Abstract
Zika virus (ZIKV) has become a threat to global health since the outbreak in Brazil in 2015. Although ZIKV is generally considered an Aedes-transmitted pathogen, new evidence has shown that parts of the virus closely resemble Culex-transmitted viruses. Therefore, it is important to evaluate the competence of Culex species for ZIKV to understand their potential as vectors. In this study, female Culex pipiens quinquefasciatus were orally exposed to ZIKV. Mosquito midguts, salivary glands and ovaries were tested for ZIKV to measure infection and dissemination at 2, 4, 6, 8, 12, 16 and 18 days post exposure (pe). In addition, saliva was collected from mosquitoes after infection and infant mice were bitten by infected mosquitoes to measure the transmission ability of Cx. p. quinquefasciatus. The results showed that the peak time of virus appearance in the salivary glands was day 8 pe, with 90% infection rate and an estimated virus titer of 3.92±0.49 lg RNA copies/mL. Eight of the nine infant mice had positive brains after being bitten by infected mosquitoes, which meant that Cx. p. quinquefasciatus could be infected with and transmit ZIKV following oral infection. These laboratory results clearly demonstrate the potential role of Cx. p. quinquefasciatus as a vector of ZIKV in China. Because there are quite different vector management strategies required to control Aedes (Stegomyia) species and Cx. p. quinquefasciatus, an integrated approach may be required should a Zika epidemic occur.
Keywords: Culex pipiens quinquefasciatus, transmission, vector competence, Zika virus (ZIKV)
INTRODUCTION
Zika virus (ZIKV) is a mosquito-borne flavivirus that was first isolated in rhesus monkeys from Uganda in 1947.1 Human infections were initially reported in the 1950s in Africa.2, 3 Outbreaks of ZIKV disease occurred in the islands of the Pacific ocean during 2007–2013.4, 5, 6 The first ZIKV infection in Brazil was confirmed in May 2015.7, 8 Thereafter, ZIKV spread throughout South America and caused unprecedented outbreaks. On 1 February 2016, the World Health Organization declared a public health emergency of international concern owing to the recent cluster of congenital microcephaly cases and other neurological disorders that have been reported in the Americas in association with the ZIKV pandemic. A total of 66 countries and territories with ZIKV transmission have been documented in the world from 1 January 2007 to 20 April 2016 (World Health Organization situation report).
ZIKV has been detected in Aedes mosquitoes, including Ae. africanus, Ae. luteocephalus, Ae. aegypti and Ae. Albopictus, from Senegal, the Ivory coast, Burkina Faso, the Central African Republic, Uganda and Asia.1, 9, 10 Ae. aegypti, Ae. albopictus and Ae. hensilli have been shown to be potential vectors of ZIKV based on experimental infection and transmission.11, 12, 13, 14, 15, 16 Although ZIKV is generally considered an Aedes-transmitted pathogen,17 it belongs to the Spondweni serocomplex, which is close to the Japanese encephalitis virus (JEV) complex according to phylogenetic analysis and E protein structures.18, 19, 20 Viruses within the JEV complex are transmitted mostly by Cx. mosquitoes, such as Cx. tritaeniorhynchus, Cx. P. quinquefasciatus and Cx. P. pipiens.21, 22 Therefore, the competence of Culex species in ZIKV transmission should be studied to understand their potential as vectors.
Cx. p. quinquefasciatus is a member of Cx. pipiens complex and is a known vector of St. Louis encephalitis and West Nile viruses in North America21 and a secondary vector of JEV in China.23, 24 Cx. p. quinquefasciatus is widely distributed south of Yangtze river, where it is the most common mosquito species in urban and rural areas of China.23 Compared with Ae. aegypti and Ae. albopictus, Cx. p. quinquefasciatus has much greater densities in human habitats.24 Although Cx. p. quinquefasciatus is an opportunistic blood feeder, humans are its common host in urban China.25 The current paper reports the experimental infection and transmission of ZIKV by Cx. p. quinquesciatus mosquitoes.
MATERIALS AND METHODS
Ethics statement
All of the experimental protocols involving animals were approved by the Laboratory Animal Center of the State Key Laboratory of Pathogen and Biosecurity, Beijing Institute of Microbiology and Epidemiology Institutional Animal Care and Use Committee (IACUC, the permit number is BIME 2011-2009). The study of animals was performed in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Mosquitoes
The Cx. p. quinquefasciatus used for experimental infection were collected as larvae from Hainan province of southern China in the summer of 2014 and reared in the laboratory. The mosquitoes were maintained under standard insectary conditions at 26±1 °C, 75±5% relative humidity and a 14 h:10 h light:dark photoperiod. Prior to the infectious feed, adult mosquitoes were provided with 8% sugar water.
Virus
The mosquitoes were exposed to a contemporary ZIKV strain SZ01 (GenBank NO. KU866423). This virus was originally isolated from the blood of a patient who returned to China from Samoa in 2016.26 The stock virus used in the current study has been passaged twice in C6/36 cells prior to the infectious feed.
Oral infection of mosquitoes
Seven-day-old female mosquitoes (n>500) were starved for 12 h prior to the infectious blood meal. The blood meal consisted of 1:1 mouse blood and virus suspension. Oral infections with ZIKV were performed with virus at a titer of 3 × 105 plaque-forming units/mL, verified by titration in a standard plaque assay.27 The mosquitoes were fed with an infectious blood meal that was warmed to 37 °C using a Hemotek membrane feeding system housed in a feeding chamber. After 30 min, the mosquitoes were cold anesthetized, and fully engorged females were transferred to 300 mL ca. plastic cups, which were maintained at 29±1 °C, 75±5% relative humidity, a 14 h/10 h light:dark photoperiod and 8% sugar water.
Mosquito processing
To determine the ZIKV infection and dissemination rates in Cx. p. quinquefasciatus, ten female mosquitoes were sampled on days 2, 4, 6, 8, 12, 16 and 18 postexposure (pe). To prevent cross-contamination of the virus across the midgut, salivary glands and ovaies of each mosquito, these organs were carefully dissected using different dissecting needles, and the organs were rinsed in phosphate-buffered saline thrice. The midguts, salivary glands and ovaries from each mosquito then were individually transferred to 1.5-mL microtubes containing 100 mL of Dulbecco's modified Eagle's medium (DMEM; GIBCO, Invitrogen, Beijing, China) supplemented with 2% fetal bovine serum (FBS). These organs were then homogenized using five-mm stainless steel grinding balls (Next Advance, Averill Park, NY, USA) in a Bullet Blender 24 mixer mill (Next Advance) set at a frequency of 12/s for one min. All dissecting needles were dipped in 80% ethanol and burned before being re-used.
Transmission experiments
To determine the ability of Cx. p. quinquefasciatus to transmit ZIKV, on day 8 pe, nine one-day-old infant mice were placed in a cage containing ~100 mosquitoes that previously were fed with the virus–blood mixture described above. After two-h exposure to mosquitoes, the infant mice were removed from the cage and reared separately. In addition, mosquitoes were anesthetized with CO2 and 18 blood-engorged females were removed for dissection. The salivary glands of each mosquito were removed and individually transferred to 1.5-mL microtubes containing 100 mL DMEM (GIBCO) supplemented with 2% FBS. On the day 10 postsucking, the nine infant mice were killed, and the brains were excised. In all, 100 mg brain tissue was individually transferred to 1.5-mL microtubes containing 100 mL DMEM (GIBCO) supplemented with 2% FBS.
To determine ZIKV transmission rates, saliva was collected from the individual mosquitoes on days 6, 8, 12 and 16 pe as previously described in Dubrulle et al.28 Briefly, the wings and legs of each mosquito were removed, and the proboscis was inserted into a quartz capillary containing five μL of FBS. After 45 min, the FBS containing saliva was expelled in 100 μL of DMEM medium for ZIKV detection.
Detection of the virus
Total RNA was isolated from the midguts, salivary glands, saliva and ovaries of the mosquitoes and the brains of the infant mice using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's recommendations. ZIKV in these samples was detected using the one-Step Detection Kit for Zika Virus RNA (Daan Gene, Guangzhou, China), which included the reverse primer 5′-TAC AAG TAC CAT CCT GAC TCC C-3′, the forward primer 5′-CYG TCA GTT GYA CTC CAT TCT C-3′ and a LNA-probe (5′-FAM-AGC TCC CCT TCY ACT GAT YTC CAC AT-BHQ1-3′). For the PCR, the following components were used: 5 μL of each RNA sample, 17 μL of PCR Mixture A, and three μL of PCR Mixture B. The final volume was 25 μL. The amplification reactions were performed in a PCR real-time system from Applied Biosystems (Carlsbad, CA, USA; ABI 7500) that was programmed for one cycle at 50 °C for 15 min, 95 °C for 15 min and 40 cycles at 94 °C for 15 s and 55 °C for 45 s.
The number of viral RNA copies was calculated by generating a standard curve from RNA isolated from uninfected mosquitoes, triturated with a known amount of seed virus, the titer of which was determined by plaque assay. The amount of RNA is expressed as lg RNA copies/mL.
Infection and transmission analysis
The infection rate (IR) of midguts, salivary glands and ovaries at each sampling day was calculated by dividing the number of infected midguts, salivary glands and ovaries by the total number of mosquitoes tested. The transmission was confirmed with appearance of brain-virus-positive in infant mouse brains that were bitten at least once by infected mosquitoes. The transmission rate was calculated by dividing the number of mosquitoes with infected saliva by the number of mosquitoes with a disseminated infection (i.e., midgut-/salivary gland-/ovary-positive). The transmission efficiency represents the proportion of mosquitoes with infectious saliva among the total number of mosquitoes tested.
RESULTS
Oral susceptibility of Cx. p. quinquefasciatus to ZIKV
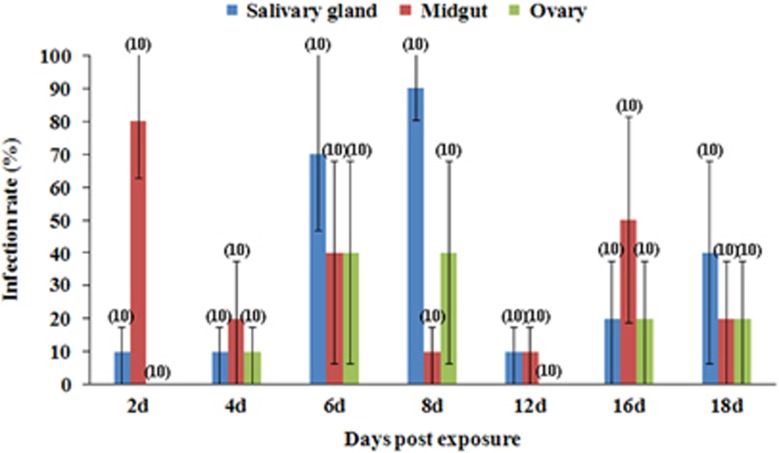
From day 2 pe onwards, after the blood meal had been completely digested, ZIKV could be detected in the mosquitoes on each of the sampling days (Figure 1). Although the IR of the midgut declined from 80% at the day 2 pe, this IR always remained above 10%. The IR of the salivary glands ascended from 10% on day 2 to 90% on day 8 pe and susequently declined to 10%–40% on days 12–18 pe. The ovary IR was relatively low, peaking at 40% on days 6–8 pe.
Figure 1.
Midgut, salivary gland and ovary ZIKV infection rates in Cx. p. quinquefasciatus at different days postexposure to the blood meal. Ten mosquitoes were sampled per day. The error bars represent the confidence intervals (95%). The number of individuals analyzed is given in parentheses. ZIKV, Zika virus.
ZIKV titers in the midguts, salivary glands and ovaries of the mosquitoes
Virus titers were calculated by a real-time reverse transcription-PCR and expressed as equivalent lg RNA copies/mL. The standard RNA used in the nucleic amplification assays was extracted from virus dilutions of a known titer, which had been determined by plaque assay.
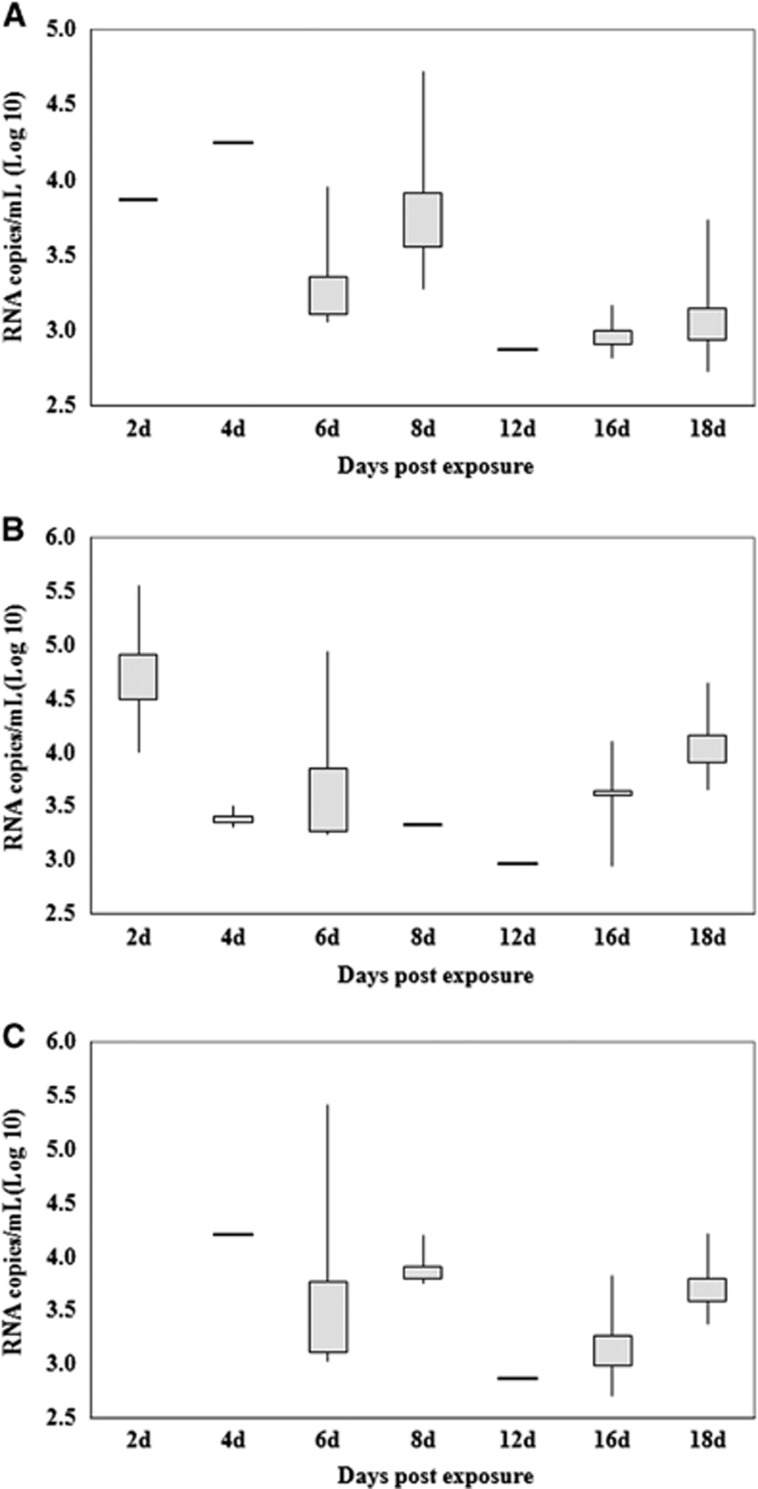
Figure 2 presents the estimated mean ZIKV titers of the midguts, salivary glands and ovaries of infected females at different days pe. In the midgut, the highest and lowest titers were detected on days 2 and 12 pe, with average values of 4.94±0.97 lg RNA copies/mL (IR=80%) and 2.98 lg RNA copies/mL (IR=10%), respectively. The virus must go through the midgut barrier and spread to the salivary glands and ovaries to cause the infection. Therefore, the times of virus detected in salivary glands and ovaries was later than the time of detection in the midgut.
Figure 2.
Quantification of Zika viral RNA copies by real-time RT-PCR detected in the salivary glands (A), midguts (B) and ovaries (C) at different days postexposure. Each box represents the median (horizontal bar), the interquartile range (box) and the full range (‘whiskers'). RT-PCR, reverse transcription-PCR.
As illustrated in Figures 1 and 2, the peak time of virus appearance in the salivary glands and ovaries of the mosquitoes was day 8 pe. Not only did the IR of the samples peak at the time (90% and 40%, respectively), but the virus titer was also at a high level (3.92±0.49 and 3.91±0.20 lg RNA copies/mL, respectively). Although the virus titers reached 4.25 and 4.22 lg RNA copies/mL in the salivary glands and ovaries, respectively, at day 4 pe, the infection efficiency of mosquitoes was less on this day than on the other days, because the IR of the salivary glands and ovaries were 10%.
Transmission of ZIKV by Cx. p. quinquefasciatus
ZIKV RNA was detected in the mosquito saliva, which indicated that this virus could be transmitted by mosquitoes. As indicated by the results in Table 1, transmission was first observed on day 8 after the infectious blood meal. At day 6 pe, although the ZIKV was detected in seven of the ten mosquito salivary glands, the viral RNA was not be detected in the saliva. At day 8 pe, RNA was detected in nine of the ten mosquito salivary glands and eight saliva samples were positive with an estimated titer of 4.90±0.56 lg RNA copies/mL. On day 12 pe, only one of the ten mosquitoes was virus RNA-positive in the salivary glands, and the saliva of this mosquito was also virus RNA-positive. However, the estimated virus titer in the saliva was only 3.88 lg RNA copies/mL. On day 16 pe, two of the ten mosquitoes were virus RNA-positive in salivary glands; however, the saliva samples were negative.
Table 1. Transmission rates of ZIKV in Cx. p. quinquefasciatus on days 6, 8, 12 and 16 postexposure to the blood meal (10 mosquitoes were sampled per day).
| Day pe | Number of mosquito tested | Number of ZIKV-positive mosquitoa | Number of ZIKV-positive mosquitoes in salivary gland | Number of ZIKV-positive mosquitoes in saliva | TR of mosquitob | TE of mosquitoc | ZIKV titer (/g RNA copies/mL) in saliva |
|---|---|---|---|---|---|---|---|
| 6 | 10 | 9 | 7 | 0 | 0.0% | 0.0% | — |
| 8 | 10 | 10 | 9 | 8 | 80.0% | 80.0% | 4.90±0.56 |
| 12 | 10 | 2 | 1 | 1 | 50.0% | 10.0% | 3.88 |
| 16 | 10 | 6 | 2 | 0 | 0.0% | 0.0% | — |
Abbreviations: transmission efficiency, TE; transmission rate, TR; Zika virus, ZIKV.
Number of ZIKV-positive mosquitoes indicates the number of mosquitoes with infected midguts, salivary glands or ovaries. The virus can be detected in any organ means that this individual is virus positive.
TR of mosquito is calculated by dividing the number of mosquitoes with infected saliva by the number of mosquitoes with a disseminated infection (i.e., midgut-/salivary gland-/ovary-positive).
TE of mosquito is calculated by dividing the number of mosquitoes with infected saliva by the total number of mosquitoes tested.
The infant mice that has been bitten by infectious mosquitoes can be infected with the virus, and the virus can break through blood–brain barrier and then replicate in the mouse brain, which is direct evidence that this mosquito species can transmit ZIKV and is a potential vector. More than one red blotch was present on the skin of all nine infant mice after removal from the virus-infected mosquito cage, which indicated that all of the infant mice were bitten by at least one mosquito. Among 18 blood-engorged mosquitoes, 15 had viral RNA in their salivary glands. On day 10 pe, eight of the nine infant mice (88.9%) had viral RNA in their brain, with an average estimated virus titer of 7.85±0.76 lg RNA copies/mL.
DISCUSSION
Zika was previously a neglected tropical disease, and similar to chikungunya fever, interest in ZIKV epidemiology was limited until the outbreak in Brazil in 2015. Currently, Zika is considered a major threat to global health in both the developing and developed countries.29 The tendency of these viruses to spread outside their known geographic range and cause large-scale epidemics relies partly on our knowledge of vector distribution. Some mosquito species have been proved to be involved in the transmission cycle of ZIKV, including Ae. aegypti, Ae. albopictus and some other Aedes species.9, 10, 11, 12, 13, 14, 15 However, the roles of mosquito species other than those in the genus Aedes remain undefined.
Cx. p. quinquefasciatus are involved in the transmission of several flaviviruses, such as West Nile and St. Louis encephalitis viruses in the Americas.21, 22 In China, Cx. p. quinquefasciatus is a secondary vector of JEV23, 24 and has been found to be a potential vector of West Nile virus.30 In recent research, sequence and structural comparisons of the ZIKV envelope (E) protein with those of other flaviviruses have revealed that parts of the protein closely resemble the neurovirulent West Nile and JEVs, while others are similar to dengue virus.20 In this study, we demonstrated that Cx. p. quinquefasciatus was able to be infected with and transmit ZIKV after oral exposure. The IR was as high as 90% in the salivary glands and 80% in the saliva on day 8 pe. The decrease in mosquito midgut infections observed over time in our study is consistent with other published studies31 and was probably due to virus clearance by the mosquito's immune system.32, 33 In the other transmission experiment, infant mice were infected after being bitten by virus-positive mosquitoes and disseminated to and replicated in the mouse brains. These findings verified the potential role of Cx. p. quinquefasciatus as a vector of ZIKV.
Cx. p. quinquefasciatus is known by the name of ‘southern house mosquito' in China, has a close relationship with humans and inhabits areas around human settlements.21 Cx. p. quinquefasciatus is the predominant mosquitoes in South China and in other tropical and subtropical areas of the world, especially in urban environments.34, 35, 36 Although Cx. p. quinquefasciatus feed on both animals and humans, humans are common blood source for this mosquito in urban habitats.25, 37, 38 Cx. p. quinquefasciatus is the primary species that enters human houses for blood meals during the night in urban areas and towns in China.25 There is a lack of animals in urban areas, and therefore Cx. p. quinquefasciatus in these areas feed mainly on humans. Even in the places with some animals, humans are an important blood source to Cx. p. quinquefasciatus. Although Cx. p. quinquefasciatus may not be the primary vector of ZIKV (Ae. aegypti occupies that role) owing to its high density, human blood-feeding behavior and vector competence shown herein, its potential importance in the transmission of ZIKV cannot be ignored, especially in urban areas and towns.
This is the first report about the potential of Cx. p. quinquefasciatus to transmit ZIKV in China. Given that Cx. p. quinquefasciatus is a potential vector of ZIKV, vector control approaches and methods may need to be updated and modified to control ZIKV. Previous vector management strategies have primarily focused on Aedes species, similar to dengue vector control programs. There are substantial differences in vector management strategies for Aedes species, i.e., Ae. aegypti and Ae. albopictus and Cx. p. quinquefasciatus.24 Source reduction needs to target more types of standing water. Additionally, more adult control methods should be implemented to control mosquitoes if a Zika epidemic occurs.
Acknowledgments
This work was funded by grants from the Infective Diseases Prevention and Cure Project of China (NO 2012ZX10004219), the National Key Technology Support Program (NO 2014BAI13B01), the National Key Research and Development Project of China (NO 2016YFD0500304) and the Zika Prevention and Control Project of China.
References
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46: 509–520. [DOI] [PubMed] [Google Scholar]
- Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 1952; 46: 521–534. [DOI] [PubMed] [Google Scholar]
- Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg 1954; 48: 139–145. [DOI] [PubMed] [Google Scholar]
- Duffy MR, Chen TH, Hancock WT et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360: 2536–2543. [DOI] [PubMed] [Google Scholar]
- Grard G, Caron M, Mombo IM et al. Zika virus in Gabon (Central Africa) - 2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis 2014; 8: e2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lormeau VM, Roche C, Teissier A et al. Zika virus, French Polynesia, South Pacific, 2013. Emerg Infect Dis 2014; 20: 1085–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis 2015; 21: 1885–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanluca C, Melo VC, Mosimann AL et al. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 2015; 110: 569–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow AJ, Williams MC, Woodall JP et al. Twelve isolations of Zika virus from Aedes (Stegomyia) africanus (Theobald) taken in and above a Uganda forest. Bull WHO 1964; 31: 57–69. [PMC free article] [PubMed] [Google Scholar]
- Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg 1969; 18: 411–415. [DOI] [PubMed] [Google Scholar]
- Chouin-Carneiro T, Vega-Rua A, Vazeille M et al. Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl Trop Dis 2016; 10: e0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P-SJ, Li MI, Chong C-S et al. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl Trop Dis 2013; 7: e2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MI, Wong PSJ, Ng LC et al. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. PLoS Negl Trop Dis 2012; 6: e1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledermann JP, Guillaumot L, Yug L et al. Aedes hensilli as a potential vector of Chikungunya and Zika viruses. PLoS Negl Trop Dis 2014; 8: e3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagne CT, Diallo D, Faye O et al. Potential of selected Senegalese Aedes spp. mosquitoes (Diptera: Culicidae) to transmit Zika virus. BMC Infect Dis 2015; 15: 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman JP, Porterfield JS. A simple technique for infection of mosquitoes with viruses; transmission of Zika virus. Trans R Soc Trop Med Hyg 1956; 50: 238–242. [DOI] [PubMed] [Google Scholar]
- Musso D, Gubler DJ. Zika virus. Clin Microbiol Rev 2016; 29: 487–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes EB. Zika virus outside Africa. Emerg Infect Dis 2009; 15: 1347–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno G, Chang GJ, Tsuchiya KR et al. Phylogeny of the genus flavivirus. J Virol 1998; 72: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuchenko VA, Lim EX, Zhang S et al. Structure of the thermally stable Zika virus. Nature 2016; 533: 425–428. [DOI] [PubMed] [Google Scholar]
- Van den Hurk AF, Ritchie SA, Mackenzie JS. Ecology and geographical expansion of Japanese encephalitis virus. Ann Rev Entomol 2009; 54: 17–35. [DOI] [PubMed] [Google Scholar]
- Turell MJ. Members of the Culex pipiens complex as vectors of virus. J Am Mosq Cont Assoc 2012; 28: 123–126. [DOI] [PubMed] [Google Scholar]
- Zhao T, Lu B. Biosystematics of Culex pipiens complex in China. Entomol Sin 1995; 2: 1–8. [Google Scholar]
- Lu BL. Mosquito Integrated Management. Science Press: Beijing, China. 1999. [Google Scholar]
- Lu BL, Li PS, Ji SH et al. Diptera: Culicidae 1. In: Fauna Sinica, Insect. Beijing: Science Press. 1997, 401. [Google Scholar]
- Deng YQ, Zhao H, Li XF et al. Isolation, identification and genomic characterization of the Asian lineage Zika virus imported to China. Sci China Life Sci 2016; 59: 428–430. [DOI] [PubMed] [Google Scholar]
- Li XF, Deng YQ, Yang HQ et al. A chimeric dengue virus vaccine using Japanese encephalitis virus vaccine strain SA14-14-2 as backbone is immunogenic and protective against either parental virus in mice and nonhuman primates. J Virol 2013; 87: 13694–13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrulle M, Mousson L, Moutailler S et al. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLoS One 2009; 4: e5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Costa F, Garcia-Blanco MA et al. Zika virus: history, emergence, biology, and prospects for control. Antiviral Res 2016; 130: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang SF, Zhang YM, Guo XX et al. Experiment studies on comparison of the potential vector competence of four species of Culex mosquitoes in China to transmit West Nile virus. J Med Entomol 2010; 47: 788–790. [DOI] [PubMed] [Google Scholar]
- Salazar MI, Richardson JH, Sanchez-Vargas I et al. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol 2007; 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vargas I, Scott JC, Poole-Smith BK et al. Dengue virus type 2 infections of Aedes aegypti are modulated by the mosquito's RNA interference pathway. PLoS Pathog 2009; 5: e1000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog 2008; 4: e1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CH, Zhao W, Wang SQ et al. Analysis of mosquito density and seasonality in urban areas of Hainan province, China in 2012. Chin J Vector Biol Control 2014; 25: 15–17. [Google Scholar]
- Wang XH, Yang XY, Zhao W et al. Analysis of surveillance data of mosquito density from 2012 to 2014 in Haikou city, Hainan, China. Chin J Vector Biol Control 2015; 26: 424–426. [Google Scholar]
- Lu RZ, Liu QY, Wu HX et al. Zoogeographical analysis of Culex pipiens pallens/Cx. pipiens quinquefasciatus in China, 2014. Chin J Vector Biol Control 2016; 27: 107–111. [Google Scholar]
- Azmi SA, Das S, Chatterjee S. Seasonal prevalence and blood meal analysis of filarial vector Culex quinquefasciatus in coastal areas of Digha, West Bengal, India. J Vector Borne Dis 2015; 52: 252–256. [PubMed] [Google Scholar]
- Samuel PP, Arunachalam N, Hiriyan J et al. Host-feeding pattern of Culex quinquefasciatus Say and Mansonia annulifera (Theobald) (Diptera: Culicidae), the major vectors of filariasis in a rural area of south India. J Med Entomol 2004; 41: 442–446. [DOI] [PubMed] [Google Scholar]