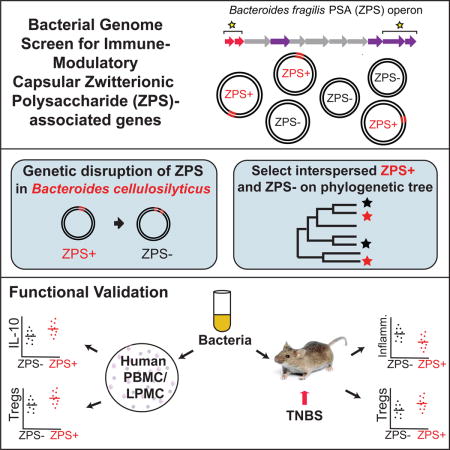
Summary
Zwitterionic capsular polysaccharides (ZPS) are bacterial products that modulate T cells, including inducing anti-inflammatory IL-10-secreting T regulatory cells (Tregs). However, only a few diverse bacteria are known to modulate the host immune system via ZPS. We present a genomic screen for bacteria encoding ZPS molecules. We identify diverse host-associated bacteria, including commensals and pathogens with known anti-inflammatory properties, with the capacity to produce ZPSs. Human mononuclear cells stimulated with lysates from putative ZPS-producing bacteria induce significantly greater IL-10 production and higher proportions of Tregs than lysates from non ZPS-encoding relatives or a commensal strain of Bacteroides cellulosilyticus in which a putative ZPS biosynthetic operon was genetically disrupted. Similarly, wild-type B. cellulosilyticus DSM 14838, but not a close relative lacking a putative ZPS, attenuated experimental colitis in mice. Collectively, this screen identifies bacterial strains that may use ZPS to interact with the host as well as those with potential probiotic properties.
eTOC
Identifying anti-inflammatory host-associated bacteria can reveal strategies to combat inflammatory disease. Neff et al. conduct a genomic screen to identify bacteria with the capability to produce capsular zwitterionic polysaccharides (ZPS). These ZPS-producing bacteria induce increased levels of anti-inflammatory IL-10 and Tregs and prevent experimental colitis in mice.
Introduction
The trillions of microorganisms that colonize the human body (the microbiota) control many aspects of both innate and adaptive immune responses (Hooper and Macpherson 2010; Molloy, Bouladoux, and Belkaid 2012) and a healthy microbiota plays a crucial role in maintaining immune homeostasis. Accordingly, dysbiosis of the gut microbiota is associated with many diseases characterized by chronic gut inflammation, including Inflammatory Bowel Diseases (Kverka et al. 2011) and HIV infection (Lozupone et al. 2014). Host-associated bacteria that influence T cell populations are of key interest, since T cells play a central role in controlling host immune status. One key class of molecules known to influence T cell populations in the context of both health and disease are capsular zwitterionic polysaccharides (ZPS)(Surana and Kasper 2012). Unlike most naturally occurring polysaccharides composed of negatively charged sugar molecules, ZPSs contain positive and negative repeating charges and can activate CD4+ T cells in complex ways (Avci and Kasper 2010; Tzianabos et al. 1993). The alternating charges are crucial, as chemical modification eliminates the ability of ZPS to modulate immune responses (Tzianabos et al. 1993). Diverse bacteria produce immune-modulatory ZPSs, including SP1 of Streptococcus pneumonia (Velez et al. 2009), CP8 of Staphylococcus aureus (O’Riordan and Lee 2004) and O-chain antigen of Morganella morganii (Young et al. 2011). The best-studied ZPS is Polysaccharide A (PSA) of the intestinal bacterium Bacteroides fragilis NCTC 9343.
Certain ZPSs are involved in disease, having the ability to induce abscess in a T cell dependent manner (Tzianabos et al. 1993). However, certain ZPSs also appear to play important and diverse roles in both maintaining and restoring intestinal immune homeostasis. For instance, studies in mice have shown that the ZPS of B. fragilis NCTC 9343 PSA, can drive a Th1-mediated response to help maintain Th1/Th2 balance in the gut (Mazmanian et al. 2005). PSA can also induce anti-inflammatory IL-10- secreting T regulatory cells (Tregs), to protect against both T-cell mediated and chemically induced colitis in mice (Mazmanian, Round, and Kasper 2008; Round and Mazmanian 2010). Finally, immune assays with human PBMC have shown that PSA can also induce CD4+ T cells with suppressive capacity in humans (Telesford et al. 2015; Kreisman and Cobb 2011).
The bacteria known to modulate the host immune system using ZPS are limited, and include phylogenetically diverse organisms that make structurally similar ZPS. Specifically, B. fragilis NCTC 9343 and S. pneumoniae make similar ZPSs called PSA and SP1 respectively (Figure 1), in which the crucial positive charge is conferred by the same amino sugar, acetamido-amino-2,4,6-trideoxygalactose (AATGal). Most strains of B. fragilis contain an AATGal-ZPS but these vary in structure, with only ~25% of strains making PSA and with strain 638R making PSA2, a more structurally complex ZPS (Figure 1) that shares similar immunological properties to PSA and a biosynthesis locus on a homologous chromosomal region (Wang et al. 2000). The biological activities of varied ZPS from different bacterial strains are generally thought to be similar (Stephen, Groneck, and Kalka-Moll 2010), although the degree to which AATGal-ZPSs may vary in function is not well understood. As an example Sp1, PSA and PSA2 all induce abscess and SP1 and PSA both induce T cell activation in an APC dependent manner (Wang et al. 2000; Kalka-Moll et al. 2002). However, PSA and not SP1 induced CD4+39+FoxP3+ cells in a Dendritic Cell (DC)-dependent manner in stimulations with human cells derived from peripheral blood (Telesford et al. 2015), which has functional implications because CD39 expression was shown to be important for PSA-mediated protection against CNS inflammation in murine models (Wang et al. 2014). Further expansion of our knowledge of the diversity of ZPSs in nature may pave the way to discovery of ZPS with therapeutic potential.
Figure 1.

PSA operon of B. fragilis NCTC 9343 and chemical structures of B. fragilis NCTC 9343 PSA, B. fragilis 638R PSA2, and S. pneumoniae SP1. A. The PSA operon of B. fragilis and the conserved properties of B. fragilis AATGal-ZPS operons, as described by Coyne et al. (Coyne et al. 2001) B. Chemical structure of PSA of B. fragilis and related ZPSs. The shaded regions indicate the amino sugar AATGal that is synthesized by the wcfR gene and transferred by the wcfS gene to a polysaccharide backbone that varies in structure between these ZPSs. Genes shared across Bacteroidales AATGal-ZPSs, and examples of other AATGal-ZPS operons are shown in Figure S2, S3, and S4.
To further expand our knowledge of the breadth of AATGal-ZPSs and the bacteria that produce them, we screened complete and draft genomes for those containing orthologues to AATGal biosynthetic genes and for a subset of these genomes, characterized the gene content of Capsular Polysaccharide Biosynthesis (CPS) operons that contained them. Furthermore, we tested the immune-modulatory properties of AATGal-ZPS encoding bacteria using human blood and tissue assays and a mouse model of colitis. The availability of thousands of sequenced genomes from a continually increasing repertoire of cultured human gut inhabitants makes such genomic screens coupled with functional experiments a powerful approach for understanding the distribution of key biological properties across human gut bacteria.
Results
Genomic screen for bacteria that encode AATGal-ZPS molecules
The PSA operon of B. fragilis NCTC 9343 had been previously cloned and sequenced (Coyne et al. 2001). This operon contains transcriptional regulators upaY and upaZ, which are conserved across CPS loci in the Bacteroides (Krinos et al. 2001; Xu et al. 2003), 4 glycosyl transferases (GT) and other key genes for CPS biosynthesis such as a flippase and polymerase (Figure 1). The key genes for the biosynthesis and attachment of the AATGal amino sugar that gives PSA its positive charges, and thus zwitterionic property, are wcfR, which encodes a protein that synthesizes AATGal and wcfS, which encodes a GT that transfers this amino sugar to the polysaccharide backbone, in an adjacent position to wcfR (Figure 1) (Coyne et al. 2001). Gene homologues to wcfR and wcfS downstream of upaY and upaZ transcriptional regulators, but not the other genes in the PSA operon, were conserved in almost all of 50 strains of B. fragilis tested, indicating that AATGal-ZPS but not canonical PSA are conserved across B. fragilis (Coyne et al. 2001).
We predicted which other bacteria may produce AATGal-ZPS by first performing a BLAST search for B. fragilis NCTC 9343 wcfR gene homologues in protein coding genes from 8065 genomes. To define a similarity threshold at which the BLAST hits were potentially orthologous to wcfR and thus produce a functionally equivalent molecule, we considered the fact that B. fragilis NCTC 9343 has a wcfR gene homologue within its genome (BLAST e-value = 3e−48) that encodes a protein with a different function, the aminotransferase DegT (ref|wp_010992163). Plotting the e-value distribution of hits to B. fragilis wcfR produced a bimodal distribution, with the DegT in the lower distribution (Figure S1A). We hypothesized BLAST hits in the upper distribution (< 1e−90 in our search) may be orthologues to wcfR. We next determined whether wcfR orthologues had other essential elements of AATGal-ZPS nearby in the genome, including a homologue to the wcfS gene and the upaY and/or upaZ transcriptional regulators (using a threshold of e-value < 1e−5).
Diverse taxa contain diverse putative AATGal-ZPS operons
Of the 8065 genomes screened, 517 had BLAST hits to the B. fragilis wcfR gene with an e-value < 1e−90, including taxonomically diverse bacteria in the Bacteroidales, Erysipelotrichales, Clostridiales, and Bacillales orders (Figure 2). In at least 409 of these 517 genomes, wcfR homologues had a wcfS homologue adjacent or in very close proximity (within 3 genes). Most genomes from bacteria in the Bacteroidales order contained homologues to the upaY and/or upaZ transcriptional regulators upstream, but wcfR homologue containing bacteria from other bacteria lineages did not. Consistent with previous reports that B. fragilis and S. pneumoniae produce AATGal-ZPS with related genes (Coyne et al. 2001), among our predicted producers of AATGal-ZPS molecules were 217 strains of S. pneumoniae (Figures 1,2). Our screen also identified the PSA2 operon of B. fragilis 638R, which has a related but more complex structure compared to PSA, with five monosaccharides rather than four (Figure 1, S3)(Wang et al. 2000). Genomes usually only contained one AATGal-ZPS operon but in rare cases could contain multiple, including B. cellulosilyticus strains DSM 14838 and CL02T12C19 and B. fragilis strains HMW 615 and YCH46 (Figure S3, S4).
Figure 2.

A neighbor joining tree of BLAST hits to the wcfR gene of B. fragilis with an e-value <1e−90. Highly related sequences from different strains of the same species (or undesignated strains that are likely the same species) are collapsed into a wedge with the number of strains (genomes) within that wedge noted. Colors indicate the taxonomic order of the bacteria from which the wcfR sequence came. The wcfR genes of bacterial strains tested in immune assays are indicated with a star, showing that tested bacteria include those with wcfR homologues throughout this phylogeny. Figure S7 shows electron micrograph evidence of a convergence in surface structures in bacteria that are very distantly related phylogenetically but share highly similar wcfR gene homologues in their genomes.
Predicted producers of AATGal-ZPS were scattered throughout the 16S rRNA phylogenetic tree of the Bacteroides genus and phylogenetically interspersed with species that did not contain predicted AATGal-ZPS operons (Figure 3A), indicating a pattern of multiple gains and losses. A similar phylogenetically interspersed pattern was also observed for predicted AATGal-ZPS and non- AATGal-ZPS producers in the Erysipelotrichales (Figure 3B) and the Clostridiales (data not shown) lineages.
Figure 3.

16S rRNA trees of all genomes that were screened in the orders Bacteroidales and Erysipelotrichales showing presence/absence of PSA-like operons. A. Strains surveyed in the Bacteroidales order and B. Strains surveyed in the Erysipelotrichales order. Species shown in red text had genes encoding both wcfR and wcfS and the genes were in adjacent or close positions within the operon. Those without a close homologue to wcfR are in black. Nodes in which all bacteria had the same color designation are collapsed into wedges with the number of strains noted in parentheses. The stars indicate predicted PSA-producing (in red) and non PSA-producing (in black) bacterial strains selected for immunologic testing.
For AATGal-ZPS encoding bacteria in the Bacteroidales order, we predicted the operon gene content based on the genes located between the upaY/upaZ transcriptional regulators and the wcfR/wcfS gene homologues and also using the Database of Prokaryotic Operons (Door2) application (Mao et al. 2009). To relate the operons to each other based on their gene content, we binned genes into families and clustered the operons that had similar collections of genes families. Interestingly, and consistent with previous reports (Coyne et al. 2001), only 4 of the 13 surveyed strains of B. fragilis had a canonical PSA operon, B. fragilis strain 638R had a unique operon consistent with its production of PSA2, and 6 strains shared an operon for a previously uncharacterized AATGal-ZPS (Figure S3). Consistent with PSA and PSA2 of B. fragilis having related but unique structures, the content of sugar biosynthesis and GT genes of the operons suggest that the AATGal-ZPSs in the Bacteroidales include variable polysaccharide backbones that generally have 4–5 sugar repeating subunits (Figure S2; Table S1).
Establishing an AATGal-ZPS immune phenotype in human blood and tissue
To establish assays in human blood for determining whether bacteria with putative AATGal-ZPS operons have anti-inflammatory properties, we first performed 3-day stimulations of peripheral blood mononuclear cells (PBMC) from healthy adults with lysate of wild-type (WT) B. fragilis NCTC 9343, and B. fragilis with the PSA operon knocked-out (B. fragilis ΔPSA). After three days of stimulation, the cell culture supernatant was subjected to IL-10 ELISA and the cell cultures to flow cytometry to assess immune cell populations (see methods).
B. fragilis WT lysate induced significantly higher IL-10 levels (p<0.0001, Figure 4A) and lower levels of the inflammatory cytokines IL-6 and TNF-α (p=0.01, p=0.02, respectively, Figure S5A) than B. fragilis ΔPSA. We also used a 24 hour Intracellular Cytokine Staining (ICCS) assay to evaluate whether any IL-10 was being produced by CD4+ T cells in these mixed cell populations. WT B. fragilis stimulations produced a significantly higher fraction of CD4+ IL-10+ cells than B. fragilis ΔPSA (p=0.0015, Figure 4B) but the vast majority of these CD4+IL-10+ cells were CD25−FoxP3− (p<0.0001, Figure 4C). We also found a significant decrease in Treg induction by B. fragilis ΔPSA compared to WT when we used CD127 and CTLA4 as additional markers to FoxP3 and CD25 (Figures 4E). Consistent with reports that CD25 and FoxP3 alone are inadequate human Treg markers (Sakaguchi et al. 2010; Gavin et al. 2006), stimulations with WT B. fragilis did not result in a significantly greater proportion of CD4+CD25+FoxP3+ cells compared to B. fragilis ΔPSA (Figure 4D).
Figure 4.

WT B. fragilis induces higher IL-10 production and proportion of Tregs compared to B. fragilis ΔPSA in human PBMC and LPMC. A. Levels of IL-10 in the supernatant of PBMC cultured with WT B. fragilis and B. fragilis ΔPSA for 3 days as determined by ELISA. B. Percent IL-10+ CD4+ T cells and C. CD4+IL-10+ T cells that are CD25+/−FoxP3+/− after one day stimulation with WT B. fragilis. D. Proportion of CD4+ T cells that are CD25+FoxP3+ in PBMC after 3 days of culture with WT B. fragilis or B. fragilis ΔPSA. E. Proportion CD4+ T cells that are CD25+FoxP3+CD127−CTLA4+ in PBMC after 3 days of culture with WT B. fragilis and B. fragilis ΔPSA. F. Levels of IL-10 in the supernatant and G. proportion of Tregs generated from purified naïve T cells mixed with bacteria stimulated purified CD14+ monocytes. H. Levels of IL-10 in the supernatant and I. proportion of CD4+ T cells that are CD25+FoxP3+CD127−CTLA4+ of LPMC after 3 days of culture with bacterial lysates. Statistical significance was calculated by paired T tests. Data for other cytokines (IL-6, TNF-α, IL-17, and IL-22) are in Figure S5A. Representative staining for CD25+FoxP3+CTLA4+CD127−Tregs in human PBMC and LPMC are in Figure S5B.
To establish an assay for determining whether AATGal-ZPS containing bacteria can stimulate naïve T cells in an APC-dependent manner as has been previously described for B. fragilis PSA, we stimulated isolated CD14+ monocytes with bacterial lysates prior to being co-cultured with purified naïve T cells. Even though no memory or effector T cells were present, WT B. fragilis induced higher levels of IL-10 and Tregs compared to B. fragilis ΔPSA (p = 0.026, p = 0.021, Figure 4F and 4G). Since fewer cells were used for this assay (two hundred thousand naive T cells) compared to whole PBMC (two million total cells), lower levels of IL-10 were observed.
Since immune cell subsets can behave differently depending on body compartment (Hu and Pasare, 2013), we also conducted assays using lamina propria mononuclear cells (LPMC) from resected gut tissue supplied to us following surgery (note that surgery was usually conducted to remove tumors. Sections of healthy tissue were used in the assays). WT B. fragilis induced more IL-10 and more CD25+FoxP3+CD127−CTLA-4+ Tregs than B. fragilis ΔPSA in LPMC (p = 0.0005, p=0.008 Figure 4H, and 4I, respectively). Taken together, our results show that an increased proportion of CD25+FoxP3+CD127−CTLA-4+ Tregs and increased IL-10 in supernatants from 3-day stimulations of PBMC and LPMC are both an indicator of PSA-like activity in bacteria.
Assaying phylogenetically interspersed ZPS encoding and non-encoding bacteria for PSA-like immunomodulatory activity
We selected 6 putative AATGal-ZPS-encoding and 7 non-encoding strains that were phylogenetically interspersed on a 16S rRNA tree of the Bacteroidales and Erysipelotrichales orders for immune assays (Figure 3). Using phylogenetically interspersed bacteria helps to reduce the effects of other genetic differences between strains that may be confounding. As an example, although any individual bacterial isolate will usually differ in many genes from even a very close phylogenetic relative, all of the putative ZPS-operon containing bacteria selected here would not be expected to share any genes to the exclusion of the phylogenetically interspersed non-ZPS encoding bacteria by chance. Finding anti-inflammatory properties established for PSA to be associated with our genomic predictions of AATGal ZPS-operon presence, would thus strongly implicate the ZPS-operons as an underlying reason. The selected bacterial strains also contained wcfR orthologues that were scattered throughout the wcfR tree (Figure 2), which allows us to test whether our selected e-value threshold is diagnostic of ZPS-like activity. Finally, they contained AATGal-ZPS operons with diverse gene content (Figure S2). Bacterial strains were grown to early stationary phase in liquid broth media, and a qPCR assay validated that the ZPS-encoding bacteria tested were expressing the wcfR gene under these growth conditions (Figure S1B).
Stimulation of human PBMC with lysates of the predicted ZPS-producers elicited significantly higher median levels of IL-10 and CD25+FoxP3+CD127−CTLA-4+ Tregs in both the Bacteroidales and the Erysipelotrichales (Figure 5 A, B). Individual predicted ZPS-producing bacteria were also each compared to its closest assayed non ZPS-producing relative with a paired T-test and in almost all cases the predicted ZPS-producer induced significantly more IL-10 and Tregs, except for a couple of case that were borderline significant (Table 1).
Figure 5.

(Left) Putative PSA producers in the Bacteroidales or Erysipelotrichales orders induce more IL-10 and Tregs compared to their non-PSA producing relatives in PBMC and LPMC. A. IL-10 levels and B. proportion of CD25+FoxP3+CTLA4+CD127−cells of CD4+ T cells in PBMC cultured with bacteria from the Bacteroidales (circles) or Erysipelotrichales order (squares) for 3 days. C. IL-10 levels and D. proportion CD25+FoxP3+CTLA4+CD127− cells of CD4+ T cells in LPMC cultured with bacteria from the Bacteroidales (circles) or Erysipelotrichales order (squares) for 3 days. Predicted PSA producers are in red and those that are not are in black. Statistical significance between medians of predicted and non-predicted PSA producers was calculated by nonparametric Mann-Whitney tests. (Right) WT B. cellulosilyticus induces higher IL-10 production and proportion of Tregs compared to a wcfR knockout in whole PBMC and purified naïve T cells. E. Levels of IL-10 and F. proportion CD4+ T cells that are CD25+FoxP3+CD127−CTLA4+ in PBMC after 3 days of culture with B. cellulosilyticus and B. cellulosilyticus ΔZPS1. G. Levels of IL-10 in the supernatant and H. proportion of Tregs generated from purified naïve T cells mixed with bacteria stimulated purified CD14+ monocytes. Statistical significance was calculated by paired T tests. Comparisons of stimulations with WT B. cellulosilyticus and the closely related B. intestinalis are in Figure S6.
Table 1.
Individual PSA-producing bacteria were compared with their closest non-PSA-producer relative for IL-10 and Treg induction in PBMC and LPMC. Statistical significance was calculated by paired T tests and the p-values for each comparison are noted in the table The 16S rRNA percent identity between pairs was calculated based on aligned sequenced in Arb (Kumar et al. 2005).
| PSA-producer | Closest non PSA-producer | 16S rRNA % Identity | PBMC IL-10 | PBMC Tregs | LPMC IL-10 | LPMC Tregs |
|---|---|---|---|---|---|---|
| Bacteroides fragilis | Bacteroides ovatus | 94 | 0.007 | 0.041 | 0.304 | 0.025 |
| Bacteroides cellulosilyticus | Bacteroides intestinalis | 97.4 | 0.003 | 0.047 | 0.002 | 0.028 |
| Bacteroides uniformis | Bacteroides stercoris | 91.1 | 0.063 | 0.009 | 0.619 | 0.260 |
| Bacteroides fluxus | Bacteroides stercoris | 93 | 0.015 | 0.034 | 0.184 | 0.495 |
| Parabacteroides distasonis | Prevotella stercorea | 84.4 | 0.002 | 0.089 | 0.611 | 0.339 |
| Clostridium spiroforme | Catenibacterium mitsuoki | 92.1 | <0.0001 | 0.003 | 0.002 | 0.225 |
Our results for stimulations in LPMC were less clear. Stimulations with LPMC showed significantly greater median IL-10 production in ZPS-producers than non-producers in the Bacteroidales (p=0.039) but not in the Erysipelotrichales (p=0.21, Figure 5C). Median Treg stimulation was not significantly higher in ZPS-producers versus non in LPMC (Figure 5D). Paired T-tests between predicted ZPS-producing bacteria and their closest assayed non ZPS-producing relative were only sometimes significant (Table 1). However, the closest non-ZPS producing relatives of the ZPS-producers had typically only a 16S rRNA % ID of ~92% ID (Table 1), indicating that differences in other genes in the genomes may be obscuring the effects of the ZPS. When we compared B. cellulosilyticus and B. intestinalis, the most highly related strains with opposite designations, statistical significance was reached for IL-10 production and Treg stimulation in both PBMC (p=0.0026, p=0.047, Figures S6A and S6B, respectively) and LPMC (p=0.0019, p=0.028, Figures S6C and S6D, respectively).
ZPS-knockout strains of Bacteroides cellulosilyticus
To further confirm that the increased IL-10 and Treg levels in these bacteria were conferred by putative ZPS operons, we disrupted a wcfR gene in B. cellulosilyticus DSM 14838 via targeted insertional mutagenesis using the pKNOCK-bla-ermGb vector as described by (Alexeyev 1999). B. cellulosilyticus DSM 14838 has two putative AATGal-ZPS operons in its genome (Figure S2), one was encoded on Scaffold 5 (ZPS1) and one was encoded on Scaffold 9 (ZPS2). The operon encoding ZPS1 was simpler than the operon encoding ZPS2, and showed greater similarity in gene content to PSA, sharing homologues to the wcfQ and wcfP GTs and to the wzx3 flippase (Figure S4). Sequencing of the cDNA produced from mRNA of WT B. cellulosilyticus DSM 14838 grown to late log phase showed that both copies of the gene were expressed by the cell population. By PCR confirmation of both genomic DNA and mRNA, we confirmed that the wcfR gene of ZPS1 and not ZPS2 was disrupted. Stimulation of PBMC with B. cellulosilyticus ΔZPS1 resulted in the production of significantly less IL-10 and Tregs compared to WT bacteria (Figure 5E and 4F). As with B. fragilis and its PSA knockout counterpart, when isolated APCs were first stimulated with B. cellulosilyticus or B. cellulosilyticus ΔZPS1 and then co-cultured with purified naïve CD4+ T cells, the knockout strain induced less IL-10 and Tregs compared to wild type (Figure 5G and H), indicating that this AATGal-ZPS of B. cellulosilyticus can also induce Tregs from naïve T cells in an APC-mediated fashion.
ZPS producing B. cellulosilyticus DSM 14838 protects against colitis in a TNBS model
To further test whether our genomic screens for immune-modulatory ZPS operons are predictive of the anti-inflammatory effects described for B. fragilis PSA, we tested the effect of gavaging mice with the putative ZPS producer B. cellulosilyticus DSM 14838 in a Trinitrobenzenesulfonic acid (TNBS) induced colitis model. In two separate trials, female C57/Bl6 mice aged 8 – 12 weeks were gavaged with 5.0 × 108 cells of bacteria in 200 μL of culture media or culture media without bacteria, once a week for 3 weeks. On the day of the third treatment, mice were challenged with TNBS.
Gavage of mice with B. cellulosilyticus led to significantly decreased weight loss induced by TNBS enema relative to vehicle control, measured by two-way repeated measures ANOVA (p = 0.014, Figure 6C). Vehicle-gavaged mice displayed a significant reduction in initial body weight by day 5 post-enema (95% ± 2.3; n = 6; p < 0.01) while those receiving B. cellulosilyticus were protected from weight loss and actually gained weight overall (102.3% ± 0.6; n = 6). In contrast, mice gavaged with B. intestinalis, a close relative of B. cellulosilyticus (97.4% identity over their aligned 16S rRNA genes) that does not encode a putative ZPS operon displayed no such protection from TNBS-induced weight loss.
Figure 6.

B. cellulosyliticus administration attenuates murine colitis in vivo. A. Representative micrographs of H&E stained colonic sections demonstrate that B. cellulosyliticus causes a decrease in intestinal inflammation relative to either vehicle controls or mice gavaged with B. intestinalis (black scale bar 200 μm). B. Histological inflammatory index scores show protection from inflammation in mice gavaged with B. cellulosilyticus relative to vehicle control or B. intestinalis. C. Weight loss curve data correlates with attenuated inflammation seen in histological scores. D. Representative contour plots showing expression of FoxP3+ T cells from the colonic lamina propria of each group, then enumerated E. * P < 0.05, ** P < 0.01.
Gavage of mice with B. cellulosilyticus also led to decreased histological evidence of inflammation (Figure 6A) as assessed in blinded manner by a trained pathologist (Figure 6B). The vehicle group of TNBS treated animals displayed histological evidence of inflammation (2.28 ± 0.26) that was significantly higher than those receiving B. cellulosilyticus (1.40 ±0.27; p = 0.02), while those receiving B. intestinalis displayed worse inflammation than vehicle controls, though the difference was not statistically significant (3.30 ± 0.55; p = 0.051). The decrease in inflammation coincided with an increase in CD4+ FoxP3+ regulatory T cells in the colonic lamina propria of the vehicle group (5.3% ± 0.77; n = 4) compared to mice gavaged with B. cellulosilyticus (7.9% ± 0.82; n = 4; p = 0.04) (Figure 6D and E). In contrast those receiving B. intestinalis displayed no significant difference in Treg frequency from vehicle controls.
DISCUSSION
Here we show that we can predict bacteria with anti-inflammatory properties in in vitro assays in humans based on the presence of predicted AATGal-ZPS operons in their genomes. Furthermore, the one putative AATGal-ZPS producer tested, B. cellulosilyticus DSM 14838, showed a robust protection of colitis in mice, indicating that this bacterial strain has potential probiotic applications. B. cellulosilyticus appears to be a benign member of the Bacteroides, especially when compared to B. fragilis. It was originally isolated from healthy human feces for its ability to degrade cellulose (Robert et al. 2007) and is a gut symbiont that is notable for its extensive glycobiome (McNulty et al. 2013). Whether it is the 2 AATGal-ZPSs encoded by B. cellulosilyticus DSM 14838 that confers this anti-colitic property remains to be elucidated. The significant reduction of Treg and IL-10 in a B. cellulosilyticus strain in which one AATGal-ZPS had been genetically disrupted supports a role for this ZPS in anti-inflammatory properties of B. cellulosilyticus DSM 14838.
A wide variety of bacteria from diverse phylogenetic lineages appear to produce AATGal-ZPSs that may modulate the immune system. We focused our analyses on bacteria within the Bacteroidales order and one isolate in the Erysipelotrichales order; Similar follow-up with a larger diversity of predicted AATGal-ZPS producing bacteria would also be useful for confirming the significance of our predictions. Our analysis of AATGal-ZPS operon gene content of bacteria in the Bacteroidales order suggests that we are identifying a diversity of ZPSs that includes PSA2 of B. fragilis strain 638R. Our immune assay results suggest that diverse AATGal-ZPS containing bacteria induce IL-10 and Tregs in stimulations of mixed immune cell populations from both PMBC and LPMC. Furthermore, assays with our B. cellulosilyticus ΔZPS1 strain indicates that for this putative AATGal-ZPS, Treg induction occurs at least in part via the APC-dependent stimulation of naïve T cells. Further work to more deeply define mechanisms of immune-modulation in these ZPSs and whether they differ between the various ZPS sub-types is needed, including experiments with AATGal-ZPSs purified from different bacteria, a greater diversity of genetic knock-out strains, human assays with more defined immune cell populations, and more validation in mouse models. Analysis of a wider diversity of AATGal-ZPSs may elucidate ones with therapeutic potential.
Even for the best-studied AATGal-ZPS, PSA of B. fragilis, the mechanisms of immune-modulation, especially in humans, are far from being completely understood. Previous work establishing anti-inflammatory effects of B. fragilis/PSA in mice and humans have shown that PSA induces Foxp3+ Treg cells that express IL-10 upon antigen presentation on DCs (Round and Mazmanian, 2010)(Telesford et al. 2015), a result which we confirm here for both B. fragilis PSA and an AATGal-ZPS in B. cellulosilyticus DSM 14838. However, we also found that PSA induced IL-10 production by CD4+ T cells in mixed cell populations was mediated mostly by FoxP3- cells in humans, which is consistent with the results of Kreisman and Cobb despite considerable differences in assay design (Kreisman and Cobb 2011). It has also been shown previously that PSA can directly induce IL-10 from FoxP3−/CD4+ T cells via TLRs in mice (Round et al. 2011). The bacterial mediation of CD4+ T cell IL-10 production by Tr1 cells in humans is not unique to B. fragilis; the probiotic Bifidobacterium breve also induces colonic IL-10 producing Tr1 cells that do not express FoxP3 (Jeon et al. 2012).
This work also evaluated the effects of PSA on immune cells in the gut compartment, and stimulations performed with mixed cell populations from LPMC from human gut tissues showed comparable results to those performed with PBMC when comparing B. fragilis to B. fragilis ΔPSA. This is an important finding since immune cells in different body compartments have the potential to respond very differently to environmental stimuli (Hu and Pasare 2013), and indicates that immune phenotypes in PBMC can be relevant to the gut compartment. However, for the broad comparison of predicted ZPS-producers to non ZPS-producers, the results for LPMC were not as strong as for PBMC with only increased IL-10 production in the Bacteroidales being modestly significant and no significant differences in Treg induction. The weaker phenotype in LPMC may be related to inherent challenges when working with these tissues. There was more variability in the responses across samples, which may be due to factors that are difficult to control for such as the presence of different bacterial populations already on the mucosal samples. Also, inherent differences in the bacteria that go beyond presence/absence of AATGal-ZPSs (e.g. production of other factors by these microbes that also influence immune phenotypes) may indicate why we had the power to observe a difference in B. fragilis versus knockout in LPMC but not in the broader comparisons of putative ZPS-producers versus non-producers. Further experiments with additional ZPS knock-out strains and purified ZPS will allow for a more robust analysis of whether diverse AATGal-ZPSs have an anti-inflammatory phenotype in human LPMC.
Our techniques identify commensal bacteria already known to have anti-inflammatory properties
Some AATGal-ZPS encoding bacteria have been shown to have anti-inflammatory properties in other studies. For instance, the crude lysate of P. distasonis, and particularly its membranous fraction, attenuated DSS-induced murine colitis while preventing increases in several pro-inflammatory cytokines and promoting significantly more CD4+CD25+FoxP3+ cells in mesenteric lymph nodes (Kverka et al. 2011). P. distasonis has also been previously shown to induce Tregs through a T cell receptor that was shared with B. uniformis, another species that we predict to produce an AATGal-ZPS (Lathrop et al. 2011). However, abolishment of function upon digestion with protease K indicated that this antigen is a protein and not a polysaccharide, signifying that P. distasonis and B. uniformis may share additional factors for stimulating Tregs.
The predicted ZPS producers also included close relatives of 2 bacteria in a 17-strain consortium previously shown to induce Tregs in mice and protect against colitis and allergic diarrhea in mouse models (Atarashi et al. 2013) (Clostridium ramosum, and Clostridum 7_3_45FAA which is highly related to Clostridium symbiosum (Figure 2). Use of a cognate antigen-driven suppressor assay determined that specific bacterial antigens likely contributed to expansion of Tregs in this model (Atarashi et al. 2013).
Our literature search also identified papers describing the convergence of surface structure properties of B. fragilis with distantly related ZPS-producers. For instance, Clostridium spiroforme, a predicted ZPS-producer in the Erysipelotrichales, had previously been reported to have a polysaccharide capsule similar to that of B. fragilis that mediates haemagglutination in both species (Baldassarri et al. 1989). Interestingly, the electron micrographs of C. spiroforme reported in (Baldassarri et al. 1989) show outer-membrane vesicles (OMVs) that are strikingly similar to those of B. fragilis (Shen et al. 2012) (Figure S7). OMVs of B. fragilis carry PSA and can induce immunomodulatory effects and prevent experimental colitis (Shen et al. 2012).
Our techniques identify human pathogens
The predicted producers of AATGal- ZPSs also include pathogenic bacteria, such as Clostridium botulinum (botulism), Clostridium perfringens (gas gangrene;bacteremias;wound infection), and Brachyspira hyodysenteriae (swine dysentery) and bacterial species that may opportunistically infect immunocompromised hosts such as C. ramosum (van der Vorm et al. 1999) and C. symbiosum (Elsayed and Zhang 2004). The presence of the AATGal-ZPS operon orthologues in pathogenic bacteria is not surprising given that ZPS producing bacteria including B. fragilis, S. pneumoniae, and S. aureus, often are commensal bacteria that can cause disease when they have escaped their normal habitats (Surana and Kasper 2012). B. fragilis is considered to be the most virulent of all of the Bacteroides species because of frequent isolation from a variety of clinical specimens including intra-abdominal abscess, blood, wound, perirectal, pelvic and other sites (Polk and Kasper 1977), and abscess formation requires PSA (Lindberg et al. 1982; Mazmanian and Kasper 2006; Onderdonk et al. 1977). S. aureus induces skin abscess in a manner that is also dependent on its ZPS (Weidenmaier, McLoughlin, and Lee 2010), and S. pneumoniae strains that produce Sp1 are the most common cause of bacterial pneumonia (Stephen, Groneck, and Kalka-Moll 2010).
Although ZPSs can also in certain contexts be pro-inflammatory, e.g. as Th17 cell inducers (Mazmanian and Kasper 2006; Surana and Kasper 2012), Treg induction itself is known to be important for many pathogens as a mechanism for evasion of immune clearance and persistence in the host (Belkaid 2007; Mills 2004). Consistent with this notion, infection with putative ZPS-producer B. hyodysenteriae, which induces a severe colitis in pigs known as swine dysentery, induces a mucosal CD4+ T-cell response. This suggests that the induction of both pro-inflammatory and regulatory responses are important in the pathogenesis of this species (Hontecillas et al. 2005).
CONCLUSIONS
Taken together this work has established a greater diversity of AATGal-ZPSs and bacteria that carry them than has been previously appreciated. Much further study will be needed to understand how this expanded class of important immune-modulatory molecules mediate interactions with the host.
EXPERIMENTAL PROCEDURES
Putative ZPS operons were identified using a BLASTP search of the wcfR, wcfS, upaY, and upaZ genes of the PSA operon of B. fragilis NCTC 9343 against the entire collection of completed and draft genomes in the NCBI database on September of 2013. The proximity of wcfR, wcfS, upaY, and upaZ gene homologues on genomic contigs were determined using custom code. Operon gene membership was further defined using DOOR2 (Mao et al. 2014) and compared as detailed in the Supplemental Methods. Phylogenetic trees of the wcfR genes and the 16S rRNA genes from the surveyed bacteria were created as described in the Supplemental Methods.
The bacterial isolates used in the immune assays were purchased from the ATCC or DSMZ and grown in rich media depending on their preferences as described in the Supplemental Methods. Bacteria were subjected to freeze/thaw or heat killing before being used in immune stimulations. Since immune stimulations with heat killed compared to freeze/thaw lysates resulted in no differences (data not shown), all reported stimulations were done with freeze/thaw lysates in order to avoid protein denaturing. The qPCR assays that were used to verify expression of wcfR in B. cellulosilyticus, B. fragilis WT, and B. uniformis are described in the Supplemental Methods and Figure S1B.
The B. cellulosilyticusΔZPS1 strain was developed using the pKNOCK-bla-ermGb vector to disrupt wcfR via targeted insertional mutagenesis as previously described (Alexeyev 1999) and as detailed in the Supplemental Methods.
To conduct the immune assays, human PBMCs were isolated by Ficol gradient centrifugation as previously described (Chain et al. 2013; Kassu et al. 2010; Neff et al. 2015) from the blood of 13 normal individuals. Informed consent was obtained and the study protocol was approved by the Colorado Multiple Institutional Review Board (COMIRB #14-1595). PBMCs were cultured with 10 μg freeze killed bacterial lysate for 3 days at 37°C. Six and 10-day stimulations were also performed and had similar results to those of 3-day stimulations (data not shown). Stimulations were performed in the presence of Streptomycin and Penicillin and in aerobic conditions and no bacterial growth was observed in the cell cultures. Unlike in the assays conducted by Kreisman and Cobb (Kreisman and Cobb 2011) in human PBMC, our stimulations were conducted in absence of exogenous IL-2; the IL-2 receptor CD25 is upregulated in the presence of IL-2, which could compromise our Treg staining. Cytokine secretion after 3-day stimulations with bacterial lysates was quantified in supernatant using ELISA Ready Set Go! (eBioscience), Tregs were enumerated using flow cytometry, and ICCS was used to determine IL-10+ cells, all as detailed in the Supplementary Methods. To determine whether induced IL-10 production and Tregs were due to restimulation of memory T cells, CD14+ monocytes were isolated by magnetic bead selection (Miltenyi Biotec), plated, stimulated with bacterial lysate for 4 hours and washed twice with PBS. Naïve T cells were negatively selected by magnetic beads (Miltenyi Biotec) and were added to the bacterial stimulated APCs. After three days supernatant was collected and subjected to IL-10 ELISA and cells were enumerated for Tregs.
Intestinal lamina propria lymphocytes were isolated from resected gut tissue of jejunum, duodenum and colon, which were obtained from patients undergoing elective abdominal surgery. All patients signed a release to allow the unrestricted use of discarded tissues for research purposes and all protected patient information was de-identified to the laboratory investigators. The lamina propria lymphocytes were isolated from these tissues as detailed in the Supplemental methods.
To test for protection in a murine TNBS colitis model, female C57/Bl6 mice aged 8 – 12 weeks were gavaged with bacteria or control PBS on days 0, 7, and 14 as described in the Supplemental Methods. Mice were cohoused prior to the intervention, and then housed in separate cages once the treatments began to prevent transmission of the introduced bacteria between mice. Mice were anesthetized, shaved and skin painted with 100μl of 1% TNBS in 100% EtOH on day 7 and received a rectal enema of 2.5% TNBS in 40% EtOH (5μl/g body weight) on day 14. Colonic tissue was prepared for histological assessment of disease as described in the Supplemental Materials or digested as previously described (Collins et al. 2013). CD4+CD25+FoxP3+ cells in colonic tissue were enumerated with flow cytometry as described in the Supplemental methods and statistical analysis was performed using GraphPad Prism. These experiments were approved by IACUC (B-104413(12)1E).
Supplementary Material
Highlights.
Genomic screen identifies bacteria with ability to produce zwitterionic polysaccharides.
Predicted bacteria display anti-inflammatory immune modulation in human cell assays.
Putative ZPS-producer Bacteroides cellulosilyticus protected mice from colitis.
ZPS-producing bacteria may have therapeutic potential.
Acknowledgments
Sources of Support
We would like to thank Ashleigh Jones for her technical assistance with the mouse experiments. We would also like to express deep gratitude to Eric Martens for sharing with us the pKNOCK-bla-ermGb vector and for his guidance in conducting insertional transposon mutagenesis. This work was funded from R01 DK104047. Dr. Lozupone was also supported by K01 DK090285. Dr. Neff was supported by NIH T32 AI007405. Dr. Collins was supported by K01 DK099403.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceptualization, C.A.L. and B.E.P.; Methodology, C.P.N., M.E.R., K.L.A., C.B.C, C.A.L., and B.E.P.; Software, M.E.R., M.S., and C.A.L.; Formal Analysis, C.P.N., M.E.R., K.L.A., C.B.C., B.E.P., and C.A.L.; Investigation, C.P.N., K.L.A., C.B.C., J.D., N.N., P.J., and J.M.S.; Resources, M.D.M. and S.K.M.; Data Curation, M.E.R., M.S., and C.P.N.; Writing – Original Draft, C.P.N., M.E.R., C.B.C., B.E.P., and C.A.L.; Writing – Review & Editing, K.L.A., M.S., S.K.M; Visualization, C.P.N., M.E.R., K.L.A., C.B.C., B.E.P., and C.A.L.; Supervision, B.E.P. and C.A.L., Project Administration, B.E.P. and C.A.L.; Funding Acquisition, C.A.L., B.E.P. and C.B.C.
References
- Alexeyev MF. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. Biotechniques. 1999;26:824–6. 28. doi: 10.2144/99265bm05. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–6. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- Avci FY, Kasper DL. How bacterial carbohydrates influence the adaptive immune system. Annu Rev Immunol. 2010;28:107–30. doi: 10.1146/annurev-immunol-030409-101159. [DOI] [PubMed] [Google Scholar]
- Baldassarri L, Pantosti A, Caprioli A, Mastrantonio P, Donelli G. Haemagglutination and surface structures in strains of Clostridium spiroforme. FEMS Microbiol Lett. 1989;51:1–4. doi: 10.1016/0378-1097(89)90066-9. [DOI] [PubMed] [Google Scholar]
- Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–88. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- Chain JL, Martin AK, Mack DG, Maier LA, Palmer BE, Fontenot AP. Impaired function of CTLA-4 in the lungs of patients with chronic beryllium disease contributes to persistent inflammation. J Immunol. 2013;191:1648–56. doi: 10.4049/jimmunol.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CB, Aherne CM, Ehrentraut SF, Gerich ME, McNamee EN, McManus MC, Lebsack MD, Jedlicka P, Azam T, de Zoeten EF, Dinarello CA, Rivera-Nieves J. Alpha-1-antitrypsin therapy ameliorates acute colitis and chronic murine ileitis. Inflamm Bowel Dis. 2013;19:1964–73. doi: 10.1097/MIB.0b013e31829292aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne MJ, Tzianabos AO, Mallory BC, Carey VJ, Kasper DL, Comstock LE. Polysaccharide biosynthesis locus required for virulence of Bacteroides fragilis. Infect Immun. 2001;69:4342–50. doi: 10.1128/IAI.69.7.4342-4350.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsayed S, Zhang K. Bacteremia caused by Clostridium symbiosum. J Clin Microbiol. 2004;42:4390–2. doi: 10.1128/JCM.42.9.4390-4392.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, Ocheltree EL, Greenberg PD, Ochs HD, Rudensky AY. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A. 2006;103:6659–64. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hontecillas R, Bassaganya-Riera J, Wilson J, Hutto DL, Wannemuehler MJ. CD4+ T-cell responses and distribution at the colonic mucosa during Brachyspira hyodysenteriae-induced colitis in pigs. Immunology. 2005;115:127–35. doi: 10.1111/j.1365-2567.2005.02124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–69. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- Hu W, Pasare C. Location, location, location: tissue-specific regulation of immune responses. J Leukoc Biol. 2013;94:409–21. doi: 10.1189/jlb.0413207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H, Tsuji NM, Kiyono H, Ma JS, Kusu T, Okumura R, Hara H, Yoshida H, Yamamoto M, Nomoto K, Takeda K. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 2012;8:e1002714. doi: 10.1371/journal.ppat.1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalka-Moll WM, Tzianabos AO, Bryant PW, Niemeyer M, Ploegh HL, Kasper DL. Zwitterionic polysaccharides stimulate T cells by MHC class II-dependent interactions. J Immunol. 2002;169:6149–53. doi: 10.4049/jimmunol.169.11.6149. [DOI] [PubMed] [Google Scholar]
- Kassu A, Marcus RA, D’Souza MB, Kelly-McKnight EA, Golden-Mason L, Akkina R, Fontenot AP, Wilson CC, Palmer BE. Regulation of virus-specific CD4+ T cell function by multiple costimulatory receptors during chronic HIV infection. J Immunol. 2010;185:3007–18. doi: 10.4049/jimmunol.1000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisman LS, Cobb BA. Glycoantigens induce human peripheral Tr1 cell differentiation with gut-homing specialization. J Biol Chem. 2011;286:8810–8. doi: 10.1074/jbc.M110.206011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinos CM, Coyne MJ, Weinacht KG, Tzianabos AO, Kasper DL, Comstock LE. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature. 2001;414:555–8. doi: 10.1038/35107092. [DOI] [PubMed] [Google Scholar]
- Kumar Y, Westram R, Behrens S, Fuchs B, Glockner FO, Amann R, Meier H, Ludwig W. Graphical representation of ribosomal RNA probe accessibility data using ARB software package. BMC Bioinformatics. 2005;6:61. doi: 10.1186/1471-2105-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kverka M, Zakostelska Z, Klimesova K, Sokol D, Hudcovic T, Hrncir T, Rossmann P, Mrazek J, Kopecny J, Verdu EF, Tlaskalova-Hogenova H. Oral administration of Parabacteroides distasonis antigens attenuates experimental murine colitis through modulation of immunity and microbiota composition. Clin Exp Immunol. 2011;163:250–9. doi: 10.1111/j.1365-2249.2010.04286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–4. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg AA, Weintraub A, Kasper DL, Lonngren J. Virulence factors in infections with bacteroides fragilis: isolation and characterization of capsular polysaccharide and lipopolysaccharide. Scand J Infect Dis Suppl. 1982;35:45–52. [PubMed] [Google Scholar]
- Lozupone CA, Rhodes ME, Neff CP, Fontenot AP, Campbell TB, Palmer BE. HIV-induced alteration in gut microbiota: driving factors, consequences, and effects of antiretroviral therapy. Gut Microbes. 2014;5:562–70. doi: 10.4161/gmic.32132. [DOI] [PubMed] [Google Scholar]
- Mao F, Dam P, Chou J, Olman V, Xu Y. DOOR: a database for prokaryotic operons. Nucleic Acids Res. 2009;37:D459–63. doi: 10.1093/nar/gkn757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Ma Q, Zhou C, Chen X, Zhang H, Yang J, Mao F, Lai W, Xu Y. DOOR 2.0: presenting operons and their functions through dynamic and integrated views. Nucleic Acids Res. 2014;42:D654–9. doi: 10.1093/nar/gkt1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Kasper DL. The love-hate relationship between bacterial polysaccharides and the host immune system. Nat Rev Immunol. 2006;6:849–58. doi: 10.1038/nri1956. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–18. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–5. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- McNulty NP, Wu M, Erickson AR, Pan C, Erickson BK, Martens EC, Pudlo NA, Muegge BD, Henrissat B, Hettich RL, Gordon JI. Effects of diet on resource utilization by a model human gut microbiota containing Bacteroides cellulosilyticus WH2, a symbiont with an extensive glycobiome. PLoS Biol. 2013;11:e1001637. doi: 10.1371/journal.pbio.1001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–55. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- Molloy MJ, Bouladoux N, Belkaid Y. Intestinal microbiota: shaping local and systemic immune responses. Semin Immunol. 2012;24:58–66. doi: 10.1016/j.smim.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff CP, Chain JL, MaWhinney S, Martin AK, Linderman DJ, Flores SC, Campbell TB, Palmer BE, Fontenot AP. Lymphocytic alveolitis is associated with the accumulation of functionally impaired HIV-specific T cells in the lung of antiretroviral therapy-naive subjects. Am J Respir Crit Care Med. 2015;191:464–73. doi: 10.1164/rccm.201408-1521OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev. 2004;17:218–34. doi: 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk AB, Kasper DL, Cisneros RL, Bartlett JG. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis. 1977;136:82–9. doi: 10.1093/infdis/136.1.82. [DOI] [PubMed] [Google Scholar]
- Polk BF, Kasper DL. Bacteroides fragilis subspecies in clinical isolates. Ann Intern Med. 1977;86:569–71. doi: 10.7326/0003-4819-86-5-569. [DOI] [PubMed] [Google Scholar]
- Robert C, Chassard C, Lawson PA, Bernalier-Donadille A. Bacteroides cellulosilyticus sp. nov., a cellulolytic bacterium from the human gut microbial community. Int J Syst Evol Microbiol. 2007;57:1516–20. doi: 10.1099/ijs.0.64998-0. [DOI] [PubMed] [Google Scholar]
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–7. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–9. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509–20. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen TL, Groneck L, Kalka-Moll WM. The modulation of adaptive immune responses by bacterial zwitterionic polysaccharides. Int J Microbiol. 2010;2010:917075. doi: 10.1155/2010/917075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surana NK, Kasper DL. The yin yang of bacterial polysaccharides: lessons learned from B. fragilis PSA. Immunol Rev. 2012;245:13–26. doi: 10.1111/j.1600-065X.2011.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telesford KM, Yan W, Ochoa-Reparaz J, Pant A, Kircher C, Christy MA, Begum-Haque S, Kasper DL, Kasper LH. A commensal symbiotic factor derived from Bacteroides fragilis promotes human CD39(+)Foxp3(+) T cells and Treg function. Gut Microbes. 2015;6:234–42. doi: 10.1080/19490976.2015.1056973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzianabos AO, Onderdonk AB, Rosner B, Cisneros RL, Kasper DL. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 1993;262:416–9. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- van der Vorm ER, von Rosenstiel IA, Spanjaard L, Dankert J. Gas gangrene in an immunocompromised girl due to a Clostridium ramosum infection. Clin Infect Dis. 1999;28:923–4. doi: 10.1086/517249. [DOI] [PubMed] [Google Scholar]
- Velez CD, Lewis CJ, Kasper DL, Cobb BA. Type I Streptococcus pneumoniae carbohydrate utilizes a nitric oxide and MHC II-dependent pathway for antigen presentation. Immunology. 2009;127:73–82. doi: 10.1111/j.1365-2567.2008.02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kalka-Moll WM, Roehrl MH, Kasper DL. Structural basis of the abscess-modulating polysaccharide A2 from Bacteroides fragilis. Proc Natl Acad Sci U S A. 2000;97:13478–83. doi: 10.1073/pnas.97.25.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Telesford KM, Ochoa-Reparaz J, Haque-Begum S, Christy M, Kasper EJ, Wang L, Wu Y, Robson SC, Kasper DL, Kasper LH. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nat Commun. 2014;5:4432. doi: 10.1038/ncomms5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenmaier C, McLoughlin RM, Lee JC. The zwitterionic cell wall teichoic acid of Staphylococcus aureus provokes skin abscesses in mice by a novel CD4+ T-cell-dependent mechanism. PLoS One. 2010;5:e13227. doi: 10.1371/journal.pone.0013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074–6. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- Young NM, Kreisman LS, Stupak J, MacLean LL, Cobb BA, Richards JC. Structural characterization and MHCII-dependent immunological properties of the zwitterionic O-chain antigen of Morganella morganii. Glycobiology. 2011;21:1266–76. doi: 10.1093/glycob/cwr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


