Abstract
The pqs quorum sensing (QS) system is crucial for Pseudomonas aeruginosa virulence both in vitro and in animal models of infection and is considered an ideal target for the development of anti-virulence agents. However, the precise role played by each individual component of this complex QS circuit in the control of virulence remains to be elucidated. Key components of the pqs QS system are 2-heptyl-4-hydroxyquinoline (HHQ), 2-heptyl-3-hydroxy-4-quinolone (PQS), 2-heptyl-4-hydroxyquinoline N-oxide (HQNO), the transcriptional regulator PqsR and the PQS-effector element PqsE. To define the individual contribution of each of these components to QS-mediated regulation, transcriptomic analyses were performed and validated on engineered P. aeruginosa strains in which the biosynthesis of 2-alkyl-4-quinolones (AQs) and expression of pqsE and pqsR have been uncoupled, facilitating the identification of the genes controlled by individual pqs system components. The results obtained demonstrate that i) the PQS biosynthetic precursor HHQ triggers a PqsR-dependent positive feedback loop that leads to the increased expression of only the pqsABCDE operon, ii) PqsE is involved in the regulation of diverse genes coding for key virulence determinants and biofilm development, iii) PQS promotes AQ biosynthesis, the expression of genes involved in the iron-starvation response and virulence factor production via PqsR-dependent and PqsR-independent pathways, and iv) HQNO does not influence transcription and hence does not function as a QS signal molecule. Overall this work has facilitated identification of the specific regulons controlled by individual pqs system components and uncovered the ability of PQS to contribute to gene regulation independent of both its ability to activate PqsR and to induce the iron-starvation response.
Author Summary
Many bacterial pathogens control virulence gene expression and the development of antibiotic-resistant biofilms via intercellular communication through ‘quorum sensing’ (QS). QS systems depend on the synthesis, secretion and perception of diffusible signalling molecules that enable bacteria to synchronize their behaviour at the population level and are considered ideal targets for the development of anti-virulence drugs. Pseudomonas aeruginosa employs several overlapping QS circuits including the pqs system to control the expression of virulence determinants. The pqs QS system relies on multiple 2-alkyl-4-quinolones (AQs), including the Pseudomonas Quinolone Signal (PQS), as signal molecules. However, the individual contributions of key AQs and the effector proteins PqsR and PqsE within the auto-regulated pqs system have not been elucidated because of their inter-dependence. By constructing P. aeruginosa strains with multiple mutations in the pqs system and determining their transcriptomes in the presence or absence of PqsR, PqsE or exogenously supplied AQs, we define the distinct regulons involved and characterize a novel PQS signalling pathway independent of PqsR and the iron-starvation response.
Introduction
Pseudomonas aeruginosa is a multi-antibiotic resistant pathogen commonly responsible for hospital-acquired infections and is the main cause of morbidity and mortality in cystic fibrosis [1]. The pathogenicity of P. aeruginosa is multifactorial and host specific relying on the coordinated production of multiple virulence factors and the formation of antibiotic tolerant biofilms [2,3]. These are controlled by a quorum sensing (QS) intercellular communication network that integrates information on population structure/dynamics and the metabolic status of the cell with environmental cues [4–7]. Since P. aeruginosa QS mutants display attenuated pathogenicity, QS is a promising target for anti-virulence agents [8].
In P. aeruginosa QS involves three major inter-linked QS signalling pathways, namely the las and rhl systems that employ N-acylhomoserine lactones and the pqs QS system that uses 2-alkyl-4-quinolones (AQs) as QS signal molecules [5]. Data from expression studies and virulence factor profiling obtained by comparing wild type with different pqs mutants have revealed the extent of the pqs regulon and its relationship with the las and rhl regulons. For example, AQs are required for full transcription of genes coding for exoenzymes, exotoxins, lectins, secondary metabolites (e.g., pyocyanin, hydrogen cyanide, rhamnolipids, pyochelin and pyoverdine) and biofilm development (reviewed in [9]). P. aeruginosa mutants defective in AQ biosynthesis or sensing are severely attenuated in plant and animal infection models [3,10,11]. Furthermore, AQs are detectable in sputum, blood and urine of individuals with cystic fibrosis and their presence correlates with clinical status [12].
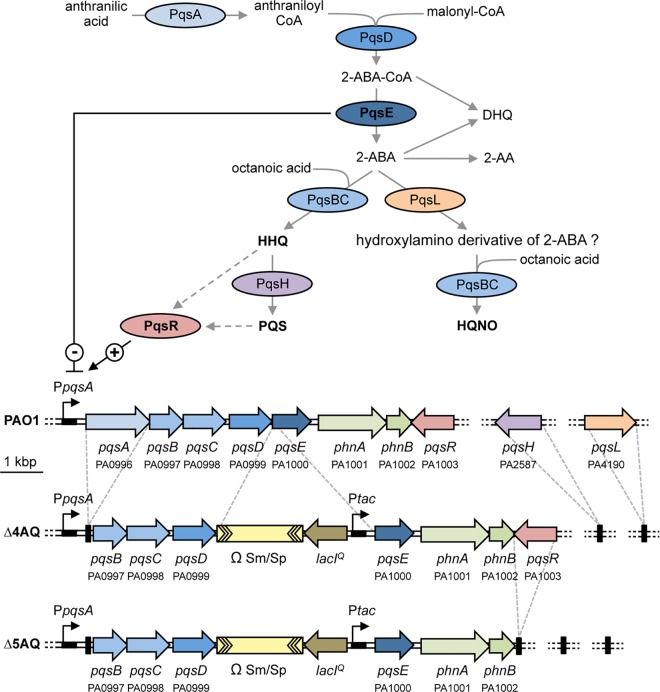
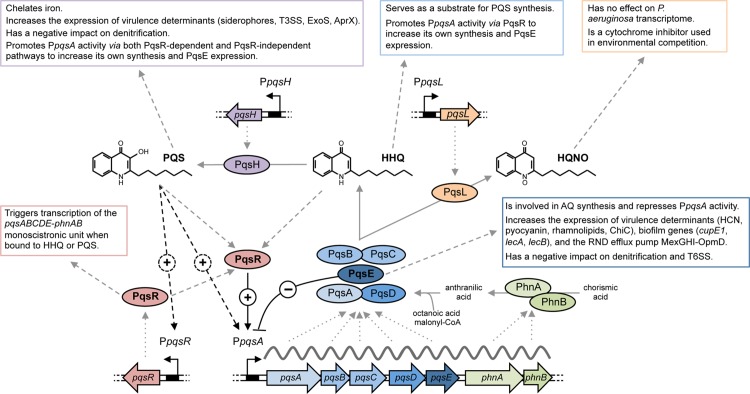
The pqs system incorporates at least four transcriptional units, with pqsABCDE (PA0996-PA1000) and pqsR (PA1003) clustering at the same genetic locus, while pqsH (PA2587) and pqsL (PA4190) are distally located [13]. Our understanding of the molecular mechanisms governing pqs-dependent QS is however limited, largely because of the inter-dependent, auto-regulatory, multi-component nature of the system (Fig 1) [9].
Fig 1. The AQ biosynthetic pathway and pqs genes.
Schematic representation of the AQ biosynthetic pathway and the pqs and phn genes in P. aeruginosa PAO1 and the isogenic ∆4AQ and ∆5AQ mutants. Main elements of the pqs QS system (HHQ, PQS, HQNO, PqsE, and PqsR) are in bold face. The PA number is indicated below the genes according to the Pseudomonas Genome Database [13]. Solid grey arrows represent biosynthesis; dashed grey arrows represent information flow; solid black arrow indicates activation (+); black T-line indicates negative regulation (-).
P. aeruginosa produces >50 different AQs [14] of which 2-heptyl-3-hydroxy-4-quinolone (also known as the Pseudomonas Quinolone Signal, PQS) and its immediate precursor 2-heptyl-4-hydroxyquinoline (HHQ) are most closely associated with QS signalling. Most of the genes required for AQ biosynthesis are located in the pqsABCDE operon (Fig 1). PqsA converts anthranilic acid to anthraniloyl-CoA that is condensed with malonyl-CoA to form 2-aminobenzoylacetyl-CoA (2-ABA-CoA) in a reaction catalysed by PqsD [15,16]. The thioesterase activity of PqsE converts 2-ABA-CoA into 2-aminobenzoylacetate (2-ABA) [17]; HHQ is formed through the condensation of octanoyl-coenzyme A and 2-ABA via the PqsBC heterodimer [18,19].
Additional enzymes are required for the biosynthesis of PQS and 2-heptyl-4-hydroxyquinoline N-oxide (HQNO) (Fig 1). Under aerobic conditions, HHQ is oxidized to PQS via the action of the monooxygenase PqsH [20]. A second monooxygenase, PqsL, is required together with the pqsABCD gene products for the synthesis of HQNO and related N-oxides [21].
The pqs system is subject to positive autoregulation, since the LysR-type transcriptional regulator PqsR (MvfR), binds to the promoter region of pqsABCDE (PpqsA) and triggers transcription once activated by HHQ or PQS (Fig 1) [22–24]. Therefore, by analogy with other QS systems, HHQ and PQS act as autoinducers by generating a positive feedback loop that accelerates their biosynthesis. Although both HHQ and PQS can function as QS signal molecules, it is not clear whether this regulatory effect is only exerted via PqsR, or also via PqsR-independent pathways. Although both HHQ and PQS activate PqsR, PQS has additional properties. For example, PQS promotes the formation of membrane vesicles (MVs) in which PQS is both bioactive and bioavailable [25], although it is not essential for MV formation [26]. The 3-hydroxy substituent also confers on PQS the ability to chelate ferric iron (Fe3+) [27]. Consequently, exogenous PQS triggers an iron-starvation response in P. aeruginosa, promoting the production of the siderophores, pyoverdine and pyochelin [27,28]. However, PQS cannot be considered as a siderophore sensu stricto, since it does not stimulate growth of a siderophore-defective P. aeruginosa mutant in iron-deficient growth conditions [27]. PQS appears instead to act as an iron trap associated with the outer membrane. In this context, the iron-chelating property of PQS may confer a survival advantage to P. aeruginosa in mixed bacterial populations by limiting the availability of iron to co-inhabitant species [29].
HQNO also contributes to the environmental competitiveness of P. aeruginosa, since it is a potent inhibitor of the cytochrome bc 1 complex [30]. At present, the role played by HQNO in P. aeruginosa physiology and the mechanism by which HQNO self-poisoning is avoided, have not been determined.
Mutations in the pqsA, pqsB, pqsC or pqsD biosynthetic genes or in the regulatory gene pqsR, all abolish AQ production, while P. aeruginosa pqsH and pqsL mutants accumulate either HHQ and HQNO or HHQ and PQS, respectively [9,14,31]. Notably, while PqsE converts 2-ABA-CoA to 2-ABA, a mutation in pqsE does not affect AQ biosynthesis [11]. This is probably because the PqsE thioesterase functionality can be provided by alternative thioesterases [17]. PqsE over-expression however completely abrogates PpqsA activity (Fig 1), and consequently AQ biosynthesis [11].
Although PqsE is dispensable for AQ biosynthesis, it is required for production of key virulence factors, such as pyocyanin, elastase, rhamnolipids, hydrogen cyanide, LecA lectin, and for biofilm maturation [11]. The activity of PqsE is also dependent on the N-butanoyl-homoserine lactone (C4-HSL) receptor RhlR, which acts downstream but in synergy with PqsE [32]. Therefore, it is likely that PqsE has, as yet unidentified, functions in addition to its thioesterase activity [17]. Transcriptomic analyses have revealed that the expression of multiple genes requires pqsE, and that full virulence in plant and animal infection models is strongly dependent on this enzyme [6,11]. Although the crystal structure of PqsE has been solved and key active site residues identified, the mechanism by which it controls P. aeruginosa virulence gene expression is not understood [17,33].
Since the HHQ- and PQS-dependent activation of pqsABCDE transcription results in increased levels of both AQs and PqsE, it is possible that functional effects previously considered to be HHQ- and/or PQS-dependent are mediated via PqsE. Alternatively, since PqsE over-expression abrogates AQ biosynthesis, some phenotypes altered as a consequence of increased pqsE expression may, at least in part, be under the control of HHQ and/or PQS. PqsE controls the expression of some virulence genes independent of contribution to AQ biosynthesis. The major reductions in LecA and pyocyanin production in an AQ-negative pqsA mutant for example could be restored fully by expressing pqsE from an inducible tac promoter [11]. Thus, the autoregulatory effect exerted by PqsE on its own transcription plays a homeostatic role in limiting AQ accumulation, thus impeding a clear understanding of the physiological role(s) played by PqsE.
Fig 1 shows how the pqs system components are interlinked. HHQ and PQS both induce transcription of the pqsABCDE operon via PqsR, increasing AQ biosynthesis and pqsE expression. The latter in turn, exerts a repressive role on both AQ production and its own expression. This complexity has obscured comprehension of the physiological roles played by specific AQs and PqsE. Characterization of the regulons controlled by individual components of the pqs system has not yet been reported. For example, the genes controlled via the pqs system have been investigated by comparing the transcriptional profiles of P. aeruginosa PA14 wild type and its pqsH isogenic mutant [23] by evaluating the effect of exogenous PQS on the P. aeruginosa PAO1 transcriptome [28] or by comparing the wild type PAO1 with pqsA or pqsE mutants [11]. In each case, numerous genes including those involved in virulence factor production, iron homeostasis and denitrification, appeared to be PQS-controlled. However, since, in the strains used, altered PQS levels led to dysregulation of HHQ and HQNO synthesis and pqsE expression, it is not possible to discriminate between the role(s) played by PQS from that of the other components of the pqs system. Similarly, it is not possible to determine whether PqsE-controlled genes in strains overexpressing this protein are controlled by PqsE itself or by the lack of AQs resulting from pqsE overexpression [11].
To circumvent these limitations, a P. aeruginosa PAO1 mutant unable to synthesize AQs or convert exogenously supplied AQs was constructed. In this strain, termed ∆4AQ, pqsE expression is chemically inducible and uncoupled from the activity of the PpqsA promoter, thus exogenous AQ provision does not alter PqsE levels. Transcriptomic analyses were performed on the ∆4AQ strain grown in the absence or in the presence of either HHQ, PQS or HQNO, or the exogenous inducer of PqsE expression (IPTG), thus enabling identification of the specific genes controlled by each pqs system component. Transcriptomic analyses were also performed on strains with pqsR-proficient or pqsR-deficient (∆5AQ) genetic backgrounds to elucidate the physiological role(s) played by the transcriptional regulator PqsR.
Results and Discussion
Characterization of HHQ, PQS, HQNO and PqsE regulons
To identify the regulons controlled individually by HHQ, PQS, HQNO and PqsE, a quadruple mutant of P. aeruginosa PAO1, named ∆4AQ, was constructed. As depicted in Fig 1, this carries in frame deletions of pqsA, pqsH and pqsL genes, and incorporates an isopropyl β-d-l-thiogalactopyranoside (IPTG)-inducible pqsE gene. Preliminary experiments were performed to validate the ∆4AQ strain. P. aeruginosa PAO1 wild type was grown in LB, while the isogenic ∆4AQ mutant was grown in LB or in LB supplemented with either HHQ, PQS, or HQNO (40 μM), or with IPTG (1 mM). All strains were grown to late exponential phase where the pqs system is maximally expressed [11]. Cell-free spent media and bacterial cells were respectively collected for determination of AQ levels by LC-MS/MS, and for quantification of pqsE mRNA levels by Real Time PCR. HHQ, PQS and HQNO were only recovered from the ∆4AQ cultures if exogenously added, and were not converted into other AQs (S1A Fig). Moreover, the P. aeruginosa ∆4AQ strain grown in the absence of IPTG showed only basal levels of pqsE RNA (∆4AQ to wild type ratio ~ 0.2), irrespective of the presence or absence of AQs while IPTG addition increased pqsE RNA levels by ~15-fold relative to the parental strain (S1B Fig) [11]. Growth of the ∆4AQ strain was not affected by exogenous provision of any AQ or IPTG (S1C Fig).
The transcriptional profiles of the ∆4AQ strain grown with 40 μM of HHQ, PQS or HQNO or with IPTG were compared by means of high-density oligonucleotide microarrays, using Affymetrix GeneChip for P. aeruginosa PAO1. This method was chosen to provide a reliable comparison with previously published data [6,10,11,23,28].
Following statistical validation of the dataset, only genes with a fold change > 2.5 and a q-value < 0.05 were considered for further analysis [34]. Table 1 lists the selected genes (see S1 Table for complete list) satisfying this cut-off and hence significantly controlled by the AQs and/or by PqsE. In brief, the RNA levels for 0, 3, 145, and 182 genes were significantly altered by HQNO, HHQ, PqsE and PQS respectively (Fig 2).
Table 1. Selected genes whose transcription is controlled by HHQ, PQS and/or PqsE.
| PA number a | Gene name a | HHQ b | PQS c | PqsE d | Product name a |
|---|---|---|---|---|---|
| PA0051 | phzH | 3.2 (3.6) | Potential phenazine-modifying enzyme | ||
| PA0083 | tssB1 | -5.1(-1.4) | TssB1 | ||
| PA0084 | tssC1 | -3.5 (-1.3) | TssC1 | ||
| PA0263 | hcpC | -4.3 | Secreted protein Hcp | ||
| PA0997* ∫ ‡ ◊ | pqsB | 6.7 | 17.5 | PqsB | |
| PA0998* ∫ ‡ ◊ | pqsC | 5.5 | 16.1 | PqsC | |
| PA0999* ∫ ‡ ◊ | pqsD | 5.8 | 15.7 | 3-oxoacyl-[acyl-carrier-protein] synthase III | |
| PA1000* ∫ ◊ | pqsE | 22.8 (140.3) | Quinolone signal response protein | ||
| PA1001* ∫ ◊ | phnA | 26.2 (ncd) | Anthranilate synthase component I | ||
| PA1002* ∫ ◊ | phnB | 22.4 (ncd) | Anthranilate synthase component II | ||
| PA1245 § ∫ | aprX | 3.9 | AprX | ||
| PA1706 | pcrV | 2.7 | Type III secretion protein PcrV | ||
| PA1707 | pcrH | 3.1 | Regulatory protein PcrH | ||
| PA1708 | popB | 5.6 | Translocator protein PopB | ||
| PA1709 | popD | 3.0 | Translocator outer membrane protein PopD precursor | ||
| PA1710 | exsC | 3.5 | ExsC exoenzyme S synthesis protein C precursor | ||
| PA1711 | exsE | 3.1 | ExsE | ||
| PA1712 | exsB | 2.6 | Exoenzyme S synthesis protein B | ||
| PA1718 | pscE | 4.3 | Type III export protein PscE | ||
| PA1901 ∫ | phzC2 | 5.5 (6.3) | Phenazine biosynthesis protein PhzC | ||
| PA1902 § | phzD2 | 7.5 (9.8) | Phenazine biosynthesis protein PhzD | ||
| PA1903 ∫ | phzE2 | 8.8 (9.6) | Phenazine biosynthesis protein PhzE | ||
| PA1904 | phzF2 | 10.3 (9.9) | Probable phenazine biosynthesis protein | ||
| PA1905 | phzG2 | 9.7 (9.6) | Probable pyridoxamine 5'-phosphate oxidase | ||
| PA2193* | hcnA | 3.6 (2.1) | Hydrogen cyanide synthase HcnA | ||
| PA2194* | hcnB | 3.1 (1.7) | Hydrogen cyanide synthase HcnB | ||
| PA2195* | hcnC | 3.0 (1.6) | Hydrogen cyanide synthase HcnC | ||
| PA2300* ∫ ◊ | chiC | 18.7 (8.2) | Chitinase | ||
| PA2426 | pvdS | 43.9 | Sigma factor PvdS | ||
| PA2570* ∫ ◊ | lecA | 26.3 (15.1) | LecA lectin | ||
| PA3361* ◊ | lecB | 8.5 (10.4) | Fucose-binding lectin LecB | ||
| PA3391 | nosR | -4.6 | -4.1 | Regulatory protein NosR | |
| PA3478* | rhlB | 3.6 (2.3) | Rhamnosyltransferase chain B | ||
| PA3479 | rhlA | 3.6 (2.3) | Rhamnosyltransferase chain A | ||
| PA3841 | exoS | 2.6 | Exoenzyme S | ||
| PA3842 | spcS | 3.9 | Specific Pseudomonas chaperone for ExoS, SpcS | ||
| PA4175 | prpL | 3.7 | PrpL, protease IV | ||
| PA4205* ∫ ◊ | mexG | 25.0 (47.2) | Hypothetical protein | ||
| PA4206* ∫ ◊ | mexH | 16.4 (28.8) | Probable RND efflux membrane fusion protein precursor | ||
| PA4207* ∫ ◊ | mexI | 18.5 (18.2) | Probable RND efflux transporter | ||
| PA4208* ∫ ◊ | opmD | 11.6 (9.3) | Probable outer membrane protein precursor | ||
| PA4209* ∫ ◊ | phzM | 4.1 (8.7) | Probable phenazine-specific methyltransferase | ||
| PA4210 ◊ | phzA1 | 10.2 (16.2) | Probable phenazine biosynthesis protein | ||
| PA4211* ◊ | phzB1 | 5.6 (10.1) | Probable phenazine biosynthesis protein | ||
| PA4217* § ◊ | phzS | 9.0 (9.3) | Flavin-containing monooxygenase | ||
| PA4227 ∫ ‡ | pchR | 11.2 | Transcriptional regulator PchR | ||
| PA4468 § | sodA | 89.4 | Superoxide dismutase | ||
| PA4470 § ∫ | fumC1 | 114.7 | Fumarate hydratase | ||
| PA4648 | cupE1 | 3.0 (2.2) | Pilin subunit CupE1 |
a PA number, gene name and product name are from the Pseudomonas Genome Database [13]. Genes previously reported as controlled by iron-starvation are in bold characters [40,41].
*, genes whose transcription was altered in the ∆pqsR mutant with respect to the wild type strain [10]
§, genes whose transcription was altered upon exogenous PQS provision [28]
∫, genes whose transcription was altered in the ∆pqsA mutant with respect to the wild type strain [11]
‡, genes whose transcription was altered upon PqsE overexpression [11]
◊, genes whose transcription was altered in the ∆pqsH mutant with respect to the wild type strain [23]. RND, Resistance-Nodulation-Cell division; MFS, major facilitator superfamily.
b Fold change in gene expression in P. aeruginosa PAO1 ∆4AQ grown in the LB supplemented with 40 μM HHQ with respect to the same strain grown in LB.
c Fold change in gene expression in P. aeruginosa PAO1 ∆4AQ grown in the LB supplemented with 40 μM PQS with respect to the same strain grown in LB.
d Fold change in gene expression in P. aeruginosa PAO1 ∆4AQ grown in the LB supplemented with 1 mM IPTG (to induce PqsE expression) with respect to the same strain grown in LB; in brackets is indicates the fold change in gene expression in P. aeruginosa PAO1 ∆pqsAHLE pUCPpqsE with respect to P. aeruginosa PAO1 ∆pqsAHLE pUCP18, both grown in LB. For the microarray analysis performed in the ∆pqsAHLE background, fold changes with a q value < 0.05 are indicated for selected virulence related genes, irrespective of the fold change. ncd, no change detected (q value > 0.05).
Fig 2. The AQ and PqsE regulons.
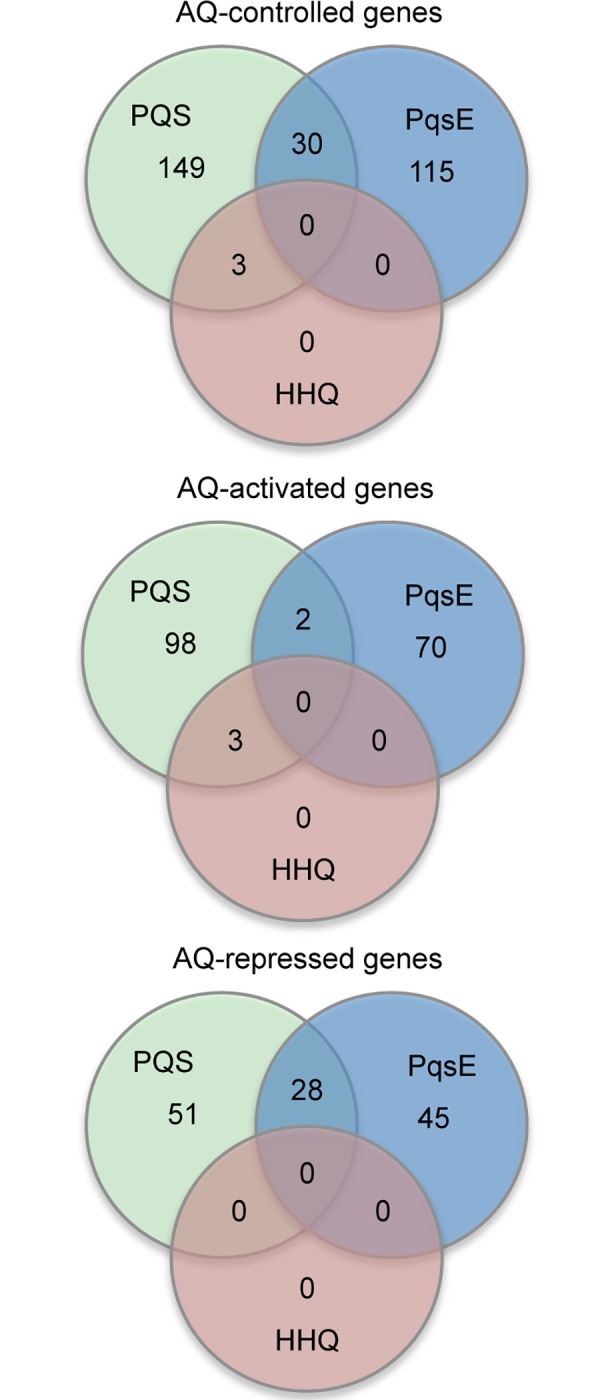
Venn diagrams showing the number of genes controlled by HHQ, PQS, and PqsE in P. aeruginosa Δ4AQ, and the overlap between the regulons.
HQNO had no effect on the P. aeruginosa ∆4AQ transcriptome indicating that this AQ does not function as a QS signal, implying an alternative role for HQNO in P. aeruginosa physiology. Since HQNO is a potent cytochrome bc 1 complex inhibitor [9], it is likely that HQNO acts primarily as a secondary metabolite that increases the environmental competitiveness of P. aeruginosa.
Notably, only 3 genes were significantly controlled by HHQ, namely pqsB, pqsC and pqsD (Table 1; Fig 2). This suggests that HHQ controls only the pqsABCDE transcriptional unit so driving the positive feedback loop. The positive effect of HHQ on PpqsA activity is mediated by PqsR [22,24], such that the primary role of HHQ as a signal is to induce the PqsR-dependent expression of the pqsABCDE transcriptional unit, ultimately resulting in increased AQ biosynthesis and pqsE expression. As expected, the pqsB, pqsC and pqsD genes were also identified among the genes up-regulated by PQS (Table 1).
In the Δ4AQ mutant background, PqsE emerges as a major effector of the pqs QS system, since the microarray analysis revealed it controls the expression of 145 genes in the ∆4AQ strain, an AQ-negative background in which PqsE is unable to down-regulate AQ production. In particular, 72 genes were up-regulated and 73 down-regulated upon IPTG-induction of pqsE expression (Fig 2; S1 Table).
The 72 genes up-regulated by pqsE expression, included the pyocyanin biosynthetic genes (phzA, phzB, phzC, phzD, phzE, phzF, phzG, phzM, phzS), the hcnABC operon required for hydrogen cyanide biosynthesis, rhlA and rhlB, required for rhamnolipid biosynthesis, and chiC, coding for the extracellular chitinase ChiC. Moreover, PqsE exerted a positive effect on the transcription of genes involved in biofilm development e.g. cupE1, lecA and lecB, explaining the positive control of PqsE on biofilm formation [11], and on the mexGHI-opmD operon, coding for a Resistance-Nodulation-Cell division (RND) efflux pump involved in antibiotic resistance that is also essential for pqs-dependent QS. This is because mexG and opmD mutants are both avirulent in plant and rat infection models and fail to produce PQS, probably as a consequence of the intracellular accumulation of a toxic AQ metabolite [35]. Furthermore, pyocyanin functions as a signal in the P. aeruginosa QS network because it induced changes in the expression of over 50 genes (23 up-regulated and 29 down-regulated) [36]. Of these only mexGHI-opmD, PA2274 and PA3250 were also up-regulated via PqsE rather than PQS (Tables 1 and S1). Hence, although PqsE controls pyocyanin biosynthesis, it only regulates a sub-set of pyocyanin-dependent genes.
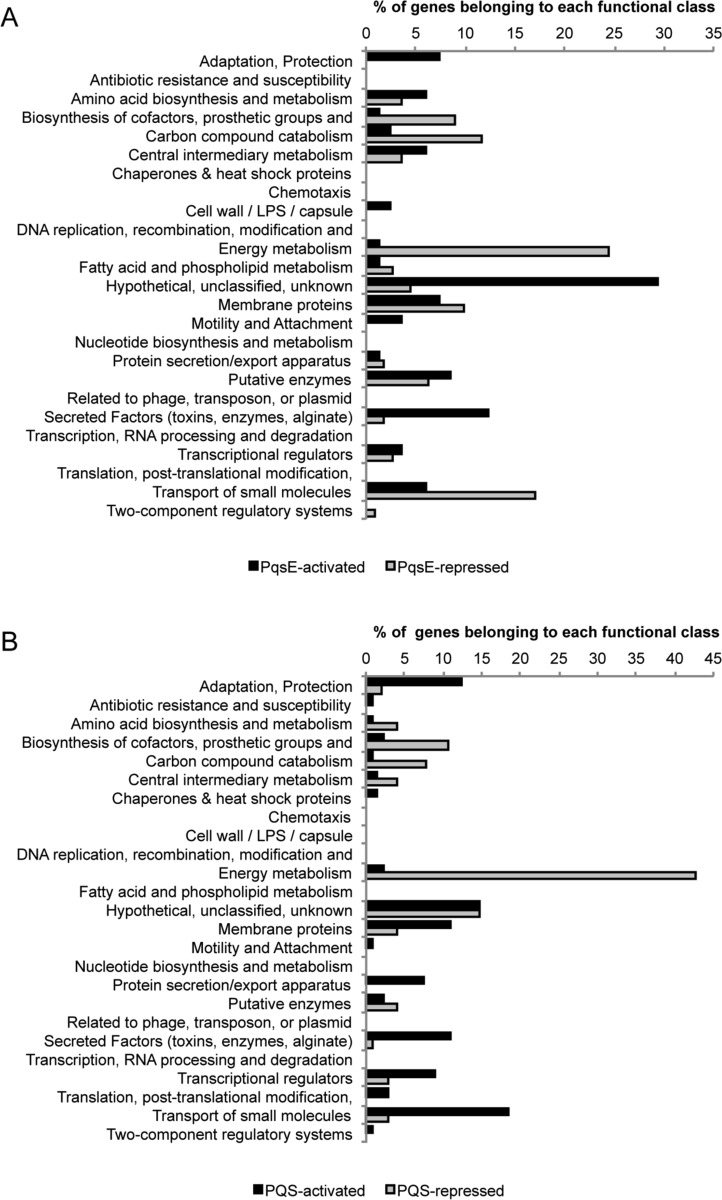
As shown in Fig 3A, many of PqsE up-regulated genes belong to the “Secreted Factors (toxins, enzymes, alginate)” and “Adaptation, Protection” functional classes (12.2% and 7.3%, respectively), highlighting the importance of PqsE in P. aeruginosa adaptive behaviour and virulence. However, most of the PqsE up-regulated genes (29.3%) are classified as “Hypothetical, unclassified, unknown”, limiting our comprehension of its physiological role.
Fig 3. Functional classes of PqsE and PQS controlled genes.
Histograms representing the distribution of (A) PqsE-controlled and (B) PQS-controlled genes according to their functional classification. Functional classes are from the Pseudomonas Genome Database [13].
With respect to the 73 genes down-regulated upon pqsE induction, they are mainly involved in energy metabolism and anaerobic respiration, including gapA, coding for glyceraldehyde 3-phosphate dehydrogenase, and almost all the nir, nor, nar, and nos genes (S1 Table). Indeed, the majority of the PqsE-repressed genes cluster in the “Energy metabolism” (24.3%), “Transport of small molecules” (17.1%), “Carbon compound catabolism” (11.7%) and “Biosynthesis of cofactors, prosthetic groups, and carriers” (9.0%) functional classes (Fig 3A). However, the physiological relevance of this repression is not clear, since the IPTG-mediated induction of PqsE does not affect bacterial growth, at least under aerobic conditions. It is also noticeable that two genes involved in type 6 secretion (T6SS; tssB1 and tssC1) are down-regulated in response to pqsE induction (Table 1).
The global effect exerted by PqsE on the P. aeruginosa transcriptome is unlikely to be direct, since this protein does not possess a DNA-binding domain [37]. Moreover, PqsE activity is not exclusively a consequence of its thioesterase activity since pqsE expression is sufficient to restore pyocyanin in an AQ-deficient (pqsA mutant) background [6,11]. Hence, the multifunctional activity of PqsE may conceivably be a consequence of a pqsE regulatory RNA acting on the expression of pqsE-controlled genes. To investigate this possibility we quantified pyocyanin production in P. aeruginosa PAO1 ∆pqsA ∆pqsE double mutant strains carrying plasmids for IPTG-inducible expression of wild type pqsE, or pqsE mutated variants lacking the first two codons (pqsE∆1–6) or with a nucleotide insertion after the ATG to alter the protein frame (pqsENoFrame). As shown in S2A Fig, pyocyanin production in the P. aeruginosa PAO1 ∆pqsA ∆pqsE strains was restored only upon complementation with wild type pqsE, despite the presence of a pqsE transcript in the mutated variants (S2B Fig). These data suggest that the activity is not due to the pqsE RNA transcript but requires the PqsE protein, a finding that suggests PqsE has independent regulatory and thioesterase enzymatic functions.
When the ∆4AQ strain was grown with IPTG, the phnAB operon was also up-regulated (Table 1). Knoten and co-workers reported that when P. aeruginosa PAO1 was grown in nutrient limiting conditions but not in LB, pqsE and phnAB were co-transcribed [38]. However, RT-PCR analysis of the PAO1 strain used in this study indicates that pqsE and phnAB are co-transcribed after growth in LB (S3 Fig), a finding consistent with the fold change increases quantified for pqsE (22.8), phnA (26.2) and phnB (22.4) upon addition of IPTG to the ∆4AQ strain (Table 1). Although anthranilate is an AQ precursor [15], pqsE-overexpression results in the abrogation of AQ production via the strong repression of PpqsA promoter [11]. This repression is not apparent in our microarray analysis as a consequence of the lack of HHQ- and PQS-dependent PpqsA activation in the ∆4AQ strain.
Anthranilate for AQ biosynthesis can be supplied by the anthranilate synthases TrpEG and PhnAB or via the kynurenine pathway that converts tryptophan into anthranilate [38]. The latter is the main source of anthranilate for PQS biosynthesis when tryptophan is present, and PhnAB appears to supply anthranilate only under nutrient-limiting conditions [38]. Consequently the increased transcription of phnAB following the IPTG-dependent induction of pqsE in the ∆4AQ strain may increase intracellular anthranilate levels and so impact on gene expression independent of PqsE. To explore this possibility, we first quantified intracellular anthranilate levels in the ∆4AQ strain grown in LB or in LB supplemented with 1 mM IPTG. S4A Fig shows that the IPTG-induced increase in phnAB expression did not result in higher levels of intracellular anthranilate. The slightly higher concentration of anthranilate in the ∆4AQ strain in the absence of IPTG may be a consequence of the PqsE-mediated increases in the expression of genes such as antR and catB that are involved in the degradation of anthranilate (S1 Table). To confirm these data, we also compared the transcriptional profiles of a P. aeruginosa PAO1 quadruple mutant strain with deletions in pqsA, pqsH, pqsL and pqsE (∆pqsAHLE) and carrying a plasmid-borne copy of pqsE or the empty vector (pUCP18). The data obtained for selected virulence related genes are summarized in Table 1. The plasmid-mediated expression of pqsE in the ∆pqsAHLE genetic background did not affect phnAB expression. In addition, the data obtained was broadly consistent with that obtained for the inducible pqsE construct with respect to the genes involved in virulence factor production, biofilm formation and antibiotic resistance (Table 1). In addition, we validated the data with respect to the pyocyanin biosynthetic genes by introducing PphzA1::lux and PphzA2::lux transcriptional fusions onto the chromosome of the ∆pqsAHLE strain, since the microarray experiments cannot discriminate between the two phz operons as they are almost identical at the DNA level [39]. The results obtained with the reporter fusions confirm the microarray data and reveal that PqsE is responsible for driving the expression of phzA1 but not phzA2 (S4B Fig).
Despite the structural similarity between HHQ and PQS and their ability to activate PqsR via the same ligand binding site [24], the microarray data revealed that, in contrast to HHQ, PQS regulates the expression of 182 genes. In particular, 103 genes were up-regulated and 79 genes were down-regulated in response to exogenous PQS (Fig 2; S1 Table). The major proportion of PQS up-regulated genes (75%) are also induced by iron-starvation [40,41]. These consist of almost all the genes involved in the biosynthesis, uptake and response to the siderophores pyoverdine and pyochelin, including the regulatory genes pvdS and pchR. Moreover, metabolic and virulence genes previously shown to be induced by iron-starvation were strongly up-regulated by PQS, including fumarate hydratase (fumC1), superoxide dismutase (sodA) and two proteases (prpL and aprX) (Table 1). These findings are consistent with the iron-chelating activity of PQS inducing an iron-starvation response [27,28]. In addition to the iron-regulated genes, PQS increased the transcription of genes involved in Type 3 secretion (T3S; pcrV, pcrH, popB, popD, exsC, exsE, exsB, and pscE), and coding for both exotoxin ExoS (exoS) and its chaperone SpcS (spcS), indicating that PQS, independent of PqsE, contributes to P. aeruginosa virulence gene regulation (Table 1). PQS production has been indirectly linked to the regulation of T3S effector secretion in P. aeruginosa at the post-transcriptional level [42]. Interestingly, the rhl QS system that represses both pqsA and pqsR also negatively regulates exoS [43]. As anticipated, pqsB, pqsC and pqsD genes were all up-regulated by PQS (Table 1).
The 79 PQS-repressed genes mainly cluster in the “Energy metabolism” (42.7%), “Biosynthesis of cofactors, prosthetic groups, and carriers” (10.7%), and “Carbon compound catabolism” (7.8%) functional classes (Fig 3B). The repression exerted by PQS on certain metabolic genes could be due to its interaction with membranes and consequent perturbation of associated energy generation. Almost all the genes involved in denitrification (nir, nor, nar, and nos genes) are also down-regulated by PQS (S1 Table). These data are in line with previous work demonstrating that PQS represses anaerobic growth of P. aeruginosa by inhibiting denitrifying enzymes [44].
A comparison of the genes regulated by PQS and PqsE revealed that they control quite distinct regulons and up-regulate different sets of virulence genes (Table 1). The PQS and PqsE regulons only share 30 genes (Fig 2). Notably, 28 genes independently down-regulated by PQS and PqsE are all involved in denitrification (nir, nor, nar, and nos genes; S1 Table), indicating that there is some redundancy in the pqs system.
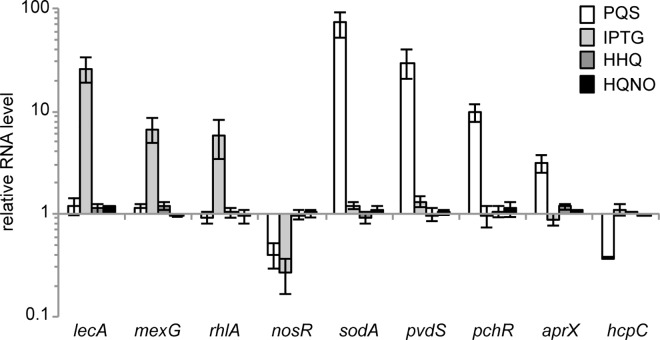
The reliability of the microarray data is supported by the observation that HHQ controls only one transcriptional unit, HQNO does not affect transcription under the growth conditions employed whereas PQS regulates 182 genes. Differential expression of selected genes by PQS or PqsE in the microarray experiment was validated by Real Time PCR analysis. A comparison between Table 1 and Fig 4 shows that the results obtained match the microarray data, since the mRNA levels of the lecA, mexG and rhlA genes increased upon IPTG-dependent induction of PqsE, while nosR decreased. Similarly, PQS increased the transcription of sodA, pvdS, pchR, and aprX but repressed nosR and hcpC. PqsE did not affect the transcript levels of PQS-controlled genes (i.e., sodA, pvdS, pchR, aprX and hcpC), and conversely PQS did not alter PqsE-regulated transcript levels (i.e., lecA, mexG and rhlA). Moreover, none of these transcripts were affected by HHQ or HQNO (Fig 4).
Fig 4. Validation of the microarray data by Real Time PCR.
Relative mRNA levels of the genes indicated quantified by Real Time PCR in the P. aeruginosa ∆4AQ strain grown in LB supplemented with 1 mM IPTG to induce PqsE expression (light-grey bars), or with 40 μM PQS (white bars), HHQ (dark-grey bars), or HQNO (black bars), with respect to the same strain grown in LB. The average of two independent analyses each performed on three technical replicates is shown with standard deviations.
The potential effects of the AQs and/or PqsE on pqsH or pqsL transcription cannot be inferred from the microarray analysis, since both genes were deleted in the ∆4AQ strain. Therefore, the expression of chromosomal pqsH and pqsL lux promoter fusions was investigated in the P. aeruginosa ∆4AQ strain. Neither promoter was influenced by exogenous HHQ, PQS or HQNO or by pqsE induction (S5 Fig). Thus, the autoregulatory activity of PQS is not directly exerted at the level of pqsH transcription, and HQNO has no effect in promoting its own biosynthesis. These data imply that a positive feedback loop exists in the pqs QS system only at the level of the pqsABCDE-phnAB transcriptional unit, with HHQ and PQS promoting their own biosynthesis by inducing PpqsA activity via PqsR.
Overall, our microarray experiments are consistent with previously published transcriptomic analyses highlighting the contributions of the pqs system to virulence factor production, ferric iron acquisition and energy metabolism [6,10,11,23,28]. However, our approach enabled us to discriminate between the physiological roles played by the distinct elements of the pqs QS system. For example, both PQS and PqsE were reported to affect iron-controlled genes, probably because in previous experimental settings, PqsE could control AQ biosynthesis [6,11]. However, our data demonstrate clearly that PQS but not PqsE, regulates the iron-regulated genes. Similarly, certain PqsE-controlled virulence factors (e.g., pyocyanin, lectins, ChiC chitinase and the MexGHI-OpmD efflux pump) were reported to be PQS-controlled, probably because in previous experiments the addition of synthetic PQS or the abrogation of PQS synthesis (in pqsR, pqsA or pqsH mutants) led to dysregulation of pqsE expression [10,23,28]. Moreover, the use of the ∆4AQ strain provides clear evidence that HQNO does not influence the P. aeruginosa transcriptome, and that HHQ exclusively regulates the pqsABCDE-phnAB transcriptional unit. Therefore, HHQ activity ultimately leads to the indirect control of specific physiological processes by increasing the expression of the effector protein PqsE and by acting as a substrate for PQS biosynthesis.
Comparison of the PQS and PqsR regulons reveals a PqsR-independent PQS regulon
Despite the structural similarity of the two AQs, HHQ controls the transcription of a single transcriptional unit (i.e., pqsABCDE-phnAB), while PQS regulates 182 genes. Since both AQs act as PqsR co-inducers [22,24], it is possible that the PqsR-HHQ complex only affects PpqsA activity, while the PqsR-PQS complex acts more globally as do other QS regulators such as LasR. However, PQS appears to influence gene expression via PqsR-dependent and PqsR-independent mechanisms, for example, by inducing an iron-starvation response [27,28].
To discriminate between PqsR-dependent and PqsR-independent PQS regulons, and to characterize the PqsR regulon itself, pqsR was deleted in the ∆4AQ strain, generating the quintuple P. aeruginosa ∆5AQ mutant (Fig 1). The transcriptomes of the ∆4AQ and ∆5AQ mutants, supplemented with PQS (40 μM), were compared. Only 4 genes were significantly down-regulated in the ∆5AQ strain with respect to the ∆4AQ mutant, namely pqsR, pqsB, pqsC, and pqsD (-101.2 < fold change < -186.2). An apparent strong down-regulation of pqsR was expected since this gene has been deleted from P. aeruginosa ∆5AQ. The down-regulation of pqsB, pqsC, and pqsD in P. aeruginosa ∆5AQ strongly suggests that in PAO1 PqsR only triggers the transcription of the pqsABCDE-phnAB operon, and thus the pqsA promoter region is the only target for the PqsR-PQS complex.
Overall, these data imply that, apart from the pqs genes, the other 179 genes identified as PQS-regulated (S1 Table) are controlled by PQS via a PqsR-independent pathway(s). This regulatory activity is likely due, at least in part, to the iron-chelating activity of PQS, consistent with the finding that 77/100 genes up-regulated by PQS in a PqsR-independent manner are known to be induced by iron-starvation [40,41]. In contrast the 79 genes down-regulated by PQS have not previously been reported to be repressed in low-iron media. PQS could conceivably also control other phenotypes in both an iron- and a PqsR-independent manner through direct interactions with the outer membrane [25] or by acting as a pro- or anti-oxidant [45].
PQS activates PpqsA via both PqsR-dependent and PqsR-independent pathways
The transcriptome analysis performed on the ∆4AQ strain indicates that PQS is more potent than HHQ in activating transcription of the pqsABCDE-phnAB operon in LB medium (Table 1). This is also consistent with a chromosomal reporter PpqsA::lux fusion in a P. aeruginosa pqsAH mutant, which cannot convert exogenously supplied HHQ to PQS, where EC50 values of 16.4±2.6 μM and 3.8±1.6 μM for HHQ and PQS respectively have been determined in LB medium [24]. However, HHQ activates the PpqsA promoter at a similar level to PQS when the bacteria are grown in an iron-deficient casamino acids (CAA) medium [27]. This suggests that PQS is more effective than HHQ in stimulating PpqsA activity because it induces an iron-starvation response. This hypothesis would be in agreement with iron-chelating activity of PQS, with the evidence that high-iron concentrations negatively impact on PpqsA activity [6].
To investigate this possibility, the effect of HHQ, PQS and 2-heptyl-3-amino-4-quinolone (3-NH2-PQS) on both PpqsA and PpqsR activation were compared in the ∆4AQ (pqsR-proficient) and ∆5AQ (pqsR-mutant) strains, grown in LB with or without 100 μM FeCl3. 3-NH2-PQS is a potent PqsR agonist (EC50 0.4±0.1 μM) isosteric with PQS but lacking iron-chelating activity [24]. In addition, although pqsR was not identified among the AQ-controlled genes in the transcriptome analysis (S1 Table) we included the pqsR promoter fusion experiments since it is not possible to exclude a regulatory effect below the fold change cut-off used (> 2.5) that has a significant effect on PpqsA activity.
Consistent with previous work, PQS was more effective than HHQ in up-regulating PpqsA in LB, while HHQ and 3-NH2-PQS induced PpqsA activity at similar levels (Fig 5A). HHQ and 3-NH2-PQS did not induce PpqsA in the pqsR mutant strain ∆5AQ, while PQS exerted a positive, ∼2 fold induction of PpqsA in this genetic background (Fig 5B). HHQ and 3-NH2-PQS had no effect on PpqsR activity, while PpqsR was induced by PQS in both the ∆4AQ and ∆5AQ strains (~1.5 fold; Fig 5C and 5D). Interestingly, the PQS-dependent induction of PpqsA in the ∆4AQ strain was strongly reduced when iron was added to the medium, showing the same activation level as that induced by HHQ and 3-NH2-PQS. Iron supplementation had no effect on PpqsA activity when the promoter was induced with HHQ or 3-NH2-PQS (Fig 5A). The reduced ability of PQS to induce PpqsA activity in the presence of 100 μM FeCl3 is likely due to its inability to induce PpqsA and PpqsR via the PqsR-independent pathway in the presence of high iron concentrations (Fig 5B–5D).
Fig 5. Interplay of HHQ, PQS, PqsR and iron in controlling PpqsA and PpqsR activity.
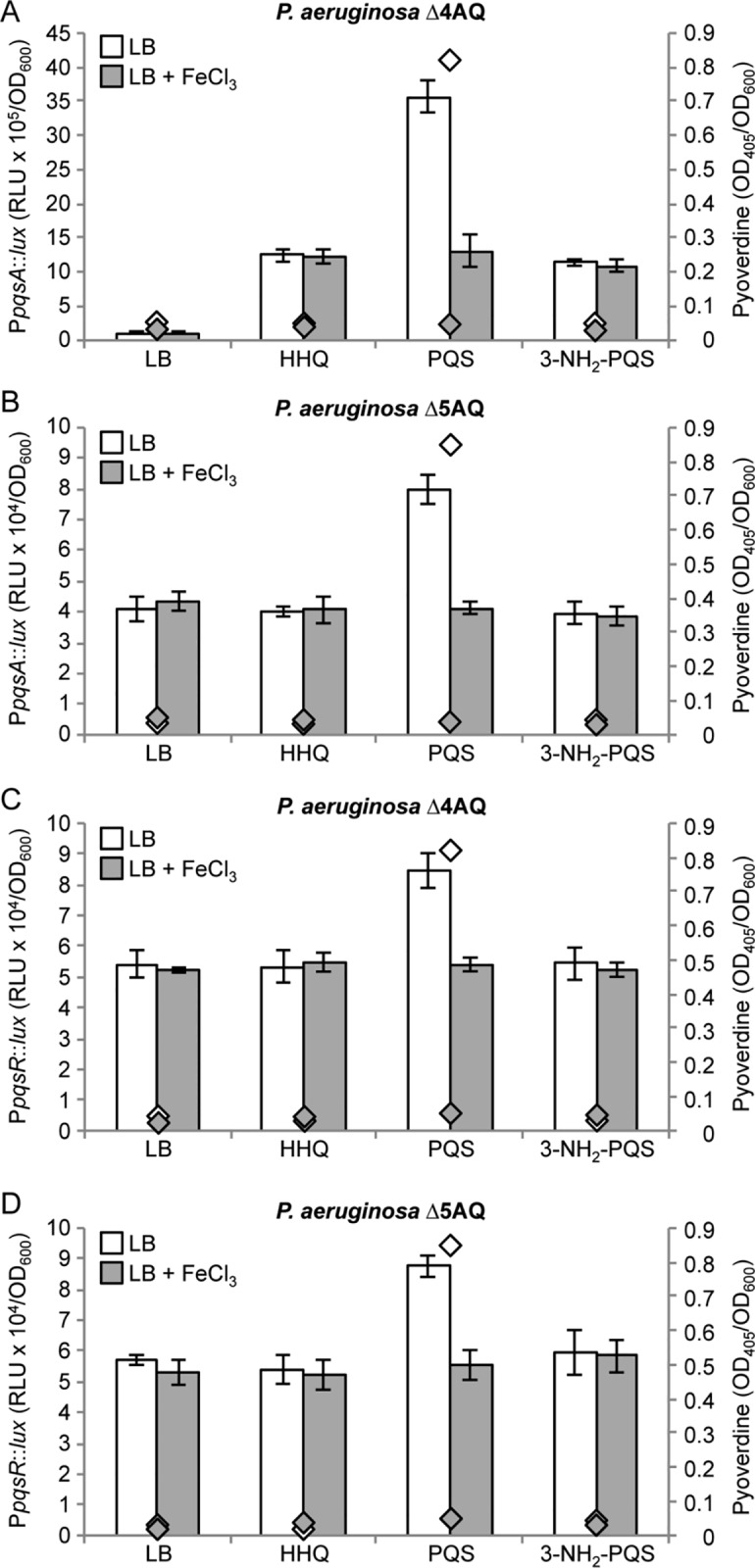
Maximal promoter activity quantified in the indicated strains carrying the transcriptional fusions PpqsA::lux (A and B) or PpqsR::lux (C and D). Strains were grown in LB or in LB supplemented with 40 μM HHQ, PQS or 3-NH2-PQS, as indicated below the graphs, in the absence (white bars) or presence (grey bars) of 100 μM FeCl3. Diamonds indicate the pyoverdine levels in the absence (white diamonds) or in the presence (grey diamonds) of 100 μM FeCl3. Promoter activity and pyoverdine level are reported as Relative Light Units (RLU) and OD405, respectively, normalized to cell density (OD600). The average of three independent experiments is reported with standard deviations.
A plausible explanation for the above results is that the iron-chelating activity of PQS decreases the levels of available iron in LB medium, triggering an iron-starvation response with consequent activation of PpqsA and PpqsR via a PqsR-independent pathway. Indeed, the siderophore pyoverdine, which is only produced under iron limiting conditions [46], is detectable when PQS is added to the ∆4AQ and ∆5AQ cultures, but not when PQS is replaced with HHQ or 3-NH2-PQS, or by excess iron (Fig 5), confirming that PQS triggers an iron-starvation response in LB. This is also in line with increased expression of the Fur-controlled iron-starvation sigma factor PvdS in the presence of PQS (Table 1).
The PqsR-independent activation exerted by PQS on PpqsA and PpqsR is not mediated by the iron-starvation response pathway
To determine whether the ability of PQS to induce the PpqsA and PpqsR promoters via a PqsR-independent pathway simply relies on its iron chelating properties, the effects of PQS and the iron chelators 2,2’-dipyridyl and deferiprone on PpqsA and PpqsR activity were compared in the P. aeruginosa strains ∆4AQ and ∆5AQ by means of transcriptional fusions. 2,2’-Dipyridyl chelates ferrous iron (Fe2+) [47], which is the prevalent intracellular iron species, while deferiprone chelates ferric iron (Fe3+), which prevails in extracellular environment, to form a 3:1 (deferiprone:Fe3+) complex [48], similar to the 3:1 ferric complexes formed by 2-hydroxy-3-alkyl-4-quinolones such as PQS [27]. Both 2,2’-dipyridyl and deferiprone induce iron-starvation in P. aeruginosa [49,50]. The results obtained show that 40 μM PQS, 500 μM 2,2’-dipyridyl or 160 μM deferiprone all triggered similar levels of pyoverdine production in LB-grown cultures. However, neither 2,2’-dipyridyl nor deferiprone induce PpqsA or PpqsR activity, irrespective of the presence of PqsR (S6 Fig). These data strongly suggest that the PqsR-independent effect exerted by PQS on PpqsA and PpqsR does not depend on the ability of PQS to induce an iron-starvation response. Consistent with these findings, the activity of the PpqsA and PpqsR promoters was unchanged in the 4AQ and ∆5AQ strains upon mutation of the pvdS gene that codes for the iron-starvation response sigma factor PvdS (S7A–S7D Fig); no differences in PpqsA and PpqsR activity were observed when these promoters were tested in P. aeruginosa PAO1 wild type and its isogenic ∆pvdS mutant (S7E Fig). Moreover, a Fur Titration Assay (FurTA) [51] revealed that the iron-response regulator Fur does not bind to the PpqsA or PpqsR promoter regions (S7F Fig).
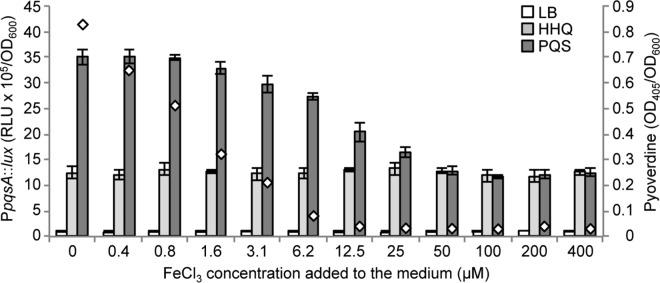
Collectively, these data demonstrate that the ability of PQS to induce PpqsA and PpqsR via a PqsR-independent pathway does not rely on the capacity of PQS to induce an iron-starvation response via the master regulators PvdS and Fur. However, the effect of PQS on both promoters is inhibited when 100 μM FeCl3 is added to the LB medium (Fig 5). To clarify this finding further, the ability of PQS to induce PpqsA activity was determined in P. aeruginosa ∆4AQ grown in LB supplemented with increasing concentrations of FeCl3 without AQs or with either PQS or HHQ. In parallel, pyoverdine levels were determined in the culture supernatants of the ∆4AQ strain grown in the presence of PQS to monitor the activation of the iron-starvation response. As shown in Fig 6, iron had no effect on the ability of HHQ to induce PpqsA activity or on the PpqsA basal level in the absence of AQs. Conversely, increasing concentrations of FeCl3 reduced the ability of PQS to promote PpqsA activity, and inhibited pyoverdine production, consistent with our previous data. Low FeCl3 concentrations (from 0.4 μM to 1.6 μM) were sufficient to decrease the iron-starvation response, as indicated by reduced pyoverdine production, without affecting the PQS-dependent induction of PpqsA. The ability of HHQ and PQS to induce PpqsA was comparable when the medium iron concentration approximated to the theoretical PQS-saturating value, ranging from 12.5 to 25 μM (considering 40 μM PQS and 3:1 ratio of the PQS-Fe3+ complex).
Fig 6. Effect of iron on the ability of PQS to stimulate PpqsA activity.
Maximal PpqsA promoter activity measured in the P. aeruginosa ∆4AQ strain carrying the transcriptional fusion PpqsA::lux, grown in LB (white bars) or in LB supplemented with 40 μM HHQ (light-grey bars) or 40 μM PQS (dark-grey bars), and FeCl3 at the concentration indicated below the graph. White diamonds indicate the pyoverdine level in the supernatants of cultures grown in the presence of PQS with or without FeCl3. Promoter activity and pyoverdine are reported as Relative Light Units (RLU) and OD405, respectively, normalized to cell density (OD600). The average of three independent experiments is reported with standard deviation.
Given that iron reduces the PqsR-independent expression of the pqsA or pqsR promoters in the absence of PvdS and that Fur does not bind to either promoter, our data suggest a regulatory role for PQS in the absence of PqsR. This regulatory activity can however be abolished by increasing the medium iron content. However, of the 179 genes regulated by PQS via PqsR-independent pathways only 77 are controlled by the iron-starvation response. The remaining 102 genes (underlined in S1 Table) are regulated via an iron-starvation-independent and PqsR-independent PQS signalling pathway(s). Most of the repressed genes are involved in energy metabolism (49%) and include the nir, nar, nos genes involved in denitrification. Of the up-regulated genes, 39% are from the protein secretion/export functional class and include T3S genes such as pcrV, exsC, exsE, exoS and spcS. The mechanism by which these genes are regulated is not yet apparent but may be due to the anti-oxidant properties of PQS since these are likely to be inhibited by excess iron [45].
Since PQS promotes PpqsA activity in P. aeruginosa at different levels depending on the availability of iron, the expression of PqsE-controlled virulence factors in iron-poor environments is likely to be higher. In this context, it is tempting to speculate that the iron chelating ability of PQS may contribute to P. aeruginosa environmental fitness both by limiting the availability of iron to competing microorganisms and by increasing the expression of specific sets of genes important in challenging iron-poor environments. This process might be relevant during the colonization of the human host, when P. aeruginosa experiences iron starvation, and implies a new role for PQS as an extracellular iron sensor.
Conclusions
Although the central role of the pqs QS system in the control P. aeruginosa infection processes has been extensively studied, the precise role played by each individual element of this complex regulatory circuit remained to be defined. Here we have filled this knowledge gap by defining the specific genome-wide regulons for HHQ, PQS and HQNO and for the effector PqsE in the presence and absence of PqsR (Fig 7). Of 145 genes regulated via PqsE only 30 were co-regulated by PQS (Fig 2). Among the key genes controlled by PqsE in the absence of AQs are those coding for the MexGHI-OpmD efflux pump and pyocyanin biosynthesis. Although biochemically PqsE functions as a thioesterase in AQ biosynthesis [17], the thioesterase-independent regulatory mechanism controlling gene expression requiring the PqsE protein remains to be elucidated. A striking feature of our transcriptome data is that signalling function of HHQ is simply to drive the expression of the pqsABCDE-phnAB transcriptional unit in a PqsR-dependent manner. These data highlight that unlike LuxR/AHL-based QS systems where the response regulator interacts with the promoters of multiple target genes, PqsR appears to target only one, the pqsABCDE-phnAB operon. Furthermore, HQNO, the N-oxide of HHQ, does not act as a signal molecule. In contrast to HHQ and HQNO, PQS is clearly a multi-functional molecule that operates via multiple PqsR-dependent and PqsR-independent pathways. In this it resembles N-(3-oxododecanoyl)homoserine lactone (3OC12-HSL), which not only modulates the P. aeruginosa transcriptome via LasR and QscR, but also in the absence of any regulators incorporating an AHL-binding domain [52].
Fig 7. Schematic representation of the pqs QS system in P. aeruginosa.
The core of the pqs QS system is composed of the pqsABCDE-phnAB operon and the pqsR gene. Proteins coded by the pqsABCDE-phnAB operon synthesize HHQ that binds to and activates PqsR. The PqsR-HHQ complex promotes PpqsA activity, thus increasing HHQ and PqsE levels. Notably, the PpqsA promoter is the only target of the PqsR-HHQ complex. Apart from its contribution to HHQ biosynthesis, PqsE influences the P. aeruginosa transcriptome via a still uncharacterized AQ-independent pathway(s). In this way, PqsE up-regulates the expression of genes involved in virulence factor production, biofilm development, and antibiotic resistance. Conversely, PqsE down-regulates PpqsA activity, AQ production and the expression of genes involved in denitrification and T6SS. The pqsH and pqsL genes are required for PQS and HQNO biosynthesis, respectively. HQNO did not affect the P. aeruginosa transcriptome, and probably contributes to environmental competition due to its cytochrome inhibitory activity. PQS chelates iron triggering the iron-starvation response and increasing the transcription of virulence factor genes coding for virulence factors such as pyoverdine, ExoS toxin and AprX protease. Moreover, PQS down-regulates genes involved in denitrification. Most of the regulatory effects exerted by PQS are PqsR-independent, since the PqsR-PQS (or PqsR-HHQ) complex only promotes PpqsA activity. However, PQS also increases PpqsA and PpqsR expression via a PqsR-independent pathway(s) that is unrelated to the iron-starvation response, but is inhibited in the presence of high-iron concentrations. Dotted grey arrows indicate gene expression; solid grey arrows represent biosynthesis; solid black arrow indicates PqsR-dependent activation (+); dashed black arrows indicate PqsR-independent activation (+); black T-line indicates negative regulation (-); dashed grey arrows represent information flow.
Materials and Methods
Bacterial strains and media
The bacterial strains used in this study are listed in S2 Table. E. coli and P. aeruginosa strains were routinely grown at 37°C in Luria-Bertani (LB) broth with aeration. When required, LB was supplemented with synthetic 40 μM HHQ, PQS, or HQNO, or with 1 mM IPTG. FeCl3, 2,2’-dipyridyl and deferiprone were used at the concentrations indicated. AQs including 3-NH2-PQS were synthesized as described previously [24]. Unless otherwise stated, antibiotics were added at the following concentrations: E. coli, 100 μg ml-1 ampicillin (Ap), 10 μg ml-1 tetracycline (Tc), or 30 μg ml-1 chloramphenicol (Cm); P. aeruginosa, 200 μg ml-1 tetracycline (Tc), 375 μg ml-1 chloramphenicol (Cm), or 400 μg ml-1 carbenicillin (Cb).
Recombinant DNA techniques
The plasmids and oligonucleotides used are listed in S2 and S3 Tables respectively. Preparation of plasmid DNA, purification of DNA fragments, restrictions, ligations, and transformations in E. coli DH5α or S17.1λpir competent cells were performed with standard procedures. DNA amplification was by Polymerase Chain Reaction (PCR) using the GoTaq Polymerase (Promega).
Construction of recombinant strains
The P. aeruginosa ∆4AQ quadruple mutant strain was constructed by allelic exchange using the suicide vectors pDM4ΔpqsEind [11] and pDM4∆pqsL in the double mutant P. aeruginosa PAO1 ∆pqsA ∆pqsH [27]. The P. aeruginosa ∆5AQ quintuple mutant was constructed using the suicide vector pDM4ΔpqsR [24] in P. aeruginosa ∆4AQ. The P. aeruginosa PAO1 ∆pqsAHLE quadruple mutant strain was generated by allelic exchange using the suicide vectors pDM4∆pqsE [11] and pDM4∆pqsL in the double mutant P. aeruginosa PAO1 ∆pqsA ∆pqsH [27]. pDM4∆pqsL was constructed by PCR amplifying the upstream and downstream fragments (~500 bp) of pqsL from PAO1 using the primers FWpqsLUP and RVpqsLUP, and FWpqsLDOWN and RVpqsLDOWN, respectively (S3 Table). The same procedures were used to introduce pvdS mutations into the P. aeruginosa wild type, ∆4AQ and ∆5AQ strains. In this case, the E. coli pEX∆pvdS strain [53] was used as donor strain in the conjugation step.
For promoter activity studies, transcriptional fusions between the promoter regions of pqsH, pqsL, pqsR, phzA1, phzA2 and the luxCDABE operon were constructed using the miniCTX-lux plasmid as previously described [27].
High-density oligonucleotide microarrays
Total RNA for the high-density oligonucleotide microarray experiments was extracted from 1 ml cultures of P. aeruginosa 4AQ, 5AQ or ∆pqsAHLE carrying the plasmid pUCP18 or pUCPpqsE, grown at 37°C with shaking at 200 rpm to an OD600 1.5 in LB or in LB supplemented with 40 μM HHQ, PQS, HQNO, or 1 mM IPTG. Cells were mixed with 2 ml of RNA Protect Bacteria Reagent (Qiagen) the cells lysed and RNA was purified using RNeasy mini-columns (Qiagen), including the on-column DNase I digestion step. In addition, we treated the eluted RNA for 1 h at 37°C with TURBO DNase (0.1 units per μg of RNA; Ambion). DNase I was removed with the RNeasy Column Purification Kit (Qiagen). RNA integrity was monitored by agarose gel electrophoresis, and the absence of contaminating chromosomal DNA was verified by PCR with primers pairs FWpqsB-RVpqsB and FW16SRT-RV16SRT ( S3 Table ).
Processing of the P. aeruginosa PAO1 Affymetrix GeneChip and statistical analysis of the dataset were performed at the Lausanne Genomic Technologies Facility, Center for Integrative Genomics, University of Lausanne, Switzerland. For each condition, two different pools of RNA were compared (biological duplicate), each containing RNAs from three independent extractions (technical triplicate). Fold changes > 2.5 with a q-value < 0.05 were considered as statistically significant. The q-value is the smallest False Discovery Rate (FDR) for which the test can be considered significant [34].
Reverse transcriptase and Real Time PCR analyses
For reverse transcriptase PCR (RT-PCR) and Real Time PCR analyses, RNA was extracted from P. aeruginosa PAO1 wild type, ∆4AQ or ∆5AQ grown to an OD600 1.5 in the same conditions as described above for the microarray experiments. cDNA synthesis was performed from 1 μg of total purified RNA by using random hexamer primers and the iScript Reverse Transcription Supermix for RT-qPCR kit (BioRad). For RT-PCR, 50 ng of cDNA were PCR amplified with the GoTaq Polymerase (Promega) and primers FWpqsERT and RVpqsERT (for pqsE), FWpqsE-phnA and RVpqsE-phnA (for transcript spanning from pqsE to phnA), or FWphnART and RVphnART (for phnA) (S3 Table). After 5 min of denaturation at 95°C, the following reaction cycle was used for 30 cycles: 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min. The PCR products were analysed on a 1% (w/v) agarose gel and stained with Midori Green DNA Stain (Nippon Genetis Europe GmbH).
Real-time PCRs were performed using the iTaq Universal SYBR Green Supermix (BioRad) and primers listed in S3 Table. Gene-specific primers employed in this analysis were designed using the Primer-Blast software (www.ncbi.nlm.nih.gov/tools/primer-blast) to avoid nonspecific amplification of P. aeruginosa DNA. The reaction procedure involved incubation at 95°C for 1 min and 40 cycles of amplification at 95°C for 10 s and 60°C for 45 s. Fluorescence was registered in the last 15 s of the 60°C step. 16S ribosomal RNA was chosen as the internal control to normalize the Real Time PCR data in each single run, and to calculate the relative fold change in gene expression by using the 2-∆∆Ct method. The analysis was performed in duplicate on three technical replicates.
Measurements of promoter activity, pyoverdine, AQ and anthranilate concentrations
Bioluminescence was determined as a function of cell density using an automated luminometer-spectrometer (GENios Pro), as previously described [27]. Pyoverdine was quantified as OD405 of culture supernatants appropriately diluted in 100 mM Tris-HCl (pH 8.0), and normalized for bacterial cell density (OD600) [53]. AQs were quantified by LC-MS/MS after extracting cultures with acidified ethyl acetate [54]. Anthranilate levels were determined using quantitative LC-MS/MS following extraction of bacterial cell pellets with 80% (v/v) methanol. MS analysis was conducted under positive electrospray conditions (+ES) with the MS in MRM (multiple reaction monitoring) mode. The precursor-product ion mass transition used for the MRM detection was m/z 138.1–120.1. The relevant chromatographic peaks were compared to those of an anthranilate standard at a range of known concentrations.
For all the assays, the average data and standard deviations were calculated from at least three independent experiments.
FUR titration assay
The binding of Fur to the PpqsA and PpqsR promoter regions was investigated by transforming the miniCTX-PpqsA::lux [27] and miniCTX-PpqsR::lux plasmids into E. coli H1717 competent cells [49]. As positive and negative controls miniCTX-PpchR::lux and miniCTX-lux plasmids respectively were used. PpchR::lux was obtained by cloning a PCR fragment amplified from PAO1 with FWPpchR and RVPpchR (S3 Table). The resulting E. coli strains were grown for 16 h in LB broth supplemented with 10 μg ml-1 Tc at 37°C, washed twice with saline, and then isolated on MacConkey agar supplemented with 10 μg ml-1 Tc and 20 μM FeSO4, as previously described [51]. Colony colour was checked after 24 h of incubation at 37°C.
Supporting Information
(PDF)
(PDF)
(PDF)
(A) Levels of HHQ (white bars), PQS (light-grey bars) and HQNO (dark-grey bars) quantified by LC-MS/MS analysis in culture supernatants of P. aeruginosa ∆4AQ grown in LB or in LB supplemented with 40 μM HHQ, PQS, or HQNO, or with 1 mM IPTG, as indicated. AQ levels were quantified in supernatants from three independent cultures grown to OD600 1.5. (B) Fold change in pqsE transcript levels measured by Real Time PCR in RNA extracted from ∆4AQ strains grown as in (A). Data were normalized to the pqsE RNA level in the wild type. (C) Growth curves of P. aeruginosa ∆4AQ grown in LB or in LB supplemented with 40 μM HHQ, PQS, or HQNO, or with 1 mM IPTG, as indicated.
(PDF)
Pyocyanin production (A) and pqsE RNA levels measured by Real Time PCR (B) in P. aeruginosa ∆pqsA ∆pqsE double mutant strains carrying the pME6032 empty vector or pME6032-derivative plasmids for IPTG-inducible expression of wild type pqsE (pME-pqsE), or pqsE mutated variants lacking the first two codons (pME-pqsE∆1–6) or with a nucleotide insertion after the ATG to alter the protein frame (pME-pqsENoFrame). Culture supernatants and total RNAs are from the indicated strains grown to an OD600 of 1.5 in LB supplemented with 1 mM IPTG. For the Real time PCR analysis, data are normalized to the pqsE RNA level measured in parallel in the P. aeruginosa PAO1 wild type.
(PDF)
Amplification of cDNAs retro-transcribed from RNA extracted from (A) P. aeruginosa PAO1 wild type grown in LB and (B) P. aeruginosa ∆4AQ grown in LB supplemented with 1 mM IPTG, to an OD600 of 1.5. A 200 bp DNA region within the pqsE gene (pqsE), a 280 bp DNA region spanning from 97 bp upstream of the pqsE stop codon to 68 bp downstream of the phnA start codon (pqsE-phnA), and a 200 bp DNA region inside the phnA gene (phnA) were amplified from: 1, PAO1 genomic DNA (positive control); 2, cDNA; 3, the corresponding RNA (negative control). L, GeneRuler 100 bp DNA Ladder Plus (MBI Fermentas).
(PDF)
(A) Anthranilate was quantified by LC-MS/MS analysis in P. aeruginosa wild type and ∆4AQ grown to an OD600 of 1.5 in LB or in LB supplemented with 1 mM IPTG. Standard deviations are based on the mean values of three parallel cultures. (B) Maximal PphzA1 and PphzA2 promoter activity measured in P. aeruginosa PAO1 wild type and in the ∆pqsAHLE strain carrying the pUCP18 empty vector or the pUCPpqsE plasmid for constitutive expression of pqsE. Strains were grown in LB or in LB supplemented with 40 μM HHQ or PQS. Promoter activity is reported as Relative Light Units (RLU)/OD600.
(PDF)
Maximal promoter activity measured in P. aeruginosa ∆4AQ strains carrying the transcriptional fusions (A) PpqsH::lux or (B) PpqsL::lux. Strains were grown in LB or in LB supplemented with 40 μM AQs or 1 mM IPTG, as indicated below the graphs. Promoter activity is reported as Relative Light Units (RLU)/OD600.
(PDF)
Maximal promoter activity in strains carrying the transcriptional fusions PpqsA::lux (A and B) or PpqsR::lux (C and D). Strains were grown in LB or in LB supplemented with 40 μM PQS, 500 μM 2,2’-dipyridyl (DIP), or 160 μM deferiprone (DEF), as indicated. Diamonds indicate the pyoverdine levels measured in parallel in culture supernatants. Promoter activity is reported as Relative Light Units (RLU)/OD600; pyoverdine levels are reported as OD405 normalized to cell density (OD600).
(PDF)
(A-D) Maximal promoter activity in the strains carrying the transcriptional fusions PpqsA::lux (A and B) or PpqsR::lux (C and D). White bars indicate the pvdS-proficient genetic backgrounds (∆4AQ and ∆5AQ); grey bars indicate the pvdS-mutant genetic backgrounds (∆4AQ∆pvdS and ∆5AQ∆pvdS). Strains were grown in LB or in LB supplemented with 40 μM HHQ or PQS, as indicated. Diamonds indicate the pyoverdine levels measured in culture supernatants in the pvdS-proficient (white diamonds) or pvdS-mutant (grey diamonds) genetic backgrounds. Promoter activity is reported as Relative Light Units (RLU)/OD600; pyoverdine levels are reported as OD405 normalized to cell density (OD600). (E) Maximal PpqsA::lux and PpqsR::lux promoter activity in the wild type (white bars) and ∆pvdS (grey bars) strains grown in LB. Promoter activity is reported as Relative Light Units (RLU)/OD600. (F) E. coli H1717 cells containing the plasmids indicated and grown for 24 h at 37°C on McConkey agar supplemented with 10 μg ml-1 Tc and 20 μM FeSO4. Red-staining indicates the ability to ferment lactose and hence the binding of Fur to the target promoter. miniCTX-PpchR::lux, positive control (red colonies); miniCTX-lux, negative control (white colonies).
(PDF)
Acknowledgments
We thank Catherine A. Ortori (Centre for Analytical Sciences, School of Pharmacy, University of Nottingham) and Nigel Halliday for the LC-MS/MS analyses, Siri Ram Chhabra and Alex Truman (Centre for Biomolecular Sciences, University of Nottingham) for AQ synthesis, and the Lausanne Genomic Technologies Facility staff (Center for Integrative Genomics, University of Lausanne, Switzerland), in particular Keith Harshman, Alexandra Paillusson, Leonore Wigger, Sylvain Pradervand and Mélanie Dupasquier, for bioinformatics assistance with the microarray analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by: Biotechnology and Biological Sciences Research Council, UK (BB/F014392/1 to PW and MC, https://www.bbsrc.ac.uk); EU-FP7 collaborative Action (grant NABATIVI, Ref 223670 to MC and PW, https://ec.europa.eu/research/fp7/index_en.cfm); Italian Ministry for University and Research (RBFR10LHD1 to GR, http://www.istruzione.it); Italian Cystic Fibrosis Research Foundation (FFC 10/2013 to LL, http://www.fibrosicisticaricerca.it); Regione Lazio (LR 13/2008 - FILAS-RU-2014-1009 to PV, http://www.regione.lazio.it/rl_main/); Swiss National Science Foundation (PBLAP3-129398/1 to EF, http://www.snf.ch/en/Pages/default.aspx). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. El Zowalaty ME, Al Thani AA, Webster TJ, El Zowalaty AE, Schweizer HP, Nasrallah GK, et al. Pseudomonas aeruginosa: arsenal of resistance mechanisms, decades of changing resistance profiles, and future antimicrobial therapies. Future Microbiol. 2015;10:1683–706. 10.2217/fmb.15.48 [DOI] [PubMed] [Google Scholar]
- 2. Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defences. Pathog Dis. 2013;67:159–73. 10.1111/2049-632X.12033 [DOI] [PubMed] [Google Scholar]
- 3. Dubern JF, Cigana C, De Simone M, Lazenby J, Juhas M, Schwager S, et al. Integrated whole-genome screening for Pseudomonas aeruginosa virulence genes using multiple disease models reveals that pathogenicity is host specific. Environ Microbiol. 2015;17:4379–93. 10.1111/1462-2920.12863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirisits MJ, Parsek MR. Does Pseudomonas aeruginosa use intercellular signalling to build biofilm communities? Cell Microbiol. 2006;8:1841–9. 10.1111/j.1462-5822.2006.00817.x [DOI] [PubMed] [Google Scholar]
- 5. Williams P, Cámara M. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr Opin Microbiol. 2009;12:182–91. 10.1016/j.mib.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 6. Hazan R, He J, Xiao G, Dekimpe V, Apidianakis Y, Lesic B, et al. Homeostatic interplay between bacterial cell-cell signaling and iron in virulence. PLoS Pathog. 2010;6(3):e1000810 10.1371/journal.ppat.1000810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee J, Zhang L. The hierarchy quorum sensing network in Pseudomonas aeruginosa . Protein Cell. 2015;6:26–41. 10.1007/s13238-014-0100-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rampioni G, Leoni L, Williams P. The art of antibacterial warfare: deception through interference with quorum sensing-mediated communication. Bioorg Chem. 2014;55:60–8. 10.1016/j.bioorg.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 9. Heeb S, Fletcher MP, Chhabra SR, Diggle SP, Williams P, Cámara M. Quinolones: from antibiotics to autoinducers. FEMS Microbiol Rev. 2010;35:247–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Déziel E, Gopalan S, Tampakaki AP, Lépine F, Padfield KE, Saucier M, et al. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-L-homoserine lactones. Mol Microbiol. 2005;55:998–1014. 10.1111/j.1365-2958.2004.04448.x [DOI] [PubMed] [Google Scholar]
- 11. Rampioni G, Pustelny C, Fletcher MP, Wright VJ, Bruce M, Rumbaugh KP, et al. Transcriptomic analysis reveals a global alkyl-quinolone-independent regulatory role for PqsE in facilitating the environmental adaptation of Pseudomonas aeruginosa to plant and animal hosts. Environ Microbiol. 2010;12:1659–73. 10.1111/j.1462-2920.2010.02214.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barr HL, Halliday N, Cámara M, Barrett DA, Williams P, Forrester DL, et al. Pseudomonas aeruginosa quorum sensing molecules correlate with clinical status in cystic fibrosis. Eur Respir J. 2015;46:1046–54. 10.1183/09031936.00225214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winsor GL, Lam DK, Fleming L, Lo R, Whiteside MD, Yu NY, et al. Pseudomonas Genome Database: improved comparative analysis and population genomics capability for Pseudomonas genomes. Nucleic Acids Res. 2011;39:596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Déziel E, Lépine F, Milot S, He J, Mindrinos MN, Tompkins RG, et al. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc Natl Acad Sci USA. 2004;101:1339–44. 10.1073/pnas.0307694100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coleman JP, Hudson LL, McKnight SL, Farrow JM 3rd, Calfee MW, Lindsey CA, et al. Pseudomonas aeruginosa PqsA is an anthranilate-coenzyme A ligase. J Bacteriol. 2008;190:1247–55. 10.1128/JB.01140-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang YM, Frank MW, Zhu K, Mayasundari A, Rock CO. PqsD is responsible for the synthesis of 2,4-dihydroxyquinoline, an extracellular metabolite produced by Pseudomonas aeruginosa . J Biol Chem. 2008;283:28788–94. 10.1074/jbc.M804555200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drees SL, Fetzner S. PqsE of Pseudomonas aeruginosa acts as pathway-specific thioesterase in the biosynthesis of alkylquinolone signaling molecules. Chem Biol. 2015;22:611–8. 10.1016/j.chembiol.2015.04.012 [DOI] [PubMed] [Google Scholar]
- 18. Dulcey CE, Dekimpe V, Fauvelle DA, Milot S, Groleau MC, Doucet N, et al. The end of an old hypothesis: the Pseudomonas signalling molecules 4-hydroxy-2-alkylquinolines derive from fatty acids, not 3-ketofatty acids. Chem Biol. 2013;20:1481–91. 10.1016/j.chembiol.2013.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drees SL, Li C, Prasetya F, Saleem M, Dreveny I, Williams P, et al. PqsBC, a condensing enzyme in the biosynthesis of the Pseudomonas aeruginosa Quinolone Signal: crystal structure, inhibition, and reaction mechanism. J Biol Chem. 2016;291:6610–24. 10.1074/jbc.M115.708453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schertzer JW, Brown SA, Whiteley M. Oxygen levels rapidly modulate Pseudomonas aeruginosa social behaviors via substrate limitation of PqsH. Mol Microbiol. 2010;77:1527–38. 10.1111/j.1365-2958.2010.07303.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lépine F, Milot S, Déziel E, He J, Rahme LG. Electrospray/mass spectrometric identification and analysis of 4-hydroxy-2-alkylquinolines (HAQs) produced by Pseudomonas aeruginosa . J Am Soc Mass Spectrom. 2004;15:862–9. 10.1016/j.jasms.2004.02.012 [DOI] [PubMed] [Google Scholar]
- 22. Wade DS, Calfee MW, Rocha ER, Ling EA, Engstrom E, Coleman JP, et al. Regulation of Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa . J Bacteriol. 2005;187:4372–80. 10.1128/JB.187.13.4372-4380.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xiao G, Déziel E, He J, Lépine F, Lesic B, Castonguay MH, et al. MvfR, a key Pseudomonas aeruginosa pathogenicity LTTR-class regulatory protein, has dual ligands. Mol Microbiol. 2006;262:1689–99. [DOI] [PubMed] [Google Scholar]
- 24. Ilangovan A, Fletcher M, Rampioni G, Pustelny C, Rumbaugh K, Heeb S, et al. Structural basis for native agonist and synthetic inhibitor recognition by the Pseudomonas aeruginosa quorum sensing regulator PqsR (MvfR). PLoS Pathog. 2013;9(7):e1003508 10.1371/journal.ppat.1003508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mashburn-Warren L, Howe J, Garidel P, Richter W, Steiniger F, Roessle M, et al. Interaction of quorum signals with outer membrane lipids: insights into prokaryotic membrane vesicle formation. Mol Microbiol. 2008;69:491–502. 10.1111/j.1365-2958.2008.06302.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Macdonald IA, Kuehn MJ. Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa . J Bacteriol. 2013;195:2971–81. 10.1128/JB.02267-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diggle SP, Matthijs S, Wright VJ, Fletcher MP, Chhabra SR, Lamont IL, et al. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem Biol. 2007;14:87–96. 10.1016/j.chembiol.2006.11.014 [DOI] [PubMed] [Google Scholar]
- 28. Bredenbruch F, Geffers R, Nimtz M, Buer J, Häussler S. The Pseudomonas aeruginosa quinolone signal (PQS) has an iron-chelating activity. Environ Microbiol. 2006;8:1318–29. 10.1111/j.1462-2920.2006.01025.x [DOI] [PubMed] [Google Scholar]
- 29. Nguyen AT, Jones JW, Ruge MA, Kane MA, Oglesby-Sherrouse AG. Iron depletion enhances production of antimicrobials by Pseudomonas aeruginosa . J Bacteriol. 2015;197:2265–75. 10.1128/JB.00072-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Voggu L, Schlag S, Biswas R, Rosenstein R, Rausch C, Götz F. Microevolution of cytochrome bd oxidase in Staphylococci and its implication in resistance to respiratory toxins released by Pseudomonas . J Bacteriol. 2006;188:8079–86. 10.1128/JB.00858-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gallagher LA, McKnight SL, Kuznetsova MS, Pesci EC, Manoil C. Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa . J Bacteriol. 2002;184:6472–80. 10.1128/JB.184.23.6472-6480.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farrow JM 3rd, Sund ZM, Ellison ML, Wade DS, Coleman JP, Pesci EC. PqsE functions independently of PqsR-Pseudomonas quinolone signal and enhances the rhl quorum-sensing system. J Bacteriol. 2008;190:7043–51. 10.1128/JB.00753-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Folch B, Déziel E, Doucet N. Systematic mutational analysis of the putative hydrolase PqsE: toward a deeper molecular understanding of virulence acquisition in Pseudomonas aeruginosa . PLoS One. 2013;8(9):e73727 10.1371/journal.pone.0073727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Storey JD, Tibshirani R. Statistical significance for genome-wide studies. Proc Natl Acad Sci USA. 2003;100:9440–5. 10.1073/pnas.1530509100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aendekerk S, Diggle SP, Song Z, Høiby N, Cornelis P, Williams P, et al. The MexGHI-OpmD multidrug efflux pump controls growth, antibiotic susceptibility and virulence in Pseudomonas aeruginosa via 4-quinolone-dependent cell-to-cell communication. Microbiology. 2005;151:1113–25. 10.1099/mic.0.27631-0 [DOI] [PubMed] [Google Scholar]
- 36. Dietrich LE, Price-Whelan A, Petersen A, Whiteley M, Newman DK. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa . Mol Microbiol. 2006;61:1308–21. 10.1111/j.1365-2958.2006.05306.x [DOI] [PubMed] [Google Scholar]
- 37. Yu S, Jensen V, Seeliger J, Feldmann I, Weber S, Schleicher E, et al. Structure elucidation and preliminary assessment of hydrolase activity of PqsE, the Pseudomonas quinolone signal (PQS) response protein. Biochemistry. 2009;48:10298–307. 10.1021/bi900123j [DOI] [PubMed] [Google Scholar]
- 38. Knoten CA, Wells G, Coleman JP, Pesci EC. A conserved suppressor mutation in a tryptophan auxotroph results in dysregulation of Pseudomonas quinolone signal synthesis. J Bacteriol. 2014;196:2413–22. 10.1128/JB.01635-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Recinos DA, Sekedat MD, Hernandez A, Cohen TS, Sakhtah H, Prince AS, Price-Whelan A, Dietrich LE. Redundant phenazine operons in Pseudomonas aeruginosa exhibit environment-dependent expression and differential roles in pathogenicity. Proc Natl Acad Sci USA. 2012; 109:19420–5. 10.1073/pnas.1213901109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ochsner UA, Wilderman PJ, Vasil AI, Vasil ML. GeneChip expression analysis of the iron starvation response in Pseudomonas aeruginosa: identification of novel pyoverdine biosynthesis genes. Mol Microbiol. 2002;45:1277–87. [DOI] [PubMed] [Google Scholar]
- 41. Palma M, Worgall S, Quadri LE. Transcriptome analysis of the Pseudomonas aeruginosa response to iron. Arch Microbiol. 2003;180:374–9. 10.1007/s00203-003-0602-z [DOI] [PubMed] [Google Scholar]
- 42. Singh G, Wu B, Baek MS, Camargo A, Nguyen A, Slusher NA, et al. Secretion of Pseudomonas aeruginosa type III cytotoxins is dependent on Pseudomonas quinolone signal concentration. Microb Pathog. 2010;49:196–203. 10.1016/j.micpath.2010.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hogardt M, Roeder M, Schreff AM, Eberl L, Heesemann J. Expression of Pseudomonas aeruginosa exoS is controlled by quorum sensing and RpoS. Microbiology. 2004;150:843–51. 10.1099/mic.0.26703-0 [DOI] [PubMed] [Google Scholar]
- 44. Toyofuku M, Nomura N, Kuno E, Tashiro Y, Nakajima T, Uchiyama H. Influence of the Pseudomonas quinolone signal on denitrification in Pseudomonas aeruginosa . J Bacteriol. 2008;190:7947–56. 10.1128/JB.00968-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Häussler S, Becker T. The Pseudomonas quinolone signal (PQS) balances life and death in Pseudomonas aeruginosa populations. PLoS Pathog. 2008;4(9):e1000166 10.1371/journal.ppat.1000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Visca P. Iron regulation and siderophore signaling in Pseudomonas aeruginosa virulence In: Ramos JL, editor. Pseudomonas, vol. 2 New York: Kluwer Academic/Plenum Publishers; 2004. p. 69–124. [Google Scholar]
- 47. Bergh A, Offenhartz P, George P, Haight GP. Complexes of 2,2′, 2″, 2-quaterpyridine with ferrous and ferric ions. J Chem Soc. 1964;1:1533–8. [Google Scholar]
- 48. Boelaert JR, Van Cutsem J, de Locht M, Schneider YJ, Crichton RR. Deferoxamine augments growth and pathogenicity of Rhizopus, while hydroxypyridinone chelators have no effect. Kidney Int. 1994;45:667–71. [DOI] [PubMed] [Google Scholar]
- 49. Liu Y, Yang L, Molin S. Synergistic activities of an efflux pump inhibitor and iron chelators against Pseudomonas aeruginosa growth and biofilm formation. Antimicrob Agents Chemother. 2010;54:3960–3. 10.1128/AAC.00463-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thompson MG, Corey BW, Si Y, Craft DW, Zurawski DV. Antibacterial activities of iron chelators against common nosocomial pathogens. Antimicrob Agents Chemother. 2012;56:5419–21. 10.1128/AAC.01197-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stojiljkovic I, Bäumler AJ, Hantke K. Fur regulon in Gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a fur titration assay. J Mol Biol. 1994;236:531–45. 10.1006/jmbi.1994.1163 [DOI] [PubMed] [Google Scholar]
- 52. Chugani S, Greenberg EP. LuxR homolog-independent gene regulation by acyl-homoserine lactones in Pseudomonas aeruginosa . Proc Natl Acad Sci USA. 2010;107:10673–8. 10.1073/pnas.1005909107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Imperi F, Massai F, Facchini M, Frangipani E, Visaggio D, Leoni L, et al. Repurposing the antimycotic drug flucytosine for suppression of Pseudomonas aeruginosa pathogenicity. Proc Natl Acad Sci USA. 2013;110:7458–63. 10.1073/pnas.1222706110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ortori CA, Dubern JF, Chhabra SR, Cámara M, Hardie K, Williams P, et al. Simultaneous quantitative profiling of N-acyl-L-homoserine lactone and 2-alkyl-4(1H)-quinolone families of quorum-sensing signaling molecules using LC-MS/MS. Anal Bioanal Chem. 2011;399:839–50. 10.1007/s00216-010-4341-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(A) Levels of HHQ (white bars), PQS (light-grey bars) and HQNO (dark-grey bars) quantified by LC-MS/MS analysis in culture supernatants of P. aeruginosa ∆4AQ grown in LB or in LB supplemented with 40 μM HHQ, PQS, or HQNO, or with 1 mM IPTG, as indicated. AQ levels were quantified in supernatants from three independent cultures grown to OD600 1.5. (B) Fold change in pqsE transcript levels measured by Real Time PCR in RNA extracted from ∆4AQ strains grown as in (A). Data were normalized to the pqsE RNA level in the wild type. (C) Growth curves of P. aeruginosa ∆4AQ grown in LB or in LB supplemented with 40 μM HHQ, PQS, or HQNO, or with 1 mM IPTG, as indicated.
(PDF)
Pyocyanin production (A) and pqsE RNA levels measured by Real Time PCR (B) in P. aeruginosa ∆pqsA ∆pqsE double mutant strains carrying the pME6032 empty vector or pME6032-derivative plasmids for IPTG-inducible expression of wild type pqsE (pME-pqsE), or pqsE mutated variants lacking the first two codons (pME-pqsE∆1–6) or with a nucleotide insertion after the ATG to alter the protein frame (pME-pqsENoFrame). Culture supernatants and total RNAs are from the indicated strains grown to an OD600 of 1.5 in LB supplemented with 1 mM IPTG. For the Real time PCR analysis, data are normalized to the pqsE RNA level measured in parallel in the P. aeruginosa PAO1 wild type.
(PDF)
Amplification of cDNAs retro-transcribed from RNA extracted from (A) P. aeruginosa PAO1 wild type grown in LB and (B) P. aeruginosa ∆4AQ grown in LB supplemented with 1 mM IPTG, to an OD600 of 1.5. A 200 bp DNA region within the pqsE gene (pqsE), a 280 bp DNA region spanning from 97 bp upstream of the pqsE stop codon to 68 bp downstream of the phnA start codon (pqsE-phnA), and a 200 bp DNA region inside the phnA gene (phnA) were amplified from: 1, PAO1 genomic DNA (positive control); 2, cDNA; 3, the corresponding RNA (negative control). L, GeneRuler 100 bp DNA Ladder Plus (MBI Fermentas).
(PDF)
(A) Anthranilate was quantified by LC-MS/MS analysis in P. aeruginosa wild type and ∆4AQ grown to an OD600 of 1.5 in LB or in LB supplemented with 1 mM IPTG. Standard deviations are based on the mean values of three parallel cultures. (B) Maximal PphzA1 and PphzA2 promoter activity measured in P. aeruginosa PAO1 wild type and in the ∆pqsAHLE strain carrying the pUCP18 empty vector or the pUCPpqsE plasmid for constitutive expression of pqsE. Strains were grown in LB or in LB supplemented with 40 μM HHQ or PQS. Promoter activity is reported as Relative Light Units (RLU)/OD600.
(PDF)
Maximal promoter activity measured in P. aeruginosa ∆4AQ strains carrying the transcriptional fusions (A) PpqsH::lux or (B) PpqsL::lux. Strains were grown in LB or in LB supplemented with 40 μM AQs or 1 mM IPTG, as indicated below the graphs. Promoter activity is reported as Relative Light Units (RLU)/OD600.
(PDF)
Maximal promoter activity in strains carrying the transcriptional fusions PpqsA::lux (A and B) or PpqsR::lux (C and D). Strains were grown in LB or in LB supplemented with 40 μM PQS, 500 μM 2,2’-dipyridyl (DIP), or 160 μM deferiprone (DEF), as indicated. Diamonds indicate the pyoverdine levels measured in parallel in culture supernatants. Promoter activity is reported as Relative Light Units (RLU)/OD600; pyoverdine levels are reported as OD405 normalized to cell density (OD600).
(PDF)
(A-D) Maximal promoter activity in the strains carrying the transcriptional fusions PpqsA::lux (A and B) or PpqsR::lux (C and D). White bars indicate the pvdS-proficient genetic backgrounds (∆4AQ and ∆5AQ); grey bars indicate the pvdS-mutant genetic backgrounds (∆4AQ∆pvdS and ∆5AQ∆pvdS). Strains were grown in LB or in LB supplemented with 40 μM HHQ or PQS, as indicated. Diamonds indicate the pyoverdine levels measured in culture supernatants in the pvdS-proficient (white diamonds) or pvdS-mutant (grey diamonds) genetic backgrounds. Promoter activity is reported as Relative Light Units (RLU)/OD600; pyoverdine levels are reported as OD405 normalized to cell density (OD600). (E) Maximal PpqsA::lux and PpqsR::lux promoter activity in the wild type (white bars) and ∆pvdS (grey bars) strains grown in LB. Promoter activity is reported as Relative Light Units (RLU)/OD600. (F) E. coli H1717 cells containing the plasmids indicated and grown for 24 h at 37°C on McConkey agar supplemented with 10 μg ml-1 Tc and 20 μM FeSO4. Red-staining indicates the ability to ferment lactose and hence the binding of Fur to the target promoter. miniCTX-PpchR::lux, positive control (red colonies); miniCTX-lux, negative control (white colonies).
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.