs on B cells requires Fas and FasL expression by T cells.
ACKNOWLEDGMENTS
We thank Dr. Juan Martinez-Barbera and Boris Reizis for Rosa26-eGFP-DTA and CD11c-cre mice, respectively. Cuiling Zhang, Yuqi Zhang and Colin Smith provided outstanding technical assistance. Kevin Nickerson assisted in our experimental response to the reviewers. We thank the Yale Animal Resources Center and the Yale Cell Sorter Facility for their excellent services. This work was supported by the National Institutes of Health grant AI073722 (to MJS), a Ruth. L. Kirschstein National Research Service Award NIH T32 AI07019-30 (to MLO), and an Arthritis Foundation Postdoctoral Fellowship (to MLO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental information includes seven figures and supplemental methods for PD-1 and Bcl-6 staining by flow cytometry.
AUTHOR CONTRIBUTIONS
M.L.O. designed and performed experiments, analyzed data, and wrote the manuscript. J.L.C., A.T-N., and J.G. performed experiments and analyzed data. M.J.S. designed experiments, supervised the study, and wrote the manuscript.
The authors declare that they have no competing financial interests.
REFERENCES
- Balázs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Birnberg T, Bar-On L, Sapoznikov A, Caton ML, Cervantes-Barragán L, Makia D, Krauthgamer R, Brenner O, Ludewig B, Brockschnieder D, et al. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29:986–997. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Boulé MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. The Journal of experimental medicine. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt D, Chan TD, Bourne K, Hermes JR, Nguyen A, Statham A, O'Reilly LA, Strasser A, Price S, Schofield P, et al. FAS Inactivation Releases Unconventional Germinal Center B Cells that Escape Antigen Control and Drive IgE and Autoantibody Production. Immunity. 2015;42:890–902. doi: 10.1016/j.immuni.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8-dendritic cells in the spleen. The Journal of experimental medicine. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AF, Gaspal F, Serre K, Mohr E, Henderson IR, Scott-Tucker A, Kenny SM, Khan M, Toellner KM, Lane PJ, MacLennan IC. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J Immunol. 2007;178:6200–6207. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- Daridon C, Loddenkemper C, Spieckermann S, Kuhl AA, Salama A, Burmester GR, Lipsky PE, Dorner T. Splenic proliferative lymphoid nodules distinct from germinal centers are sites of autoantigen stimulation in immune thrombocytopenia. Blood. 2012;120:5021–5031. doi: 10.1182/blood-2012-04-424648. [DOI] [PubMed] [Google Scholar]
- Di Niro R, Lee SJ, Vander Heiden JA, Elsner RA, Trivedi N, Bannock JM, Gupta NT, Kleinstein SH, Vigneault F, Gilbert TJ, et al. Salmonella Infection Drives Promiscuous B Cell Activation Followed by Extrafollicular Affinity Maturation. Immunity. 2015;43:120–131. doi: 10.1016/j.immuni.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do RK, Hatada E, Lee H, Tourigny MR, Hilbert D, Chen-Kiang S. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. The Journal of experimental medicine. 2000;192:953–964. doi: 10.1084/jem.192.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckl-Dorna J, Batista FD. BCR-mediated uptake of antigen linked to TLR9 ligand stimulates B-cell proliferation and antigen-specific plasma cell formation. Blood. 2009;113:3969–3977. doi: 10.1182/blood-2008-10-185421. [DOI] [PubMed] [Google Scholar]
- Gilbert MR, Carnathan DG, Cogswell PC, Lin L, Baldwin AS, Vilen BJ. Dendritic cells from lupus-prone mice are defective in repressing immunoglobulin secretion. J Immunol. 2007;178:4803–4810. doi: 10.4049/jimmunol.178.8.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giltiay NV, Lu Y, Cullen JL, Jorgensen TN, Shlomchik MJ, Li X. Spontaneous loss of tolerance of autoreactive B cells in Act1-deficient rheumatoid factor transgenic mice. J Immunol. 2013;191:2155–2163. doi: 10.4049/jimmunol.1300152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez SF, Lukacs-Kornek V, Kuligowski MP, Pitcher LA, Degn SE, Kim Y, Cloninger MJ, Martinez-Pomares L, Gordon S, Turley SJ, Carroll MC. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat Immunol. 2010;11:427–434. doi: 10.1038/ni.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory MS, Hackett CG, Abernathy EF, Lee KS, Saff RR, Hohlbaum AM, Moody KS, Hobson MW, Jones A, Kolovou P, et al. Opposing roles for membrane bound and soluble Fas ligand in glaucoma-associated retinal ganglion cell death. PLoS One. 2011;6:e17659. doi: 10.1371/journal.pone.0017659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum LG, Ni D, Haberman AM, Weigert MG, Shlomchik MJ. A disease-related rheumatoid factor autoantibody is not tolerized in a normal mouse: implications for the origins of autoantibodies in autoimmune disease. The Journal of experimental medicine. 1996;184:1269–1278. doi: 10.1084/jem.184.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Duncan GS, Seagal J, Su Y, Hong C, Haight J, Chen N, Elia A, Wakeham A, Li WY, et al. Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity. 2008;29:615–627. doi: 10.1016/j.immuni.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebel K, Griewank K, Inamine A, Chang HD, Müller-Hilke B, Fillatreau S, Manz RA, Radbruch A, Jung S. Plasma cell differentiation in T-independent type 2 immune responses is independent of CD11c(high) dendritic cells. Eur J Immunol. 2006;36:2912–2919. doi: 10.1002/eji.200636356. [DOI] [PubMed] [Google Scholar]
- Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlands RA, William J, Hershberg U, Shlomchik MJ. Anti-chromatin antibodies drive in vivo antigen-specific activation and somatic hypermutation of rheumatoid factor B cells at extrafollicular sites. Eur J Immunol. 2007;37:3339–3351. doi: 10.1002/eji.200737752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, Hiepe F, Manz RA. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. The Journal of experimental medicine. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova A, Signore M, Caro N, Greene ND, Copp AJ, Martinez-Barbera JP. In vivo genetic ablation by Cre-mediated expression of diphtheria toxin fragment A. Genesis. 2005;43:129–135. doi: 10.1002/gene.20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- Lee SK, Rigby RJ, Zotos D, Tsai LM, Kawamoto S, Marshall JL, Ramiscal RR, Chan TD, Gatto D, Brink R, et al. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. The Journal of experimental medicine. 2011;208:1377–1388. doi: 10.1084/jem.20102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losman MJ, Fasy TM, Novick KE, Monestier M. Monoclonal autoantibodies to subnucleosomes from a MRL/Mp(−)+/+ mouse. Oligoclonality of the antibody response and recognition of a determinant composed of histones H2A, H2B, and DNA. J Immunol. 1992;148:1561–1569. [PubMed] [Google Scholar]
- Lu L, Qian S, Hershberger PA, Rudert WA, Lynch DH, Thomson AW. Fas ligand (CD95L) and B7 expression on dendritic cells provide counter-regulatory signals for T cell survival and proliferation. J Immunol. 1997;158:5676–5684. [PubMed] [Google Scholar]
- Mabrouk I, Buart S, Hasmim M, Michiels C, Connault E, Opolon P, Chiocchia G, Lévi-Strauss M, Chouaib S, Karray S. Prevention of autoimmunity and control of recall response to exogenous antigen by Fas death receptor ligand expression on T cells. Immunity. 2008;29:922–933. doi: 10.1016/j.immuni.2008.10.007. [DOI] [PubMed] [Google Scholar]
- MacLennan I, Vinuesa C. Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity. 2002;17:235–238. doi: 10.1016/s1074-7613(02)00398-9. [DOI] [PubMed] [Google Scholar]
- Maclennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zúñiga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- Mohr E, Serre K, Manz RA, Cunningham AF, Khan M, Hardie DL, Bird R, Maclennan IC. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J Immunol. 2009;182:2113–2123. doi: 10.4049/jimmunol.0802771. [DOI] [PubMed] [Google Scholar]
- Neves P, Lampropoulou V, Calderon-Gomez E, Roch T, Stervbo U, Shen P, Kühl AA, Loddenkemper C, Haury M, Nedospasov SA, et al. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 2010;33:777–790. doi: 10.1016/j.immuni.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Nundel K, Green NM, Shaffer AL, Moody KL, Busto P, Eilat D, Miyake K, Oropallo MA, Cancro MP, Marshak-Rothstein A. Cell-intrinsic expression of TLR9 in autoreactive B cells constrains BCR/TLR7-dependent responses. J Immunol. 2015;194:2504–2512. doi: 10.4049/jimmunol.1402425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. The Journal of experimental medicine. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. The Journal of experimental medicine. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Cooke MP, Ho WY, Grein J, Townsend SE, Davis MM, Goodnow CC. CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature. 1995;376:181–184. doi: 10.1038/376181a0. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C. Dendritic cells in a mature age. Nature reviews. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- Rothstein TL, Wang JK, Panka DJ, Foote LC, Wang Z, Stanger B, Cui H, Ju ST, Marshak-Rothstein A. Protection against Fas-dependent Th1-mediated apoptosis by antigen receptor engagement in B cells. Nature. 1995;374:163–165. doi: 10.1038/374163a0. [DOI] [PubMed] [Google Scholar]
- Sage PT, Sharpe AH. T follicular regulatory cells in the regulation of B cell responses. Trends Immunol. 2015;36:410–418. doi: 10.1016/j.it.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang A, Niu H, Cullen J, Choi SC, Zheng YY, Wang H, Shlomchik MJ, Morel L. Activation of rheumatoid factor-specific B cells is antigen dependent and occurs preferentially outside of germinal centers in the lupus-prone NZM2410 mouse model. J Immunol. 2014;193:1609–1621. doi: 10.4049/jimmunol.1303000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos L, Draves KE, Boton M, Grewal PK, Marth JD, Clark EA. Dendritic cell-dependent inhibition of B cell proliferation requires CD22. J Immunol. 2008;180:4561–4569. doi: 10.4049/jimmunol.180.7.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SJ, Fields ML, Buckler JL, Reed AJ, Mandik-Nayak L, Nish SA, Noelle RJ, Turka LA, Finkelman FD, Caton AJ, Erikson J. The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity. 2002;16:535–546. doi: 10.1016/s1074-7613(02)00298-4. [DOI] [PubMed] [Google Scholar]
- Shlomchik MJ. Activating systemic autoimmunity: B's, T's, and tolls. Curr Opin Immunol. 2009;21:626–633. doi: 10.1016/j.coi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhava VJ, Tuna H, Gachuki BW, DiLillo DJ, Avdiushko MG, Onami TM, Tedder TF, Cohen DA, Bondada S. Bone marrow dendritic cell-mediated regulation of TLR and B cell receptor signaling in B cells. J Immunol. 2012;189:3355–3367. doi: 10.4049/jimmunol.1101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, Hartig H, Sundberg JP, Servick S, Kaufmann G, et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Süss G, Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. The Journal of experimental medicine. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Christensen SR, Harris ML, Shupe J, Sutherland JL, Shlomchik MJ. A new site-directed transgenic rheumatoid factor mouse model demonstrates extrafollicular class switch and plasmablast formation. Autoimmunity. 2010;43:607–618. doi: 10.3109/08916930903567500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Ols ML, Cullen JL, Milam AV, Yagita H, Shlomchik MJ. Facultative role for T cells in extrafollicular Toll-like receptor-dependent autoreactive B-cell responses in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7932–7937. doi: 10.1073/pnas.1018571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Ohta H, Takemori T. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity. 2001;14:181–192. doi: 10.1016/s1074-7613(01)00100-5. [DOI] [PubMed] [Google Scholar]
- Teichmann LL, Cullen JL, Kashgarian M, Dong C, Craft J, Shlomchik MJ. Local Triggering of the ICOS Coreceptor by CD11c(+) Myeloid Cells Drives Organ Inflammation in Lupus. Immunity. 2015;42:552–565. doi: 10.1016/j.immuni.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann LL, Ols ML, Kashgarian M, Reizis B, Kaplan DH, Shlomchik MJ. Dendritic Cells in Lupus Are Not Required for Activation of T and B Cells but Promote Their Expansion, Resulting in Tissue Damage. Immunity. 2010;33:967–978. doi: 10.1016/j.immuni.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton CM, Fucile CF, Darce J, Chida A, Ichikawa T, Gregoretti I, Schieferl S, Hom J, Jenks S, Feldman RJ, et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol. 2015;16:755–765. doi: 10.1038/ni.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini-Siegel J, Zhang W, Altmeyer A, Hatada EN, Do RKG, Yagita H, Chen-Kiang S. TRAIL/Apo-2 ligand induces primary plasma cell apoptosis. J Immunol. 2002;169:5505–5513. doi: 10.4049/jimmunol.169.10.5505. [DOI] [PubMed] [Google Scholar]
- Van Parijs L, Abbas AK. Role of Fas-mediated cell death in the regulation of immune responses. Curr Opin Immunol. 1996;8:355–361. doi: 10.1016/s0952-7915(96)80125-7. [DOI] [PubMed] [Google Scholar]
- Wang H, Shlomchik MJ. Autoantigen-specific B cell activation in Fas-deficient rheumatoid factor immunoglobulin transgenic mice. The Journal of experimental medicine. 1999;190:639–649. doi: 10.1084/jem.190.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- William J, Euler C, Leadbetter E, Marshak-Rothstein A, Shlomchik MJ. Visualizing the onset and evolution of an autoantibody response in systemic autoimmunity. J Immunol. 2005a;174:6872–6878. doi: 10.4049/jimmunol.174.11.6872. [DOI] [PubMed] [Google Scholar]
- William J, Euler C, Shlomchik MJ. Short-lived plasmablasts dominate the early spontaneous rheumatoid factor response: differentiation pathways, hypermutating cell types, and affinity maturation outside the germinal center. J Immunol. 2005b;174:6879–6887. doi: 10.4049/jimmunol.174.11.6879. [DOI] [PubMed] [Google Scholar]
- Zammit DJ, Cauley LS, Pham QM, Lefrançois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenay S, Keller AM, Whitney PG, Schraml BU, Deddouche S, Rogers NC, Schulz O, Sancho D, Sousa CRE. The dendritic cell receptor DNGR-1 controls endocytic handling of necrotic cell antigens to favor cross-priming of CTLs in virus-infected mice. Journal of Clinical Investigation. 2012;122:1615–1627. doi: 10.1172/JCI60644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.