Abstract
The immune synapse (IS) is a specialized structure established between different immune cells that fulfills several functions, including a role as a communication bridge. This intimate contact between a T cell and an antigen-presenting cell promotes the proliferation and differentiation of lymphocytes involved in the contact. T-cell activation requires the specific triggering of the T-cell receptor (TCR), which promotes the activation of different signaling pathways inducing the polarization of the T cell. During this process, different adhesion and signaling receptors reorganize at specialized membrane domains, concomitantly to the polarization of the tubulin and actin cytoskeletons, forming stable polarization platforms. The centrosome also moves toward the IS, driving the movement of different organelles, such as the biosynthetic, secretory, degrading machinery, and mitochondria, to sustain T-cell activation. A proper orchestration of all these events is essential for T-cell effector functions and the accomplishment of a complete immune response.
1. Introduction
Immune responses protect the organism against nonself-threats through cell- and molecular-based mechanisms. These mechanisms may be subdivided into innate and adaptive immune responses, which are executed by cells of different lineages. These two responses are interdependent: innate cells are essential triggers of adaptive responses, for example, through MHC-dependent antigenic presentation; conversely, cells that mediate adaptive responses enhance and amplify the innate arm of the immune system, for example, through cell–cell contacts and cytokine secretion.
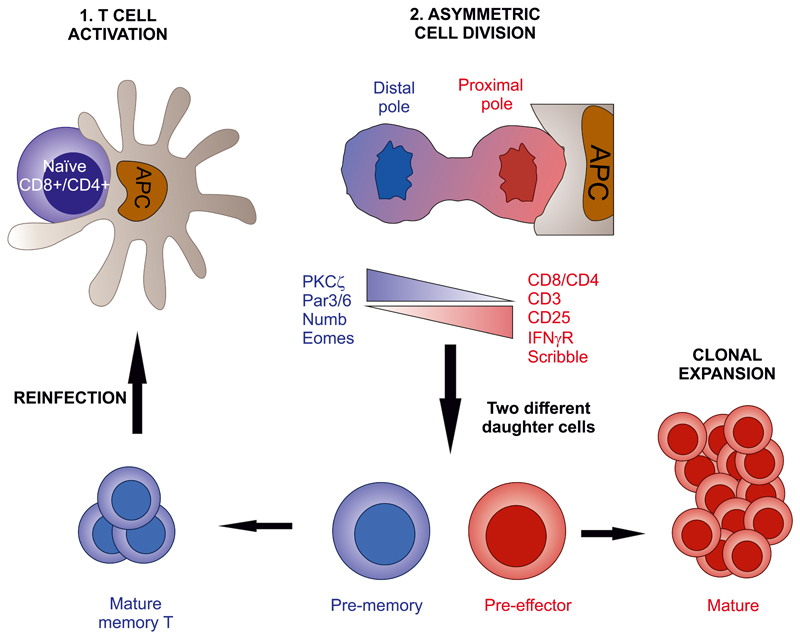
The first encounter of a T lymphocyte with an antigen bearing sufficient affinity for its TCR to trigger its activation depends on the interaction of the T cell with an antigen-presenting cell (APC) that presents the antigen associated to its MHC molecules. Such contact takes place at lymph nodes that drain most peripheral tissues. During infection, draining lymph nodes attract dendritic cells bearing pathogenic antigens to present the antigen to specific T cells, generating an antigen-specific, that is, adaptive, response (Fig. 1). The contact between the T cell and the APC takes a very specific shape, termed the IS. This structure acts as a transient, cell-to-cell communication structure between the T cell and the APC, which is a hallmark of the adaptive immune response (Monks et al., 1998). APCs can be myeloid cells, such as dendritic cells or macrophages; lymphoid, for example, B lymphocytes, or nonimmune cells, such as target cells that have been infected by virus or bacteria or are transformed into tumorigenic cells, activated endothelial cells, and some others (Friedl et al., 2005). T cells scan the surface of the APC, during which the αβTCR “probes” the peptide–MHC complex expressed by the APC. If the affinity of the TCR for the peptide–MHC complex is sufficient, the TCR undergoes conformational changes that activate different signaling pathways, leading to cytoskeletal reorganization and organelle polarization to the contact area with the APC. The stability of the IS is sustained by the TCR-dependent transactivation of adhesion molecules, for example, integrins, which maintain the IS over time and seal the extracellular space between the T cell and the APC. In this manner, the T:APC space adopts cleft shape, not unlike those observed in neuronal synapses. The IS structure is classically described as an eye-shaped molecular assembly. It is formed by a central SMAC (cSMAC; supramolecular activation clusters) that contains TCR microclusters with associated molecules (TCR signalosomes). The cSMAC is surrounded by the peripheral SMAC (pSMAC), which comprises adhesion molecules such as integrins (Davis and van der Merwe, 2006). This structure establishes an intimate contact between the T cell and the APC that increases the relative concentration of secreted molecules, thereby facilitating the exchange of signals between them.
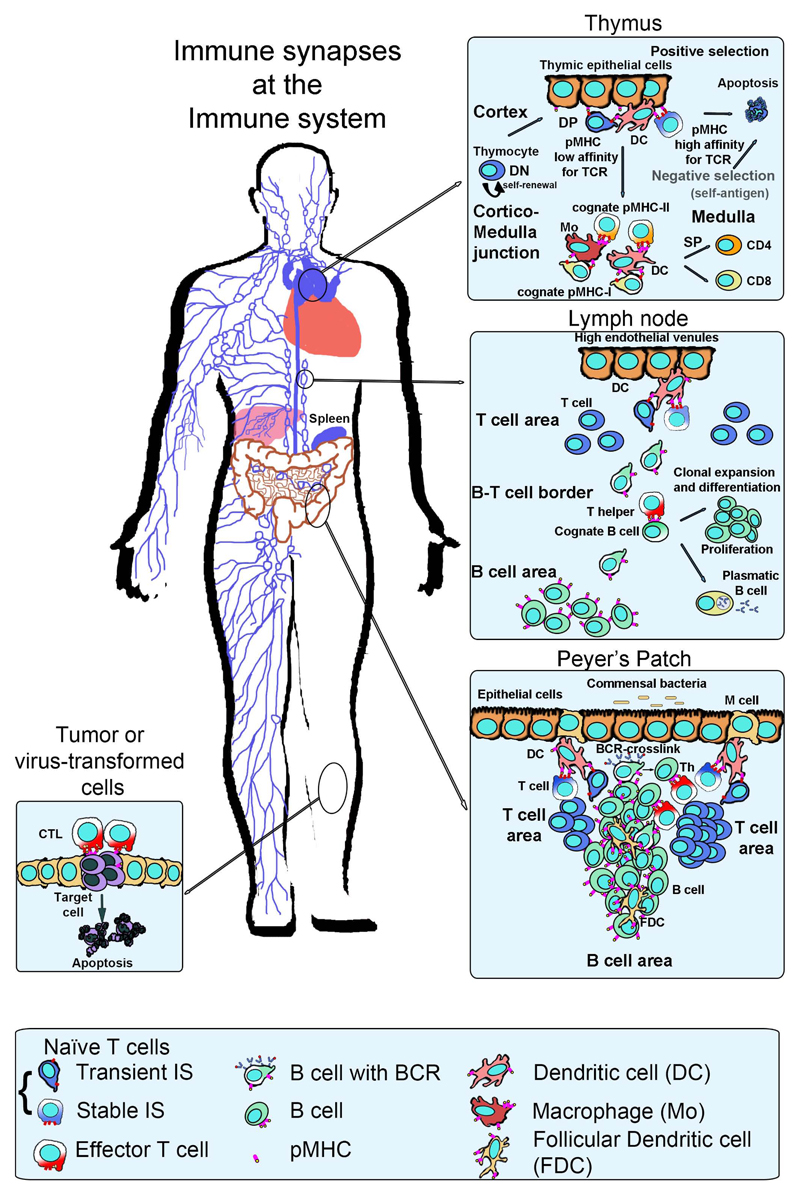
Figure 1. Immune synapses along the immune system.
Left, distribution of the immune system in the human body. Lymphoid organs such as the thymus and spleen are indicated; secondary lymphoid organs (SLO) such as lymph nodes or Peyer’s patches at the intestinal mucosa appear as loops and are interconnected by lymphatics (lines) and blood vessels (not shown). Top inset, T cells differentiate in the thymus into two major populations defined by the expression of the CD4 and CD8 co-receptors through the establishment of immune synapses with the thymic epithelia or dcs. Middle inset, CD4+or CD8+ bearing, naive T cells can differentiate into memory or effector T cells, called Th (helper) cells through immune synapse formation in lymph nodes. Effector CD8+ T cells are also known as cytotoxic T lymphocytes (ctls). T cells recognizing antigens migrate to the T-B frontier to form immune synapses and costimulate B cells. Bottom right inset, Peyer’s patches at the ileum mucosa respond against antigens that enter the body through the oral route and organize T and B areas similar to the lymph node. Bottom left inset, ctls destroy virus-infected or tumor cells by inducing apoptosis of the target cell.
In this review we offer an updated perspective of the changes evoked by the formation of the IS in the T cell; the mechanisms used by T cells to regulate these changes; and the functional consequences of the correct building of the IS in T cell–mediated responses.
2. Membrane Microdomains and Nanoclusters Orchestrate Cell–Cell Contacts
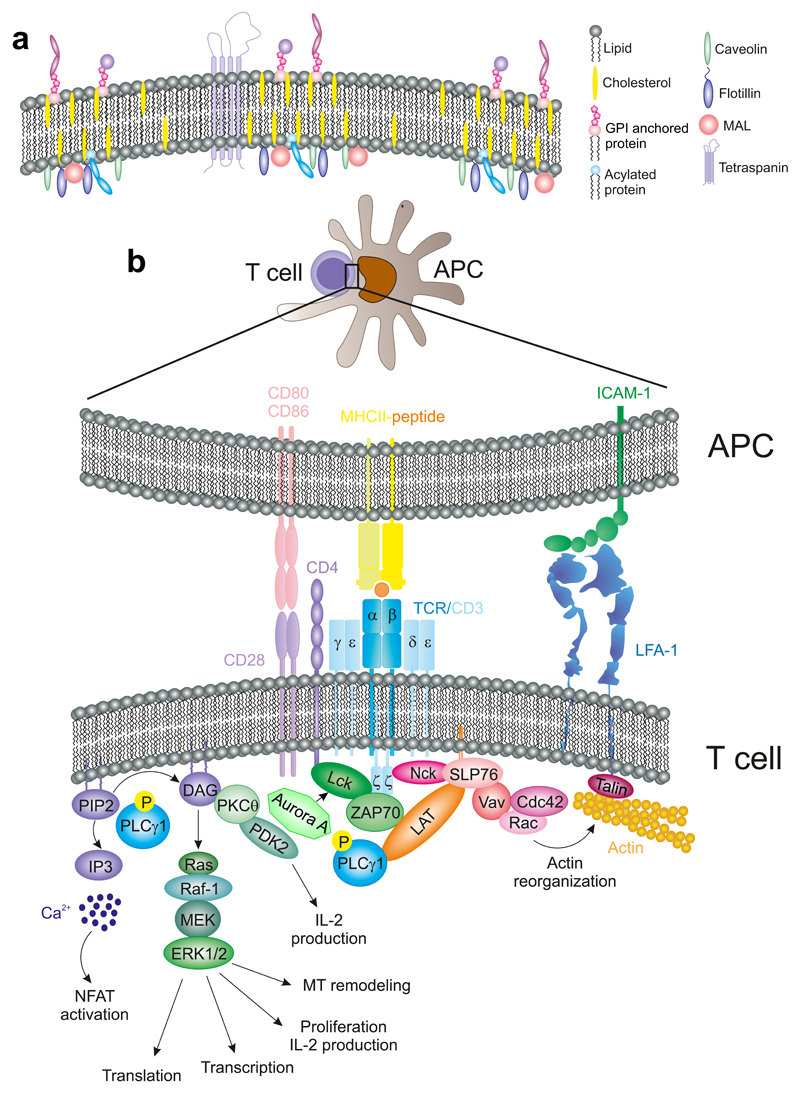
The formation of the IS implies that the membrane of the T cell and that of the APC are in close proximity to each other. The plasma mem- brane is not entirely homogeneous [Fig. 2(A)]; separate domains can be defined according to their different composition of lipids and proteins (Brown, 1998). In terms of lipids, the plasma membrane is formed by different specific domains distinguished by their solubility or insolubility in nonionic detergents. Insoluble, cholesterol-rich domains are classically termed “rafts” (Simons and Ikonen, 1997). Since different adaptors and signaling intermediates display a preference for localizing in raft or nonraft domains, lipid-based regions can be considered as platforms for signaling molecules involved in T-cell activation. In this view, these platforms boost or block signaling; and these signals are coordinated by the action of different lipid-associated proteins that are the backbone of these specific microdomains. The localization of TCR/CD3 complexes at preformed nanoclusters or “protein islands” has been proposed as a strategy in naive cells to form TCR signalosomes that promote rapid and effective T-cell activation upon TCR triggering (Beck-Garcia et al., 2015; Lillemeier et al., 2010). Indeed, in memory T cells, these nanoclusters are larger than those of naive T cells (Kumar et al., 2011). Despite this evidence, the specific function of the domain-forming lipoproteins during TCR clustering is still under study.
Figure 2. Membrane microdomains in T cell activation.
(A) Diagram showing plasma membrane subdomains regulated by the action of proteins like tetraspanins, caveolins, MAL, and Flotillins. GPI- and acyl-proteins also localize to specific microdomains at the membrane. (B) TCR-activation pathways. Upon TCR recognition of a peptide-charged MHC, the TCR/CD3 complex undergoes conformational changes (not depicted) that initiate its activation.
The composition of the lipid rafts is similar among mammalian cells. They are rich in sphingolipids and cholesterol. Regarding protein moieties, rafts preferentially contain two major classes of proteins, based on the strength of their interactions with the membrane: integral or intrinsic proteins, which are tightly bound to the membrane; and peripheral, or extrinsic, proteins that are weakly bound (Nicolson, 2014). Some proteins are modified with saturated fatty acyl chains, such as glycosylphosphatidylinositol (GPI), which is attached to the outer surface of the plasma membrane. Numerous proteins involved in TCR signaling are attached to the inner face of the plasma membrane through posttranslational modification such as palmitoylation and myristoylation, for example, the Src-family kinases Lck and Fyn (Rodgers et al., 1994). Others display specific transmembrane domains, for example, linker for activation of T cells (LAT) (Zhang et al., 1998).
Several families of membrane subdomains generator and nucleators include:
Myelin and lymphocyte protein (MAL): It is an intrinsic membrane protein that contains a MARVEL (MAL and Related proteins for Vesicle trafficking and membrane Link) domain that binds cholesterol [Fig. 2(A)]. MAL family includes MAL, MAL2, and BENE. All of them have a role in polarized intracellular transport. MAL controls apical transport in epithelial cells; in T cells, it has a crucial role in the traffic at membrane for Lck (Anton et al., 2008). BENE is involved in cholesterol transport in endothelial cells and MAL2 controls basolateral-to- apical transcytosis pathway in hepatoma cells (Llorente et al., 2004). MAL is rapidly redistributed to the cSMAC during IS formation; its depletion blocks the redistribution of TCR, Lck, and LAT. MAL recruits Lck to specific membrane microdomains at the IS, thereby controlling T-cell activation (Anton et al., 2008, 2011). Another protein in MAL- dependent domains is caveolin-1, a member of the caveolin family (Llorente et al., 2004). Caveolin-1 was found at membrane invaginations, named caveolae, enriched in cholesterol and glycosphingolipids. Caveolin-2 is also associated with caveolin-1 in most of the cell types studied. Caveolae act as signaling platforms; acting from these platforms, caveolin-1 recruits and activates different signaling molecules. The abro- gation of caveolin-1 expression prevents Lck relocation at the TCR/CD3 complexes, decreasing the phosphorylation levels of the tyrosine residues of the CD3 ITAMs (immunoreceptor tyrosine-based activation motifs) and therefore the activation of downstream molecules (Schonle et al., 2016; Tomassian et al., 2011).
Flotillins (also known as reggies): These proteins are asymmetrically distributed before T-cell polarization or activation [Fig. 2(A)]. Flotillins stabilize caveolin-1-dependent domains at the membrane. The flotillin– caveolin complex is involved in uropod formation during T-cell migration (Rajendran et al., 2009). In T cells, flotillins are associated with Lck and Fyn, and also with LAT. Importantly, flotillin-2 (reggie-1) absence blocks the polarization of detergent-insoluble microdomains and the localization of the GEF protein Vav, causing defects in actin cytoskeleton reorganiza- tion during T-cell activation (Langhorst et al., 2006; Zhao et al., 2011).
Tetraspanins (TM4): TM4 is a family of small molecules with four transmembrane domains present in the plasma membrane and intracellular vesicles. TM4 also act as signaling platforms in T cells [Fig. 2(A)] (Yanez-Mo et al., 2009). These proteins lend their name to tetraspanin-enriched membrane microdomains (TEMs), which contain signaling and adhesion receptors. TM4 proteins facilitate this process due to the establishment of lateral interactions with integrins and other receptors at the plasma membrane. TEMs are also enriched in cholesterol. Several TM4 proteins, CD9, CD53, CD81, and CD82, display costimulatory function in T cells. Specifically, CD81 is expressed in T and B lymphocytes, and it is essential for proper T-cell activation and Th2 responses. In B cells, TEMs participate in antigen recognition by the B- cell receptor (BCR) and are also involved in protein processing and peptide loading into the MHC (Rocha-Perugini et al., 2015). CD81 accumulates at the cSMAC, where they colocalize with TCR/CD3ζ complexes during the early stages of IS formation. CD81 deficiency decreases the number of TCR/CD3ζ complexes at the cSMAC; and the phosphorylation of CD3ζ and other downstream molecules, for example, the tyrosine kinase zeta-associated protein 70 (ZAP-70) and LAT (Rocha-Perugini et al., 2013). Also, CD81 regulates the localization of ICAM-1 at the pSMAC to enable the full maturation of the IS (Rocha-Perugini et al., 2013; Yanez-Mo et al., 2009).
2.1. T Cell–APC Contact: Initiating Immune Synapse
Inside the lymph node, naive CD4 or CD8 T cells scan the surface of dendritic cells. Such scanning aims to permit the interaction of a few TCR molecules with peptide-loaded MHC (p-MHC) complexes. Establishment of a productive interaction delivers a stop signal that prevents the detachment of the T cell from the APC and favors the formation of the IS (Dustin et al., 1997a). A similar effect can be caused by large concentrations of chemokines, which act through G-protein coupled receptors and trigger their internalization. Indeed, chemokines can enhance the contact of the T cell with the APC, reshaping the cell to promote the concentration of TCR-based nanoclusters at the T side of the IS (Krummel and Davis, 2002). This first step of the T cell–APC contact is mediated by the interac- tion of LFA-1 integrin with low-afinity ligands, specifically ICAM-3 (Montoya et al., 2002) and VLA-4, which is the integrin receptor for VCAM-1 during leukocyte extravasation, is also recruited to the IS and participates in the differentiation of T cells toward Th1 responses indepen- dently of VCAM-1 expression by the APC (Mittelbrunn et al., 2004). VLA-4 acts in concert with tetraspanins CD9 and CD151 to localize at the IS (Rocha-Perugini et al., 2014). Other receptors involved in the establishment of initial interactions are CD2 and CD58 that interact with their counterpart receptors on the APC, promoting adhesion [Fig. 2(B)]. Some of these molecules, for example, CD2 and its ligand (CD48), are also associated with specific membrane microdomains (Dustin et al., 1997b).
The coalescence of specialized membrane microdomains at the IS requires the dynamic rearrangement of the actin and tubulin cytoskeletons. Both cytoskeletal systems are heavily involved in the movement and segregation of membrane and intracellular components. For example, they participate in the accumulation of the TCR at the IS that accounts for a higher clustering of the TCR than that predicted by models of passive diffusion. In this regard, passive lateral diffusion of receptors is actin-dependent. A model of actin-dependent TCR accumulation envisions the pSMAC as a contractile actin-myosin ring that allows retrograde flow of actin and centripetal movements that direct the TCR/CD3 nanoclusters to the center of the IS (Ilani et al., 2009). Nanoclusters merge into microclusters before reaching the cSMAC (Lillemeier et al., 2010; Varma et al., 2006). A proposed model is that TCR/CD3 is translocated into specific cholesterol-enriched microdomains, where it is activated by Lck (He and Marguet, 2008). CD28, a major costimulation receptor that binds to CD80 and CD86 also concentrates at the cSMAC, controlling the ability of PKCθ to activate transcription factors, for example, NF-κB (Yokosuka et al., 2010). The absence of CD28 results in T-cell anergy, preventing full activation of the T cell. The cognate recognition of pMHC by the αβTCR subunits of the TCR–CD3 complex promotes a conformational change in the heterodimers containing the CD3ε subunit (CD3 γε and δε) prior to the phosphorylation on its own ITAM and the three ITAMs at each CD3ζ subunit of the homodimers that are part of the CD3 complex [Fig. 2(B)]. Nck is rapidly recruited to the TCR/CD3 complex upon exposure of a proline-rich, Nck- binding sequence (PRS) in CD3ε. Nck then binds to the adaptor protein Src homology 2 (SH) domain-containing leukocyte protein of 76 kD (SLP76) and Vav, which in turn promotes the reorganization of the actin cytoskeleton (Gil et al., 2002). In addition to this conformational change, ITAM phosphorylation by the Src-family kinase Lck and/or Fyn enables the recruitment of proteins containing SH2 domains, such as ZAP70. CD3 and ZAP70 coupling predates the recruitment of CD4 or CD8 coreceptors, which are bound to Lck. CD4 or CD8 remain in close proximity to the TCR–CD3 complex, enabling lateral binding to the corresponding MHC (CD4- MHCII; CD8-MHCI). This interaction stabilizes the TCR-p-MHC inter- action, while Lck keeps phosphorylating ITAMs that are being recruited to the macromolecular complex (Gascoigne et al., 2011), [Figs. 2(B) and 3)]. It has been recently reported the role of a well know mitotic protein, Aurora Kinase A in this process. The absence of Aurora A prevents CD3 ITAMs phosphorylation, through the regulation of Lck location and activation, and therefore T-cell activation (Blas-Rus et al., 2016).
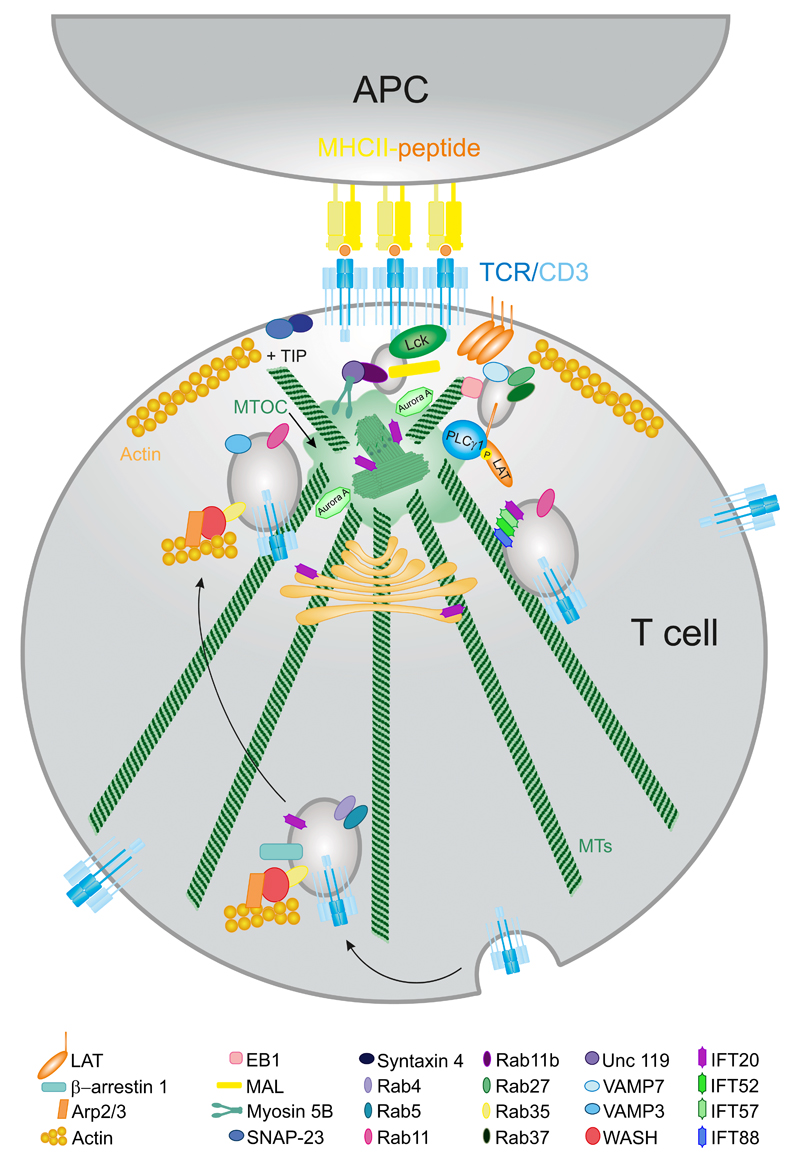
Figure 3. Intracellular traffic and activation at the IS.
TCR recycling is important for a sustained T-cell signaling. TCR complex is endocytosed by the formation of Rab4- and Rab11-bearing vesicles. They are transported along the MTs toward the MTOC or centrosome. Once in the proximity of the IS they fuse in a VAMP3-dependent manner. Lck and LAT also have an intracellular pool that docks at the IS. Different IFT proteins form large complexes that are in charge of TCR/CD3 recycling through Rab11- bearing vesicles.
Active ZAP70 phosphorylates LAT, which is a scaffold protein bearing multiple tyrosine residues. Phosphorylated LAT acts as a docking site for different proteins. LAT forms two spatially segregated pools; one appears at the plasma membrane and it is involved in the amplification of the initial TCR signal. A second pool localizes to intracellular compartments (Bonello et al., 2004). LAT interacts with SLP76, which recruits multimolecular complexes that converge on the cytoskeletal regulators CDC42/Rac and Nck and Vav, thereby controlling the remodeling of T-cell actin cytoskeleton (Martin-Cofreces et al., 2014; Pauker et al., 2012) [Fig. 2(B)]. One molecule of these complexes is PLCγ1, which binds to LAT at phospho-Y132 and hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to produce inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). DAG is an activator of different PKCs (serine/threonine kinases) while IP3 causes a sustained increase in intracellular Ca2+ concentrations from endogenous reservoirs, which is crucial for NFAT activation [Fig. 2(B) (Balagopalan et al., 2010)].
Production of DAG is a critical step for the propagation of signals emanating from the TCR and centrosome polarization to the IS (Quann et al., 2009). DAG binds to and activates PKD2. PKD2 also requires PKC-dependent phosphorylation on S707 and S711 for its complete activation [Fig. 2(B)]. This process promotes the amplification of PKC and PKD2 activation and mediates the production of cytokines that stimulate T-cell proliferation and T cell–dependent inflammation, such as interleukin 2 (IL-2) and interferon gamma (IFNγ), respectively (Navarro et al., 2012). Additionally, DAG participates in the activation of Ras through binding to the serine/threonine kinase Raf-1. Ras activation initiates the mitogen-activated protein kinases signaling cascade (MAPK). This results in the phosphorylation and activation of the serine/threonine kinases ERK1 and ERK2, which in turn phosphorylate and activate transcription factors, for example, ELK-1, SAP-1, and SAP-2. As a result, ERK1 and 2 regulate the expression of early activation genes such as c-Fos and Jun in T cells [Fig. 2(B)]. Furthermore, ERK1 and 2 also play a role in microtubule (MT) remodeling through the regulation and phosphorylation of stathmin (Filbert et al., 2012). Ribosomal S6 kinase (RSK) is also regulated by ERK1 and 2, promoting cell cycle progression and cytokine production in T cells. Finally, other proteins regulated by the ERK1/2 pathway are MNK1 and MNK2, which participate in the phosphorylation of the eukaryotic translation initiation factor eIF4E. (Navarro and Cantrell, 2014; Romeo et al., 2012).
2.2. Intracellular Traffic of TCR/CD3, LAT, and Lck
The concentration of TCR/CD3 complexes at the IS feeds from two sources. One is lateral membrane mobility. The other is the recycling of the complexes via endosomes (Fig. 3). In general, the endosomal system is used by the cells to maintain a metabolic steady-state, enabling the rapid reutilization of pre-synthesized molecules. Endosomes are characterized by the localization of different Rab GTPases bound to their membrane, for example, Rab11. These molecular switches act as spatial and temporal coordinators of recycling (Baetz and Goldenring, 2013). Endosomes also contain signaling molecules and adaptor proteins. Finally, they are essential for plasma membrane replenishment at the IS. This is a crucially important event because the interaction between the T cell and the APC through the IS continuously triggers TCR/CD3 internalization. However, for sustained signaling to take place, the amount of TCR/CD3 complexes at the T-cell surface of the IS need to be relatively constant (Varma et al., 2006), hence the internalized fraction needs to be renewed immediately (Das et al., 2002). Not surprisingly, the endosomal TCR/CD3 pool constitutes an important fraction of the total amount of TCR/CD3 that is recycled back to the IS (Fig. 3). The amount of internalization/recycling events at the IS functionally result in heavy and localized vesicular trafficking, leading to the definition of the IS as an “active zone”. Mechanistically, recycling is enabled by membrane fusogenic factors of the SNARE (N-ethylmaleimide-sensitive factor attachment protein receptors) family. Specifically, vesicles carry specific vesicle (v-) SNARE and target membranes contain target (t-) SNAREs. Vesicle docking and priming is enabled by the formation of a complex between two t-SNAREs (syntaxin-3 or -4 and SNAP-23 in non-neuronal cells) and one v-SNARE, for example, VAMP3 (Hay, 2001). This process requires the presence of high Ca2+ (Hay, 2001) and depends on MTs (Beemiller et al., 2012).
Although actin dynamics may contribute to TCR/CD3 complex con- centration at the IS, mainly due to retrograde flow (Beemiller et al., 2012), the recycling pool is mostly driven by MT and MT-based motors. In this regard, a recent work from our group has described that Aurora A blockade disrupts MT growth during IS (Fig. 3). Thus, MT-dependent trafficking of CD3ζ-bearing vesicles is abolished as a result (Blas-Rus et al., 2016). Moreover, dynein, a minus-end directed MT motor protein, accumulates at the pSMAC, where it associates with adhesion and degranulation pro- moting adaptor protein (ADAP). This interaction may generate the pulling force needed to polarize the centrosome to the IS (Combs et al., 2006). This can be graphically described as “reeling a fish,” in which the dynein–ADAP complex acts as a fixed reel, the MTs would be the line and the centrosome would be the fish. However, dynein/dynactin activity was also found essential for sustained T-cell activation, which likely means that dynein is not only involved in the reeling of the centrosome, but also in its long-term maintenance in the contact zone (Hashimoto-Tane et al., 2011; Martin-Cofreces et al., 2008) (Fig. 3).
On the signaling front, continuous internalization and recycling of TCR/CD3 depends on the phosphorylation of a di-leucine motif present on the CD3γ chain mediated by PKC, which is then recruited by the clathrin adaptor protein AP-2 (Monjas et al., 2004). TCR/CD3 complexes that enter this pathway are directed to recycling endosomes positive for Rab4 and Rab11. Rab4-endosomes are early endosomes involved in the rapid shuttling of internalized receptors to the plasma membrane in an MT-independent manner. On the other hand, endosomes marked by Rab11 aggregate in more distal locations inside the cell, following a slower route and moving along the MT in order to return to the plasma membrane. Other Rab GTPases, for example, Rab35, are also involved in the regulation of endosomal trafficking as well as in actin polymerization through WASp (Wiskott–Aldrich syndrome protein and SCAR homolog). WASp activates Arp2/3 complex and also interacts with tubulin cytoskeleton in both early and late endosomes, conjoining both networks to promote efficient endosome shuttling back to the IS (Finetti et al., 2015b) (Fig. 3).
TCR/CD3-containing endosomes can also be degraded by fusion with lysosomes (endolysosomal system). This is important to modulate TCR-dependent signaling. TCR/CD3 internalization and subsequent degradation requires a TCR ligand, and it also involves Lck and ZAP-70. Additional signaling adaptors required for this process include Cbl (E3 ligase Casitas B-lineage Lymphoma), which is recruited to the engaged TCR and promotes the lysosomal targeting of internalized receptors upon ubiquitination of the CD3γ and ζ chains (Naramura et al., 2002). Lck is also implicated in a constitutive TCR internalization by phosphorylating clathrin heavy chains (Crotzer et al., 2004). Despite these data, there is also evidence of tyrosine kinase-independent TCR internalization and downregulation.
Regarding the type of membrane-dependent mechanism involved in TCR/CD3 internalization, it has been proposed that non-engaged, bystander TCRs are internalized in clathrin-coated pits, while engaged TCRs are internalized in a cholesterol-enriched domains–dependent manner (Monjas et al., 2004). More recently, GPCR-interacting β-arrestin-1, which is a multiple-subunit receptor without intrinsic enzymatic activity, has been identified as a new ligand that can bind to phosphorylated ITAMs. This fact situates TCR/CD3 complexes also in the GPCRs-driven, arrestin-dependent internalization pathway. TCR/CD3 ligation promotes its PKC-dependent phosphorylation at S163, which in turn promotes β-arrestin-1 recruitment. In this manner, bystander, co-internalized TCRs are directly recycled back to the plasma membrane, while engaged, internalized TCRs are targeted to lysosomes for degradation (Fernandez-Arenas et al., 2014).
In contrast to TCR/CD3 vesicles, which are controlled by VAMP3, the endosomal recruitment and docking of LAT to the cortical region of the IS depend on the v-SNARE protein VAMP7. It is, however, important to note that CD3 vesicles may also interact with VAMP7. Endosomal pools of LAT are localized in different subpopulations of recycling endosomes positive for Rab27 and Rab37 (Fig. 3). The main difference between CD3- and LAT-containing vesicles is that LAT vesicles do not fuse with the plasma membrane. This suggests that early phosphorylation of LAT upon TCR activation depends on the clustering of the LAT pool at the plasma membrane, rather than on the LAT subset at endosomes. The latter pool is likely more important to stabilize signaling mediators close to the TCR (Larghi et al., 2013; Soares et al., 2013a). Analysis of end-binding protein 1 (EB1) by total internal reflection fluorescence microscopy (TIRFm) showed that MT growing mediated by EB1 favors the movement of cortical vesicles underneath the TCR/CD3 microclusters at the plasma membrane. EB1 also favors the proximity of LAT- and TCR/CD3-harboring vesicles at the IS, thereby facilitating the sustained activation of LAT and PLCγ1 upon TCR triggering (Martin-Cofreces et al., 2012). Moreover, a Rab11b- and MAL-positive endosomal pool containing Lck also contributes to T-cell activation. In this pool, MAL enables the association of Lck to the plasma membrane at the IS, whereas Rab11b interacts with myosin 5B through the adaptor protein uncoordinated 119 (Unc-119), promoting the movement of the vesicles from the pericentrosomal region to the IS (Martin-Cofreces et al., 2014; Soares et al., 2013b) (Fig. 3).
2.3. Negative Regulatory Signals
Negative regulatory signals at the IS tune down the intensity of the TCR signaling, likely to prevent apoptosis by over-activation. Negative regulators include the inhibitory receptor CTLA-4. CTLA-4 microclusters appear at the cSMAC, pushing CD28 and PKCθ away from the cSMAC. In this manner, CTLA-4 blocks CD28-mediated co-stimulation, down-modulating T-cell activation (Yokosuka et al., 2010). In addition, one study reported significant trans-endocytosis of CTLA-4 coupled to its counterpart receptors in T cells, which would decrease the functional levels of CD80 and CD86 at the APC (Qureshi et al., 2011). This kind of regulation has also been described for the αβTCR/CD3 at the IS, which would decrease the number of pMHC-II complexes at the APC, also dampening the intensity of the signal (Martinez-Martin et al., 2011).
Another negative regulatory signal is ubiquitination, which is very prominent at the IS (Vardhana et al., 2010). Ubiquitination targets receptors for degradation (Varshavsky, 2012), hence Ubiquitin-labeled TCR/CD3 complexes targeted for degradation need to be replenished with intracellular pools as described earlier for sustained T-cell activation (Das et al., 2004). Eventually, an imbalance toward degradation is likely to be involved in signal down- regulation and/or termination. (Vardhana et al., 2010)
2.4. Intraflagellar Transport System
The intraflagellar transport (IFT) system is an unconventional molecular complex recently implicated in TCR recycling at the IS (Fig. 3). The IFT comprises several multimeric protein complexes involved in the biogenesis and maintenance of the primary cilium. Although T cells lack primary cilia, they contain IFT proteins. Also, the IS and both primary cilia share several structural and compositional features. For instance, both structures depend on the polarization of the GA and the centrosome, which acts as an MT-organizing center (MTOC) that directs the traffic of vesicles toward the plasma membrane. These structures act as platforms for signal integration through the enrichment of receptors and signaling mediators. Also, their constituent membranes show similar lipid composition, which is highly enriched in cholesterol and sphingolipids (Finetti et al., 2015a).
The similarities between the IFT and the IS go beyond the composition of the plasma membrane. For example, both processes display vigorous tubule scission from the GA, which is mediated by spastin, which is an MT-severin protein that interacts with the ESCRT complex (Allison et al., 2013). Also, cryotomography and transmission electron microscopy have revealed the existence of tubulin rails similar to those observed in primary cilia at the IS near organella such as mitochondria and endoplasmic reticulum (ER) (Baixauli et al., 2011; Calabia-Linares et al., 2011; Ueda et al., 2011). This is in agreement with the notion that the IS share components and steps with the formation of the primary cilium (Finetti et al., 2015a).
Other common elements include AKAP450, which mediates the formation of the basal body of the primary cilium; but it is also required for TCR/CD3 and integrin activation and clustering at the IS (Robles-Valero et al., 2010). After TCR triggering, IFT20, an IFT protein primarily located in cilium and GA in ciliated cells, is recruited to the IS in association with the GA and centrioles. IFT20 is not only involved in T-cell activation, it also carries out its function in conjunction with other IFT proteins such as IFT88, IFT52, and IFT57 (Finetti et al., 2014) (Fig. 3). IFT20 is not essential for the proper GA and MTOC translocation to the IS, but it is required for sustained TCR clustering and signaling. Although it is unclear how IFT20 identifies the internalized TCR/CD3 complexes associated with early endosomes, it is known that this protein acts at an early step of the TCR/CD3 recycling pathway by coupling internalized TCR/CD3 complexes with Rab5 in early endosomes and promotes their transit to recycling endosomes. Moreover, IFT20 colocalizes with Rab11- and Rab4-positive endosomes, indicating that it may remain associated with TCR/CD3 during other steps in the recycling pathway. Furthermore, IFT20 may also interact with the transferrin receptor (TfR), another protein that is recycled in a polarized manner upon TCR activation (Batista et al., 2004; Finetti et al., 2009, 2014) (Fig. 3).
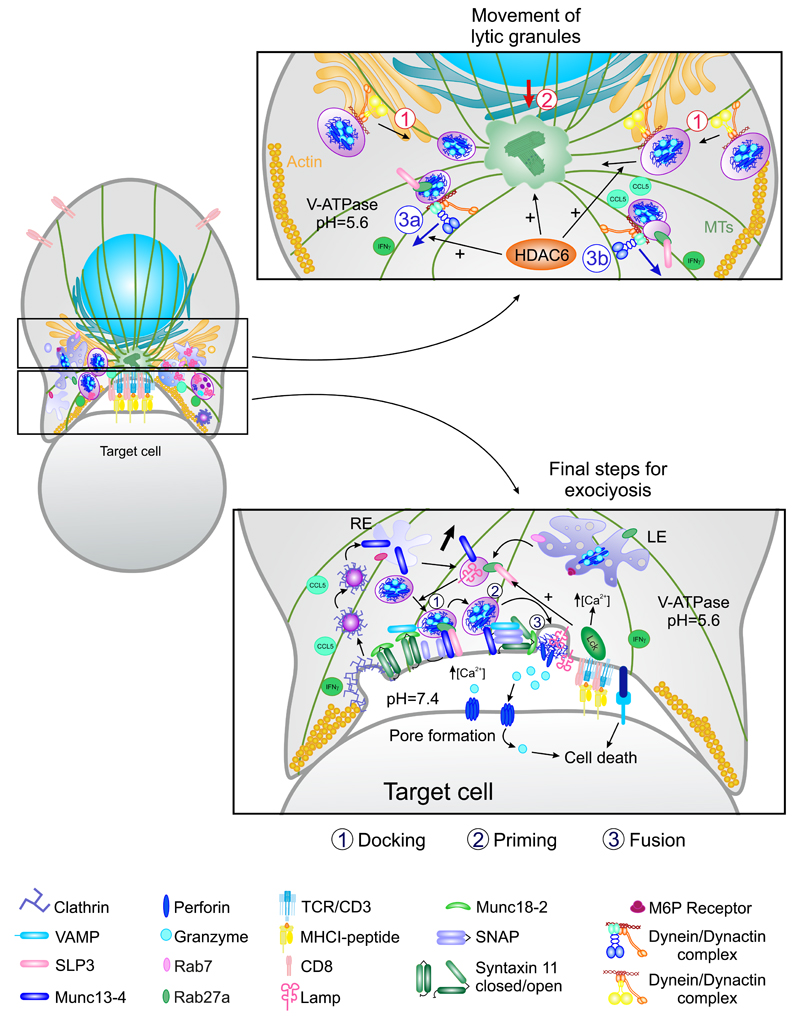
The correct biogenesis of the basal body of the cilium requires the formation of a complex containing cenexin-centriolin-Rab11a-Rabin8- Rab8 that enables the movement of vesicles along MTs through dynein and kinesin molecular motors for retrograde and anterograde transport, respectively. These molecules are also important for recycling at the IS: Rab8 interacts with VAMP3 for TCR recycling (Finetti et al., 2015b); dynein is relevant for SMACs formation at the IS and sustained T-cell activation (Combs et al., 2006; Martin-Cofreces et al., 2008) and kinesins are important to deliver lytic granules at the IS in CTLs (Kurowska et al., 2012). In addition, Casein kinase 1 (CK1δ) regulates ciliogenesis and causes dissociation of Rab8a and Rab11a from the pericentriolar region (Greer et al., 2014). In this regard, the docking of CK1δ at the centrosome is mediated by AKAP450 (Sillibourne et al., 2002) and AKAP450 inhibition delocalizes the centrosome from the IS (Robles-Valero et al., 2010). CK1δ also controls centrosome positioning at the IS through EB1 (Zyss et al., 2011); however, silencing EB1 does not prevent centrosome positioning at the IS, but it abrogates TCR signaling mediated by the LAT/ PLCγ1 signalosome, and it also regulates the traffic of CD3ζ vesicles at the IS (Martin-Cofreces et al., 2012). The role of EB1 in the formation of the cilium has also been related to vesicular transport (Schroder et al., 2011). In addition, histone deacetylase 6 (HDAC6), which also has a role in cilium disassembly (Mergen et al., 2013) influences CD4+ T-cell activation at the IS. Its overexpression precludes centrosome positioning and the interaction of signaling molecules from the TCR pathway with MTs (Serrador et al., 2004). Conceivably, these signaling molecules might be transported in vesicles found at the IS (Martin-Cofreces et al., 2012; Purbhoo et al., 2010; Soares et al., 2013a). In this sense, HDAC6 promotes the movement from the centrosome and exocytosis of lytic granules at the IS in a kinesin-dependent manner (Nunez-Andrade et al., 2016). HDAC6 is important for lymphocyte migration as a scaffold protein (Cabrero et al., 2006), and its role in migration in other cell types has been linked to EB1 protein (Li et al., 2011).
3. Centrosome as Organelle-Organizing Center
In different immune cell types, cytoskeletal remodeling underlies functional polarization. This process is important for many highly specialized and polarized cells, such as the neurons, in which the localization of the centrosome determines the number of neurites that initially sprout from the cell body, and which of these becomes the axon (de Anda et al., 2005). MTOC reorientation also promotes the movement of other cellular organelles such as the GA, the mitochondria, and the recycling and secretory apparatus (Figs. 3 and 4). These events also occur in T cells in response to antigen recognition by the TCR. TCR triggering in a polarized, migrating T cell trumps migratory polarity, and the leading edge evolves into a structure containing radially symmetric lamellipodium that spreads over the APC (Dustin et al., 2010). The MTOC relocates to the cSMAC, along with many other cellular components and organelles (Huse et al., 2008). MTOC polarization promotes a directional secretion of cytokines and vesicles toward the APC, which is very important for the specificity of the CD4+ T cell–dependent response (Mittelbrunn et al., 2011). Moreover, MTOC translocation is crucial for the function of cytotoxic T lymphocytes and NK cells, enabling the polarized secretion of granules containing perforin, granzymes, and cathepsins that kill target cells (Stinchcombe and Griffiths, 2007).
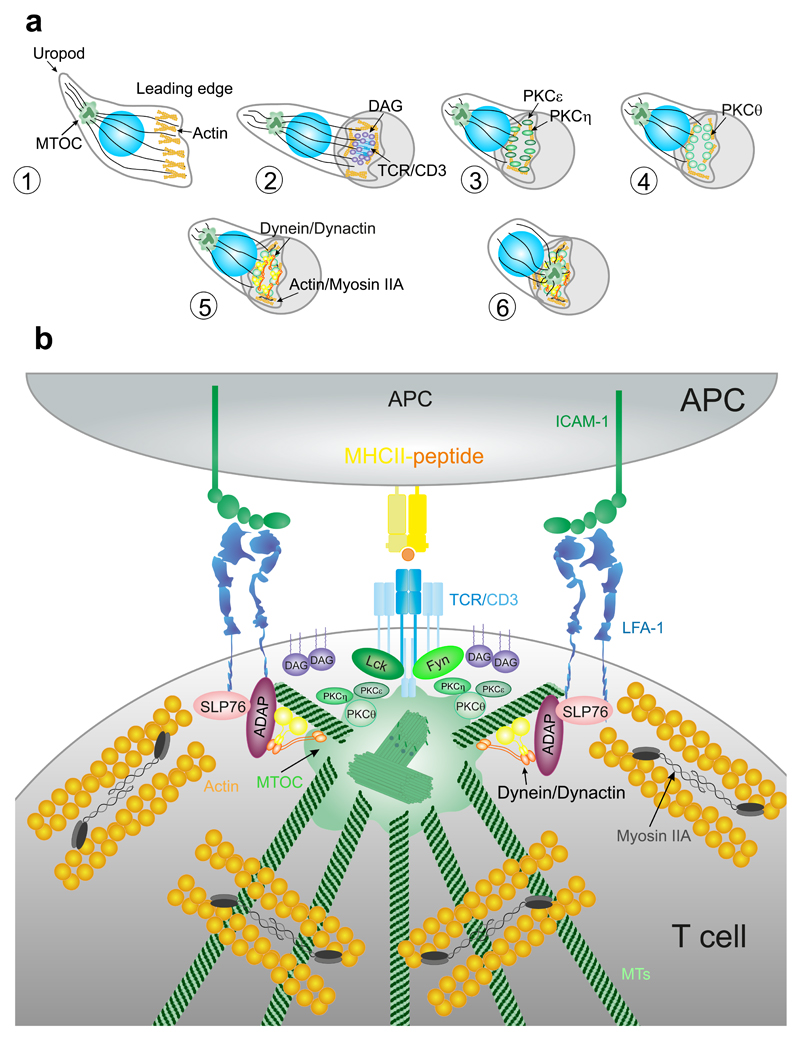
Figure 4. Centrosome translocation and docking at the IS.
(A) Contact between the APC and T cell triggers a rapid translocation of the MTOC toward the IS (2–5 min). Migrating lymphocytes show a polarized shape. Cognate contact with a specific APC promotes clustering of TCR/CD3 complexes and production of local DAG. PKCε and η cluster to DAG and help actin polymerization. PKCθ clusters to actin cytoskeleton and regulates its dynamics. Dynein/dynactin complexes and Myosin IIA help the coalescence of TCR/CD3 microcluster and MTOC translocation to the IS in formation. (B) Activation of TCR/CD3 through the phosphorylation of the ITAMs by Lck and Fyn members of src family of kinases promotes MTOC translocation to the IS. This movement depends on the dynein/ dynactin complex and also requires the interaction of ADAP with the integrins to generate the pulling force toward the IS.
The Src family of Tyr-kinase proteins, for example, Lck, is involved in centrosome translocation and docking to the membrane upon TCR activation [Fig. 4(B)]. An early study demonstrated that Lck-dependent ITAM phosphorylation in the TCR/CD3 complex was essential for centrosome polarization (Martin-Cofreces et al., 2014). However, later evidence showed that Lck-deficient cells do polarize the centrosome around the nucleus, but cannot maintain the centrosome at the IS, suggesting that this protein is not involved in centrosome translocation per se, but it participates in its stabilization at the IS (Tsun et al., 2011). On the other hand, Fyn-deficient cells do not polarize the centrosome properly; interestingly, Fyn does not compensate the lack of Lck, which indicates that both proteins are important for centrosome translocation and docking (Martin-Cofreces et al., 2006).
Another important factor is DAG (Fig. 4). DAG is produced by PLCγ1 at the IS during its formation, promoting the recruitment of several proteins containing DAG-binding C1 domains to the membrane. MTOC polarization is preceded by the accumulation of DAG at activated TCR microclusters, suggesting that DAG guides centrosome positioning. Indeed, the absence of DAG blocks centrosome translocation (Huse, 2012). The equilibrium between DAG production and catalysis into phosphatidic acid (PA) by DAG kinases is important for maintaining the clusters of DAG (Zhong et al., 2008). T cells likely use both DAG and PIP3 to decouple lamellipodial dynamics from centrosome movement. The clustering of DAG and PIP3 conversion by lipid phosphatases may be important for the transition between migratory and synaptic morphologies (Huse, 2012).
Accumulation of DAG at the IS engages the MT-based dynein motor complex (Quann et al., 2009). Dynein is a multisubunit protein composed of two heavy chains that contain the motor and MT-binding domains and several accessory light chains that provide structural integrity and support interactions with other proteins. One of these proteins is dynactin, a multisubunit complex that enhances dynein processivity and controls its localization (Fu and Holzbaur, 2014; Kikkawa, 2013). The recruitment of SLP-76 and ADAP into the integrin ring at the pSMAC facilitates dynein movement to the IS (Combs et al., 2006). The dynein/dynactin complex is involved in the polarization of the MTOC to the IS in human T cells (Fig. 4). Disruption of dynein/dynactin complex prevents the correct localization of the centrosome (Martin-Cofreces et al., 2008), while ADAP depletion prevents both dynein accumulation and MTOC translocation (Combs et al., 2006). ADAP interacts with MTs and also with dynein, suggesting that accumulation of ADAP at the pSMAC generates tension along MTs that support the MTOC reeling mechanism toward the IS (Combs et al., 2006). This suggests that dynein/dynactin complex may help dock the MTOC to the IS by interacting with Fyb/ADAP (Martin-Cofreces et al., 2014) (Fig. 4). However, in mouse primary T cells, depletion or inhibition of dynein was not enough to block the translocation of the MTOC; although it slowed the dynamics of TCR/CD3 microclusters (Hashimoto-Tane et al., 2011). These results suggest that a dynein-independent pathway may also mediate MTOC reorientation; or that these mechanisms differ from human to mouse lymphocytes.
Coupling between actin and MT networks seems essential for MTOC translocation (Martin-Cofreces et al., 2011; Obino et al., 2016). Several proteins involved in coupling MT and actin cytoskeletons include the scaffolding molecule IQGAP1 and the diaphanous formins. These molecules do participate in MTOC polarization (Gomez et al., 2007). Indeed, INF2, a formin-related protein is important for centrosome translocation and modification of MT, and for Lck transport to the plasma membrane (Andres-Delgado et al., 2010, 2012). In fibroblasts, MTOC polarization by actin dynamics is controlled by myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK) and myosin II (Gomez et al., 2005). Based on this, a recent study has shown that both dynein heavy chain depletion or blebbistatin treatment modestly reduce centrosome polarization. However, when T cells are treated with blebbistatin and also depleted of dynein heavy chain, the effect on MTOC polarization is stronger, suggesting that myosin IIA and dynein could act together in this process (Liu et al., 2013). No correlation has been observed between myosin II and dynein behavior during MTOC translocation. In human T cells, dynein accumulates at the pSMAC (Martin-Cofreces et al., 2008), but in mouse cells, dynein is located in the TCR zone, while myosin II localizes at the opposite side of cells, forming clusters behind the MTOC. This model suggests that dynein may pull the MT cytoskeleton, while myosin II may push it from behind, although the role of myosin II still remains unclear (Liu et al., 2013) (Fig. 4). However, a myosin II ring has also been observed at the pSMAC, either in human or mouse cells (Baixauli et al., 2011; Ilani et al., 2009). Therefore, much work is still needed to elucidate the crosstalk between actin and tubulin-based motors in MTOC polarization.
As mentioned earlier, DAG promotes the recruitment of DAG-binding proteins to the IS. Some of these proteins are members of the PKC family (Fig. 4), which are involved in many TCR-induced responses such as proliferation and secretion of cytokines (Baier and Wagner, 2009). The PKC family can be divided into three subfamilies based on their N-terminal regulatory regions. Conventional PKCs (cPKCs) display a DAG-binding C1 and a C2 domain and both DAG and Ca2+ regulate them. Novel PKCs (nPKCs) contain a C2 domain and are strongly dependent on DAG but not on Ca2+. Finally, atypical PKCs (aPKCs) contain only a C1 domain and lack the ability to bind DAG or Ca2+; rather, they are mainly regulated by protein–protein interactions (Newton, 2010). Ca2+ does not seem to be involved in centrosome translocation during the IS (Quann et al., 2009), which suggests that cPKCs may not be involved directly in this process. Due to the DAG-dependent activation of nPKCs, studies of MTOC polarization have focused on these proteins. Three out of the four isoforms that compose the nPKCs subfamily are recruited to the TCR prior to centrosome polarization: PKCε, PKCη, and PKCθ. In contrast, PKCδ localizes at intracellular granules (Quann et al., 2011). The depletion of PKCθ or the combined depletion of PKCε and PKCη prevent MTOC polarization. PKCε and PKCη share a 60% of sequence and therefore can compensate for each other (Gruber et al., 2005). The depletion of both proteins blocks the recruitment of PKCθ, suggesting that PKCε and PKCη are upstream of PKCθ in the process of MTOC translocation to the IS. These data are consistent with the early accumulation of PKCε and PKCη observed at the IS, which is followed by the subsequent accumulation of PKCθ at the peripheral actin ring and the polarization of the centrosome (Quann et al., 2011). The differences in the timing of recruitment of these proteins can be explained by their affinities for DAG; PKCε and PKCη bind to DAG with higher affinity than PKCθ (Huse et al., 2013). Therefore, affinity for DAG and the interaction with other lipids or proteins could potentially tune the localization of different PKCs.
PKC inhibition blocks the accumulation of dynein and the delocalization of myosin II from the IS (Liu et al., 2013). nPKCs likely regulate the distribution of cortical myosin II through the phosphorylation of regulatory light chain (RLC) in T cells, reducing its motor activity and also altering its subcellular localization (Huse et al., 2013). RLC is phosphorylated and activated by the Ser/Thr kinases ROCK and MLCK. ROCK inhibition also impairs MTOC polarization, supporting the role of myosin II in MTOC translocation. Moreover, there is another pool of myosin II that localizes at the edge of the actin ring at the pSMAC that could promote the centripetal movement of signaling microclusters containing TCR/CD3 complex during early T-cell activation (Huse et al., 2013; Ilani et al., 2009). These data support a model in which there are multiple pools of myosin II that contribute in different ways to MTOC reorientation or are involved in actin reorganization to the IS.
4. Endoplasmic Reticulum and Golgi Apparatus
The study of the role of the secretory apparatus in the formation of the IS in T cells has classically focused on the Golgi apparatus (GA), due to the early detection of its translocation to the IS in CTLs together with the centrosome. A remarkable feature is also its ability to organize a rapid and direct secretion from the Trans-Golgi Network (TGN) to the plasma mem- brane in cells from multiple lineages (Kienzle and von Blume, 2014). The GA sorts the differential secretion toward endosomes (basolateral transport); direct transport to the plasma membrane (apical transport); and also participates in the accumulation of secretory vesicles (storage). The localization of the GA is also readily observed at the IS in CD4+ T cells. GA accompanies the centrosome and other membranous organelles, such as the ER, early and late endosomes and vesicles (Baixauli et al., 2011; Calabia-Linares et al., 2011; Ueda et al., 2011).
The most studied role of the GA localization at the IS is the polarized secretion of diverse molecules, for example, cytokines. At least in CD4+ T cells, IL-2 and IFNγ are secreted into the synapse cleft, whereas TNFα is not (Huse et al., 2006). In CD8+ CTLs, the lytic granules, lysosomal-derived organelles shaped from the GA and accumulated during differentiation of naive CD8+ cells upon activation, are also directed toward the IS formed with the target cell (de Saint Basile et al., 2010). In CD4+ T cells, MAL, which is involved in the apical transport from the GA in polarized cells such as epithelial cells, promotes the transport of Lck from the GA to the plasma membrane (Anton et al., 2008). Therefore, part of the transport from the GA to the IS may be comparable to apical transport in polarized epithelial cells. The organization of the secretory vesicles that drive different stimulatory proteins such as LAT (Bonello et al., 2004) from intracellular compartments to the plasma membrane and the mechanism regulating their fusion and delivery at the plasma membrane have been widely studied recently. Different species of vesicles are defined depending on their content and the proteins involved in their sorting (Soares et al., 2013a,b). The GA may serve as a scaffold for signaling at the IS. One example is the GA-resident endothelial nitric oxide synthase (eNOS), which activates the N-Ras/Erk pathway upon IS formation and GA polarization toward the APC (Ibiza et al., 2008). Moreover, since NO is a regulator of cytokine production in the IS, eNOS activation during GA translocation toward the IS and the subsequent production of NO has a differential impact on IFNγ and IL-2 production and T-cell activation (Ibiza et al., 2006). In this regard, PLC activation upon TCR promotes the production of DAG and the rapid localization of RasGRP1, a guanine exchange factor (GEF) of the Ras family of proteins that contain a C1 domain, in internal membranes (Ebinu et al., 2000). The C1RasGRP1 domain preferentially binds to saturated forms of DAG. It is found at the GA, probably driving activation of the Ras pathway at this location (Carrasco and Merida, 2004). Therefore, both eNOS and RasGRP1 may synergize to activate the Ras/Erk pathway at the GA during IS formation.
Concerning the biosynthetic route, the GA is linked to the ER, which constitutes a first step due to its relationship with ribosomes and protein synthesis (Venditti et al., 2014). The ER is acquiring an increasing relevance, due to its role on the synthesis, glycosylation and sorting of proteins, and more recently, in the equilibrium of intracellular calcium and the relationship with mitochondria and lipid droplet formation. Sorting from the ER depends on the coat protein complex II (COPII), which is confined to dedicated sites in the ER surface, named ERESs (ER exit sites). Sorting vesicles are formed by the initial recruitment of the small GTPase Sar1 by the transmembrane GTP Exchange Factor Sec12. Sar1-GTP promotes membrane curvature; it recruits the Sec23/24 complex and it also induces the subsequent coating of the budding vesicle with of Sec13/31. Sec23, acting as a GTPase-activating protein (GAP), promotes the activation of the GTPase moiety of Sar1, which is enhanced by the tetrameric complex, releasing Sar1-GDP from the forming vesicle and enabling the closure of the membrane (Zanetti et al., 2012). Sec16, a core component of the COPII that interacts with Sec12 and Sec13, may serve as a scaffold for the whole system (Montegna et al., 2012; Whittle and Schwartz, 2010). Sec16 is phosphorylated by Erk2, which enhances the activity of COPII complexes (Farhan et al., 2010). Therefore, the control of Ras/Erk activity at the GA by the TCR activation may act on the components of the COPII complex to enhance sorting from the ER, toward the plasma membrane, endosomes, or the GA.
The vesicles formed by the budding of ER membranes at ERESs can be targeted to the GA. In rat basophil leukemia cells, 3D for the ERESs revealed a layer of COPII budding areas near to vesicular-tubular components that contain COPI near to the GA stacks. The COPI complex mediates anterograde transport from the Cis-Golgi to the ER (Lord et al., 2013). The GTPase ARF1 is involved in the formation of COPI-dependent vesicles. Its activation by GA-resident GEFs initiates COPI vesicle formation, whereas GAPs activity leads to the uncoating of COPI after vesicle formation. These conclusions stemmed from the observations of the effect of brefeldin A blocking the formation of COPI vesicles through inhibition of ARF1 GEFs (Lippincott-Schwartz et al., 1991). Conversely, GTPγS promoted the accumulation of COPI-coated vesicles (Nickel et al., 1998). Well-characterized cargo proteins of COPI vesicles include KDEL receptors. Recently, a point mutation in the KDELR1 gene in mice demonstrated that this protein is needed for the homeostasis of T cells and antigen-specific T-cell responses in vivo. KDELR1 co-immunoprecipitated with the phosphatase PP1 (Kamimura et al., 2015). TCR-dependent signals in these mutant mice alter T-cell development by multiple effects on the intracellular transport routes. It would be of interest to assess whether the retrograde transport from the GA to the ER during IS formation can affect the reprogramming of naive T cells. In this regard, depletion of DAG in cells results in accumulation of the KDELR at the GA by preventing GA-ER transport. This effect is probably mediated by the inhibition and mislocalization of ARFGAP1 (Fernandez-Ulibarri et al., 2007). ARFGAP1 regulates the formation of the COPI-coated vesicles (Hsu et al., 2009) and its activation depends, at least partially, on specific DAGs (Antonny et al., 1997).
The small GTPase Cdc42 has well-characterized functions in actin polymerization and centrosome polarization in different cell types (Cau and Hall, 2005). In the context of the GA, TCR activation signaling activates Cdc42, which in turn participates in the clearance of actin from the IS, facilitating the secretion of IFNγ in CD4+ T cells. However, its silencing does not prevent MT or cytokine-containing vesicle polarization to the IS (Chemin et al., 2012). IQGAP1, an effector of Cdc42, exerts a similar effect on actin clearance in CD8+ T cells during synaptic secretion (Stinchcombe et al., 2006). Cdc42 is an essential, resident protein in the GA that participates in the overall organization of the GA structure together with GM130 (Kodani et al., 2009) in a process sensitive to brefeldin A. Cdc42 is able disturb the interaction of the COPI complex with its cargo, thereby regulating both anterograde and retrograde transport (Park et al., 2015), which may underlie its role on cytokine secretion in T cells. Additionally, it can also interact with the CIP–AKAP450 complex at the TGN, which participates in cisternae organization (Larocca et al., 2004). AKAP450 organizes MT polymerization from the GA in cooperation with GM130 (Rivero et al., 2009). It also has a role in the correct activation of T cells and the polarization of the centrosome to the IS (Robles-Valero et al., 2010). It is therefore conceivable that Cdc42, in concert with different proteins such as AKAP450, promotes secretion from the polarized centrosome and associated GA toward the IS in T cells upon TCR activation. This is in agreement with the need of MT growth for IFNγ secretion in CD4+ T cells (Chemin et al., 2012) and for the CD3ζ-enriched vesicles traffic at the IS (Martin-Cofreces et al., 2012).
On the other hand, vesicles stemming from the GA may also be involved in the termination of the signal and resolution of the IS, such as the CTLA-4-enriched vesicles that are ready-to-go upon T-cell activation through the TCR (Linsley et al., 1996). These vesicles have been defined as secretory lysosomes. Recently, Ras-related GTPase Rab8 has been implicated in the production of these vesicles from the GA through its interaction with TRIM and LAX proteins (Banton et al., 2014; Catalfamo et al., 2008; Guntermann and Alexander, 2002). A complex between PKCη-PIX-GIT2 is essential to direct these vesicles to the IS, specifically to adhesion sites, in a PAK-dependent manner (Kong et al., 2014).
Another negative regulator of T-cell activation is BTLA (B and T lymphocyte attenuator). BTLA interacts with HVEM (herpes virus entry mediator) expressed by the APC (Watanabe et al., 2003). BTLA is found at the GA and it accumulates in secretory lysosomes in resting T cells. These secretory lysosomes redelivered to the IS at late stages of the process (Owada et al., 2010). Therefore, the secretion of negative regulators together with the endocytosis of stimulatory molecules likely shapes and regulates the IS. In this regard, the formation of the SMACs and their role have been the focus of intense debate, specifically whether the cSMAC is an activation node or a site for downregulation and termination of the signal, or whether its function shifts from activating to terminating over time (Lee et al., 2003; Martin- Cofreces et al., 2014; Mossman et al., 2005).
5. Multivesicular Bodies in Secretion, Recycling, and Renewal of Immune Synapse Components
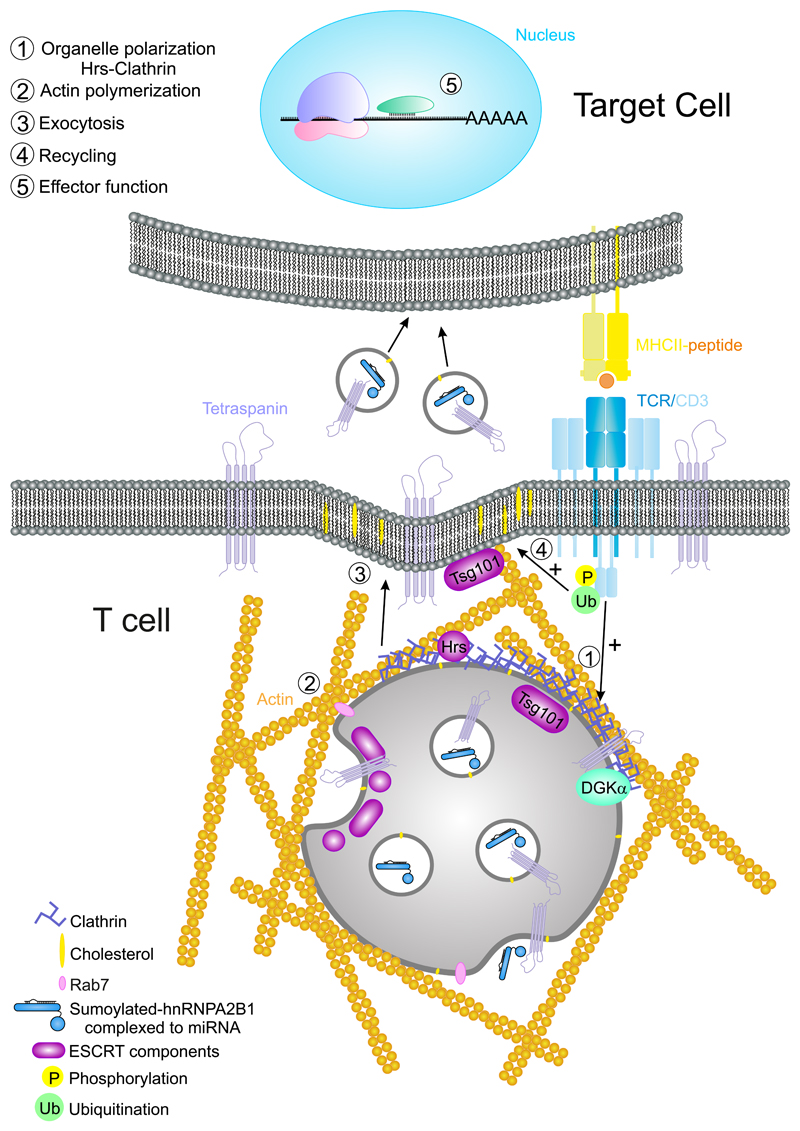
Clathrin-coated vesicles formed in the TGN are sorted to early endosomes. These can be transformed into lysosomes or late endosomes and form intraluminal vesicles (ILVs) by the invagination of their limiting membrane (Kienzle and von Blume, 2014). These organelles belong to the recycling endosomes that sort and re-export different cellular components from the plasma membrane to intracellular compartments and vice versa (Goldenring, 2015). Late endosomes enriched in ILVs are also called multivesicular bodies (MVB; Fig. 5). The endosomal content may be directed either to degradation (through fusion with lysosomes), or to secretion (by fusion with the plasma membrane). Upon fusion with the plasma membrane, there is a patch or “island” coming from the internal limiting membrane from the endosomes, with a different composition of lipids and proteins that may serve as a signal for recycling (Denzer et al., 2000).
Figure 5. Multivesicular bodies at the IS.
The activation of the TCR/CD3 complex promotes the phosphorylation of the ITAMs and subsequent ubiquitination of CD3 subunits. The MVBs are directed to the plasma membrane and released to the IS to establish a unidirectional communication between T cells and APC through exosomes. Effector function: at the APC, the content of exosomes such as specific miRNAs with the required binding motif for hnRNPA2B1, may be delivered to the cytosol and prevent translation of their target mRNA.
Exosomes are small vesicles (50–200 nm in mammals), with a parallel biogenesis to that of ILVs. Exosomes are released to the extracellular medium and serve as cell–cell communication devices in diverse functions. Exosomes could be considered an evolution of retroviruses—the “Trojan exosome hypothesis” might explain the ability of retroviruses to escape from the immune system (Gould et al., 2003). It was proposed that exosomes and HIV Gag emerged from the same sites at T-cell membranes (Booth et al., 2006) and that the budding of the virus on macrophages hijacked the exosome release pathway (Nguyen et al., 2003). However, recent evidence has demonstrated that HIV-1 particles can be released independently of exosomes (Park and He, 2010).
Two routes are involved in extracellular vesicles biogenesis; immediate, from endosomal patches at the plasma membrane; or delayed, from the limiting membrane of discrete endosomes (Gould et al., 2003). ARF6 protein is involved in the plasma membrane–derived exosomes (Muralidharan-Chari et al., 2009) together with Tsg101 (ESCRT 1) and ARMMS (arrestin domain-containing protein 1) (Nabhan et al., 2012). The biogenesis of ILVs has been analyzed by targeting different factors such as monoubiquitination, N-terminal myristoylation, and fatty acid acylation and aggregation. In particular, MHC-II sorting requires the participation of different components of the ESCRT machinery, such as STAM (ESCRT 0) and Tsg101; in contrast, CHMP4C (ESCRT III) inactivation increases ILVs production, whereas ALIX and VSP4B are not essential (Colombo et al., 2013). In sum, the ESCRT machinery together with HRS dock to ubiquitinated proteins (K63) at the endosomal membranes, promoting their invagination and the formation of ILVs (Bache et al., 2003; Hurley, 2008) (Fig. 5). More recently, exosomes are being considered asthe vesicles originated from the MVB (Mittelbrunn and Sanchez-Madrid, 2012; Villarroya-Beltri et al., 2014) (Fig. 5). Exosomal content is skewed toward specific components and it is thus different from the rest of the cell, including apoptotic bodies. Their membrane is enriched in cholesterol, glycosphingolipids, and GPI-anchored proteins, as well as tetraspanins and LAMPs (Yanez-Mo et al., 2015). Once released, exosomes can be used as information-harboring shuttles that mediate cell–cell communication by their fusion with membranes of neighboring cells, releasing its content into the target cell’s cytoplasm (Fig. 5). Exosomes are also found in extracellular fluids, protecting information and enabling its communication to distant anatomical locations. In this context, they are important for lymphocyte activation and induction of tolerance (Bobrie et al., 2011; Gutierrez-Vazquez et al., 2013; Yanez-Mo et al., 2015).
During CD4+ T-cell activation, MVB are directed to the IS. Their high content in clathrin and associated components, such as Hrs, cooperates with actin polymerization and reorganization factors during establishment of the actin-rich ring in the IS (Calabia-Linares et al., 2011) (Fig. 5). This structure participates in the formation of the adhesive pSMAC that seals the intracellular space between the T cell and the APC (Martin-Cofreces et al., 2014). The secretion from MVB at the synaptic cleft allows the delivery of exosomes that are subsequently captured by the APC, bearing the potential to modify the behavior of the APC (Mittelbrunn et al., 2011). MVB contain lysobiphosphatidic acid (LBPA), which is found at the IS and supports MVB fusion at this location (Varma et al., 2006). Specific stimulation of CD8+ T cells by dendritic cells produce different kinds of exosomes that can be taken up by distant dendritic cells and exert an immunosuppressive effect on CD8+ T-cell responses through FAS-related apoptosis and downregulation of pMHC-I surface expression on dendritic cells (DC) (Xie et al., 2010). Therefore, exosomes may affect APC through a directional pathway, or instead regulate other immune cells. The use of the IS to transfer exosomes to the APC is a potentially efficient way of cell communication. Indeed, specific microRNAs (miRNA) are sorted into exosomes through interaction of specific sequences with proteins such as hRNPA2B1 (Villarroya-Beltri et al., 2013). miRNAs are specialized RNAs that modulate the expression of target messenger RNA in cells, regulating the expression of the target proteins at the posttranscriptional level (Murchison and Hannon, 2004) (Fig. 5). The polarized release of micro-vesicles has also been observed using lipid bilayers (Choudhuri et al., 2014). The content of MVB increases with T-cell activation. Diacylglycerol kinase (DGKα) has a dual role in this process, since it is translocated to MVB upon activation, but its inhibition increases the number of mature MVB and the secretion of exosomes (Alonso et al., 2011). DGK has a negative role in T-cell activation through the production of phosphatidic acid from DAG. Its absence prevents T-cell anergy when CD3 stimulation lacks co-stimulation (Joshi and Koretzky, 2013). This may be a route to negatively regulate the recycling of receptors and signaling molecules at the IS. Chemical inactivation or genetic ablation of PKD1 and PKD2, mediators of DGKα, increase the size of the MVB, which are consequently less effective in releasing exosomes bearing FASL. Overexpression of a constitutively active form of PKD1 increases the localization of MVB at the IS and inactivation of DGKα promoted major activation of PKD1. Finally, lack of PKD2 decreased the cytotoxicity of CTLs as well as AICD (activation- induced cell death) (Mazzeo et al., 2016). Therefore, it seems clear that exosomes from MVB may have negative effects on their cellular targets. This is in agreement with the finding of FasL and APO2L/TRAIL- decorated exosomes released by activated T cells that can prime rapid cell death during immune regulation in an autocrine or paracrine manner (Monleon et al., 2001). Indeed, PKD2 protein has an important role on CD8+ T cells, by controlling the response of naive cells to weak or strong signals in a digital manner. PKD2 therefore determines the percentage of differentiation into effector cells (CTLs), acting as an amplifier of PKC and a marker of DAG production at the plasma membrane (Navarro et al., 2014). This function of PKD2 can also have a role in the control of cell death through homeostasis of CD8+ T cells.
6. Lysosomes and Autophagy: More than Degradation
Lysosomes are ubiquitous and the primary degradative organelle in the cell. Their morphology is heterogeneous, appearing as globular, or tubular, vacuoles. They have an electron-dense lumen and membrane sheets, often containing ILVs. Their biogenesis is still unclear. Their components may be directly targeted from the TGN, or recycled from the plasma membrane through the endosomal system. They are enriched in acid hydrolases transported to lysosomes by the mannose-6 phosphate receptor (M6PR). They have an acidic pH due to the action of the V-ATPase proton pump. Different transmembrane proteins are typical of lysosomes, for example, LAMP1, 2, and 3 (lysosome-associated membrane protein; CD107a, CD107b, and CD63); although their targeting to lysosomal membranes is not completely understood (Saftig and Klumperman, 2009). Major insights regarding lysosome biology have emerged from studying human diseases and mutations in lysosome-related genes, which have important consequences on immune responses.
The endosomal system uses MVB to reach the lumen of lysosomes and degrade extracellular cargo and nutrients, together with constituents of the plasma membrane, integral membrane proteins and receptors, and hydrolytic enzymes. For this purpose, MVB fuse or deliver their content into lysosomes. Indeed, autophagy also serves to recycle cellular materials such as damaged organelles, aggregated proteins, and invading pathogens. Although the autophagy and MVB pathways deliver most components to be degraded by lysosomes, there are different vesicular and non-vesicular pathways that also target cargoes to lysosomes. As an example, both microautophagy (MA) and chaperone-mediated autophagy are used to selectively deliver cargo into lysosomes (Huber and Teis, 2016; Parkinson-Lawrence et al., 2010).
The autophagosome is the major organelle used in autophagy to regulate cellular homeostasis. This organelle is made of membranes stemming from the ER (Tooze and Yoshimori, 2010), the endosomal system (Puri et al., 2013), and lipid droplets (Dupont et al., 2014), among others. Autophagosome assembly has been studied in yeasts, and homologs for major proteins have been identified in mammals. The Atg1/ULK complexes form independently of starvation, and serve as a scaffold for protein localization to the phagophore assembly site (PAS). PAS is formed by the fusion of Atg9-enriched vesicles emerging from the TGN that coalesce to form the phagophore membrane. The autophagosome is complete when the phagophore membrane undergoes a tightly regulated closure process that encases specific components (Stanley et al., 2014). The activation of the Atg1/ULK complex is dependent on mTOR (mammalian target of rapamycin) and AMPK that inactivate or activate, respectively, the function of ULK-kinase through direct phosphorylation (Kim et al., 2011). ULK phosphorylation of Beclin-1 (Russell et al., 2013) and additional, complementary, phosphorylations by AMPK (Kim et al., 2013) promote autophagy.
The components included in the autophagosome may also be captured by specific receptors. For example, entire mitochondrial targeting to the autophagosome (mitophagy) requires the concurrence of mitochondrial damage with loss of the mitochondrial membrane potential Δψ. This causes the accumulation of PINK1 (PTEN-induced putative kinase protein 1) at the outer membrane, and the subsequent recruitment of Parkin. Parkin ubiquitinates the damaged mitochondria and targets it to the phagophore for degradation upon fusion with lysosomes (Narendra et al., 2012). There is some controversy on whether p62 at the phagophore is the sole receptor for ubiquitinated proteins at the mitochondrial mem- brane (Youle and Narendra, 2011). In this regard, HDAC6 is also able to bind to ubiquitinilated substrates and accumulates at the surface of damaged mitochondria. HDAC6 is also required for parkin-mediated mitophagy (Lee et al., 2010). Mitochondria location at the IS relies on the fission factor Drp1 (dynamin-related protein 1) (Baixauli et al., 2011). However, Drp1 is not related directly to mitophagy (Youle and Narendra, 2011). Despite its participation in a complex with Milton and PINK1 at the mitochondria, Miro-1 is not related to mitophagy, either (Weihofen et al., 2009). However, its degradation upon activation of Parkin promotes mitophagy (Liu et al., 2012). Miro-1 has a specific role in maintaining the mitochondria network around the MTOC during lymphocyte adhesion to activated endothelia and transmigration due to its interaction with dynein/dynactin motors (Morlino et al., 2014). The active relocalization of mitochondria near the centrosome upon parkin/PINK1 inactivation in mammalian cells, rather than their pause and subsequent mitophagy (Vives-Bauza et al., 2010), suggests an important role for dynein motors in the transport toward minus-ends of MTs, centrosome docking, and for the centrosome itself as a center for autophagy organization. In this sense, the IS congregates the essential machinery required for specific autophagy, including the ER, GA, MVB, lysosomes, and mitochondria. This hypothesis requires additional examination. However, since different authors have observed that the blockade of autophagy increases T-cell activation (Paul and Schaefer, 2012), an attractive hypothesis would be that polarized autophagy serves as a negative regulator of TCR activation and organization at the IS.
7. Mitochondria: Powering Immune Synapse
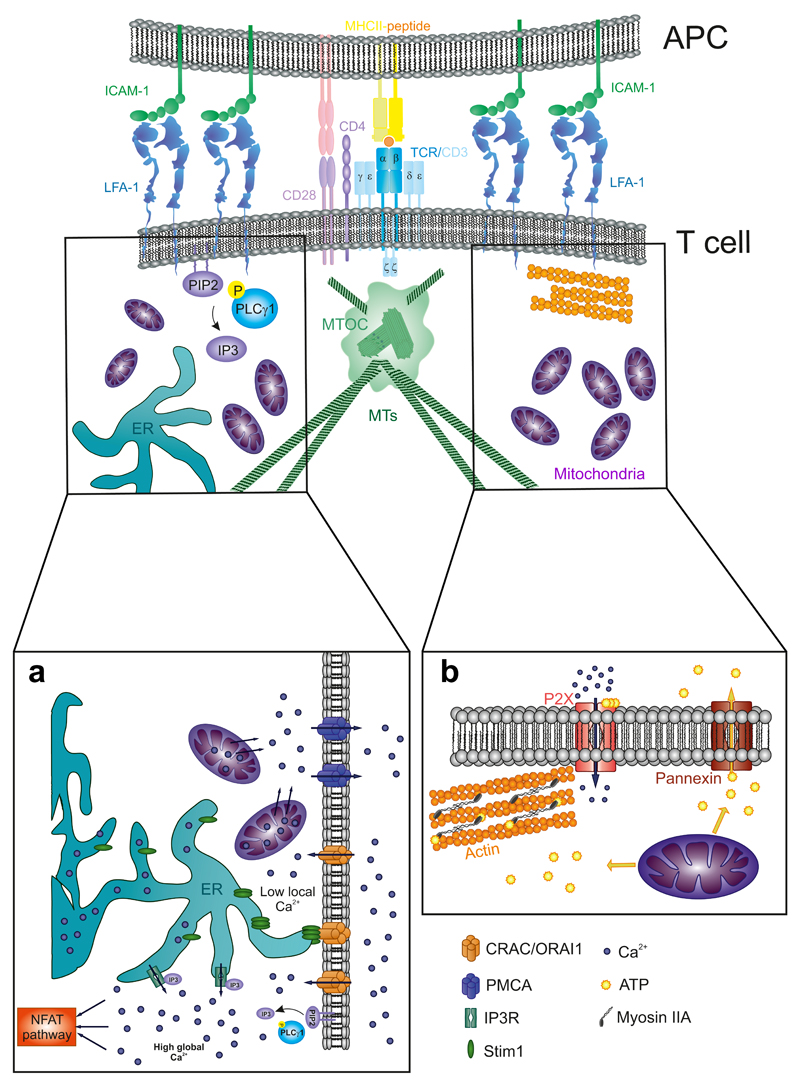
Mitochondria are essential organelles of every eukaryotic cell. They participate in many different processes, acting as the main energy-producing organelles and also controlling cell metabolism. Moreover, they also play an essential role in apoptosis and calcium buffering (Kasahara and Scorrano, 2014). In T cells, mitochondria also participate in T-cell migration and the maintenance of the architecture of the IS (Fig. 6). During IS formation, most mitochondria translocate to the proximity of T cell–APC contact (Quintana et al., 2007), specifically to the subplasma membrane region corresponding to the p-SMAC (Baixauli et al., 2011). Once there, they control local and global calcium signaling and provide the ATP necessary for autocrine signaling and actin dynamics.
Figure 6. Mitochondrial regulation of calcium and ATP at the IS.
(A) Mitochondria- dependent local and global control of [Ca2+] at the IS are regulated by the relationship between the plasma membrane, the mitochondria, and the endoplasmic reticulum (ER). (B) Mitochondria generation of ATP gradients at the IS promotes the sorting of ATP to the extracellular milieu through the Pannexin-1 shuttles. This ATP acts as an autocrine signal and also serves to fuel the phosphorylation of myosin light chain of Myosin IIA, therefore activating it.
7.1. Mitochondria Translocation to Immune Synapse
Quintana et al., reported differences in the calcium flux of T cells activated with a non-polarizing stimulus in comparison with a proper IS formation. They observed that a majority of the T-cell mitochondria accumulated near the IS (Quintana et al., 2007). Years later, other studies have corroborated and extended this observation, defining the subjacent region of the p-SMAC as major clustering area (Baixauli et al., 2011). Besides, mitochondrial accumulation also occurred in other kind of synapses, for example, those that form between an NK cell and a tumor cell (Abarca- Rojano et al., 2009). It is not well established yet how mitochondria move to the IS and accumulate there. A two-step model has been proposed: the model postulates a first approximation dependent on MTs and kinesin or dynein motors; and the subsequent use of actin filaments as tracks to get closer to the IS, probably in a myosin II-dependent manner. Different actin depolymerizing drugs such as cytochalasin D totally disrupt mitochondria translocation. Conversely, nocodazole treatment disrupts the tubulin cyto- skeleton but does not perturb mitochondrial polarization at the IS (Quintana et al., 2007). Recent mitochondria transport studies conducted in neurons can also be relevant for IS formation. In neurons, mitochondria initiate their movement in the soma (where they are generated) until they reach the end of the axon near the neural presynaptic terminal. In order to cover all this distance—even centimeters—mitochondria are transported through the MTs by a complex formed of two proteins: Miro-1 and Milton (Macaskill et al., 2009). Miro-1 has a C-terminal transmembrane domain that binds to the outer mitochondrial membrane (OMM) (Fransson et al., 2003), while Milton interacts with KIF5B, a kinesin heavy chain, which propels mitochondria translocation on MTs (Glater et al., 2006). Ca2+ concentration regulates mitochondria movement through the two EF domains present in Miro-1 that act as calcium sensors (Fransson et al., 2003). Upon Ca2+ binding to EF domains, Miro-1—Milton complex uncouples from kinesin motors and therefore stops mitochondria (Macaskill et al., 2009). Milton can also detect changes in glucose concentration, which regulates its binding to kinesins (Pekkurnaz et al., 2014).
Part of these mechanisms may be also present in lymphocytes. We have recently shown that Miro-1 controls mitochondria movement and rearrangement during endothelia–lymphocyte interaction for extravasation from blood vessels (Morlino et al., 2014). The localization of calcium signals at the cell–cell contact controls mitochondria positioning via Miro-1. Therefore, although no molecular mechanism has been described yet for mitochondria translocation to the IS, a plausible hypothesis is that they move toward the IS region using the MTs as trails in a Miro-1—Milton—Kinesin- dependent manner. Once in the vicinity of the IS the high calcium flux stops their movement, enabling their switch to the actin cytoskeleton (Quintana et al., 2007), which moves them closer to the subcortical region of the IS. Another important factor in mitochondria movement is their size and organization in intracellular networks. Mitochondria undergo fission and fusion, and these processes control network plasticity and remodeling. While fusion is normally conducted by mitofusins and Optic Athrophy 1 (OPA1) proteins, fission is a process mainly mediated by Drp1 (Kasahara and Scorrano, 2014). Mitochondrial fission is essential for a proper and equal share of the mitochondrial mass between daughter cells upon cell division, as well as for elimination of damaged mitochondria through mitophagy (Kasahara and Scorrano, 2014; Mishra and Chan, 2014). Indeed, mitochondrial fission influences the tubular mitochondrial network, producing smaller mitochondria that facilitates their transport. In this regard, it has been demonstrated that targeting Drp-1 abrogates mitochondrial translocation in T cells, but without affecting MTOC polarization to the IS. However, this treatment caused severe defects in T-cell signaling, calcium regulation, and ATP levels (Baixauli et al., 2011). Therefore, the regulation of mitochondrial size through fusion–fission cycles is essential for their physiology and accumulation at synapses.
Mitochondria transport and functionality are regulated by their contact with other organelles, such as the membrane of the ER as well as the plasma membrane. In the case of the ER, these tethering regions are known as mitochondria-associated membranes (MAMs), which are essential for lipid metabolism, mitochondria fusion–fission, and calcium exchange (Rowland and Voeltz, 2012). The ERMES complex mediates these contacts in yeasts, whereas mitofusin-2 plays a major role in mitochondria-ER tethering. Also, proteins involved in transport such as Miro-1 are detected at MAMs in vertebrates (Klecker et al., 2014). MAMs are also closely related to the autophagy and mitophagy processes by forming lipid-based platforms that serve as scaffolds for the phagophore. Besides, mitochondria are physically related to lipid droplets, a required contact for exchange of fatty acids exclusively metabolized in the mitochondria (Barbosa et al., 2015). Therefore, the interaction of mitochondria with other organelles seems to be closely related to the formation of other lipid-based structures. It may also be considered a form of regulation for their intracellular localization.
Most published studies agree that mitochondrial translocation in T cells is triggered by TCR activation (Baixauli et al., 2011; Quintana et al., 2007, 2011). In NK cells, triggering would be executed by the NKG2D receptor, since they do not possess a TCR (Abarca-Rojano et al., 2009). Nevertheless, a recent study claimed that mitochondrial translocation in T cells is TCR- independent and exclusively due to the action of integrin receptors like LFA-1 (Contento et al., 2010). Preactivation of LFA-1 by inside-out signaling upon chemokine binding to corresponding receptors, such as the pair CXCL12-CXCR4 and the subsequent interaction of LFA-1 with its ligands (ICAM-1 or -2) during the scanning of the APC surface leads to mitochon- dria translocation, even in the absence of TCR stimulation. According to Contento and coworkers, LFA-1 interaction with ICAM-1 or -2 but not ICAM-3 produces a PI3K-dependent signaling cascade that induces mitochondrial polarization toward the cell–cell contact. Although these authors suggested that differences with other studies are due to LFA-1 pre-activated state by APC-secreted chemokines, further research is required to determine the specific signals and motors that mediate mitochondria translocation. In this regard, a recent work proved that signaling by soluble CXCL12 enhances the process of conjugation and IS formation at the T cell, but also shows that the CXCL12 produced by the DC is present in its surface, rather than soluble (Cascio et al., 2015).
7.2. Calcium Buffering During T-Cell Activation: Local Control at Immune Synapse
Calcium signaling plays a major role during T-cell activation. Calcium release–activated channels (CRACs) are the main calcium transporters in T cells. In these, the main subunit is ORAI1. These channels coordinately act with STIM1, a transmembrane protein from the ER (Kummerow et al., 2009). TCR activation leads to PLCγ1 recruitment to the IS to produce IP3, which binds to its receptor at the ER, promoting the opening of CRAC/ORAI1 channels through STIM1 [Fig. 6(A)]. This causes a massive influx of Ca2+ into the cytoplasm from intracellular compartments as well as the extracellular milieu. This elevation of the cytoplasmic concentration of Ca2+ promotes the triggering of several signaling pathways. For example, activation of Calcineurin induces NFAT dephosphorylation and translocation to the nucleus, favoring IL-2 gene expression and therefore late T-cell activation and proliferation (Kummerow et al., 2009). A tight control of calcium levels at the IS regulates proper T-cell activation; mitochondria positioning at the IS also enables the existence of regionally different levels of intracellular calcium, higher at the IS and lower elsewhere in the cell. In this sense, preventing mitochondria polarization at the IS does not lower the global levels of Ca2+ in T cells (Baixauli et al., 2011). Polarized mitochondria dock at CRAC/ORAI1 and PMCA (a calcium sorting pump)-enriched regions; high local concentrations of calcium stop this process. High local Ca2+ leads to the inactivation of CRAC/ORAI1 channels and the activation of PMCA (Quintana et al., 2011). Once there, active calcium mitochondria-uptake avoids local excess of calcium and CRAC/ORAI1 inactivation. Mitochondria redistribute Ca2+ to PMCA regions to allow extracellular shuttling (Quintana et al., 2011). These processes enable a sustained high global calcium flux by uninterrupted influx through CRAC/ORAI1 channels, while low local calcium prevents CRAC/ ORAI1 inactivation and impairs T-cell signaling. Mitochondria polarization is therefore essential for proper T-cell activation in terms of Ca2+-flux regulation (Baixauli et al., 2011; Quintana et al., 2011). Although there are numerous reports describing deficiencies in calcium signaling by preventing mitochondria action, not so much is known about how mitochondria take up Ca2+ and redistribute it. With the advent of new calcium sensors and techniques for local calcium detection such as TIRFm and super-resolution microscopy, new pieces of this puzzle will be available soon.
7.3. ATP Reservoirs for Signaling
As for many other cell types, mitochondria constitute a major source of ATP in T cells, that is used to maintain the cellular metabolism or for signaling. They act as the fueling machinery of the cell. In T cells, ATP is fundamental for autocrine signaling and actin polymerization and contraction at the IS. ATP-dependent autocrine signaling is essential to maintain calcium influx upon TCR activation [Fig. 6(B)]. The ATP channel Pannexin 1 accumulates at the IS and induces a rapid release of cytosolic ATP to the extracellular cleft with the APC. Extracellular cleft ATP acts as a ligand of autocrine purinergic receptor like P2X4 or P2X1, which also accumulate at the IS. This induces a higher calcium flux, sustaining T-cell activation (Junger, 2011). Apart from this initial, extracellular release of ATP a local, intracellular increase of ATP from mitochondria is detected, but not from new synthesis (Ledderose et al., 2014). Defects in mitochondria polarization to the IS prevent the local increase in ATP and the subsequent extracellular sorting and autocrine stimulation. Besides, ATP regulates actin polymerization and acto-myosin retrograde flow at the pSMAC of the IS. Upon TCR activation, actin rapidly polymerizes and myosin IIA clusters and activates at the p-SMAC (Ilani et al., 2009; Vicente-Manzanares and Sanchez-Madrid, 2004). This leads to the centripetal movement of TCR microclusters and their associated signalosomes, “pushing” them to the c-SMAC for recycling and T-cell signal decay (Ilani et al., 2009). Mitochondrial impairment by inhibiting Drp-1 causes a reduction in the clustering and recycling of CD3, and enhances TCR-dependent signaling (Baixauli et al., 2011). Hence, mitochondria act as ATP reservoirs that promote autocrine signaling through P2X receptors but also to provide the chemical energy that fuels the acto-myosin retrograde flow used for TCR recycling and signaling decay at the IS.
8. Lytic Granules: Polarizing Killing Machinery
The lytic granules are lysosome-related organelles (LRO) that can degrade proteins and other cellular components. They also secrete cytotoxic effector molecules into the synaptic cleft formed between a cytotoxic lymphocyte (CTL) or NK (natural killer) cell and a target cell, which acts as an APC in this context. These lytic mediators promote the death of the target cell. Typical target cells include stressed, tumorigenic, or virus-infected cells. CTLs recognize them by the antigens presented on their surface, in the context of MHC molecules (Fig. 7). NK cells recognize target cells by the absence of MHC-I molecules (downregulated by viruses trying to avoid CTL-mediated responses) or an overexpression of stress- induced ligands for activating receptors, for example, MIC-A or MIC-B. The systematic analysis of lytic granule–derived diseases enabled the identification of key proteins either in human or mice that are components of the lytic machinery or their biogenesis routes. Lytic granules have an acidic pH similar to conventional lysosomes, but they contain specialized proteolytic enzymes that are inactive at low pH. By electron microscopy, lytic proteins that include granzymes and perforins are localized to a dense core domain in the lytic granules. These proteins are bound to proteoglycans, such as chondroitin sulfate, linked to a serglycin lattice, which helps to maintain them in an inactive state and protects the CTL from self- degradation. CTLs may also kill through other methods, such as FAS-L ligation to its receptor in the target cell, or the secretion of pro-inflammatory cytokines such as TNFα (tumor necrosis factor) or IFNγ (interferon). However, the localization of their lytic granules at the IS formed with the target cell and the release of the perforin, granzymes, and cathepsins is a hallmark of their cytotoxic activity in the context of antiviral and/or antitumor immune surveillance (de Saint Basile et al., 2010; Pachlopnik-Schmid et al., 2010; Stinchcombe and Griffiths, 2007). Component sorting to lytic granules is still under study. Part of their contents stem from the TGN and the endosomal M6P-dependent route. These components are then released into lysosomes that will mature into lytic granules (Fig. 7). Granzymes A and B use M6P to sort to lysosomes, but can also sort in other manners. In this context, cells of patients suffering from I-cell disease (mucolipidosis II; deficiency in the N-acetylglucosamine-1-phosphotransferase gene) are still able to load granzymes and cathepsin D into lysosomal components with the M6P tag. Indeed, a tyrosine-based motif and a di-leucine based motif drive the lysosomal sorting of different transmembrane proteins, such as CD63, CD3γ, CD3δ, and TIA. Serglycin is essential for normal lytic granule biogenesis and granzyme B storage, but not for granzyme A or perforin inclusion. Perforin is a member of the MACPF family of proteins (membrane-attack- complex-perforin). This protein self-assembles into pores in cellular mem- branes (de Saint Basile et al., 2010). Perforin sorting is M6P-independent; it has been proposed that it selectively aggregates at the TGN due to low pH and high Ca2+. This seems sufficient to sort it into lysosomes (Chanat and Huttner, 1991). Perforin deficiency causes the FHL2 disease (familial hemophagocytic lymphohistiocytosis), which is an autosomal, often lethal, recessive disorder with occurrence of HLH (hemophagocytic lymphohistiocytic syndrome). At a cellular level, FHL2 is characterized by an excessive activation of CD8+ T cells. These hyperactive CTL are not able to kill their targets, but instead they secrete IFNγ; therefore, they efficiently activate macrophages, which can phagocytose blood components and infiltrate into different tissues, causing severe damage. Perforin forms stable pores in the membrane of the target cell at neutral pH in a concentration- and Ca2+-dependent manner (Fig. 7). There has been much controversy on whether the pores were large enough to allow granzymes entry into the target cell, and a mixed endocytosis/intracellular delivery was proposed (Pipkin and Lieberman, 2007). Recent work has shown that perforin exocytosis is rapid upon increase in intracellular Ca2+ flux at the CTL and forms transient pores of about 12–17 nm of internal diameter. These pores close as quickly as 20 s after formation, but this is enough to allow granzyme entry into the target cell (Lopez et al., 2013). The localization of lytic granules at the IS is normal in PRF-/- CTLs. Unexpectedly, perforin-containing DCs play a regulatory role by controlling T-cell repertory in autoimmune disease (Zlotnikov-Klionsky et al., 2015).
Figure 7. Target cell killing.
(A) The formation of a lytic immune synapse involves the movement of the lytic granules (LG) toward the target cell on a microtubule-based manner. CTL activation involves a first step (1) to congregate LG at the centrosome through dynein-based transport; (2) the centrosome polarizes toward the target cell, and (3) LG move from the centrosome to IS through a Kinesin-1-rab27a-slp3 complex. (B) In CTLs, secretion of lytic granules and cytokines seems to follow different routes. LG secretion involves an additional step that entails the fusion of exocytic vesicles coming from recycling and late endosomes to provide the LG with the docking and priming machinery needed to delivery at the synaptic cleft.
The movement of lytic granules has been extensively analyzed in both CTLs and NK cells (Fig. 7). They are recruited to the centrosome (Stinchcombe et al., 2006) before the translocation to the IS of the centro- some from the CTL. At least in NK cells, this movement is dependent on dynein (Mentlik et al., 2010). Also, kinesin-dependent movement of lytic granules on MTs was described in vitro (Burkhardt et al., 1993) and more recently in vivo (Kurowska et al., 2012). The terminal transport of lytic granules from the centrosome to the plasma membrane for fusion critically depends on a complex that includes Rab27a and slp3 (Kurowska et al., 2012).
HDAC6 also has a role in the terminal delivery of the lytic granules at the IS. HDAC6 coordinates kinesin motors. In HDAC6-deficient CTLs, lytic granule exocytosis and degranulation of LAMP1 was attenuated; however, production and release of IFNγ were normal, similar to CTLs from some FHL patients (Nunez-Andrade et al., 2016). The HDAC6-deficient mouse does not seem to develop an HLH-like phenotype upon infection with vaccinia virus. However, the proliferation of CD8+ T cells is more prominent than in the wild-type at late stages, which is also a hallmark of the disease, due to the inability of the CD8+ T cells to adequately kill the infected cells, leading to the termination of the immune response (Fig. 7). Novel insights into the function of different HDACs, for example, HDAC8, which disrupts the centrosome and prevents correct endolysosomal biogenesis, are being addressed in different cell types (Yamauchi et al., 2011). These could also be important for CTL function.
The movement of lytic granules depends on the intensity of the TCR- dependent signaling in CTLs, pointing to an important role of the proximal signaling in the localization of the lytic granules at the IS. It also suggests that the antigen presented tunes this response. In general, stronger activation implies more polarization at the central secretory domain (Beal et al., 2009). Furthermore, actin clearance at the central domain upon activation with strong versus weak antigenic peptides (Jenkins et al., 2009) is needed for correct docking of lytic granules at the IS (Ritter et al., 2015). In fact, centrosome translocation is observed even toward APCs loaded with antagonist peptides that do not activate the exocytosis of lytic mediators. Instead, these peptides activate Vav1, which is probably ultimately responsible for centrosomal translocation together with members of the Src kinase family (Martin-Cofreces et al., 2006). In this regard, Lck has been proposed to maintain the centrosome docked at the IS in CTLs (Tsun et al., 2011), whereas Fyn-deficient cells display ineffective centrosome translocation in the presence of agonist peptides, which is fully abrogated if antagonist peptides are used (Martin-Cofreces et al., 2006). Therefore, both the movement of the lytic granules and their biogenesis determine the ability of the CTLs for correct formation of the IS and cytotoxic activity, which helps the clearance of target cells in different scenarios important for the immune response.
The FHL3 disease is caused by defects in the UNC13D gene that encodes the priming factor Munc 13-4. This factor is crucial in the secretion of the contents of the lytic granules through their fusion with the plasma membrane at the IS (Fig. 7). It also decreases the levels of LAMP1 (CD107a) in the plasma membrane of the CTL, but it does not affect IFNγ secretion (Feldmann et al., 2003; Hong, 2005; Huse et al., 2006; Marcenaro et al., 2006). Munc13-4 is also needed to form endosomal vesicles that move parallel to the lytic granules toward the IS and help them to fuse with the plasma membrane (Menager et al., 2007). Interestingly, Munc13-4 also appears polarized to the IS in CD4+ T cells (Huse et al., 2006). Therefore, not all the elements needed for the fusion of lytic granules with the plasma membrane are present in them. Rather, they seem to use a different pathway than cytokine-containing vesicles. This fact has also been observed in NK cells, in which different lytic granules contain perforin and either Munc13-4 or Rab27a (Wood et al., 2009). Importantly, Rab27a deficiency underlies the Griscelli syndrome type 2 (Menasche et al., 2000). These mechanisms may only apply to CTLs and NK, therefore allowing the well-known iter- ative cytotoxicity of these cells (serial killers). In this context, FHL3 patients do not present respiratory tract disease with normal mucus secretion, despite expression of Munc13-4 in goblet cells (Pachlopnik Schmid et al., 2010). Rab27a deficiency prevents the localization of the lytic granules to the IS in CTLs (Stinchcombe et al., 2001); Rab27a is not initially associated with the lytic granules, but appears enriched in the endosomal vesicles that also bear Munc13-4 and subsequently fuse with the lytic granules before docking at the plasma membrane (Menager et al., 2007). The association of Rab27 with slp3 (synaptotagmin-like protein 3) and the tubulin-based kinesin 1 molec- ular motor enables the transport of the lytic granules to the IS along MTs (Kurowska et al., 2012). The existence of complementary vesicles bearing different elements that help the process of membrane fusion may be consid- ered as a compartmentalization control system aimed at avoiding self-deg- radation and killing of nontarget cells.
The deficiency in the STX11 gene prevents the fusion of lytic granules with the plasma membrane, but not their localization at the IS. This gene encodes syntaxin 11, a v-SNARE involved in fusion events through the interaction of t-SNAREs at the target membrane and the SNAPs (synaptosomal-associated proteins). The molecular mechanism involves the formation of a highly stable and parallel four-helix bundle that triggers the formation of double membrane intermediates and fusion upon Ca2+ increase. Syntaxin 11 is mainly expressed in immune cells such as CTLs and NKs. It is essential for the correct function of CTLs, and syntaxin 11- defective cells do not release lytic mediators to the target cell. However, NKs are only mildly affected, and inhibition is relieved by treatment with IL-2 (Bryceson et al., 2007; Mills et al., 2005; Zur Stadt et al., 2006). The STXBP2 gene encodes Munc18-2. This protein underlies the defects found in the FHL5 disease. Munc18-2, together with syntaxin 11, participates in the fusion of lytic granules in primed CTLs. Therefore, its deficiency prevents perforin and granzymes release to the synaptic cleft. Again, this defect is critical for CTL function, but NK functionality is recovered by IL-2 treatment (Cote et al., 2009; zur Stadt et al., 2009).
Lysosomal trafficking regulator (LYST) deficiencies cause the Chediak–Higashi syndrome (CHS), an autosomal recessive disorder characterized by the presence of giant lysosome-like organelles in different cell types. The molecular function of LYST is still being elucidated (Sepulveda et al., 2015). In cells deficient for LYST, the enlarged compartment is a hybrid organelle that contains Rab11 from recycling endosomes; Rab27a and Rab7 from late endosomes, but not EEA1 from early endosomes. In these cells, degranulation is not observed in CTLs, whose cytotoxic activity is largely impaired. However, granules may be observed at the contact area with the target cell. The coexpression of proteins involved in the biogenesis and exocytosis of the lytic granules such as Munc13-4, slp3, and Rab27a restored, at least partially, the lytic activity of CTLs. However, the size of lytic granules did not improve (Sepulveda et al., 2015).
9. Asymmetric Cell Division: Versus Clonal Expansion
9.1. Nucleus Regulation: INSIDE OUT
The nucleus is a critical organelle. From a biophysical standpoint, the size and position of the nucleus controls gene expression at the transcriptional level. In this context, chromatin condensation constitutes both a protective and regulatory strategy. In T-cell activation, controlling the expression of some genes, for example, CD69, CD25, or IL-2 is essential to ensure the proper function of an activated T cell. Although the main role of the T-cell nucleus implicates later events, some of its associated proteins also modulate TCR triggering and early T-cell activation in different ways.
9.2. Nuclear Pores and Nucleoskeleton
Nuclear-cytoplasmic shuttling is fundamental for the proper translocation of some transcription factors and adaptor proteins, for example, NFAT or NF- κB. It also controls the export of transcriptional messengers, for example, mRNAs or microRNAs. The nuclear structure that mediates import– export is known as the nuclear pore complex (NPC). The NPC is formed by 30 different types of proteins, usually called nucleosporins (Nups). These proteins congregate forming a structure that comprises a central framework interconnected to cytoplasmic filaments, and a nuclear basket (Hoelz et al., 2011). Apart from the core of proteins that form the NPC, the cytoplasmic filaments also interact with RanGTPases that control the direc- tionality of the nuclear transport (Quimby and Dasso, 2003). Ran-GTPases show a very low GTPase activity per se and require the action of a GAP complex formed by Ran-GAP1 and Ran-BP2 (Bischoff et al., 1994; Hutten et al., 2008). GTP hydrolysis tilts cargo transport toward the nucleus, that is, nuclear import (Quimby and Dasso, 2003). Ran-GAP1 localizes to the nuclear envelope upon sumoylation, which is required for its interaction with the NPC and RanGTPases (Mahajan et al., 1998). A recent study demonstrated that SLP-76 binds to sumoylated Ran-GAP1 in T cells, favor- ing its location at the NPC, where it exerts its GAP activity. This interaction helps the nuclear import of NF-ATc1 and p65/RelA (Liu et al., 2015). SLP-76 would therefore link TCR activation with the control of NPC transport. In addition to the regulation of the molecules imported or exported from the nucleus, the structural backbone of the nucleus (nucleoskeleton) exerts an “inside-out” control over T-cell activation through the protein Lamin A/C (Gonzalez-Granado et al., 2014). Gonzalez-Granado and coworkers showed that Lamin A/C expression increases after TCR activation, and that this favors late T-cell activation. Surprisingly, the low amount of Lamin A/C previously present in T cells also affects early T-cell activation by enhancing CD3 clustering, centrosome translocation, and early TCR signaling (Gonzalez-Granado et al., 2014). The nucleoskeleton connects with the cytoskeleton through a bridging complex formed by SUN (Sad1 and UNC-84) and KASH (Klarsicht, ANC-1, and Sync/Nesprin homology) proteins (Starr and Fridolfsson, 2010). Lamin A effect over T cell activation is dependent on this connection, since disruption of the SUN-KASH linker complex decoupled lamins from T-cell activation (Gonzalez-Granado et al., 2014). The connection between the nucleoskeleton and the cytoskeleton also participates in additional steps after activation. For example, this interaction might provide signals to define a polarity axis for naive T cells undergoing mitosis upon activation, acting as a determinant factor in the asymmetric distribution of some cellular components (see Section 9.3).
9.3. Asymmetric Cell Division: Versus Clonal Expansion
The population of naive T cells responsive to a given antigen in homeostatic conditions is very low. Pathogen infections promote cognate naive T-cell activation, leading to an enormous increase of this responsive pool of cells to fight efficiently against the threat. Clonal expansion is followed by a con- traction phase to eliminate these cells. However, a small subset of long-term responding cells remain after activation. These cells are the basis of immunological memory and are called memory T cells. Memory T cells are divided into effector-memory T cells (Tem), which recirculate and respond faster in case of reinfection, and central-memory T cells (Tcm), which remain quiescent in peripheral lymphoid organs. This polarization is tightly controlled by many cytokines, including IL-2, IL-15, and IL-7 (Ahmed et al., 2009; Schluns and Lefrancois, 2003). Although the burst of effector T-cell pools upon IS formation and lymphocyte activation is due to clonal expansion, recent studies demonstrate that naive T cells undergo a first asymmetric division that generates two divergent precursor daughter cells: one originates the effector pool, whereas the other engenders the memory subset (Chang et al., 2007).
9.4. Asymmetric Cell Division and Cell Fate
Asymmetric cell division (ACD) is a well-conserved mechanism that plays a role in many physiological processes, for example, epithelial regeneration, stem cell renewal or development (Chang and Reiner, 2008; Knoblich, 2008). A naive T cell undergoes ACD in vivo during its first division cycle in response to cognate-antigenic activation and IS formation (Chang et al., 2007). Both CD8 and CD4 subsets are able to perform ACD to generate two different daughter cells: a pre-memory and a pre-effector cell (Fig. 8). Upon stimulation, the parent T cell undergoes an uneven polarization event, localizing some components preferentially to one pole. Some effector markers, for example, CD8, CD25, IFNγR, or LFA-1 accumulate at the proximal pole (near the IS) to generate the future effector T cell, while other molecules such as PKCθ or Eomesodermin (Eomes) accumulate at the distal pole, where the putative memory T cell will form (Chang et al., 2007). The first asymmetric division generates two daughter populations that are differentiated according to their levels of CD8: CD8high cells correspond to the effector pool and they also express effector-related markers like T-bet or CD25. Conversely, CD8low cells constitute the memory pool and express memory-like markers (Chang et al., 2007). Afterward, other studies have shown the asymmetric distribution of other proteins to the distal pole, like Numb or Par3, and to the proximal one, such as Scribble (Oliaro et al., 2010) (Fig. 8).
Figure 8. Dynamics of T cell asymmetric cell division and clonal expansion.
The activation of a naive T cell (stage 1) involves the asymmetric sharing of some cell determinants during its first division (stage 2). This asymmetry defines the generation of two different subsets: a prememory and a preeffector daughter cell. The preeffector undergoes a burst of proliferation (clonal expansion) to respond against a specific threat. The prememory cell maturates into a quiescent pool of cells ready to respond to the specific antigen that promoted their differentiation. This pool of cells can asymmetrically divide again in case of reinfection.
Proper ACD depends mainly on two factors: TCR engagement and T cell–APC adhesive interactions. TCR activation is essential for ACD since ACD does not occur in the absence of stimuli or in the presence of non- specific stimuli (Chang et al., 2007). The affinity of the TCR for its ligand is a crucial determinant for ACD; high-affinity ligands trigger ACD, while low affinity ones do not (King et al., 2012). Besides, T cell-APC contact is also needed for correct ACD because short-term conjugates or defects in LFA-1-ICAM-1 interaction lead to impaired ACD (Chang et al., 2007; Oliaro et al., 2010). This is supported by studies using artificial lipid layers, in which naive T cells are activated on ICAM-1-covered surfaces with foci of acti- vating signals (anti-CD3/anti-CD28 antibodies) of different sizes and sepa- ration (Jung et al., 2014). This study concluded that only one pole of activation is needed for proper ACD, and that both TCR/costimulatory (anti-CD3/anti-CD28) and adhesion (ICAM-1) signals are required. The proposed model states that a low number of dendritic cells presenting a small number of antigens lead to ACD (similar to what is seen in a pathogen infection), while many dendritic cells presenting the same antigen would preclude ACD to prevent the formation of memory T cells against this particular antigen. This is the case of autoantigens, and this is thought to avoid the development of autoreactive T-cell clones (Jung et al., 2014). This is also in line with the observation that only high-affinity ligands but not low- affinity ones (autoantigens) lead to ACD (King et al., 2012).
While the exact mechanism still remains unclear, several clues have been found to explain T-cell ACD. For example, T-bet accumulation at the proximal pole is due to a distal accumulation of the proteasome, which mediates an asymmetric degradation of the transcription factor, higher in the distal area (Chang et al., 2007). This mechanism can also explain the differential distribution of other proteins regulated by ubiquitination and degradation. Besides, another group suggested that the contact with the APC establishes the initial asymmetric axis by polarizing the mitotic spindle to mark the orientation of the mitotic axis. This is a conserved mechanism of ACD observed in other cellular systems (Oliaro et al., 2010). In this sense, the maintenance of the polarized state during the activation might be an essential cue for the early establishment of the ACD axis. Different aPKCs, such as PKCζ, interact with adaptor molecules like PAR3 and PAR6 to stabilize the polarized state of the cell in other ACD systems (Nelson, 2003). In T cells, PAR3 and phosphorylated and activated PKCζ accumulate at the IS after 20–30 min upon T cell-APC contact, but Scribble and Disc-large (Dlg) colocalize at the back of the cell (Bertrand et al., 2010). The PAR and Scribble-Dlg complexes antagonize each other’s assembly to enhance and stabilize the polarized shape (Nelson, 2003). These proteins may be non- compulsory for MTOC initial polarization, but necessary for the subsequent stabilization of the polarized state, since blocking PKCζ prevents the correct localization of the MTOC at the IS at 20 min (Bertrand et al., 2010). Together, all the results to date support a biphasic model of MTOC polarization: first, fast reorientation depends on DAG and nPKCs; long-term stabilization of the polarized MTOC depends on the PAR3-PAR6-aPKC and Scribble-Dlg complexes (Huse, 2012). In line with these mechanisms of MTOC polarization, atypical PKCζ and PKCλ/ι regulate ACD in CD8 T cells at a late stage, since gene-specific knockout mice show normal MTOC and LFA-1 redistribution to the IS, but reduced ACD, which is prevented by symmetrical distribution of the proteasome and therefore similar amount of T-bet in each daughter cell. PKCλ/ι deficiency promoted stronger effects than that of PKCζ. Deficiency in PKCζ or PKCλ/ι produces less memory T cells independent of long- or short-lived effector T cells (Metz et al., 2015). Also, the disruption of the PKC–Par interaction has a drastic effect on ACD (Oliaro et al., 2010).
There are many parallels between the process of MTOC polarization and ACD. In this view, the contact with the APC and the initial polarization of the MTOC would define an initial asymmetry, while the asymmetric accu- mulation of PAR3-PAR6-aPKC and Scribble-Dlg complexes would main- tain asymmetry and trigger ACD (Arsenio et al., 2015). Although ACD was first demonstrated in naive CD8 and CD4 T cells, memory T cells resulting from this first division are also able to undergo another round of ACD upon rechallenge and reinfection to ensure that proper renewal occurs (Ciocca et al., 2012). In the case of B lymphocytes, the existence of such a mechanism remains unclear. Some groups have proven that some proteins, for example, Bcl6 and IL21-R or the BCR distribute asymmetrically (Barnett et al., 2012; Thaunat and Batista, 2012), while other studies did not detect asymmetry in the distribution of the BCR, PKCζ, or Scribble (Hawkins et al., 2014).
9.5. Clonal T-Cell Expansion
The study of different adjuvants to produce vaccination has shown that proliferation of specific T-cell clones is regulated by the affinity they display toward pMHC complexes, and not by the amount of antigen present in the system. This fact leads to the generation of a more specific pool of responsive T cells against a particular threat (Malherbe et al., 2008). Although TCR affinity regulates a proper selection of the responsive clones, the proliferative burst is mainly driven by IL-2 secretion as well as by the interaction with its receptor (Malek and Castro, 2010). The IL-2 receptor (IL2R) is a trimeric complex with a specific α-chain (CD25; IL2Rα), a β-chain (CD122), and a γc-chain (CD132). To form a high-affinity receptor, a quaternary complex with IL-2 is needed. The binding of IL2Rα to IL-2 favors the binding of the other two subunits, the formation of this complex, and the initiation of the subsequent signaling cascade (Wang et al., 2005). When a T-cell activates, several transcription factors like NFAT are triggered and translocate to the nucleus, enhancing IL-2 expression and secretion. T-cell activation leads to a highly-focused secretion of IL-2 toward the IS, which favors autocrine and paracrine signaling (Huse et al., 2008). Once the IL2R binds IL2, Jak-1 and -3 (Janus Kinase) phosphorylate different residues of the receptor to promote the docking of members of the MAPK and PI3K pathways (Gaffen, 2001). Although the MAPK pathway leads to some proliferative signals, PI3K is more important for a sustained clonal expansion and cell survival (Lali et al., 2004). Stat5 is also essential for clonal expansion, but its activation requires Shc for IL2R proliferative signals (Lockyer et al., 2007). TCR activation drives proliferation, but also makes the effector T cell switch to a more glycolytic metabolism. This sustains the subsequent cell growth and division (Pearce and Pearce, 2013). The IL-2 pathway is also involved in this effect, since IL2Rβ activates c-Myc through Jak-1 signaling (Gaffen, 2001). In addition to IL-2 signaling, IRF4 is also required for clonal expansion of CD8+ T cells. In IRF4-deficient mice, antigen-specific clones are less responsive after infection. Only high-affinity ligands of the TCR promote IRF4 overexpression, allowing clonal expansion. In this sense, IRF4 acts as a sensor of TCR affinity. IRF4 is also responsible for the metabolic switch in effector T cells through the induction of c-Myc expression during effector differentiation (Man et al., 2013).
In summary, when a T cell becomes specifically activated, it first under- goes an asymmetric T-cell division that leads to two daughter cells with different fates. The pre-effector T cell inherits most of the IL2Rα, favoring its responsiveness to IL-2. This facilitates its rapid clonal expansion in response to the cytokine to expand the effector pool to respond efficiently to the threat. The pre-memory T cell shows lower responsiveness to IL-2 due to its lower content of IL2Rα. Conversely, it is more responsive to other cytokines like IL-15 or IL-7. Their receptors are formed by specific α-chains (IL15Rα for the IL-15 receptor; CD127 for the IL-7 receptor) and other chains shared with the IL2R, specifically CD122 and CD132 for the IL15 receptor and CD132 alone for the IL7 receptor. This ensures the specific Surveillance and quiescent state essential for a possible, posterior re-challenge. Therefore, the combination of a first asymmetric division and the subsequent clonal expansion of effector T cells is essential to assure a proper effector and memory T-cell pool.
10. Concluding Remarks
The organization of the different intracellular compartments and their interrelations, that is, signaling spread or components exchange, is important for T-cell functions. The biosynthetic and recycling pathways are interconnected and cross-regulated, forming a very convoluted network that finely tunes T-cell activation and differentiation. In one clear example, autophagy permits the survival of T cells by preventing the pro-apoptotic effects of FAS/ FASL signaling that follows TCR activation (He and He, 2013), and proper maturation of naive T cells depends on autophagy to reduce their mitochondrial content (Pua et al., 2009). Indeed, MVB formation and exosome delivery at the IS likely regulate the extent of activation of the APC and a possible differentiation toward other phenotypes (Mittelbrunn et al., 2011). The effector functions of T cells may be mediated by the secretion of soluble cytokines, some of which are polarized to the IS, as well as lytic granules in the case of CTL (Huse et al., 2008). An emerging new concept is that not only the biogenesis of the organelles is linked to their localization in the cell, but also its function. There are proteins with dual roles in these processes that can be targeted for therapeutics at different levels.
Recent data closely relate the function of mitochondria with other organelles suggesting the existence of a very regulated and interconnected network of organelles that control the function of the T cells and the overall immune system. In this regard, a relationship between mitochondria dysfunction and aberrant lysosome function accompanied by altered autophagy during T-cell activation has been related to the content of TFAM (Transcription Factor A, Mitochondrial), a mitochondrial (mt) transcription factor encoded by the nucleus that also participates in the repair of damaged mtDNA, that binds to and regulates the amount of mitochondrial DNA in T cells (Baixauli et al., 2015). This study points to the relationship of the different organelles in T-cell homeostasis. In this regard, a recent study in CD8+ T cells demonstrated that autophagy is essential for cell survival and control of viremia during a chronic infection; specific CD8+ T cells differentiate into exhausted T cells in these situations. This work points to autophagy as a form of maintaining the survival of exhausted CD8+ T cells to promote immunologic memory (Xu et al., 2014). Autophagosomes have not been detected in resting naive CD4 T cells, but they appear in Th1 or Th2 differentiated CD4+ T cells upon TCR stimulation. Interestingly, Th2 cells seem more prone to undergo autophagy than Th1 cells (Li et al., 2006), which may support the major susceptibility to cell death of Th1 compared to Th2.
Apart from the initial asymmetric distribution of some determinants essential for the differentiation into a pre-memory or a pre-effector T cell, one of the main factors that also sustains the posterior clonal expansion and that defines the specific type of effector or memory subset in which the T cell is going to differentiate, is the miRNAs repertoire of the T cell. As stated before, one of the most surprising and important role of miRNAs in T-cell activation relies on their transport in exosomes toward the APC (Mittelbrunn et al., 2011; Valadi et al., 2007). However, they are essential in processes dependent on TCR triggering. Wide range genomic studies have identified a differential signature in the pattern of expression of at least 71 miRNAs upon T-cell activation (Grigoryev et al., 2011). The miRNAs cluster (miRNAs 17~92) is upregulated upon TCR triggering and favors T-cell survival and proliferation upon activation (Jiang et al., 2011; Podshivalova and Salomon, 2013). In contrast, some miRNAs are downregulated upon T-cell activation, such as miRNA 181 that blocks the expression of the IL2 mRNA (Xue et al., 2011). To induce T-cell proliferation, some miRNAs are differentially expressed during T-cell activation to allow cell cycle progression and block either the extrinsic (miRNA-98) or the intrinsic (miRNA 17~92 cluster) apoptosis pathway (Podshivalova and Salomon, 2013). miRNAs differentially expressed also control the differentiation of T helper cells into the different subsets of effector cells (Th1, Th2, Th17, or Treg) (Baumjohann and Ansel, 2013). TH1 polarization is inhibited by miR-29 and -21 expression; miR-29 seems to differentially control IFNγ mRNA expression directly in T cells (Ma et al., 2011) while miR-21 inhibits IL-12 secretion by dendritic cells, an essential cytokine for Th1 polarization (Lu et al., 2011). miRNAs normally act by impairing Th1 polarization rather than targeting genes involved in Th2 polarization (Baumjohann and Ansel, 2013). Also, the Th17/Treg equilibrium is tightly regulated by miRNA differential signatures, such as miR-155 (O’Connell et al., 2010). In summary, miRNAs play critical roles in T-cell activation and in T-cell differentiation. They constitute a very complex and regulated network that tunes differential gene expression, conferring particular signatures to the different subsets of T cells generated upon activation.
Acknowledgments
We thank Dr. Miguel Vicente-Manzanares and Dr. Manuel Gómez for their critical reading of the manuscript and editing. This study was supported by grants SAF2014-55579-R from the Spanish Ministry of Economy and Competitiveness, INDISNET-S2011/BMD-2332 from the Comunidad de Madrid, ERC-2011-AdG294340-GENTRIS from EU, Red Cardiovascular RD12-0042-0056, and grant PIE13/00041 BIOIMID from Instituto Salud Carlos III (ISCIII). The Centro Nacional de InvestigacionesCardiovasculares (CNIC, Spain) is supported by the Spanish Ministry of Science and Innovation and the Pro-CNIC Foundation.
References
- Abarca-Rojano E, Muniz-Hernandez S, Moreno-Altamirano MM, Mondragon-Flores R, Enriquez-Rincon F, Sanchez-Garcia FJ. Re-organization of mitochondria at the NK cell immune synapse. Immunol Lett. 2009;122(1):18–25. doi: 10.1016/j.imlet.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: models and controversies. Nat Rev Immunol. 2009;9(9):662–668. doi: 10.1038/nri2619. [DOI] [PubMed] [Google Scholar]
- Allison R, Lumb JH, Fassier C, Connell JW, Ten Martin D, Seaman MN, Hazan J, Reid E. An ESCRT-spastin interaction promotes fission of recycling tubules from the endosome. J Cell Biol. 2013;202(3):527–543. doi: 10.1083/jcb.201211045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso R, Mazzeo C, Rodriguez MC, Marsh M, Fraile-Ramos A, Calvo V, Avila- Flores A, Merida I, Izquierdo M. Diacylglycerol kinase alpha regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 2011;18(7):1161–1173. doi: 10.1038/cdd.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres-Delgado L, Anton OM, Bartolini F, Ruiz-Saenz A, Correas I, Gundersen GG, Alonso MA. INF2 promotes the formation of detyrosinated microtubules necessary for centrosome reorientation in T cells. J Cell Biol. 2012;198(6):1025–1037. doi: 10.1083/jcb.201202137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres-Delgado L, Anton OM, Madrid R, Byrne JA, Alonso MA. Formin INF2 regulates MAL-mediated transport of Lck to the plasma membrane of human T lymphocytes. Blood. 2010;116(26):5919–5929. doi: 10.1182/blood-2010-08-300665. [DOI] [PubMed] [Google Scholar]
- Anton O, Batista A, Millan J, Andres-Delgado L, Puertollano R, Correas I, Alonso MA. An essential role for the MAL protein in targeting Lck to the plasma membrane of human T lymphocytes. J Exp Med. 2008;205(13):3201–3213. doi: 10.1084/jem.20080552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton OM, Andres-Delgado L, Reglero-Real N, Batista A, Alonso MA. MAL protein controls protein sorting at the supramolecular activation cluster of human T lymphocytes. J Immunol. 2011;186(11):6345–6356. doi: 10.4049/jimmunol.1003771. [DOI] [PubMed] [Google Scholar]
- Antonny B, Huber I, Paris S, Chabre M, Cassel D. Activation of ADP-ribosylation factor 1 GTPase-activating protein by phosphatidylcholine-derived diacylglycerols. J Biol Chem. 1997;272(49):30848–30851. doi: 10.1074/jbc.272.49.30848. [DOI] [PubMed] [Google Scholar]
- Arsenio J, Metz PJ, Chang JT. Asymmetric cell division in T lymphocyte fate diversification. Trends Immunol. 2015;36(11):670–683. doi: 10.1016/j.it.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bache KG, Brech A, Mehlum A, Stenmark H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J Cell Biol. 2003;162(3):435–442. doi: 10.1083/jcb.200302131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetz NW, Goldenring JR. Rab11-family interacting proteins define spatially and temporally distinct regions within the dynamic Rab11a-dependent recycling system. Mol Biol Cell. 2013;24(5):643–658. doi: 10.1091/mbc.E12-09-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier G, Wagner J. PKC inhibitors: potential in T cell-dependent immune diseases. Curr Opin Cell Biol. 2009;21(2):262–267. doi: 10.1016/j.ceb.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Baixauli F, Acin-Perez R, Villarroya-Beltri C, Mazzeo C, Nunez-Andrade N, Gabande- Rodriguez E, Ledesma MD, Blazquez A, Martin MA, Falcon-Perez JM, Redondo JM, et al. Mitochondrial respiration controls lysosomal function during inflammatory T cell responses. Cell Metab. 2015;22(3):485–498. doi: 10.1016/j.cmet.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baixauli F, Martin-Cofreces NB, Morlino G, Carrasco YR, Calabia-Linares C, Veiga E, Serrador JM, Sanchez-Madrid F. The mitochondrial fission factor dynamin- related protein 1 modulates T-cell receptor signalling at the immune synapse. EMBO J. 2011;30(7):1238–1250. doi: 10.1038/emboj.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagopalan L, Coussens NP, Sherman E, Samelson LE, Sommers CL. The LAT story: a tale of cooperativity, coordination, and choreography. Cold Spring Harb Perspect Biol. 2010;2(8):a005512. doi: 10.1101/cshperspect.a005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banton MC, Inder KL, Valk E, Rudd CE, Schneider H. Rab8 binding to immune cell-specific adaptor LAX facilitates formation of trans-Golgi network-proximal CTLA-4 vesicles for surface expression. Mol Cell Biol. 2014;34(8):1486–1499. doi: 10.1128/MCB.01331-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa AD, Savage DB, Siniossoglou S. Lipid droplet–organelle interactions: emerging roles in lipid metabolism. Curr Opin Cell Biol. 2015;35:91–97. doi: 10.1016/j.ceb.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Barnett BE, Ciocca ML, Goenka R, Barnett LG, Wu J, Laufer TM, Burkhardt JK, Cancro MP, Reiner SL. Asymmetric B cell division in the germinal center reaction. Science. 2012;335(6066):342–344. doi: 10.1126/science.1213495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista A, Millan J, Mittelbrunn M, Sanchez-Madrid F, Alonso MA. Recruitment of transferrin receptor to immunological synapse in response to TCR engagement. J Immunol. 2004;172(11):6709–6714. doi: 10.4049/jimmunol.172.11.6709. [DOI] [PubMed] [Google Scholar]
- Baumjohann D, Ansel KM. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat Rev Immunol. 2013;13(9):666–678. doi: 10.1038/nri3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal AM, Anikeeva N, Varma R, Cameron TO, Vasiliver-Shamis G, Norris PJ, Dustin ML, Sykulev Y. Kinetics of early T cell receptor signaling regulate the pathway of lytic granule delivery to the secretory domain. Immunity. 2009;31(4):632–642. doi: 10.1016/j.immuni.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck-Garcia K, Beck-Garcia E, Bohler S, Zorzin C, Sezgin E, Levental I, Alarcon B, Schamel WW. Nanoclusters of the resting T cell antigen receptor (TCR) localize to non-raft domains. Biochim Biophys Acta. 2015;1853(4):802–809. doi: 10.1016/j.bbamcr.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Beemiller P, Jacobelli J, Krummel MF. Integration of the movement of signaling microclusters with cellular motility in immunological synapses. Nat Immunol. 2012;13(8):787–795. doi: 10.1038/ni.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand F, Esquerre M, Petit AE, Rodrigues M, Duchez S, Delon J, Valitutti S. Activation of the ancestral polarity regulator protein kinase C zeta at the immuno- logical synapse drives polarization of Th cell secretory machinery toward APCs. J Immunol. 2010;185(5):2887–2894. doi: 10.4049/jimmunol.1000739. [DOI] [PubMed] [Google Scholar]
- Bischoff FR, Klebe C, Kretschmer J, Wittinghofer A, Ponstingl H. RanGAP1 induces GTPase activity of nuclear Ras-related Ran. Proc Natl Acad Sci USA. 1994;91(7):2587–2591. doi: 10.1073/pnas.91.7.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blas-Rus N, Bustos-Morán E, Pérez de Castro I, de Cárcer G, Borroto A, Camafeita E, Jorge I, Vázquez J, Alarcón A, Malumbres M, Martín-Cófreces NB, et al. Aurora A drives early signalling and vesicle dynamics during T-cell activation. Nat Commun. 2016;7:11389. doi: 10.1038/ncomms11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- Bonello G, Blanchard N, Montoya MC, Aguado E, Langlet C, He HT, Nunez- Cruz S, Malissen M, Sanchez-Madrid F, Olive D, Hivroz C, et al. Dynamic recruitment of the adaptor protein LAT: LAT exists in two distinct intracellular pools and controls its own recruitment. J Cell Sci. 2004;117(Pt 7):1009–1016. doi: 10.1242/jcs.00968. [DOI] [PubMed] [Google Scholar]
- Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172(6):923–935. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE. Sphingolipid organization in biomembranes: what physical studies of model membranes reveal. J Cell Sci. 1998;111(Pt 1):1–9. doi: 10.1242/jcs.111.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, Rudd E, Zheng C, Edner J, Ma D, Wood SM, Bechensteen AG, Boelens JJ, Celkan T, Farah RA, Hultenby K, et al. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohis- tiocytosis 4 (FHL4) patients. Blood. 2007;110(6):1906–1915. doi: 10.1182/blood-2007-02-074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt JK, McIlvain JM, Jr, Sheetz MP, Argon Y. Lytic granules from cytotoxic T cells exhibit kinesin-dependent motility on microtubules in vitro. J Cell Sci. 1993;104(Pt 1):151–162. doi: 10.1242/jcs.104.1.151. [DOI] [PubMed] [Google Scholar]
- Cabrero JR, Serrador JM, Barreiro O, Mittelbrunn M, Naranjo-Suarez S, Martin- Cofreces N, Vicente-Manzanares M, Mazitschek R, Bradner JE, Avila J, Valenzuela- Fernandez A, et al. Lymphocyte chemotaxis is regulated by histone deacetylase 6, independently of its deacetylase activity. Mol Biol Cell. 2006;17(8):3435–3445. doi: 10.1091/mbc.E06-01-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabia-Linares C, Robles-Valero J, de la Fuente H, Perez-Martinez M, Martin- Cofreces N, Alfonso-Perez M, Gutierrez-Vazquez C, Mittelbrunn M, Ibiza S, Urbano-Olmos FR, Aguado-Ballano C, et al. Endosomal clathrin drives actin accumulation at the immunological synapse. J Cell Sci. 2011;124(Pt 5):820–830. doi: 10.1242/jcs.078832. [DOI] [PubMed] [Google Scholar]
- Carrasco S, Merida I. Diacylglycerol-dependent binding recruits PKCtheta and RasGRP1 C1 domains to specific subcellular localizations in living T lymphocytes. Mol Biol Cell. 2004;15(6):2932–2942. doi: 10.1091/mbc.E03-11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio G, Martin-Cofreces NB, Rodriguez-Frade JM, Lopez-Cotarelo P, Criado G, Pablos JL, Rodriguez-Fernandez JL, Sanchez-Madrid F, Mellado M. CXCL12 Regulates through JAK1 and JAK2 formation of productive immunological synapses. J Immunol. 2015;194(11):5509–5519. doi: 10.4049/jimmunol.1402419. [DOI] [PubMed] [Google Scholar]
- Catalfamo M, Tai X, Karpova T, McNally J, Henkart PA. TcR-induced regu- lated secretion leads to surface expression of CTLA-4 in CD4+CD25+ T cells. Immunology. 2008;125(1):70–79. doi: 10.1111/j.1365-2567.2008.02822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau J, Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J Cell Sci. 2005;118(Pt 12):2579–2587. doi: 10.1242/jcs.02385. [DOI] [PubMed] [Google Scholar]
- Chanat E, Huttner WB. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol. 1991;115(6):1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, Killeen N, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315(5819):1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- Chang JT, Reiner SL. Asymmetric division and stem cell renewal without a permanent niche: lessons from lymphocytes. Cold Spring Harb Symp Quant Biol. 2008;73:73–79. doi: 10.1101/sqb.2008.73.008. [DOI] [PubMed] [Google Scholar]
- Chemin K, Bohineust A, Dogniaux S, Tourret M, Guegan S, Miro F, Hivroz C. Cytokine secretion by CD4+ T cells at the immunological synapse requires Cdc42- dependent local actin remodeling but not microtubule organizing center polarity. J Immunol. 2012;189(5):2159–2168. doi: 10.4049/jimmunol.1200156. [DOI] [PubMed] [Google Scholar]
- Choudhuri K, Llodra J, Roth EW, Tsai J, Gordo S, Wucherpfennig KW, Kam LC, Stokes DL, Dustin ML. Polarized release of T-cell-receptor-enriched micro-vesicles at the immunological synapse. Nature. 2014;507(7490):118–123. doi: 10.1038/nature12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocca ML, Barnett BE, Burkhardt JK, Chang JT, Reiner SL. Cutting edge: asymmetric memory T cell division in response to rechallenge. J Immunol. 2012;188(9):4145–4148. doi: 10.4049/jimmunol.1200176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Thery C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- Combs J, Kim SJ, Tan S, Ligon LA, Holzbaur EL, Kuhn J, Poenie M. Recruitment of dynein to the Jurkat immunological synapse. Proc Natl Acad Sci USA. 2006;103(40):14883–14888. doi: 10.1073/pnas.0600914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contento RL, Campello S, Trovato AE, Magrini E, Anselmi F, Viola A. Adhesion shapes T cells for prompt and sustained T-cell receptor signalling. EMBO J. 2010;29(23):4035–4047. doi: 10.1038/emboj.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote M, Menager MM, Burgess A, Mahlaoui N, Picard C, Schaffner C, Al- Manjomi F, Al-Harbi M, Alangari A, Le Deist F, Gennery AR, et al. Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest. 2009;119(12):3765–3773. doi: 10.1172/JCI40732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotzer VL, Mabardy AS, Weiss A, Brodsky FM. T cell receptor engagement leads to phosphorylation of clathrin heavy chain during receptor internalization. J Exp Med. 2004;199(7):981–991. doi: 10.1084/jem.20031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das V, Nal B, Dujeancourt A, Thoulouze MI, Galli T, Roux P, Dautry-Varsat A, Alcover A. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity. 2004;20(5):577–588. doi: 10.1016/s1074-7613(04)00106-2. [DOI] [PubMed] [Google Scholar]
- Das V, Nal B, Roumier A, Meas-Yedid V, Zimmer C, Olivo-Marin JC, Roux P, Ferrier P, Dautry-Varsat A, Alcover A. Membrane-cytoskeleton interactions during the formation of the immunological synapse and subsequent T-cell activation. Immunol Rev. 2002;189:123–135. doi: 10.1034/j.1600-065x.2002.18911.x. [DOI] [PubMed] [Google Scholar]
- Davis SJ, van der Merwe PA. The kinetic-segregation model: TCR triggering and beyond. Nat Immunol. 2006;7(8):803–809. doi: 10.1038/ni1369. [DOI] [PubMed] [Google Scholar]
- de Anda FC, Pollarolo G, Da Silva JS, Camoletto PG, Feiguin F, Dotti CG. Centrosome localization determines neuronal polarity. Nature. 2005;436(7051):704–708. doi: 10.1038/nature03811. [DOI] [PubMed] [Google Scholar]
- de Saint Basile G, Menasche G, Fischer A. Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat Rev Immunol. 2010;10(8):568–579. doi: 10.1038/nri2803. [DOI] [PubMed] [Google Scholar]
- Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113(Pt 19):3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- Dupont N, Chauhan S, Arko-Mensah J, Castillo EF, Masedunskas A, Weigert R, Robenek H, Proikas-Cezanne T, Deretic V. Neutral lipid stores and lipase PNPLA5 contribute to autophagosome biogenesis. Curr Biol. 2014;24(6):609–620. doi: 10.1016/j.cub.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci USA. 1997a;94(8):3909–3913. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Chakraborty AK, Shaw AS. Understanding the structure and func- tion of the immunological synapse. Cold Spring Harb Perspect Biol. 2010;2(10):a002311. doi: 10.1101/cshperspect.a002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Golan DE, Zhu DM, Miller JM, Meier W, Davies EA, van der Merwe PA. Low affinity interaction of human or rat T cell adhesion molecule CD2 with its ligand aligns adhering membranes to achieve high physiological affinity. J Biol Chem. 1997b;272(49):30889–30898. doi: 10.1074/jbc.272.49.30889. [DOI] [PubMed] [Google Scholar]
- Ebinu JO, Stang SL, Teixeira C, Bottorff DA, Hooton J, Blumberg PM, Barry M, Bleakley RC, Ostergaard HL, Stone JC. RasGRP links T-cell receptor sig- naling to Ras. Blood. 2000;95(10):3199–3203. [PubMed] [Google Scholar]
- Farhan H, Wendeler MW, Mitrovic S, Fava E, Silberberg Y, Sharan R, Zerial M, Hauri HP. MAPK signaling to the early secretory pathway revealed by kinase/ phosphatase functional screening. J Cell Biol. 2010;189(6):997–1011. doi: 10.1083/jcb.200912082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, Lambert N, Ouachee-Chardin M, Chedeville G, Tamary H, Minard-Colin V, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lym- phohistiocytosis (FHL3) Cell. 2003;115(4):461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- Fernandez-Arenas E, Calleja E, Martinez-Martin N, Gharbi SI, Navajas R, Garcia-Medel N, Penela P, Alcami A, Mayor F, Jr, Albar JP, Alarcon B. beta- Arrestin-1 mediates the TCR-triggered re-routing of distal receptors to the immunolog- ical synapse by a PKC-mediated mechanism. EMBO J. 2014;33(6):559–577. doi: 10.1002/embj.201386022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ulibarri I, Vilella M, Lazaro-Dieguez F, Sarri E, Martinez SE, Jimenez N, Claro E, Merida I, Burger KN, Egea G. Diacylglycerol is required for the formation of COPI vesicles in the Golgi-to-ER transport pathway. Mol Biol Cell. 2007;18(9):3250–3263. doi: 10.1091/mbc.E07-04-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbert EL, Le Borgne M, Lin J, Heuser JE, Shaw AS. Stathmin regulates microtubule dynamics and microtubule organizing center polarization in activated T cells. J Immunol. 2012;188(11):5421–5427. doi: 10.4049/jimmunol.1200242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finetti F, Onnis A, Baldari CT. Regulation of vesicular traffic at the T cell immune synapse: lessons from the primary cilium. Traffic. 2015a;16(3):241–249. doi: 10.1111/tra.12241. [DOI] [PubMed] [Google Scholar]
- Finetti F, Paccani SR, Riparbelli MG, Giacomello E, Perinetti G, Pazour GJ, Rosenbaum JL, Baldari CT. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat Cell Biol. 2009;11(11):1332–1339. doi: 10.1038/ncb1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finetti F, Patrussi L, Galgano D, Cassioli C, Perinetti G, Pazour GJ, Baldari CT. The small GTPase Rab8 interacts with VAMP-3 to regulate the delivery of recycling T-cell receptors to the immune synapse. J Cell Sci. 2015b;128(14):2541–2552. doi: 10.1242/jcs.171652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finetti F, Patrussi L, Masi G, Onnis A, Galgano D, Lucherini OM, Pazour GJ, Baldari CT. Specific recycling receptors are targeted to the immune synapse by the intraflagellar transport system. J Cell Sci. 2014;127(Pt 9):1924–1937. doi: 10.1242/jcs.139337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson A, Ruusala A, Aspenstrom P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem. 2003;278(8):6495–6502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- Friedl P, den Boer AT, Gunzer M. Tuning immune responses: diversity and adaptation of the immunological synapse. Nat Rev Immunol. 2005;5(7):532–545. doi: 10.1038/nri1647. [DOI] [PubMed] [Google Scholar]
- Fu MM, Holzbaur EL. Integrated regulation of motor-driven organelle transport by scaffolding proteins. Trends Cell Biol. 2014;24(10):564–574. doi: 10.1016/j.tcb.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL. Signaling domains of the interleukin 2 receptor. Cytokine. 2001;14(2):63–77. doi: 10.1006/cyto.2001.0862. [DOI] [PubMed] [Google Scholar]
- Gascoigne NR, Casas J, Brzostek J, Rybakin V. Initiation of TCR phosphorylation and signal transduction. Front Immunol. 2011;2:72. doi: 10.3389/fimmu.2011.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil D, Schamel WW, Montoya M, Sanchez-Madrid F, Alarcon B. Recruitment of Nck by CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109(7):901–912. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mito- chondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173(4):545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenring JR. Recycling endosomes. Curr Opin Cell Biol. 2015;35:117–122. doi: 10.1016/j.ceb.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TS, Hamann MJ, McCarney S, Savoy DN, Lubking CM, Heldebrant MP, Labno CM, McKean DJ, McNiven MA, Burkhardt JK, Billadeau DD. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nat Immunol. 2005;6(3):261–270. doi: 10.1038/ni1168. [DOI] [PubMed] [Google Scholar]
- Gomez TS, Kumar K, Medeiros RB, Shimizu Y, Leibson PJ, Billadeau DD. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity. 2007;26(2):177–190. doi: 10.1016/j.immuni.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Granado JM, Silvestre-Roig C, Rocha-Perugini V, Trigueros-Motos L, Cibrian D, Morlino G, Blanco-Berrocal M, Osorio FG, Freije JM, Lopez-Otin C, Sanchez-Madrid F, et al. Nuclear envelope lamin-A couples actin dynamics with immunological synapse architecture and T cell activation. Sci Signal. 2014;7(322):ra37. doi: 10.1126/scisignal.2004872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SJ, Booth AM, Hildreth JE. The Trojan exosome hypothesis. Proc Natl Acad Sci USA. 2003;100(19):10592–10597. doi: 10.1073/pnas.1831413100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer YE, Westlake CJ, Gao B, Bharti K, Shiba Y, Xavier CP, Pazour GJ, Yang Y, Rubin JS. Casein kinase 1delta functions at the centrosome and Golgi to promote ciliogenesis. Mol Biol Cell. 2014;25(10):1629–1640. doi: 10.1091/mbc.E13-10-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryev YA, Kurian SM, Hart T, Nakorchevsky AA, Chen C, Campbell D, Head SR, Yates JR, III, Salomon DR. MicroRNA regulation of molecular networks mapped by global microRNA, mRNA, and protein expression in activated T lymphocytes. J Immunol. 2011;187(5):2233–2243. doi: 10.4049/jimmunol.1101233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber T, Thuille N, Hermann-Kleiter N, Leitges M, Baier G. Protein kinase Cepsilon is dispensable for TCR/CD3-signaling. Mol Immunol. 2005;42(3):305–310. doi: 10.1016/j.molimm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Guntermann C, Alexander DR. CTLA-4 suppresses proximal TCR signaling in resting human CD4(+) T cells by inhibiting ZAP-70 Tyr(319) phosphorylation: a poten- tial role for tyrosine phosphatases. J Immunol. 2002;168(9):4420–4429. doi: 10.4049/jimmunol.168.9.4420. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Vazquez C, Villarroya-Beltri C, Mittelbrunn M, Sanchez-Madrid F. Transfer of extracellular vesicles during immune cell–cell interactions. Immunol Rev. 2013;251(1):125–142. doi: 10.1111/imr.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Tane A, Yokosuka T, Sakata-Sogawa K, Sakuma M, Ishihara C, Tokunaga M, Saito T. Dynein-driven transport of T cell receptor microclusters regulates immune synapse formation and T cell activation. Immunity. 2011;34(6):919–931. doi: 10.1016/j.immuni.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Hawkins ED, Oliaro J, Ramsbottom KM, Ting SB, Sacirbegovic F, Harvey M, Kinwell T, Ghysdael J, Johnstone RW, Humbert PO, Russell SM. Lethal giant larvae 1 tumour suppressor activity is not conserved in models of mammalian T and B cell leukaemia. PloS One. 2014;9(1):e87376. doi: 10.1371/journal.pone.0087376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay JC. SNARE complex structure and function. Exp Cell Res. 2001;271(1):10–21. doi: 10.1006/excr.2001.5368. [DOI] [PubMed] [Google Scholar]
- He HT, Marguet D. T-cell antigen receptor triggering and lipid rafts: a matter of space and time scales. Talking point on the involvement of lipid rafts in T-cell activation. EMBO Rep. 2008;9(6):525–530. doi: 10.1038/embor.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He MX, He YW. A role for c-FLIP(L) in the regulation of apoptosis, autophagy, and necroptosis in T lymphocytes. Cell Death Differ. 2013;20(2):188–197. doi: 10.1038/cdd.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelz A, Debler EW, Blobel G. The structure of the nuclear pore complex. Annu Rev Biochem. 2011;80:613–643. doi: 10.1146/annurev-biochem-060109-151030. [DOI] [PubMed] [Google Scholar]
- Hong W. Cytotoxic T lymphocyte exocytosis: bring on the SNAREs! Trends Cell Biol. 2005;15(12):644–650. doi: 10.1016/j.tcb.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Hsu VW, Lee SY, Yang JS. The evolving understanding of COPI vesicle formation. Nat Rev Mol Cell Biol. 2009;10(5):360–364. doi: 10.1038/nrm2663. [DOI] [PubMed] [Google Scholar]
- Huber LA, Teis D. Lysosomal signaling in control of degradation pathways. Curr Opin Cell Biol. 2016;39:8–14. doi: 10.1016/j.ceb.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol. 2008;20(1):4–11. doi: 10.1016/j.ceb.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M. Microtubule-organizing center polarity and the immunological synapse: protein kinase C and beyond. Front Immunol. 2012;3:235. doi: 10.3389/fimmu.2012.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M, Le Floc’h A, Liu X. From lipid second messengers to molecular motors: microtubule-organizing center eorientation in T cells. Immunol Rev. 2013;256(1):95–106. doi: 10.1111/imr.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7(3):247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- Huse M, Quann EJ, Davis MM. Shouts, whispers and the kiss of death: directional secretion in T cells. Nat Immunol. 2008;9(10):1105–1111. doi: 10.1038/ni.f.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutten S, Flotho A, Melchior F, Kehlenbach RH. The Nup358–RanGAP complex is required for efficient importin alpha/beta-dependent nuclear import. Mol Biol Cell. 2008;19(5):2300–2310. doi: 10.1091/mbc.E07-12-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibiza S, Perez-Rodriguez A, Ortega A, Martinez-Ruiz A, Barreiro O, Garcia-Dominguez CA, Victor VM, Esplugues JV, Rojas JM, Sanchez-Madrid F, Serrador JM. Endothelial nitric oxide synthase regulates N-Ras activation on the Golgi complex of antigen-stimulated T cells. Proc Natl Acad Sci USA. 2008;105(30):10507–10512. doi: 10.1073/pnas.0711062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibiza S, Victor VM, Bosca I, Ortega A, Urzainqui A, O’Connor JE, Sanchez- Madrid F, Esplugues JV, Serrador JM. Endothelial nitric oxide synthase regulates T cell receptor signaling at the immunological synapse. Immunity. 2006;24(6):753–765. doi: 10.1016/j.immuni.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Ilani T, Vasiliver-Shamis G, Vardhana S, Bretscher A, Dustin ML. T cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nat Immunol. 2009;10(5):531–539. doi: 10.1038/ni.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MR, Tsun A, Stinchcombe JC, Griffiths GM. The strength of T cell receptor signal controls the polarization of cytotoxic machinery to the immunological synapse. Immunity. 2009;31(4):621–631. doi: 10.1016/j.immuni.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Li C, Olive V, Lykken E, Feng F, Sevilla J, Wan Y, He L, Li QJ. Molecular dissection of the miR-17-92 cluster’s critical dual roles in promoting Th1 responses and preventing inducible Treg differentiation. Blood. 2011;118(20):5487–5497. doi: 10.1182/blood-2011-05-355644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi RP, Koretzky GA. Diacylglycerol kinases: regulated controllers of T cell activation, function, and development. Int J Mol Sci. 2013;14(4):6649–6673. doi: 10.3390/ijms14046649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HR, Song KH, Chang JT, Doh J. Geometrically controlled asymmetric division of CD4+ T cells studied by immunological synapse arrays. PloS One. 2014;9(3):e91926. doi: 10.1371/journal.pone.0091926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11(3):201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura D, Katsunuma K, Arima Y, Atsumi T, Jiang JJ, Bando H, Meng J, Sabharwal L, Stofkova A, Nishikawa N, Suzuki H, et al. KDEL receptor 1 regulates T-cell homeostasis via PP1 that is a key phosphatase for ISR. Nat Commun. 2015;6:7474. doi: 10.1038/ncomms8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara A, Scorrano L. Mitochondria: from cell death executioners to regulators of cell differentiation. Trends Cell Biol. 2014;24(12):761–770. doi: 10.1016/j.tcb.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Kienzle C, von Blume J. Secretory cargo sorting at the trans-Golgi network. Trends Cell Biol. 2014;24(10):584–593. doi: 10.1016/j.tcb.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Kikkawa M. Big steps toward understanding dynein. J Cell Biol. 2013;202(1):15–23. doi: 10.1083/jcb.201304099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, Liu R, Zhong Q, Guan KL. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152(1–2):290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CG, Koehli S, Hausmann B, Schmaler M, Zehn D, Palmer E. T cell affinity regulates asymmetric division, effector cell differentiation, and tissue pathology. Immunity. 2012;37(4):709–720. doi: 10.1016/j.immuni.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klecker T, Bockler S, Westermann B. Making connections: interorganelle contacts orchestrate mitochondrial behavior. Trends Cell Biol. 2014;24(9):537–545. doi: 10.1016/j.tcb.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132(4):583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Kodani A, Kristensen I, Huang L, Sutterlin C. GM130-dependent control of Cdc42 activity at the Golgi regulates centrosome organization. Mol Biol Cell. 2009;20(4):1192–1200. doi: 10.1091/mbc.E08-08-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong KF, Fu G, Zhang Y, Yokosuka T, Casas J, Canonigo-Balancio AJ, Becart S, Kim G, Yates JR, III, Kronenberg M, Saito T, et al. Protein kinase C-eta controls CTLA-4-mediated regulatory T cell function. Nat Immunol. 2014;15(5):465–472. doi: 10.1038/ni.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel MF, Davis MM. Dynamics of the immunological synapse: finding, establishing and solidifying a connection. Curr Opin Immunol. 2002;14(1):66–74. doi: 10.1016/s0952-7915(01)00299-0. [DOI] [PubMed] [Google Scholar]
- Kumar R, Ferez M, Swamy M, Arechaga I, Rejas MT, Valpuesta JM, Schamel WW, Alarcon B, van Santen HM. Increased sensitivity of antigen-experienced T cells through the enrichment of oligomeric T cell receptor complexes. Immunity. 2011;35(3):375–387. doi: 10.1016/j.immuni.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Kummerow C, Junker C, Kruse K, Rieger H, Quintana A, Hoth M. The immunological synapse controls local and global calcium signals in T lymphocytes. Immunol Rev. 2009;231(1):132–147. doi: 10.1111/j.1600-065X.2009.00811.x. [DOI] [PubMed] [Google Scholar]
- Kurowska M, Goudin N, Nehme NT, Court M, Garin J, Fischer A, de Saint Basile G, Menasche G. Terminal transport of lytic granules to the immune synapse is mediated by the kinesin-1/Slp3/Rab27a complex. Blood. 2012;119(17):3879–3889. doi: 10.1182/blood-2011-09-382556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lali FV, Crawley J, McCulloch DA, Foxwell BM. A late, prolonged activation of the phosphatidylinositol 3-kinase pathway is required for T cell proliferation. J Immunol. 2004;172(6):3527–3534. doi: 10.4049/jimmunol.172.6.3527. [DOI] [PubMed] [Google Scholar]
- Langhorst MF, Reuter A, Luxenhofer G, Boneberg EM, Legler DF, Plattner H, Stuermer CA. Preformed reggie/flotillin caps: stable priming platforms for macrodomain assembly in T cells. FASEB J. 2006;20(6):711–713. doi: 10.1096/fj.05-4760fje. [DOI] [PubMed] [Google Scholar]
- Larghi P, Williamson DJ, Carpier JM, Dogniaux S, Chemin K, Bohineust A, Danglot L, Gaus K, Galli T, Hivroz C. VAMP7 controls T cell activation by regulating the recruitment and phosphorylation of vesicular Lat at TCR-activation sites. Nat Immunol. 2013;14(7):723–731. doi: 10.1038/ni.2609. [DOI] [PubMed] [Google Scholar]
- Larocca MC, Shanks RA, Tian L, Nelson DL, Stewart DM, Goldenring JR. AKAP350 interaction with cdc42 interacting protein 4 at the Golgi apparatus. Mol Biol Cell. 2004;15(6):2771–2781. doi: 10.1091/mbc.E03-10-0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledderose C, Bao Y, Lidicky M, Zipperle J, Li L, Strasser K, Shapiro NI, Junger WG. Mitochondria are gate-keepers of T cell function by producing the ATP that drives purinergic signaling. J Biol Chem. 2014;289(37):25936–25945. doi: 10.1074/jbc.M114.575308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mito- phagy. J Cell Biol. 2010;189(4):671–679. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, Burack WR, Wu H, Wang J, Kanagawa O, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302(5648):1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- Li C, Capan E, Zhao Y, Zhao J, Stolz D, Watkins SC, Jin S, Lu B. Autophagy is induced in CD4+ T cells and important for the growth factor-withdrawal cell death. J Immunol. 2006;177(8):5163–5168. doi: 10.4049/jimmunol.177.8.5163. [DOI] [PubMed] [Google Scholar]
- Li D, Xie S, Ren Y, Huo L, Gao J, Cui D, Liu M, Zhou J. Microtubule- associated deacetylase HDAC6 promotes angiogenesis by regulating cell migration in an EB1-dependent manner. Protein Cell. 2011;2(2):150–160. doi: 10.1007/s13238-011-1015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010;11(1):90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engage- ment. Immunity. 1996;4(6):535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner RD. Brefeldin A’s effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67(3):601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Liu H, Schneider H, Recino A, Richardson C, Goldberg MW, Rudd CE. The immune adaptor SLP-76 binds to SUMO-RANGAP1 at nuclear pore complex filaments to regulate nuclear import of transcription factors in T cells. Mol Cell. 2015;59(5):840–849. doi: 10.1016/j.molcel.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Sawada T, Lee S, Yu W, Silverio G, Alapatt P, Millan I, Shen A, Saxton W, Kanao T, Takahashi R, et al. Parkinson’s disease-associ- ated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria. PLoS Genet. 2012;8(3):e1002537. doi: 10.1371/journal.pgen.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kapoor TM, Chen JK, Huse M. Diacylglycerol promotes centrosome polarization in T cells via reciprocal localization of dynein and myosin II. Proc Natl Acad Sci USA. 2013;110(29):11976–11981. doi: 10.1073/pnas.1306180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente A, de Marco MC, Alonso MA. Caveolin-1 and MAL are located on prostasomes secreted by the prostate cancer PC-3 cell line. J Cell Sci. 2004;117(Pt 22):5343–5351. doi: 10.1242/jcs.01420. [DOI] [PubMed] [Google Scholar]
- Lockyer HM, Tran E, Nelson BH. STAT5 is essential for Akt/p70S6 kinase activity during IL-2-induced lymphocyte proliferation. J Immunol. 2007;179(8):5301–5308. doi: 10.4049/jimmunol.179.8.5301. [DOI] [PubMed] [Google Scholar]
- Lopez JA, Susanto O, Jenkins MR, Lukoyanova N, Sutton VR, Law RH, Johnston A, Bird CH, Bird PI, Whisstock JC, Trapani JA, et al. Perforin forms transient pores on the target cell plasma membrane to facilitate rapid access of granzymes during killer cell attack. Blood. 2013;121(14):2659–2668. doi: 10.1182/blood-2012-07-446146. [DOI] [PubMed] [Google Scholar]
- Lord C, Ferro-Novick S, Miller EA. The highly conserved COPII coat complex sorts cargo from the endoplasmic reticulum and targets it to the Golgi. Cold Spring Harb Perspect Biol. 2013;5(2):a013367. doi: 10.1101/cshperspect.a013367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TX, Hartner J, Lim EJ, Fabry V, Mingler MK, Cole ET, Orkin SH, Aronow BJ, Rothenberg ME. MicroRNA-21 limits in vivo immune response-mediated activation of the IL-12/IFN-gamma pathway, Th1 polarization, and the severity of delayed-type hypersensitivity. J Immunol. 2011;187(6):3362–3373. doi: 10.4049/jimmunol.1101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M, Hua M, Li N, Yao H, Cao X. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma. Nat Immunol. 2011;12(9):861–869. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61(4):541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan R, Gerace L, Melchior F. Molecular characterization of the SUMO-1 modification of RanGAP1 and its role in nuclear envelope association. J Cell Biol. 1998;140(2):259–270. doi: 10.1083/jcb.140.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33(2):153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe L, Mark L, Fazilleau N, McHeyzer-Williams LJ, McHeyzer-Williams MG. Vaccine adjuvants alter TCR-based selection thresholds. Immunity. 2008;28(5):698–709. doi: 10.1016/j.immuni.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man K, Miasari M, Shi W, Xin A, Henstridge DC, Preston S, Pellegrini M, Belz GT, Smyth GK, Febbraio MA, Nutt SL, et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat Immunol. 2013;14(11):1155–1165. doi: 10.1038/ni.2710. [DOI] [PubMed] [Google Scholar]
- Marcenaro S, Gallo F, Martini S, Santoro A, Griffiths GM, Arico M, Moretta L, Pende D. Analysis of natural killer-cell function in familial hemophagocytic lymp- hohistiocytosis (FHL): defective CD107a surface expression heralds Munc13-4 defect and discriminates between genetic subtypes of the disease. Blood. 2006;108(7):2316–2323. doi: 10.1182/blood-2006-04-015693. [DOI] [PubMed] [Google Scholar]
- Martin-Cofreces NB, Alarcon B, Sanchez-Madrid F. Tubulin and actin interplay at the T cell and antigen-presenting cell interface. Front Immunol. 2011;2:24. doi: 10.3389/fimmu.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Cofreces NB, Baixauli F, Lopez MJ, Gil D, Monjas A, Alarcon B, Sanchez- Madrid F. End-binding protein 1 controls signal propagation from the T cell receptor. EMBO J. 2012;31(21):4140–4152. doi: 10.1038/emboj.2012.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Cofreces NB, Baixauli F, Sanchez-Madrid F. Immune synapse: conductor of orchestrated organelle movement. Trends Cell Biol. 2014;24(1):61–72. doi: 10.1016/j.tcb.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Cofreces NB, Robles-Valero J, Cabrero JR, Mittelbrunn M, Gordon- Alonso M, Sung CH, Alarcon B, Vazquez J, Sanchez-Madrid F. MTOC translocation modulates IS formation and controls sustained T cell signaling. J Cell Biol. 2008;182(5):951–962. doi: 10.1083/jcb.200801014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Cofreces NB, Sancho D, Fernandez E, Vicente-Manzanares M, Gordon-Alonso M, Montoya MC, Michel F, Acuto O, Alarcon B, Sanchez-Madrid F. Role of Fyn in the rearrangement of tubulin cytoskeleton induced through TCR. J Immunol. 2006;176(7):4201–4207. doi: 10.4049/jimmunol.176.7.4201. [DOI] [PubMed] [Google Scholar]
- Martinez-Martin N, Fernandez-Arenas E, Cemerski S, Delgado P, Turner M, Heuser J, Irvine DJ, Huang B, Bustelo XR, Shaw A, Alarcon B. T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity. 2011;35(2):208–222. doi: 10.1016/j.immuni.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzeo C, Calvo V, Alonso R, Merida I, Izquierdo M. Protein kinase D1/2 is involved in the maturation of multivesicular bodies and secretion of exosomes in T and B lymphocytes. Cell Death Differ. 2016;23(1):99–109. doi: 10.1038/cdd.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menager MM, Menasche G, Romao M, Knapnougel P, Ho CH, Garfa M, Raposo G, Feldmann J, Fischer A, de Saint Basile G. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat Immunol. 2007;8(3):257–267. doi: 10.1038/ni1431. [DOI] [PubMed] [Google Scholar]
- Menasche G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, de Saint Basile G. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000;25(2):173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- Mentlik AN, Sanborn KB, Holzbaur EL, Orange JS. Rapid lytic granule convergence to the MTOC in natural killer cells is dependent on dynein but not cytolytic commitment. Mol Biol Cell. 2010;21(13):2241–2256. doi: 10.1091/mbc.E09-11-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergen M, Engel C, Muller B, Follo M, Schafer T, Jung M, Walz G. The nephronophthisis gene product NPHP2/Inversin interacts with Aurora A and interferes with HDAC6-mediated cilia disassembly. Nephrol Dial Transplant. 2013;28(11):2744–2753. doi: 10.1093/ndt/gft316. [DOI] [PubMed] [Google Scholar]
- Metz PJ, Arsenio J, Kakaradov B, Kim SH, Remedios KA, Oakley K, Akimoto K, Ohno S, Yeo GW, Chang JT. Regulation of asymmetric division and CD8+ T lymphocyte fate specification by protein kinase Czeta and protein kinase Clambda/iota. J Immunol. 2015;194(5):2249–2259. doi: 10.4049/jimmunol.1401652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills PB, Surtees RA, Champion MP, Beesley CE, Dalton N, Scambler PJ, Heales SJ, Briddon A, Scheimberg I, Hoffmann GF, Zschocke J, et al. Neonatal epileptic encephalopathy caused by mutations in the PNPO gene encoding pyridox(am)ine 50 -phosphate oxidase. Hum Mol Genet. 2005;14(8):1077–1086. doi: 10.1093/hmg/ddi120. [DOI] [PubMed] [Google Scholar]
- Mishra P, Chan DC. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol. 2014;15(10):634–646. doi: 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M, Molina A, Escribese MM, Yanez-Mo M, Escudero E, Ursa A, Tejedor R, Mampaso F, Sanchez-Madrid F. VLA-4 integrin concentrates at the peripheral supramolecular activation complex of the immune synapse and drives T helper 1 responses. Proc Natl Acad Sci USA. 2004;101(30):11058–11063. doi: 10.1073/pnas.0307927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M, Sanchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13(5):328–335. doi: 10.1038/nrm3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monjas A, Alcover A, Alarcon B. Engaged and bystander T cell receptors are down-modulated by different endocytotic pathways. J Biol Chem. 2004;279(53):55376–55384. doi: 10.1074/jbc.M409342200. [DOI] [PubMed] [Google Scholar]
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimen- sional segregation of supramolecular activation clusters in T cells. Nature. 1998;395(6697):82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Monleon I, Martinez-Lorenzo MJ, Monteagudo L, Lasierra P, Taules M, Iturralde M, Pineiro A, Larrad L, Alava MA, Naval J, Anel A. Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J Immunol. 2001;167(12):6736–6744. doi: 10.4049/jimmunol.167.12.6736. [DOI] [PubMed] [Google Scholar]
- Montegna EA, Bhave M, Liu Y, Bhattacharyya D, Glick BS. Sec12 binds to Sec16 at transitional ER sites. PloS One. 2012;7(2):e31156. doi: 10.1371/journal.pone.0031156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya MC, Sancho D, Vicente-Manzanares M, Sanchez-Madrid F. Cell adhesion and polarity during immune interactions. Immunol Rev. 2002;186:68–82. doi: 10.1034/j.1600-065x.2002.18607.x. [DOI] [PubMed] [Google Scholar]
- Morlino G, Barreiro O, Baixauli F, Robles-Valero J, Gonzalez-Granado JM, VillaBellosta R, Cuenca J, Sanchez-Sorzano CO, Veiga E, Martin-Cofreces NB, Sanchez-Madrid F. Miro-1 links mitochondria and microtubule Dynein motors to control lymphocyte migration and polarity. Mol Cell Biol. 2014;34(8):1412–1426. doi: 10.1128/MCB.01177-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman KD, Campi G, Groves JT, Dustin ML. Altered TCR signaling from geometrically repatterned immunological synapses. Science. 2005;310(5751):1191–1193. doi: 10.1126/science.1119238. [DOI] [PubMed] [Google Scholar]
- Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D’Souza-Schorey C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19(22):1875–1885. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison EP, Hannon GJ. miRNAs on the move: miRNA biogenesis and the RNAi machinery. Curr Opin Cell Biol. 2004;16(3):223–229. doi: 10.1016/j.ceb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci USA. 2012;109(11):4146–4151. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naramura M, Jang IK, Kole H, Huang F, Haines D, Gu H. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat Immunol. 2002;3(12):1192–1199. doi: 10.1038/ni855. [DOI] [PubMed] [Google Scholar]
- Narendra D, Walker JE, Youle R. Mitochondrial quality control mediated by PINK1 and Parkin: links to parkinsonism. Cold Spring Harb. Perspect Biol. 2012;4(11):a011338. doi: 10.1101/cshperspect.a011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro MN, Cantrell DA. Serine-threonine kinases in TCR signaling. Nat Immunol. 2014;15(9):808–814. doi: 10.1038/ni.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro MN, Feijoo-Carnero C, Arandilla AG, Trost M, Cantrell DA. Protein kinase D2 is a digital amplifier of T cell receptor-stimulated diacylglycerol signaling in naive CD8(+) T cells. Sci Signal. 2014;7(348):ra99. doi: 10.1126/scisignal.2005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro MN, Sinclair LV, Feijoo-Carnero C, Clarke R, Matthews SA, Cantrell DA. Protein kinase D2 has a restricted but critical role in T-cell antigen receptor signalling in mature T-cells. Biochem J. 2012;442(3):649–659. doi: 10.1042/BJ20111700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422(6933):766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab. 2010;298(3):E395–E402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DG, Booth A, Gould SJ, Hildreth JE. Evidence that HIV budding in primary macrophages occurs through the exosome release pathway. J Biol Chem. 2003;278(52):52347–52354. doi: 10.1074/jbc.M309009200. [DOI] [PubMed] [Google Scholar]
- Nickel W, Malsam J, Gorgas K, Ravazzola M, Jenne N, Helms JB, Wieland FT. Uptake by COPI-coated vesicles of both anterograde and retrograde cargo is inhibited by GTPgammaS in vitro. J Cell Sci. 1998;111(Pt 20):3081–3090. doi: 10.1242/jcs.111.20.3081. [DOI] [PubMed] [Google Scholar]
- Nicolson GL. The Fluid-Mosaic model of membrane structure: still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim Biophys Acta. 2014;1838(6):1451–1466. doi: 10.1016/j.bbamem.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Nunez-Andrade N, Iborra S, Trullo A, Moreno-Gonzalo O, Calvo E, Catalan E, Menasche G, Sancho D, Vazquez J, Yao TP, Martin-Cofreces NB, et al. HDAC6 regulates the dynamics of lytic granules in cytotoxic T lymphocytes. J Cell Sci. 2016;129(7):1305–1311. doi: 10.1242/jcs.180885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33(4):607–619. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obino D, Farina F, Malbec O, Saez PJ, Maurin M, Gaillard J, Dingli F, Loew D, Gautreau A, Yuseff MI, Blanchoin L, et al. Actin nucleation at the centrosome controls lymphocyte polarity. Nat Commun. 2016;7:10969. doi: 10.1038/ncomms10969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliaro J, Van Ham V, Sacirbegovic F, Pasam A, Bomzon Z, Pham K, Ludford-Menting MJ, Waterhouse NJ, Bots M, Hawkins ED, Watt SV, et al. Asymmetric cell division of T cells upon antigen presentation uses multiple conserved mechanisms. J Immunol. 2010;185(1):367–375. doi: 10.4049/jimmunol.0903627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owada T, Watanabe N, Oki M, Oya Y, Saito Y, Saito T, Iwamoto I, Murphy TL, Murphy KM, Nakajima H. Activation-induced accumulation of B and T lymphocyte attenuator at the immunological synapse in CD4+ T cells. J Leukoc Biol. 2010;87(3):425–432. doi: 10.1189/jlb.0309138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachlopnik Schmid J, Cote M, Menager MM, Burgess A, Nehme N, Menasche G, Fischer A, de Saint Basile G. Inherited defects in lymphocyte cytotoxic activity. Immunol Rev. 2010;235(1):10–23. doi: 10.1111/j.0105-2896.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- Park IW, He JJ. HIV-1 is budded from CD4+ T lymphocytes independently of exosomes. Virol J. 2010;7:234. doi: 10.1186/1743-422X-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Yang JS, Schmider AB, Soberman RJ, Hsu VW. Coordinated regulation of bidirectional COPI transport at the Golgi by CDC42. Nature. 2015;521(7553):529–532. doi: 10.1038/nature14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson-Lawrence EJ, Shandala T, Prodoehl M, Plew R, Borlace GN, Brooks DA. Lysosomal storage disease: revealing lysosomal function and physiology. Physiology (Bethesda) 2010;25(2):102–115. doi: 10.1152/physiol.00041.2009. [DOI] [PubMed] [Google Scholar]
- Pauker MH, Hassan N, Noy E, Reicher B, Barda-Saad M. Studying the dynamics of SLP-76, Nck, and Vav1 multimolecular complex formation in live human cells with triple-color FRET. Sci Signal. 2012;5(221):rs3. doi: 10.1126/scisignal.2002423. [DOI] [PubMed] [Google Scholar]
- Paul S, Schaefer BC. Selective autophagy regulates T cell activation. Autophagy. 2012;8(11):1690–1692. doi: 10.4161/auto.21581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quies- cence. Immunity. 2013;38(4):633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkurnaz G, Trinidad JC, Wang X, Kong D, Schwarz TL. Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase. Cell. 2014;158(1):54–68. doi: 10.1016/j.cell.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipkin ME, Lieberman J. Delivering the kiss of death: progress on understanding how perforin works. Curr Opin Immunol. 2007;19(3):301–308. doi: 10.1016/j.coi.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Podshivalova K, Salomon DR. MicroRNA regulation of T-lymphocyte immunity: modulation of molecular networks responsible for T-cell activation, differentiation, and development. Crit Rev Immunol. 2013;33(5):435–476. doi: 10.1615/critrevimmunol.2013006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pua HH, Guo J, Komatsu M, He YW. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J Immunol. 2009;182(7):4046–4055. doi: 10.4049/jimmunol.0801143. [DOI] [PubMed] [Google Scholar]
- Purbhoo MA, Liu H, Oddos S, Owen DM, Neil MA, Pageon SV, French PM, Rudd CE, Davis DM. Dynamics of subsynaptic vesicles and surface microclusters at the immunological synapse. Sci Signal. 2010;3(121):ra36. doi: 10.1126/scisignal.2000645. [DOI] [PubMed] [Google Scholar]
- Puri C, Renna M, Bento CF, Moreau K, Rubinsztein DC. Diverse autophagosome membrane sources coalesce in recycling endosomes. Cell. 2013;154(6):1285–1299. doi: 10.1016/j.cell.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quann EJ, Liu X, Altan-Bonnet G, Huse M. A cascade of protein kinase C isozymes promotes cytoskeletal polarization in T cells. Nat Immunol. 2011;12(7):647–654. doi: 10.1038/ni.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quann EJ, Merino E, Furuta T, Huse M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol. 2009;10(6):627–635. doi: 10.1038/ni.1734. [DOI] [PubMed] [Google Scholar]
- Quimby BB, Dasso M. The small GTPase Ran: interpreting the signs. Curr Opin Cell Biol. 2003;15(3):338–344. doi: 10.1016/s0955-0674(03)00046-2. [DOI] [PubMed] [Google Scholar]
- Quintana A, Pasche M, Junker C, Al-Ansary D, Rieger H, Kummerow C, Nunez L, Villalobos C, Meraner P, Becherer U, Rettig J. Calcium microdomains at the immunological synapse: how ORAI channels, mitochondria and calcium pumps generate local calcium signals for efficient T-cell activation. EMBO J. 2011;30(19):3895–3912. doi: 10.1038/emboj.2011.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Schwindling C, Wenning AS, Becherer U, Rettig J, Schwarz EC, Hoth M. T cell activation requires mitochondrial translocation to the immunological synapse. Proc Natl Acad Sci USA. 2007;104(36):14418–14423. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran L, Beckmann J, Magenau A, Boneberg EM, Gaus K, Viola A, Giebel B, Illges H. Flotillins are involved in the polarization of primitive and mature hematopoietic cells. PloS One. 2009;4(12):e8290. doi: 10.1371/journal.pone.0008290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter AT, Asano Y, Stinchcombe JC, Dieckmann NM, Chen BC, Gawden-Bone C, van Engelenburg S, Legant W, Gao L, Davidson MW, Betzig E, et al. Actin depletion initiates events leading to granule secretion at the immunological synapse. Immunity. 2015;42(5):864–876. doi: 10.1016/j.immuni.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero S, Cardenas J, Bornens M, Rios RM. Microtubule nucleation at the cis- side of the Golgi apparatus requires AKAP450 and GM130. EMBO J. 2009;28(8):1016–1028. doi: 10.1038/emboj.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Valero J, Martin-Cofreces NB, Lamana A, Macdonald S, Volkov Y, Sanchez-Madrid F. Integrin and CD3/TCR activation are regulated by the scaffold protein AKAP450. Blood. 2010;115(21):4174–4184. doi: 10.1182/blood-2009-12-256222. [DOI] [PubMed] [Google Scholar]
- Rocha-Perugini V, Gonzalez-Granado JM, Tejera E, Lopez-Martin S, Yanez-Mo M, Sanchez-Madrid F. Tetraspanins CD9 and CD151 at the immune synapse support T-cell integrin signaling. Eur J Immunol. 2014;44(7):1967–1975. doi: 10.1002/eji.201344235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Perugini V, Sanchez-Madrid F, Martinez Del Hoyo G. Function and dynamics of tetraspanins during antigen recognition and immunological synapse formation. Front Immunol. 2015;6:653. doi: 10.3389/fimmu.2015.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Perugini V, Zamai M, Gonzalez-Granado JM, Barreiro O, Tejera E, Yanez- Mo M, Caiolfa VR, Sanchez-Madrid F. CD81 controls sustained T cell activation signaling and defines the maturation stages of cognate immunological synapses. Mol Cell Biol. 2013;33(18):3644–3658. doi: 10.1128/MCB.00302-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers W, Crise B, Rose JK. Signals determining protein tyrosine kinase and glycosyl-phosphatidylinositol-anchored protein targeting to a glycolipid-enriched mem-brane fraction. Mol Cell Biol. 1994;14(8):5384–5391. doi: 10.1128/mcb.14.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo Y, Zhang X, Roux PP. Regulation and function of the RSK family of protein kinases. Biochem J. 2012;441(2):553–569. doi: 10.1042/BJ20110289. [DOI] [PubMed] [Google Scholar]
- Rowland AA, Voeltz GK. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol. 2012;13(10):607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, Kim H, Neufeld TP, Dillin A, Guan KL. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15(7):741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10(9):623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3(4):269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- Schonle A, Hartl FA, Mentzel J, Noltner T, Rauch KS, Prestipino A, Wohlfeil SA, Apostolova P, Hechinger AK, Melchinger W, Fehrenbach K, et al. Caveolin-1 regulates TCR signal strength and regulatory T cell differentiation into alloreactive T cells. Blood. 2016;127:1930–1939. doi: 10.1182/blood-2015-09-672428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder JM, Larsen J, Komarova Y, Akhmanova A, Thorsteinsson RI, Grigoriev I, Manguso R, Christensen ST, Pedersen SF, Geimer S, Pedersen LB. EB1 and EB3 promote cilia biogenesis by several centrosome-related mechanisms. J Cell Sci. 2011;124(Pt 15):2539–2551. doi: 10.1242/jcs.085852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda FE, Burgess A, Heiligenstein X, Goudin N, Menager MM, Romao M, Cote M, Mahlaoui N, Fischer A, Raposo G, Menasche G, et al. LYST controls the biogenesis of the endosomal compartment required for secretory lysosome function. Traffic. 2015;16(2):191–203. doi: 10.1111/tra.12244. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Cabrero JR, Sancho D, Mittelbrunn M, Urzainqui A, Sanchez-Madrid F. HDAC6 deacetylase activity links the tubulin cytoskeleton with immune synapse organization. Immunity. 2004;20(4):417–428. doi: 10.1016/s1074-7613(04)00078-0. [DOI] [PubMed] [Google Scholar]
- Sillibourne JE, Milne DM, Takahashi M, Ono Y, Meek DW. Centrosomal anchoring of the protein kinase CK1delta mediated by attachment to the large, coiled-coil scaffolding protein CG-NAP/AKAP450. J Mol Biol. 2002;322(4):785–797. doi: 10.1016/s0022-2836(02)00857-4. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Soares H, Henriques R, Sachse M, Ventimiglia L, Alonso MA, Zimmer C, Thoulouze MI, Alcover A. Regulated vesicle fusion generates signaling nano- territories that control T cell activation at the immunological synapse. J Exp Med. 2013a;210(11):2415–2433. doi: 10.1084/jem.20130150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares H, Lasserre R, Alcover A. Orchestrating cytoskeleton and intracellular vesicle traffic to build functional immunological synapses. Immunol Rev. 2013b;256(1):118–132. doi: 10.1111/imr.12110. [DOI] [PubMed] [Google Scholar]
- Stanley RE, Ragusa MJ, Hurley JH. The beginning of the end: how scaffolds nucleate autophagosome biogenesis. Trends Cell Biol. 2014;24(1):73–81. doi: 10.1016/j.tcb.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol. 2010;26:421–444. doi: 10.1146/annurev-cellbio-100109-104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe JC, Barral DC, Mules EH, Booth S, Hume AN, Machesky LM, Seabra MC, Griffiths GM. Rab27a is required for regulated secretion in cyto- toxic T lymphocytes. J Cell Biol. 2001;152(4):825–834. doi: 10.1083/jcb.152.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe JC, Griffiths GM. Secretory mechanisms in cell-mediated cytotox- icity. Annu Rev Cell Dev Biol. 2007;23:495–517. doi: 10.1146/annurev.cellbio.23.090506.123521. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443(7110):462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- Thaunat O, Batista FD. Daughter B cells are not created equal: asymmetric segre- gation of antigen during B cell division. Cell Cycle. 2012;11(12):2219–2220. doi: 10.4161/cc.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassian T, Humphries LA, Liu SD, Silva O, Brooks DG, Miceli C. Caveolin-1 orchestrates TCR synaptic polarity, signal specificity, and function in CD8 T cells. J Immunol. 2011;187(6):2993–3002. doi: 10.4049/jimmunol.1101447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze SA, Yoshimori T. The origin of the autophagosomal membrane. Nat Cell Biol. 2010;12(9):831–835. doi: 10.1038/ncb0910-831. [DOI] [PubMed] [Google Scholar]
- Tsun A, Qureshi I, Stinchcombe JC, Jenkins MR, de la Roche M, Kleczkowska J, Zamoyska R, Griffiths GM. Centrosome docking at the immunological synapse is controlled by Lck signaling. J Cell Biol. 2011;192(4):663–674. doi: 10.1083/jcb.201008140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Morphew MK, McIntosh JR, Davis MM. CD4+ T-cell synapses involve multiple distinct stages. Proc Natl Acad Sci USA. 2011;108(41):17099–17104. doi: 10.1073/pnas.1113703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Vardhana S, Choudhuri K, Varma R, Dustin ML. Essential role of ubiquitin and TSG101 protein in formation and function of the central supramolecular activation cluster. Immunity. 2010;32(4):531–540. doi: 10.1016/j.immuni.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramo- lecular activation cluster. Immunity. 2006;25(1):117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. The ubiquitin system, an immense realm. Annu Rev Biochem. 2012;81:167–176. doi: 10.1146/annurev-biochem-051910-094049. [DOI] [PubMed] [Google Scholar]
- Venditti R, Wilson C, De Matteis MA. Exiting the ER: what we know and what we don’t. Trends Cell Biol. 2014;24(1):9–18. doi: 10.1016/j.tcb.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Sanchez-Madrid F. Role of the cytoskeleton during leukocyte responses. Nat Rev Immunol. 2004;4(2):110–122. doi: 10.1038/nri1268. [DOI] [PubMed] [Google Scholar]
- Villarroya-Beltri C, Baixauli F, Gutierrez-Vazquez C, Sanchez-Madrid F, Mittelbrunn M. Sorting it out: regulation of exosome loading. Semin Cancer Biol. 2014;28:3–13. doi: 10.1016/j.semcancer.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sanchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, Magrane J, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA. 2010;107(1):378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Rickert M, Garcia KC. Structure of the quaternary complex of inter- leukin-2 with its alpha, beta, and gammac receptors. Science. 2005;310(5751):1159–1163. doi: 10.1126/science.1117893. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, Murphy TL, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4(7):670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- Weihofen A, Thomas KJ, Ostaszewski BL, Cookson MR, Selkoe DJ. Pink1 forms a multiprotein complex with Miro and Milton, linking Pink1 function to mito- chondrial trafficking. Biochemistry. 2009;48(9):2045–2052. doi: 10.1021/bi8019178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle JR, Schwartz TU. Structure of the Sec13-Sec16 edge element, a template for assembly of the COPII vesicle coat. J Cell Biol. 2010;190(3):347–361. doi: 10.1083/jcb.201003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SM, Meeths M, Chiang SC, Bechensteen AG, Boelens JJ, Heilmann C, Horiuchi H, Rosthoj S, Rutynowska O, Winiarski J, Stow JL, et al. Different NK cell-activating receptors preferentially recruit Rab27a or Munc13-4 to perforin-containing granules for cytotoxicity. Blood. 2009;114(19):4117–4127. doi: 10.1182/blood-2009-06-225359. [DOI] [PubMed] [Google Scholar]
- Xie Y, Zhang H, Li W, Deng Y, Munegowda MA, Chibbar R, Qureshi M, Xiang J. Dendritic cells recruit T cell exosomes via exosomal LFA-1 leading to inhibition of CD8+ CTL responses through downregulation of peptide/MHC class I and Fas ligand-mediated cytotoxicity. J Immunol. 2010;185(9):5268–5278. doi: 10.4049/jimmunol.1000386. [DOI] [PubMed] [Google Scholar]
- Xu X, Araki K, Li S, Han JH, Ye L, Tan WG, Konieczny BT, Bruinsma MW, Martinez J, Pearce EL, Green DR, et al. Autophagy is essential for effector CD8(+) T cell survival and memory formation. Nature Immunol. 2014;15(12):1152–1161. doi: 10.1038/ni.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q, Guo ZY, Li W, Wen WH, Meng YL, Jia LT, Wang J, Yao LB, Jin BQ, Wang T, Yang AG. Human activated CD4(+) T lymphocytes increase IL-2 expression by downregulating microRNA-181c. Mol Immunol. 2011;48(4):592–599. doi: 10.1016/j.molimm.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Boukari H, Banerjee I, Sbalzarini IF, Horvath P, Helenius A. Histone deacetylase 8 is required for centrosome cohesion and influenza A virus entry. PLoS Pathog. 2011;7(10):e1002316. doi: 10.1371/journal.ppat.1002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19(9):434–446. doi: 10.1016/j.tcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka T, Kobayashi W, Takamatsu M, Sakata-Sogawa K, Zeng H, Hashimoto- Tane A, Yagita H, Tokunaga M, Saito T. Spatiotemporal basis of CTLA-4 costimulatory molecule-mediated negative regulation of T cell activation. Immunity. 2010;33(3):326–339. doi: 10.1016/j.immuni.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12(1):9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. COPII and the regulation of protein sorting in mammals. Nat Cell Biol. 2012;14(1):20–28. doi: 10.1038/ncb2390. [DOI] [PubMed] [Google Scholar]
- Zhang W, Trible RP, Samelson LE. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9(2):239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- Zhao F, Zhang J, Liu YS, Li L, He YL. Research advances on flotillins. Virol J. 2011;8:479. doi: 10.1186/1743-422X-8-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XP, Guo R, Zhou H, Liu C, Wan CK. Diacylglycerol kinases in immune cell function and self-tolerance. Immunol Rev. 2008;224:249–264. doi: 10.1111/j.1600-065X.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnikov-Klionsky Y, Nathansohn-Levi B, Shezen E, Rosen C, Kagan S, Bar-On L, Jung S, Shifrut E, Reich-Zeliger S, Friedman N, Aharoni R, et al. Perforin-positive dendritic cells exhibit an immuno-regulatory role in metabolic syndrome and autoimmunity. Immunity. 2015;43(4):776–87. doi: 10.1016/j.immuni.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Zur Stadt U, Beutel K, Kolberg S, Schneppenheim R, Kabisch H, Janka G, Hennies HC. Mutation spectrum in children with primary hemophagocytic lymphohistiocytosis: molecular and functional analyses of PRF1, UNC13D, STX11, and RAB27A. Hum Mutat. 2006;27(1):62–68. doi: 10.1002/humu.20274. [DOI] [PubMed] [Google Scholar]
- Zur Stadt U, Rohr J, Seifert W, Koch F, Grieve S, Pagel J, Strauss J, Kasper B, Nurnberg G, Becker C, Maul-Pavicic A, et al. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. Am J Hum Genet. 2009;85(4):482–492. doi: 10.1016/j.ajhg.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyss D, Ebrahimi H, Gergely F. Casein kinase I delta controls centrosome positioning during T cell activation. J Cell Biol. 2011;195(5):781–797. doi: 10.1083/jcb.201106025. [DOI] [PMC free article] [PubMed] [Google Scholar]