Abstract
The formation of spores is critical for the survival of Clostridium difficile outside the host gastrointestinal tract. Persistence of C. difficile spores greatly contributes to the spread of C. difficile infection (CDI), and the resistance of spores to antimicrobials facilitates the relapse of infection. Despite the importance of sporulation to C. difficile pathogenesis, the molecular mechanisms controlling spore formation are not well understood. The initiation of sporulation is known to be regulated through activation of the conserved transcription factor Spo0A. Multiple regulators influence Spo0A activation in other species; however, many of these factors are not conserved in C. difficile and few novel factors have been identified. Here, we investigated the function of a protein, CD1492, that is annotated as a kinase and was originally proposed to promote sporulation by directly phosphorylating Spo0A. We found that deletion of CD1492 resulted in increased sporulation, indicating that CD1492 is a negative regulator of sporulation. Accordingly, we observed increased transcription of Spo0A-dependent genes in the CD1492 mutant. Deletion of CD1492 also resulted in decreased toxin production in vitro and in decreased virulence in the hamster model of CDI. Further, the CD1492 mutant demonstrated effects on gene expression that are not associated with Spo0A activation, including lower sigD and rstA transcription, suggesting that this protein interacts with factors other than Spo0A. Altogether, the data indicate that CD1492 negatively affects sporulation and positively influences motility and virulence. These results provide further evidence that C. difficile sporulation is regulated differently from that of other endospore-forming species.
INTRODUCTION
Clostridium difficile causes severe diarrheal infections that are difficult to treat and easily transmitted. C. difficile enters the host as a dormant spore, which then germinates in the presence of bile salts to form a vegetative cell (1, 2). The vegetative form of C. difficile then grows and divides in the host gastrointestinal tract, producing toxins that cause the symptoms of disease (3, 4). During infection, a subset of C. difficile vegetative cells initiates the process of sporulation and morphologically transforms into spores (5, 6). These spores are metabolically dormant and highly resistant to oxygen, heat, and chemicals that would destroy the vegetative form of C. difficile (7–9).
Although the signals that activate C. difficile sporulation have not been identified, it is expected that the master regulatory factor Spo0A must be phosphorylated for the sporulation gene expression program to begin (10–12). Once phosphorylated, active Spo0A binds DNA, promoting the expression of early sporulation-specific genes and initiating spore formation (10, 13). In the extensively studied spore former Bacillus subtilis, activation of Spo0A is accomplished through a phosphorelay that is composed of sensor histidine kinases and a series of phosphotransfer proteins that tightly control the phosphorylation state of Spo0A (14, 15). The C. difficile genome does not encode an apparent phosphorelay but does contain three putative sensor histidine kinase proteins that are anticipated to directly phosphorylate and activate Spo0A (11, 16). One of these histidine kinase proteins, CD2492, was shown to positively affect C. difficile sporulation, and another, CD1579, was shown to interact directly with and transfer phosphate to Spo0A in vitro (11). The function of the third putative sporulation histidine kinase, CD1492, is not known.
In this study, we investigated the role of the putative sporulation kinase CD1492 in C. difficile sporulation. We examined the sporulation-specific gene expression and resulting phenotypes of a CD1492 deletion mutant and strains overexpressing wild-type or mutated CD1492 alleles. Our results indicate that CD1492 is involved in the initiation of sporulation, but contrary to its proposed function, this protein plays a role in preventing spore formation. In addition, we found that the CD1492 null mutant exhibited changes in gene expression that are not directly dependent on Spo0A activation or sporulation, including decreased production of TcdA and motility regulators. Furthermore, the CD1492 mutant was significantly less virulent in a hamster model of infection.
MATERIALS AND METHODS
Cultivation of bacteria.
C. difficile cultures were grown in an anaerobic chamber (Coy Laboratory Products) containing an atmosphere of 85% nitrogen, 10% hydrogen, and 5% CO2 at 37°C as described previously (17). C. difficile strains were cultured in brain heart infusion (BHI) medium supplemented with 2% yeast extract (BHIS medium) as broth or 1.5% agar medium (18). Escherichia coli were grown at 37°C in L broth (19) or agar plates or in BHIS medium supplemented with 20 μg/ml chloramphenicol or 100 μg/ml ampicillin as needed. Thiamphenicol (2 to 10 μg/ml) was used for selection of C. difficile plasmids, and kanamycin (50 μg/ml) was utilized for counterselection against E. coli as previously detailed (20–22). Taurocholate (Sigma-Aldrich) was added to cultures at 0.1% to induce spore germination (23).
Strain and plasmid construction.
The plasmids and bacterial strains used in this study are listed in Table 1, and the details of vector constructions are outlined in File S1 in the supplemental material. Primer design was based on the C. difficile strain 630 genomic sequence (GenBank accession no. NC_009089.1), and the 630Δerm derivative was used for PCR amplification and cloning (Table 2). Plasmid DNA isolation, PCR, and cloning were performed by using standard protocols. Plasmid sequences were verified prior to use (Eurofins MWG Operon). C. difficile genomic DNA was isolated as previously described (24, 25). C. difficile-E. coli conjugations and gene deletions were carried out as previously described (22, 23, 26).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or features | Source, construction, and/or reference |
|---|---|---|
| E. coli strains | ||
| HB101 | F− mcrB mrr hsdS20 (rB− mB−) recA13 leuB6 ara-14 proA2 lacY1 galK2 xyl-5 mtl-1 rpsL20 | B. Dupuy |
| MC277 | HB101 containing pRK24 and pMC211 | 28 |
| MC527 | HB101 containing pRK24 and pMC381 | This study |
| MC559 | HB101 containing pRK24 and pMC386 | This study |
| MC779 | HB101 containing pRK24 and pMC539 | This study |
| C. difficile strains | ||
| 630 | Clinical isolate | 65 |
| 630Δerm | Erms derivative of strain 630 | N. Minton, 66 |
| MC282 | 630Δerm pMC211 | 28 |
| MC587 | 630Δerm pMC386 | This study |
| MC674 | 630Δerm ΔCD1492 | This study |
| MC729 | 630Δerm ΔCD1492 pMC211 | This study |
| MC730 | 630Δerm ΔCD1492 pMC386 | This study |
| MC771 | 630Δerm ΔCD1492 pMC539 | This study |
| Plasmids | ||
| pRK24 | Tra+ Mob+ bla tet | 67 |
| pUC19 | Cloning vector, bla | 68 |
| pMTL-SC7315 | For allelic exchange in nonepidemic C. difficile strains | N. Minton, 26 |
| pMC123 | E. coli-C. difficile shuttle vector, bla catP | 69 |
| pMC211 | pMC123 PcprA | 28 |
| pMC381 | pMTL7315 ΔCD1492 cassette | This study |
| pMC386 | pMC211 with CD1492 | This study |
| pMC538 | pUC19 CD1492 H668A | This study |
| pMC539 | pMC211 with CD1492 H668A | This study |
TABLE 2.
Oligonucleotides used in this study
| Primer | Sequence (5′→3′) | Use and/or source |
|---|---|---|
| oMC44 | 5′-CTAGCTGCTCCTATGTCTCACATC-3′ | 69 |
| oMC45 | 5′-CCAGTCTCTCCTGGATCAACTA-3′ | 69 |
| oMC112 | 5′-GGCAAATGTAAGATTTCGTACTCA-3′ | tcdB (CD0660) qPCR, 28 |
| oMC113 | 5′-TCGACTACAGTATTCTCTGAC-3′ | tcdB (CD0660) qPCR, 28 |
| oMC189 | 5′-TGCCTCTTGTAAAGAGTATAGCA-3′ | sigD (CD0266) qPCR, 24 |
| oMC190 | 5′-GCATCAATCAATCCAATGACTCCAC-3′ | sigD (CD0266) qPCR, 24 |
| oMC331 | 5′-CTCAAAGCGCAATAAATCTAGGAGC-3′ | spo0A (CD1214) qPCR, 28 |
| oMC332 | 5′-TTGAGTCTCTTGAACTGGTCTAGG-3′ | spo0A (CD1214) qPCR, 28 |
| oMC333 | 5′-AGTAAGGGTATGGGCAAAGTATTACA-3′ | CD1579 qPCR, 24 |
| oMC334 | 5′-CCACTTCATTTGAGAACAACTCTTTG-3′ | CD1579 qPCR, 24 |
| oMC335 | 5′-ACTTGTAAGAAGTGCTGAAGGTGGTA-3′ | CD1492 qPCR, 24 |
| oMC336 | 5′-GTCATATCGACCAAATCACTTGAAACAC-3′ | CD1492 qPCR, 24 |
| oMC337 | 5′-CAGGAATTTGTGACTATCTGGGAAATGG-3′ | CD2492 qPCR, 24 |
| oMC338 | 5′-TCCCATTTGCCTTTATTTGAACTTGA-3′ | CD2492 qPCR, 24 |
| oMC339 | 5′-GGGCAAATATACTTCCTCCTCCAT-3′ | sigE (CD2643) qPCR, 28 |
| oMC340 | 5′-TGACTTTACACTTTCATCTGTTTCTAGC-3′ | sigE (CD2643) qPCR, 28 |
| oMC355 | 5′-CTGTTGGAATATCTAGGCGATAAGC-3′ | rstA (CD3668) qPCR, 24 |
| oMC356 | 5′-TGGTCCTCAGCCTTGTTTAATTC-3′ | rstA (CD3668) qPCR, 24 |
| oMC365 | 5′-GGAAGTAACTGTTGCCAGAGAAGA-3′ | sigF (CD0772) qPCR, 28 |
| oMC366 | 5′-CGCTCCTAACTAGACCTAAATTGC-3′ | sigF (CD0772) qPCR, 28 |
| oMC547 | 5′-TGGATAGGTGGAGAAGTCAGT-3′ | tcdA qPCR (CD0663), 28 |
| oMC548 | 5′-GCTGTAATGCTTCAGTGGTAGA-3′ | tcdA qPCR (CD0663), 28 |
| oMC569/tcdRqF | 5′-AGCAAGAAATAACTCAGTAGATGATT-3′ | tcdR qPCR (CD0659), 40 |
| oMC570/tcdRqR | 5′-TTATTAAATCTGTTTCTCCCTCTTCA-3′ | tcdR qPCR (CD0659), 40 |
| oMC897 | 5′-GCCATGGATCCTTGGAAGAATTGTGGTAACATATTTATAG-3′ | CD1492 cloning |
| oMC898 | 5′-GATGCCTGCAGACGCATCAAATACAACTAAAGTAATAAA-3′ | CD1492 cloning |
| oMC911 | 5′-GCGCGGCCGCGAGTAGGAAATCTGGCTTAT-3′ | CD1492 deletion construct |
| oMC912 | 5′-ACAACTAAAGCACTTCTTCATTTTTATATAGTTTTACC-3′ | CD1492 deletion construct |
| oMC913 | 5′-TGAAGAAGTGCTTTAGTTGTATTTGATGCGTTTTA-3′ | CD1492 deletion construct |
| oMC914 | 5′-GCGCGGCCGCCAGCCTTGTCATTTTTTAGATTG-3′ | CD1492 deletion construct |
| fliCqF | 5′-TACAAGTTGGAGCAAGTTATGGAAC-3′ | 40 |
| fliCqR | 5′-GTTGTTATACCAGCTGAAGCCATTA-3′ | 40 |
Single nucleotide polymorphism (SNP) analysis.
C. difficile genomic DNA was prepared as previously described (24, 25). Genomic DNA was quantitated with a NanoDrop (Thermo Scientific), and 1 ng of DNA was used for library preparation. Libraries were generated with the Nextera XT DNA Library Preparation kit (Illumina); dual barcoding and sequencing primers were added according to the manufacturer's protocol. Libraries were validated by microelectrophoresis, quantified, pooled, and clustered on an Illumina MiSeq instrument in 150-bp reads. Per-sample reads were mapped to the C. difficile 630 reference genome (GenBank no. AM180355). All variant analysis was performed and annotated with CLC Genomics Workbench v9.0, with the “Fixed Ploidy Variant Detection” and “Annotate with Overlap Information” tools.
Sporulation efficiency assays and phase-contrast microscopy.
C. difficile cultures were started in BHIS medium supplemented with 0.1% taurocholate to allow for germination of spores within the starting inoculum and 0.2% fructose to prevent sporulation. When cultures reached an optical density at 600 nm (OD600) of 0.5, 250 μl was applied evenly to 70:30 agar medium and incubated anaerobically at 37°C (27). Samples were scraped from the plates at the time points indicated for each experiment and evaluated for sporulation frequency by both phase-contrast counting and enumeration of ethanol-resistant spores. Samples for phase-contrast microscopy were resuspended in BHIS broth and applied to slides as previously described (28). Phase-contrast microscopy was performed with a Nikon Eclipse Ci-L microscope with an X100 Ph3 oil immersion objective, and images were acquired with a DS-Fi2 camera. Two or more fields of view were captured for each strain, and at least 1,000 cells were assessed and enumerated per experiment. The percentage of spores present was calculated as the number of spores divided by the total number of cells. The mean percentage of spores and the standard error of the mean were calculated from at least three independent experiments (24).
To determine the number of viable spores in the total viable population, C. difficile cultures were grown on 70:30 sporulation agar as described above and ethanol resistance assays were performed. After 24 h of growth, cells were resuspended in BHIS medium to an OD600 of 1.0, serially diluted in BHIS medium, and plated onto BHIS agar medium to enumerate vegetative cells. A 0.5-ml aliquot of culture was then mixed with 95% ethanol and water to a final concentration of 28.5%. Ethanol-treated cells were vortexed, incubated at room temperature for 15 min, serially diluted in 1× phosphate-buffered saline containing 0.1% taurocholate, and then plated onto BHIS medium plates containing 0.1% taurocholate to enumerate spore outgrowth. Plates were incubated for a minimum of 24 h, and the number of CFU per milliliter of starting culture was calculated. The sporulation frequency was calculated as the number of ethanol-resistant spores divided by the total number of cells (combined counts of spores and vegetative cells per milliliter). A spo0A mutant (MC310) was used as a control to ensure vegetative cell death in ethanol.
qRT-PCR.
C. difficile cultures were harvested from 70:30 sporulation agar, resuspended in a cold solution of 1.5:1.5:3 ethanol-acetone-distilled H2O, and stored immediately at −80°C. RNA was purified from the cell cultures, and cDNA was synthesized as described previously (28, 29). A 50- or 200-ng sample of cDNA was used per reaction mixture for standard or cecal quantitative PCR (qPCR) analysis, respectively. Quantitative reverse transcription-PCR (qRT-PCR) analysis was performed with the Bioline SensiFAST SYBR and Fluorescein kit and a Roche LightCycler 96 instrument. Reaction mixtures without reverse transcriptase were included for each primer set to detect genomic DNA contamination. qPCR primers were designed with the IDT PrimerQuest program (Table 2). qPCR was performed in technical triplicate for each cDNA sample and primer pair combination. Primer efficiencies were calculated for each primer set prior to assays. Results were calculated by the comparative cycle threshold method, normalized to the internal control transcript rpoC (30). At least four biological replicates were assessed. Relative-expression results are presented as the means and standard errors of the means. The two-tailed Student t test was performed to assess the statistical significance of differences between the expression ratios of the control and test groups.
Animal studies.
Spores were prepared and enumerated for animal experiments as previously described (28). Female Syrian golden hamsters (Mesocricetus auratus) weighing 70 to 110 g were obtained from Charles River Laboratories and maintained in an animal biosafety level 2 room within the Division of Animal Resources at Emory University. Animals were housed individually and provided standard rodent chow and water ad libitum. Hamsters were administered a single dose of clindamycin (30 mg/kg of body weight) by oral gavage 7 days prior to infection (day −7). At day 0, hamsters were administered approximately 5,000 spores of a single C. difficile strain and monitored several times per day for display of disease symptoms (weight loss, lethargy, diarrhea, or a wet tail). All animals were weighed at least once per day, and fecal samples were collected daily for determination of the total number of cells throughout the experiment. Experiments were performed two times with cohorts of five or six animals per C. difficile strain tested. Additional animals that received clindamycin but were not administered C. difficile served as negative controls in each experiment. Animals were considered moribund and were euthanized if (i) they lost 15% or more of their body weight or (ii) if they exhibited diarrhea and lethargy. Hamsters were euthanized by CO2 asphyxiation followed by a thoracotomy. Following euthanasia, animals were necropsied and cecal contents were collected for enumeration of C. difficile bacteria and RNA isolation. Cecal samples used for RNA isolation were stored in 1:1 ethanol-acetone at −80°C. Strain-specific differences in the numbers of C. difficile CFU recovered from feces and cecal contents were determined by single-factor analysis of variance (ANOVA; GraphPad Prism 6) and by two-tailed Student t test (Excel; Microsoft). Differences in hamster survival for animals infected with MC674 (CD1492) or 630Δerm were assessed with the log rank test (GraphPad Prism 6).
SDS-PAGE and Western blot analysis.
C. difficile strains 630Δerm, MC674 (CD1492), MC282 (630Δerm Pcpr), MC729 (CD1492 Pcpr), MC730 (CD1492 Pcpr::CD1492), and MC771 (CD1492 Pcpr::CD1492 H668A) were grown in TY medium for 24 h at 37°C as previously described (24), except that strains were cultivated in BHIS medium overnight. Total protein was quantitated with the Pierce Micro BCA Protein Assay kit (Thermo Scientific), and 8 μg of total protein was loaded onto precast TGX 4 to 15% gradient gels (Bio-Rad), separated by electrophoresis, and subsequently transferred to 0.45-μm nitrocellulose membranes (Bio-Rad). Western blot analysis was conducted with mouse anti-TcdA antibodies (Novus Biologicals), followed by a goat anti-mouse Alexa Fluor 488 secondary antibody (Life Technologies). Imaging and densitometry were performed with a ChemiDoc and Image Lab Software (Bio-Rad), and three biological replicates were analyzed for each strain. The Student two-tailed t test and a one-way ANOVA, followed by Dunnett's multiple-comparison test, were performed to assess statistical differences in TcdA protein levels between the mutant and parent strains and the complemented strains, respectively (GraphPad Prism 6). A representative Western blot image is shown.
Motility studies.
Strains were grown in BHIS medium to an OD600 of 0.5, and 5 μl of culture was spotted into the center of one-half-concentration BHI plates containing 0.3% agar. Swimming diameters were measured every 24 h for a total of 168 h. Results represent the mean values and the standard errors of the means for a minimum of three independent experiments. A two-tailed Student t test was performed to determine statistically significant differences in outcomes between the mutant and parent strains.
RESULTS
Deletion of the predicted orphan kinase gene CD1492 results in increased spore formation.
The genome of C. difficile strain 630 encodes three putative orphan histidine kinases that have been implicated as sporulation sensor kinases: CD1492, CD1579, and CD2492 (11). Previous investigations found that disruption of the CD2492 kinase results in a significant decrease in spore formation, while the CD1579 kinase was shown to affect Spo0A phosphorylation in vitro (11). CD1492, the third suspected sporulation sensor kinase, has not been directly linked to a sporulation phenotype or phosphorylation of Spo0A in vitro. As outlined in Fig. 1, CD1492 and CD2492 both have multiple predicted transmembrane segments, while CD1579 is an apparent cytosolic protein. All three proteins have predicted histidine kinase catalytic domains of typical sensor histidine kinases.
FIG 1.

In silico analysis of sporulation-associated histidine kinases. (A) Tan boxes represent predicted transmembrane domains; the H+ ATPase region includes the kinase catalytic domain (56, 57). DHpt, dimerization and histidine phosphotransferase; aa, amino acids. (B) Alignment of the dimerization and histidine phosphotransfer subdomains of C. difficile, B. subtilis, and B. anthracis sporulation kinases and E. coli EnvZ. The known or suspected conserved histidine is underlined. Asterisks represent the residues involved in direct interactions with the cognate response regulators (11, 61, 62), and shaded residues are highly conserved in C. difficile. E.c., E. coli; B.s., B. subtilis; B.a., Bacillus anthracis.
To investigate the potential influence of CD1492 on sporulation, we deleted the coding sequence by double crossover by markerless allelic exchange (see Fig. S2 in the supplemental material) (26). Expression of CD1492 in the CD1492 null mutant MC674 was ablated, as expected, and no growth defect was observed in any medium tested (data not shown). Whole-genome SNP analysis by Illumina next-generation sequencing revealed no additional nucleotide changes in the CD1492 mutant (see Materials and Methods). The CD1492 mutant was tested for the ability to sporulate on 70:30 sporulation agar (27). As demonstrated in Fig. 2A and Table 3, the CD1492 mutant produced significantly more spores than the parent strain, having a sporulation frequency approximately 2.4-fold higher than that of the parent strain, as determined by phase-contrast microscopy (630Δerm, 23.4% ± 5.1%; MC674, 56.4% ± 6.8%). Similarly, the production of ethanol-resistant spores was 3.4-fold higher for the CD1492 mutant than for the parent strain (Fig. 2B; 630Δerm, 23.0% ± 8.0%; MC674, 79.1% ± 5.3%), demonstrating that the spores produced by the mutant are fully formed and viable. The high-spore-forming phenotype of the CD1492 mutant suggests that, unlike many other sporulation histidine kinases, CD1492 is a negative regulator of sporulation initiation. On the basis of the mutant phenotype, CD1492 is unlikely to function solely as a sporulation sensor kinase that activates Spo0A by phosphorylation, as was predicted through in silico analyses. Alternatively, CD1492 may act as a phosphatase that inactivates Spo0A to prevent sporulation or it may activate a sporulation-repressing function.
FIG 2.
The ΔCD1492 mutant has a hypersporulation phenotype. (A) Representative phase-contrast micrographs of the parent strain (630Δerm) and the ΔCD1492 mutant (MC674) grown on 70:30 sporulation agar for 24 h. Open arrowheads indicate phase-bright spores. (B) Ethanol-resistant spore formation frequency per total viable cells of the 630Δerm and ΔCD1492 mutant strains collected from 70:30 sporulation agar after 24 h. Sporulation frequencies were calculated as described for each assay in Materials and Methods. The mean values and the standard errors of the means are shown. *, P ≤ 0.05 by a two-tailed Student t test.
TABLE 3.
The C. difficile CD1492 mutant forms more ethanol-resistant spores on 70:30 sporulation agar
| Strain | No. of CFU/ml |
Sporulation frequencyd | % Sporulation | ||
|---|---|---|---|---|---|
| Vegetative cellsa | Sporesb | Total cellsc | |||
| 630Δerm | 1.49 × 108 ± 2.13 × 107e | 3.60 × 107 ± 6.18 × 106 | 1.85 × 108± 1.61 × 107 | 2.23 × 10−1 ± 7.28 × 10−2 | 22.3 ± 7.28 |
| ΔCD1492 | 1.64 × 107 ± 3.88 × 106 | 5.33 × 107 ± 5.98 × 106 | 6.97 × 107 ± 8.03 × 106 | 7.76 × 10−1 ± 5.18 × 10−2 | 77.6 ± 5.18 |
The numbers of vegetative cells are the numbers of CFU recovered on BHIS medium plates.
The number of spores is the total number of CFU that survived ethanol treatment as described in Materials and Methods and were recovered on BHIS medium plates supplemented with 0.1% taurocholate.
The total cell numbers include both vegetative cells and spores.
The sporulation frequency was calculated as the number of ethanol-resistant spores divided by the total number of cells.
Values are means and standard errors of the means.
The CD1492 mutant has elevated sporulation-specific gene expression.
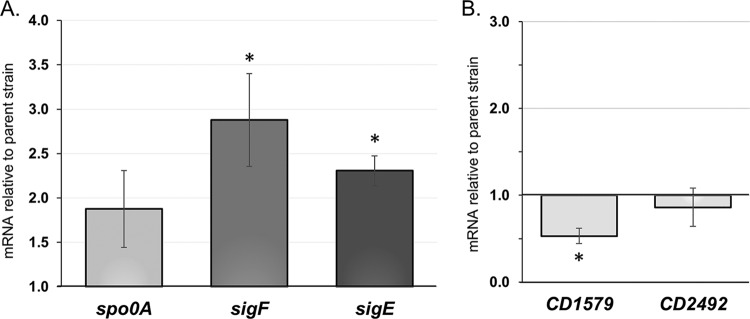
To determine the effect of the CD1492 mutation on sporulation initiation, the abundance of key early sporulation transcripts, relative to the parent strain, was measured during growth on sporulation medium. Transcription of the master sporulation regulator spo0A was slightly higher in the CD1492 mutant after 12 h on sporulation medium, but this difference did not reach statistical significance (Fig. 3A). Further, the transcription of the Spo0A-dependent sigma factors sigF and sigE was >2-fold higher in the CD1492 mutant strain (P ≤ 0.05). SigF and SigE are required for forespore and mother cell-specific sporulation gene expression, respectively (31). The greater sporulation frequencies and higher early sporulation gene expression of the CD1492 mutant suggest that a greater proportion of these cells enters the sporulation pathway.
FIG 3.
Expression of key early sporulation regulators and putative sporulation kinases in the ΔCD1492 mutant. Transcriptional analysis of spo0A, sigF, and sigE (A) and predicted sporulation kinase CD1579 and CD2492 expression (B) in the ΔCD1492 mutant (MC674) relative to that in parent strain 630Δerm. Cultures were grown on 70:30 sporulation agar for 12 h, RNA was harvested, cDNA was prepared, and qRT-PCR was performed with gene-specific primers as outlined in Materials and Methods. The mean values and the standard errors of the means of at least three biological replicates are shown. *, P ≤ 0.05 by two-tailed Student t test.
As mentioned previously, CD1492 is one of three orphan kinases that are proposed to function as sporulation sensor kinases. We found that expression of one of the suspected sporulation kinases, CD1579, was 2-fold lower in the CD1492 mutant, while expression of CD2492 was similar to that in the parent strain (Fig. 3B). Thus, CD1492 has a modest effect on the expression of one of the other suspected kinases.
In the model spore former B. subtilis, the sporulation sensor kinases facilitate sporulation initiation through the sporulation phosphorelay, which consists of the intermediate proteins Spo0F and Spo0B that enable phosphotransfer to Spo0A (32). As initiators of sporulation, the B. subtilis kinases are expressed prior to the onset of sporulation and their expression wanes in a sporulating population as sporulation progresses (32). We investigated the expression of CD1492 during growth on sporulation medium to determine how the timing of its transcription relates to sporulation initiation. Samples of the parent strain were taken during growth on 70:30 medium and assessed for CD1492 and sigE expression over time (see Fig. S3 in the supplemental material). Relative to transcription at 6 h after transfer to sporulation medium (H6, logarithmic phase), the transcription of CD1492 increased about 5-fold at 8 h postinoculation (H8). CD1492 transcript levels declined at later time points, even as levels of the early mother cell sigma factor sigE remained elevated. The decline in CD1492 expression during early sporulation implies that CD1492 is involved prior to the initiation of sporulation. The hypersporulation phenotype of the CD1492 mutant and the timing of CD1492 expression suggest that CD1492 acts as a negative regulator of sporulation before initiation.
A conserved catalytic histidine residue is required for CD1492 function.
To confirm that the CD1492 deletion was responsible for the mutant phenotypes, complementation was performed with a wild-type CD1492 allele under the control of the nisin-inducible cprA promoter (20, 28). As illustrated in Fig. 4, complementation of the CD1492 mutant with an inducible CD1492 allele (CD1492 pPcprA::CD1492, MC730) resulted in reduced spore formation. The restoration of the parental sporulation phenotype in the complemented strain demonstrates that the CD1492 mutation is responsible for the increased sporulation frequency of the mutant and suggests that the timing of CD1492 expression is not critical to its function as a negative sporulation regulator (Fig. 4; see Fig. S3 and S4 in the supplemental material). In addition, expression of exogenous CD1492 in the parent strain (630Δerm pPcprA::CD1492, MC587, Fig. 4) decreased the number of ethanol-resistant spores formed, suggesting that overexpression of CD1492 can reduce sporulation in an otherwise wild-type strain. This is similar to the sporulation sensor kinases of Bacillus and Clostridium species that can affect sporulation when overexpressed (33–35). To determine if CD1492 functions as a predicted sensor histidine kinase, we created a site-directed mutation at the conserved histidine residue, substituting an alanine (H668A). Histidine-to-alanine substitutions in the conserved histidine catalytic residues of sensor kinases result in an inability of the protein to transfer phosphate signals, thereby rendering them nonfunctional (15, 36). When the CD1492 H668A mutated allele was used to complement the CD1492 mutant (CD1492 pPcprA::CD1492-H668A, MC771), the sporulation frequency did not decrease relative to that of the mutant (Fig. 4). The inability of the CD1492 H668A allele to restore the mutant phenotype strongly suggests that the phosphotransfer capability of CD1492 is essential to its function as an inhibitor of sporulation.
FIG 4.

Complementation of ΔCD1492 and site-directed mutagenesis of conserved sensor kinase residues. Sporulation frequencies calculated from the ratios of spores to vegetative cells by phase-contrast micrographs obtained at H24 (black bars) or from cells before and after treatment with ethanol (gray bars). Strains 630Δerm pPcpr (MC282, vector control), 630Δerm pPcprA::CD1492 (MC587), ΔCD1492 pPcpr (MC729, vector control), ΔCD1492 pPcprA::CD1492 (MC730), and ΔCD1492 pPcprA::CD1492-H668A (MC771) were grown on 70:30 sporulation agar plates supplemented with 2 μg ml−1 thiamphenicol and 1 μg ml−1 nisin, and sporulation frequency assays were performed as described in Materials and Methods. The mean values and the standard errors of the means of at least four biological replicates are shown. *, P ≤ 0.05 by two-tailed Student t test.
Deletion of CD1492 results in decreased virulence in an animal model of infection.
Although the CD1492 mutant exhibits a higher sporulation frequency in vitro, the function of CD1492 in sporulation in the intestine and the effect of CD1492 on pathogenesis are not known. To this end, we examined the CD1492 mutant in a hamster model of C. difficile infection. Female Syrian golden hamsters were infected by oral gavage with approximately 5,000 spores of either the CD1492 mutant or parent strain 630Δerm. Following inoculation, animals were monitored for disease symptoms and fecal samples were acquired every 24 h postinfection for enumeration of C. difficile bacteria. As shown in Fig. 5A, hamsters infected with CD1492 mutant spores became moribund much more slowly than animals infected with the parent strain (mean times to morbidity: 630Δerm, 45.5 ± 3.5 h; ΔCD1492, 107.9 ± 52.5 h; P < 0.01, log rank test). Determination of the total number of C. difficile bacteria shed in the feces of infected animals revealed no significant differences between the CFU counts of the CD1492 mutant and parent strain infections, indicating that the mutant strain does not have an observable growth defect in vivo (Fig. 5B). Likewise, C. difficile CFU counts were similar in the cecal contents of CD1492 mutant and parent strain-infected animals postmortem (Fig. 5C). These data indicate that the decreased virulence observed in CD1492 mutant infections was not caused by in vivo defects in the outgrowth of spores or vegetative cells.
FIG 5.
Deletion of CD1492 results in decreased virulence in the hamster model of infection. (A) Kaplan-Meier survival plot of the survival times of Syrian golden hamsters infected with 5,000 spores of C. difficile strain 630Δerm (n = 12) or MC674 (ΔCD1492; n = 12). The mean times to morbidity were as follows: 630Δerm, 45.5 ± 3.5 h; ΔCD1492, 107.9 ± 52.5 h. (P < 0.01, log rank test). The total number of CFU of C. difficile recovered from feces at 24 h postinfection (B) or per milliliter of cecal content recovered postmortem (C) is shown. The solid lines in panels B and C represent the median CFU count of each strain, and the dotted lines denote the limit of detection (LOD; 2 × 101 CFU/g or CFU/ml). Statistical significance was assessed by one-way ANOVA. Animals infected with 630Δerm served as positive infection controls that were shown in a parallel study (22).
The CD1492 mutant produces less TcdA and has lower expression of toxin-associated regulators and motility genes.
On the basis of the decreased virulence and lack of a growth defect of the CD1492 strain in vivo, we hypothesized that this mutant may have lower expression of the two major virulence factors toxins TcdA and TcdB (3). To investigate the impact of CD1492 on toxin production, we analyzed the expression of toxin in vitro and in vivo. The expression of tcdA was 2-fold lower in CD1492 mutant cultures grown on sporulation agar (Fig. 6A), but no change in tcdB expression was observed. Supporting these results, a similar decrease in TcdA production was detected in strains that did not have a functional CD1492 gene by Western blotting of in vitro cultures with anti-TcdA antibody (Fig. 6B). Examination of toxin transcripts from the cecal contents of animals that succumbed to infection revealed considerable variability in tcdB transcript production in CD1492 mutant-infected animals, but differences in toxin expression did not achieve statistical significance (Fig. 6C).
FIG 6.
Toxin production in the ΔCD1492 mutant. (A) Transcriptional analysis of the primary toxins, tcdA and tcdB in the ΔCD1492 mutant (MC674) relative to the parent strain, 630Δerm. Cultures were grown on 70:30 agar medium for 12 h, RNA was harvested, cDNA was prepared, and qRT-PCR was performed with gene-specific primers as outlined in Materials and Methods. WT, wild type. (B) A representative Western blot analysis of TcdA in 630Δerm, ΔCD1492 (MC674), 630Δerm pPcpr (MC282, vector control), ΔCD1492 pPcpr (MC729, vector control), ΔCD1492 pPcprA::CD1492 (MC730), and ΔCD1492 pPcprA::CD1492-H668A (MC771) grown in TY medium for 24 h. The mean values and the standard errors of the means of three independent experiments are shown at the bottom; bold values are statistically significantly different from those of the parent strain by a two-tailed Student t test or by a one-way ANOVA, followed by Dunnett's multiple-comparison test, as described in Materials and Methods. (C) qRT-PCR analysis of tcdA and tcdB transcript levels in cecal contents of hamsters infected with 630Δerm (n = 5) or MC674 (ΔCD1492; n = 5). The mean values and the standard errors of the means are shown (*, P ≤ 0.05 by two-tailed Student t test).
Although the CD1492 mutant produces less tcdA transcript in vitro, it is unlikely that CD1492 is a direct regulator of toxin transcription. Toxin expression in C. difficile is affected by multiple regulatory factors that integrate complex cellular nutritional signals to control nutrient acquisition and motility (37). TcdA and TcdB are directly transcribed by the toxin-specific sigma factor TcdR, which in turn is transcribed by the motility sigma factor SigD (FliA) (38–40). Multiple negative regulators can also repress tcdR transcription, thereby preventing toxin production (41, 42). We investigated the expression of the positive regulator of toxin transcription sigD to determine if its transcription was affected in the CD1492 mutant. As shown in Fig. 7A, sigD expression is >6-fold lower in the CD1492 mutant grown on sporulation agar. A corresponding decrease in fliC transcription, which is SigD dependent, was also observed in the CD1492 mutant, indicating that SigD activity is also reduced. Decreased SigD activity is known to dramatically lower tcdA and tcdB expression, as well as flagellum production and motility (20, 39, 40). Accordingly, we compared the swimming motility of the CD1492 mutant to that of the parent strain on soft agar medium (Fig. 7B) but observed no significant difference in motility in vitro. However, examination of C. difficile fliC transcription from the ceca of infected animals revealed lower fliC expression in hamsters infected with the CD1492 mutant, demonstrating that SigD activity is decreased in vivo (Fig. 7C). The timing and expression of sigD and flagellar genes during infection affect colonization by and the virulence of C. difficile and other motile pathogens and thus probably contribute to the decreased virulence of the CD1492 mutant in the animal model (43–47).
FIG 7.
The ΔCD1492 mutant has decreased expression of toxin, motility, and sporulation regulators. (A) qRT-PCR analysis of the motility and toxin-associated sigma factor sigD, the sigD-dependent gene fliC, and the sporulation and sigD regulator rstA in the ΔCD1492 mutant relative to those in parent strain 630Δerm. Cultures were grown on 70:30 agar medium for 12 h, RNA was harvested, cDNA was prepared, and qPCR was performed with gene-specific primers as outlined in Materials and Methods. (B) Motility of 630Δerm, ΔCD1492 (MC674), and sigD (RT1075, negative control) mutant strains in one-half-concentration BHI medium with 0.3% agar. Swimming diameters were measured every 24 h for a total of 168 h. (C) qRT-PCR analysis of fliC transcript levels in the cecal contents of hamsters infected with 630Δerm (n = 5) or MC674 (ΔCD1492; n = 5). The mean values and the standard errors of the means are shown (*, P ≤ 0.05 by two-tailed Student t test).
The specific factors that control sigD transcription and activity have not been fully elucidated for C. difficile in vitro, and even less is known about the regulation of SigD in vivo. A few negative regulators of SigD have been identified, including the early sporulation effector RstA, the stationary-phase sigma factor SigH, and the anti-sigma factor FlgM (24, 39, 48, 49). We examined the transcription of rstA on sporulation medium and found that its expression was more than 3-fold lower in the CD1492 mutant than in the parent strain (Fig. 7A). This finding is intriguing, as an rstA null mutant has higher sigD expression and, accordingly, RstA negatively affects sigD transcription (24). Further investigation of the expression profiles for the CD1492 and rstA mutants revealed that the gene expression and sporulation profiles of these mutants are reversed (Table 4). The inverse correlation of gene expression and phenotypes between the rstA and CD1492 mutants strongly suggests that the activities of these factors are linked within a regulatory pathway, likely with CD1492 functioning upstream of RstA. Although it is possible that CD1492 acts directly on Spo0A as a phosphatase, the effects of CD1492 on sigD and rstA expression suggest that CD1492 functions at least partially independently of Spo0A (10).
TABLE 4.
Comparison of gene expression and phenotypes of rstA and CD1492 null mutants
| Trait | Product | Fold changef vs parent |
|
|---|---|---|---|
| CD1492 | rstAa | ||
| Phenotypeb | |||
| Sporulation frequencyc | ↑ 2.4 | ↓ 20.1 | |
| Virulenced | ↓ 2.4 | ↑ 1.3 | |
| Gene expressionb | |||
| CD2492 | Sporulation sensor kinase | ↓ 1.2 | ↑ 2.4 |
| CD1579 | Sporulation sensor kinase | ↓ 1.9 | ↑ 4.9 |
| CD1492 | Sporulation sensor kinase | —e | ↑ 2.4 |
| rstA | Sporulation/sigD regulator | ↓ 3.6 | —e |
| spo0A | Sporulation master regulator | ↑ 1.9 | ↓ 1.4 |
| sigF | Sporulation sigma factor | ↑ 2.9 | ↓ 2.2 |
| sigE | Sporulation sigma factor | ↑ 2.3 | ↓ 6.4 |
| sigD | Motility sigma factor | ↓ 6.3 | ↑ 2.5 |
| fliC | Flagellar component | ↓ 3.1 | ↑ 3.5 |
| tcdR | Toxin sigma factor | 0.0 | ↑ 2.9 |
| tcdA | Toxin A | ↓ 2.0 | ↑ 4.0 |
| tcdB | Toxin B | ↓ 1.1 | ↑ 3.5 |
rstA values obtained under the same experimental conditions, previously reported (24).
All mean fold changes reported are relative to parent strain 630Δerm. Bold values are statistically significantly different (P ≤ 0.05 by two-tailed Student t test).
As determined by phase-contrast microscopy.
Defined here as fold change in time to morbidity in hamsters.
No significant transcript levels were detected in null mutants.
Ratios of the mean relative transcript levels for mutant and wild-type strains.
DISCUSSION
The formation of endospores is critical for the survival of C. difficile outside the host and for dissemination of the bacterium to new hosts (50). The basic morphological programs for producing a dormant spore are similar in C. difficile and well-characterized spore formers such as Bacillus subtilis, but the specific factors that regulate entry into sporulation are not well conserved (5, 10, 16, 31, 51, 52). The specific signals that activate sporulation are not known, but it is anticipated that, like other sporulating members of the phylum Firmicutes, the sporulation-initiating and -inhibiting signals for C. difficile are transmitted through predicted sporulation sensor histidine kinases, including CD1492. Our investigation found that the predicted sporulation kinase CD1492 inhibits sporulation initiation. Moreover, CD1492 affects the function of factors other than its anticipated target, Spo0A, including the expression of the sporulation regulator RstA and the motility and toxin regulator SigD. As a result, CD1492 impacts both sporulation and motility during the infection of a host.
In the spore-forming members of the phylum Firmicutes, the master regulator of sporulation Spo0A is activated by phosphorylation and inactivated by dephosphorylation (16, 53). In the sporulating anaerobes that have been studied, most of the predicted orphan kinases that affect sporulation initiation function as Spo0A activators (34, 35). Mutation of the catalytic histidine residue in CD1492 resulted in a loss of activity and failure to restore the mutant phenotype (CD1492 pPcprA::CD1492-H668A, MC771). Therefore, CD1492 may function as a kinase on a target other than Spo0A but more likely acts as a phosphatase on Spo0A. In addition to CD1492 of C. difficile, other predicted sensor kinases were found to negatively impact sporulation in two anaerobic species, Clostridium acetobutylicum and “Ruminiclostridium (formerly Clostridium) thermocellum.” In C. acetobutylicum, the predicted kinase Ca_C0437 represses sporulation and can catalyze ATP-dependent dephosphorylation of Spo0A∼P in vitro (35) (see Fig. S5 in the supplemental material). In vitro phosphotransfer data suggest that Ca_C0437 acts as a phosphatase, playing a role similar to that of the Spo0E and Rap proteins that inactivate Spo0A in B. subtilis (54, 55). A similar sporulation histidine kinase-like protein, Clo1313_1973, was identified in “R. thermocellum” (34) (see Fig. S5). The role of these histidine kinase-like phosphatases in preventing initiation is further evidence that the anaerobic spore formers evolved strategies that are distinct from the Bacillus model but still achieve the same goal of Spo0A inactivation.
In C. acetobutylicum, three positive-acting and one negative-acting sporulation kinase-like proteins have been identified, while in “R. thermocellum,” four positive regulators and one negative regulator were found (34, 35) (see Fig. S5). Analysis of these sporulation sensor mutants uncovered the existence of two genetic pathways that can lead to activation of sporulation in each of these species (34, 35). C. difficile has three putative sporulation histidine kinase-like proteins: CD1492 and CD2492, which are predicted membrane proteins, and CD1579, which is likely cytosolic (11) (Fig. 1). Comparing the sporulation sensors of C. difficile, “R. thermocellum,” and C. acetobutylicum (smart.embl-heidelberg.de); (56, 57), there is tremendous variability in sensor architecture and there are no known or apparent structural features that are predictive of the role these factors play in the initiation cascade (Fig. 1; see Fig. S5). BLAST analysis also revealed that orthologs of CD1492 are encoded in closely related species such as C. sordellii, “C. dakarense,” and C. mangenotii, but these factors have not been characterized. On the basis of the protein structures and what is known about the initiation pathways, it appears that each species evolved independent means of processing the signals that stimulate or inhibit sporulation, which is expected since these distant relatives inhabit very different ecological niches.
The phosphatase functions of sensor kinases are known to contribute to the activation state of their cognate response regulators (58–60). The roles of Ca_C0437, Clo1313_1973, and CD1492 as phosphatases are supported by the hypersporulation phenotypes observed in the respective null mutants, though the mechanisms of phosphatase activity have not been characterized. Traditionally, the specific functions of sporulation phosphotransfer proteins have been examined through in vitro phosphotransfer assays, which demonstrate the ability of potential interacting partners to give or receive phosphate (11, 14, 35, 61, 62). While in vitro phosphotransfer assays can demonstrate interactions between individual sporulation initiation factors, phosphotransfer between these proteins often occurs in both directions in vitro, whereas in vivo, one direction of transfer is favored (14, 32). Consequently, the results of these assays are often not reliable indicators of the direction of transfer or the order of pathway components. However, a combination of phosphotransfer assays, genetic analyses, overexpression phenotypes, and characterization of multiple null mutants in pathway components can help unravel the roles of individual factors, which we plan to perform in future studies (32, 34).
In addition to defining the specific role of CD1492 in Spo0A activation, determining how CD1492 affects RstA and SigD will help to reveal how these factors interact to control both sporulation and pathogenesis. Previous studies of sporulation-defective spo0A and rstA mutants have observed different effects on virulence and have revealed indirect links between sporulation and pathogenesis (12, 24, 63, 64). Although the spore-forming anaerobes are currently thought to have a simplified mechanism for initiating sporulation, the discovery of interactions between regulators of initiation, toxin production, and motility indicates that the initiation process in these species is far from simple. The data suggest that there are multiple layers of transcriptional and posttranslational regulation, as well as protein-protein interactions that control these processes. Further defining the complex genetic pathways, the protein interactions and the signals that activate sporulation in C. difficile could provide clues about how to manipulate this process and prevent the spread of disease.
Supplementary Material
ACKNOWLEDGMENTS
We give special thanks to Charles Moran and members of the McBride lab for helpful suggestions and discussions during the course of this work. In addition, we thank Gregory Tharp, Nirav Patel, and Steven Bosinger of the Yerkes Genomics Core Laboratory for assistance with the next-generation sequencing experiments and data analysis.
The Genomics Core receives financial support from P51 OD011132 (Support of the Yerkes National Primate Research Center). This research was supported by the U.S. National Institutes of Health through research grants DK087763, DK101870, AI109526, and AI116933 to S.M.M.; T32 GM008169 to E.C.W.; and T32 AI106699 to K.L.N. and S.E.A.
The content of this report is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00735-16.
REFERENCES
- 1.Wilson KH, Kennedy MJ, Fekety FR. 1982. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J Clin Microbiol 15:443–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorg JA, Sonenshein AL. 2008. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol 190:2505–2512. doi: 10.1128/JB.01765-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voth DE, Ballard JD. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev 18:247–263. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theriot CM, Young VB. 2015. Interactions between the gastrointestinal microbiome and Clostridium difficile. Annu Rev Microbiol 69:445–461. doi: 10.1146/annurev-micro-091014-104115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McBride SM. 2014. More than one way to make a spore. Microbe 9:153–157. doi: 10.1128/microbe.9.153.1. [DOI] [Google Scholar]
- 6.Koenigsknecht MJ, Theriot CM, Bergin IL, Schumacher CA, Schloss PD, Young VB. 2015. Dynamics and establishment of Clostridium difficile infection in the murine gastrointestinal tract. Infect Immun 83:934–941. doi: 10.1128/IAI.02768-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubberke E. 2012. Strategies for prevention of Clostridium difficile infection. J Hosp Med 7(Suppl 3):S14–S17. doi: 10.1002/jhm.1908. [DOI] [PubMed] [Google Scholar]
- 8.Vohra P, Poxton IR. 2011. Efficacy of decontaminants and disinfectants against Clostridium difficile. J Med Microbiol 60:1218–1224. doi: 10.1099/jmm.0.030288-0. [DOI] [PubMed] [Google Scholar]
- 9.Ali S, Moore G, Wilson AP. 2011. Spread and persistence of Clostridium difficile spores during and after cleaning with sporicidal disinfectants. J Hosp Infect 79:97–98. doi: 10.1016/j.jhin.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Fimlaid KA, Bond JP, Schutz KC, Putnam EE, Leung JM, Lawley TD, Shen A. 2013. Global analysis of the sporulation pathway of Clostridium difficile. PLoS Genet 9:e1003660. doi: 10.1371/journal.pgen.1003660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Underwood S, Guan S, Vijayasubhash V, Baines SD, Graham L, Lewis RJ, Wilcox MH, Stephenson K. 2009. Characterization of the sporulation initiation pathway of Clostridium difficile and its role in toxin production. J Bacteriol 191:7296–7305. doi: 10.1128/JB.00882-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettit LJ, Browne HP, Yu L, Smits WK, Fagan RP, Barquist L, Martin MJ, Goulding D, Duncan SH, Flint HJ, Dougan G, Choudhary JS, Lawley TD. 2014. Functional genomics reveals that Clostridium difficile Spo0A coordinates sporulation, virulence and metabolism. BMC Genomics 15:160. doi: 10.1186/1471-2164-15-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonenshein AL. 2000. Control of sporulation initiation in Bacillus subtilis. Curr Opin Microbiol 3:561–566. doi: 10.1016/S1369-5274(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 14.Burbulys D, Trach KA, Hoch JA. 1991. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell 64:545–552. doi: 10.1016/0092-8674(91)90238-T. [DOI] [PubMed] [Google Scholar]
- 15.Hoch JA. 2000. Two-component and phosphorelay signal transduction. Curr Opin Microbiol 3:165–170. doi: 10.1016/S1369-5274(00)00070-9. [DOI] [PubMed] [Google Scholar]
- 16.Paredes CJ, Alsaker KV, Papoutsakis ET. 2005. A comparative genomic view of clostridial sporulation and physiology. Nat Rev Microbiol 3:969–978. doi: 10.1038/nrmicro1288. [DOI] [PubMed] [Google Scholar]
- 17.Edwards AN, Suárez JM, McBride SM. 2013. Culturing and maintaining Clostridium difficile in an anaerobic environment. J Vis Exp 79:e50787. doi: 10.3791/50787:e50787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith CJ, Markowitz SM, Macrina FL. 1981. Transferable tetracycline resistance in Clostridium difficile. Antimicrob Agents Chemother 19:997–1003. doi: 10.1128/AAC.19.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luria SE, Burrous JW. 1957. Hybridization between Escherichia coli and Shigella. J Bacteriol 74:461–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purcell EB, McKee RW, McBride SM, Waters CM, Tamayo R. 2012. Cyclic diguanylate inversely regulates motility and aggregation in Clostridium difficile. J Bacteriol 194:3307–3316. doi: 10.1128/JB.00100-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorg JA, Dineen SS. 2009. Laboratory maintenance of Clostridium difficile. Curr Protoc Microbiol Chapter 9:Unit9A.1. doi: 10.1002/9780471729259.mc09a01s12. [DOI] [PubMed] [Google Scholar]
- 22.McBride SM, Sonenshein AL. 2011. The dlt operon confers resistance to cationic antimicrobial peptides in Clostridium difficile. Microbiology 157:1457–1465. doi: 10.1099/mic.0.045997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouillaut L, McBride SM, Sorg JA. 2011. Genetic manipulation of Clostridium difficile. Curr Protoc Microbiol Chapter 9:Unit 9A.2. doi: 10.1002/9780471729259.mc09a02s20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards AN, Tamayo R, McBride SM. 2016. A novel regulator controls Clostridium difficile sporulation, motility and toxin production. Mol Microbiol 100:954–971. doi: 10.1111/mmi.13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harju S, Fedosyuk H, Peterson KR. 2004. Rapid isolation of yeast genomic DNA: bust n' grab. BMC Biotechnol 4:8. doi: 10.1186/1472-6750-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cartman ST, Kelly ML, Heeg D, Heap JT, Minton NP. 2012. Precise manipulation of the Clostridium difficile chromosome reveals a lack of association between the tcdC genotype and toxin production. Appl Environ Microbiol 78:4683–4690. doi: 10.1128/AEM.00249-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Putnam EE, Nock AM, Lawley TD, Shen A. 2013. SpoIVA and SipL are Clostridium difficile spore morphogenetic proteins. J Bacteriol 195:1214–1225. doi: 10.1128/JB.02181-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards AN, Nawrocki KL, McBride SM. 2014. Conserved oligopeptide permeases modulate sporulation initiation in Clostridium difficile. Infect Immun 82:4276–4291. doi: 10.1128/IAI.02323-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dineen SS, McBride SM, Sonenshein AL. 2010. Integration of metabolism and virulence by Clostridium difficile CodY. J Bacteriol 192:5350–5362. doi: 10.1128/JB.00341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 31.Pereira FC, Saujet L, Tome AR, Serrano M, Monot M, Couture-Tosi E, Martin-Verstraete I, Dupuy B, Henriques AO. 2013. The spore differentiation pathway in the enteric pathogen Clostridium difficile. PLoS Genet 9:e1003782. doi: 10.1371/journal.pgen.1003782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang M, Shao W, Perego M, Hoch JA. 2000. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol Microbiol 38:535–542. doi: 10.1046/j.1365-2958.2000.02148.x. [DOI] [PubMed] [Google Scholar]
- 33.Perego M, Cole SP, Burbulys D, Trach K, Hoch JA. 1989. Characterization of the gene for a protein kinase which phosphorylates the sporulation-regulatory proteins Spo0A and Spo0F of Bacillus subtilis. J Bacteriol 171:6187–6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mearls EB, Lynd LR. 2014. The identification of four histidine kinases that influence sporulation in. Clostridium thermocellum. Anaerobe 28:109–119. doi: 10.1016/j.anaerobe.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Steiner E, Dago AE, Young DI, Heap JT, Minton NP, Hoch JA, Young M. 2011. Multiple orphan histidine kinases interact directly with Spo0A to control the initiation of endospore formation in. Clostridium acetobutylicum. Mol Microbiol 80:641–654. doi: 10.1111/j.1365-2958.2011.07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suárez JM, Edwards AN, McBride SM. 2013. The Clostridium difficile cpr locus is regulated by a noncontiguous two-component system in response to type A and B lantibiotics. J Bacteriol 195:2621–2631. doi: 10.1128/JB.00166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouillaut L, Dubois T, Sonenshein AL, Dupuy B. 2015. Integration of metabolism and virulence in Clostridium difficile. Res Microbiol 166:375–383. doi: 10.1016/j.resmic.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mani N, Dupuy B. 2001. Regulation of toxin synthesis in Clostridium difficile by an alternative RNA polymerase sigma factor. Proc Natl Acad Sci U S A 98:5844–5849. doi: 10.1073/pnas.101126598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Meouche I, Peltier J, Monot M, Soutourina O, Pestel-Caron M, Dupuy B, Pons JL. 2013. Characterization of the SigD regulon of C. difficile and its positive control of toxin production through the regulation of tcdR. PLoS One 8:e83748. doi: 10.1371/journal.pone.0083748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKee RW, Mangalea MR, Purcell EB, Borchardt EK, Tamayo R. 2013. The second messenger cyclic di-GMP regulates Clostridium difficile toxin production by controlling expression of sigD. J Bacteriol 195:5174–5185. doi: 10.1128/JB.00501-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antunes A, Martin-Verstraete I, Dupuy B. 2011. CcpA-mediated repression of Clostridium difficile toxin gene expression. Mol Microbiol 79:882–899. doi: 10.1111/j.1365-2958.2010.07495.x. [DOI] [PubMed] [Google Scholar]
- 42.Dineen SS, Villapakkam AC, Nordman JT, Sonenshein AL. 2007. Repression of Clostridium difficile toxin gene expression by CodY. Mol Microbiol 66:206–219. doi: 10.1111/j.1365-2958.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- 43.Aubry A, Hussack G, Chen W, KuoLee R, Twine SM, Fulton KM, Foote S, Carrillo CD, Tanha J, Logan SM. 2012. Modulation of toxin production by the flagellar regulon in Clostridium difficile. Infect Immun 80:3521–3532. doi: 10.1128/IAI.00224-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scaria J, Janvilisri T, Fubini S, Gleed RD, McDonough SP, Chang YF. 2011. Clostridium difficile transcriptome analysis using pig ligated loop model reveals modulation of pathways not modulated in vitro. J Infect Dis 203:1613–1620. doi: 10.1093/infdis/jir112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dingle TC, Mulvey GL, Armstrong GD. 2011. Mutagenic analysis of the Clostridium difficile flagellar proteins, FliC and FliD, and their contribution to virulence in hamsters. Infect Immun 79:4061–4067. doi: 10.1128/IAI.05305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baban ST, Kuehne SA, Barketi-Klai A, Cartman ST, Kelly ML, Hardie KR, Kansau I, Collignon A, Minton NP. 2013. The role of flagella in Clostridium difficile pathogenesis: comparison between a non-epidemic and an epidemic strain. PLoS One 8:e73026. doi: 10.1371/journal.pone.0073026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janoir C. 2016. Virulence factors of Clostridium difficile and their role during infection. Anaerobe 37:13–24. doi: 10.1016/j.anaerobe.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Janoir C, Deneve C, Bouttier S, Barbut F, Hoys S, Caleechum L, Chapeton-Montes D, Pereira FC, Henriques AO, Collignon A, Monot M, Dupuy B. 2013. Adaptive strategies and pathogenesis of Clostridium difficile from in vivo transcriptomics. Infect Immun 81:3757–3769. doi: 10.1128/IAI.00515-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saujet L, Monot M, Dupuy B, Soutourina O, Martin-Verstraete I. 2011. The key sigma factor of transition phase, SigH, controls sporulation, metabolism, and virulence factor expression in Clostridium difficile. J Bacteriol 193:3186–3196. doi: 10.1128/JB.00272-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deakin LJ, Clare S, Fagan RP, Dawson LF, Pickard DJ, West MR, Wren BW, Fairweather NF, Dougan G, Lawley TD. 2012. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect Immun 80:2704–2711. doi: 10.1128/IAI.00147-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saujet L, Pereira FC, Serrano M, Soutourina O, Monot M, Shelyakin PV, Gelfand MS, Dupuy B, Henriques AO, Martin-Verstraete I. 2013. Genome-wide analysis of cell type-specific gene transcription during spore formation in Clostridium difficile. PLoS Genet 9:e1003756. doi: 10.1371/journal.pgen.1003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edwards AN, McBride SM. 2014. Initiation of sporulation in Clostridium difficile: a twist on the classic model. FEMS Microbiol Lett 358:110–118. doi: 10.1111/1574-6968.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown DP, Ganova-Raeva L, Green BD, Wilkinson SR, Young M, Youngman P. 1994. Characterization of spo0A homologues in diverse Bacillus and Clostridium species identifies a probable DNA-binding domain. Mol Microbiol 14:411–426. doi: 10.1111/j.1365-2958.1994.tb02176.x. [DOI] [PubMed] [Google Scholar]
- 54.Perego M, Hoch JA. 1991. Negative regulation of Bacillus subtilis sporulation by the spo0E gene product. J Bacteriol 173:2514–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perego M. 2001. A new family of aspartyl phosphate phosphatases targeting the sporulation transcription factor Spo0A of Bacillus subtilis. Mol Microbiol 42:133–143. [DOI] [PubMed] [Google Scholar]
- 56.Schultz J, Milpetz F, Bork P, Ponting CP. 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci U S A 95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Letunic I, Doerks T, Bork P. 2015. SMART: recent updates, new developments and status in 2015. Nucleic Acids Res 43:D257–260. doi: 10.1093/nar/gku949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kenney LJ. 2010. How important is the phosphatase activity of sensor kinases? Curr Opin Microbiol 13:168–176. doi: 10.1016/j.mib.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raivio TL, Silhavy TJ. 1997. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J Bacteriol 179:7724–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Wulf P, Lin EC. 2000. Cpx two-component signal transduction in Escherichia coli: excessive CpxR-P levels underlie CpxA* phenotypes. J Bacteriol 182:1423–1426. doi: 10.1128/JB.182.5.1423-1426.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stephenson K, Hoch JA. 2002. Evolution of signalling in the sporulation phosphorelay. Mol Microbiol 46:297–304. doi: 10.1046/j.1365-2958.2002.03186.x. [DOI] [PubMed] [Google Scholar]
- 62.Skerker JM, Perchuk BS, Siryaporn A, Lubin EA, Ashenberg O, Goulian M, Laub MT. 2008. Rewiring the specificity of two-component signal transduction systems. Cell 133:1043–1054. doi: 10.1016/j.cell.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mackin KE, Carter GP, Howarth P, Rood JI, Lyras D. 2013. Spo0A differentially regulates toxin production in evolutionarily diverse strains of Clostridium difficile. PLoS One 8:e79666. doi: 10.1371/journal.pone.0079666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenbusch KE, Bakker D, Kuijper EJ, Smits WK. 2012. C. difficile 630Δerm Spo0A regulates sporulation, but does not contribute to toxin production, by direct high-affinity binding to target DNA. PLoS One 7:e48608. doi: 10.1371/journal.pone.0048608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wüst J, Hardegger U. 1983. Transferable resistance to clindamycin, erythromycin, and tetracycline in Clostridium difficile. Antimicrob Agents Chemother 23:784–786. doi: 10.1128/AAC.23.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hussain HA, Roberts AP, Mullany P. 2005. Generation of an erythromycin-sensitive derivative of Clostridium difficile strain 630 (630Deltaerm) and demonstration that the conjugative transposon Tn916DeltaE enters the genome of this strain at multiple sites. J Med Microbiol 54:137–141. doi: 10.1099/jmm.0.45790-0. [DOI] [PubMed] [Google Scholar]
- 67.Thomas CM, Smith CA. 1987. Incompatibility group P plasmids: genetics, evolution, and use in genetic manipulation. Annu Rev Microbiol 41:77–101. doi: 10.1146/annurev.mi.41.100187.000453. [DOI] [PubMed] [Google Scholar]
- 68.Yanisch-Perron C, Vieira J, Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 69.McBride SM, Sonenshein AL. 2011. Identification of a genetic locus responsible for antimicrobial peptide resistance in Clostridium difficile. Infect Immun 79:167–176. doi: 10.1128/IAI.00731-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.