Abstract
The kidney plays an essential role in maintaining homeostasis of blood ion concentrations. Because the concentration gradient of potassium across the cell membrane is a key determinant of the membrane potential of cells, even small deviations in serum potassium from the normal setpoint can lead to severe muscle dysfunction, resulting in respiratory failure and cardiac arrest. Less severe hypo- and hyperkalemia are also associated with morbidity and mortality across various patient populations. In addition, deficiencies in potassium intake have been associated with hypertension and adverse cardiovascular and renal outcomes. This is likely due in part to the interrelated handling of sodium and potassium by the kidney. Here, data on the beneficial effects of potassium on blood pressure and cardiovascular and renal outcomes will be reviewed, along with the physiological basis for these effects. In some patient populations, however, potassium excess is deleterious. Risk factors for the development of hyperkalemia will be reviewed, as well as the risks and benefits of existing and emerging therapies for hyperkalemia.
Keywords: potassium homeostasis, potassium intake, hypokalemia, hyperkalemia, renal physiology, sodium polystyrene sulfonate, patiromer, ZS-9
Introduction
Both hypokalemia and hyperkalemia are associated with adverse consequences. In addition, inadequate potassium intake, even without an abnormality in serum potassium concentration, has also been associated with adverse cardiovascular and renal outcomes. The goal of this review is to discuss publications on the potential benefits and harms of potassium intake on human health. Recent advances illuminating the physiology underlying the beneficial effects of potassium will be reviewed. In some patients, excess potassium intake may result in hyperkalemia; the physiology of this electrolyte disorder will be reviewed, along with clinical risk factors. New data on existing therapies for hyperkalemia management, as well as studies on emerging therapies, will be reviewed. Understanding these principles will guide the clinician in optimizing recommendations for potassium intake in different patient populations. Additionally, clinicians will gain understanding of the risks and benefits of existing and novel therapies for hyperkalemia.
Potassium: Friend – The Beneficial Effects of Potassium Intake
High blood pressure is the largest threat to human health worldwide [1]. The beneficial effects of potassium salts in promoting natriuresis and diuresis have long been appreciated [2, 3]. Recent studies have provided further evidence of the beneficial effects of potassium intake on blood pressure and clinical outcomes. In a meta-analysis of 21 randomized controlled trials, Aburto et al. found that higher potassium intake resulted in blood pressure lowering in the overall population studied, with more pronounced effects in patients with hypertension or consuming a high sodium diet (Table 1) [4]. Furthermore, analysis of 11 cohort studies with a total of 127,038 participants showed that potassium intake in the range of 90–120 mmol/day was associated with a decreased risk of stroke (RR 0.79, 95% confidence interval 0.68 – 0.93). On the basis of these findings, the World Health Organization recommends daily potassium intake of at least 90 mmol/day (3.5 g/day) [4], while the Institute of Medicine recommends an intake of at least 155 mmol/day (4.5 g/day) for children aged 9–13 years, and 120 mmol/day (4.7 g/day) for older children and adults [5].
Table 1. Effect of higher potassium intake on blood pressure.
Created using data from [4]; further details in text
| SBP (mmHg) | DBP (mmHg) | |
|---|---|---|
| Overall population | −3.49 | −1.96 |
| Hypertensive subjects | −5.32 | −3.10 |
| High Na+ (> 4g/day) intake | −6.91 | −2.87 |
SBP: systolic blood pressure, DBP: diastolic blood pressure
The PURE (Prospective Urban Rural Epidemiology) study adds further support to this idea. A global population comprising ~102,000 adults was studied. 24 hour urinary potassium was estimated from spot urine samples. This study found that for any given level of sodium intake, higher potassium intake was associated with decreased blood pressure, and vice versa. As in the Aburto study, the modifying effect of potassium was greatest in those individuals consuming the highest sodium diets [6]. This is particularly relevant given high sodium consumption worldwide. In the United States, median sodium intake is 3.4 g/day, with fewer than 10 % of individuals consuming < 2.3 g/day [7], and worldwide, mean sodium intake is 4.9 g, with 3.3 % of individuals consuming < 2.3 g/day [6]. Furthermore, in PURE, high potassium intake, as inferred from urinary excretion, was associated with decreased risk of death or cardiovascular events, while low potassium intake was associated with increased risk of death and cardiovascular events [8]. Low potassium intake also increases the risk of poor renal outcomes: an analysis of patients enrolled in the ONTARGET and TRANSCEND studies found an inverse correlation between dietary potassium intake and estimated glomerular filtration rate (eGFR) decline ≥30 % or chronic dialysis, or proteinuria, with decreased odds of these adverse outcomes at higher levels of dietary potassium, and increased odds with lower potassium intake [9]. Similarly, in a prospective study of Japanese patients with type 2 diabetes who initially had eGFR ≥60 ml/min, higher urinary potassium excretion was associated with a lower risk of renal and cardiovascular events [10], although this was not the case in a cohort with established chronic kidney disease (CKD) [11]. Two prospective cohort studies in pediatric subjects also found associations between higher potassium intake and lower blood pressure [12, 13].
Despite the beneficial effects of potassium, median potassium intake in the United States is estimated to be 66 mmol (2.6 g)/day [7], while worldwide the median potassium intake is 54 mmol (2.12 g)/day [6]. The National Kidney Foundation provides information on the potassium content of various foods at https://www.kidney.org/atoz/content/potassium, and a partial list of high potassium foods is provided in Table 2.
Table 2. Partial list of high potassium foods.
Created using data from https://www.kidney.org/atoz/content/potassium and https://ndb.nal.usda.gov
| High Potassium Foods | mEq K+ per 100 g |
|---|---|
| apricots | 7 |
| cantaloupe | 7 |
| mango | 4 |
| prunes | 19 |
| butternut squash | 7 |
| beets, boiled | 8 |
| carrots, raw | 8 |
| spinach, cooked | 12 |
| beans, baked | 9 |
| potatoes, baked | 15 |
| milk | 5 |
| yogurt | 4 |
| nuts (eg peanut butter) | 14 |
Why is Potassium Beneficial? Recent Advances
It has long been appreciated that potassium has natriuretic and diuretic effects, and therefore the potential to lower blood pressure. In 1935, Keith and Binger reported a series of 60 patients, of whom 80 % had a diuretic response to potassium salts. They demonstrated an increase in urinary sodium excretion in several patients [2]. Subsequent studies further elucidated the relationship between urinary sodium and potassium handling. Womersley and Darragh, studying themselves, demonstrated that severely depleting the diet of potassium (2 mEq K+/day) caused sodium and water retention in a dietary sodium-dependent fashion [14]. Similarly, in a group of ten healthy men, a low potassium diet (10 mEq K+/day) resulted in decreased urinary sodium excretion, increased blood pressure, and salt sensitivity [15].
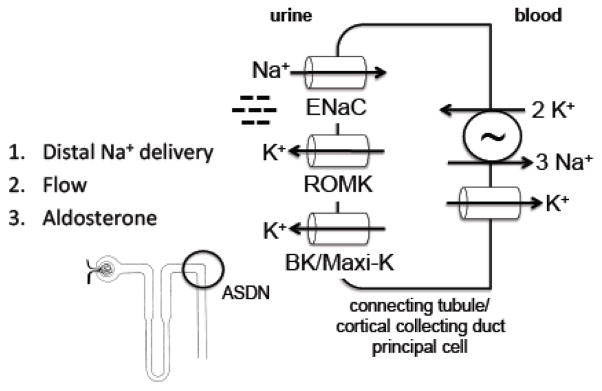
Potassium is freely filtered at the glomerulus and ~2/3 is reabsorbed in the proximal tubule, and an additional ~25 % in the thick ascending limb of the loop of Henle [16]. Fine-tuning of renal potassium excretion occurs in the aldosterone-sensitive distal nephron (ASDN), comprising the late distal convoluted tubule (DCT), connecting tubule (CNT) and cortical collecting duct (CCD) [17]. In the ASDN, three key factors promote potassium secretion into the tubular lumen: sodium delivery; tubular fluid flow rate; and aldosterone [16]. Sodium reabsorption through the epithelial sodium channel (ENaC) generates the negative luminal charge that drives potassium secretion (Figure 1) [16]. Changes in sodium reabsorption in segments proximal to the ASDN (proximal tubule, thick ascending limb, and DCT) influence sodium delivery to the ASDN, and therefore reabsorption through ENaC and generation of the lumen-negative charge, thereby altering potassium secretion in this segment.
Figure 1.
Key determinants of potassium secretion in the aldosterone-sensitive distal nephron (ASDN). Sodium reabsorption through the epithelial sodium channel (ENaC) generates a lumen-negative charge that drives potassium secretion through the ROMK and BK potassium channels. Therefore, distal sodium delivery is a key determinant of potassium secretion. Tubular lumen flow stimulates ENaC and BK, and effectively lowers luminal potassium concentration, thereby increasing the driving force for potassium secretion. BK expression is low in neonates, limiting flow-stimulated potassium secretion and facilitating potassium retention at this stage of development [117]. Aldosterone upregulates ENaC and the Na+/K+-ATPase, and has additional effects, such as stimulating medullary potassium recycling, that enhance potassium secretion.
Studies performed in the 1970s and 1980s established that potassium influences proximal sodium reabsorption. For example, intravenous infusions of KCl decrease sodium reabsorption in the proximal tubule [18], while bathing the thick ascending limb in a high potassium bath, as might occur during medullary potassium recycling, decreases sodium reabsorption in that segment [19]. Subsequent studies showed that administration of a high potassium diet or aldosterone to experimental animals results in increased medullary potassium recycling, with increased potassium concentration in the medullary interstitium and decreased sodium reabsorption in the thick ascending limb [20,21]. Contemporary studies have also confirmed that high dietary potassium decreases sodium reabsorption in the thick ascending limb [22].
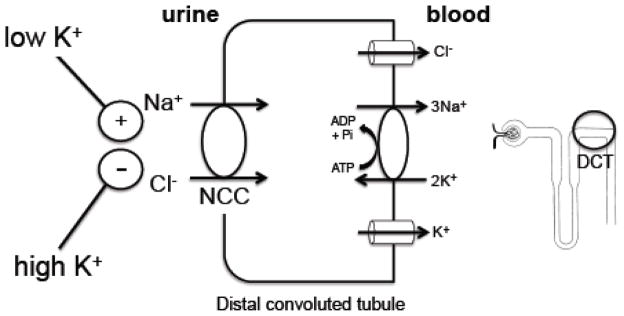
Effects of potassium on the distal convoluted tubule sodium chloride cotransporter (NCC)
In the past several years, multiple investigators have examined the effect of varying dietary potassium intake on the thiazide-sensitive sodium chloride cotransporter, NCC, which mediates sodium chloride reabsorption in the DCT. These studies have focused on the abundance of total and apical NCC as well as NCC phosphorylation, since NCC phosphorylation increases transport of sodium chloride [23, 24]. A consistent finding has been that high dietary potassium inhibits NCC expression and phosphorylation [25–28], whereas low dietary potassium has the opposite effect, increasing NCC expression and phosphorylation [29, 30, 28, 31]. In fact, even a single oral potassium “meal” is sufficient to rapidly suppress NCC and increase urinary sodium excretion [32].
Like potassium, a high sodium diet suppresses NCC, while a low sodium diet activates the transporter [29, 33]. How does the kidney handle the combination of high potassium/low sodium or low potassium/high sodium, in which there are opposing signals on NCC? In both cases, the potassium signal dominates. On a high potassium/low sodium diet, NCC is suppressed [26]. This is consistent with human data showing that increasing dietary potassium increases urinary sodium excretion, even in subjects on a very low sodium diet [34]. In animals on a low potassium/high sodium diet, NCC is activated [35,36], and this is also observed in human subjects, as determined by examination of phospho- and total NCC in urinary exosomes [36]. Because the typical Western diet is both high in sodium and low in potassium, the stimulation of NCC by a low potassium diet, even in the face of a high sodium intake, leads to increased NaCl reabsorption by the kidney and hypertension [36].
To summarize, high potassium inhibits sodium chloride reabsorption through NCC in the distal convoluted tubule, shunting sodium downstream to the aldosterone-sensitive distal nephron, where reabsorption through the epithelial sodium channel generates a lumen-negative charge that drives potassium secretion. Conversely, low potassium activates sodium chloride reabsorption in the distal convoluted tubule (Figure 2). In individuals consuming a low potassium/high sodium diet, as is typical in modern society, this leads to plasma volume expansion and increased blood pressure.
Figure 2.
Summary of dietary potassium effects on the thiazide-sensitive sodium chloride cotransporter (NCC). High dietary potassium intake inhibits NCC, increasing sodium delivery to the more distal aldosterone-sensitive distal nephron (ASDN) where potassium secretion occurs (Figure 1), while low dietary potassium stimulates the transporter. This occurs in both low and high dietary sodium conditions. Thus, in the face of a low potassium/high sodium diet, NCC is activated and NaCl reabsorption is increased, resulting in extracellular volume expansion and hypertension.
Modes of sodium reabsorption in the aldosterone-sensitive distal nephron
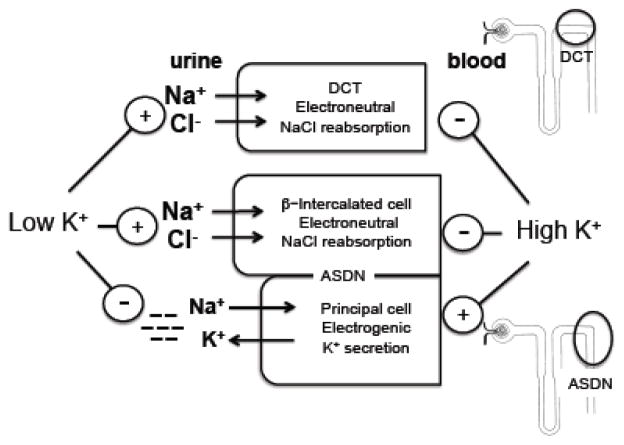
In the aldosterone-sensitive distal nephron, there are at least three “modes” of sodium reabsorption, which influence the degree of potassium secretion. Electrogenic reabsorption of sodium by the principal cell, which generates a negative charge in the tubule lumen, can either drive potassium secretion, as described above, or, alternatively, can drive paracellular chloride reabsorption [16]. Claudin-4 and claudin-8 are components of the paracellular pathway that mediate chloride flux [37]. Knockout of either claudin-4 or claudin-8 in mice results in renal salt wasting, and, in the case of claudin-8 knockout, hypokalemia [38, 39], suggesting that decreasing paracellular chloride reabsorption increases potassium secretion. A third pathway is electroneutral sodium chloride reabsorption through non-α-non-β- and β-intercalated cells [40] [41,42] [43], which are also known as “pendrin-positive intercalated cells” based on the presence of the pendrin transporter. Non-electrogenic sodium reabsorption through this pathway is expected to spare potassium secretion.
In conditions of volume depletion or low sodium intake, all modes of sodium reabsorption in the distal nephron are stimulated: electroneutral sodium chloride reabsorption by the sodium chloride cotransporter in the distal convoluted tubule [44]; electroneutral sodium chloride reabsorption by the pendrin-positive intercalated cell [43]; and sodium reabsorption by the epithelial sodium channel in the principal cell of the aldosterone-sensitive distal nephron [45, 46]. However, sodium reabsorption by more upstream segments typically reduces sodium delivery to the principal cell, thereby limiting potassium excretion in the ASDN during low sodium conditions. Like low sodium, low potassium also stimulates sodium chloride reabsorption by the DCT, as discussed above. The pendrin-positive intercalated cell is also potassium-sensitive, with higher expression of transporters on relatively lower potassium diets [47]. In contrast, epithelial sodium channel activity in the ASDN principal cell is suppressed in conditions of low dietary potassium, even when dietary sodium is also low (Figure 3) [30], thereby sparing potassium secretion. However, in low potassium/high sodium conditions, stimulation of the electroneutral pathways in the distal convoluted tubule and through the pendrin-positive intercalated cells will lead to excess sodium retention, plasma volume expansion, and increased blood pressure.
Figure 3.
Effects of potassium intake on electroneutral vs. electrogenic sodium reabsorption. Similar to a low sodium diet, low dietary potassium intake stimulates electroneutral NaCl reabsorption, decreasing the availability of sodium to ENaC in the aldosterone-sensitive distal nephron (ASDN) principal cell and thereby limiting potassium secretion. A low potassium diet also directly inhibits ENaC activity. High dietary potassium has the opposite effect.
DCT: distal convoluted tubule
Intrarenal paracrine regulation of the distal nephron by the proximal tubule
What homeostatic responses occur in the kidney as a result of volume depletion and hypokalemia? A recent paper illustrates an integrated response and unveils interesting paracrine regulation between the proximal tubule and distal nephron [48]. In this study, STE20/SPS1-related proline/alanine-rich kinase (SPAK) was knocked out. SPAK is a key activator of NCC, as discussed below, and the SPAK knockout mice have a Gitelman’s-like phenotype, with renal salt wasting, volume contraction, and mild hypokalemia (~3.4 mEq/L) [49, 50, 48]; this phenotype is similar to chronic thiazide administration. In response to volume depletion, there is upregulation of the pendrin-positive intercalated cell: the number of these cells increases relative to α-intercalated cells, and there is upregulation of the transport machinery [48]. Ammoniagenesis is upregulated in the proximal cell, presumably in response to hypokalemia, with metabolism of glutamine to α-ketoglutarate. In the SPAK knockout mice, the proximal tubule transport machinery favors transport of the α-ketoglutarate into the lumen, with a three-fold increase in urinary α-ketoglutarate levels [48]. Prior work showed that α-ketoglutarate acts on the seven transmembrane receptor, Oxgr1, to stimulate sodium chloride reabsorption by the pendrin-positive intercalated cell, and inhibits sodium reabsorption through ENaC in the principal cell. These effects will decrease potassium secretion by favoring sodium reabsorption through the electroneutral pathway in the pendrin-positive intercalated cell, and decreasing sodium reabsorption through the electrogenic pathway in the principal cell [51]. Indeed, Oxgr1 is upregulated in the pendrin-positive intercalated cell in the SPAK knockout mice [48]. Since the pendrin-positive intercalated cell also secretes bicarbonate, metabolic alkalosis is also limited [51].
The importance of the pendrin-positive intercalated cell is also illustrated by findings from mice lacking the sodium chloride cotransporter. Mice in which both NCC and pendrin are knocked out have a more dramatic degree of renal salt wasting, volume depletion, and metabolic alkalosis than is seen when either transporter is knocked out individually [52]. Similarly, individuals with Pendred syndrome, carrying mutations in pendrin, have goiter and deafness, but typically do not have renal manifestations at baseline. However, upon challenge with a thiazide diuretic, a child with Pendred syndrome rapidly developed severe volume depletion and hypokalemic metabolic alkalosis [53].
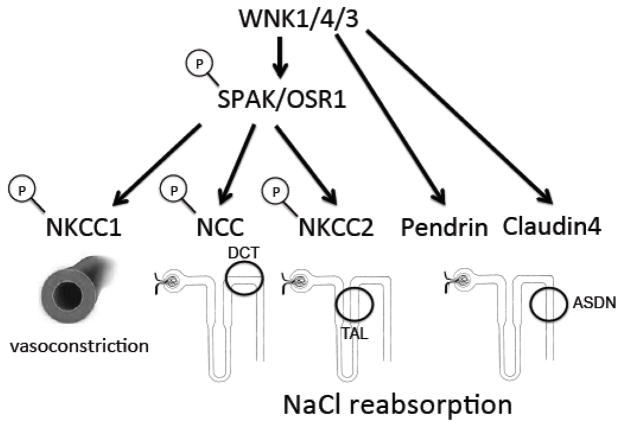
The role of the WNK-SPAK/OSR1 signaling pathway
The first With-no-lysine (WNK) kinase was cloned in 2000 by Cobb and colleagues [54]. The following year, Lifton’s group showed that WNK1 and WNK4 are mutated in a human disorder, pseudohypoaldosteronism type II (also called Gordon’s syndrome or familial hyperkalemia with hypertension), characterized by hypertension and hyperkalemia [55]. Subsequent work showed that WNKs phosphorylate and activate two related downstream kinases, SPAK and oxidative-stress response (OSR1) (reviewed in [56]). SPAK and OSR1 then phosphorylate the N-termini of the related sodium-coupled chloride cotransporters, which include NCC and the sodium-potassium-2-chloride cotransporters, NKCC1 and NKCC2, resulting in transporter activation [56]. In fact, WNK-SPAK/OSR1 regulation of ion flux through NKCCs is evolutionarily ancient: this pathway regulates transepithelial potassium flux and fluid secretion in the Drosophila renal tubule [57, 58]. WNK1 and WNK4 also have a positive regulatory effect on the pendrin-positive intercalated cell [47] and may also positively regulate the paracellular Cl− reabsorption pathway in the ASDN [56]. In vivo studies have confirmed the importance of WNK-SPAK/OSR1 signaling in stimulating sodium reabsorption through NCC in the distal convoluted tubule and NKCC2 in the thick ascending limb [56]. In addition, WNK1 and WNK3 regulate vascular contractility through their regulation of NKCC1 in the vasculature (reviewed in [59]). Thus, WNKs overall promote renal NaCl reabsorption in multiple nephron segments, as well as vasoconstriction (Figure 4), explaining the hypertensive phenotype of gain-of-function alleles of WNK1 and WNK4. The stimulation of electroneutral sodium chloride reabsorption, as well as effects on K+ secretory channels in the ASDN [56], explains hyperkalemia in these patients.
Figure 4.
WNK kinases stimulate vasoconstriction and NaCl reabsorption. WNK kinases phosphorylate and activate two related kinases, SPAK and OSR1. SPAK and OSR1 phosphorylate the N-terminus of the related sodium-coupled chloride cotransporters, NKCC1 in the vasculature, NCC in the distal convoluted tubule, and NKCC2 in the thick ascending limb of the loop of Henle. Phosphorylation results in transporter activation, leading to vasoconstriction and increased sodium chloride reabsorption in multiple nephron segments. In addition, WNKs have positive regulatory effects on pendrin in the β-intercalated cells and paracellular chloride transport in the ASDN, mediated by claudin-4, which again will increase renal sodium chloride reabsorption. The overall effect of WNK signaling is to promote vasoconstriction and NaCl reabsorption.
DCT: distal convoluted tubule, TAL: thick ascending limb, ASDN: aldosterone-sensitive distal nephron
Because WNK-SPAK/OSR1 signaling is a key regulator of NCC, recent work has focused on the effects of dietary potassium on the WNK-SPAK/OSR1 pathway. A low potassium diet increases WNK phosphorylation [36], as well as SPAK phosphorylation [36, 31, 28], which will increase NCC phosphorylation and activity.
An unsolved mystery is the mechanism by which the WNK-SPAK/OSR1 pathway senses changes in dietary potassium. One proposal is that changes in extracellular potassium alter the voltage of the distal convoluted cell, driving changes in intracellular Cl− [36]. A crystallographic study proved that WNK is a chloride-sensitive kinase: chloride binds directly to the active site of WNK, inhibiting the autophosphorylation required for activation [60]. WNK4, the dominant NCC-regulating WNK in the DCT, is particularly Cl−-sensitive in vitro, and there is a correlation between plasma potassium concentration and NCC phosphorylation [61]. In cells, WNK activation occurs when intracellular Cl− is lowered. Whether this also occurs in the DCT is not currently known, but modeling studies support the hypothesis that changes in intracellular Cl− could explain alterations in transepithelial flux in the DCT in the presence of varying serum potassium [36].
Effects of aldosterone – recent findings
Although the importance of aldosterone in promoting renal potassium excretion has long been understood, new mechanisms for this have been demonstrated. Phosphorylation of the mineralocorticoid receptor on serine 843 occurs in the intercalated cell of the distal nephron and renders the mineralocorticoid receptor insensitive to aldosterone [47]. According to this model, aldosterone upregulates the transport machinery of the pendrin-positive intercalated cell, which would increase electroneutral NaCl reabsorption, as discussed above. In conditions of volume depletion, angiotensin II signaling, or activation of WNK1 and WNK4, Ser 843 is dephosphorylated and the mineralocorticoid receptor is responsive to aldosterone [47]. In contrast, in high potassium conditions, Ser 843 is phosphorylated and the intercalated cell is unresponsive to aldosterone [47], decreasing electroneutral sodium chloride reabsorption as compared to the electrogenic sodium reabsorption that promotes potassium secretion.
Potassium: Foe – Deleterious Effects of Potassium Excess
Despite the benefits of potassium intake outlined above, potassium excess can be problematic in patients with impaired potassium excretion. The most dreaded complication is ventricular fibrillation [62] leading to sudden death. Therefore, the clinician must understand factors which predispose patients to developing hyperkalemia, and manage this electrolyte complication appropriately when it arises. Fortunately, novel therapies are in development that may advance treatment of hyperkalemia.
Risk factors for hyperkalemia
Potassium is the most abundant intracellular cation and its concentration in the extracellular space is low. This is due to the action of the Na+/K+-ATPase, which pumps three Na+ ions out of the cell in exchange for two K+ ions. Thus, 98 % of total body potassium (~3400 mEq) is found in intracellular stores, chiefly in muscle, with smaller amounts in red blood cells, liver, and the remaining cells of the body. Only 2 % (~65 mEq) of total body potassium is in the extracellular space [63]. 90 % of ingested potassium is excreted through the kidney, whereas 10 % is excreted in stool [16]. Thus, most cases of hyperkalemia are due either to abnormal shifts of potassium from the intracellular compartment to the extracellular compartment (eg rhabdomyolysis, tumor lysis), or to dysfunction of renal potassium excretion [64].
Because of the importance of aldosterone in maximizing renal potassium excretion, medications and conditions, including congenital disorders, that impair the renin-angiotensin-aldosterone system (RAAS) are frequent culprits in the development of hyperkalemia [65] (Table 3). Because 90 % of potassium excretion occurs through the kidney, decreased GFR is also a powerful predictor of hyperkalemia [66–71].
Table 3.
| Drugs and Conditions which Increase Risk for Hyperkalemia |
|---|
| Diabetes mellitus |
| Advanced age |
| Beta-blockers |
| Nonsteroidal anti-inflammatory drugs |
| Angiotensin converting enzyme inhibitors |
| Angiotensin receptor blockers |
| Adrenal insufficiency, congenital adrenal hyperplasia |
| Aldosterone synthase deficiency or inhibitors (heparin, ketoconazole) |
| Loss-of-function mutations in the mineralocorticoid receptor or antagonists (spironolactone, eplerenone, drosperinone) |
| Loss-of-function mutations in the epithelial sodium channel or inhibitors (amiloride, triamterene, trimethoprim, pentamidine) |
| Decreased glomerular filtration rate |
Recent studies have highlighted the ways in which multiple risk factors for hyperkalemia often exist in patients who develop overt hyperkalemia. Trimethoprim, which inhibits the epithelial sodium channel, is illustrative. In a randomized controlled trial of 97 outpatients treated for various infections with trimethoprim-sulfamethoxazole (TMP) vs. other antibiotics, serum potassium increased in 81 % of the TMP group [72]. This was not clinically significant in most of the patients. However, three patients with additional risk factors for hyperkalemia (older age, impaired GFR, or diabetes mellitus) developed a serum potassium concentration of >6 mEq/L [72]. A quartet of studies has examined the risk of hyperkalemia in older patients (>66 yo) prescribed an ACE (angiotensin converting enzyme) inhibitor, ARB (angiotensin receptor blocker) or the mineralocorticoid receptor antagonist spironolactone, who were subsequently prescribed TMP. Using a nested case-control design, the investigators demonstrated that TMP increased the risk of both hyperkalemia and sudden death in this population when compared to the control antibiotic, amoxicillin [73–76]. Thus, deleterious effects are seen in the presence of a combination of three risk factors for hyperkalemia – older age; use of an ACE inhibitor, ARB or spironolactone; and use of trimethoprim. Another study found that, of patients admitted to an emergency room with hyperkalemia, 95 % of patients with a serum potassium of >7 mEq/L were taking at least one medication that interferes with potassium secretion, 75 % were taking two such drugs, and 90 % had an impaired GFR [77]. The concept of additive risks – either with dual RAAS blockade, or RAAS blockade in the context of congestive heart failure or CKD – is also consistent with findings from clinical trials [78, 79].
Aldosterone-independent potassium secretion
Two recent studies highlight that the risk of hyperkalemia from RAAS blockade is most pronounced within the first month of initiation. In the first study, 6575 hypertensive patients were studied. Spironolactone was added at a mean dose of 42 mg (in non-CKD patients) or 36 mg (in CKD patients). At 4 weeks, serum potassium increased in both CKD and non-CKD patients, but by 8 weeks, serum potassium normalized in both groups. The incidence of hyperkalemia (serum potassium >5 mEq/L) was 43 % in the non-CKD group at 4 weeks and 50 % in the CKD group, but returned to baseline levels of 3–4 % by 8 weeks [70]. A second study with a different design, a population-based case-control study in a cohort of patients with newly diagnosed congestive heart failure, also found that the odds of hyperkalemia were highest within the first month of initiation of an ACE inhibitor or spironolactone and decreased over time [68].
Several conclusions can be drawn from these studies. First, clinicians should monitor serum potassium early after initiation of RAAS blockers, with timely follow-up of abnormal results – an area that has been identified as a safety concern [80, 81]. Second, if hyperkalemia is mild, it may be possible to continue the RAAS blocker, as long as serum potassium is carefully monitored. The improvement in serum potassium that occurs over time is likely due to adaptive changes in the kidney. Although aldosterone is an important mediator of renal (and colonic) potassium excretion, abundant literature describes aldosterone-independent potassium secretion [82–87].
Indeed, a recent paper examined potassium handling in aldosterone synthase knockout mice, which lack the ability to synthesize aldosterone. These mice could tolerate a moderate dietary potassium load, but died on a very high potassium diet. The sodium chloride cotransporter was downregulated to promote distal sodium delivery, and both ENaC and ROMK were upregulated. Interestingly, angiotensin signaling appeared to play a permissive role for potassium secretion, since treatment with losartan rendered the mice unable to handle moderate dietary potassium [88]. This is consistent with clinical data showing that dual RAAS blockade raises serum potassium more than single RAAS blockade [89, 78]. The aldosterone synthase knockout study also found that colonic potassium secretion was entirely aldosterone-dependent [88], consistent with clinical data showing that hyperkalemia complicates the use of eplerenone in patients on hemodialysis [90].
Preventing hyperkalemia in high-risk patients
The prevention of hyperkalemia in high-risk patients has previously been reviewed [65]. Management includes discontinuation of NSAIDs and hyperkalemic herbal preparations; use of diuretics; correcting metabolic acidosis; and prescribing a low potassium diet. If these measures are unsuccessful, lower doses or discontinuation of medications that cause hyperkalemia may be necessary [65].
While hyperkalemia is problematic, hypokalemia is also associated with worse outcomes in patients with CKD. For example, a potassium concentration below 4 mEq/L, even in the range of 3.5 – 3.9 mEq/L, was associated with increased mortality in a population with CKD and congestive heart failure [91]. Two other studies also found a U-shaped association between serum potassium and mortality in patients with CKD, with increased mortality, cardiovascular events, and end-stage renal disease in patients with a serum potassium below 4 mEq/L [69, 92]. Thus, even mild degrees of hypokalemia confer increased risk of mortality. Therefore, a low potassium diet should not routinely be prescribed in patients with normal serum potassium concentrations, particularly if they are on loop or thiazide diuretics that result in renal potassium wasting. However, there is a linear association between the risk of hyperkalemia and dietary potassium intake in patients with CKD, so patients should be appropriately monitored [9].
Hyperkalemia management
Management of hyperkalemia has been reviewed [93]. Acutely, an electrocardiogram should be immediately obtained to assess for cardiac toxicity, and intravenous calcium administered if electrocardiographic changes are present. The second step involves shifting potassium from the extracellular space to the intracellular space using insulin and beta agonist therapy. These increase uptake of potassium through the Na+/K+-ATPase into muscle. Interestingly, even in patients with “insulin resistance” in terms of glucose uptake in response to insulin, uptake of potassium is not impaired [94]. Finally, potassium must be definitively eliminated either through the kidney, the gut, or dialysis. Because most potassium excretion is through the kidney, in a patient who is not oliguric or anuric, loop diuretics can be effective in eliminating potassium by increasing distal delivery of sodium and distal flow rates. Large doses of a loop diuretic may be needed in patients with impaired GFR. For patients with hypovolemia, concomitant administration of isotonic fluids can help prevent worsening volume depletion, and in patients with a prerenal decrease in GFR due to hypovolemia, saline alone may be sufficient to improve GFR and resolve hyperkalemia.
Until recently, sodium polystyrene sulfonate (SPS) was the only approved potassium-binding resin in the United States for gastrointestinal elimination of potassium. However, the safety of this medication was called into question when reports began to emerge of cases of colonic necrosis [95], and SPS with 70 % sorbitol now carries a black box warning [96]. The estimated incidence of this complication, based on the largest available study, is 0.14 %, with a number needed to harm of 1395 [97]. Another potential adverse effect of SPS is extracellular volume expansion: a 60 g dose of SPS contains 65 mEq of sodium.
A second question that has been raised is whether SPS is effective in lowering potassium [95, 93]. A small randomized controlled study was recently published, in which 33 outpatients with CKD and baseline serum potassium of 5.0 – 5.9 mEq/L were randomized to placebo or 30 g SPS daily. At the end of 1 week, serum potassium in the SPS group was 1.04 mEq/L lower than the placebo group. However, hypocalcemia and hypomagnesemia occurred in a substantial fraction of the SPS group (19 % and 31 % respectively) [98]. A larger retrospective study of inpatients evaluated the response to a single dose of SPS. The investigators observed a dose-dependent decrease in serum potassium, ranging from 0.82 ± 0.48 mEq/L for the 15 g dose, to a 1.40 ± 0.42 mEq/L decrease for the 60 g dose. 94 % of patients normalized serum potassium with a single dose, though the mean starting potassium concentration was <6 mEq/L in all four groups [99]. A second retrospective study also demonstrated dose-dependent decreases in serum potassium concentration after SPS administration [100].
RAAS blockers are effective for the treatment of conditions which simultaneously predispose to hyperkalemia, such as congestive heart failure and diabetic nephropathy [101–105]. Given the limitations of SPS outlined above, intense effort has been focused on developing new potassium-lowering drugs. Two agents are in advanced clinical trials. Patiromer, also known as RLY5016, is a non-absorbed polymer that binds potassium in the gastrointestinal tract. Results from the PEARL-HF [106], OPAL-HK [107] and AMETHYST-DN [108] studies are summarized in Table 4. Patiromer effectively decreases serum potassium concentrations in high-risk patients on RAAS blockers, including those with heart failure, CKD, and diabetic nephropathy. The most frequent adverse events are hypomagnesemia and gastrointestinal side effects, but the number of patients studied (648) has been relatively small and the longest follow-up to date has been one year. Furthermore, whether patiromer has beneficial effects on clinical outcomes beyond hyperkalemia is unknown. Nevertheless, patiromer was approved by the Food and Drug Administration in October 2015, albeit with a black box warning that it may bind other orally administered drugs and decrease their intestinal absorption [109]. Therefore, the warning recommends that patiromer should be administered six hours apart from other orally administered medications – a challenge for patients with multiple comorbidities on many medications. Simultaneously, the FDA also required further studies on SPS to determine whether it also binds orally administered medications [110].
Table 4. Summary of 3 clinical trials examining patiromer safety and efficacy.
| Study | Patients | Drug | Outcome | Adverse effect |
|---|---|---|---|---|
| PEARL-HF [106] | 105 pts with CHF+CKD or prior discontinuation of RAAS blocker or beta blocker due to hyperkalemia | Spironolactome + patiromer or placebo, 4 weeks | Placebo: 25% K > 5.5 mEq/L Patiromer: 7% K > 5.5 mEq/L |
24% of patiromer patients Mg < 1.8 mg/dL |
| OPAL-HK [107] | 237 pts with CKD stages 3/4 on RAAS blockade | Patiromer, 8 weeks Dose adjustments required in 60% of patients, mostly on days 3 and 7 |
After drug withdrawal: Hyperkalemia in 90% in placebo group vs. 43% in patiromer group Able to continue RAAS blocker: 44% in placebo group vs. 94% in patiromer group |
9 patients in patiromer group required Mg replacement |
| AMETHYST-DN [108] | 306 pts with diabetic nephropathy on ACE-I/ARB + spironolactone | Patiromer, 1 year | Significant decrease in serum K+ throughout the study period | Hypomagnesemia; constipation; 30% of patients discontinued treatment |
CHF, congestive heart failure. CKD, chronic kidney disease. RAAS blocker, renin-angiotensin-aldosterone system blocker. ACE-I, angiotensin converting enzyme inhibitor. ARB, angiotensin receptor blocker.
Sodium zirconium cyclosilicate, also known as ZS-9, is a non-absorbed microporous compound whose pore size renders it highly selective for potassium ions as compared to calcium or magnesium ions [111]. ZS-9 has been evaluated in three clinical trials to date [112] [113] [114], which are summarized in Table 5. ZS-9 rapidly lowered serum potassium concentrations, and the effect was maintained for up to 4 weeks. Hypomagnesemia was not observed, presumably because of the high selectivity of ZS-9 for potassium, but GI side effects and edema were seen in patients on higher doses of the drug. Finally, the investigators of the prior trials analyzed 45 patients with baseline serum potassium of 6.0 mEq/L who received a 10 g dose of ZS-9. They reported that after a single 10 g dose, serum potassium decreased by 0.4 mEq/L at 1 hour, 0.6 mEq/L at 2 hours, and 0.7 mEq/L at 4 hours. The median time to a serum potassium level <6.0 mEq/L was 1.07 hours, and the median time to a serum potassium level <5.5 mEq/L was 4 hours [115]. In contrast, after an 8.4 g dose of patiromer, the first statistically significant reduction in serum potassium occurred after 7 hours, and was modest – 0.21 mEq/L. The median time to a serum potassium <5.5 mEq/L (from a starting point of 5.93 mEq/L) was 12.7 hours, occurring after a second dose of patiromer was administered at hour 10. Thus, patiromer appears to act less rapidly than ZS-9 in lowering serum potassium acutely [116]. Like patiromer, the effects of ZS-9 on clinical outcomes beyond serum potassium concentration have not been evaluated.
Table 5. Summary of 3 clinical trials examining safety and efficacy of sodium zirconium cyclosilicate (ZS-9).
| Study | Patients | Drug | Outcome | Adverse effect |
|---|---|---|---|---|
| Phase 2, double-blind, placebo controlled dose-escalation study [112] | 90 pts with CKD stage 3 | ZS-9: 0.3 g, 3 g, or 10 g × 48 hours | K+ lowering 2 days:
|
GI side effects, esp at 10 g dose |
| Phase 3, two-stage, double-blind, placebo-controlled [113] | 753 pts with hyperkalemia, K+ 5.0–6.5 mEq/L CKD - 75% DM - 60% CHF - 40% RAAS blockers - 67% |
ZS-9: placebo, 1.25 g, 2.5 g, 5 g or 10 g × 2 days. If K 3.5–4.9 mEq/L after 2 days (543 pts), received same ZS-9 dose vs. placebo × 2 weeks. |
Dose-dependent lowering of serum K+ in first 48 hours. Days 2–14, maintenance of serum K+ in ZS-9 groups vs increase in placebo groups |
Similar between placebo and ZS-9 groups |
| HARMONIZE [114] | 258 pts with hyperkalemia, serum K+ ≥ 5.1 mEq/L CKD - 66% DM - 66% CHF - 36% RAAS blockers - 70% |
ZS-9 10 g × 2 days (open-label), then placebo or ZS-9 5 g, 10 g or 15 g × 4 weeks | 92% achieved normokalemia during the first 2 days of ZS-9 10 g. Median time to normalization, 2.2h. During 4 week maintenance phase, mean serum K+ by dose:
|
Hypokalemia in ~10% of patients in 10 g and 15 g groups; edema in 2.4% of placebo group, 2.2% of 5 g group, 5.9% of 10 g group and 14.3 % of 15 g groups |
CKD, chronic kidney disease. GI, gastrointestinal. DM, diabetes mellitus. CHF, congestive heart failure. RAAS, renin-angiotensin-aldosterone system.
Summary
Dietary potassium intake lowers blood pressure and is associated with decreased risks of cardiovascular morbidity, overall mortality and progression of renal disease. Some of the benefits from potassium are likely due to the interrelated handling of sodium and potassium in the kidney. Specifically, potassium inhibits sodium reabsorption by the kidney, while a low potassium diet enhances renal sodium reabsorption, even with a concomitant high sodium diet. Therefore, current guidelines recommend dietary potassium intake in the range of 90 to 120 mmol/day, well above usual intake in American and worldwide populations. In some patients, however, excess dietary potassium intake results in hyperkalemia. Patients at risk include older patients and those with CKD, congestive heart failure, or diabetes mellitus, especially with concomitant use of medications that inhibit the RAAS, whether as an on-target effect (eg ACE inhibitors, ARBs or mineralocorticoid receptor antagonists), or as an off-target effect (eg trimethoprim). Appropriate monitoring is required when using these beneficial medications in high-risk populations. Concerns about the toxicity of SPS for hyperkalemia management have led to the development of two new drugs: patiromer, which has recently been approved in the United States, and sodium zirconium cyclosilicate (ZS-9), for which the Food and Drug Administration has accepted a new drug application filing.
Acknowledgments
The author would like to thank Dr. Jyothsna Gattineni for helpful discussion. ARR is supported by NIH grants DK091316 and DK106350.
Footnotes
Conflict of interest: The author declares no conflict of interest.
References
- 1.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 2.Keith NM, Binger MW. Diuretic action of potassium. J Am Med Assoc. 1935;105:1584–1591. [Google Scholar]
- 3.Barker M. Edema as influenced by a low ratio of sodium to potassium intake: Clinical observations. J Am Med Assoc. 1932;98:2193–2197. [Google Scholar]
- 4.Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ. 2013;346:f1378. doi: 10.1136/bmj.f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. The National Academies Press; Washington, DC: 2005. [Google Scholar]
- 6.Mente A, O’Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, Morrison H, Li W, Wang X, Di C, Mony P, Devanath A, Rosengren A, Oguz A, Zatonska K, Yusufali AH, Lopez-Jaramillo P, Avezum A, Ismail N, Lanas F, Puoane T, Diaz R, Kelishadi R, Iqbal R, Yusuf R, Chifamba J, Khatib R, Teo K, Yusuf S PURE Investigators. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371:601–611. doi: 10.1056/NEJMoa1311989. [DOI] [PubMed] [Google Scholar]
- 7.Cogswell ME, Zhang Z, Carriquiry AL, Gunn JP, Kuklina EV, Saydah SH, Yang Q, Moshfegh AJ. Sodium and potassium intakes among US adults: NHANES 2003–2008. Am J Clin Nutr. 2012;96:647–657. doi: 10.3945/ajcn.112.034413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, Rosengren A, Lopez-Jaramillo P, Diaz R, Avezum A, Lanas F, Yusoff K, Iqbal R, Ilow R, Mohammadifard N, Gulec S, Yusufali AH, Kruger L, Yusuf R, Chifamba J, Kabali C, Dagenais G, Lear SA, Teo K, Yusuf S PURE Investigators. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–623. doi: 10.1056/NEJMoa1311889. [DOI] [PubMed] [Google Scholar]
- 9.Smyth A, Dunkler D, Gao P, Teo KK, Yusuf S, O’Donnell MJ, Mann JF, Clase CM ONTARGET and TRANSCEND investigators. The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int. 2014;86:1205–1212. doi: 10.1038/ki.2014.214. [DOI] [PubMed] [Google Scholar]
- 10.Araki S, Haneda M, Koya D, Kondo K, Tanaka S, Arima H, Kume S, Nakazawa J, Chin-Kanasaki M, Ugi S, Kawai H, Araki H, Uzu T, Maegawa H. Urinary Potassium Excretion and Renal and Cardiovascular Complications in Patients with Type 2 Diabetes and Normal Renal Function. Clin J Am Soc Nephrol. 2015;10:2152–2158. doi: 10.2215/CJN.00980115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He J, Mills KT, Appel LJ, Yang W, Chen J, Lee BT, Rosas SE, Porter A, Makos G, Weir MR, Hamm LL, Kusek JW Chronic Renal Insufficiency Cohort Study Investigators. Urinary Sodium and Potassium Excretion and CKD Progression. J Am Soc Nephrol. 2016;27:1202–1212. doi: 10.1681/ASN.2015010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geleijnse JM, Grobbee DE, Hofman A. Sodium and potassium intake and blood pressure change in childhood. BMJ. 1990;300:899–902. doi: 10.1136/bmj.300.6729.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buendia JR, Bradlee ML, Daniels SR, Singer MR, Moore LL. Longitudinal effects of dietary sodium and potassium on blood pressure in adolescent girls. JAMA Pediatr. 2015;169:560–568. doi: 10.1001/jamapediatrics.2015.0411. [DOI] [PubMed] [Google Scholar]
- 14.Womersley RA, Darragh JH. Potassium and Sodium Restriction in the Normal Human. J Clin Invest. 1955;34:456–461. doi: 10.1172/JCI103094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishna GG, Miller E, Kapoor S. Increased Blood-Pressure during Potassium- Depletion in Normotensive Men. N Engl J Med. 1989;320:1177–1182. doi: 10.1056/NEJM198905043201804. [DOI] [PubMed] [Google Scholar]
- 16.Malnic G, Giebisch G, Muto S, Wang W, Bailey MA, Satlin LM. Chapter 49 - Regulation of K+ Excretion. In: Caplan RJAWM, editor. Seldin and Giebisch’s The Kidney. 5. Academic Press; 2013. pp. 1659–1715. [Google Scholar]
- 17.Welling PA. Regulation of renal potassium secretion: molecular mechanisms. Seminars in nephrology. 2013;33:215–228. doi: 10.1016/j.semnephrol.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Brandis M, Keyes J, Windhager EE. Potassium-induced inhibition of proximal tubular fluid reabsorption in rats. Am J Physiol. 1972;222:421–427. doi: 10.1152/ajplegacy.1972.222.2.421. [DOI] [PubMed] [Google Scholar]
- 19.Stokes JB. Consequences of potassium recycling in the renal medulla. Effects of ion transport by the medullary thick ascending limb of Henle’s loop. J Clin Invest. 1982;70:219–229. doi: 10.1172/JCI110609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Battilana CA, Dobyan DC, Lacy FB, Bhattacharya J, Johnston PA, Jamison RL. Effect of chronic potassium loading on potassium secretion by the pars recta or descending limb of the juxtamedullary nephron in the rat. J Clin Invest. 1978;62:1093–1103. doi: 10.1172/JCI109215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higashihara E, Kokko JP. Effects of aldosterone on potassium recycling in the kidney of adrenalectomized rats. Am J Physiol. 1985;248:F219–227. doi: 10.1152/ajprenal.1985.248.2.F219. [DOI] [PubMed] [Google Scholar]
- 22.Cheng CJ, Truong T, Baum M, Huang CL. Kidney-specific WNK1 inhibits sodium reabsorption in the cortical thick ascending limb. Am J Physiol Renal Physiol. 2012;303:F667–673. doi: 10.1152/ajprenal.00290.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pacheco-Alvarez D, Cristobal PS, Meade P, Moreno E, Vazquez N, Munoz E, Diaz A, Juarez ME, Gimenez I, Gamba G. The Na+:Cl− cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem. 2006;281:28755–28763. doi: 10.1074/jbc.M603773200. [DOI] [PubMed] [Google Scholar]
- 24.Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR. Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121:675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]
- 25.Wade JB, Fang L, Coleman RA, Liu J, Grimm PR, Wang T, Welling PA. Differential regulation of ROMK (Kir1.1) in distal nephron segments by dietary potassium. Am J Physiol Renal Physiol. 2011;300:F1385–1393. doi: 10.1152/ajprenal.00592.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AH, Fenton RA, Zietse R, Hoorn EJ. K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl− cotransporter. Am J Physiol Renal Physiol. 2013;305:F1177–1188. doi: 10.1152/ajprenal.00201.2013. [DOI] [PubMed] [Google Scholar]
- 27.Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA. Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol. 2014;306:F1059–1068. doi: 10.1152/ajprenal.00015.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castaneda-Bueno M, Cervantes-Perez LG, Rojas-Vega L, Arroyo-Garza I, Vazquez N, Moreno E, Gamba G. Modulation of NCC activity by low and high K(+) intake: insights into the signaling pathways involved. Am J Physiol Renal Physiol. 2014;306:F1507–1519. doi: 10.1152/ajprenal.00255.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na+-Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol. 2009;297:F704–712. doi: 10.1152/ajprenal.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frindt G, Houde V, Palmer LG. Conservation of Na+ vs. K+ by the rat cortical collecting duct. Am J Physiol Renal Physiol. 2011;301:F14–20. doi: 10.1152/ajprenal.00705.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wade JB, Liu J, Coleman R, Grimm PR, Delpire E, Welling PA. SPAK-mediated NCC regulation in response to low-K+ diet. Am J Physiol Renal Physiol. 2015;308:F923–931. doi: 10.1152/ajprenal.00388.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int. 2013;83:811–824. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 33.Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S. Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int. 2008;74:1403–1409. doi: 10.1038/ki.2008.451. [DOI] [PubMed] [Google Scholar]
- 34.Turban S, Thompson CB, Parekh RS, Appel LJ. Effects of sodium intake and diet on racial differences in urinary potassium excretion: results from the Dietary Approaches to Stop Hypertension (DASH)-Sodium trial. Am J Kidney Dis. 2013;61:88–95. doi: 10.1053/j.ajkd.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 35.Vitzthum H, Seniuk A, Schulte LH, Muller ML, Hetz H, Ehmke H. Functional coupling of renal K+ and Na+ handling causes high blood pressure in Na+ replete mice. J Physiol. 2014;592:1139–1157. doi: 10.1113/jphysiol.2013.266924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015;21:39–50. doi: 10.1016/j.cmet.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci U S A. 2010;107:18010–18015. doi: 10.1073/pnas.1009399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong Y, Wang J, Yang J, Gonzales E, Perez R, Hou J. KLHL3 regulates paracellular chloride transport in the kidney by ubiquitination of claudin-8. Proc Natl Acad Sci U S A. 2015;112:4340–4345. doi: 10.1073/pnas.1421441112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong Y, Yu M, Yang J, Gonzales E, Perez R, Hou M, Tripathi P, Hering-Smith KS, Hamm LL, Hou J. The Cap1-claudin-4 regulatory pathway is important for renal chloride reabsorption and blood pressure regulation. Proc Natl Acad Sci U S A. 2014;111:E3766–3774. doi: 10.1073/pnas.1406741111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terada Y, Knepper MA. Thiazide-sensitive NaCl absorption in rat cortical collecting duct. Am J Physiol. 1990;259:F519–528. doi: 10.1152/ajprenal.1990.259.3.F519. [DOI] [PubMed] [Google Scholar]
- 41.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Corniere N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R. Renal beta-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest. 2013;123:4219–4231. doi: 10.1172/JCI63492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chambrey R, Kurth I, Peti-Peterdi J, Houillier P, Purkerson JM, Leviel F, Hentschke M, Zdebik AA, Schwartz GJ, Hubner CA, Eladari D. Renal intercalated cells are rather energized by a proton than a sodium pump. Proc Natl Acad Sci U S A. 2013;110:7928–7933. doi: 10.1073/pnas.1221496110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest. 2010;120:1627–1635. doi: 10.1172/JCI40145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellison DH, Velazquez H, Wright FS. Adaptation of the distal convoluted tubule of the rat. Structural and functional effects of dietary salt intake and chronic diuretic infusion. J Clin Invest. 1989;83:113–126. doi: 10.1172/JCI113847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nesterov V, Dahlmann A, Krueger B, Bertog M, Loffing J, Korbmacher C. Aldosterone-dependent and -independent regulation of the epithelial sodium channel (ENaC) in mouse distal nephron. Am J Physiol Renal Physiol. 2012;303:F1289–1299. doi: 10.1152/ajprenal.00247.2012. [DOI] [PubMed] [Google Scholar]
- 46.Frindt G, Sackin H, Palmer LG. Whole-cell currents in rat cortical collecting tubule: low-Na diet increases amiloride-sensitive conductance. Am J Physiol. 1990;258:F562–567. doi: 10.1152/ajprenal.1990.258.3.F562. [DOI] [PubMed] [Google Scholar]
- 47.Shibata S, Rinehart J, Zhang J, Moeckel G, Castaneda-Bueno M, Stiegler AL, Boggon TJ, Gamba G, Lifton RP. Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab. 2013;18:660–671. doi: 10.1016/j.cmet.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grimm PR, Lazo-Fernandez Y, Delpire E, Wall SM, Dorsey SG, Weinman EJ, Coleman R, Wade JB, Welling PA. Integrated compensatory network is activated in the absence of NCC phosphorylation. J Clin Invest. 2015;125:2136–2150. doi: 10.1172/JCI78558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol. 2010;21:1868–1877. doi: 10.1681/ASN.2009121295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grimm PR, Taneja TK, Liu J, Coleman R, Chen YY, Delpire E, Wade JB, Welling PA. SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem. 2012;287:37673–37690. doi: 10.1074/jbc.M112.402800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tokonami N, Morla L, Centeno G, Mordasini D, Ramakrishnan SK, Nikolaeva S, Wagner CA, Bonny O, Houillier P, Doucet A, Firsov D. alpha-Ketoglutarate regulates acid-base balance through an intrarenal paracrine mechanism. J Clin Invest. 2013;123:3166–3171. doi: 10.1172/JCI67562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soleimani M, Barone S, Xu J, Shull GE, Siddiqui F, Zahedi K, Amlal H. Double knockout of pendrin and Na-Cl cotransporter (NCC) causes severe salt wasting, volume depletion, and renal failure. Proc Natl Acad Sci U S A. 2012;109:13368–13373. doi: 10.1073/pnas.1202671109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pela I, Bigozzi M, Bianchi B. Profound hypokalemia and hypochloremic metabolic alkalosis during thiazide therapy in a child with Pendred syndrome. Clin Nephrol. 2008;69:450–453. doi: 10.5414/cnp69450. [DOI] [PubMed] [Google Scholar]
- 54.Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem. 2000;275:16795–16801. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- 55.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 56.Hadchouel J, Ellison DH, Gamba G. Regulation of Renal Electrolyte Transport by WNK and SPAK-OSR1 Kinases. Annu Rev Physiol. 2016;78:367–389. doi: 10.1146/annurev-physiol-021115-105431. [DOI] [PubMed] [Google Scholar]
- 57.Rodan AR, Baum M, Huang CL. The Drosophila NKCC Ncc69 is required for normal renal tubule function. Am J Physiol Cell Physiol. 2012;303:C883–894. doi: 10.1152/ajpcell.00201.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Y, Schellinger JN, Huang CL, Rodan AR. Hypotonicity Stimulates Potassium Flux through the WNK-SPAK/OSR1 Kinase Cascade and the Ncc69 Sodium-Potassium-2-Chloride Cotransporter in the Drosophila Renal Tubule. J Biol Chem. 2014;289:26131–26142. doi: 10.1074/jbc.M114.577767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dbouk HA, Huang CL, Cobb MH. Hypertension: The Missing WNKs. Am J Physiol Renal Physiol. 2016 doi: 10.1152/ajprenal.00358.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ. Chloride Sensing by WNK1 Involves Inhibition of Autophosphorylation. Sci Signal. 2014;7:ra41. doi: 10.1126/scisignal.2005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int. 2015 doi: 10.1038/ki.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petrov DB. Images in clinical medicine. An electrocardiographic sine wave in hyperkalemia. N Engl J Med. 2012;366:1824. doi: 10.1056/NEJMicm1113009. [DOI] [PubMed] [Google Scholar]
- 63.Cheng CJ, Kuo E, Huang CL. Extracellular potassium homeostasis: insights from hypokalemic periodic paralysis. Semin Nephrol. 2013;33:237–247. doi: 10.1016/j.semnephrol.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palmer BF. A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am J Kidney Dis. 2010;56:387–393. doi: 10.1053/j.ajkd.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 65.Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004;351:585–592. doi: 10.1056/NEJMra035279. [DOI] [PubMed] [Google Scholar]
- 66.Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, Weir MR, Fink JC. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156–1162. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jain N, Kotla S, Little BB, Weideman RA, Brilakis ES, Reilly RF, Banerjee S. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;109:1510–1513. doi: 10.1016/j.amjcard.2012.01.367. [DOI] [PubMed] [Google Scholar]
- 68.Michel A, Martin-Perez M, Ruigomez A, Garcia Rodriguez LA. Risk factors for hyperkalaemia in a cohort of patients with newly diagnosed heart failure: a nested case-control study in UK general practice. Eur J Heart Fail. 2015;17:205–213. doi: 10.1002/ejhf.226. [DOI] [PubMed] [Google Scholar]
- 69.Korgaonkar S, Tilea A, Gillespie BW, Kiser M, Eisele G, Finkelstein F, Kotanko P, Pitt B, Saran R. Serum potassium and outcomes in CKD: insights from the RRI-CKD cohort study. Clin J Am Soc Nephrol. 2010;5:762–769. doi: 10.2215/CJN.05850809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gwoo S, Kim YN, Shin HS, Jung YS, Rim H. Predictors of hyperkalemia risk after hypertension control with aldosterone blockade according to the presence or absence of chronic kidney disease. Nephron Clin Pract. 2014;128:381–386. doi: 10.1159/000369138. [DOI] [PubMed] [Google Scholar]
- 71.Weinberg JM, Appel LJ, Bakris G, Gassman JJ, Greene T, Kendrick CA, Wang X, Lash J, Lewis JA, Pogue V, Thornley-Brown D, Phillips RA African American Study of Hypertension and Kidney Disease Collaborative Research Group. Risk of hyperkalemia in nondiabetic patients with chronic kidney disease receiving antihypertensive therapy. Arch Intern Med. 2009;169:1587–1594. doi: 10.1001/archinternmed.2009.284. [DOI] [PubMed] [Google Scholar]
- 72.Alappan R, Buller GK, Perazella MA. Trimethoprim-sulfamethoxazole therapy in outpatients: is hyperkalemia a significant problem? Am J Nephrol. 1999;19:389–394. doi: 10.1159/000013483. [DOI] [PubMed] [Google Scholar]
- 73.Antoniou T, Gomes T, Juurlink DN, Loutfy MR, Glazier RH, Mamdani MM. Trimethoprim-sulfamethoxazole-induced hyperkalemia in patients receiving inhibitors of the renin-angiotensin system: a population-based study. Arch Intern Med. 2010;170:1045–1049. doi: 10.1001/archinternmed.2010.142. [DOI] [PubMed] [Google Scholar]
- 74.Antoniou T, Gomes T, Mamdani MM, Yao Z, Hellings C, Garg AX, Weir MA, Juurlink DN. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. BMJ. 2011;343:d5228. doi: 10.1136/bmj.d5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Antoniou T, Hollands S, Macdonald EM, Gomes T, Mamdani MM, Juurlink DN Canadian Drug Safety and Effectiveness Research Network. Trimethoprim-sulfamethoxazole and risk of sudden death among patients taking spironolactone. CMAJ. 2015;187:E138–143. doi: 10.1503/cmaj.140816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fralick M, Macdonald EM, Gomes T, Antoniou T, Hollands S, Mamdani MM, Juurlink DN Canadian Drug Safety and Effectiveness Research Network. Co-trimoxazole and sudden death in patients receiving inhibitors of renin-angiotensin system: population based study. BMJ. 2014;349:g6196. doi: 10.1136/bmj.g6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muschart X, Boulouffe C, Jamart J, Nougon G, Gerard V, de Canniere L, Vanpee D. A determination of the current causes of hyperkalaemia and whether they have changed over the past 25 years. Acta Clin Belg. 2014;69:280–284. doi: 10.1179/0001551214Z.00000000077. [DOI] [PubMed] [Google Scholar]
- 78.Weir MR, Rolfe M. Potassium homeostasis and renin-angiotensin- aldosterone system inhibitors. Clin J Am Soc Nephrol. 2010;5:531–548. doi: 10.2215/CJN.07821109. [DOI] [PubMed] [Google Scholar]
- 79.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 80.Moore C, Lin J, McGinn T, Halm E. Factors associated with time to follow-up of severe hyperkalemia in the ambulatory setting. Am J Med Qual. 2007;22:428–437. doi: 10.1177/1062860607305245. [DOI] [PubMed] [Google Scholar]
- 81.Moore CR, Lin JJ, O’Connor N, Halm EA. Follow-up of markedly elevated serum potassium results in the ambulatory setting: implications for patient safety. Am J Med Qual. 2006;21:115–124. doi: 10.1177/1062860605285047. [DOI] [PubMed] [Google Scholar]
- 82.Field MJ, Stanton BA, Giebisch GH. Differential acute effects of aldosterone, dexamethasone, and hyperkalemia on distal tubular potassium secretion in the rat kidney. J Clin Invest. 1984;74:1792–1802. doi: 10.1172/JCI111598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hirsch D, Kashgarian M, Boulpaep EL, Hayslett JP. Role of aldosterone in the mechanism of potassium adaptation in the initial collecting tubule. Kidney Int. 1984;26:798–807. doi: 10.1038/ki.1984.221. [DOI] [PubMed] [Google Scholar]
- 84.Stanton B, Pan L, Deetjen H, Guckian V, Giebisch G. Independent effects of aldosterone and potassium on induction of potassium adaptation in rat kidney. J Clin Invest. 1987;79:198–206. doi: 10.1172/JCI112783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wingo CS, Seldin DW, Kokko JP, Jacobson HR. Dietary modulation of active potassium secretion in the cortical collecting tubule of adrenalectomized rabbits. J Clin Invest. 1982;70:579–586. doi: 10.1172/JCI110650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Young DB. Quantitative analysis of aldosterone’s role in potassium regulation. Am J Physiol. 1988;255:F811–822. doi: 10.1152/ajprenal.1988.255.5.F811. [DOI] [PubMed] [Google Scholar]
- 87.Frindt G, Palmer LG. K+ secretion in the rat kidney: Na+ channel-dependent and -independent mechanisms. Am J Physiol Renal Physiol. 2009;297:F389–396. doi: 10.1152/ajprenal.90528.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Todkar A, Picard N, Loffing-Cueni D, Sorensen MV, Mihailova M, Nesterov V, Makhanova N, Korbmacher C, Wagner CA, Loffing J. Mechanisms of renal control of potassium homeostasis in complete aldosterone deficiency. J Am Soc Nephrol. 2015;26:425–438. doi: 10.1681/ASN.2013111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Buren PN, Adams-Huet B, Nguyen M, Molina C, Toto RD. Potassium handling with dual renin-angiotensin system inhibition in diabetic nephropathy. Clin J Am Soc Nephrol. 2014;9:295–301. doi: 10.2215/CJN.07460713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walsh M, Manns B, Garg AX, Bueti J, Rabbat C, Smyth A, Tyrwhitt J, Bosch J, Gao P, Devereaux PJ, Wald R. The Safety of Eplerenone in Hemodialysis Patients: A Noninferiority Randomized Controlled Trial. Clin J Am Soc Nephrol. 2015;10:1602–1608. doi: 10.2215/CJN.12371214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bowling CB, Pitt B, Ahmed MI, Aban IB, Sanders PW, Mujib M, Campbell RC, Love TE, Aronow WS, Allman RM, Bakris GL, Ahmed A. Hypokalemia and outcomes in patients with chronic heart failure and chronic kidney disease: findings from propensity-matched studies. Circ Heart Fail. 2010;3:253–260. doi: 10.1161/CIRCHEARTFAILURE.109.899526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luo J, Brunelli SM, Jensen DE, Yang A. Association between Serum Potassium and Outcomes in Patients with Reduced Kidney Function. Clin J Am Soc Nephrol. 2016;11:90–100. doi: 10.2215/CJN.01730215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kovesdy CP. Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol. 2014;10:653–662. doi: 10.1038/nrneph.2014.168. [DOI] [PubMed] [Google Scholar]
- 94.Nguyen TQ, Maalouf NM, Sakhaee K, Moe OW. Comparison of insulin action on glucose versus potassium uptake in humans. Clin J Am Soc Nephrol. 2011;6:1533–1539. doi: 10.2215/CJN.00750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21:733–735. doi: 10.1681/ASN.2010010079. [DOI] [PubMed] [Google Scholar]
- 96.Kayexelate (sodium polystyrene sulfonate) powder. Detailed view: safety labeling changes approved by FDA Center for Drug Evaluation and Research (CDER). (2011)
- 97.Watson MA, Baker TP, Nguyen A, Sebastianelli ME, Stewart HL, Oliver DK, Abbott KC, Yuan CM. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis. 2012;60:409–416. doi: 10.1053/j.ajkd.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 98.Lepage L, Dufour AC, Doiron J, Handfield K, Desforges K, Bell R, Vallee M, Savoie M, Perreault S, Laurin LP, Pichette V, Lafrance JP. Randomized Clinical Trial of Sodium Polystyrene Sulfonate for the Treatment of Mild Hyperkalemia in CKD. Clin J Am Soc Nephrol. 2015;10:2136–2142. doi: 10.2215/CJN.03640415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kessler C, Ng J, Valdez K, Xie H, Geiger B. The use of sodium polystyrene sulfonate in the inpatient management of hyperkalemia. J Hosp Med. 2011;6:136–140. doi: 10.1002/jhm.834. [DOI] [PubMed] [Google Scholar]
- 100.Fordjour KN, Walton T, Doran JJ. Management of hyperkalemia in hospitalized patients. Am J Med Sci. 2014;347:93–100. doi: 10.1097/MAJ.0b013e318279b105. [DOI] [PubMed] [Google Scholar]
- 101.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 102.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 103.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 104.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 105.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 106.Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ PEARL-HF Investigators. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32:820–828. doi: 10.1093/eurheartj/ehq502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, Wittes J, Christ-Schmidt H, Berman L, Pitt B OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 108.Bakris GL, Pitt B, Weir MR, Freeman MW, Mayo MR, Garza D, Stasiv Y, Zawadzki R, Berman L, Bushinsky DA AMETHYST-DN Investigators. Effect of Patiromer on Serum Potassium Level in Patients With Hyperkalemia and Diabetic Kidney Disease: The AMETHYST-DN Randomized Clinical Trial. JAMA. 2015;314:151–161. doi: 10.1001/jama.2015.7446. [DOI] [PubMed] [Google Scholar]
- 109. [Accessed 2/27/2016];FDA approves new drug to treat hyperkalemia. 2015 http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm468546.htm.
- 110. [Accessed 2/27/2016];FDA drug safety communication: FDA requires drug interaction studies with potassium-lowering drug Kayexelate (sodium polystyrene sulfonate) 2015 http://www.fda.gov/Drugs/DrugSafety/ucm468035.htm.
- 111.Stavros F, Yang A, Leon A, Nuttall M, Rasmussen HS. Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS One. 2014;9:e114686. doi: 10.1371/journal.pone.0114686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ash SR, Singh B, Lavin PT, Stavros F, Rasmussen HS. A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int. 2015;88:404–411. doi: 10.1038/ki.2014.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Packham DK, Rasmussen HS, Lavin PT, El-Shahawy MA, Roger SD, Block G, Qunibi W, Pergola P, Singh B. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372:222–231. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]
- 114.Kosiborod M, Rasmussen HS, Lavin P, Qunibi WY, Spinowitz B, Packham D, Roger SD, Yang A, Lerma E, Singh B. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312:2223–2233. doi: 10.1001/jama.2014.15688. [DOI] [PubMed] [Google Scholar]
- 115.Kosiborod M, Peacock WF, Packham DK. Sodium zirconium cyclosilicate for urgent therapy of severe hyperkalemia. N Engl J Med. 2015;372:1577–1578. doi: 10.1056/NEJMc1500353. [DOI] [PubMed] [Google Scholar]
- 116.Bushinsky DA, Williams GH, Pitt B, Weir MR, Freeman MW, Garza D, Stasiv Y, Li E, Berman L, Bakris GL. Patiromer induces rapid and sustained potassium lowering in patients with chronic kidney disease and hyperkalemia. Kidney Int. 2015;88:1427–1433. doi: 10.1038/ki.2015.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Satlin LM. Developmental regulation of expression of renal potassium secretory channels. Curr Opin Nephrol Hypertens. 2004;13:445–450. doi: 10.1097/01.mnh.0000133979.17311.21. [DOI] [PubMed] [Google Scholar]