Abstract
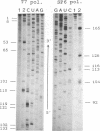
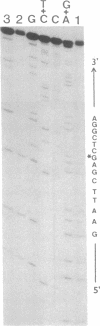
A DNA restriction fragment with convergent SP6 and T7 promoters has undergone reaction with cis-diamminedichloroplatinum(II) (cis-DDP) and was then used as a template for RNA synthesis in vitro. The T7 and SP6 RNA polymerases generate fragments of defined sizes. Analysis of the RNA fragments shows that the polymerases are mainly blocked at the level of the d(GG) and d(AG) sites and to a lesser extent at the level of the d(GC) sites. The adducts at the d(GC) sites are more resistant to cyanide ion attack than those at the major sites and are identified as interstrand cross-links. The formation of an interstrand cross-link between the N-7 atoms of two guanine residues at the d(GC) sites was further confirmed by chemical modifications.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod V. D., Kramer F. R. Transcription from bacteriophage T7 and SP6 RNA polymerase promoters in the presence of 3'-deoxyribonucleoside 5'-triphosphate chain terminators. Biochemistry. 1985 Oct 8;24(21):5716–5723. doi: 10.1021/bi00342a005. [DOI] [PubMed] [Google Scholar]
- Castleman H., Hanau L. H., Erlanger B. F. Stabilization of (dG-dC)n.(dG-dC)n in the Z conformation by a crosslinking reaction. Nucleic Acids Res. 1983 Dec 10;11(23):8421–8429. doi: 10.1093/nar/11.23.8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda Y., Job C., Anin M. F., Leng M., Job D. Transcription by eucaryotic and procaryotic RNA polymerases of DNA modified at a d(GG) or a d(AG) site by the antitumor drug cis-diamminedichloroplatinum(II). Biochemistry. 1991 Jan 8;30(1):222–230. doi: 10.1021/bi00215a032. [DOI] [PubMed] [Google Scholar]
- Eastman A. Interstrand cross-links and sequence specificity in the reaction of cis-dichloro(ethylenediamine)platinum(II) with DNA. Biochemistry. 1985 Sep 10;24(19):5027–5032. doi: 10.1021/bi00340a011. [DOI] [PubMed] [Google Scholar]
- Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther. 1987;34(2):155–166. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- Gralla J. D., Sasse-Dwight S., Poljak L. G. Formation of blocking lesions at identical DNA sequences by the nitrosourea and platinum classes of anticancer drugs. Cancer Res. 1987 Oct 1;47(19):5092–5096. [PubMed] [Google Scholar]
- Hemminki K., Thilly W. G. Binding of cisplatin to specific sequences of human DNA in vitro. Mutat Res. 1988 Nov;202(1):133–138. doi: 10.1016/0027-5107(88)90174-1. [DOI] [PubMed] [Google Scholar]
- Htun H., Dahlberg J. E. Single strands, triple strands, and kinks in H-DNA. Science. 1988 Sep 30;241(4874):1791–1796. doi: 10.1126/science.3175620. [DOI] [PubMed] [Google Scholar]
- Lippard S. J., Hoeschele J. D. Binding of cis- and trans-dichlorodiammineplatinum(II) to the nucleosome core. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6091–6095. doi: 10.1073/pnas.76.12.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinge J. M., Schwartz A., Leng M. Characterization of the ternary complexes formed in the reaction of cis-diamminedichloroplatinum (II), ethidium bromide and nucleic acids. Nucleic Acids Res. 1987 Feb 25;15(4):1779–1797. doi: 10.1093/nar/15.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrot L., Leng M. Chemical probes of the conformation of DNA modified by cis-diamminedichloroplatinum(II). Biochemistry. 1989 Feb 21;28(4):1454–1461. doi: 10.1021/bi00430a005. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A. L., Lippard S. J. Sequence-dependent termination of in vitro DNA synthesis by cis- and trans-diamminedichloroplatinum (II). Proc Natl Acad Sci U S A. 1985 Jul;82(14):4616–4619. doi: 10.1073/pnas.82.14.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson C. M., Eastman A. Influence of cis-diamminedichloroplatinum(II) on DNA synthesis and cell cycle progression in excision repair proficient and deficient Chinese hamster ovary cells. Cancer Res. 1988 Dec 1;48(23):6703–6707. [PubMed] [Google Scholar]
- Sorenson C. M., Eastman A. Mechanism of cis-diamminedichloroplatinum(II)-induced cytotoxicity: role of G2 arrest and DNA double-strand breaks. Cancer Res. 1988 Aug 15;48(16):4484–4488. [PubMed] [Google Scholar]
- Villani G., Hübscher U., Butour J. L. Sites of termination of in vitro DNA synthesis on cis-diamminedichloroplatinum(II) treated single-stranded DNA: a comparison between E. coli DNA polymerase I and eucaryotic DNA polymerases alpha. Nucleic Acids Res. 1988 May 25;16(10):4407–4418. doi: 10.1093/nar/16.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]