Abstract
Approximately 20% of the population is persistently colonized by Staphylococcus aureus in the nares. Th17-like immune responses mediated by the interleukin-17 (IL-17) family of cytokines and neutrophils are becoming recognized as relevant host defense mechanisms for resolution of S. aureus mucocutaneous infections. Since antimicrobial peptides are regulated by the IL-17 cytokines, we sought to determine the role of IL-17 cytokines in production of antimicrobial peptides in a murine model of S. aureus nasal carriage. We discovered that nasal tissue supernatants have antistaphylococcal activity, and mice deficient in both IL-17A and IL-17F lost the ability to clear S. aureus nasal colonization. IL-17A was found to be sufficient for nasal mBD-3 production ex vivo and was required for CRAMP, mBD-3, and mBD-14 expression in response to S. aureus colonization in vivo. These data were confirmed in a clinical study of nasal secretions in which elevated levels of the human forms of these antimicrobial peptides were found in nasal secretions from healthy human subjects when they were colonized with S. aureus but not in secretions from noncolonized subjects. Together, these data provide evidence for the importance of IL-17A regulation of antimicrobial peptides and IL-17F in the clearance of S. aureus nasal carriage.
INTRODUCTION
It is increasingly recognized that antimicrobial peptides (AMPs) play an important role in the immune system of humans. AMPs are present at all human body sites normally exposed to microbes, such as the skin and mucosa (1). The most prominent mammalian AMPs are the defensins (2). Four human β-defensins (hBD-1 to hBD-4) have been characterized that are produced by mucosa and epithelial cells (3). However, with the exception of hBD-3, their antimicrobial activity is antagonized by increasing concentrations of monovalent or divalent ions found at many body sites (4). In humans, only one cathelicidin, LL-37, is present, characterized by a conserved N-terminal domain that is proteolytically cleaved to generate the mature, active peptide contained within the C terminus (5, 6), and it is mainly produced in leukocytes and epithelial cells (7).
Human cathelicidin and β-defensins have implications for host defense against Staphylococcus aureus colonization. LL-37 was determined to be expressed in corneal epithelial cells and to have potent activity against S. aureus (8). Expression of hBD-2 mRNA by human keratinocytes was significantly induced by contact with S. aureus (9). In conjunction, the activities of hBD-3 and CAP18, the precursor of LL-37, against S. aureus were found to be greater than those of hBD-1 and hBD-2 (9). Activity of hBD-2 against S. aureus is present but at higher concentrations (10). hBD-3 has a stronger lethal effect on both planktonic and biofilm-grown S. aureus than vancomycin and other antibiotics at low concentrations (11, 12). In another study, hBD-3 and hBD-2 were induced in patients with lesional S. aureus skin infections but not in healthy patients (13). Additionally, healthy individuals with deficient hBD-3 expression in keratinocytes are more prone to persistent nasal colonization with S. aureus (14). The reasons for differences in AMP expression at epithelial surfaces and their relation to nasal colonization remain elusive. Multiple studies have determined that defensin gene polymorphisms, both in sequence and in gene copy numbers, do not seem to be involved in S. aureus carriage predisposition (15, 16). In contrast, polymorphisms in the DEFB1 gene promoter region, a regulator of hBD-1 and hBD-3, were associated with lower hBD-3 expression and persistent S. aureus nasal colonization (17).
Cathelicidin and β-defensin antimicrobial peptides are also produced in mouse. Cathelin-related antimicrobial peptide, or CRAMP, is the murine homolog to human LL-37 (18). Analogous to LL-37, neutrophil-derived CRAMP displays effective S. aureus killing (19). Mouse β-defensin-3 and -14 correspond to human β-defensin-2 and -3, respectively (20, 21). S. aureus cells treated with recombinant mBD-3 showed growth inhibition and morphological and structural changes, including delamination and perforation of the peripheral cell walls, porosity, and release of the cytoplasmic contents (22, 23). mBD-14 is expressed in a wide variety of tissues, including spleen, colon, and tissues of the upper and lower respiratory tract (24), is induced in osteoblasts upon stimulation with S. aureus supernatants, and exhibits antistaphylococcal activity (25).
Previously, we have shown that clearance of S. aureus nasal colonization requires IL-17A-mediated neutrophil influx (26). In addition, we previously found another IL-17 family member, IL-17F, to be upregulated, but its importance during nasal colonization has not been defined. The IL-17 family of cytokines is reported to induce epithelial AMP production in response to S. aureus insult (27). Therefore, we hypothesized that IL-17A and IL-17F expression during nasal carriage induces AMPs, and these AMPs promote decolonization. In particular, we were interested in the role of CRAMP, mBD-3, and mBD-14 antimicrobial peptides in S. aureus nasal colonization due to their antistaphylococcal activity and inducible tissue expression at epithelial surfaces.
Here, we show that nasal tissue supernatants exhibited antistaphylococcal activity. In addition, IL-17A- and IL-17F-deficient mice are unable to clear nasal colonization and displayed a more severe phenotype than IL-17A-deficient mice alone. IL-17A was found to be required for upregulation of the antimicrobial peptides CRAMP, mBD-3, and mBD-14 during S. aureus nasal colonization and to be sufficient for mBD-3 expression in nasal tissue. This observation was confirmed by demonstrating statistically significant upregulation of AMP expression in nasal secretions from healthy subjects colonized with S. aureus. This is the first study to provide direct evidence that S. aureus induces AMP expression in the nares, suggesting an involvement of antimicrobial peptides in the defense against S. aureus nasal colonization.
MATERIALS AND METHODS
Ethics statement.
All animals were handled in strict accordance with good animal practice as defined in the federal regulations set forth in the Animal Welfare Act (AWA), the 1996 Guide for the Care and Use of Laboratory Animals (28), the Public Health Service Policy on Humane Care and Use of Laboratory Animals (29), as well as UMB's policies and procedures as set forth in the UMB animal care and use training manual, and all animal work was approved by the UMB Dental Institutional Animal Care and Use Committee (number 12-10-01).
The collection of human nasal secretion samples and S. aureus strain SA1108, isolated from the nares of a patient, were approved by the University of Pennsylvania and University of Maryland Baltimore Institutional Review Boards, respectively. Adult subjects provided informed written consent, and no children subjects were used in these studies. In addition, collected human nasal secretion samples and strain SA1108 were deidentified before use. Strain SA1108 was not collected specifically for this study but was received from the Antibodies in S. aureus Bacteremia collection at UMB. Only two time points were chosen for collecting patient samples, since patients are often lost to follow-up for multiple sampling due to subject discomfort and the extended time required for the collection of nasal secretions. This two-time-point protocol has been successfully used by Nouwen et al. to effectively determine S. aureus colonization rates (30).
Animals.
C57BL/6J wild-type (WT) mice were purchased from The Jackson Laboratories. C57BL/6J IL-17A and IL-17A/F knockout (KO) mice were generous gifts from Yoichiro Iwakura (University of Tokyo, Japan). Animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Maryland, Baltimore Dental School. Female mice aged 6 to 8 weeks were used for each study.
S. aureus strain and nasal colonization model.
S. aureus strain SA1108 was isolated from the nares of a colonized patient enrolled in an epidemiological carriage study. SA1108 was grown overnight in 15 ml tryptic soy broth and diluted to an optical density (OD) at 600 nm of 0.1 (equal to 109 CFU/ml). After centrifugation, the pellet was resuspended in sterile phosphate-buffered saline (PBS) to a final density of 1010 CFU/ml.
Prior to inoculation, mice were anesthetized with isoflurane, followed by intraperitoneal (i.p.) injection with 0.1 ml of a ketamine (20 mg/ml) and xylazine (2 mg/ml) cocktail. An inoculum of 10 μl (108 CFU) was presented intranasally without trauma to the nares.
To harvest nasal tissue, mice were decapitated using surgical scissors (Roboz, Gaithersburg, MD). The facial tissue and lower jaw were dissected along with the nasal-associated lymphoid tissue (NALT) located on the upper palate. The posterior cranium was removed by dissection along the NALT tissue line. The remaining nasal tissue was placed in 1 ml RNAlater or ice-cold sterile RPMI (plus l-glutamine and HEPES) with 10% fetal bovine serum (FBS) and 100 μg/ml streptomycin.
Ex vivo stimulation.
Tissue placed in sterile RPMI (plus l-glutamine and HEPES) with 10% FBS and 100 μg/ml streptomycin was incubated in a cell incubator (37°C and 5% CO2) for 1 h to eliminate endogenous bacteria. Nasal tissue was then washed twice in sterile RPMI (plus l-glutamine and HEPES) with 10% FBS and no streptomycin. After being washed, nasal tissue was placed in 1 ml fresh RPMI (plus l-glutamine and HEPES) with 10% FBS in a 24-well plate. To stimulate tissue, 20 ng/ml of mouse recombinant IL-17A (rIL-17A), rIL-17F, or both was added. Nonstimulated tissue acted as negative controls. At 24 h poststimulation, supernatants were collected and centrifuged at 14,800 rpm and 4°C for 5 min and stored at −20°C until needed. Nasal tissue was washed three times in sterile PBS to remove RPMI medium. Nasal tissue then was placed in RNAlater until needed.
Nasal secretion killing assay.
S. aureus SA1108 was grown overnight in sterile RPMI (plus l-glutamine and HEPES) with 10% FBS. SA1108 was diluted to an OD of 0.1 (∼108 CFU/ml) in RPMI. A 100-μl aliquot was added to a clean Eppendorf tube and resuspended in 1 ml RPMI or 1 ml sterile double-distilled water (ddH2O) (107 CFU/ml). Ten microliters of bacterial suspension was added to a 96-well plate. Bacterial suspension was cocultured with either 90 μl nasal secretion or 10 μl nasal secretion and 80 μl sterile ddH2O. Sterile ddH2O was utilized to dilute salt concentrations into a range effective for antistaphylococcal activity of antimicrobial peptides. Alternatively, secretions were heat inactivated by autoclaving for 15 min and cooled on ice prior to incubation with SA1108. This provided a final bacterial concentration of 106 CFU/ml. RPMI or 1:10 RPMI and RPMI plus streptomycin acted as negative and positive controls, respectively. The bacterial suspension was incubated at 37°C and rotated at 200 rpm for the indicated time. The initial concentration of SA1108 was plated on tryptic soy agar (TSA) to confirm 1 × 106 CFU/ml. After a set amount of time, 10 μl of bacterial suspension was serially diluted in sterile PBS and plated on TSA to measure CFU per milliliter.
RT-PCR.
For reverse transcription-PCR (RT-PCR), RNA from nasal tissue immersed in RNAlater was purified with an RNeasy minikit (Qiagen). cDNA was produced from 500 ng RNA with a QuantiTect RT kit (Qiagen). PCR was performed in a 25-μl reaction mixture containing 1 μl of 1:10-diluted cDNA, 12.5 μl SYBR green quantitative PCR (qPCR) master mix (Invitrogen), 10.7 μl diethyl pyrocarbonate-treated water, and 0.4 μl of 50 nM forward (F) and reverse (R) primers. To detect specific antimicrobial peptides, primers for mBD-14 (F, 5′-GTATTCCTCATCTTGTTCTTGG-3′; R, 5′-AAGTACAGCACACCGGCCAC-3′), CRAMP (F, 5′-GCCGCTGATTCTTTTGACAT-3′; R, 5′-ATTCTTCTCCCCACCTTTGC-3′), and mBD-3 (F, 5′-CTTTGCATTTCTCCTGGTGC-3′; R, 5′-GCCTCCTTTCCTCAAACAACT-3′) were utilized. RPL-19 served as the ribosomal housekeeping gene (primers: F, 5′-GCATCCTCATGGAGCACAT-3′; R, 5′-CTGGTCAGCCAGGAGCTT-3′). Real-time PCR was performed on an ABI 7500 Fast system (Applied Biosystems, USA) under the following conditions: 95°C for 10 min and then 40 cycles of 95°C for 10 s and 60°C for 30 s. This was followed by the default dissociation cycle for melting curve analysis. The ΔΔCT method was utilized by software to calculate the relative expression.
Patient S. aureus nasal colonization determination.
Nasal swabs collected from patients were placed in 1 ml sterile PBS and vigorously vortexed. Patients were deemed positive for S. aureus colonization if they were positive with either a culture- or PCR-based method. To determine S. aureus positivity by culture, a 100-μl aliquot was plated on CHROMagar-S. aureus for 24 h at 37°C. A sample was considered positive for S. aureus if red colonies were present after incubation. To determine S. aureus positivity by PCR, DNA from the remaining sample was purified with the QIAamp DNA minikit and lysostaphin (200 μg/ml) by the manufacturer's protocol. PCR was performed with 1 μM primers specific for the S. aureus protein A (spa) gene (spa-1113f, 5′-TAAAGACGATCCTTCGGTGAGC-3′; spa-1514r, 5′-CAGCAGTAGTGCCGTTTGCTT-3′) (31) under the following conditions: 95°C for 15 min; 30 cycles of 95°C for 30 s, 59°C for 1 min, and 72°C for 1 min; and a final step at 72°C for 10 min (32). PCR products were run on a 1% Tris-acetate-EDTA gel containing 0.5 μg/ml ethidium bromide, and bands were visualized with an Alpha Innotech FluorChem 8900 imager. A sample was considered positive for S. aureus if a PCR product was visualized.
ELISAs.
This was an institutional review board-approved study prospectively collecting nasal secretions from 60 patients, as described before (33). All patients were greater than 18 years of age and had no active upper respiratory infection. Exclusion criteria included active smoking history, recent antibiotic use (in the last month), a history of chronic rhinosinusitis, or active use of nasal steroid, antihistamine, or saline sprays or saline lavage. Briefly, using a sterile bayonet forceps, a Merocel pope ear wick (catalog number 400141; Medtronic) was placed bilaterally along the nasal cavity between the nasal septum and inferior turbinate under endoscopic guidance. These were left in place for 5 min. They were then removed and spun down at 4°C and >14,000 × g for 10 min in Eppendorf tubes to separate nasal secretions from wicks. Nasal secretions were snap-frozen in liquid nitrogen and kept at −80°C until utilized. Secretion samples were normalized for total protein, and enzyme-linked immunosorbent assays (ELISAs) for hBD-2 (catalog number 900-M172; Peprotech), hBD-3 (catalog number 900-M210; Peprotech), and LL-37 (catalog number HK321-01; Hycult Biotech) were utilized to measure protein levels in nasal secretions and done by following the manufacturers' protocols. This procedure was subsequently performed in the same manner 6 weeks from the initial collection date.
Statistical analysis.
The data were analyzed by t test (one-tailed), Fisher's exact test, or Mann-Whitney test as indicated, utilizing GraphPad Prism version 5.0 (GraphPad Software, Inc.). Results are expressed as means ± standard errors of the means (SEM). A P value of <0.05 was considered significant.
RESULTS
Ex vivo supernatants have antistaphylococcal activity that is salt and heat sensitive.
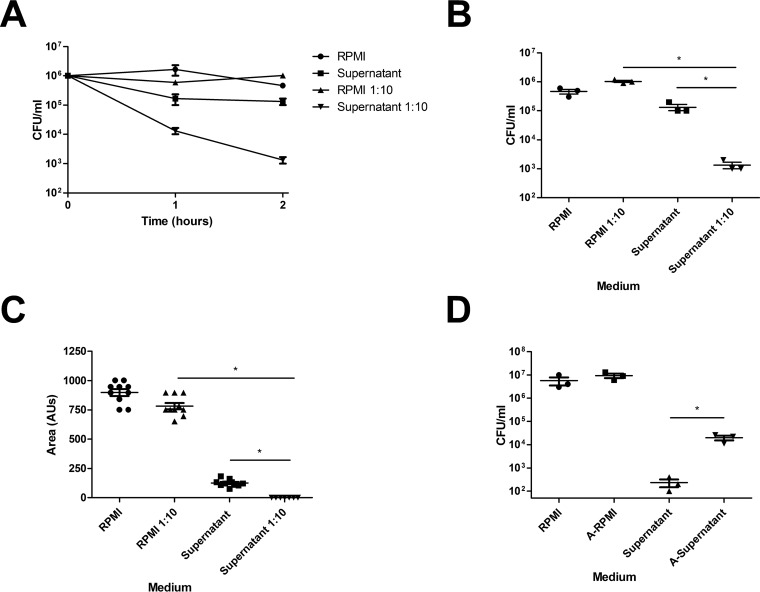
In order to determine the antimicrobial peptide response against S. aureus nasal colonization, we developed an S. aureus killing assay with supernatants from ex vivo nasal tissue. Due to the salt sensitivity of most antimicrobial peptide activity, we included a group of 1:10-diluted supernatants in sterile ddH2O (34). Ex vivo supernatants were able to diminish S. aureus over time (Fig. 1A); however, dilution of supernatants 1:10 in sterile ddH2O resulted in enhanced killing of S. aureus (Fig. 1A and B). Additionally, S. aureus colonies incubated in diluted supernatants were 1/5 the size of diluted controls (Fig. 1C), and heat inactivation of diluted supernatants partially restored CFU counts to those of diluted controls (Fig. 1D).
FIG 1.
Nasal tissue supernatants have antistaphylococcal activity that is solute and heat sensitive. Naive nasal tissue was harvested from WT C57BL/6J mice. Tissue was incubated in RPMI for 24 h. Tissue supernatants were utilized for in vitro killing assays. (A) S. aureus (106 CFU) was cultured in nasal tissue supernatants or supernatants diluted 1:10 in ddH2O for various amounts of time. RPMI and RPMI diluted 1:10 in ddH2O served as controls. (B) CFU counts at 2 h postinduction. (C) S. aureus was incubated for 2 h in RPMI or tissue supernatants and plated on TSA. Colony area was assessed by visualization on an Alpha Innotech FluorChem 8900 imager. AUs, arbitrary units. (D) S. aureus was incubated for 2 h in diluted RPMI or tissue supernatant or for 2 h in previously autoclaved RPMI (A-RPMI) or supernatant (A-Supernatant). n = 3 nasal tissue samples per group. Data are representative of two independent experiments. Statistical analysis was performed with a one-tailed t test (*, P < 0.05).
IL-17A and IL-17F expression is critical for nasal decolonization.
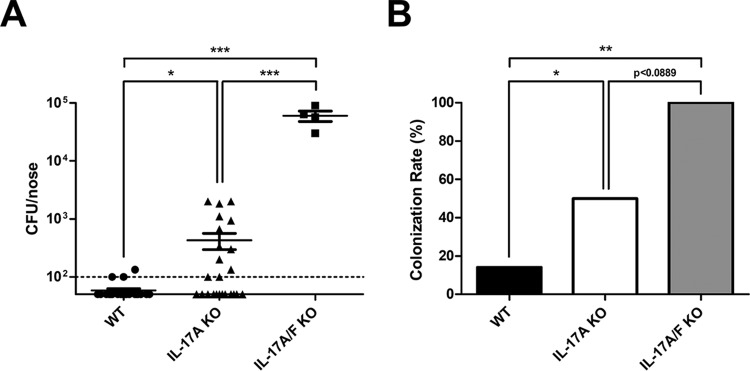
We previously reported that IL-17A-deficient mice have a defect in nasal decolonization and IL-17F is upregulated in response to S. aureus nasal carriage (26). Since both IL-17A and IL-17F can induce AMP expression in epithelial cells and IL-17A and IL-17F deficiency can promote spontaneous S. aureus abscesses in vivo (35), we hypothesized that mice deficient in IL-17A and IL-17F (IL-17A/F KO) would be defective in clearance and have a more severe phenotype than IL-17A-deficient mice alone. In order to determine the combined importance of IL-17A and IL-17F during colonization, WT, IL-17A KO, and IL-17A/F KO mice were inoculated intranasally with S. aureus. IL-17A and IL-17A/F KO mice were found to have a significant increase in intranasal S. aureus CFU compared to WT mice and an intermediate or complete inability to clear colonization, respectively (Fig. 2A and B). IL-17A/F KO mice have a significantly higher S. aureus burden and a trend toward increased colonization rate compared to IL-17A KO mice. In conjunction, the inability to clear colonization in IL-17A/F mice is similar to the phenotype of SCID and β/δ-TCR KO mice based on our previously published data (26).
FIG 2.
IL-17A and IL-17F are required for clearance of S. aureus nasal carriage. C57BL/6J WT, IL-17A KO, and IL-17A/F KO mice were inoculated intranasally with S. aureus clinical isolate SA1108. (A) CFU counts/nose 28 days postinoculation (dpi). Nasal tissue was harvested at 28 dpi, homogenized, and plated on CHROMagar-S. aureus to determine CFU counts. The detection limit was 100 CFU. (B) Colonization rate 28 days postinoculation. Colonization rate was determined by the number of mice positive for S. aureus out of the total number of mice. n = 4 to 9 C57BL/6J mice per group. Data are combined from one or two independent experiments. Statistical analyses are in comparison to results for WT mice or between groups, as indicated by lines, and were performed with a one-tailed t test or Fisher's exact test (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
IL-17A directly upregulates mBD-3 expression ex vivo.
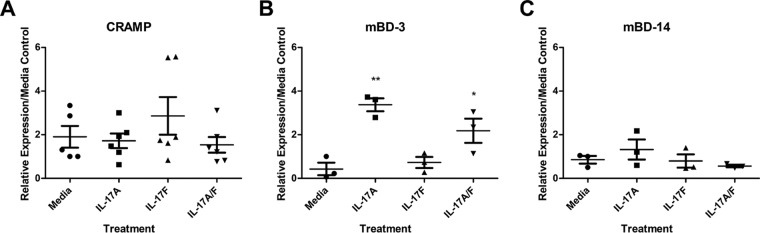
Since IL-17A and IL-17F are crucial for in vivo clearance of S. aureus nasal carriage, we wanted to determine their ability to induce AMP expression in an ex vivo nasal tissue model. Nasal tissue from naive mice were harvested and placed in RPMI containing streptomycin to remove endogenous microflora. A concentration of 20 ng/ml of IL-17A, IL-17F, or both IL-17A and IL-17F was added to cultured nasal tissue and then incubated at 37°C and 5% CO2 for 24 h. Addition of sterile PBS served as a nonstimulated control. Following incubation, RNA was purified from nasal tissue, converted to cDNA, and analyzed by RT-PCR for relative expression of mouse CRAMP, β-defensin-3, and β-defensin-14. Under the conditions tested, mBD-3 was the only AMP upregulated compared to results with the medium control, and it was upregulated by IL-17A alone (Fig. 3B). IL-17F did not promote expression of CRAMP, mBD-3, or mBD-14 (Fig. 3A to C). Stimulation by a combination of IL-17A and IL-17F did not have a synergistic effect but may induce a slight but not statistically significant antagonistic effect on mBD-3 expression over IL-17A treatment alone (Fig. 3B).
FIG 3.
IL-17A is sufficient for expression of mBD-3 in ex vivo nasal tissue. Naive nasal tissue was harvested from WT C57BL/6J mice. Tissue was incubated in RPMI for 24 h with 20 ng/ml of mouse rIL-17A, rIL-17F, or both rIL-17A and rIL-17F. Nonsupplemented medium acted as a negative control. Nasal tissue RNA was purified, converted to cDNA, and used for RT-PCR assays. Levels of mouse CRAMP (A), mBD-3 (B), and mBD-14 (C) were measured. Data are presented as RNA expression relative to that of medium (nonsupplemented) controls. n = 3 to 6 C57BL/6J mice per time point. Data are representative of two independent experiments. Statistical analyses are in comparison to medium control samples and were performed with a one-tailed t test (*, P < 0.05; **, P < 0.01).
Nasal tissue AMP expression is upregulated by S. aureus and is IL-17A dependent.
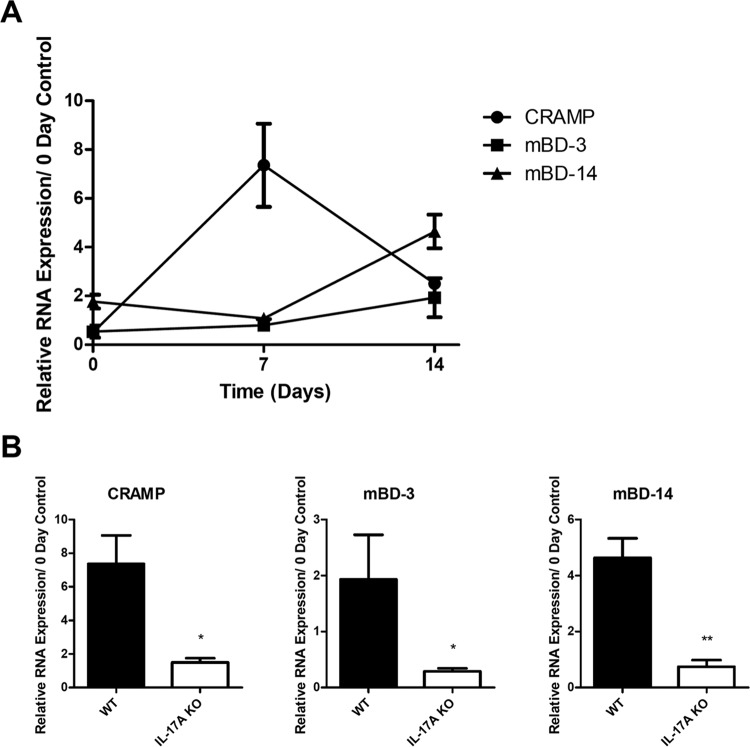
We have shown that nasal tissue supernatants have antistaphylococcal properties and can produce AMPs when stimulated with IL-17A ex vivo; however, it is unknown if AMPs are induced upon S. aureus nasal colonization in vivo. In order to determine the antimicrobial peptide response against S. aureus nasal colonization, C57BL/6J mice were inoculated intranasally, and nasal tissue was harvested at various time points. AMP expression analysis displayed an upregulation of all antimicrobial peptides tested (Fig. 4A). CRAMP expression was enhanced 7 days postinoculation, whereas mBD-3 and mBD-14 were upregulated 14 days postinoculation.
FIG 4.
IL-17A is required for AMP expression induced by S. aureus nasal colonization. WT C57BL/6J and IL-17A KO mice were inoculated intranasally with S. aureus clinical isolate SA1108. Nasal tissue RNA was purified, converted to cDNA, and used for RT-PCR assays. (A) Levels of mouse CRAMP, mBD-3, and mBD-14 were measured in WT mice at various time points. (B) Expression levels of CRAMP, mBD-3, and mBD-14 were compared between WT and IL-17A KO mice at either day 7 or day 14. Data are presented as RNA expression relative to that of day 0 (noninoculated) controls. n = 2 to 5 C57BL/6J or IL-17A KO mice per time point. Data are representative of at least two independent experiments. Statistical analysis was performed with a one-tailed t test (*, P < 0.05; **, P < 0.01).
Since IL-17A was found to induce expression of mBD-3 under ex vivo conditions, we decided to test the importance of IL-17A on AMP expression in vivo. Therefore, we inoculated IL-17A KO mice with S. aureus and harvested nasal tissue for expression analysis as previously described. In contrast to WT C57BL/6J mice, IL-17A KO mice were deficient in upregulation of CRAMP, mBD-3, and mBD-14 at the time points tested (Fig. 4B).
Nasal tissue AMP production correlates with human nasal S. aureus carriage state.
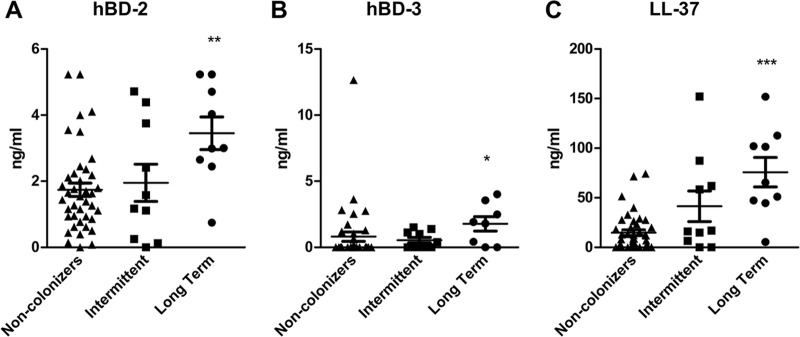
In order to translate whether AMP production correlates with S. aureus colonization in humans, we acquired human nasal secretion samples from healthy volunteers and screened them by culture- and PCR-based methods for the presence of S. aureus. Two samples were collected over a 6-week period from each subject and grouped by carriage state into noncolonizers (both samples S. aureus negative), intermittent colonizers (one sample positive/one sample negative), and long-term colonizers (both samples positive). Since the sampling occurred only over a 6-week period and only two samples were obtained, we could not designate these long-term colonizers as persistently colonized, which are defined as S. aureus culture-positive nasal swabs collected on 5 to 10 separate occasions during a 6-month period, and subjects are labeled as persistent carriers if >80% of the cultures are positive (36–38). In our studies with S. aureus, we found that 68% of patients were noncolonizers, 17% were intermittent colonizers, and 15% were long-term colonizers (data not shown). Nasal secretion protein levels of human β-defensin-2, β-defensin-3, and LL-37, the human orthologs of mouse β-defensin-3, β-defensin-14, and CRAMP, were measured by ELISAs and grouped by carriage state (Fig. 5A to C). These results showed a significant increase in AMP levels in long-term carriers compared to noncolonizers; however, no statistical difference was found between intermittent colonizers and the other groups. The largest increase in protein expression between noncolonizers and long-term carriers was in LL-37, followed by hBD-2 and hBD-3.
FIG 5.
Human S. aureus nasal carriage promotes AMP production. Human nasal secretions were determined by culture and PCR to be either from noncolonizers, intermittent colonizers, or long-term S. aureus colonizers, and levels of hBD-2 (A), hBD-3 (B), and LL-37 (C) were elucidated by ELISAs. n = 41 noncolonizers; n = 10 intermittent colonizers; n = 9 long-term colonized human samples. Statistical analyses are in comparison to results for the noncolonizer group and were performed with the Mann-Whitney test (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
DISCUSSION
There is growing evidence that innate antimicrobial peptides are an important and often required component of the immune system for resolution of infection. Additionally, recent reports support the role of AMPs in host defense against mucocutaneous S. aureus infections (39, 40). Here, we show that nasal tissue supernatants have solute and heat-dependent antistaphylococcal activity. Furthermore, we have demonstrated that IL-17F, in conjunction with IL-17A, is required for clearance of S. aureus nasal carriage. IL-17A stimulation was sufficient for upregulation of mBD-3 in nasal tissue, and the antimicrobial peptides CRAMP, mBD-3, and mBD-14 were upregulated in an IL-17A-dependent fashion during S. aureus nasal carriage. Importantly, human nasal secretions were found to have increased protein levels of hBD-2, hBD-3, and LL-37, the human orthologs of mBD-3, mBD-14, and CRAMP, in patients with long-term S. aureus colonization compared to noncolonizers.
Cultured explant nasal tissue supernatant displayed strong antistaphylococcal activity that was solute dependent and heat sensitive (Fig. 1). Our results mimic a study that displayed reduced killing of S. aureus in heat-inactivated human nasal secretions (41). As mentioned previously, most antimicrobial peptide activity is sensitive to high salt concentrations. Therefore, the solute-dependent killing effect observed in our ex vivo nasal tissue supernatants may be explained by the presence of antimicrobial peptides.
It is important to note that in a previous study, IL-17A-deficient mice had only a partial clearance defect, whereas NOD-SCID mice displayed a complete defect in decolonization (26). We found that depletion of both IL-17A and IL-17F was required to recapitulate the severe clearance defect in NOD-SCID mice, suggesting that IL-17A and IL-17F are critical and nonredundant effector cytokines for elimination of S. aureus nasal colonization. Interestingly, a study found that mice deficient in both IL-17A and IL-17F developed spontaneous S. aureus mucocutaneous abscesses around the nose and mouth; however, mice depleted of only IL-17A or IL-17F did not produce abscesses (35). Therefore, our data and the literature suggest that both IL-17A and IL-17F are required for host defense and have nonredundant roles against S. aureus mucocutaneous infections.
Our data displayed an IL-17A-dependent induction of CRAMP, mBD-3, and mBD-14 upon S. aureus carriage. This is not unexpected, as an array of studies currently support the link between Th17/IL-17A and AMP expression (42–44). Interestingly, out of all AMPs and treatments tested, only mBD-3 was upregulated, and it was upregulated only in the presence of IL-17A in our ex vivo stimulation experiment. Human airway epithelial cells, upon treatment with IL-17A, had significant upregulation of hBD-2 (mBD-3 equivalent) (45). In addition, stimulation of human airway epithelial cells with a myriad of innate cytokines displayed IL-17A as the most potent inducer of hBD-2. This study validates our data showing specific mBD-3 upregulation upon stimulation with IL-17A in murine ex vivo nasal tissue.
When given together, IL-17A and IL-17F did not display an additive or synergistic effect on AMP expression. The deficient production of CRAMP and mBD-14 in IL-17A- and IL-17F-stimulated nasal tissue may be due to lack of a costimulatory agent. Peric et al. found that human keratinocytes stimulated with supernatants from T cells isolated from lesional psoriatic skin increased expression of cathelicidin when stimulated in the presence of 1,25-dihydroxyvitamin D3 (1,25D3; active form of vitamin D) (46). In vitro, IL-17A-enhanced cathelicidin mRNA and peptide expression in keratinocytes was dependent on the presence of 1,25D3. Another study elucidated that IL-17A and IL-17F, in conjunction with IL-22, synergistically induced the expression of β-defensin-2 and S100A9 and additively enhanced the expression of S100A7 and S100A8 in human keratinocytes (42). Therefore, IL-17A or IL-17F alone may not be sufficient to induce CRAMP and mBD-14 production. The observed upregulation of IL-1β in our previous studies may be a sufficient costimulatory cytokine when combined with IL-17A or IL-17F (26). Liu et al. discovered that IL-1β was required for expression of human β-defensin-4 (47). The inability of IL-17A and IL-17F to induce CRAMP and mBD-14 expression ex vivo, while IL-17A is required for their expression in vivo, suggests that IL-17A, and possibly IL-17F, are required but not sufficient for AMP expression. Further studies need to be performed in order to determine the cytokine milieu sufficient for CRAMP and mBD-14 production.
Although we demonstrated the induction of CRAMP, mBD-3, and mBD-14 in nasal tissue, we have not determined the source of these peptides. These peptides have broad inducible tissue expression. Human LL-37 (mouse CRAMP) is produced in neutrophil granules, in addition to inflamed skin, lung epithelia, and squamous epithelia of human mouth, tongue, esophagus, cervix, and vagina (48–51). The source of CRAMP expression may originate from nasal lumen-associated neutrophils or nasal epithelial cells that have been activated in response to S. aureus insult. Human β-defensin-2 and β-defensin-3 (mBD-3 and mBD-14) are present in oral tissue, gastric mucosa, skin, lung epithelia, infected kidney, and trachea (3, 52, 53). The most prominent source of mBD-3 and mBD-14 assembly is likely stimulated nasal mucosa. Further studies are necessary to elucidate the cellular origin of nasal AMP expression in response to S. aureus colonization.
These data show that human nasal secretions have increased AMP protein production in response to S. aureus carriage and validate our mouse model of AMP expression in response to S. aureus nasal colonization. Those with intermittent colonization (culture positive for S. aureus at time zero and culture negative 6 weeks later) may have been in the final stages of clearance and had intermediate AMP expression levels. This is in contrast to those patients with long-term S. aureus colonization (culture positive for S. aureus at time zero and 6 weeks later). However, since human nasal secretion samples were only taken at two time points, the patient S. aureus nasal carriage status and AMP secretion levels should be further validated with clinical data involving more frequent sample collection.
These patients had a continuously activated immune response against colonizing S. aureus with sustained AMP-upregulated expression. This upregulated AMP expression was shown to be effective at the slow but eventual elimination of S. aureus carriage in mice (26). In fact, elevated AMP levels were able to ultimately eliminate S. aureus from the nares in greater than 70% of the mice by 28 days after colonization initiation using the controlled environment of the murine model of colonization (26). The sustained colonization seen in humans, even with elevated AMP levels, may be due to a longer time course required for colonization elimination in human subjects. It may also be due to recolonization from the patient's local environment, pets, or human cohabitation. However, since patients were not sampled at later time points, it is unknown if these elevated AMP levels eventually cleared staphylococcal colonization. If these patients were never able to clear S. aureus and were persistently colonized, the ineffectiveness of the host may be due to other factors, such as having a suboptimal neutrophil response, a uniquely fit strain of colonizing S. aureus, or a permissive nares microbiome. Importantly, we do not have data on AMP levels of noncolonizers given S. aureus. It is possible that noncolonizers induce AMPs more robustly than patients colonized long term in response to S. aureus carriage or do so in a transient manner.
In conclusion, our data show that IL-17A and IL-17F have nonredundant roles in the clearance of S. aureus nasal carriage. Additionally, nasal tissue supernatants display IL-17A-dependent and AMP-mediated antistaphylococcal activity, and patients with long-term nasal colonization have enhanced AMP production. Promotion of antistaphylococcal antimicrobial peptides may be therapeutically beneficial in the elimination of S. aureus nasal colonization and thereby prevent distal infections originating from nasal carriage.
REFERENCES
- 1.Bardan A, Nizet V, Gallo RL. 2004. Antimicrobial peptides and the skin. Expert Opin Biol Ther 4:543–549. doi: 10.1517/14712598.4.4.543. [DOI] [PubMed] [Google Scholar]
- 2.Selsted ME, Harwig SS, Ganz T, Schilling JW, Lehrer RI. 1985. Primary structures of three human neutrophil defensins. J Clin Investig 76:1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehmann J, Retz M, Harder J, Krams M, Kellner U, Hartmann J, Hohgrawe K, Raffenberg U, Gerber M, Loch T, Weichert-Jacobsen K, Stockle M. 2002. Expression of human beta-defensins 1 and 2 in kidneys with chronic bacterial infection. BMC Infect Dis 2:20. doi: 10.1186/1471-2334-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jenssen H, Hamill P, Hancock RE. 2006. Peptide antimicrobial agents. Clin Microbiol Rev 19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doss M, White MR, Tecle T, Hartshorn KL. 2010. Human defensins and LL-37 in mucosal immunity. J Leukoc Biol 87:79–92. doi: 10.1189/jlb.0609382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanetti M. 2004. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol 75:39–48. [DOI] [PubMed] [Google Scholar]
- 7.Boman HG. 2003. Antibacterial peptides: basic facts and emerging concepts. J Intern Med 254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 8.Gordon YJ, Huang LC, Romanowski EG, Yates KA, Proske RJ, McDermott AM. 2005. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr Eye Res 30:385–394. doi: 10.1080/02713680590934111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Midorikawa K, Ouhara K, Komatsuzawa H, Kawai T, Yamada S, Fujiwara T, Yamazaki K, Sayama K, Taubman MA, Kurihara H, Hashimoto K, Sugai M. 2003. Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes. Infect Immun 71:3730–3739. doi: 10.1128/IAI.71.7.3730-3739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, Wilson JM. 1998. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Investig 102:874–880. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harder J, Bartels J, Christophers E, Schroder JM. 2001. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem 276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 12.Huang Q, Yu HJ, Liu GD, Huang XK, Zhang LY, Zhou YG, Chen JY, Lin F, Wang Y, Fei J. 2012. Comparison of the effects of human beta-defensin 3, vancomycin, and clindamycin on Staphylococcus aureus biofilm formation. Orthopedics 35:e53–e60. [DOI] [PubMed] [Google Scholar]
- 13.Zanger P, Holzer J, Schleucher R, Scherbaum H, Schittek B, Gabrysch S. 2010. Severity of Staphylococcus aureus infection of the skin is associated with inducibility of human beta-defensin 3 but not human beta-defensin 2. Infect Immun 78:3112–3117. doi: 10.1128/IAI.00078-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanger P, Nurjadi D, Vath B, Kremsner PG. 2011. Persistent nasal carriage of Staphylococcus aureus is associated with deficient induction of human beta-defensin 3 after sterile wounding of healthy skin in vivo. Infect Immun 79:2658–2662. doi: 10.1128/IAI.00101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Belkum A, Emonts M, Wertheim H, de Jongh C, Nouwen J, Bartels H, Cole A, Cole A, Hermans P, Boelens H, Toom NL, Snijders S, Verbrugh H, van Leeuwen W. 2007. The role of human innate immune factors in nasal colonization by Staphylococcus aureus. Microbes Infect 9:1471–1477. doi: 10.1016/j.micinf.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Fode P, Stegger M, Andersen PS. 2011. Human beta-defensin 3 (DEFB103) and its influence on Staphylococcus aureus nasal carriage. Int J Infect Dis 15:e388–e394. doi: 10.1016/j.ijid.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Nurjadi D, Herrmann E, Hinderberger I, Zanger P. 2013. Impaired beta-defensin expression in human skin links DEFB1 promoter polymorphisms with persistent Staphylococcus aureus nasal carriage. J Infect Dis 207:666–674. doi: 10.1093/infdis/jis735. [DOI] [PubMed] [Google Scholar]
- 18.Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, Merluzzi L, Gennaro R. 1997. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem 272:13088–13093. doi: 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- 19.Jann NJ, Schmaler M, Kristian SA, Radek KA, Gallo RL, Nizet V, Peschel A, Landmann R. 2009. Neutrophil antimicrobial defense against Staphylococcus aureus is mediated by phagolysosomal but not extracellular trap-associated cathelicidin. J Leukoc Biol 86:1159–1169. doi: 10.1189/jlb.0209053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bals R, Wang X, Meegalla RL, Wattler S, Weiner DJ, Nehls MC, Wilson JM. 1999. Mouse beta-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect Immun 67:3542–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinrichsen K, Podschun R, Schubert S, Schroder JM, Harder J, Proksch E. 2008. Mouse beta-defensin-14, an antimicrobial ortholog of human beta-defensin-3. Antimicrob Agents Chemother 52:1876–1879. doi: 10.1128/AAC.01308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burd RS, Furrer JL, Sullivan J, Smith AL. 2002. Murine beta-defensin-3 is an inducible peptide with limited tissue expression and broad-spectrum antimicrobial activity. Shock 18:461–464. doi: 10.1097/00024382-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y, Yi X, Li M, Wang T, Qi T, She X. 2012. Antimicrobial activities of recombinant mouse beta-defensin 3 and its synergy with antibiotics. J Mater Sci Mater Med 23:1723–1728. doi: 10.1007/s10856-012-4645-z. [DOI] [PubMed] [Google Scholar]
- 24.Rohrl J, Yang D, Oppenheim JJ, Hehlgans T. 2008. Identification and biological characterization of mouse beta-defensin 14, the orthologue of human beta-defensin 3. J Biol Chem 283:5414–5419. [DOI] [PubMed] [Google Scholar]
- 25.Zhu C, Qin H, Cheng T, Tan HL, Guo YY, Shi SF, Chen DS, Zhang XL. 2013. Staphylococcus aureus supernatant induces the release of mouse beta-defensin-14 from osteoblasts via the p38 MAPK and NF-kappaB pathways. Int J Mol Med 31:1484–1494. [DOI] [PubMed] [Google Scholar]
- 26.Archer NK, Harro JM, Shirtliff ME. 2013. Clearance of Staphylococcus aureus nasal carriage is T cell dependent and mediated through interleukin-17A expression and neutrophil influx. Infect Immun 81:2070–2075. doi: 10.1128/IAI.00084-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolls JK, McCray PB Jr, Chan YR. 2008. Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol 8:829–835. doi: 10.1038/nri2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC. [Google Scholar]
- 29.National Institutes of Health. 2002. Public Health Service policy on humane care and use of laboratory animals. Office of Laboratory Animal Welfare, National Institutes of Health, Bethesda, MD. [Google Scholar]
- 30.Nouwen JL, Ott A, Kluytmans-Vandenbergh MF, Boelens HA, Hofman A, van Belkum A, Verbrugh HA. 2004. Predicting the Staphylococcus aureus nasal carrier state: derivation and validation of a “culture rule”. Clin Infect Dis 39:806–811. [DOI] [PubMed] [Google Scholar]
- 31.Strommenger B, Kettlitz C, Weniger T, Harmsen D, Friedrich AW, Witte W. 2006. Assignment of Staphylococcus isolates to groups by spa typing, SmaI macrorestriction analysis, and multilocus sequence typing. J Clin Microbiol 44:2533–2540. doi: 10.1128/JCM.00420-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsen AR, Stegger M, Sorum M. 2008. spa typing directly from a mecA, spa and pvl multiplex PCR assay-a cost-effective improvement for methicillin-resistant Staphylococcus aureus surveillance. Clin Microbiol Infect 14:611–614. doi: 10.1111/j.1469-0691.2008.01995.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee RJ, Kofonow JM, Rosen PL, Siebert AP, Chen B, Doghramji L, Xiong G, Adappa ND, Palmer JN, Kennedy DW, Kreindler JL, Margolskee RF, Cohen NA. 2014. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Investig 124:1393–1405. doi: 10.1172/JCI72094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganz T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 35.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y. 2009. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 36.van Belkum A, Verkaik NJ, de Vogel CP, Boelens HA, Verveer J, Nouwen JL, Verbrugh HA, Wertheim HF. 2009. Reclassification of Staphylococcus aureus nasal carriage types. J Infect Dis 199:1820–1826. doi: 10.1086/599119. [DOI] [PubMed] [Google Scholar]
- 37.Kluytmans J, van Belkum A, Verbrugh H. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 10:505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sollid JU, Furberg AS, Hanssen AM, Johannessen M. 2014. Staphylococcus aureus: determinants of human carriage. Infect Genet Evol 21:531–541. doi: 10.1016/j.meegid.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 39.Zanger P, Holzer J, Schleucher R, Steffen H, Schittek B, Gabrysch S. 2009. Constitutive expression of the antimicrobial peptide RNase 7 is associated with Staphylococcus aureus infection of the skin. J Infect Dis 200:1907–1915. doi: 10.1086/648408. [DOI] [PubMed] [Google Scholar]
- 40.Krishna S, Miller LS. 2012. Innate and adaptive immune responses against Staphylococcus aureus skin infections. Semin Immunopathol 34:261–280. doi: 10.1007/s00281-011-0292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cole AM, Dewan P, Ganz T. 1999. Innate antimicrobial activity of nasal secretions. Infect Immun 67:3267–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang F, Kao CY, Wachi S, Thai P, Ryu J, Wu R. 2007. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J Immunol 179:6504–6513. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 44.Pietrella D, Rachini A, Pines M, Pandey N, Mosci P, Bistoni F, d'Enfert C, Vecchiarelli A. 2011. Th17 cells and IL-17 in protective immunity to vaginal candidiasis. PLoS One 6:e22770. doi: 10.1371/journal.pone.0022770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. 2004. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol 173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 46.Peric M, Koglin S, Kim SM, Morizane S, Besch R, Prinz JC, Ruzicka T, Gallo RL, Schauber J. 2008. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol 181:8504–8512. doi: 10.4049/jimmunol.181.12.8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, Vazirnia A, Zhang X, Steinmeyer A, Zugel U, Hollis BW, Cheng G, Modlin RL. 2009. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One 4:e5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bals R, Wang X, Zasloff M, Wilson JM. 1998. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A 95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cowland JB, Johnsen AH, Borregaard N. 1995. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett 368:173–176. doi: 10.1016/0014-5793(95)00634-L. [DOI] [PubMed] [Google Scholar]
- 50.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, Gudmundsson GH. 1997. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem 272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 51.Heilborn JD, Nilsson MF, Kratz G, Weber G, Sorensen O, Borregaard N, Stahle-Backdahl M. 2003. The cathelicidin anti-microbial peptide LL-37 is involved in reepithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Investig Dermatol 120:379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 52.Dunsche A, Acil Y, Dommisch H, Siebert R, Schroder JM, Jepsen S. 2002. The novel human beta-defensin-3 is widely expressed in oral tissues. Eur J Oral Sci 110:121–124. doi: 10.1034/j.1600-0722.2002.11186.x. [DOI] [PubMed] [Google Scholar]
- 53.Uehara N, Yagihashi A, Kondoh K, Tsuji N, Fujita T, Hamada H, Watanabe N. 2003. Human beta-defensin-2 induction in Helicobacter pylori-infected gastric mucosal tissues: antimicrobial effect of overexpression. J Med Microbiol 52:41–45. doi: 10.1099/jmm.0.04985-0. [DOI] [PubMed] [Google Scholar]