Summary
Progress in clinical and affective neuroscience is redefining psychiatric illness as symptomatic expression of cellular/molecular dysfunctions in specific brain circuits. Post-traumatic stress disorder (PTSD) has been an exemplar of this progress, with improved understanding of neurobiological systems subserving fear learning, salience detection, and emotion regulation explaining much of its phenomenology and neurobiology. However, many features remain unexplained and a parsimonious model that more fully accounts for symptoms and the core neurobiology remains elusive. Contextual processing is a key modulatory function of hippocampal-prefrontal-thalamic circuitry, allowing organisms to disambiguate cues and derive situation-specific meaning from the world. We propose that dysregulation within this context-processing circuit is at the core of PTSD pathophysiology, accounting for much of its phenomenology and most of its biological findings. Understanding core mechanisms like this, and their underlying neural circuits, will sharpen diagnostic precision and understanding of risk factors, enhancing our ability to develop preventive and “personalized” interventions.
Introduction
Historical perspective on the evolution of the PTSD concept
The past two decades have witnessed a fundamental transformation in our understanding and conceptualization of psychopathology. Cultural, clinical and scientific perspectives increasingly converge on a view of psychiatric disorders as dysfunction within specific components of the central nervous system, rather than “functional” disorders unrelated to neurobiological substrates. This shift has forced theoretical approaches to grapple with the biological underpinning of psychological processes, grounding our models of illness in growing understanding of neural circuits, cellular mechanisms and molecular processes. This conceptual shift has been challenging but also transformational in the study of post-traumatic stress disorder (PTSD), which has seemed like a quintessentially psychological illness because it develops in reaction to specific environmental events.
The deleterious effects of severe trauma on the human psyche have been documented for centuries, generally depicted as “normative” responses to extraordinary circumstances. During the 19th and early 20th century, with emergence of psychiatry and psychology as clinical disciplines, the more debilitating consequences of trauma were re-conceptualized as clinical conditions, amenable to scientific inquiry and treatment (Jones, 2006). Conceptual challenges persisted, however, in sometimes controversial efforts to differentiate true psychopathology from normative responses to extreme circumstances. Resolution of these controversies awaits empirical documentation of causative neurobiological pathways, which will facilitate an understanding of how brain-based vulnerabilities lead to dysregulated psychological responses, and emergence of disorder-specific neurobiological abnormalities. PTSD has been studied intensively in recent decades; but despite important breakthroughs, we still lack a comprehensive, integrated model that coherently and parsimoniously explains both its phenomenology and its neurobiology. In this Perspective, we will describe PTSD as a clinical entity and touch on its well-established neurobiological findings. We will then try to organize emerging findings within existing conceptual frameworks, highlighting the value and limitations of those frameworks. Finally, we will describe a new framework, focused on the neural circuitry of context processing, which adds additional explanatory power and can potentially better integrate more of what we currently know about this important disorder.
PTSD as a clinical phenomenon
In the absence of scientific understanding of the pathophysiologic processes involved in PTSD development, the “gold standard” for defining it has been expert consensus as reflected in DSM criteria. These criteria have changed as clinical perspectives and scientific understanding have evolved, but they have remained phenomenological at their core since they are not yet grounded in biological processes and CNS neuro-circuitry. Despite periodic and sometimes substantial changes, there has been consistent agreement on three sets of symptom clusters considered characteristic of PTSD -- the intrusive, avoidant and hyperarousal clusters. These encompass unwanted and repeatedly re-experienced memories, sensations or dreams associated with the trauma (intrusive cluster); behavioral avoidance of trauma reminders; and excessive physiological arousal both in response to trauma cues or independently (e.g., sleep problems). These core clusters have been embraced in DSM III, IV, and 5, but other symptoms or criteria have been added (negative affect or reckless behavior in DSM-5), deleted (trauma outside of usual human experience), or included descriptively (shame/guilt, dissociation). Shifting criteria create scientific challenges to sample homogeneity across time and undermine clinical efficacy when targets for intervention are imprecise and continuously moving. As clinician-scientists, we will do better when we can target precisely defined dysregulations within specific neural circuits sub-serving particular, mechanistically relevant brain functions. As we identify underlying mechanisms rooted in specific neural circuits, diagnostic precision will sharpen and our ability to “personalize” interventions will be enhanced. For PTSD, we will also be able to go beyond treatment and apply preventive interventions before or immediately after trauma exposure.
PTSD pathophysiology -- early studies
Prior to development of in vivo functional neuroimaging and valid animal models, allowing direct examination of CNS structure and function, biological study of PTSD focused on identification of abnormal peripheral markers. The best replicated of these have been psychophysiological indices of autonomic nervous system (ANS) function, and altered blood, saliva and urine concentration of stress hormones and catecholamines. Noradrenergic hyper-reactivity was the most reliably replicated finding in PTSD (Pitman et al., 2012), along with altered function of the hypothalamic-pituitary adrenal (HPA) axis, where hypersensitivity of glucocorticoid receptors was accompanied by unaltered or decreased levels of circulating cortisol (Yehuda, 2001).
ANS abnormalities have been seen at rest and in reaction to trauma cue exposure (Agorastos et al., 2013; Cohen et al., 2000; Lee and Theus, 2012; Minassian et al., 2014; Shah et al., 2013) (for meta-analysis see Pole, 2007). Heart rate variability (HRV) is a sensitive index of ANS function (Bilchick and Berger, 2006; Malik and Camm, 1995) and reductions in it -- which suggest arousal dysregulation and autonomic inflexibility due to sympathetic overdrive and/or parasympathetic insufficiency (Shah et al., 2013; Cohen et al., 2000) -- predict PTSD development whether measured prior to (Minassian et al., 2015) or immediately after trauma exposure (Shaikh al arab et al., 2012). PTSD is also associated with exaggerated catecholamine responses to trauma cues (Liberzon, Abelson, et al., 1999), and elevated levels of NE secretion in 24-hour urine collections (Wingenfeld et al., 2015). PTSD may thus reflect a “hyper-adrenergic state” (Krystal and Neumeister, 2009). Dysregulation is also evident in HPA axis function, but this appears more complex. The more consistent abnormalities involve enhanced dexamethasone suppression of cortisol (Yehuda et al., 1993), often attributed to glucocorticoid (GC) receptor hyper-sensitivity (Yehuda, 2001). This is supported by in vivo assay evidence of GC receptor hyper-sensitivity on lymphocytes (Yehuda, Boisoneau, et al., 1995). Measurement of cortisol levels in PTSD has generally suggested normal (Baker et al., 1999) or reduced levels (Yehuda et al., 1990; Yehuda, Kahana, et al., 1995; Yehuda et al., 1996), though there are some inconsistent reports (Meewisse et al., 2007; Lindley et al., 2004; Pitman and Orr, 1990). Reduced levels would be consistent with GC receptor hypersensitivity and early HPA axis “shutdown.”
Additional peripheral biological abnormalities linked to PTSD require replication, but are consistent with the changes reported above. These include alterations in serotonin systems (Southwick et al., 1999), DHEA (Yehuda et al., 2006), neuropeptide Y (Rasmusson et al., 2000), neurosteroids (Morris et al., 2012), endocannabinoids (Wilker et al., 2016) and endogenous opioids (van der Kolk et al., 1989). Genetic work is rapidly expanding, and has identified PTSD-linked polymorphisms in genes encoding elements within the HPA axis (FKBP5 (Binder et al., 2008) and CRHR1 (Amstadter et al., 2011; White et al., 2013; Etkin et al., 2015)), and the sympatho-adrenal system (ADRB2 (Liberzon et al., 2014) and COMT (Boscarino et al., 2011)). These require confirmation in large-scale GWAS studies, but are consistent with the peripheral findings and suggest that PTSD is characterized by ANS hypersensitivity, associated with or leading to hyperadrenergic states, and a hypersensitive glucocorticoid receptor system. However, potential linkages between these abnormalities and the central mechanisms that drive them remain unknown.
PTSD Neurocircuits
The development of in vivo neuroimaging methodologies and emergence of affective neuroscience have shifted the focus of contemporary neuropsychiatric inquiry to specific neural circuits that might govern the development of PTSD. Converging evidence from human imaging studies and animal models of complex behaviors like fear associated learning has generated powerful, neurocircuitry-based models of its pathophysiology. In the following sections, we selectively review the evolving picture, organizing it within dominant emergent models, identified as (1) an abnormal fear learning model (FL), which encompasses altered fear conditioning, fear extinction and fear generalization hypotheses; (2) an exaggerated threat detection model (TD) which postulates altered attention, anticipation, or “alarm” functions; and (3) a diminished emotional regulation/executive function (EF) model which postulates deficient regulatory capacity of cognitive/executive function regions over emotion generating limbic structures. These models are distinct in their focus on different aspects of PTSD psychopathology and different brain circuits, and may be driven by different cellular and molecular processes. However, they are not necessarily mutually exclusive. Each may explain different aspects of the disorder and a different subset of its biological abnormalities. We then identify important aspects of PTSD that are not explained by these models. Together they can explain much, but they invoke at least three independent pathophysiological processes while leaving some key symptoms and findings unexplained. This is not very parsimonious or conceptually satisfying. It is possible, or even likely, that PTSD is not a single homogeneous disorder but rather is a syndrome that “houses” within it a number of sub-categories with somewhat different presentations, endophenotypes, and pathophysiological mechanisms. Nevertheless the fact that important parts remain unexplained suggests a need for additional, alternative, or more mechanistically expansive models, which might parsimoniously explain both the complex symptomatology of PTSD and its existing neurobiological findings. Following discussion of the FL, TD and ER/EF models, we present a novel model that is both more general and more parsimonious, explaining multiple, not previously explained symptoms, and integrating neurobiological findings within the framework of a focal pathophysiologic process – altered contextual processing (CP). The CP model focuses our attention on dysfunction within specific brain circuits governing the critical adaptive function of contextualization – involving hippocampus (Hpc) prefrontal cortex (PFC), thalamic circuits that modulate activity in amygdala and other limbic and cortical regions. Prior work has identified clear abnormalities in PTSD in the Hpc, PFC, and amygdala, and we suggest that dysfunction within this circuit may be a key pathophysiologic process underlying expression of various PTSD symptoms, in a way that may be able to integrate the contributions of the altered noradrenergic and glucocorticoid functions that have been so consistently documented in this disorder.
Models of Pathophysiology
Abnormal Fear Learning
Learning what is threatening and what is safe is a critical, highly conserved function that had been extensively studied neurobiologically using fear associated learning paradigms and fear conditioning, fear extinction, and fear renewal, processes. PTSD has been conceptualized as heightened fear reactivity that develops in response to exposure to a threatening event, thus abnormal fear learning was a logical initial place to look in searching for its neurobiology. Fear and safety learning studies have focused mechanistically on a key structure governing these processes – the amygdala – and the complex interplay within the amygdaloid complex (Figure 1) between various nuclei and cell types. Additional processes clearly participate in fear/safety learning, like memory consolidation, fear generalization, and extinction retention, and these involve additional brain mechanisms and regions (e.g., sensory cortex, thalamus, hippocampus, vmPFC). However, within the fear-associated learning framework, these processes and regions have been seen through the prism of their impact on amygdala function and output, rather than as key psychological/pathophysiologic processes shaping PTSD development. Other conceptual models (Threat Detection, Emotional Regulation and Contextual Processing) focus on these extra-amygdala processes as key contributors to PTSD pathophysiology, and they are discussed in subsequent sections.
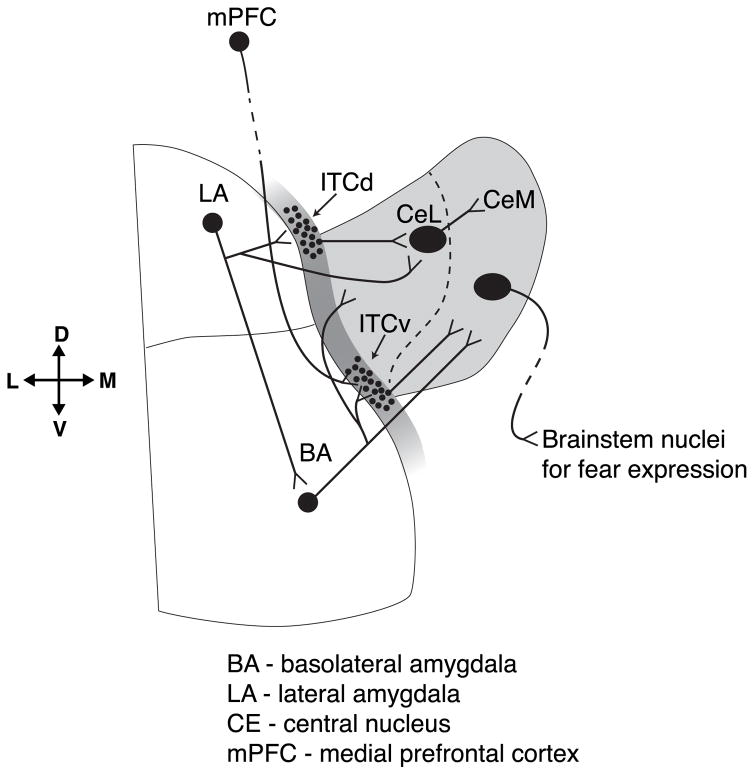
Figure 1.
Neural circuits within the Basolateral Complex (BLC) of the Amygdala. Altered functioning within BLC circuits has been implicated in the Abnormal Fear Learning model of PTSD (see text). Interrupted lines represent distal inputs to and outputs from BLC.
Fear conditioning/acquisition
The basic circuits of fear conditioning/extinction have been well-worked out. They involve perceptual inputs to thalamus, then to amygdala and to effector systems (LeDoux et al., 1990). Within the amygdala, the basolateral complex (BLC), composed of the lateral (LA), basolateral (BLA), and accessory basal (AB) nuclei, provides the main input to the central nucleus (CE), which constitutes the “final relay” in coordinated outputs to physiological and behavioral effector systems that “express fear” (e.g., Campeau and Davis, 1995). The BLC is the region where associative fear learning, extinction learning, consolidation, and expression are thought to take place (Davis et al., 1994; Fanselow and LeDoux, 1999; Davis, 1992). The cellular substrates for associative learning are long-term potentiation (LTP) and long-term depression (LTD) (Eichenbaum, 1996; Matynia et al., 2002), whereby associative LTP at BLC principal neurons (Blair et al., 2001; Goosens and Maren, 2002) creates a neural link between an unconditioned stimulus (US) and a conditioned stimulus (CS). The phenomenology of PTSD readily suggests disruption in this process, since so many “CSs” (e.g., a backfiring car) seem to be inappropriately linked to the sense of threat carried by traumatic memories. However, BLC sub-networks also have specific connections with prefrontal cortical regions that modulate fear expression, including prelimbic and infralimbic areas. Specific BLC neurons can activate prelimbic PFC during fear learning, while others target infralimbic PFC during extinction (Senn et al., 2014). The BLC can thus provide higher level processors with “information” about what is dangerous and what is safe, potentially for use in future situations that are ambiguous but carry threat potential.
Though intuitively appealing, evidence that PTSD is a disorder of fear conditioning itself is sparse. Trauma exposure itself is readily thought of as an explicit conditioning episode (Elzinga and Bremner, 2002; Jovanovic and Ressler, 2010; VanElzakker et al., 2014), but evidence for abnormalities in fear acquisition in PTSD is conflicting. In typical differential fear conditioning paradigms, where subjects are presented with a US-predicting CS (CS+) and a non-US-predicting CS (CS−), which acts as a safety signal (Lissek et al., 2005), PTSD patients have difficulty differentiating safety from threat (Grillon et al., 1998; Grillon, 2002), but they generally do not show abnormal acquisition of the conditioned response (to CS+) itself (Garfinkel et al., 2014; Milad et al., 2009). Enhanced fear potentiated startle or skin conductance responses have been reported during fear learning by some (Morgan et al., 1995; Orr et al., 2000; Peri et al., 2000), but not others (Blechert et al., 2007; Milad et al., 2008; Milad et al., 2009). Trauma that can cause PTSD is much more severe than the simple US “threats” used in laboratory studies, but these studies do suggest that the basic ability to acquire new fear conditioned responses is not inherently abnormal in PTSD.
Fear Extinction
Alternatively, it has been proposed that fear associative learning deficits in PTSD stem from abnormalities in extinction or safety learning rather than fear conditioning. A key feature of PTSD is inability to learn that cues once associated with trauma are no longer dangerous -- a process akin to fear extinction in animal models. Dysregulation of extinction, undermining ability to learn that something once dangerous is now safe, may contribute to safety learning deficits in PTSD (Jovanovic et al., 2012). In general, extinction is less robust than conditioning and does not erase an established fear memory. Rather, it creates a safety memory trace that “overlays” the fear memory trace. It is context specific, decays with time, and involves learned inhibition of fear expression. It is mediated by distinct and dedicated pathways within BLC (Jasnow et al., 2013). Dysregulation in the topographically organized BLC output systems may induce imbalances within the larger fear-on and extinction circuits, leading to extinction deficits and persistence of fear memories. Indeed, a number of fear extinction studies report heightened autonomic reactions during extinction learning in PTSD, including increased skin conductance and heart rate and sustained fear-potentiated startle (Orr et al., 2000; Peri et al., 2000; Blechert et al., 2007; Norrholm et al., 2011).
Problems with safety signal learning and inability to modulate fear responses with safety cues (Jovanovic et al., 2009; Jovanovic et al., 2012) also suggest impairment in inhibition of fear pathways. However, other work suggests intact extinction learning in PTSD, but impaired recall of fear extinction memories 24 hours later (Garfinkel et al., 2014; Milad et al., 2009). Intact extinction recall, however, requires cortical inputs from vmPFC to amygdala (Quirk and Mueller, 2008), and intact safety learning after fear conditioning involves inputs from vmPFC and hippocampus (Corcoran and Quirk, 2007). Prefrontal regions have well-traced inputs to the amygdala through which they can amplify (prelimibic) or inhibit (infralimbic) fear expression (Sierra-Mercado et al., 2011). The human homologue of IL (vmPFC) is linked to extinction recall and is smaller in volume and hypo-responsive in PTSD patients (Bremner et al., 2008). Reduced activation of this area is associated with impaired fear inhibition (Jovanovic et al., 2012), raising the possibility that core pathophysiologic processes contributing to deficits in extinction retention may be located in regions outside amygdala and involve neuro-behavioral functions beyond basic fear associated learning processes.
Fear generalization
Fear generalization is another aspect of fear learning implicated in PTSD. Fear generalization occurs when, following conditioning, stimuli other than the CS, but which share some similarities with CS, elicit a fear-related response. This is an adaptive process that facilitates protective responses to situations that are similar to but not identical with situations previously learned to be dangerous (Shepard, 1987). Disruption in generalization (e.g., over-generalization) could contribute to excessive fear expression in PTSD (Lissek and van Meurs, 2015). Generalization is demonstrated by stimulus “response gradients” -- if during fear conditioning, the CS+ and CS− are chosen to represent two ends along a continuum, fear responses occur to the CS+ and to stimuli close to it on the continuum, with a declining response gradient as the cues approach the CS- end (Lissek et al., 2008). Importantly, the intensity of the US impacts the breadth of generalization beyond the CS+ (Baldi et al., 2004; Ghosh and Chattarji, 2015). A very strong US produces broad generalization. During PTSD-generative traumatic exposure the US (e.g., threat to life) may be sufficient to lead to broad generalization, undermining discrimination between dangerous and safe cues, contributing to high reactivity to “reminders” that bear any resemblance to trauma cues.
Neurobiological studies of generalization in fear conditioning paradigms have been sparse (Likhtik and Paz, 2015). Typical fear conditioning work uses easily discriminable stimuli to ensure strong CS+ vs. CS− differentiation, avoiding potential for generalization (Genud-Gabai et al., 2013). Generalization studies demonstrate neuronally instantiated “tuning curves” that differentiate tones of differing frequencies within auditory cortex, auditory thalamus, and amygdala (Bordi and LeDoux, 1994; Resnik and Paz, 2015; Bordi and LeDoux, 1992). Multiple amygdalar regions are involved, including CE (Ciocchi et al., 2010), other subnuclei (Shaban et al., 2006), and LA (Ghosh and Chattarji, 2015). The amygdala appears to play a role in generating graded generalization curves (Schechtman et al., 2010; Resnik et al., 2011; Laufer and Paz, 2012), and amygdala neurons show tuning properties that could account for behavioral generalization (Resnik and Paz, 2015), perhaps contributing to the broad fear generalization gradients suggested for PTSD. However, amygdala changes in these studies could be due to inputs coming from other brain regions. For example, there is conflicting evidence about the role of auditory cortex in stimulus discrimination and in overgeneralization (Aizenberg and Geffen, 2013; Letzkus et al., 2011). Complex interconnections make it difficult to isolate specific behavioral dysfunction, like overgeneralization, to disruptions at highly specific locations, and the complete circuit that mediates generalization of more complex real-life stimuli might involve interactions between microcircuits within the amygdala (Duvarci and Pare, 2014), the prefrontal-cortex (Likhtik et al., 2014; Klavir et al., 2013; Chavez et al., 2009; Dunsmoor et al., 2011; Courtin et al., 2014), hippocampus (Xu and Sudhof, 2013; Bergado-Acosta et al., 2008; Kaouane et al., 2012), and other regions.
Neuroimaging work has begun to examine the human neurocircuitry of fear generalization, revealing that activity in the amygdala and insula correlate selectively with generalized SCRs, and that functional connectivity between the amygdala and extra-striate visual cortex was selectively enhanced for a perceptually similar cue of high emotional intensity that was never paired with US (Dunsmoor et al., 2011). This suggests that amygdala-cortical communication might be active during generalization. Lissek et al. (2013) reported that gradients of fMRI activity are present in insula and dmPFC, with activity levels declining as similarity to CS+ declined, with a reverse gradient in vmPFC and hippocampus, where activity is greatest to the safety cue. Clinically, some evidence shows that PTSD patients show a failure to discriminate CS+ (threat) from CS− (safety), perhaps due to overgeneralization (Lissek et al., 2005; Jovanovic et al., 2012). However, there is very little experimental evidence specifically demonstrating overly broad generalization gradients in PTSD (Lissek and van Meurs, 2015). It is often assumed that patients with PTSD would exhibit broad generalization gradients based on their symptom profiles, but empirical documentation is still needed to substantiate this model.
Exaggerated Threat Detection (TD)
Disruptions in Fear/Safety learning or fear generalization can increase reactivity to potentially threatening stimuli. However, threat hyper-reactivity can also stem from processes independent of fear learning. One alternative potential source is in threat detection systems. An ability to detect “salience” in the environment, whether in the realm of threat or reward, is essential for survival, which requires a rapid detection system that can focus attention on salient cues, recruit multiple homeostatic systems to respond to them, and trigger automatic motor routines (Phelps and LeDoux, 2005). Hypersensitive salience detection could heighten vigilance and threat reactivity in PTSD, and activate fear and protective responses disproportionate to actual threats. Exaggerated threat detection has in fact been implicated in PTSD pathophysiology by some investigators (for review, see Shin and Liberzon, 2010; Sripada et al., 2012).
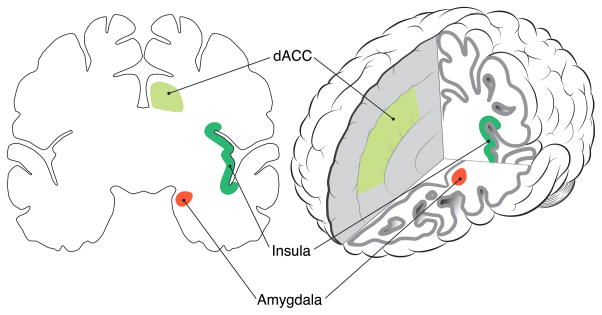
Functional neuroimaging and resting state studies have identified an interconnected network of brain regions, including amygdala, dorsal anterior cingulate cortex (dACC) and insula/operculum (Seeley et al., 2007) that activate when salience is detected in the environment (Figure 2). The amygdala’s role in threat detection and fear expression (Ohman, 2005) is well established (see above), but human fMRI work has also demonstrated amygdala reactivity to positive, rewarding or social stimuli (e.g., human faces; Javanbakht et al., 2015). Similarly, dACC is implicated in error and conflict detection, and in autonomic arousal (Critchley, 2004); and anterior insula/operculum activity is associated with anticipation of meaningful events (Simmons et al., 2004), monitoring of internal states (Craig, 2009), and pain perception (Craig, 2003). Functional connectivity between regions of this network creates a circuit, identified as a Salience Network (SN), which activates in response to emotionally arousing information (Seeley et al., 2007). Hyper-reactivity in the nodes of this network, or enhanced connectivity between these nodes, could contribute to hypervigilance, exaggerated threat detection, exaggerated physiological reactivity and other core symptoms of PTSD.
Figure 2.
Brain regions of the Salience Network. Abnormal function within Salience Network regions is implicated in the Exaggerated Threat Detection model of PTSD (see text).
Enhanced threat sensitivity expressed as hypervigilance is indeed a fundamental feature of PTSD. PTSD patients are biased to attend to threat (Buckley et al., 2000), at both supraliminal (Bryant and Harvey, 1995) and subliminal (Harvey et al., 1996) levels. Insula appears to be hyperactive in PTSD during anticipation (Lanius et al., 2007; R. J. L. Lindauer et al., 2008; Aupperle et al., 2012); and increased activation by threat cues is seen in the amygdala (Liberzon, Taylor, et al., 1999; Shin et al., 2004; Bryant et al., 2008). There is evidence that the SN might be hypersensitive to threat in PTSD patients even before trauma exposure, and this sensitivity can predict subsequent symptom development (Admon et al., 2009). Increased dACC reactivity is also seen in PTSD in response to conditioned fear cues (Rougemont-Bucking et al., 2011), demonstrating heightened sensitivity in all of the main nodes of the salience network. Excessive activity in all three nodes (dACC, insula and amygdala) predicted poor response to treatment (van Rooij et al., 2016), suggesting that enhanced SN activity might be involved in symptom persistence. Resting state connectivity studies have also demonstrated hyper-connectivity within SN regions, involving amygdala, insula, and ACC (Sripada et al., 2012). Such connectivity could enhance processing of threat cues. There thus may be sensitivities at multiple levels within the SN that could contribute to enhanced fear behaviors.
On the cellular/molecular level there is little information on specific processes supporting exaggerated Salience or Threat Detection. Neuronal sensitization within the amygdala to repeated cue exposure is one potential pathway to amygdala hyper-reactivity (Sandi and Richter-Levin, 2009; Li et al., 2010), but this has not yet been directly linked to enhanced Threat Detection or altered SN activity. A key challenge is the lack of animal models of SN hyperactivity, with amygdala reactivity models primarily focusing on conditioning and extinction processes. Another potential pathway to enhanced threat detection in PTSD patients could involve their peripheral ANS abnormalities. For example, low heart rate variability, which may reflect sympathetic-parasympathetic imbalance, is associated with increased threat detection (Melzig et al., 2009; Sevenster et al., 2015) and with risk for development of PTSD (Minassian et al., 2015). Abnormalities in adrenergic tone or glucocorticoids could also contribute to enhanced Threat Detection, as noradrenergic hyperactivity is linked to increased amygdala reactivity (Neumeister et al., 2006) and glucocorticoid receptors, which are abundant in amygdala, have been linked to enhanced anxiety and fear (Schulkin et al., 2005), but direct evidence for a mechanistic pathway through these systems to enhanced threat detection in PTSD is still missing.
Diminished Executive Function and Emotion Regulation
An additional mechanism that could contribute to PTSD pathophysiology involves regulatory processes that modulate responses to emotionally evocative stimuli like threat. Successful navigation in daily life, including avoidance of danger, requires holding information in mind, resisting distractors, switching between tasks, and planning -- tasks that are considered components of Executive Function (EF). EF is a family of capacities by which we manage cognitive processes, and includes mechanisms of working memory, attention, inhibition, and task shifting. Deficits in multiple EF domains are seen in psychiatric disorders and associated with poor social and occupational functioning (Chen and Etkin, 2013). The same mechanisms, and their neural substrates, are also involved in regulation of emotions (ER), in that these cognitive resources are used to modulate emotionally driven behavior. There are multiple aspects of PTSD that could stem from deficits in EF/ER, including memory deficits, exaggerated emotional responses to salient cues, irritability and impulsivity.
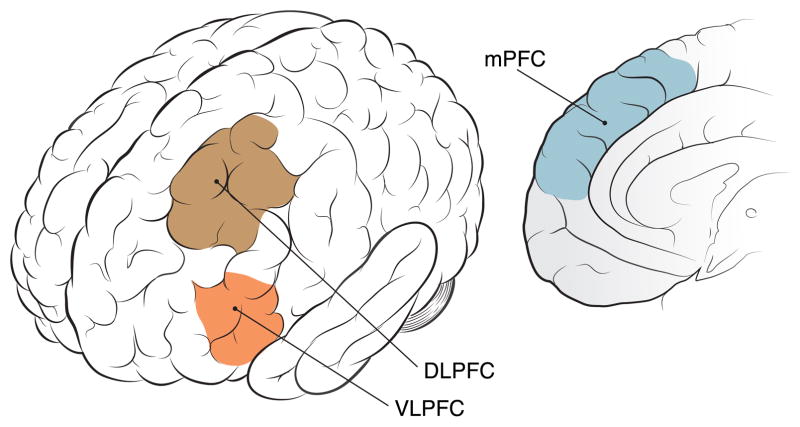
Neural activity in dorsal cortical networks, like fronto-parietal attentional networks, has been linked to EF (Niendam et al., 2012). EF participates in shifting neural engagement from a default mode network (DMN), involved in internally-oriented self-directed mentation, to salience and task networks. Imbalance and discoordination between DMN and task networks may contribute to lapses in attention and decrements in performance (Anticevic et al., 2012), as well as to psychiatric disorders (Sheline et al., 2009; Chen and Etkin, 2013; Bartova et al., 2015). ER involves some of the same cognitive control systems, such as memory and attention (Ochsner et al., 2002; Ochsner et al., 2012), supporting the notion of a common set of prefrontal regions are implicated in the regulation of affective and non-affective responses (Figure 3). For example, the lPFC and mPFC regions activated by emotional regulation (Ochsner et al., 2002) overlap with those activated in working memory and response selection tasks (Miller and Cohen, 2001; Smith and Jonides, 1999; Buhle et al., 2014; Kohn et al., 2014). The most commonly studied type of emotion regulation is reappraisal, which involves conscious, volitional efforts to modulate emotional responses by imposing a “less emotional” cognitive frame on them. In concert with notion of common EF/ER mechanisms, reappraisal activates overlapping regions including dlPFC, vlPFC, dmPFC, dACC, lateral orbitofrontal cortex (OFC), and the posterior parietal lobe (Buhle et al., 2014; Phan et al., 2005).
Figure 3.
Brain regions involved in executive function and emotional regulation. Abnormal function in these regions has been implicated in the Diminished Executive Function and Emotional Regulation model of PTSD (see text).
There is considerable face validity to the idea that deficits in cognitive control and associated top-down inhibitory modulation of autonomic and emotional activation areas might play a role in PTSD. Deficits in EF and/or ER may contribute to functional impairment following trauma and underlie some manifestations of PTSD, including biased attention to trauma cues, heightened emotionality, deficits in memory processes, irritability and impulsivity (Powers et al., 2015). There is in fact growing evidence of dysfunction within EF/ER circuits in PTSD patients. For example, EF-related networks show impaired within-network connectivity in dorsal attention frontoparietal regions in PTSD (Huemer et al., Network architectural and gene expression mechanisms of cognitive dysfunction: insights from post-traumatic stress disorder; under review) and others have reported altered connectivity within and between EF networks in PTSD (Sripada et al., 2012; Chen and Etkin, 2013; Du et al., 2015; Dunkley et al., 2015; Kaiser et al., 2015; Zhang et al., 2015). However, few PTSD studies have examined volitional ER. Only two imaging studies examined cognitive reappraisal in PTSD, with evidence suggesting generally reduced lateral and medial prefrontal activation during reappraisal (Rabinak et al., 2014; New et al., 2009). Reappraisal-related changes were also examined before and after PTSD treatment, with evidence that treatment with selective serotonin inhibitors can ameliorate initial deficits in ER prefrontal brain function, and pretreatment ER-region deficits negatively predict treatment-related gains (MacNamara et al., 2016).
The cellular/molecular mechanisms involved in deficient ER/EF are unknown, largely due to the fact that valid animal models of volitional (and involuntary) emotional regulation are yet to be developed. Noradrenergic hyper-reactivity, postulated to be characteristic of PTSD, has been linked to diminished frontal lobe-mediated executive capacities (e.g., working memory; Arnsten, 2009), and could contribute to diminished EF. Changes in ANS have been associated with reduced performance in a number of EF tasks (e.g., working memory, attention) (Thayer et al., 2009). Whether the peripheral changes “drive” these deficits in CNS function or are downstream effects that feed back is not known. In either case, they could be contributing to EF abnormalities in PTSD. However, while ER deficits are intuitively appealing and somewhat promising based on pilot data, direct evidence for them as contributing factors to PTSD pathophysiology, using established ER paradigms, remains sparse.
Explanatory Gaps and Remaining Questions
The Fear Learning/Generalization, Threat Detection and Emotional Regulation models can account for many of the signs and symptoms of PTSD. Abnormal Fear Learning and generalization readily account for key features like the dominance of fear over safety memories, generalization of fear responses to any “trauma-related” stimuli, exaggerated psychophysiologic and adrenergic reactivity, and hypervigilance. Insofar as avoidance of trauma reminders represents a “defensive” strategy to prevent recurrence of these strong fear responses, avoidance symptoms could be explained as bi-products of altered fear learning. The Abnormal Fear Learning model, however, does not offer satisfying explanations for other key PTSD features such as spontaneous (non cue-triggered) intrusions, nightmares and other sleep disturbances, emotional numbing or pervasive negative affect, or the behavioral recklessness often seen in PTSD, leading to re-traumatization. Emotional numbing and re-exposure to traumatic environments are in fact particularly difficult to reconcile with a view of PTSD as a disorder of exaggerated fear and diminished safety learning.
Exaggerated Threat Detection and Diminished EF/ER models, on the other hand, offer plausible explanation for the presence of threat-focused attentional bias, working memory deficits and hyperarousal in PTSD, which could lead to hypervigilance, and exaggerated emotional and physiologic responses. Exaggerated emotional responses and failure to regulate emotions could contribute to pervasive anger and impulsivity. They could also generate avoidance behavior and contribute to the emergence of intrusive memories in response to traumatic reminder cues. These models, however, have difficulty explaining the pervasiveness of trauma memories and, like the FL model, can not explain spontaneous recollections and intrusions, sleep abnormalities, emotional numbing or re-traumatization.
The main PTSD symptoms left unexplained by these three models are spontaneous (non-cued) recollections, nightmares and other sleep abnormalities, emotional numbing and reckless behavior leading to re-traumatization. Neurobiologically, they can account for exaggerated psychophysiologic reactivity and a hyperadrenergic state, triggered by trauma or trauma-like cues. However, they cannot readily integrate other neurobiologic abnormalties, such as structural hippocampal deficits, HPA axis dysregulation, genetic risk factors like FKBP5 or ADRB2, and altered sleep physiology. These models also require a number of independent pathophysiologic processes in several areas of the CNS to explain as much as they can explain, while leaving important symptoms and findings unexplained. Furthermore, as described above, existing empirical evidence in support of the proposed mechanisms is limited in some places and often contradictory. PTSD could actually be a collection of somewhat distinct neurobiological entities. If so, the FL, TD and EF models might correspond to specific disorder subtypes. However, given the empirical gaps noted and important phenomenological and neurobiological features still unexplained, ongoing pursuit of a more general and parsimonious explanation of PTSD’s pathophysiology is clearly still warranted.
A New Model: Deficient Context Processing
In search of a more comprehensive and parsimonious explanation of the full range of PTSD symptoms and the disorder’s neurobiology, we have hypothesized a core pathophysiological deficit within neural circuits that process the contextual information that is used to modulate emotional responses (Liberzon and Sripada, 2008; Maren et al., 2013). This model is firmly rooted in prior work but it expands upon previous models, by reframing them through the lens of context processing, in an effort to better integrate existing understanding and evidence with the “missing pieces” that remain unexplained.
Functionally, the concept of context has been used to refer to general cognitive, semantic or ‘emotional’ backgrounds that allow one to derive situation-informed meaning from the world (Maren et al., 2013). Contexts are composed of many stimulus elements that are assembled into configural, or ‘contextual’, representations, perceived as a “gestalt” and acquired rapidly (Maren et al., 2013). Salient cues may require radically different responses in different situations – the critical function of context processing is to match cued responses to the “needs” of a given situation or context (Maren et al., 2013). For example, spotting a mountain lion in one’s back yard is life threatening, but in a zoo it is exciting (even though the safety features can be well hidden). Contextual information allows us to freeze, flee, or enjoy the view of the lion, depending on the situation. Considering the central role of context in the flexible representation and retrieval of information and in resolving ambiguity, contextual processing deficits would lead to cue-focused behavioral reactivity, which is less flexible and more likely to be situationally inappropriate, potentially contributing to a broad range of PTSD symptoms.
The neural circuits involved in context processing have been extensively studied, largely through studies of associative learning (Bouton, 1993, 1994), building foundation for CP models of psychopathology by revealing the fundamental mechanisms whereby contexts are encoded and how they modulate memory retrieval (Corcoran and Maren, 2001). These studies have shown that the hippocampus plays a critical role in contextual learning and memory (Holland and Bouton, 1999), which is consistent with its role in episodic memory and spatial representation. Unique contextual representations are established in the hippocampus by population coding of spatial properties of the environment, integrating them with non-spatial information (e.g., time, prior experience, internal states) into a gestalt that becomes context (Hayman et al., 2003). Converging findings from animal and human studies (Alvarez et al., 2008; Marschner et al., 2008) indicate that the role of the hippocampus is critical, although not exclusive, in context encoding, and that hippocampal and prefrontal cortical regions interact in context retrieval (Figure 4). The hippocampus establishes new memory representations, minimizing overlap with previous memories, but it also helps to retrieve old memory based on partial information. These processes are known as pattern separation and pattern completion, respectively (see Yassa and Stark, 2011 for review). The ability to differentiate the zoo’s diorama from a real mountain landscape, even though the former was designed to invoke the latter, depends upon this process of pattern separation, helping to differentiate safety from threat. In contrast, pattern completion processes allow one to retrieve memory based on partial or incomplete information, for example, allowing one to take quick, protective action with just a glimpse of a “lion-like” silhouette in the backyard. These processes have clear relevance to PTSD, where a bias towards pattern completion in the face of a partial threat cue, and difficulty “reading” the contextual nuances needed for pattern separation, would make it difficult to discriminate threat from safety and would foster threat generalization (Kheirbek and Hen, 2014; Kheirbek et al., 2012), contributing to hypervigilance and hyperarousal. Impairment in hippocampal subregions involved in pattern separation (i.e., the dentate gyrus) could result in recurrent retrieval of trauma memories in response to partial cues, leading to generalized fear responses that are incongruous with the current context.
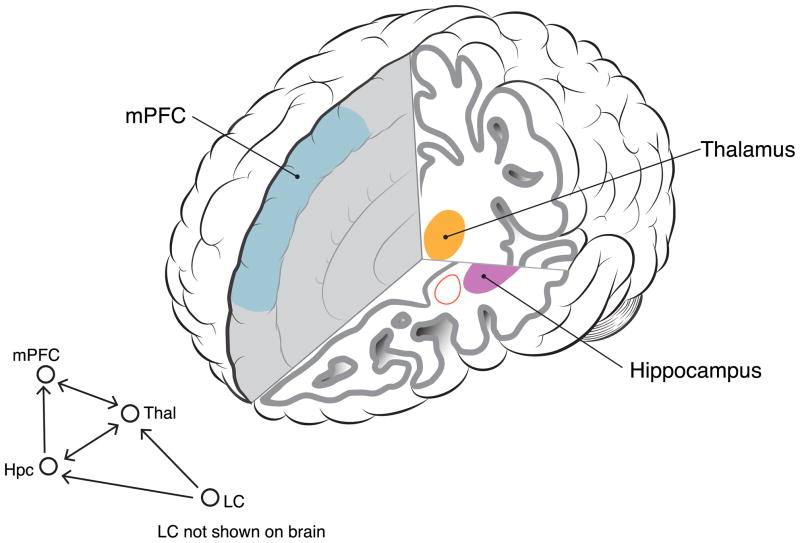
Figure 4.
Brain circuits involved in contextual processing functions. Abnormal function within these circuits is implicated in the Deficient Contextual Processing model of PTSD (see text). Information flow in this circuit includes Locus Coeruleus (LC) adrenergic neurons (shown in schematic but not visible in depicted brain slices).
The mPFC is another critical component of context processing circuitry. It is highly interconnected with hippocampus, and bidirectional interactions between hippocampus and mPFC have been implicated in contextual memory and in PTSD (Jin and Maren, 2015). Ensembles of mPFC neurons track environmental contexts (Hyman et al., 2012) and integrate contextual recognition and the context-US association during fear associated learning (Zelikowsky et al., 2014). Lesions in mPFC disrupt remote context memory (Quinn et al., 2008), suggesting that mPFC helps maintain context representations over time, since the role of the hippocampus in this function appears to be time-limited (see Sutherland and Lehmann, 2011). While ventral hippocampus is thought to encode non-spatial contextual information such as odors, bodily states, and emotions (Pennartz et al., 2011), mPFC creates associations between events, contexts, locations, and emotional responses (Euston et al., 2012). Preston & Eichenbaum (2013) suggest that while hippocampus forms novel memories, the prefrontal cortex “…accumulates features of related memories that compose the ‘context’ of a set of connected experiences” (p. 766). In this role, mPFC both guides appropriate memory retrieval by using the context to resolve conflicting information, and participates in memory formation by resolving conflicts between pre-existing schemas and new events. This process of organizing multiple features into a single, coherent representation, integrating temporal and spatial information, involves theta-oscillations that functionally bind hippocampus and PFC (Herweg et al., 2016). If this system is not working well, (being a bit “unmoored” in time and space) it could create vulnerability to over-react to potential indicators of threat, enhancing vigilance, providing face validity for potential relevance to PTSD.
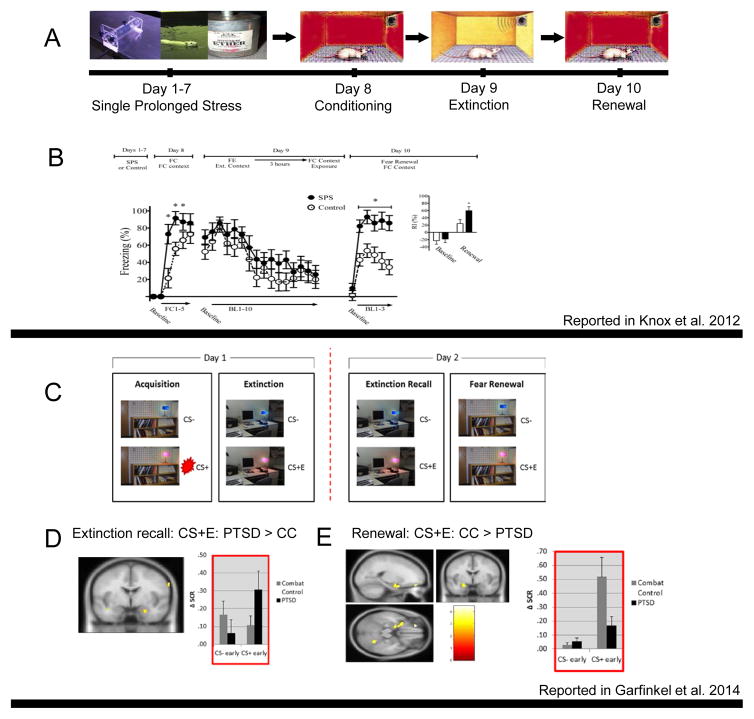
Indeed, evidence is accumulating that dysfunction within this interconnected context processing circuitry -- which involves hippocampus, prefrontal cortex, thalamus, and amygdala -- may play a central role in the pathophysiology of PTSD. With direct relevance to fear and safety learning, human studies have confirmed the engagement of mPFC regions (including subgenual ACC, vmPFC and OFC) (Gottfried and Dolan, 2004; Phelps et al., 2004; Lang et al., 2009), dACC, rostral mPFC (LaBar et al., 1998; Veit et al., 2002; Gottfried and Dolan, 2004; Knight et al., 2004; Phelps et al., 2004; Milad et al., 2007; Lang et al., 2009), and hippocampus (Knight et al., 2004) in extinction of fear memory, a process that is inherently context dependent. At the same time, diminished mPFC signal had been repeatedly demonstrated in PTSD and diminished vmPFC signal is linked to PTSD deficits in extinction recall/retention (Shin and Liberzon, 2010; Etkin and Wager, 2007). In concert, abnormalities in processing of contextual information can lead to cue-elicited fear responses, in association with hypo-activation in vmPFC and hyper-activation of dACC (Rougemont-Bucking et al., 2011). Diminished vmPFC activation is also seen during a Go/NoGo “inhibitory” task in PTSD subjects, and linked to enhanced fear-potentiated startle responses during safety signal learning (Jovanovic et al., 2013). Together, this emerging evidence suggested to us that abnormalities seen in PTSD in retention of extinction or in fear renewal, or failure to learn or recall safety cues, could all stem from a general deficit in contextual processing, rooted in a hippocampal-prefrontal-thalamic circuitry dysfunction. As an initial test of this hypothesis, we extended a standard conditioning/extinction paradigm into a renewal phase, making the counter-intuitive prediction that PTSD patients would fail to detect “danger” signals, despite their continuous hypervigilance for threat, if those signals were contextual in nature. We hypothesized that extinction recall deficits and fear renewal deficits would both be present due to disrupted hippocampal functioning, since both extinction recall and fear renewal are context- and hippocampal-dependent processes. If PTSD patients indeed exhibit such a general deficit, then when presented again with the original conditioning context in a renewal test, they would “fail” to display an appropriate return of the conditioned fear response, similar to their reported inability to recall extinction when exposed to the extinction context. This is exactly what happened (Figure 5, bottom panel). PTSD patients failed to “properly” recall extinction and failed to renew conditioned fear, and they also displayed reduced activation in the hippocampus and vmPFC (Garfinkel et al., 2014).
Figure 5.
Contextual processing abnormalities in the Single Prolonged Stress animal model of PTSD (Panels A and B) and in PTSD patients (Panels C, D and E). Panel A – Schematic depiction of the SPS paradigm, which involves multiple stressors delivered sequentially in one day and then a 7 day “rest” period, followed by fear conditioning, extinction and renewal testing. Panel B – data using this paradigm show abnormal fear renewal in SPS animals (seen on the right, in line graph and summary bar graph). Panel C – Schematic depiction (including screen shots) of the paradigm used to examine fear conditioning, extinction, extinction recall and fear renewal in fMRI experiments with humans. Panels D and E – data using this paradigm with PTSD patients and combat control (CC) subjects show abnormal extinction recall (D) and abnormal fear renewal (E) in PTSD (skin conductance data shown on right in both panels). Differences in fMRI activations in PTSD compared to controls, in response to previously conditioned and then extinguished stimuli (CS+E), are shown on the left in both panels.
Subsequent studies from other laboratories have corroborated contextual processing deficits in PTSD. In a similar SCR fear conditioning paradigm, PTSD patients failed to “properly” renew conditioned fear when re-exposed to the conditioning context following full extinction that occurred 24 hours after the conditioning phase (Shvil et al., 2014: Impaired contextual modulation in post-traumatic stress disorder; presented at the 34th annual meeting of the Anxiety & Depression Association of America, March 28th, 2014). Another laboratory further corroborated these results by showing that PTSD patients failed to utilize a contextual “warning” cue in a stop signal anticipation task, supporting the idea that the context processing deficit in PTSD is general and not isolated to fear-processing (van Rooij et al., 2015).
In addition to modulating fear associated learning, contextual processing plays a key role in shaping memory processes, emotional responses and regulation, and in shaping flexible behavioral choices. As such, a context processing deficit based in dysfunction of a hippocampal-prefrontal-thalamic network could contribute to multiple aspect of post-traumatic psychopathology, beyond the documented specific deficits in extinction retention and fear renewal, and their associated symptomatology. For example, mPFC-Hpc circuitry creates associations between contexts, events, and corresponding emotional responses (Euston et al., 2012; Preston and Eichenbaum, 2013), so dysfunction in this system could link erroneous emotional contexts to a given situation, triggering emotional responses that are “inappropriate”. For example erroneous retrieval of an intensely negative emotional context of loss and pain during normally joyful/happy events will prevent experiencing the joyful event as happy, which can be experienced as “emotional numbing”. Similarly, excessive anger can emerge, following relatively innocuous triggers, if the context is perceived as dangerous or threatening. If context is identified as “unsafe”, attention is focused on potential threat cues, leading to hypervigilance; but if contextual information that should alert one to danger is missed, this might lead to recklessness and retraumatization. Being “unmoored” from current contexts that keeps trauma memories from emerging in response to partial cues can lead to the emergence of intrusive memories. These hypothesized links between CP and a broad range of PTSD symptomology are speculative, and extensive work is needed to test these ideas, but initial support linking connectivity within the CP circuit to specific PTSD symptoms has been emerging. For example, we have shown that diminished vmPFC/hippocampus connectivity at rest was associated with the severity of each of the three PTSD symptom clusters (i.e. intrusive, avoidant and hyperarousal), and that enhanced connectivity of these regions with SN regions was associated specifically with enhanced hyperarousal symptoms (Sripada et al., 2012). A more recent resting state connectivity study replicated these findings, reporting that re-experiencing symptoms in PTSD are linked to “disconnection” of hippocampus from right PFC, interpreted by the authors as a failure of contextualization and associated overgeneralization of trauma memories (Spielberg et al., 2015).
In addition to providing a broad and parsimonious explanation of PTSD symptoms, the CP model also provides a robust framework for integrating much of what is known, neurobiologically, about this disorder. The cellular and molecular processes that underlie context-processing have been revealed in animal studies (Maren et al., 2013). These data, coupled with data from animal models of PTSD (Knox et al., 2012) and human genetic and genomic studies support the relevance of context processing and its neurobiology to PTSD pathophysiology. For example, mediation of contextual fear depends upon activation of glucocorticoid receptors in prefrontal cortex (Reis et al., 2015). Glucocorticoid receptor (GR) hypersensitivity (with enhanced HPA negative feedback) has been repeatedly demonstrated in PTSD. Diminished mPFC function has also been consistently reported in PTSD (Shin and Liberzon, 2010) and confirmed by meta-analyses (Etkin and Wager, 2007). In our PTSD animal model (SPS), which successfully replicates the enhanced HPA negative feedback seen in patients (Yamamoto et al., 2009), abnormalities in fear renewal are also seen (Figure 5, top panel), and upregulation of glucocorticoid receptors in mPFC has been shown to be critical for expression of the PTSD-like phenotype (George et al., 2015). Similarly, glucocorticoid signaling in Hpc plays a critical role in pattern separation and can induce PTSD-like memory abnormalities in a mouse model (Kaouane et al., 2012); and in our SPS rat model, upregulation of GR in Hpc also appears critical to emergence of the PTSD-like phenotype (Knox et al., 2012). In concert, the emerging genetic and genomic findings in PTSD implicate molecular pathways that might alter hippocampal-prefrontal-thalamic circuitry – perhaps impacting hippocampal volume or context-relevant hippocampal function like neurogenesis and LTP. For example, polymorphisms in BDNF (Gatt et al., 2009; Aas et al., 2014), ADBR2 and FKBP5 (Fani et al., 2013), or methylation of SLC6A4 (Dannlowski et al., 2014) or FKBP5 (Fani et al., 2013), all have hippocampal effects, and each has evidence for association with PTSD. These findings resonate with reports of hippocampal size differences seen in PTSD, which may constitute a risk factor for PTSD development (Gilbertson et al., 2002; Bremner et al., 2003; Kitayama et al., 2005). Indeed, PTSD patients exhibit impaired performance on hippocampal-dependent tasks including visual memory recall tests and verbal memory tasks (R. J. Lindauer et al., 2006; Hayes et al., 2011), and these also may predate PTSD onset. Together these converging findings suggest a potential causative link between genetic and developmental factors that shape hippocampal-mPFC circuitry and the development of “signature” HPA abnormalities, and lead to contextual processing deficits that can create much of PTSD’s phenomenology.
The CP model also nicely resonates with recent electrophysiologic and sleep findings. Hippocampal-prefrontal communication involves additional nodes in the midline thalamus, such as the reuniens and rhomboid nuclei, which participate in consolidation of enduring memories at a systems level (Pereira de Vasconcelos and Cassel, 2015). This process requires sleep-specific field-potential oscillations occurring during slow-wave rapid eye movement sleep (REM), which, in turn, rely upon specific function of locus coeruleus (LC) neurons (Vanderheyden et al., 2014). Thus effective encoding, consolidation, updating, and retrieval of contextual memory within hippocampal-prefrontal-thalamic circuitry likely require appropriate modulation by LC firing. This raises the possibility that adrenergic dysregulation, LC firing, and sleep problems might be contributing to disruption of the appropriate encoding and retrieval of contextual information, by affecting hippocampal-prefrontal communication. This is obviously only one potential etiologic pathway to mPFC-Hpc dysfunction (see below) and associated CP abnormalities, but these linkages pull adrenergic/autonomic function and sleep disruption into our context processing model, suggesting how sleep problems can play a key role in the emergence or perpetuation of CP deficits in a way that is consistent with sleep neurobiology in PTSD. Normal rewiring of memory networks in the hippocampus (including contextual memories) during sleep requires bidirectional plasticity (both synaptic strengthening (LTP) and weakening (DP) (Kemp and Manahan-Vaughan, 2004)) specifically during REM and transition to REM (TR). During REM and TR, to allow bi-directional plasticity (Poe et al., 2010), the noradrenergic (NE) neurons in the LC must turn off, which is essential for synaptic depotentiation (Thomas et al., 1996; Booth and Poe, 2006). If a hyperadrenergic state is present during REM and TR, the necessary synaptic depotentiation would be impaired, disrupting the rewiring of memory networks in the hippocampus (Vanderheyden et al., 2014), and potentially contributing to emergence of contextual processing abnormalities. The origins of the “hyperadrenergic state” could be partly genetic, given the ADRB2 findings in PTSD (Liberzon et al., 2014), which may thus contribute to development of deficits in hippocampal-prefrontal-thalamic circuit function. Indeed, there is evidence of linkage between disrupted REM and memory in PTSD (Lipinska et al., 2014) and evidence that REM abnormalities may play a mechanistic role in PTSD development (Marshall et al., 2014). It is also possible that pre-existing CP deficits, rooted in Hpc-mPFC circuit disruption after trauma exposure (recent or past), undermine adaptive, post-traumatic processing and contribute to the nocturnal hyperadrenergic state, disrupting sleep-dependent synaptic remodeling, and perpetuating and exacerbating the CP deficits. There are thus multiple potential etiologic pathways to Hpc-mPFC dysfunction and CP deficits, but the CP model does offer a parsimonious way to integrate longstanding evidence that PTSD involves sleep, ANS and HPA axis abnormalities, noradrenergic/HPA axis interaction effects on memory processes (Kukolja et al., 2011), and understanding of specific roles played by hippocampal-prefrontal-thalamic circuits in shaping episodic and contextual memory.
So what are the immediate implications and the next steps for development of the CP model, beyond improved conceptual coherence and explanatory power? A set of refutable hypothesis will need to be tested to enhance confidence in the model and demonstrate its utility in shaping future studies. First step will be to further document that the PTSD deficits seen in fear associated learning paradigms are also present in a broader range of tasks that involve contextual processing and modulation. These include replication of fear renewal findings, and examination of fear reinstatement, contextual conditioning, and contextual modulation of responses to reward cues. Second, the hypothesized key role of Hpc-mPFC dysfunction should be examined by testing other Hpc-mPFC dependent functions in PTSD. These should include tasks that probe pattern separation and pattern completion capacities, visual-spatial navigation tasks, and tasks that require utilization of internal context (e.g., hunger, arousal). Finally the integrative, organizing concept of the CP deficit role predicts that other aspects of PTSD symptomatology, like emotional numbing, anger outbursts hypervigilance, etc. should be associated with CP deficits in both human studies and animal models.
Animal work will also be critical in determining the implications and value of this model, and it will require studies that directly examine contextual processing and Hpc-mPFC circuits. To date, animal studies have largely utilized paradigms rooted in fear conditioning/extinction with a focus on amygdala function. These paradigms have face-validity and have contributed a great deal, but it may be time to develop models rooted in other theory-based constructs like CP. These models may offer great value in translational and back-translational efforts. Indeed some steps in this direction have already been taken by various groups studying contextual processing (Jin and Maren, 2015), PTSD models (Knox et al., 2012) and glucocorticoid modulation of memory processes (Kaouane et al., 2012).
Conclusions and Implications
The identification of neural circuits involved in PTSD pathophysiology and the development of comprehensive models -- to link dysfunction in these circuits to existing established findings (genetic, neuroendocrine, immunolological, psychophysiologic) and to mechanisms of symptom development and disease maintenance -- is an ongoing effort that has gathered substantial momentum in the past decade. This signifies both a paradigm shift and remarkable progress in the trauma field, and clinical neuroscience in general, as similar brain-based comprehensive models of psychopathology have yet to be developed in the fields of schizophrenia, depression or bipolar disorder research, despite longer and better funded efforts. Our progress promises both early translational and longer-term conceptual and more fundamental scientific benefits.
Among the early translational/clinical benefits are potential advancements in development of more specific, mechanism-based treatments. If, as we suggest, PTSD is actually a collection of somewhat distinct neurobiological entities, with the FL, TD and EF models perhaps representing somewhat different endophenotypes, then identification of the dominant phenotype in a given patient might lead to a specific type of treatment, moving us towards a personalized medicine approach. For a patient with an “Altered FL” phenotype, enhancing extinction training by using a pharmacological enhancer (e.g., D-cycloserine) in conjunction with prolong exposure might be the most promising avenue. For an individual with a CP phenotype, use of glucocorticoid/adrenergic agents in combination with contextual processing “retraining” might be more promising. Individually tailored treatment packages could be further augmented by development of behavioral treatments specifically designed to strengthen contextualizing skills via enhanced function in Hpc-mPFC circuitry.
Improved pathophysiological models can also perhaps lead to better use of existing treatment strategies. For example, evidence for noradrenergic abnormalities in PTSD, along with evidence for their effects on memory, has led to efforts to treat or prevent PTSD using the beta receptor antagonist propranolol. Propranolol was administered immediately or soon after trauma exposure, to try to prevent consolidation of traumatic memories. While initial studies appeared promising (Pitman et al., 2002), subsequent reports did not support value of this approach (Sijbrandij et al., 2015). If, as the CP deficit model suggests, hyperadrenergic activity is most relevant to PTSD development during sleep, and REM in particular, the failure of some propranolol trials could be due to the wrong timing of propranolol administration. Adrenergic interventions might have to be directed toward nocturnal hyperadrenergic states. Similar logic might apply to attempts to treat/prevent PTSD using glucocorticoid administration. Glucocorticoids dose-dependently impacts learning processes with an inverted U-shaped dose-response curve (Salehi et al., 2010). Knowledge of a trauma exposed individual’s glucocorticoid “status” could be essential to insuring beneficial effects, since “over-treatment” in some cases could enhance trauma memory consolidation or contribute to fear learning generalization. On the other hand, with appropriate understanding of the brain mechanisms involved, glucocorticoid administration could perhaps be used to enhance context processing capacities, allowing traumatic memory to be better contextualized, in a flexible way, facilitating an appropriate balance between mechanisms of pattern separation and pattern completion in the hippocampus. Indeed evidence that glucocorticoid administration may enhance efficacy of prolonged exposure therapy for PTSD has been recently reported, but efficacy depends on the individual’s GC receptor sensitivity (Yehuda et al., 2015).
The long-term implications of this progress are more fundamental and more extensive. Developing comprehensive brain based models that describe abnormalities in memory deposition and retrieval as the result of specific neurobiological processes and molecular risk factors significantly advances our understanding of basic “cold” and “hot” memory mechanisms. It also deepens understanding of stress response systems and brain mechanisms underlying generation and modulation of emotions. This work has implications beyond PTSD translationally as well. The key brain pathways and physiological processes involved in PTSD pathophysiology likely have relevance to our understanding of other disorders, as well as stress sensitivity and resilience more generally. It is very unlikely that any psychiatric disorder can be fully explained by dysfunction of a single neurobiological circuit; and given the interconnected nature of these circuits, the disruption in functions of any particular circuit is likely to be seen in disorders, whose pathophysiology intersects with Hpc-mPFC circuits. Substance Dependence is one example, where contextual factors have already been invoked to explain relapse vulnerability (Crombag and Shaham, 2002). Exaggerated reactivity to drug cues, rather than trauma cues, may stem from CP deficits, in this case leading to abnormal approach behavior rather than the avoidance seen in PTSD. Determining the origins and roles of a particular Hpc-mPFC dysfunction in the etiological pathway of a specific disorder will require additional research, but examining CP broadly as a key brain function of relevance to a broad range of psychopathologies may facilitate progress.
As this work moves forward, we will be better able to define our disorders based on specific disruptions within particular brain circuits leading to impaired function, and this more precise, neurobiologically based understanding, will allow increasingly efficient, impactful, and individually tailored treatments. This progress will also slowly put to rest age-old debates about “nature vs. nurture” and “psychological vs. biological” in our study and understanding of psychiatric disorders, and thereby help to combat stigmatization and further open the door to better treatments for all who need them.
Existing models of PTSD pathophysiology implicate neurocircuits subserving fear learning, salience detection, and emotion regulation. However, much remains unexplained. Liberzon and Abelson offer a novel, parsimonious model of hippocampal-prefrontal-thalamic dysregulation creating context processing deficits that contribute to PTSD symptom development.
Acknowledgments
The writing of this manuscript was supported by grants to one or both authors from the Department of Defense (W81XWH-13-1-0377) and the National Institute of Mental Health (R24 MH075999 and RO1 MH093486). We also thank Jony Sheynin, PhD for invaluable help in preparation of this manuscript for publication, and Arieh Shalev, MD for help with conceptual development.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aas M, Haukvik UK, Djurovic S, Tesli M, Athanasiu L, Bjella T, Hansson L, Cattaneo A, Agartz I, Andreassen OA, et al. Interplay between childhood trauma and BDNF val66met variants on blood BDNF mRNA levels and on hippocampus subfields volumes in schizophrenia spectrum and bipolar disorders. Journal of psychiatric research. 2014;59:14–21. doi: 10.1016/j.jpsychires.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, Hendler T. Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14120–14125. doi: 10.1073/pnas.0903183106. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agorastos A, Boel JA, Heppner PS, Hager T, Moeller-Bertram T, Haji U, Motazedi A, Yanagi MA, Baker DG, Stiedl O. Diminished vagal activity and blunted diurnal variation of heart rate dynamics in posttraumatic stress disorder. Stress. 2013;16:300–310. doi: 10.3109/10253890.2012.751369. [DOI] [PubMed] [Google Scholar]
- Aizenberg M, Geffen MN. Bidirectional effects of aversive learning on perceptual acuity are mediated by the sensory cortex. Nat Neurosci. 2013;16:994–996. doi: 10.1038/nn.3443. [DOI] [PubMed] [Google Scholar]
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J Neurosci. 2008;28:6211–6219. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter AB, Nugent NR, Yang BZ, Miller A, Siburian R, Moorjani P, Haddad S, Basu A, Fagerness J, Saxe G, et al. Corticotrophin-releasing hormone type 1 receptor gene (CRHR1) variants predict posttraumatic stress disorder onset and course in pediatric injury patients. Dis Markers. 2011;30:89–99. doi: 10.3233/DMA-2011-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends in Cognitive Sciences. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, Allard CB, Grimes EM, Simmons AN, Flagan T, Behrooznia M, Cissell SH, Twamley EW, Thorp SR, Norman SB, et al. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Archives of general psychiatry. 2012;69:360–371. doi: 10.1001/archgenpsychiatry.2011.1539. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD., Jr Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- Baldi E, Lorenzini CA, Bucherelli C. Footshock intensity and generalization in contextual and auditory-cued fear conditioning in the rat. Neurobiol Learn Mem. 2004;81:162–166. doi: 10.1016/j.nlm.2004.02.004. [Article] [DOI] [PubMed] [Google Scholar]
- Bartova L, Meyer BM, Diers K, Rabl U, Scharinger C, Popovic A, Pail G, Kalcher K, Boubela RN, Huemer J, et al. Reduced default mode network suppression during a working memory task in remitted major depression. J Psychiatr Res. 2015;64:9–18. doi: 10.1016/j.jpsychires.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergado-Acosta JR, Sangha S, Narayanan RT, Obata K, Pape HC, Stork O. Critical role of the 65-kDa isoform of glutamic acid decarboxylase in consolidation and generalization of Pavlovian fear memory. Learn Mem. 2008;15:163–171. doi: 10.1101/lm.705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilchick KC, Berger RD. Heart rate variability. J Cardiovasc Electrophysiol. 2006;17:691–694. doi: 10.1111/j.1540-8167.2006.00501.x. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther. 2007;45:2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Booth V, Poe GR. Input source and strength influences overall firing phase of model hippocampal CA1 pyramidal cells during theta: relevance to REM sleep reactivation and memory consolidation. Hippocampus. 2006;16:161–173. doi: 10.1002/hipo.20143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi F, LeDoux J. Sensory tuning beyond the sensory system: an initial analysis of auditory response properties of neurons in the lateral amygdaloid nucleus and overlying areas of the striatum. J Neurosci. 1992;12:2493–2503. doi: 10.1523/JNEUROSCI.12-07-02493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi F, LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amgydala. 1. acoustic discharge patterns and frequency receptive-fields. [Article] Experimental Brain Research. 1994;98:261–274. doi: 10.1007/BF00228414. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, Hoffman SN, Rukstalis M, Stewart WF. Association of FKBP5, COMT and CHRNA5 polymorphisms with PTSD among outpatients at risk for PTSD. Psychiatry Research. 2011;188:173–174. doi: 10.1016/j.psychres.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, Ambiguity, and Classical-Conditioning. Current Directions in Psychological Science. 1994;3:49–53. [Google Scholar]
- Bremner JD, Elzinga B, Schmahl C, Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog Brain Res. 2008;167:171–186. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Harvey AG. Processing threatening information in posttraumatic stress disorder. Journal of Abnormal Psychology. 1995;104:537–541. doi: 10.1037//0021-843x.104.3.537. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, Gordon E, Williams LM. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Human brain mapping. 2008;29:517–523. doi: 10.1002/hbm.20415. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TC, Blanchard EB, Neill WT. Information processing and PTSD: a review of the empirical literature. Clin Psychol Rev. 2000;20:1041–1065. doi: 10.1016/s0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez CM, McGaugh JL, Weinberger NM. The basolateral amygdala modulates specific sensory memory representations in the cerebral cortex. Neurobiol Learn Mem. 2009;91:382–392. doi: 10.1016/j.nlm.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Etkin A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Cohen H, Benjamin J, Geva AB, Matar MA, Kaplan Z, Kotler M. Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Research. 2000;96:1–13. doi: 10.1016/s0165-1781(00)00195-5. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Recalling safety: cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectr. 2007;12:200–206. doi: 10.1017/s1092852900020915. [DOI] [PubMed] [Google Scholar]
- Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, Abdi A, Baufreton J, Bienvenu TC, Herry C. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014;505:92–96. doi: 10.1038/nature12755. [DOI] [PubMed] [Google Scholar]
- Craig AD. A new view of pain as a homeostatic emotion. Trends in neurosciences. 2003;26:303–307. doi: 10.1016/s0166-2236(03)00123-1. [Research Support, U.S. Gov’t, P.H.S. Review] [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. [Research Support, Non-U.S. Gov’t]. Nature reviews. Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD. The human cortex responds to an interoceptive challenge. [Comment] Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6333–6334. doi: 10.1073/pnas.0401510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Kugel H, Redlich R, Halik A, Schneider I, Opel N, Grotegerd D, Schwarte K, Schettler C, Ambree O, et al. Serotonin transporter gene methylation is associated with hippocampal gray matter volume. Human brain mapping. 2014;35:5356–5367. doi: 10.1002/hbm.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Du MY, Liao W, Lui S, Huang XQ, Li F, Kuang WH, Li J, Chen HF, Kendrick KM, Gong QY. Altered functional connectivity in the brain default-mode network of earthquake survivors persists after 2 years despite recovery from anxiety symptoms. Soc Cogn Affect Neurosci. 2015 doi: 10.1093/scan/nsv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley BT, Doesburg SM, Jetly R, Sedge PA, Pang EW, Taylor MJ. Characterising intra- and inter-intrinsic network synchrony in combat-related post-traumatic stress disorder. Psychiatry Res. 2015 doi: 10.1016/j.pscychresns.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Prince SE, Murty VP, Kragel PA, LaBar KS. Neurobehavioral mechanisms of human fear generalization. Neuroimage. 2011;55:1878–1888. doi: 10.1016/j.neuroimage.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Learning from LTP: a comment on recent attempts to identify cellular and molecular mechanisms of memory. Learn Mem. 1996;3:61–73. doi: 10.1101/lm.3.2-3.61. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J Affect Disord. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Buchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. 2015;16:693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. [Comparative Study Meta-Analysis] The American journal of psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Gutman D, Tone EB, Almli L, Mercer KB, Davis J, Glover E, Jovanovic T, Bradley B, Dinov ID, et al. FKBP5 and attention bias for threat: associations with hippocampal function and shape. JAMA Psychiatry. 2013;70:392–400. doi: 10.1001/2013.jamapsychiatry.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, Liberzon I. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci. 2014;34:13435–13443. doi: 10.1523/JNEUROSCI.4287-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, Gordon E, Kemp AH, Williams LM. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009;14:681–695. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Genud-Gabai R, Klavir O, Paz R. Safety signals in the primate amygdala. J Neurosci. 2013;33:17986–17994. doi: 10.1523/JNEUROSCI.1539-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SA, Rodriguez-Santiago M, Riley J, Rodriguez E, Liberzon I. The effect of chronic phenytoin administration on single prolonged stress induced extinction retention deficits and glucocorticoid upregulation in the rat medial prefrontal cortex. Psychopharmacology (Berl) 2015;232:47–56. doi: 10.1007/s00213-014-3635-x. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Chattarji S. Neuronal encoding of the switch from specific to generalized fear. Nature Neuroscience. 2015;18:112–120. doi: 10.1038/nn.3888. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Long-term potentiation as a substrate for memory: evidence from studies of amygdaloid plasticity and Pavlovian fear conditioning. Hippocampus. 2002;12:592–599. doi: 10.1002/hipo.10099. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci. 2004;7:1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biol Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, 3rd, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biol Psychiatry. 1998;44:1027–1036. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Bryant RA, Rapee RM. Preconscious processing of threat in posttraumatic stress disorder. Cognitive Therapy and Research. 1996;20:613–623. [Google Scholar]
- Hayes JP, LaBar KS, McCarthy G, Selgrade E, Nasser J, Dolcos F, Morey RA. Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. J Psychiatr Res. 2011;45:660–669. doi: 10.1016/j.jpsychires.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman RM, Chakraborty S, Anderson MI, Jeffery KJ. Context-specific acquisition of location discrimination by hippocampal place cells. Eur J Neurosci. 2003;18:2825–2834. doi: 10.1111/j.1460-9568.2003.03035.x. [DOI] [PubMed] [Google Scholar]
- Herweg NA, Apitz T, Leicht G, Mulert C, Fuentemilla L, Bunzeck N. Theta-Alpha Oscillations Bind the Hippocampus, Prefrontal Cortex, and Striatum during Recollection: Evidence from Simultaneous EEG-fMRI. J Neurosci. 2016;36:3579–3587. doi: 10.1523/JNEUROSCI.3629-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Bouton ME. Hippocampus and context in classical conditioning. Curr Opin Neurobiol. 1999;9:195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Ma L, Balaguer-Ballester E, Durstewitz D, Seamans JK. Contextual encoding by ensembles of medial prefrontal cortex neurons. Proc Natl Acad Sci U S A. 2012;109:5086–5091. doi: 10.1073/pnas.1114415109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Ehrlich DE, Choi DC, Dabrowska J, Bowers ME, McCullough KM, Rainnie DG, Ressler KJ. Thy1-expressing neurons in the basolateral amygdala may mediate fear inhibition. J Neurosci. 2013;33:10396–10404. doi: 10.1523/JNEUROSCI.5539-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javanbakht A, King AP, Evans GW, Swain JE, Angstadt M, Phan KL, Liberzon I. Childhood Poverty Predicts Adult Amygdala and Frontal Activity and Connectivity in Response to Emotional Faces. Frontiers in behavioral neuroscience. 2015;9:154. doi: 10.3389/fnbeh.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Maren S. Prefrontal-Hippocampal Interactions in Memory and Emotion. Front Syst Neurosci. 2015;9:170. doi: 10.3389/fnsys.2015.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. Historical approaches to post-combat disorders. [Historical Article] Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2006;361:533–542. doi: 10.1098/rstb.2006.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, Norrholm SD, Bradley B, Ressler KJ. Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex. 2013;49:1884–1891. doi: 10.1016/j.cortex.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62:695–704. doi: 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Davis M, Duncan EJ. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res. 2009;167:151–160. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaouane N, Porte Y, Vallee M, Brayda-Bruno L, Mons N, Calandreau L, Marighetto A, Piazza PV, Desmedt A. Glucocorticoids Can Induce PTSD-Like Memory Impairments in Mice. Science. 2012;335:1510–1513. doi: 10.1126/science.1207615. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci U S A. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Hen R. Add neurons, subtract anxiety. Sci Am. 2014;311:62–67. doi: 10.1038/scientificamerican0714-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nature Neuroscience. 2012;15:1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Klavir O, Genud-Gabai R, Paz R. Functional Connectivity between Amygdala and Cingulate Cortex for Adaptive Aversive Learning. Neuron. 2013;80:1290–1300. doi: 10.1016/j.neuron.2013.09.035. [DOI] [PubMed] [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. J Neurosci. 2004;24:218–228. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Nault T, Henderson C, Liberzon I. Glucocorticoid receptors and extinction retention deficits in the single prolonged stress model. Neuroscience. 2012;223:163–173. doi: 10.1016/j.neuroscience.2012.07.047. [DOI] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation--an ALE meta-analysis and MACM analysis. Neuroimage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Neumeister A. Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Res. 2009;1293:13–23. doi: 10.1016/j.brainres.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukolja J, Klingmuller D, Maier W, Fink GR, Hurlemann R. Noradrenergic-glucocorticoid modulation of emotional memory encoding in the human hippocampus. Psychological medicine. 2011;41:2167–2176. doi: 10.1017/S0033291711000183. [Randomized Controlled Trial Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lang S, Kroll A, Lipinski SJ, Wessa M, Ridder S, Christmann C, Schad LR, Flor H. Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. Eur J Neurosci. 2009;29:823–832. doi: 10.1111/j.1460-9568.2009.06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Frewen PA, Girotti M, Neufeld RWJ, Stevens TK, Densmore M. Neural correlates of trauma script-imagery in posttraumatic stress disorder with and without comorbid major depression: A functional MRI investigation. Psychiatry Research-Neuroimaging. 2007;155:45–56. doi: 10.1016/j.pscychresns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Laufer O, Paz R. Monetary loss alters perceptual thresholds and compromises future decisions via amygdala and prefrontal networks. J Neurosci. 2012;32:6304–6311. doi: 10.1523/JNEUROSCI.6281-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci. 1990;10:1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EA, Theus SA. Lower heart rate variability associated with military sexual trauma rape and posttraumatic stress disorder. Biol Res Nurs. 2012;14:412–418. doi: 10.1177/1099800412454453. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Luthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Li CQ, Zhang JW, Dai RP, Wang J, Luo XG, Zhou XF. Surgical incision induces anxiety-like behavior and amygdala sensitization: effects of morphine and gabapentin. Pain research and treatment. 2010;2010:705874. doi: 10.1155/2010/705874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Abelson JL, Flagel SB, Raz J, Young EA. Neuroendocrine and psychophysiologic responses in PTSD: a symptom provocation study. Neuropsychopharmacology. 1999;21:40–50. doi: 10.1016/S0893-133X(98)00128-6. [DOI] [PubMed] [Google Scholar]
- Liberzon I, King AP, Ressler KJ, Almli LM, Zhang P, Ma ST, Cohen GH, Tamburrino MB, Calabrese JR, Galea S. Interaction of the ADRB2 gene polymorphism with childhood trauma in predicting adult symptoms of posttraumatic stress disorder. JAMA Psychiatry. 2014;71:1174–1182. doi: 10.1001/jamapsychiatry.2014.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. [Review] Progress in brain research. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Paz R. Amygdala-prefrontal interactions in (mal)adaptive learning. Trends in Neurosciences. 2015 doi: 10.1016/j.tins.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Stujenske JM, MAT, Harris AZ, Gordon JA. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 2014;17:106–113. doi: 10.1038/nn.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer RJ, Olff M, van Meijel EP, Carlier IV, Gersons BP. Cortisol, learning, memory, and attention in relation to smaller hippocampal volume in police officers with posttraumatic stress disorder. Biol Psychiatry. 2006;59:171–177. doi: 10.1016/j.biopsych.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Lindauer RJL, Booij J, Habraken JBA, van Meijel EPM, Uylings HBM, Olff M, Carlier IVE, den Heeten GJ, van Eck-Smit BLF, Gersons BPR. Effects of psychotherapy on regional cerebral blood flow during trauma imagery in patients with post-traumatic stress disorder: a randomized clinical trial. Psychological Medicine. 2008;38:543–554. doi: 10.1017/S0033291707001432. [DOI] [PubMed] [Google Scholar]
- Lindley SE, Carlson EB, Benoit M. Basal and dexamethasone suppressed salivary cortisol concentrations in a community sample of patients with posttraumatic stress disorder. Biol Psychiatry. 2004;55:940–945. doi: 10.1016/j.biopsych.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Lipinska M, Timol R, Kaminer D, Thomas KG. Disrupted rapid eye movement sleep predicts poor declarative memory performance in post-traumatic stress disorder. J Sleep Res. 2014;23:309–317. doi: 10.1111/jsr.12122. [DOI] [PubMed] [Google Scholar]
- Lissek S, Biggs AL, Rabin SJ, Cornwell BR, Alvarez RP, Pine DS, Grillon C. Generalization of conditioned fear-potentiated startle in humans: experimental validation and clinical relevance. Behav Res Ther. 2008;46:678–687. doi: 10.1016/j.brat.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Bradford DE, Alvarez RP, Burton P, Espensen-Sturges T, Reynolds RC, Grillon C. Neural substrates of classically conditioned fear-generalization in humans: a parametric fMRI study. Soc Cogn Affect Neurosci. 2013 doi: 10.1093/scan/nst096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav Res Ther. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lissek S, van Meurs B. Learning models of PTSD: Theoretical accounts and psychobiological evidence. Int J Psychophysiol. 2015;98:594–605. doi: 10.1016/j.ijpsycho.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Rabinak CA, Kennedy AE, Fitzgerald DA, Liberzon I, Stein MB, Phan KL. Emotion Regulatory Brain Function and SSRI Treatment in PTSD: Neural Correlates and Predictors of Change. Neuropsychopharmacology. 2016;41:611–618. doi: 10.1038/npp.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M, Camm AJ. Heart rate variability. New York: Futura Publishing; 1995. [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. [Research Support, N.I.H., Extramural Review]. Nature reviews. Neuroscience. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Buchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci. 2008;28:9030–9036. doi: 10.1523/JNEUROSCI.1651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall AJ, Acheson DT, Risbrough VB, Straus LD, Drummond SP. Fear conditioning, safety learning, and sleep in humans. J Neurosci. 2014;34:11754–11760. doi: 10.1523/JNEUROSCI.0478-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matynia A, Kushner SA, Silva AJ. Genetic approaches to molecular and cellular cognition: a focus on LTP and learning and memory. Annu Rev Genet. 2002;36:687–720. doi: 10.1146/annurev.genet.36.062802.091007. [DOI] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Melzig CA, Weike AI, Hamm AO, Thayer JF. Individual differences in fear-potentiated startle as a function of resting heart rate variability: implications for panic disorder. Int J Psychophysiol. 2009;71:109–117. doi: 10.1016/j.ijpsycho.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Minassian A, Geyer MA, Baker DG, Nievergelt CM, O’Connor DT, Risbrough VB Marine Resiliency Study T. Heart rate variability characteristics in a large group of active-duty marines and relationship to posttraumatic stress. Psychosom Med. 2014;76:292–301. doi: 10.1097/PSY.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Maihofer AX, Baker DG, Nievergelt CM, Geyer MA, Risbrough VB Marine Resiliency Study T. Association of Predeployment Heart Rate Variability With Risk of Postdeployment Posttraumatic Stress Disorder in Active-Duty Marines. JAMA Psychiatry. 2015;72:979–986. doi: 10.1001/jamapsychiatry.2015.0922. [DOI] [PubMed] [Google Scholar]
- Morgan CA, 3rd, Grillon C, Southwick SM, Davis M, Charney DS. Fear-potentiated startle in posttraumatic stress disorder. Biol Psychiatry. 1995;38:378–385. doi: 10.1016/0006-3223(94)00321-S. [DOI] [PubMed] [Google Scholar]
- Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32:301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister A, Drevets WC, Belfer I, Luckenbaugh DA, Henry S, Bonne O, Herscovitch P, Goldman D, Charney DS. Effects of a [alpha]2C-Adrenoreceptor Gene Polymorphism on Neural Responses to Facial Expressions in Depression. Neuropsychopharmacology. 2006;31:1750–1756. doi: 10.1038/sj.npp.1301010. [DOI] [PubMed] [Google Scholar]
- New AS, Fan J, Murrough JW, Liu X, Liebman RE, Guise KG, Tang CY, Charney DS. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry. 2009;66:656–664. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30:953–958. doi: 10.1016/j.psyneuen.2005.03.019. [Research Support, Non-U.S. Gov’t Review] [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- Pennartz CM, Ito R, Verschure PF, Battaglia FP, Robbins TW. The hippocampal-striatal axis in learning, prediction and goal-directed behavior. Trends Neurosci. 2011;34:548–559. doi: 10.1016/j.tins.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Pereira de Vasconcelos A, Cassel JC. The nonspecific thalamus: A place in a wedding bed for making memories last? Neurosci Biobehav Rev. 2015;54:175–196. doi: 10.1016/j.neubiorev.2014.10.021. [DOI] [PubMed] [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP. Twenty-four hour urinary cortisol and catecholamine excretion in combat-related posttraumatic stress disorder. Biol Psychiatry. 1990;27:245–247. doi: 10.1016/0006-3223(90)90654-k. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Cahill L, Orr SP. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51:189–192. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- Poe GR, Walsh CM, Bjorness TE. Cognitive neuroscience of sleep. Prog Brain Res. 2010;185:1–19. doi: 10.1016/B978-0-444-53702-7.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol Bull. 2007;133:725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Powers A, Etkin A, Gyurak A, Bradley B, Jovanovic T. Associations Between Childhood Abuse, Posttraumatic Stress Disorder, and Implicit Emotion Regulation Deficits: Evidence From a Low-Income, Inner-City Population. Psychiatry. 2015;78:251–264. doi: 10.1080/00332747.2015.1069656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23:R764–773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Ma QD, Tinsley MR, Koch C, Fanselow MS. Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learn Mem. 2008;15:368–372. doi: 10.1101/lm.813608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, MacNamara A, Kennedy AE, Angstadt M, Stein MB, Liberzon I, Phan KL. Focal and aberrant prefrontal engagement during emotion regulation in veterans with posttraumatic stress disorder. Depress Anxiety. 2014;31:851–861. doi: 10.1002/da.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Hauger RL, Morgan CA, Bremner JD, Charney DS, Southwick SM. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol Psychiatry. 2000;47:526–539. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- Reis FM, Almada RC, Fogaca MV, Brandao ML. Rapid Activation of Glucocorticoid Receptors in the Prefrontal Cortex Mediates the Expression of Contextual Conditioned Fear in Rats. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv103. [DOI] [PubMed] [Google Scholar]
- Resnik J, Paz R. Fear generalization in the primate amygdala. Nat Neurosci. 2015;18:188–190. doi: 10.1038/nn.3900. [DOI] [PubMed] [Google Scholar]
- Resnik J, Sobel N, Paz R. Auditory aversive learning increases discrimination thresholds. Nat Neurosci. 2011;14:791–796. doi: 10.1038/nn.2802. [DOI] [PubMed] [Google Scholar]
- Rougemont-Bucking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Rauch SL, Pitman RK, Milad MR. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS neuroscience & therapeutics. 2011;17:227–236. doi: 10.1111/j.1755-5949.2010.00152.x. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B, Cordero MI, Sandi C. Learning under stress: the inverted-U-shape function revisited. Learning & memory. 2010;17:522–530. doi: 10.1101/lm.1914110. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Sandi C, Richter-Levin G. From high anxiety trait to depression: a neurocognitive hypothesis. Trends in neurosciences. 2009;32:312–320. doi: 10.1016/j.tins.2009.02.004. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Schechtman E, Laufer O, Paz R. Negative valence widens generalization of learning. J Neurosci. 2010;30:10460–10464. doi: 10.1523/JNEUROSCI.2377-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulkin J, Morgan MA, Rosen JB. A neuroendocrine mechanism for sustaining fear. [Review] Trends in neurosciences. 2005;28:629–635. doi: 10.1016/j.tins.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Grundemann J, Fadok JP, Muller C, Letzkus JJ, Luthi A. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014;81:428–437. doi: 10.1016/j.neuron.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Sevenster D, Hamm A, Beckers T, Kindt M. Heart rate pattern and resting heart rate variability mediate individual differences in contextual anxiety and conditioned responses. Int J Psychophysiol. 2015 doi: 10.1016/j.ijpsycho.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S, Barbieri S, van der Putten H, Kaupmann K, Bettler B, et al. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat Neurosci. 2006;9:1028–1035. doi: 10.1038/nn1732. [DOI] [PubMed] [Google Scholar]
- Shah AJ, Lampert R, Goldberg J, Veledar E, Bremner JD, Vaccarino V. Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biol Psychiatry. 2013;73:1103–1110. doi: 10.1016/j.biopsych.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh al arab A, Guedon-Moreau L, Ducrocq F, Molenda S, Duhem S, Salleron J, Chaudieu I, Bert D, Libersa C, Vaiva G. Temporal analysis of heart rate variability as a predictor of post traumatic stress disorder in road traffic accidents survivors. J Psychiatr Res. 2012;46:790–796. doi: 10.1016/j.jpsychires.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard RN. Toward a universal law of generalization for psychological science. Science. 1987;237:1317–1323. doi: 10.1126/science.3629243. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. [Review] Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of general psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijbrandij M, Kleiboer A, Bisson JI, Barbui C, Cuijpers P. Pharmacological prevention of post-traumatic stress disorder and acute stress disorder: a systematic review and meta-analysis. Lancet Psychiatry. 2015;2:413–421. doi: 10.1016/S2215-0366(14)00121-7. [DOI] [PubMed] [Google Scholar]
- Simmons A, Matthews SC, Stein MB, Paulus MP. Anticipation of emotionally aversive visual stimuli activates right insula. Neuroreport. 2004;15:2261–2265. doi: 10.1097/00001756-200410050-00024. [Comparative Study Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Paige S, Morgan CA, 3rd, Bremner JD, Krystal JH, Charney DS. Neurotransmitter alterations in PTSD: catecholamines and serotonin. Semin Clin Neuropsychiatry. 1999;4:242–248. doi: 10.153/SCNP00400242. [DOI] [PubMed] [Google Scholar]
- Spielberg JM, McGlinchey RE, Milberg WP, Salat DH. Brain network disturbance related to posttraumatic stress and traumatic brain injury in veterans. Biol Psychiatry. 2015;78:210–216. doi: 10.1016/j.biopsych.2015.02.013. [DOI] [PubMed] [Google Scholar]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, Liberzon I. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med. 2012;74:904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, Lehmann H. Alternative conceptions of memory consolidation and the role of the hippocampus at the systems level in rodents. Curr Opin Neurobiol. 2011;21:446–451. doi: 10.1016/j.conb.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 2009;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Moody TD, Makhinson M, O’Dell TJ. Activity-dependent beta-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron. 1996;17:475–482. doi: 10.1016/s0896-6273(00)80179-8. [DOI] [PubMed] [Google Scholar]
- van der Kolk BA, Greenberg MS, Orr SP, Pitman RK. Endogenous opioids, stress induced analgesia, and posttraumatic stress disorder. Psychopharmacol Bull. 1989;25:417–421. [PubMed] [Google Scholar]
- van Rooij SJ, Geuze E, Kennis M, Rademaker AR, Vink M. Neural correlates of inhibition and contextual cue processing related to treatment response in PTSD. Neuropsychopharmacology. 2015;40:667–675. doi: 10.1038/npp.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJ, Kennis M, Vink M, Geuze E. Predicting Treatment Outcome in PTSD: A Longitudinal Functional MRI Study on Trauma-Unrelated Emotional Processing. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2016;41:1156–1165. doi: 10.1038/npp.2015.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderheyden WM, Poe GR, Liberzon I. Trauma exposure and sleep: using a rodent model to understand sleep function in PTSD. [Review] Experimental brain research. 2014;232:1575–1584. doi: 10.1007/s00221-014-3890-4. [DOI] [PubMed] [Google Scholar]
- VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, Birbaumer N. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neurosci Lett. 2002;328:233–236. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- White S, Acierno R, Ruggiero KJ, Koenen KC, Kilpatrick DG, Galea S, Gelernter J, Williamson V, McMichael O, Vladimirov VI, et al. Association of CRHR1 variants and posttraumatic stress symptoms in hurricane exposed adults. J Anxiety Disord. 2013;27:678–683. doi: 10.1016/j.janxdis.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilker S, Pfeiffer A, Elbert T, Ovuga E, Karabatsiakis A, Krumbholz A, Thieme D, Schelling G, Kolassa IT. Endocannabinoid concentrations in hair are associated with PTSD symptom severity. Psychoneuroendocrinology. 2016;67:198–206. doi: 10.1016/j.psyneuen.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Wingenfeld K, Whooley MA, Neylan TC, Otte C, Cohen BE. Effect of current and lifetime posttraumatic stress disorder on 24-h urinary catecholamines and cortisol: results from the Mind Your Heart Study. Psychoneuroendocrinology. 2015;52:83–91. doi: 10.1016/j.psyneuen.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Sudhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339:1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, Liberzon I. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety. 2009;26:1110–1117. doi: 10.1002/da.20629. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry. 2001;62(Suppl 17):41–46. [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Pratchett LC, Lehrner A, Koch EC, Van Manen JA, Flory JD, Makotkine I, Hildebrandt T. Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: Randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology. 2015;51:589–597. doi: 10.1016/j.psyneuen.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Boisoneau D, Lowy MT, Giller EL., Jr Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry. 1995;52:583–593. doi: 10.1001/archpsyc.1995.03950190065010. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Brand SR, Golier JA, Yang RK. Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatr Scand. 2006;114:187–193. doi: 10.1111/j.1600-0447.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Kahana B, Binder-Brynes K, Southwick SM, Mason JW, Giller EL. Low urinary cortisol excretion in Holocaust survivors with posttraumatic stress disorder. Am J Psychiatry. 1995;152:982–986. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry. 1993;150:83–86. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Nussbaum G, Wahby V, Giller EL, Jr, Mason JW. Low urinary cortisol excretion in patients with posttraumatic stress disorder. J Nerv Ment Dis. 1990;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry. 1996;40:79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Hersman S, Chawla MK, Barnes CA, Fanselow MS. Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. J Neurosci. 2014;34:8462–8466. doi: 10.1523/JNEUROSCI.3624-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu F, Chen H, Li M, Duan X, Xie B, Chen H. Intranetwork and internetwork functional connectivity alterations in post-traumatic stress disorder. J Affect Disord. 2015;187:114–121. doi: 10.1016/j.jad.2015.08.043. [DOI] [PubMed] [Google Scholar]