Abstract
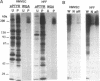
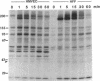
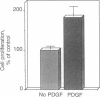
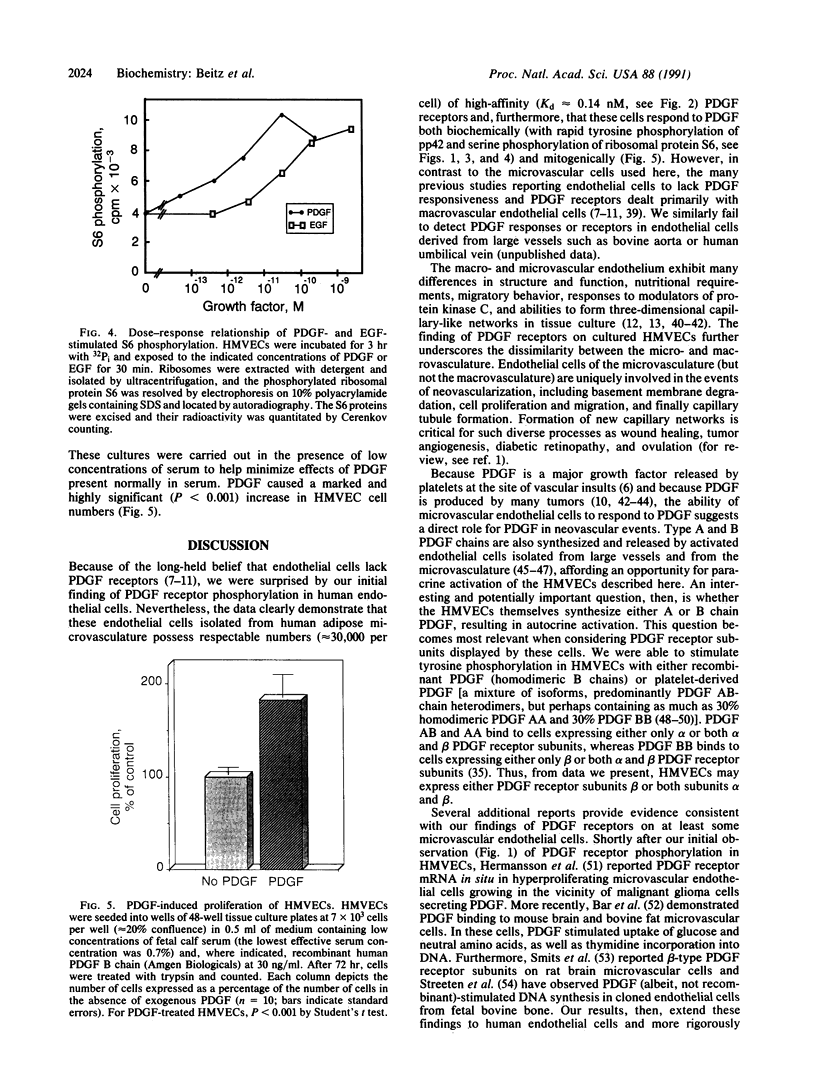
Endothelial cells have been widely thought to be unresponsive to platelet-derived growth factor (PDGF, a major growth factor released from stimulated platelets at the sites of vascular insults) and devoid of PDGF receptors. Nevertheless, in examining the growth-factor responses of microvascular endothelial cells isolated from human omental adipose tissue, we were surprised to detect PDGF-induced tyrosine phosphorylation of a 180-kDa glycoprotein, subsequently identified as the cellular receptor for PDGF by specific immunoprecipitation. Scatchard analysis of 125I-labeled PDGF binding to human microvascular endothelial cells revealed 30,000 PDGF receptors per cell with a Kd of 0.14 nM. PDGF stimulated tyrosine phosphorylation of PDGF receptors and other cellular proteins in a dose- and time-dependent manner, with half-maximal receptor phosphorylation occurring at 0.3 nM recombinant human PDGF (B chain) and a less than or equal to 1-min exposure to PDGF. Normal cellular consequences of receptor activation were also observed, including tyrosine phosphorylation of a 42-kDa protein and serine phosphorylation of ribosomal protein S6. Furthermore, PDGF was mitogenic for these cells. Microvascular endothelial cells play a central role in neovascularization required for wound healing and solid tumor growth. Thus, the discovery of functional PDGF receptors on human microvascular endothelial cells suggests a direct role for PDGF in this process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausprunk D. H., Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977 Jul;14(1):53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- Bar R. S., Boes M., Booth B. A., Dake B. L., Henley S., Hart M. N. The effects of platelet-derived growth factor in cultured microvessel endothelial cells. Endocrinology. 1989 Apr;124(4):1841–1848. doi: 10.1210/endo-124-4-1841. [DOI] [PubMed] [Google Scholar]
- Barrett T. B., Gajdusek C. M., Schwartz S. M., McDougall J. K., Benditt E. P. Expression of the sis gene by endothelial cells in culture and in vivo. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6772–6774. doi: 10.1073/pnas.81.21.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Hart C. E., Seifert R. A. Sera and conditioned media contain different isoforms of platelet-derived growth factor (PDGF) which bind to different classes of PDGF receptor. J Biol Chem. 1989 Feb 15;264(5):2502–2508. [PubMed] [Google Scholar]
- Bowen-Pope D. F., Ross R. Platelet-derived growth factor. II. Specific binding to cultured cells. J Biol Chem. 1982 May 10;257(9):5161–5171. [PubMed] [Google Scholar]
- Chung-Welch N., Patton W. F., Yen-Patton G. P., Hechtman H. B., Shepro D. Phenotypic comparison between mesothelial and microvascular endothelial cell lineages using conventional endothelial cell markers, cytoskeletal protein markers and in vitro assays of angiogenic potential. Differentiation. 1989 Oct;42(1):44–53. doi: 10.1111/j.1432-0436.1989.tb00606.x. [DOI] [PubMed] [Google Scholar]
- Claesson-Welsh L., Eriksson A., Westermark B., Heldin C. H. cDNA cloning and expression of the human A-type platelet-derived growth factor (PDGF) receptor establishes structural similarity to the B-type PDGF receptor. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4917–4921. doi: 10.1073/pnas.86.13.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson-Welsh L., Hammacher A., Westermark B., Heldin C. H., Nistér M. Identification and structural analysis of the A type receptor for platelet-derived growth factor. Similarities with the B type receptor. J Biol Chem. 1989 Jan 25;264(3):1742–1747. [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Major substrate for growth factor-activated protein-tyrosine kinases is a low-abundance protein. Mol Cell Biol. 1985 Nov;5(11):3304–3309. doi: 10.1128/mcb.5.11.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. F., Ross R. Mediation of pinocytosis in cultured arterial smooth muscle and endothelial cells by platelet-derived growth factor. J Cell Biol. 1978 Dec;79(3):663–671. doi: 10.1083/jcb.79.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doctrow S. R., Folkman J. Protein kinase C activators suppress stimulation of capillary endothelial cell growth by angiogenic endothelial mitogens. J Cell Biol. 1987 Mar;104(3):679–687. doi: 10.1083/jcb.104.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek B., Heldin C. H. Characterization of a tyrosine-specific kinase activity in human fibroblast membranes stimulated by platelet-derived growth factor. J Biol Chem. 1982 Sep 10;257(17):10486–10492. [PubMed] [Google Scholar]
- Escobedo J. A., Keating M. T., Ives H. E., Williams L. T. Platelet-derived growth factor receptors expressed by cDNA transfection couple to a diverse group of cellular responses associated with cell proliferation. J Biol Chem. 1988 Jan 25;263(3):1482–1487. [PubMed] [Google Scholar]
- Escobedo J. A., Navankasatussas S., Cousens L. S., Coughlin S. R., Bell G. I., Williams L. T. A common PDGF receptor is activated by homodimeric A and B forms of PDGF. Science. 1988 Jun 10;240(4858):1532–1534. doi: 10.1126/science.2836953. [DOI] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. Angiogenesis in vitro. Nature. 1980 Dec 11;288(5791):551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Ross A. H., Eisen H. N. Characterization and use of monoclonal antibodies for isolation of phosphotyrosyl proteins from retrovirus-transformed cells and growth factor-stimulated cells. Mol Cell Biol. 1983 Aug;3(8):1343–1352. doi: 10.1128/mcb.3.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Tremble P. M., Williams L. T. Evidence for the platelet-derived growth factor-stimulated tyrosine phosphorylation of the platelet-derived growth factor receptor in vivo. Immunopurification using a monoclonal antibody to phosphotyrosine. J Biol Chem. 1984 Jun 25;259(12):7909–7915. [PubMed] [Google Scholar]
- Hammacher A., Hellman U., Johnsson A., Ostman A., Gunnarsson K., Westermark B., Wasteson A., Heldin C. H. A major part of platelet-derived growth factor purified from human platelets is a heterodimer of one A and one B chain. J Biol Chem. 1988 Nov 5;263(31):16493–16498. [PubMed] [Google Scholar]
- Hart C. E., Bailey M., Curtis D. A., Osborn S., Raines E., Ross R., Forstrom J. W. Purification of PDGF-AB and PDGF-BB from human platelet extracts and identification of all three PDGF dimers in human platelets. Biochemistry. 1990 Jan 9;29(1):166–172. doi: 10.1021/bi00453a022. [DOI] [PubMed] [Google Scholar]
- Haudenschild C. C., Zahniser D., Folkman J., Klagsbrun M. Human vascular endothelial cells in culture. Lack of response to serum growth factors. Exp Cell Res. 1976 Mar 1;98(1):175–183. doi: 10.1016/0014-4827(76)90477-8. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Specific receptors for platelet-derived growth factor on cells derived from connective tissue and glia. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3664–3668. doi: 10.1073/pnas.78.6.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansson M., Nistér M., Betsholtz C., Heldin C. H., Westermark B., Funa K. Endothelial cell hyperplasia in human glioblastoma: coexpression of mRNA for platelet-derived growth factor (PDGF) B chain and PDGF receptor suggests autocrine growth stimulation. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7748–7752. doi: 10.1073/pnas.85.20.7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn R. D., Posner M. R., Rayter S. I., Foulkes J. G., Frackelton A. R., Jr Cell lines and peripheral blood leukocytes derived from individuals with chronic myelogenous leukemia display virtually identical proteins phosphorylated on tyrosine residues. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4408–4412. doi: 10.1073/pnas.84.13.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Flier J. S., Grunfeld C., Harmon J. T., Harrison L. C., Karlsson F. A., Kasuga M., King G. L., Lang U. C. Insulin receptors, receptor antibodies, and the mechanism of insulin action. Recent Prog Horm Res. 1981;37:477–538. doi: 10.1016/b978-0-12-571137-1.50015-3. [DOI] [PubMed] [Google Scholar]
- Kavanaugh W. M., Harsh G. R., 4th, Starksen N. F., Rocco C. M., Williams L. T. Transcriptional regulation of the A and B chain genes of platelet-derived growth factor in microvascular endothelial cells. J Biol Chem. 1988 Jun 15;263(17):8470–8472. [PubMed] [Google Scholar]
- Kazlauskas A., Cooper J. A. Protein kinase C mediates platelet-derived growth factor-induced tyrosine phosphorylation of p42. J Cell Biol. 1988 Apr;106(4):1395–1402. doi: 10.1083/jcb.106.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskas A., DiCorleto P. E. Cultured endothelial cells do not respond to a platelet-derived growth-factor-like protein in an autocrine manner. Biochim Biophys Acta. 1985 Sep 30;846(3):405–412. doi: 10.1016/0167-4889(85)90013-8. [DOI] [PubMed] [Google Scholar]
- Keating M. T., Harryman C. C., Williams L. T. Platelet-derived growth factor receptor inducibility is acquired immediately after translation and does not require glycosylation. J Biol Chem. 1989 Jun 5;264(16):9129–9132. [PubMed] [Google Scholar]
- Keating M. T., Williams L. T. Processing of the platelet-derived growth factor receptor. Biosynthetic and degradation studies using anti-receptor antibodies. J Biol Chem. 1987 Jun 5;262(16):7932–7937. [PubMed] [Google Scholar]
- Kern P. A., Knedler A., Eckel R. H. Isolation and culture of microvascular endothelium from human adipose tissue. J Clin Invest. 1983 Jun;71(6):1822–1829. doi: 10.1172/JCI110937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knedler A., Eckel R. H., Kern P. A., Ham R. G. Microvascular endothelial cell cultures from human omental adipose tissue. In Vitro Cell Dev Biol. 1989 Oct;25(10):863–864. doi: 10.1007/BF02623995. [DOI] [PubMed] [Google Scholar]
- Knedler A., Ham R. G. Optimized medium for clonal growth of human microvascular endothelial cells with minimal serum. In Vitro Cell Dev Biol. 1987 Jul;23(7):481–491. doi: 10.1007/BF02628418. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Williams S. K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Heidaran M., Miki T., Popescu N., La Rochelle W., Kraus M., Pierce J., Aaronson S. Isolation of a novel receptor cDNA establishes the existence of two PDGF receptor genes. Science. 1989 Feb 10;243(4892):800–804. doi: 10.1126/science.2536956. [DOI] [PubMed] [Google Scholar]
- Montesano R., Orci L. Phorbol esters induce angiogenesis in vitro from large-vessel endothelial cells. J Cell Physiol. 1987 Feb;130(2):284–291. doi: 10.1002/jcp.1041300215. [DOI] [PubMed] [Google Scholar]
- Nishimura J., Huang J. S., Deuel T. F. Platelet-derived growth factor stimulates tyrosine-specific protein kinase activity in Swiss mouse 3T3 cell membranes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4303–4307. doi: 10.1073/pnas.79.14.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistér M., Heldin C. H., Wasteson A., Westermark B. A glioma-derived analog to platelet-derived growth factor: demonstration of receptor competing activity and immunological crossreactivity. Proc Natl Acad Sci U S A. 1984 Feb;81(3):926–930. doi: 10.1073/pnas.81.3.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike L. J., Bowen-Pope D. F., Ross R., Krebs E. G. Characterization of platelet-derived growth factor-stimulated phosphorylation in cell membranes. J Biol Chem. 1983 Aug 10;258(15):9383–9390. [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Rossomando A. J., Payne D. M., Weber M. J., Sturgill T. W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariban E., Sitaras N. M., Antoniades H. N., Kufe D. W., Pantazis P. Expression of platelet-derived growth factor (PDGF)-related transcripts and synthesis of biologically active PDGF-like proteins by human malignant epithelial cell lines. J Clin Invest. 1988 Oct;82(4):1157–1164. doi: 10.1172/JCI113712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Seifert R. A., Hart C. E., Phillips P. E., Forstrom J. W., Ross R., Murray M. J., Bowen-Pope D. F. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J Biol Chem. 1989 May 25;264(15):8771–8778. [PubMed] [Google Scholar]
- Smits A., Hermansson M., Nistér M., Karnushina I., Heldin C. H., Westermark B., Funa K. Rat brain capillary endothelial cells express functional PDGF B-type receptors. Growth Factors. 1989;2(1):1–8. doi: 10.3109/08977198909069076. [DOI] [PubMed] [Google Scholar]
- Streeten E. A., Ornberg R., Curcio F., Sakaguchi K., Marx S., Aurbach G. D., Brandi M. L. Cloned endothelial cells from fetal bovine bone. Proc Natl Acad Sci U S A. 1989 Feb;86(3):916–920. doi: 10.1073/pnas.86.3.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill T. W., Ray L. B., Erikson E., Maller J. L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988 Aug 25;334(6184):715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Terracio L., Rönnstrand L., Tingström A., Rubin K., Claesson-Welsh L., Funa K., Heldin C. H. Induction of platelet-derived growth factor receptor expression in smooth muscle cells and fibroblasts upon tissue culturing. J Cell Biol. 1988 Nov;107(5):1947–1957. doi: 10.1083/jcb.107.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T. Stimulation of paracrine and autocrine pathways of cell proliferation by platelet-derived growth factor. Clin Res. 1988 Jan;36(1):5–10. [PubMed] [Google Scholar]
- Zetter B. R. The endothelial cells of large and small blood vessels. Diabetes. 1981;30(Suppl 2):24–28. doi: 10.2337/diab.30.2.s24. [DOI] [PubMed] [Google Scholar]