Abstract
Objectives
To determine the relationship of the time from surgery to intraperitoneal (IP) chemotherapy (TSIC) initiation with survival of patients with stage III epithelial ovarian cancer (EOC) patients using ancillary data from cooperative group clinical trials.
Methods
Data from 420 patients with stage III EOC treated with IP chemotherapy under GOG-0114 and 172 were reviewed. The Cox proportional hazards model was used to evaluate independent prognostic factors and estimate their covariate-adjusted effects on PFS and OS.
Results
The median TSIC was 62.5 days (interquartile range 28-83). The median TSIC was longer for patients in GOG-0114 vs those in GOG-172 (83 vs 26 days, p <0.001). TSIC was significantly associated (P = 0.049) with PFS: each 10% increase in TSIC (days) decreases the risk of progression by 3%. TSIC was not significantly associated with OS in this model. In a linear regression model, gross residual disease was significantly associated with shorter TSIC (R2 -0.141, 95%CI -0.217, -0.064, p < 0.001). When only data from GOG-172 were considered, no statistical significant association was found between TSIC and PFS or OS.
Conclusions
In this ancillary data study, TSIC was not associated with improved OS in patients with stage III epithelial ovarian cancer. TSIC was significantly associated with PFS for the entire cohort, suggesting increase in PFS with longer TSIC. However, this was not found when only data from GOG 172 or GOG 114 were analyzed separately. Hence, the relationship between IP chemotherapy initiation and time from surgery needs to be studied further.
Introduction
Ovarian carcinoma is the leading cause of death from gynecological malignancy in the United States [1]. Advances in survival have been achieved, with the one of the longest median survival reported to date at 66 months for stage III epithelial ovarian cancer (EOC) patients treated with intraperitoneal (IP) chemotherapy [2]. However, even though there is high overall clinical response rates achieved with platinum-based therapy (up to 80%), < 30% of patients will remain free of disease [3].
Evaluation of variables that could influence both progression free survival (PFS) and overall survival (OS) is imperative to achieve improvement in outcomes for EOC patients. Cytoreductive surgery has been shown to be the principal determinant of prognosis in advanced EOC [4]. Emphasis has been placed in the achievement of complete gross resection as the optimal goal of cytoreduction, for which radical surgical procedures may be required [5]. The use of radical surgical procedures to treat patients with advanced ovarian cancer has resulted in acceptable morbidity rates [6]. However, these procedures may result in prolonged postoperative recovery and delay in initiation of adjuvant chemotherapy. For this reason, time from surgery to chemotherapy initiation has been evaluated as a variable that could impact both PFS and OS.
The association of time from surgery to chemotherapy initiation with survival has been evaluated with conflicting results [7-13]. The optimal interval between cytoreductive surgery and initiation of chemotherapy has not been defined; however, most clinical trials allow delays of 6-8 weeks. Given that increased metastatic growth after tumor removal was found, in vivo studies suggest a decrease in survival after a delayed start of chemotherapy [7]. Retrospective studies have failed to show time to chemotherapy initiation as a determinant prognostic factor for advanced stage ovarian cancer [8]. An analysis of prospectively collected data from 371 women with stage IIC-IV treated with primary cytoreduction followed by platinum-based chemotherapy in Norway, also failed to show an impact of time from surgery to chemotherapy initiation on short term survival [9]. On the other hand, a recent data analysis of 3 prospective randomized phase III trials showed that early initiation of chemotherapy might result in slight improvement in survival for patients with complete cytoreduction [14]. In their analysis, Mahnner et al found that residual tumor after surgery was associated with significantly earlier start of chemotherapy in multivariate Cox regression. Also, in patients with no residual disease after surgery, longer time to initiation of chemotherapy was associated with a trend towards earlier progression (per week delay HR 1.038, 95% CI 0.973- 1.106, p= 0.257) and was associated with shorter OS (per week delay 1.087, 95% CI 1.005-1.176, p= 0.038). Tewari KS et al recently published their results of Gynecology Oncology Group ancillary data study, in which a negative impact in survival was associated with > 25 day from surgery to initiation of chemotherapy in advanced ovarian cancer [13]. All these studies indicate the need to evaluate the association of time from surgery to chemotherapy initiation further.
Although there is conflicting data, recent studies do suggest that there is a negative impact on survival by a delay in chemotherapy initiation. To date, all studies both retrospective and prospective have evaluated timing of initiation of intravenous (IV) chemotherapy. However, the effect of time to chemotherapy initiation on the prognosis of advanced-stage ovarian cancer has not been evaluated for those receiving IP chemotherapy. The optimal timing for IP catheter placement is also still under debate, especially in those patients that undergo extensive cytoreductive procedures and bowel resections. Given that IV and IP chemotherapy administration have inherent pharmacokinetic differences, the impact of timing of IP chemotherapy initiation should also be evaluated. Some of the concerns with the use of IP chemotherapy for the treatment of EOC are that there might be a delay in initiation of therapy due to performance of bowel resections. The aim of this ancillary data analysis is to review pooled data collected from GOG trials using IP chemotherapy [2,15] and analyze the time from surgery to of first-line IP chemotherapy initiation (TSIC) and its subsequent relationship with survival. As a secondary objective, we evaluated factors associated with delay in chemotherapy initiation.
Methods
A retrospective review of data collected from patients with EOC treated with IP chemotherapy on randomized clinical trials conducted by the GOG, protocols 114 [15] and 172 [2], was performed. Clinicopathologic and survival data were abstracted from electronic patient records from each protocol maintained at the GOG Statistics & Data Center in Buffalo, NY. All patients underwent optimal cytoreductive surgery defined as residual disease less than 1 cm. TSIC was defined as the time from surgery to initiation of IP chemotherapy, in days. Patients with incomplete data for TSIC were excluded from the study. The primary end points for both studies were progression free survival (PFS) and overall survival (OS). PFS was calculated from the date of enrollment to the date of recurrence, death or most recent follow up visit. OS was calculated from the date of enrollment to date of death or last contact. The PFS and OS of patients with different durations of TSIC were considered. TSIC was also analyzed as a categorical variable split near its median.
Both of these studies evaluated the use of IP chemotherapy for the treatment of ovarian cancer compared to traditional IV regimens (Figure S1), but with different treatment regimens. In both trials the IV regimen consisted of IV paclitaxel 135 mg/m2 over 24 hours followed by IV cisplatin 75 mg/m2 every 3 weeks. In GOG-0114 patients received two cycles of IV chemotherapy (Carboplatin AUC 9 IV every 28 days) prior to initiation of IP chemotherapy. Both of these trials also administered IV paclitaxel 135 mg/m2 over 24 hours on day 1 followed by IP regimen (GOG 114: cisplatin 100 mg/m2 on day 2 every 3 weeks; GOG 172: cisplatin 100 mg/m2 on day 2 and 60 mg/m2 of IP paclitaxel on day 8). In order to have a higher number of patients with long term follow up and that received IP chemotherapy we used the data of both trials. We conducted the analysis for each separate trial as well, given the inherent differences in trial design,.
Categorical variables were compared between the patient subgroups by the Pearson chi-square test [16] and continuous variables by the Wilcoxon–Mann–Whitney test 1 [17]. Survival was estimated using the Kaplan–Meier method [18]. The Cox proportional hazards model [19] was used to evaluate independent prognostic factors and to estimate their covariate-adjusted effects on PFS and OS. The nonlinearity of the effect of continuous variables was assessed using restricted cubic splines [20]. Multicollinearity was assessed through the method of variance inflation factors (VIF) described by Marquardt [21] and found unproblematic. Except where noted, all statistical tests were two-tailed with the significance level set at α = 0.05. Statistical analyses were performed using the R programming language and environment [22] by the GOG statistical office team.
Results
Patient characteristics
Four hundred and forty patients received the IP chemotherapy regimen of GOG-0114 (n= 235) and 172 (n= 205). Due to incomplete data, 20 patients were excluded from this study. Data from 420 patients enrolled in the IP arm of GOG-0114 (n= 220) and 172 (n= 200) with complete TSIC values were included in this study. Table 1 summarizes the patient demographics and clinical characteristics. The median age was 57 years (interquartile range, 49 to 64), 91% were white, and 71.4% had a performance status of 0. Of these patients, 50% had poorly differentiated tumors and 70% had serous histology. Gross residual disease was found in 64% after primary cytoreductive surgery. The median TSIC was 63 days (interquartile range, 28 to 83). The median number of completed cycles of IP chemotherapy was 6 (interquartile range, 2-6).
Table 1.
Eligible patient demographics and clinical characteristics (N = 420). Describes clinicopatholigic characteristics of this cohort of patients.
| N | |
|---|---|
| Age (years) | 48.9 57.1 64.8 |
| BMI (kg/m2) | 21.5 24.3 28.5 |
| Race/Ethnicity | |
| White | 91% (384) |
| Black | 4.5% (9) |
| Performance status | |
| 0 | 71% (300) |
| 1 | 24% (99) |
| Grade (differentiation) | |
| good | 11.4% (48) |
| moderate | 37.9% (159) |
| poor | 49.5% (208) |
| Histology | |
| serous adenocarcinoma | 69.8% (293) |
| endometrioid adenocarcinoma | 11.7% (49) |
| mixed epithelial carcinoma | 8.1% (34) |
| clear-cell carcinoma | 4.3% (18) |
| Gross residual disease | |
| no | 36% (153) |
| yes | 64% (267) |
| Surgical interval days | 28 63 83 |
| No. complete IP cycles | 2 6 6 |
| Protocol number | |
| GOG 114 | 52% (220) |
| GOG 172 | 48% (200) |
a b c represent the lower quartile a, the median b, and the upper quartile c for continuous variables.
N is the number of non–missing values.
Numbers after percents are frequencies.
Table 2 summarizes the similarity of clinicopathological characteristics of patients in each trial. All the patients enrolled on GOG-172 that received IP chemotherapy had a performance status of 0, while only 46% of the patients from GOG 114 had that performance status (p <0.001). The median TSIC for patients on GOG 172 was significantly shorter than the TSIC for patients in GOG 114 (26 vs 83 days, p <0.001). However, the median number of completed IP cycles was higher in patient treated under GOG 114 than those under GOG 172 (6 vs 4, p <0.001).
Table 2.
Patient characteristics by GOG protocol (N = 420). Summarized the similarity of clinicopathologic characteristics of the patients that received IP chemotherapy under GOG 114 and those in the IP arm of GOG 172.
| GOG 114 | GOG 172 | p value | |
|---|---|---|---|
| Age years | 48.3 57.1 64.7 | 49.3 57.3 65.2 | 0.305 |
| BMI kg/m2 | 21.5 24.1 28.4 | 21.6 24.8 28.6 | 0.676 |
| Race/Ethnicity | |||
| White | 91% (202) | 91% (182) | 0.062 |
| Black | 6% (13) | 3% (6) | |
| Performance status | |||
| 0 | 46% (100) | 100% (200) | <0.001 |
| 1 | 45% (99) | 0% (0) | |
| Grade (differentiation) | 0.641 | ||
| good | 10.5% (23) | 12.5% (25) | |
| moderate | 40.5% (89) | 35.0% (70) | |
| poor | 48.2% (106) | 51.0% (102) | |
| Histology | 0.002 | ||
| serous adenocarcinoma | 63% (139) | 77.0% (154) | |
| endometrioid adenocarcinoma | 14.5% (32) | 8.5% (17) | |
| mixed epithelial carcinoma | 9.5% (21) | 6.5% (13) | |
| clear-cell carcinoma | 3.2% (7) | 5.5% (11) | |
| Gross residual disease | 0.296 | ||
| no | 34% (75) | 39% (78) | |
| yes | 66% (145) | 61% (122) | |
| Surgical interval days | 70 83 93 | 19 26 36 | <0.001 |
| No. complete IP cycles | 4 6 6 | 1 4 6 | <0.001 |
a b c represent the lower quartile a, the median b, and the upper quartile c for continuous variables.
N is the number of non–missing values.
Numbers after percents are frequencies.
Tests used: 1Wilcoxon test; 2Pearson test
Survival
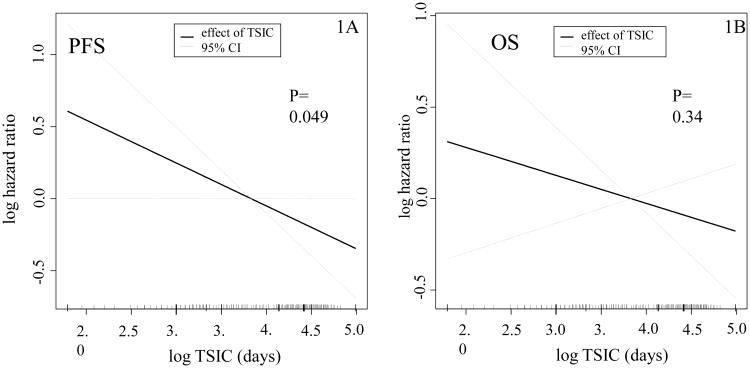
The median PFS for the ancillary-study IP patients was 26 months. Table 3 summarizes the results of a Cox regression model of PFS fitted to the clinicopathologic variables of the study patients. In this model, TSIC is weakly associated (AHR 0.97, 95% CI 0.94-1.00, P = 0.049) with PFS and each 10% increase in TSIC (days) decreases the risk of progression by 3% (Figure 1A). This result, barely statistically significant, is nonetheless surprising; however, the effect of TSIC was not significant in separate subgroup analyses of GOG-0114 and 172. Also, in this model, clear cell histology was associated with a significant higher risk of progression (AHR 2.38, 1.31-4.35, p <0.014) as compared to serous histology. Gross residual disease was also significantly associated with higher risk of progression (AHR 1.51, 1.17-1.94, p=0.001). TSIC is not significantly associated with OS in the Cox regression model (Figure 1B). Gross residual disease (AHR 1.72, 1.31-2.26, p <0.001), age (AHR 1.02, 1.00-1.03, p 0.008) and clear cell histology (AHR 3.69, 1.99-6.85, p <0.001) were significantly associated with higher risk of death in this model (Table S1). Given the significant association of residual disease with PFS and OS, as well as the inherent differences between the trials, we evaluated if residual disease was more important in on trial versus the other. We evaluated this by incorporating the interaction term between residual disease and protocol into the survival model. We found that the interaction term was not significant, therefore, residual disease was not associated with more risk in one protocol over the other.
Table 3.
Multivariate Progression-Free Survival Analysis. Summarizes the results of a Cox regression model of progression-free survival (PFS) fitted to the covariate data of the study patients.
| Covariate | AHR | 95% CI | P* |
|---|---|---|---|
| Age(years)† | 1.01 | 1.00-1.02 | P=0.287 |
| BMI (kg/m2) ‡ | 0.99 | 0.94-1.05 | P=0.824 |
| Race/Ethnicity | |||
| White | 1.00 | referent | P=0.321 |
| Black | 0.84 | 0.48-1.49 | |
| other | 0.61 | 0.31-1.21 | |
| Performance Status | |||
| 0 | 1.00 | referent | P=0.340 |
| 1 | 1.08 | 0.79-1.48 | |
| 2 | 1.46 | 0.88-2.42 | |
| Grade (differentiation) | |||
| Good | 1.00 | referent | P= 0.146 |
| Moderate | 1.61 | 1.08-2.41 | |
| Poor | 1.48 | 0.99-2.19 | |
| Not graded | 1.57 | 0.48-5.14 | |
| Histology | |||
| Serous adenocarcinoma | 1.00 | referent | P=0.014 |
| Endometriod adenocarcinoma | 0.70 | 0.47-1.05 | |
| Mixed | 1.05 | 0.69 -1.60 | |
| Clear-cell carcinoma | 2.38 | 1.31- 4.35 | |
| Other | 1.01 | 0.64- 1.58 | |
| Gross residual disease | |||
| No | 1.00 | referent | P 0.001 |
| Yes | 1.51 | 1.17-1.94 | |
| TSIC (days) § | 0.97 | 0.94-1.00 | P= 0.049 |
The p-values are from the overall test of significance of each covariate in the model.
The AHR denotes the change in risk of progression or death associated with an increase of 1 year of age.
The AHR denotes the change in risk of progression or death associated with a 10% increase in BMI (kg/m2).
The AHR denotes the change in risk of progression or death associated with a 10% increase in TSIC (days).
Figure 1.
Plot of the partial effect of TSIC on the log hazard ratio of the progression-free (1A) and overall survival models (1B) in GOG 114 and GOG 172. These are the plots of the partial effect of log TSCI on the log hazard ratio of PFS (1A) and OS (1B) models, respectively. They represent the risk contribution of continues variables (TSCI) in a survival model.
TSIC association with other clinicopathologic variables
In order to attempt to evaluate possible factors associated with delay in IP chemotherapy initiation we evaluated available clinicopathologic characteristics and their relationship with TSIC in a linear regression model. In this linear regression model for log TSIC as a function of the patient's baseline variables gross residual disease was associated with shorter TSIC (Table S2). Also, each 10% increase in BMI was associated with a 2% decrease in TSIC. We could not find a correlation between BMI and gross residual disease. None of the other evaluated variables, such as age, performance status, or histology, were noted to be significantly associated with TSIC. However, other interesting variables such as radicality of the surgery or postoperative complications were not able to be evaluated given lack of data.
GOG 172 analysis
Given that GOG 114 required administration of 2 cycles of IV carboplatin prior to beginning of IP therapy, which could confound the analysis of TSCI, independent analysis of GOG 172 and GOG 114 data was performed. This data was analyzed as a continuous variable for both trials and as categorical variable for GOG 172, dichotomizing the data by those who received IP chemotherapy within 25 days and those who received it in or after 25 days from the surgery (the median was 26 days).Table 4 summarizes the patient demographics and clinical characteristics. The proportion of patients that had residual disease was significantly higher in those who received the first cycle of IP <25 days from surgery than those that received > 25 days (69.8% vs 54.4%, p= 0.27).
Table 4.
Eligible patient demographics and clinical characteristics GOG 172. Summarizes the similarity of clinicopathologic characteristics of the patients that received IP chemotherapy under IP arm of GOG 172 prior to 25 days from surgery and those that received it in or after 25 days from surgery.
|
|
|||
|---|---|---|---|
| <25 N=86 | ≥ 25 N=114 | Test statistics | |
|
|
|||
| Age (yrs) | 49.3 59.9 66.4 | 49.4 55.2 64.6 | P=0.3331 |
| BMI (kg/m2) | 22.8 25.6 29.1 | 21.0 24.0 28.3 | P=0.0771 |
| Race/Ethnicity | P=0.8832 | ||
| White | 91.9% | 90.4% | |
| Black | 2.3% | 3.5% | |
| Performance status | P= -2 | ||
| 0 | 100% (86) | 100%(114) | |
| 1 | 0% (0) | 0% (0) | |
| Grade (differentiation) | P=0.5572 | ||
| Good | 15.1% (13) | 10.5%(12) | |
| Moderate | 31.4% (27) | 37.7%(43) | |
| Poor | 51.2% (44) | 50.9%(58) | |
| Histology | P=0.8222 | ||
| Serous adenocarcinoma | 76.7% (66) | 77.2%(88) | |
| Endometriod | 9.3% (8) | 7.9% (9) | |
| Mixed | 4.7% (4) | 7.9% (9) | |
| Clear-cell carcinoma | 5.8% (5) | 5.3% (6) | |
| Gross residual disease | P=0.0272 | ||
| No | 30.2% (26) | 45.6%(52) | |
| Yes | 69.8% (60) | 54.4%(62) | |
| No. completed IP cycles | 1 3 6 | 1 5 6 | P= 0.4811 |
a b c represent the lower quartile a, the median b, and the upper quartile c for continuous variables.
N is the number of non–missing values.
Numbers after percents are frequencies.
Tests used: 1Wilcoxon test; 2Pearson test
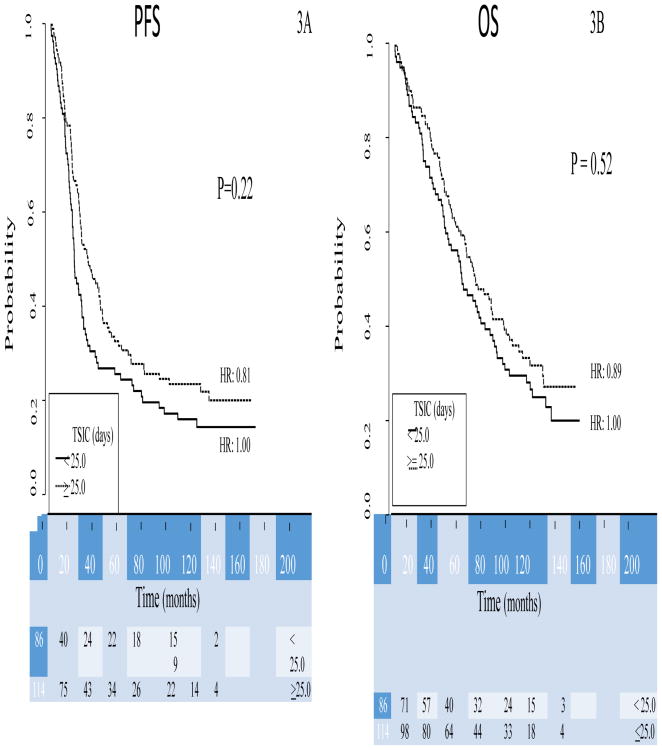
There was no association of TSIC with survival, PFS (HR 0.748, 0.53-1.04, p= 0.086) or OS (HR 0.878, 0.62-1.25, p= 0.470) (Figure S2). The only variable significantly associated with TSIC in the linear regression model was presence of gross residual disease, with shorter TSIC seen in those patients with residual disease (HR -0.263, -0.42- -0.11, p <0.001). When analyzed as a categorical variable, there was not an association of TSIC with survival, PFS (p=0.22) or OS (p=0.52) (Figure 2). This result was maintained when TSIC was analyzed as quartile intervals as well. As for the entire cohort, the only variable that was significantly associated with survival was residual disease after surgery in the patients that received IP chemotherapy as part of GOG 172 (AHR 1.98, 1.33-2.96, p<0.001) (Table S3, online).
Figure 2.
Kaplan–Meier curves of progression-free (2A) and overall (2B) survival for study patients from GOG-0172, stratified by TSIC groups. The TSIC groups are those patients that received IP chemotherapy under IP arm of GOG 172 prior to 25 days from surgery and those that received it in or after 25 days from surgery. Figures below months indicate the numbers of patients at risk. The p-value is from the Wald test to compare hazard ratios between the subgroups in the multivariate model.
GOG 114 analysis
Given that patients in GOG 114 received 2 cycles of high dose carboplatin IV (AUC 9) prior to the initiation of the IP chemotherapy, we also analyzed this trial separately. We first wanted to evaluate if there was a relationship between the time to initiation of the IV carboplatin and both PFS and OS. We noted that time to initiation of IV carboplatin was not significantly associated with either PFS or OS (P=0.64 and 0.87, respectively). We also analyzed the relationship between time to initiation of IP chemotherapy (TSIC) in this group. The TSIC for this group was also not significantly associated with PFS or OS.
Conclusion
IP chemotherapy has been shown to provide the longest survival to date for patients with stage III epithelial ovarian cancer with no gross residual disease (127 months in patients with no gross residual disease treated with IP on GOG 172) [24]. However, despite the clinical announcement encouraging the use of IP chemotherapy in these patients by the National Cancer Institute (NCI) in 2006, this modality has not been widely adopted. Wright et al reported that fewer than 50% of eligible patients treated at six comprehensive cancer centers in USA received IP chemotherapy [23]. They also showed a significant improvement in overall survival in those patients treated with IP vs IV chemotherapy (3 year OS 81% vs 71%, HR 0.66, 95% CI 0.47-0.92, p=0.02). Hence, further investigation on factors affecting adoption of this modality is warranted.
Since there is no consensus on the timing of IP catheter placement or initiation of IP chemotherapy, especially in those patients undergoing aggressive cytoreduction, TSIC is a variable that should be investigated. In our study, time from surgery to initiation of IP chemotherapy (TSIC) was not associated with improved OS in patients with stage III epithelial ovarian cancer. However, TSIC was significantly associated with PFS for the entire cohort, suggesting a decrease risk of progression with longer TSIC (p=0.049). This relationship between PFS and TSIC is contrary to the hypothesis that a delay in initiation of adjuvant chemotherapy may lead to increase tumor growth and, therefore, worse outcomes. This finding may be due to differences between GOG 114 and GOG 172, given that 2 cycles of IV chemotherapy were administered prior to IP on GOG 114. Patients in GOG 114 started IP chemotherapy significantly later than those patients in GOG 172. Therefore, we decided to conduct the analysis for each trial separately. No association was found for either OS or PFS when only data from GOG 172 or GOG 114 were analyzed. However, the proportion of patients with residual disease was higher in those patients that started IP chemotherapy prior to 25 days in the GOG 172 group. This difference may be a confounder in our study. We also evaluated the possible relationship between the time from surgery to initiation of the first IV chemotherapy in GOG 114 and survival. Again, no association was found for either OS or PFS and time of chemotherapy initiation. Hence, there may be other confounders unaccounted for that could explain the findings for PFS when the entire cohort was analyzed.
“We found that TSIC was shorter in those patients with residual disease. This could be due to many different factors. For example, patients with less radical surgery could have undergone less radical surgery and therefore started chemotherapy sooner. However, given the available data we cannot explain it with certainty. One limitation of the trial is that we were not able to evaluate factors such as radicality of the surgical procedure performed, amount of tumor burden prior to surgery (that will in part account for tumor biology), or postoperative complications. These data were not collected in the studied trials, therefore not available for review. Such variables could be related to TSIC. They could also represent confounders present within each trial as well as in the entire cohort as a hole”.
For the entire cohort, as well as for the patients treated on either trial, the most important variable for survival, both PFS and OS, is residual disease. This parameter is likely of greater importance for patients treated with IP chemotherapy, given that the penetrance of the drugs is limited to a few millimeters. Residual disease might limit the effectiveness of this adjuvant regimen. Since the administration of IV chemotherapy in GOG 114 prior to the IP regimen could reduce the tumor burden, potentially modify the efficacy of the IP therapy and the impact of residual disease on survival, we analyzed the trials separately. In our analysis residual disease was not associated with more risk in one trial over the other. Therefore, presence of residual disease most likely is also related to other variables such as initial tumor burden and tumor biology.
In contemporary practices, some practitioners start with IV chemotherapy and then transition to IP chemotherapy. This is partially due to concern that awaiting for post op recovery or placement of IP catheter might increase risk of progression and decrease survival. Our results do not support this stategy, since no association between survival and TSIC was found. Further studies would be required to clarify the role of such strategy”.
Recent results of GOG 252 presented at the annual meeting of the Society of Gynecologic Oncology don't show an advantage in PFS with IP chemotherapy, however conclusions of the value of IP chemotherapy maybe premature. Given the OS of 127 months reported on patients with no gross residual disease treated with IP regimen on GOG 172 [24], the utilization of this regimen should still considered in this patient population.
Further studies are warranted to better elucidate the association between survival and TSIC. Our study is the first investigating this variable for IP chemotherapy administration, however, it is an ancillary data study of two prospective clinical trials. These trials were not designed to answer this question and, therefore, the study might not be powered to detect a small difference in outcomes. However, the data used for the study was prospectively collected and included a large number of patients treated in multiple institutions. Our results suggest that the IP chemotherapy benefit is independent of time from surgery to its initiation. Therefore, IP chemotherapy should be considered even to those patients with no gross residual disease that may require delayed insertion of the IP catheter for adjuvant treatment initiation.
Supplementary Material
Figure S1: Treatment Schema of GOG 114 and GOG 172 trials. Schematic representation of the treatment regimens investigated under GOG 114 and GOG 172. The principal differences of the trials were noted in bold
Figure S2: Plot of the partial effect of TSIC on the log hazard ratio of the progression-free and overall survival model in GOG-0172 cohort. These are the plots of the partial effect of log TSCI on the log hazard ratio of PFS (S1A) and OS (S1B) models, respectively, for those patients treated under IP arm of GOG 172.
Table S1: Multivariate Overall Survival Analysis. Summarizes the results of a Cox regression model of overall survival (OS) fitted to the covariate data of the study patients.
Table S2: Results of a linear model for log TSIC as a function of covariates (Wald statistics). Summarizes the results of a liner regression model for log TSIC as a function of the patients' baseline characteristics.
Table S3: Multivariate Progression-Free Survival Analysis (GOG-0172). Summarizes the results of a Cox regression model of progression-free survival (PFS) fitted to the covariate data of the patients that received the IP arm of GOG 172
Highlights.
Time from surgery to IP chemotherapy initiation (TSIC) did not impact survival
Gross residual disease was significantly associated with shorter TSIC
Gross residual disease significantly associated with higher risk of progression
Acknowledgments
The following National Cancer Institute grants also supported this study: NRG Oncology Operations grant number U10 CA 180868 as well as NRG SDMC grant U10 CA180822, Gynecologic Oncology Group (GOG) Administrative Office and GOG Tissue Bank (CA 114793).
Footnotes
Parts of this study were presented in abstract form at the Society of Gynecologic Oncology annual meeting held on 3/28/2015.
The following Gynecologic Oncology Group member institutions participated in this study: University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, Walter Reed Army Medical Center, University of Minnesota Medical School, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group P.C., University of California at Los Angeles, University of Washington, University of Pennsylvania Cancer Center, Milton S. Hershey Medical Center, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke's Medical Center, University of Kentucky, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Columbus Cancer Council, University of Massachusetts Medical School, Women's Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, University of Chicago, Tacoma General Hospital, Thomas Jefferson University Hospital, Mayo Clinic, Case Western Reserve University, Tampa Bay Cancer Consortium, North Shore University Hospital, Gynecologic Oncology Network, Ellis Fischel Cancer Center, and Fletcher Allen Health Care.
Conflicts of Interest: The authors report no conflicts of interest with the exception of Dr. Angeles Alvarez Secord who wishes to acknowledge that she received funds from Janssen, Clovis Oncology, Genentech and AstraZeneca. Additionally, Dr. Alvarez Secord reports research grant funding from Astellas Pharma Inc, Genentech, Amgen, Endocyte, Exelixis, Boehringher Ingelheim, Astex Pharmaceuticals Inc., Prima Biomed, Tesaro, Astra Zeneca, Eisai Morphotek, Bristol Myers Squibb and Incyte. These funds were distributed to Duke University Medical Center to support research including salary support for Dr. Secord. In the last 36 months, she has served on Advisory Boards for Janssen, Clovis Oncology, Genentech and AstraZeneca, outside the submitted work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
James J Java, Email: james.j.java@gmail.com.
Wilberto Nieves Neira, Email: nwilberto@hotmail.com.
J Matthew Pearson, Email: mpearson@med.miami.edu.
David E. Cohn, Email: David.Cohn@osumc.edu.
Shashikant B Lele, Email: Shashi.Lele@RoswellPark.org.
Krishnansu S Tewari, Email: ktewari@uci.edu.
Joan L Walker, Email: joan-walker@ouhsc.edu.
Angeles Alvarez Secord, Email: angeles.secord@duke.edu.
Deborah K Armstrong, Email: ARMSTDE@jhmi.ed.
Larry J Copeland, Email: larry.copeland@osumc.edu.
References
- 1.Siegel RL, Milller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 3.Markman M, Markman J, Webster K, Zanotti K, Kulp B, Peterson G, et al. Duration of response to second-line, platinum-based chemotherapy for ovarian cancer: Implications for patient management and clinical trial design. J Clin Oncol. 2004;22:3120–25. doi: 10.1200/JCO.2004.05.195. [DOI] [PubMed] [Google Scholar]
- 4.Randall TC, Rubin SC. Cytoreductive surgery for ovarian cancer. Surg Clin North Am. 2001;81:871–83. doi: 10.1016/s0039-6109(05)70171-7. [DOI] [PubMed] [Google Scholar]
- 5.Chi DS, Franklin CC, Levine DA, Akselrod F, Sabbatini P, Jarnagin WR, et al. Improved optimal cytoreduction rates for stage IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: a change in surgical approach. Gynecol Oncol. 2004;94:650–4. doi: 10.1016/j.ygyno.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Chi DS, Zivanovic O, Levinson KL, Kolev V, Huh J, Dottino J, et al. The incidence of major complications after the performance of extensive upper abdominal surgical procedures during primary cytoreduction of advanced ovarian, tubal and peritoneal carcinomas. Gynecol Oncol. 2010;119:38–42. doi: 10.1016/j.ygyno.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Gunduz N, Saffer EA. Influence of interval between primary tumor removal and chemotherapy on kinetics and growth of metastases. Cancer Res. 1983;43:1488–92. [PubMed] [Google Scholar]
- 8.Aletti GD, Long HJ, Podratz KC, Cliby WA. Is time to chemotherapy a determinant of prognosis in advanced-stage ovarian cancer? Gynecol Oncol. 2007;104:212–16. doi: 10.1016/j.ygyno.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 9.Paulsen T, Kaern J, Kjaerheim K, Haldorsen T, Trope C. Influence of interval between primary surgery and chemotherapy on short-term survival of patients with advanced ovarian, tubal or peritoneal cancer. Gynecol Oncol. 2006;102:447–52. doi: 10.1016/j.ygyno.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 10.Warwick J, Kehoe S, Earl H, Luesley D, Redman C, Chan KK. Long-term follow-up of patients with advanced ovarian cancer treated in randomised clinical trials. Br J Cancer. 1995;72:1513–7. doi: 10.1038/bjc.1995.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn PM, Paul J, Cruickshank DJ Scottish Gynaecological Cancer Trials Group. Does the interval from primary surgery to chemotherapy influence progression-free survival in ovarian cancer? Gynecol Oncol. 2002;86:354–7. doi: 10.1006/gyno.2002.6750. [DOI] [PubMed] [Google Scholar]
- 12.Wright JD, Herzog TJ, Neugut AI, Burke WM, Lu YS, Lewin SN, et al. Effect of radical cytoreductive suregery on omission and delay of chemotherapy for advanced-stage ovarian cancer. Obstet Gynecol. 2012;120:871–81. doi: 10.1097/AOG.0b013e31826981de. [DOI] [PubMed] [Google Scholar]
- 13.Tewari KS, J J, Eskander RN, Monk BJ, Burger RA. Early initiation of chemotherapy following complete resection of advanced ovarian cancer associated with improved survival: An NRG Oncology/Gynecologic Oncology Group study. Ann Oncol. 2016;27:114–21. doi: 10.1093/annonc/mdv500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manhner S, Eulenburg C, Staehle A, Wegscheider K, Reuss A, Pujade-Lauraine E, et al. Prognostic impact of the time interval between surgery and chemotherapy in advanced ovarian cancer: Analysis of prospective randomised phase III trials. Eur J Cancer. 2013;49:142–9. doi: 10.1016/j.ejca.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–7. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 16.Pearson K. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Philosophical Magazine Series 5. 1900;50:157–75. [Google Scholar]
- 17.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 1947;18:50–60. [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. JAMA. 1958;53:457–481. [Google Scholar]
- 19.Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society Series B (Methodological) 1972;34:187–220. [Google Scholar]
- 20.Harrell Frank E. Regression modeling strategies: with applications to linear models, logistic regression and survival analysis. Springer; 2001. [Google Scholar]
- 21.Marquardt DW. Generalized Inverses, ridge regression, biased linear estimation, and nonlinear estimation. Technometrics. 1970;12:591–612. [Google Scholar]
- 22.Team RC. A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- 23.Wright AA, Cronin A, Milne DE, Bookman MA, Burger RA, Cohn DE, et al. Use and effectiveness of intraperitoneal chemotherapy for treatment of ovarian cancer. J Clin Oncol. 2015;33:2841–7. doi: 10.1200/JCO.2015.61.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tewari D, J J, Salani R, Armstrong DK, Markman M, Herzog T, Monk BJ, Chan J. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: A Gynecoloy Oncology Group study. J Clin Oncol. 2015;33(13):1460–66. doi: 10.1200/JCO.2014.55.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Treatment Schema of GOG 114 and GOG 172 trials. Schematic representation of the treatment regimens investigated under GOG 114 and GOG 172. The principal differences of the trials were noted in bold
Figure S2: Plot of the partial effect of TSIC on the log hazard ratio of the progression-free and overall survival model in GOG-0172 cohort. These are the plots of the partial effect of log TSCI on the log hazard ratio of PFS (S1A) and OS (S1B) models, respectively, for those patients treated under IP arm of GOG 172.
Table S1: Multivariate Overall Survival Analysis. Summarizes the results of a Cox regression model of overall survival (OS) fitted to the covariate data of the study patients.
Table S2: Results of a linear model for log TSIC as a function of covariates (Wald statistics). Summarizes the results of a liner regression model for log TSIC as a function of the patients' baseline characteristics.
Table S3: Multivariate Progression-Free Survival Analysis (GOG-0172). Summarizes the results of a Cox regression model of progression-free survival (PFS) fitted to the covariate data of the patients that received the IP arm of GOG 172