Abstract
Genus Xanthomonas comprises many economically important plant pathogens that affect a wide range of hosts. Indeed, fourteen Xanthomonas species/pathovars have been regarded as official quarantine bacteria for imports in China. To date, however, a rapid and accurate method capable of identifying all of the quarantine species/pathovars has yet to be developed. In this study, we therefore evaluated the capacity of DNA barcoding as a digital identification method for discriminating quarantine species/pathovars of Xanthomonas. For these analyses, 327 isolates, representing 45 Xanthomonas species/pathovars, as well as five additional species/pathovars from GenBank (50 species/pathovars total), were utilized to test the efficacy of four DNA barcode candidate genes (16S rRNA gene, cpn60, gyrB, and avrBs2). Of these candidate genes, cpn60 displayed the highest rate of PCR amplification and sequencing success. The tree-building (Neighbor-joining), ‘best close match’, and barcode gap methods were subsequently employed to assess the species- and pathovar-level resolution of each gene. Notably, all isolates of each quarantine species/pathovars formed a monophyletic group in the neighbor-joining tree constructed using the cpn60 sequences. Moreover, cpn60 also demonstrated the most satisfactory results in both barcoding gap analysis and the ‘best close match’ test. Thus, compared with the other markers tested, cpn60 proved to be a powerful DNA barcode, providing a reliable and effective means for the species- and pathovar-level identification of the quarantine plant pathogen Xanthomonas.
Introduction
The bacterial genus Xanthomonas comprises a large number of plant pathogens that are responsible for diseases of many economically important crops, including citrus, cassava, mangos, bananas, rice, wheat, sugarcane, beans, cruciferous vegetables, and many others. Notably, of the known species/pathovars of Xanthomonas, 14 are considered quarantine bacteria in China: X. albilineans, X. arboricola pv. celebensis, X. axonopodis pv. betlicola, X. axonopodis pv. citri, X. axonopodis pv. manihotis, X. axonopodis pv. vasculorum, X. campestris pv. mangiferaeindicae, X. campestris pv. musacearum, X. cassavae, X. fragariae, X. hyacinthi, X. oryzae pv. oryzae, X. oryzae pv. oryzicola, and X. populi. These quarantine species comprise a serious threat to the production of fruits and other commercially important food crops, as they are highly destructive to their respective host plants. Moreover, several of the aforementioned species/pathovars have been included in the European and Mediterranean Plant Protection Organization (EPPO) Pest Lists or are regarded as quarantine pests on a global scale. For these reasons, they are rigorously monitored by quarantine authorities, although the complexity of the genus makes it difficult to resolve this genus at the species or pathovar level. Indeed, while several methods, including isolation on semi-selective media, serology, and PCR-based methods, have been developed to identify these pathogens, the majority of these approaches are capable of identifying only one or two species/pathovars, and therefore do not have much versatility. As such, the objective of this study was to establish a new method for the efficient identification of all quarantine species and pathovars of Xanthomonas.
DNA barcoding is a molecular technique that was developed as a rapid and practical method for identifying organisms at the species level using specific DNA sequences [1]. Indeed, this technique has already been utilized for the molecular identification of multiple animal and plant species [2,3,4,5,6]. Currently, the identification of bacterial species is most commonly achieved via sequencing of the 16S rRNA gene, which encodes one of the structural RNA molecules in the small ribosomal subunit. Despite the positive features that have led to its preference as the barcode gene for bacteria, 16S rRNA gene sequencing often fails to provide sufficient information for species-level identification [7]. Therefore, several other candidate markers have been proposed, including DNA gyrase subunit B (gyrB), RNA polymerase genes (rpoB, rpoD, etc.), heat shock protein-related genes (cpn60, hsp70, etc.), and the bacterial DNA recombination protein gene recA, as have various combinations of markers. An ideal gene locus that meets the barcode criteria and is suitable for the identification and differentiation of microorganisms would be a powerful tool for differentiation of bacterial species/pathovars.
Genus Xanthomonas has been the subject of numerous taxonomical and phylogenetic studies, and the species-level classification of these organisms has been based largely on DNA–DNA hybridization, fatty acid profiling, repetitive element palindromic (rep)-PCR, and sequencing analysis of the 16S rRNA, dnaK, fyuA, gyrB, and rpoD genes, etc. [8,9,10,11]. However, species- and pathovar-level identification of Xanthomonas remains a significant challenge for diagnostic laboratories. The term pathovar is used to refer to a strain or set of strains with the same or similar characteristics, differentiated at infrasubspecific level from other strains of the same species or subspecies on the basis of distinctive pathogenicity to one or more plant hosts. Usually, pathovars are distinguished in terms of proved differences in host range [12]. However, Hajri et al. (2009) [13] found that there is a correlation between the composition of type III secretion system effector (T3E) repertoires and pathovars of X. axonopodis. Therefore, ‘Developing DNA barcode identification for Q-organisms Quarantine’ (QBOL, http://www.qbol.org/en/qbol.htm) has recommended that a ribosomal RNA gene (16S rRNA gene), a DNA gyrase gene (gyrB), and a T3E gene (avrBs2) be utilized as core barcoding genes for Xanthomonas. While cpn60, which encodes the 60 kDa chaperonin protein of bacteria and eukaryotes, is also considered an effective barcode gene for bacteria [14], there is little information to indicate whether this gene is suitable for barcoding of Xanthomonas.
Therefore, in this study, we investigated the DNA barcoding of quarantine species and pathovars of Xanthomonas in the context of biosecurity. Here, we selected the 16S rRNA gene, gyrB, avrBs2, and cpn60 as candidate barcode genes. The primary objectives of the study were (1) to determine the universality of the primers used and quantify their amplification and sequencing success rates, and (2) to test the effectiveness of these DNA barcode candidates for species- and pathovar-level identification. The results of these analyses could provide a new digital identification method for the quarantine plant pathogen Xanthomonas.
Materials and Methods
Bacterial strains
All Xanthomonas strains used in this study were obtained from international culture collections [including the American Type Culture Collection (ATCC); National Collection of Plant Pathogenic Bacteria (NCPPB); Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ); International Collection Of Micro-organisms (ICMP); and Laboratory of Microbiology Gent Bacteria Collection (BCCM/LMG)], or were provided by research scientists from universities and research institutes in China (S1 Table). In total, 327 strains, comprising 45 species or pathovars and representing the majority of important Xanthomonas species, were collected for use in this study. All strains were routinely cultivated in nutrient broth (NB) at 28°C.
DNA extraction, amplification, and sequencing
DNA was prepared from suspensions of freshly grown Xanthomonas cultures by boiling in a water bath for 10 min, followed by rapid cooling on ice for 5 min. After cooling, the bacterial lysates were centrifuged at 8,000 × g for 2–3 min and the DNA-containing supernatants were transferred to new centrifuge tubes and frozen at -20°C prior to use.
The following DNA regions were amplified for use as barcodes: 16S rRNA gene, amplified with primer pair 16sF/16sR (designed in our laboratory); cpn60, amplified with primer pair H1594/H1595 [15]; gyrB, amplified with primer pair gyr-f/gyr-r [16]; and avrBs2, amplified with primer pair AvrBs2-F/AvrBs2-R [13]. The gyrB and avrBs2 primers were recommended by QBOL, and all primer sequences are shown in Table 1. The sequences for the commercially available M13 (24 bp) sequencing primers were added to the 5' end of each primer (indicated by underlined nucleotides).
Table 1. Primers and PCR conditions used for DNA sequence amplifications in this study.
| Gene | Primer name | Amplification primers (5'-3') | PCR Conditions | Amplicon size |
|---|---|---|---|---|
| 16S rDNA | 16s F | CGCCAGGGTTTTCCCAGTCACGACGCGTAGAGTTTGATCCTGGCTCAG | 94°C 5 min; 94°C 1 min, 60°C 1 min, 72°C 1 min, 35 cycles; 72°C 10 min | 1200 bp |
| 16s R | AGCGGATAACAATTTCACACAGGAGACGGGCGGTGTGTRCA | |||
| cpn60 | H1594 | CGCCAGGGTTTTCCCAGTCACGACGACGTCGCCGGTGACGGCACCACCAC | 94°C 5 min; 94°C 30 s, 57°C 30 s, 72°C 45 s, 35 cycles; 72°C 10 min | 555 bp |
| H1595 | AGCGGATAACAATTTCACACAGGACGACGGTCGCCGAAGCCCGGGGCCTT | |||
| gyrB | gyr-f | CGCCAGGGTTTTCCCAGTCACGACAAGCAGGGCAAGAGCGAGCTGTA | 94°C 2.5 min; 94°C 30 s, 50°C 45 s, 72°C 1 min, 35 cycles; 72°C 7 min | 600 bp |
| gyr-r | AGCGGATAACAATTTCACACAGGACAAGGTGCTGAAGATCTGGTC | |||
| avrBs2 | AvrBs2-F | CGCCAGGGTTTTCCCAGTCACGACGGACTAGTCCTGCCGGTGTTGATGCACGA | 94°C 2 min; 94°C 1 min, 60°C 1 min, 72°C 1 min, 35 cycles; 72°C 7 min | 780 bp |
| AvrBs2-R | AGCGGATAACAATTTCACACAGGACCGCTCGAGCGGTGATCGGTCAACAGGCTTTC |
PCR amplification of the four candidate barcodes was performed in 50 μL reaction mixtures containing 25 μL of 2× PCR Master Mix (Biomed Biotechnology, Beijing, China), 19 μL of ddH2O, 2 μL of each primer (10 μM), and 2 μL of template DNA. The amplification conditions for each region are provided in Table 1. PCR products were examined by 1.5% agarose gel electrophoresis. Purification and bidirectional sequencing were completed by Biomed Biotechnology, using the sequencing primers M13-47 and M13-48.
Data analysis
Tree-based methods (Neighbor-joining; NJ) and distance-based methods (the ‘best close match’ and barcoding gap analysis) were applied to test the efficacy of the four DNA barcode candidates for the identification of quarantine Xanthomonas species/pathovars. Sequences were assembled and edited using DNAMAN version 7.0 software (Lynnon Corporation, Quebec, Canada). Multiple sequence alignments were performed using the ClustalW tool from MEGA 6.0 [17], with the default parameters (gap opening penalty of 15 and gap extension penalty of 6.66).
Tree-based methods
Species/pathovar discrimination was evaluated through tree-based analysis of each gene. NJ trees, which were recommended as the standard barcoding method [1], were adopted and constructed using MEGA 6.0 software, based on the Kimura 2-parameter (K2P) model. Branch support was evaluated with 1,000 bootstrap replicates.
Distance-based methods
Barcoding gap and ‘best close match’ analyses were conducted for each respective gene. Pairwise distances were calculated separately to determine the intra- and inter-taxon variation using Spider [18,19], Brown et al.’s DNA barcode analysis package for R, with the uncorrected K2P method. The presence of ‘barcoding gap’ between intra- and inter-taxon distances was evaluated using frequency histograms based on the uncorrected K2P distance. The distance-based criteria for ‘best close match’, which was proposed by Meier et al. (2006) [20], were also evaluated using Spider to estimate the identification success rate. The threshold similarity value was calculated separately for each matrix (all genes separately), below which 95% of all intra-taxon distances are found. In such tests, results can be classified as follows: if all matches of the query sequence belong to the same species/pathovar, the barcode assignment is recorded as a ‘correct’ identification; if a query sequence does not encounter a sequence of the same species/pathovar within the threshold, the test is recorded as an ‘incorrect’ identification; if the matches of the query sequence were equally good, but correspond to a mixture of species/pathovars (including the correct one) within the threshold, it is recorded as ‘ambiguous’; and no match within the threshold is reported as ‘no identification’.
To evaluate the DNA barcode candidates at the pathovar-level, the seven species included in our study that have been defined only to species-level were treated as equivalent to the other 43 pathovars. In other words, the 50 Xanthomonas species/pathovars were treated as 50 individual taxons. Thus, comparisons made within the same pathovar or species (only those seven that were not defined to the pathovar level) were considered as “intra-”, whereas comparisons made between the 50 individual taxons [e.g. between different pathovars (whether or not they are of the same species) or different species (only those seven that were not defined to the pathovar level), or between pathovars and those seven species] were considered as “inter-”.
Results
Amplification, sequencing success, and marker features
Each of the 327 strains was subjected to PCR analysis for the four DNA barcode candidates (16S rRNA gene, cpn60, gyrB, and avrBs2), respectively. The sequence information is provided in Table 2. The amplification reactions were performed with high success (>90%) for all four candidate genes. Specifically, cpn60 exhibited the highest success rate (amplified from 100% of the strains tested), while gyrB (98%) and the 16S rRNA gene (97%) exhibited intermediate efficiency. In contrast, avrBs2 showed the lowest efficiency, with a 93% success rate. High-quality sequences were obtained from all of the amplified DNA samples, and the four DNA regions chosen were effectively amplified and sequenced from the majority of the species/pathovars tested. Additionally, we obtained the 16S rRNA, cpn60, gyrB, and avrBs2 gene sequences for 17 species/pathovars from GenBank (accession numbers shown in S2 Table), thereby generating a database of 1,380 sequences; however, it was not possible to obtain sequences for all of the DNA regions for all strains. All sequence files are available at the DNA Barcode Appraisal System on Chinese Quarantine Pests: http://www.qbol.org.cn/. Users can find or retrieve the data by searching for a species name in the integrated query link after registration for the service. Moreover, we also uploaded all the sequences to Figshare (https://figshare.com/s/31f44679024e443bc45f).
Table 2. Sample sizes, success rates of amplification and sequencing, and sequence characteristics of the four DNA regions in the Xanthomonas species assessed in this study.
| Gene | Success rate of amplification (%) | Success rate of sequencing (%) | Alignment Length (bp) | No. of conserved sites | No. of variable sites | No. of parsimony informative sites |
|---|---|---|---|---|---|---|
| 16S rRNA | 97 | 100 | 1169 | 1120 | 49 | 39 |
| cpn60 | 100 | 100 | 555 | 423 | 132 | 127 |
| gyrB | 98 | 100 | 623 | 357 | 266 | 260 |
| avrBs2 | 93 | 100 | 797 | 448 | 349 | 311 |
For the quarantine Xanthomonas, cpn60 was successfully amplified and sequenced from all 14 quarantine species/pathovars, whereas the 16S rRNA and gyrB genes were amplified from only 12 species/pathovars (failing for X. albilineans and X. populi) and the avrBs2 gene from only eight species/pathovars (failing for X. albilineans, X. axonopodis pv. manihotis, X. campestris pv. musacearum, X. fragariae, X. hyacinthi, and X. populi).
For analysis convenience, the sequences for each candidate gene were trimmed to a uniform length that provided 100% coverage for each strain. The lengths of the aligned DNA fragments of the 16S rRNA, cpn60, gyrB, and avrBs2 genes were 1,169, 555, 623, and 797 bp, respectively. Of the four regions amplified and sequenced, the 16S rRNA gene was the most highly conserved (1120/1169 nucleotides), based on both sequence length and the number of conserved sites. Meanwhile, the target region of avrBs2 provided the largest number of variable sites (349) and parsimony-informative sites (311), followed by gyrB (266 and 260, respectively), cpn60 (132 and 127, respectively), and the 16S rRNA gene (49 and 39, respectively).
Phylogenetic tree-based discrimination of Xanthomonas at the species and pathovar level
We conducted a phylogenetic tree-based analysis to evaluate the effectiveness of the four DNA barcode candidate genes for species- and pathovar-level discrimination of Xanthomonas strains. Due to the large number of sequences used, the NJ tree is too large to be displayed in its entirety. We therefore used the “Compress Subtree” tool within the MEGA package to compress identical sequences within the same branch, respectively. As a result, such sequences are represented only once in Figs 1, 2, 3 and 4.
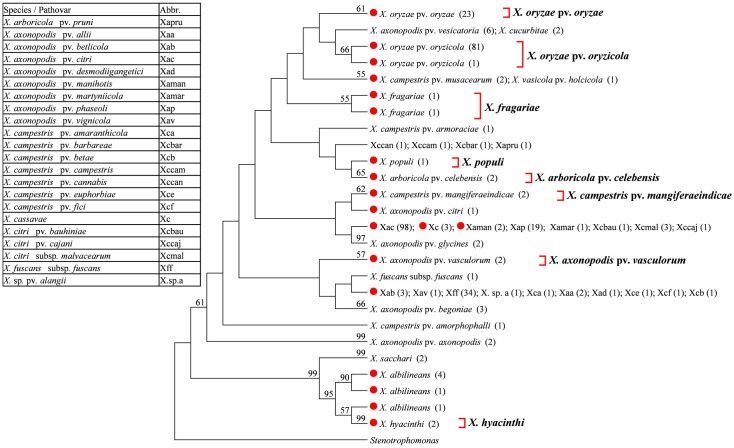
Fig 1. Neighbor-joining tree based on 16S rRNA gene.
Bootstrap values (>50%) are shown above the branches. Identical sequences are represented only once. Numbers following taxon names indicate the number of isolates. Red dots indicate quarantine Xanthomonas species/pathovars. Species/pathovars that were successfully identified are shown on the right.
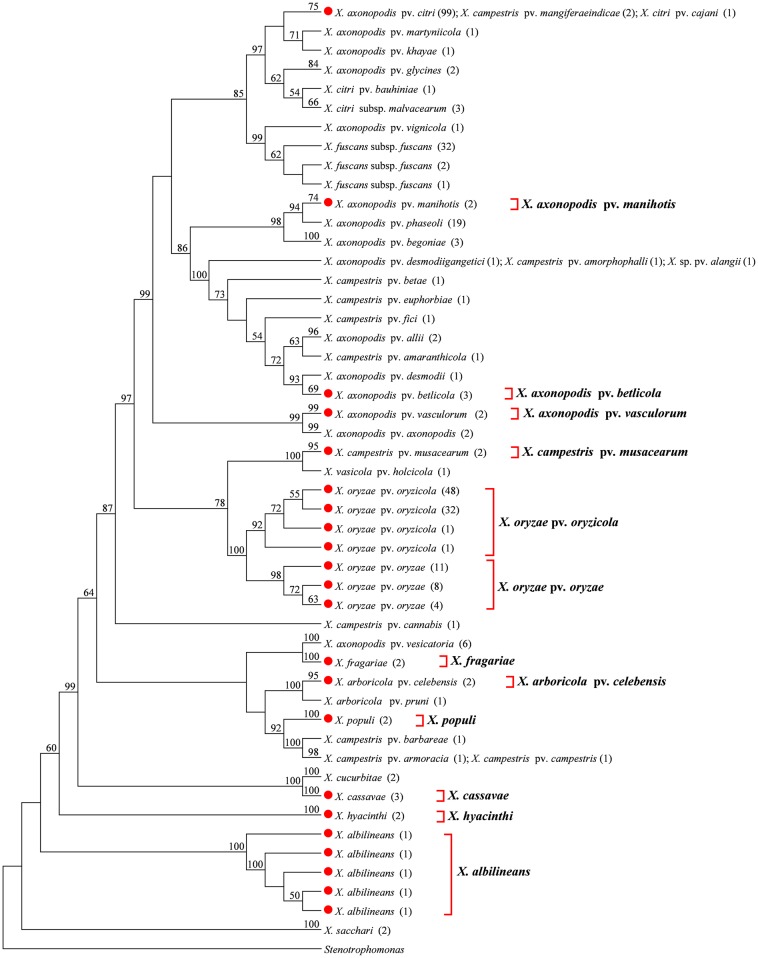
Fig 2. Neighbor-joining tree based on gyrB.
Bootstrap values (>50%) are shown above the branches. Identical sequences are represented only once. Numbers following taxon names indicate the number of isolates. Red dots indicate quarantine Xanthomonas species/pathovars. Species/pathovars that were successfully identified are shown on the right.
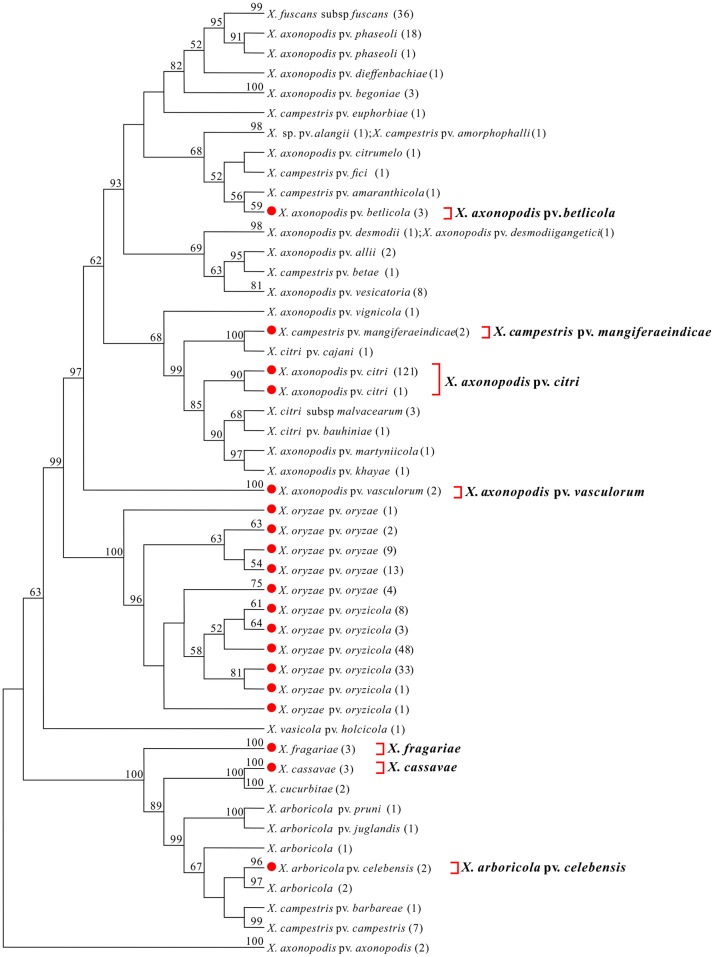
Fig 3. Neighbor-joining tree based on avrBs2.
Bootstrap values (>50%) are shown above the branches. Identical sequences are represented only once. Numbers following taxon names indicate the number of isolates. Red dots indicate quarantine Xanthomonas species/pathovars. Species/pathovars that were successfully identified are shown on the right.
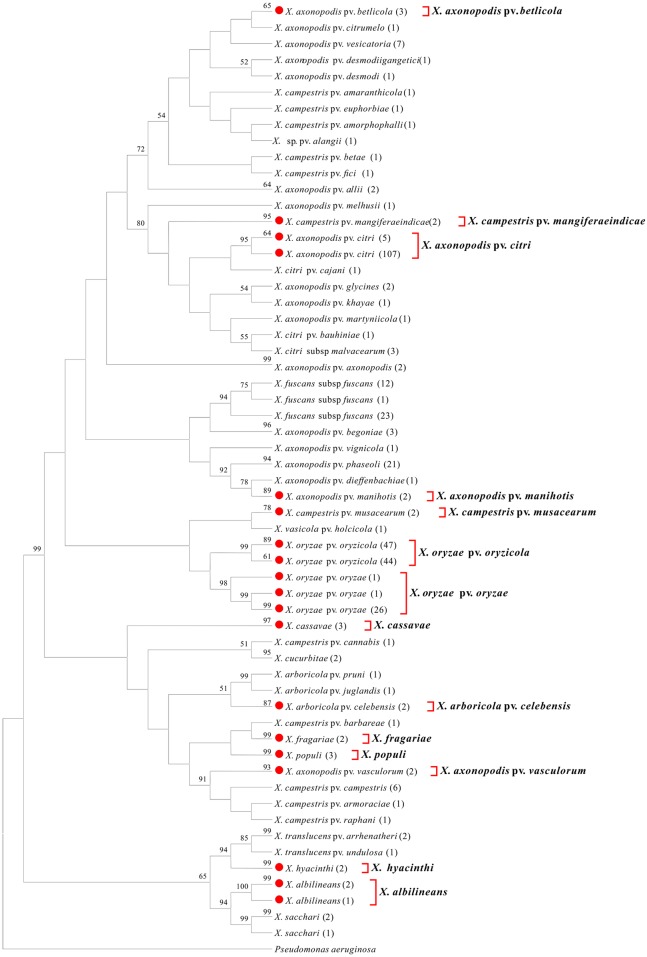
Fig 4. Neighbor-joining tree based on cpn60.
Bootstrap values (>50%) are shown above the branches. Identical sequences are represented only once. Numbers following taxon names indicate the number of isolates. Red dots indicate quarantine Xanthomonas species/pathovars. Species/pathovars that were successfully identified are shown on the right.
16S rRNA
Amplification of 16S rRNA gene was unsuccessful for two quarantine Xanthomonas species, X. albilineans and X. populi. We therefore obtained an additional seven 16S rRNA gene sequences for these two species from GenBank. In total, 324 16S rRNA gene sequences, representing 39 Xanthomonas species/pathovars, were used in the present study. The 16S rRNA gene sequence from a species of Stenotrophomonas was chosen as the outgroup. NJ analysis successfully identified 8 of target quarantine species/pathovars, as these isolates were clustered together into a monophyletic group (Fig 1). However, sequences of X. axonopodis pv. citri (with the exception of one isolate), X. axonopodis pv. manihotis, and X. cassavae were identified as identical to five non-quarantine pathovars, while X. campestris pv. musacearum sequences were identical to X. vasicola pv. holcicola, and X. axonopodis pv. betlicola sequences were identical to 9 other non-quarantine pathovars. In these cases, quarantine and non-quarantine pathovars could not be distinguished. In addition, six X. albilineans isolates were assigned to distinct branches, meaning that they could not be clustered together into a monophyletic group.
gyrB
We failed to amplify gyrB from two quarantine Xanthomonas species, X. albilineans and X. populi. We therefore obtained an additional seven gyrB sequences for these two species from GenBank. Thus, a total of 326 gyrB sequences, representing 43 Xanthomonas species/pathovars, were used in the present study. The gyrB sequence of Stenotrophomonas was used as an out-group. While NJ analysis successfully identified 12 of the target quarantine species/pathovars, as isolates from these species/pathovars clustered together into a monophyletic group (Fig 2), X. axonopodis pv. citri and X. campestris pv. mangiferaeindicae represented complex pathovar clusters containing both quarantine and non-quarantine pathovars that could not be clearly distinguished.
avrBs2
Amplification of avrBs2 was unsuccessful for six quarantine Xanthomonas species/pathovars: X. albilineans, X. axonopodis pv. manihotis, X. campestris pv. musacearum, X. fragariae, X. hyacinthi, and X. populi. Notably, only the avrBs2 sequences of X. fragariae could be obtained from GenBank. However, we also obtained an additional 59 avrBs2 sequences for 11 species/pathovars from GenBank. Thus, a total of 364 avrBs2 sequences, representing 37 Xanthomonas species/pathovars (containing nine quarantine species/pathovars), were subjected to NJ analysis; the tree was rooted using avrBs2 sequences from X. axonopodis pv. axonopodis. These analyses successfully identified seven target quarantine species/pathovars (Fig 3). However, the sequences from X. oryzae pv. oryzae and X. oryzae pv. oryzicola were clustered together into one group, which could not be clearly distinguished.
cpn60
Notably, we achieved successful amplification of cpn60 from all 14 quarantine Xanthomonas species/pathovars. Moreover, an additional 43 cpn60 sequences from 14 species/pathovars were obtained from GenBank. In total, 370 cpn60 sequences, representing 50 Xanthomonas species/pathovars, were used in the present study. The cpn60 sequence from Pseudomonas aeruginosa was chosen as an outgroup. NJ analysis successfully identified each of the 14 target quarantine species/pathovars, as the isolates of these species/pathovars clustered together into a highly supported monophyletic group (>90% bootstrap support, with the exception of X. axonopodis pv. betlicola, X. axonopodis pv. manihotis, and X. campestris pv. musacearum, which displayed bootstrap support values of 65%, 89%, and 78%, respectively), separating them from their closest relatives (Fig 4). Moreover, all Xanthomonas species/pathovars with multiple isolates were recovered as monophyletic. These data suggest that cpn60 may be the most suitable target gene for use as a DNA barcode to distinguish quarantine species/pathovars of Xanthomonas.
Distance-based discrimination of Xanthomonas at the species and pathovar level
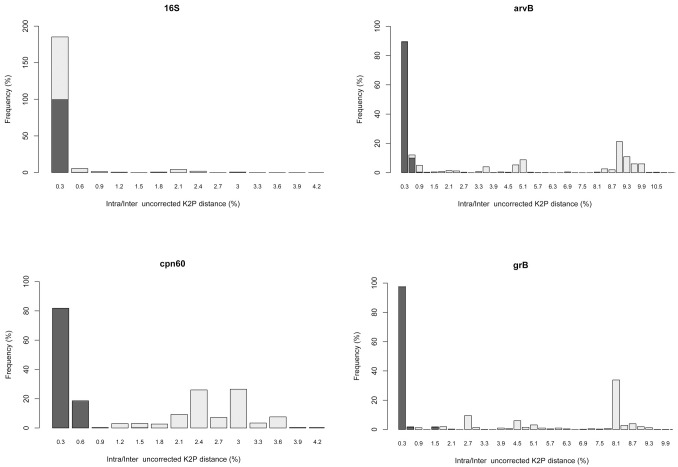
For a robust DNA barcode, it is essential that genetic variation between species be much larger than variation within species. Via calculation of uncorrected K2P distances, variations in intra- and inter-species/pathovar distances were observed among the four DNA barcode candidates. Meanwhile, the presence or absence of a barcoding gap for individual genes was represented by the frequency distribution of the genetic distance between intra-species/pathovar sequences and between inter-species/pathovar sequences (Fig 5). Among the four candidates, cpn60 presented the best barcode gap performance, with 99.73% of pairwise intra-species/pathovar K2P distances lower than 0.006, and 99.25% of pairwise inter-species/pathovar K2P distances greater than 0.006. In addition, avrBs2 performed quite well, providing the highest values for inter-species/pathovar genetic distances, with a range varying from zero to a maximum of 0.326. Notably, however, there was still a relatively high frequency of low distances at the inter-species/pathovar level, with only 97.57% of pairwise inter-species/pathovar K2P distances greater than 0.006, and 99.31% of pairwise intra-species/pathovar K2P distances lower than 0.006. Conversely, 16S rRNA gene showed the worst performance, with a total overlap of intra- and inter-species/pathovar variation, and the gyrB also performed unsatisfactorily.
Fig 5. Histograms of the frequencies (y-axes) of pairwise intra-species/pathovar (dark gray bars) and inter-species/pathovar (light gray bars) divergences based on the uncorrected K2P distance (x-axes) for each candidate gene.
The results of the ‘best close match’ test for each single gene revealed avrBs2 and cpn60 as the best at species/pathovars identification (94.94% and 92.96%, respectively; Table 3). In contrast, gyrB and the 16S rRNA gene exhibited poor rates of correct identification (62.77% and 39.63%, respectively) and larger proportions of ambiguous identifications (31.08% and 54.49%, respectively). Incorrect identification and no identification rates were relatively low (< 6%) for all of the genes tested. Our data contained a number of singleton species/pathovars, which likely led to a lower identification success rate for the candidate genes. To evaluate how the data might behave once fully sampled, we also performed a ‘best close match’ test for each single gene after removing these singletons. The results revealed a higher correct identification rate and a lower incorrect identification rate for all of the individual genes. In particular, the rate of correct identification for cpn60 and avrBs2 were both >99%. Additionally, we also tested the effectiveness of selected gene combinations (cpn60 + gyrB, cpn60 + gyrB + avrBs2, and cpn60 + gyrB + avrBs2 + 16S rRNA) for species identification using the distance-based methods. While each of the three combinations performed satisfactorily in the barcoding gap analysis and the ‘best close match’ test (data not shown), neither provided a decided advantage over the cpn60 gene gene alone.
Table 3. Identification success based on “best close match” method.
| best close match (include singleton) | best close match (exclude singleton) | |||||||
|---|---|---|---|---|---|---|---|---|
| Gene | Correct | Incorrect | Ambiguous | No id | Correct | Incorrect | Ambiguous | No id |
| 16S rRNA | 39.63% | 5.58% | 54.49% | 0.31% | 41.83% | 0.33% | 57.52% | 0.33% |
| cpn60 | 92.96% | 5.97% | 0.00% | 1.09% | 99.14% | 0.00% | 0.00% | 0.87% |
| gyrB | 62.77% | 4.31% | 31.08% | 1.85% | 66.45% | 0.00% | 32.9% | 0.66% |
| avrBs2 | 94.94% | 3.86% | 0.00% | 1.66% | 99.42% | 0.00% | 0.00% | 0.58% |
Discussion
Development of a method for accurate DNA barcoding of Xanthomonas is essential, as it would provide a rapid and accurate means for discrimination of Xanthomonas strains and would assist in screening for quarantine pathogens. In general, there were notable differences in the abilities of the different candidate gene sequences to distinguish between different groups of bacteria. For Xanthomonas, QBOL recommends using the 16S rRNA, gyrB, and avrBs2 genes as core barcode genes for ensuring accurate identification. Of these, the 16S rRNA gene is the most commonly used for bacterial identification. Molecular phylogenetics based on this ribosomal RNA gene has played a vital role in the classification of the genus Xanthomonas; however, the results of our study demonstrate that it is not suitable for identification below the species level due to its low resolution. Specifically, while our data show that this gene can be used to successfully distinguish Xanthomonas spp. from the closely related genus Stenotrophomonas, it failed to provide sufficient information for species-level identification. Moreover, this gene also demonstrated poor performance in the barcoding gap analysis and the ‘best close match’ test.
In previous studies, Parkinson et al. (2008 & 2009) [10,16] evaluated the applicability of gyrB sequences for species and intra-species identification in Xanthomonas, noting that the gyrB sequences used were sufficiently distinct to discriminate between the majority of established Xanthomonas species, thereby offering greater resolution than the 16S rRNA gene [21]. To further evaluate this locus for quarantine species/pathovar identification, we tested a large number of quarantine species/pathovar strains via the NJ method. Our results demonstrate that, based on a partial sequence of the gyrB gene (approximately 700 bp), it is possible to identify most of the quarantine species/pathovars (X. albilineans, X. arboricola pv. celebensis, X. axonopodis pv. betlicola, X. axonopodis pv. manihotis, X. axonopodis pv. vasculorum, X. campestris pv. musacearum, X. cassavae, X. fragariae, X. hyacinthi, X. oryzae pv. oryzae, X. oryzae pv. oryzicola, and X. populi). However, this gene was incapable of identifying Xanthomonas axonopodis pv. citri and X. campestris pv. mangiferaeindicae, which were grouped within complex pathovar clusters containing both quarantine and non-quarantine pathovars. Additional genes would therefore be necessary to identify the quarantine pathovars within the complex clusters. Moreover, gyrB performed markedly worse than cpn60 and avrBs2 in the barcoding gap analysis and ‘‘best close match” test. Together, these data demonstrate that gyrB does not live up to our expectations for pathovar-level identification of Xanthomonas strains.
The T3E gene avrBs2 is broadly distributed among Xanthomonas strains. Hajri et al. (2009) [13] detected a correlation between the composition of T3E repertoires and pathovars of X. axonopodis. We therefore further evaluated the applicability of this locus for use in quarantine species/pathovars identification. Despite multiple attempts, we were unable to amplify the avrBs2sequence from five quarantine species/pathovars (X. albilineans, X. axonopodis pv. manihotis, X. campestris pv. musacearum, X. hyacinthi, and X. populi), resulting in a markedly lower reaction efficiency for quarantine species/pathovars than the other loci tested. This effect could be due to the high sequence variability observed for this gene in our study. Notably, however, avrBs2 still performed better than the 16S rRNA and gyrB genes for species- and pathovar-level identification of Xanthomonas. Specifically, we successfully identified seven of the nine quarantine species/pathovars tested (X. arboricola pv. celebensis, X. axonopodis pv. betlicola, X. axonopodis pv. citri, X. axonopodis pv. vasculorum, X. campestris pv. mangiferaeindicae, X. cassavae, and X. fragariae) via phylogenetic analysis using a partial sequence of the avrBs2 gene (approximately 800 bp). Moreover, avrBs2 performed satisfactorily in the barcoding gap analysis and the ‘best close match’ test, exhibiting the highest inter-species/pathovar genetic distance values, a small overlap of intra- and inter-species/pathovar variation, and the highest correct identification rate. While the efficacy of avrBs2 for species and pathovar identification indicates that the this gene might comprise an ideal candidate for DNA barcoding, the lack of a set of efficient universal primers for amplification avrBs2 constitutes a major limitation for the application of this locus for such testing. Therefore, further research should be conducted to develop efficient universal primers for the avrBs2 gene.
cpn60, which encodes the 60 kDa chaperonin protein, has been established as a target for the detection, identification, and classification of many microorganisms [22,23,24,25,26,27,28,29,30]. Previously, Links et al. (2010) [14] performed a barcode analysis of cpn60 and several regions of the 16S rRNA gene, and found that the cpn60 universal target exhibited a much larger barcode gap than 16S rRNA gene, suggesting cpn60 as a preferred barcode gene for bacteria. In this study, we evaluated the usefulness of this locus for the identification of quarantine Xanthomonas species/pathovars. The efficiency of PCR amplification and sequencing is an important index for evaluating a candidate DNA barcode. In this regard, the cpn60 gene exhibited the highest success rate of amplification (100%) and sequencing (100%) of the four genes tested in our study. Moreover, NJ tree analysis of a region of this gene (approximately 555 bp) enabled successful identification of all 14 target quarantine species/pathovars, as isolates were clustered together into a highly supported monophyletic group, separating them from their closest relatives. Furthermore, along with the quarantine species/pathovars, another 12 non-quarantine species/pathovars, represented by multiple isolates, were recovered as monophyletic. Meanwhile, the remaining 24 species/pathovars, represented by only one isolate each, were all located in independent branch. Moreover, the cpn60 gene provided the most efficient identification of Xanthomonas species/pathovars, exhibiting the best barcode gap performance and a high identification rate (99.14% with the singletons removed) when using all of the sequences obtained (n = 370). Thus, it can be inferred that the cpn60 gene might be suitable for use as a DNA barcode for all species/pathovars of the genus Xanthomonas. However, further research will be required to verify this assertion. Besides, cpn60 alone yielded similar results to combinations of candidate barcode genes, indicating that this locus is sufficient for rapid and accurate discrimination of Xanthomonas strains. Coupled with the low amplification success rate for the other three genes, we feel that it is not necessary to use combinations of genes as a DNA barcode for quarantine Xanthomonas strains.
In summary, an ideal DNA barcode should be universal, reliable, and show good discriminatory power. Based on the amplification and sequencing efficiency, the intra- and inter-species/pathovar divergence patterns, the ‘best close match’ test results, and the ability to recover species/pathovar-specific clusters in phylogenetic trees observed in this study, we recommend cpn60 for use in barcode identification of the quarantine pathogen Xanthomonas.
Supporting Information
(PDF)
(PDF)
Acknowledgments
We thank professors Gongyou Chen (Shanghai Jiao Tong University) and Zhendong Zhu (Chinese Academy of Agricultural Sciences) for providing bacteria strains.
Data Availability
All relevant data are available within the paper, the supporting information files, and the stable public repository Figshare. The data hosted at Figshare can be found at the following DOI: 10.6084/m9.figshare.4083378.
Funding Statement
This work is financially supported by National Science and Technology Ministry (2012BAK11B02). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hebert PD, Cywinska A, Ball SL, Dewaard JR (2003) Biological identifications through DNA barcodes. Proceedings Biological Sciences 270: 313–321. 10.1098/rspb.2002.2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hebert PD, Stoeckle MY, Zemlak TS, Francis CM (2004) Identification of Birds through DNA Barcodes. Plos Biology 2: e312 10.1371/journal.pbio.0020312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PDN (2005) DNA barcoding Australia's fish species. Philosophical Transactions of the Royal Society of London 360: 1847–1857. 10.1098/rstb.2005.1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajibabaei M, Janzen DH, Burns JM, Hallwachs W, Hebert PDN (2006) DNA barcodes distinguish species of tropical Lepidoptera. Proceedings of the National Academy of Sciences 103: 968–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kress WJ, Wurdack KJ, Zimmer EA, Weigt LA, Janzen DH (2005) Use of DNA barcodes to identify flowering plants. Proceedings of the National Academy of Sciences of the United States of America 102: págs. 8369–8374. 10.1073/pnas.0503123102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen SL, Yao H, Han JP, Liu C, Song JY, Shi LC, et al. (2010) Validation of the ITS2 Region as a Novel DNA Barcode for Identifying Medicinal Plant Species. Plos One 5: e8613–e8613. 10.1371/journal.pone.0008613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeigler DR (2003) Gene sequences useful for predicting relatedness of whole genomes in bacteria. International Journal of Systematic & Evolutionary Microbiology 53: 1893–1900. [DOI] [PubMed] [Google Scholar]
- 8.Stead DE (1989) Grouping of Xanthomonas campestris pathovars of cereals and grasses by fatty acid profiling 1. Bulletin Oepp/eppo Bulletin 19: 57–68. [Google Scholar]
- 9.Vauterin L, Swings J, Kersters K, Gillis M, Mew TW, Schroth MN, et al. (1990) Towards an improved taxonomy of Xanthomonas. International Journal of Systematic Bacteriology 40: 312–316. [Google Scholar]
- 10.Parkinson N, Cowie C, Heeney J, Stead D (2009) Phylogenetic structure of Xanthomonas determined by comparison of gyrB sequences. International Journal of Systematic & Evolutionary Microbiology 59: 264–274. [DOI] [PubMed] [Google Scholar]
- 11.Young JM, Park DC, Shearman HM, Fargier E (2008) A multilocus sequence analysis of the genus Xanthomonas ☆. Systematic & Applied Microbiology 31: 366–377. [DOI] [PubMed] [Google Scholar]
- 12.Dye DW, Bradbury JF, Goto M, Hayward AC, Lelliott RA, Schroth MN (1980) International standards for naming pathovars of phytopathogenic bacteria and a list of pathovar names and pathotype strains. Review of Plant Pathology 59: 153–168. [Google Scholar]
- 13.Hajri A, Brin C, Hunault G, Lardeux F, Lemaire C, Manceau C, et al. (2009) A «Repertoire for Repertoire» Hypothesis: Repertoires of Type Three Effectors are Candidate Determinants of Host Specificity in Xanthomonas. Plos One 4: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Links MG, Dumonceaux TJ, Hemmingsen SM, Hill JE (2012) The chaperonin-60 universal target is a barcode for bacteria that enables de novo assembly of metagenomic sequence data. Plos One 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahin N, Gonzalez JM, Iizuka T, Hill JE (2010) Characterization of two aerobic ultramicrobacteria isolated from urban soil and a description of Oxalicibacterium solurbis sp. nov. Fems Microbiology Letters 307: 25–29. 10.1111/j.1574-6968.2010.01954.x [DOI] [PubMed] [Google Scholar]
- 16.Parkinson N, Aritua V, Heeney J, Cowie C, Bew J, Stead D (2008) Phylogenetic analysis of Xanthomonas species by comparison of partial gyrase B gene sequences. International Journal of Systematic & Evolutionary Microbiology 57: 2881–2887. [DOI] [PubMed] [Google Scholar]
- 17.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology & Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown SD, Collins RA, Boyer S, Lefort MC, Malumbres-Olarte J, Vink CJ, et al. (2012) Spider: An R package for the analysis of species identity and evolution, with particular reference to DNA barcoding. Molecular Ecology Resources 12: 562–565. 10.1111/j.1755-0998.2011.03108.x [DOI] [PubMed] [Google Scholar]
- 19.Paradis E, Claude J, Strimmer K (2004) APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics 20: 289–290. [DOI] [PubMed] [Google Scholar]
- 20.Meier R, Shiyang K, Vaidya G, Ng PK (2006) DNA barcoding and taxonomy in Diptera: a tale of high intraspecific variability and low identification success. Systematic Biology 55: 715–728. 10.1080/10635150600969864 [DOI] [PubMed] [Google Scholar]
- 21.Hauben L, Vauterin L, Swings J, Moore ER (1997) Comparison of 16S ribosomal DNA sequences of all Xanthomonas species. International Journal of Systematic Bacteriology 47: 328–335. 10.1099/00207713-47-2-328 [DOI] [PubMed] [Google Scholar]
- 22.Brousseau R, Hill JE, Préfontaine G, Goh SH, Harel J, Hemmingsen SM (2001) Streptococcus suis Serotypes Characterized by Analysis of Chaperonin 60 Gene Sequences. Applied & Environmental Microbiology 67: 4828–4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill JE, Paccagnella A, Law K, Melito PL, Woodward DL, Price L, et al. (2006) Identification of Campylobacter spp. and discrimination from Helicobacter and Arcobacter spp. by direct sequencing of PCR-amplified cpn60 sequences and comparison to cpnDB, a chaperonin reference sequence database. Journal of Medical Microbiology 55: 393–399. 10.1099/jmm.0.46282-0 [DOI] [PubMed] [Google Scholar]
- 24.Goh SH, Facklam RR, Chang M, Hill JE, Tyrrell GJ, Burns EC, et al. (2000) Identification of Enterococcus species and phenotypically similar Lactococcus and Vagococcus species by reverse checkerboard hybridization to chaperonin 60 gene sequences. Journal of Clinical Microbiology 38: 3953–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumonceaux TJ, Hill JE, Briggs SA, Amoako KK, Hemmingsen SM, Van Kessel AG (2006) Enumeration of specific bacterial populations in complex intestinal communities using quantitative PCR based on the chaperonin -60 target. Journal of Microbiological Methods 64: 46–62. 10.1016/j.mimet.2005.04.006 [DOI] [PubMed] [Google Scholar]
- 26.Chaban B, Ngeleka M, Hill JE (2010) Detection and quantification of 14 Campylobacter species in pet dogs reveals an increase in species richness in feces of diarrheic animals. Bmc Microbiology 10: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miñana-Galbis D, Urbizu-Serrano A, Farfán M, Fusté MC, Lorén JG (2009) Phylogenetic analysis and identification of Aeromonas species based on sequencing of the cpn60 universal target. International Journal of Systematic & Evolutionary Microbiology 59: 1976–1983. [DOI] [PubMed] [Google Scholar]
- 28.Sakamoto M, Ohkuma M (2010) Usefulness of the hsp60 gene for the identification and classification of Gram-negative anaerobic rods. Journal of Medical Microbiology 59: 1293–1302. 10.1099/jmm.0.020420-0 [DOI] [PubMed] [Google Scholar]
- 29.Kwok AY, Chow AW (2003) Phylogenetic study of Staphylococcus and Macrococcus species based on partial hsp60 gene sequences. International Journal of Systematic & Evolutionary Microbiology 53: 87–92. [DOI] [PubMed] [Google Scholar]
- 30.Paramel JT, Schellenberg JJ, Hill JE (2012) Resolution and characterization of distinct cpn60-based subgroups of Gardnerella vaginalis in the vaginal microbiota. Plos One 7: e43009–e43009. 10.1371/journal.pone.0043009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data are available within the paper, the supporting information files, and the stable public repository Figshare. The data hosted at Figshare can be found at the following DOI: 10.6084/m9.figshare.4083378.