Abstract
Background & objectives:
Though newer antiepileptic drugs are considered safer than conventional antiepileptics, the effects of lamotrigine, levetiracetam and topiramate on neurobehavioural functions are yet to be established. This study evaluated neurobehavioural parameters and oxidative stress markers in brain tissue of rats treated with lamotrigine, levetiracetam and topiramate compared to sodium valproate.
Methods:
Five groups of male Wistar rats were treated respectively with normal saline (control), sodium valproate (370 mg/kg), lamotrigine (50 mg/kg), levetiracetam (310 mg/kg) and topiramate (100 mg/kg) for 45 days. Neurobehavioural parameters were assessed using elevated plus maze (EPM), actophotometer, rotarod, passive avoidance and Morris water maze (MWM) at baseline and at the end of treatment. Oxidative stress parameters [malondialdehyde (MDA), reduced glutathione (GSH) and superoxide dismutase (SOD)] were estimated in rat brain at the end of treatment.
Results:
Valproate and lamotrigine showed no significant effect on learning and memory in passive avoidance and MWM tests. However, levetiracetam and topiramate reduced retention memory significantly as compared to control (P<0.01) and lamotrigine (P<0.05) groups. Performances on EPM, rotarod and actophotometer were not significantly different between the groups. In comparison to control group, MDA was higher in the levetiracetam and topiramate (360.9 and 345.9 nmol/g of homogenized brain tissue, respectively) groups. GSH and SOD activity were significantly reduced by valproate and levetiracetam treatment. Lamotrigine did not induce significant oxidative stress.
Interpretation & conclusions:
Long-term and therapeutic dose treatment with levetiracetam and topiramate significantly impaired learning and memory, which was not seen with valproate and lamotrigine in rats. Levetiracetam, topiramate and valproate augmented oxidative stress, whereas lamotrigine has little effect on it. These antiepileptic drugs are used in clinical practice, hence pharmacovigilance studies are required to evaluate their safety profile.
Keywords: Lamotrigine, levetiracetam, Morris water maze, neurobehavioural, oxidative stress, topiramate, valproate
The treatment of epilepsy is preferably initiated with conventional antiepileptic drugs (AEDs) like phenytoin (PHT), phenobarbitone (PB), carbamazepine (CBZ), and sodium valproate (VPA) since these are less expensive and the side effects with long-term use are well known1. Despite development of several new antiepileptic drugs, the treatment of epilepsy remains a challenge, because of high incidence of adverse drug reactions (ADRs) of AEDs and drug resistance in 20-30 per cent patients2. Many AEDs are metabolized to generate reactive metabolites with the capability of covalent binding to macromolecules as proteins or other vital biomolecules and hence eliciting systemic toxicity3.
Conventional AEDs are known to cause cutaneous and hair changes, behaviour alteration, mood and cognitive dysfunction, blurring of vision, peripheral neuropathy, gastrointestinal side effects, loss of appetite and body weight, anaemia and thrombocytopenia, which are at least in part due to oxidative stress2,3. There is claim of better tolerability profiles providing a beneficial edge to these newer drugs2. However, recently there is an increased concern of neurobehavioural ADRs with the newer AEDs as evidenced through case reports like lamotrigine induced sleep behaviour disturbance4, psychotropic side effects associated with levetiracetam5, impaired cognition and irritability with topiramate treatment6 and cognitive delays in children due to antiepileptic treatment of mothers7. There are reports suggesting no association of antiepileptic on the suicidal behaviour8 and no effect of lamotrigine on movement scores of foetuses7. There is still a lack of documentation of safety aspects regarding many of these drugs. Therefore, this study was done to evaluate neurobehavioural parameters and oxidative stress in brain tissue of rats on chronic treatment with newer antiepileptic drugs, i.e. lamotrigine, levetiracetam and topiramate in comparison with sodium valproate, a conventional antiepileptic drug.
Material & Methods
Male Wistar rats weighing 100-200 g were procured from central animal facility, All India Institute of Medical Sciences (AIIMS), New Delhi and acclimatized for a period of seven days before the start of experiments. Animals were housed under standard laboratory conditions with food and water ad libitum. All animal experiments were performed at the Department of Pharmacology, All India Institute of Medical Sciences (AIIMS), New Delhi, India, and were initiated after the approval by the Institutional Animal Ethics Committee.
All the chemicals used in this study were of analytical grade and purchased from Sigma (Sigma Chemicals Co., USA). The chemicals used were sodium phosphate, sodium dodecyl sulphate, acetic acid, thiobarbituric acid, n-butanol, pyridine, malondialdehyde bis-dimethyl acetal, trichloroacetic acid, 5’5-dithiobis (2-nitrobenzoic acid), reduced glutathione (GSH), pyrogallol, HCl and ethylenediaminetetraacetic acid.
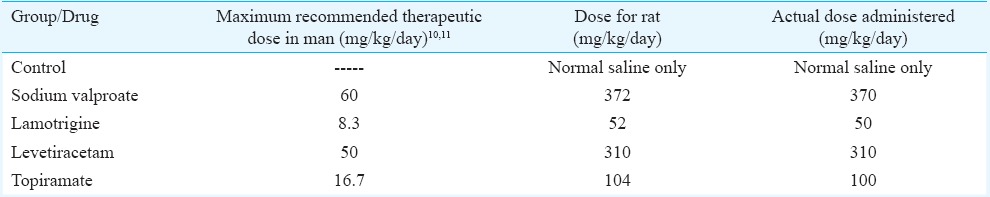
Drug treatment: Animals were divided into five groups (n=6) according to the drugs administered, i.e. (a) control (normal saline treatment), (b) sodium valproate (active control), (c) lamotrigine, (d) levetiracetam and (e) topiramate. The drugs (powders of active ingredients) were obtained as a gift from Torrent Pharmaceuticals Limited, Gujarat, India. The animals were administered with freshly prepared suspension of drugs in normal saline by oral gavage cannula at volumes not greater than 1.0 ml/100 g body weight daily at 0900 h. The drugs were administered for 45 days to see the effect of chronic treatment based on a previous study9. The dose administered in rats was equivalent to maximum recommended human therapeutic dose, so that the effects produced in rats will have better extrapolation10,11. The reference weight (human) considered was 60 kg. The calculated rat doses12 are given in the Table.
Table.
Calculated doses of antiepileptics administered to the rats
After seven days of acclimatization, animals were assessed for baseline neurobehavioural functions in the next five days. The drug treatment was continued and from 40th to 45th day of drug treatment, post-treatment neurobehavioural assessment was performed. Animals were euthanized 24 h after the last dose of drug and tissue samples were collected for oxidative stress parameters estimation.
Assessment of behavioural parameters: Assessments were performed in order of increasing stress i.e. (i) anxiolytic activity by elevated plus maze test; (ii) locomotor activity and motor coordination by actophotometer and rotarod test; and (iii) learning and memory function by passive avoidance and Morris water maze test. On the day of behavioural testing, the rats were brought to a sound-attenuated testing room to acclimate for one hour before each test.
Elevated plus maze test: Elevated plus maze test was done as described by Walf and Frye13, with little modification. Animals were placed individually at the end of one open arm facing away from central platform. The video-tracking system recorded the number of entries made by the rodent onto the open and closed arms and the time spent on the open arm and closed arm. At the end of the 5-min test, the rodent was removed and maze was cleaned.
Actophotometer test: Locomotor activity was measured using digital actophotometer (Techno Electronics, Lucknow, India). Animal was kept for free movement inside actophotometer and the number of times it crosses the beam of light was displayed digitally.
Rotarod test: Muscle coordination in rats was assessed by rotarod (Ugo Basile, Italy) using the method described by Rogers et al14. Each animal received a training session on the rotarod at a constant speed of 8 rpm. During the test, animal was kept on accelerating rotarod and time of stay on rod was noted.
Passive avoidance test: Memory retention was evaluated in a passive avoidance apparatus (Ugo Basile, Italy) as described earlier by Sharma and Gupta15. On the acquisition trial, animal was placed for 30 sec in lighted chamber and the door separating the light and dark chambers was opened. Animals exhibiting an initial latency time of more than 60 sec to enter the dark chamber were excluded. After entry into dark chamber, the door was closed and an electric foot shock (75 V, 0.2 mA, 50 Hz) was delivered for 3 sec. Animal was removed from the dark chamber 10 sec later. Twenty four hours later, retention latency time was taken in the same way as acquisition trial, but foot shock was not delivered, and latency time was recorded up to a maximum of 300 sec.
Morris water maze test: Morris water maze is used to test the spatial memory of the animals as described by Morris16, with little modification. Briefly, the water tank was divided into four equal quadrants (1, 2, 3 and 4) and the platform was kept into 4th quadrant. The animal was placed into the pool in one quadrant, facing the wall of tank. The trial was terminated automatically as soon as the animal reached the platform or when 120 sec were elapsed. Animals were allowed to stay on the platform for 15 sec. Thus four trials (one in each quadrant) each day with an inter-trial interval of at least 20 min were performed during four days of acquisition sessions. On the fifth day, the platform was removed. Animal was introduced from the quadrant opposite to that where the platform was initially placed. The path of each rat was analyzed by using the Any-maze video tracking system (Catterpillar Instrumentation Pvt., India). The parameters determined were latency to reach the platform zone and time spent in the platform zone during 60 sec of this spatial probe trial.
Assessment of oxidative stress parameters: The animals were euthanized by decapitation after 24 h from the end of drug treatment and their brains were dissected on ice to segregate the frontal cortex and striatum parts. These were stored at -70 °C for the evaluation of oxidative stress parameters, i.e., malondialdehyde (MDA), reduced glutathione (GSH) levels and superoxide dismutase (SOD) enzyme activity within next seven days. Brain tissue samples (frontal cortex and striatum) were thawed and 10 per cent (w/v) homogenate was made with ice-cold 0.1 M phosphate buffer (pH 7.4). The oxidative stress parameters were estimated from the aliquots of homogenates and were expressed per g of brain tissue.
MDA measurements: MDA in tissue was analyzed by the method of Ohkawa et al17. The absorbance was read at 532 nm using a spectrophotometer (Specord 200, Analytik Jena, Germany). Malondialdehyde bis-dimethyl acetal (Sigma, USA) was used as the external standard. Results were expressed as nmol of MDA/g of brain tissue.
GSH measurements: GSH content was measured by the method of Ellman18 with minor modifications. An equal quantity of the homogenate was mixed with 5 per cent trichloroacetic acid and centrifuged at 600 g for 10 min to separate out the proteins. Supernatant 0.01 ml was added to 2 ml of 0.3 M phosphate buffer (pH 8.4), 0.5 ml of 5’5-dithiobis (2-nitrobenzoic acid) and 0.4 ml of double-distilled water. The mixture was vortexed and the absorbance was read at 412 nm within 15 min. The GSH content was expressed as μg/g of brain tissue. GSH (Sigma, USA) was used as the external standard.
Estimation of SOD activity: Activity of SOD was estimated by the method of Marklund and Marklund19. Changes in absorbance at 420 nm were recorded at one min interval for three min. Data were expressed as SOD activity in terms of % inhibition of pyrogallol oxidation compared with the blank reading.
Statistical analysis: Data analysis was performed using SPSS 16 software (IBM corporation, Chicago, USA). All results were expressed as mean ± SEM. For statistical analysis, group means were compared using one way analysis of variance (ANOVA) followed by Bonferroni post hoc test. Linear discriminant analysis (LDA) was used to categorize different antiepileptic drugs according to their effect on neurocognitive functions and also their role in oxidative stress. All predictor variables were entered together for analysis. Wilks’ lambda (λ) test was used to find out the variable which contributed significance in discriminant function. To find multicolinearity of data tolerance was also tested. Fisher's classification function coefficients were used for direct classification. The Wilks’ lambda test was applied to verify which canonical discriminant functions were significant. Also a leaving-one-out cross-validation procedure was carried out to assess the model performance.
Results
Assessment of behavioural parameters: The baseline assessments of the behavioural parameters did not show significant difference among the different groups. In elevated plus maze test there was no significant difference in time spent in the open arms and number of entries into the open arms (Fig. 1A, B). There were no significant differences across the groups in locomotor activity as revealed in actophotometer test (Fig. 1C) and motor coordination as revealed in rotarod test (Fig. 1D).
Fig. 1.
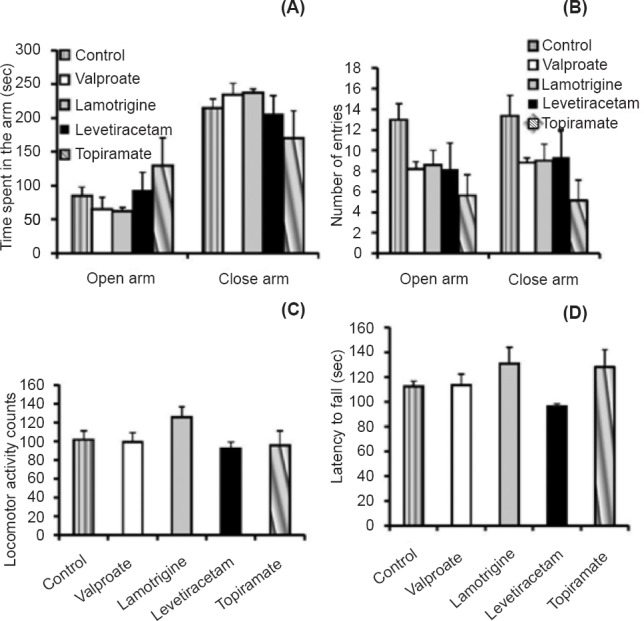
(A and B). Effect of valproate, lamotrigine, levetiracetam and topiramate on anxiety behaviour in rats, using elevated plus maze apparatus (A- Time for which the rat stayed in the open and closed arm, B- Number of entries in the open and closed arm); C- Effects of drugs on locomotor activity in rats, using actophotometer apparatus; D- Effects of drugs on muscle relaxant activity in rats, studied using rotarod apparatus. Values are expressed as mean ± SEM (n=6).
Passive avoidance: Both levetiracetam and topiramate groups exhibited a significantly shorter latency to enter the dark compartment (mean ± SEM, 224.4 ± 10.5 and 229.3 ± 7.9 sec, respectively) than control (P<0.01) and lamotrigine (P<0.05) groups (286.6 ± 10.4 and 277.4 ± 10.1 sec, respectively), demonstrating that fear learning and memory were affected by levetiracetam and topiramate treatment. (Fig. 2A).
Fig. 2.
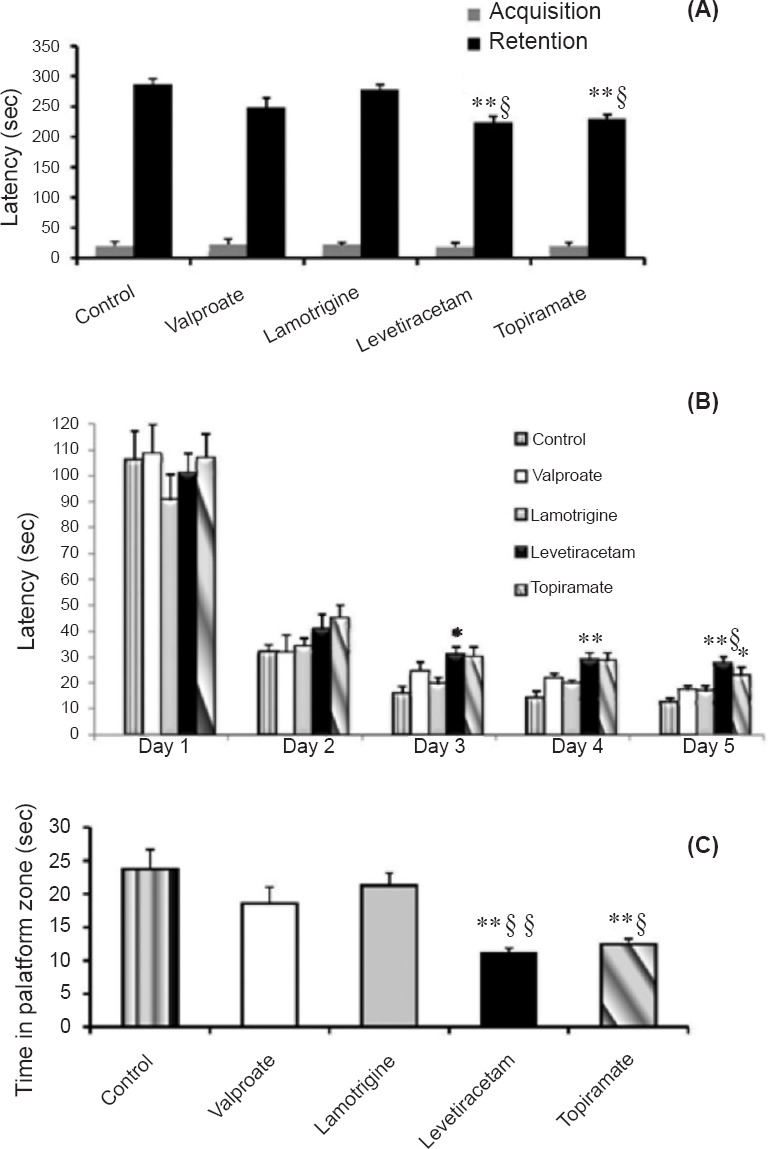
(A). Effect of antiepileptic drugs on the latency in retention of fear learning and memory using passive avoidance apparatus. (B and C). Effect of antiepileptic drugs on spatial learning and memory using Morris water maze apparatus. B- Latency to find the platform during acquisition and probe trial; C- Time spent during the probe trial in the platform zone). Values are expressed as the mean ± SEM (n=6). P*<0.05 and **<0.01 versus the corresponding value of control group on the same day; P§<0.05 and §§<0.01 versus the corresponding value of lamotrigine group on the same day.
Morris water maze: Control animals learned the location of the hidden platform in the Morris water maze, as shown by a decrease in escape latency during training. Animals exposed to lamotrigine and valproate showed similar learning curves and had no significant difference in escape latencies compared with the control group. Levetiracetam and topiramate treated group had significantly longer escape latencies on day 3 and 4 (P<0.05 and <0.01, respectively) than control group. The latency time on day 5 of levetiracetam group was significantly higher than lamotrigine group (P<0.05) and control group (P<0.01). The topiramate group had significant difference (P<0.05) with the control group on day 5. (Fig. 2B).
On the probe trial, levetiracetam and topiramate groups displayed a significantly less time spent in the platform zone (P<0.01) as compared to control animals. Lamotrigine group had spent more time in platform zone than levetiracetam group (P<0.01) and topiramate group (P<0.05). Though valproate group had a lesser time spent in platform zone, there was no significant difference between the control, valproate and lamotrigine groups. (Fig. 2C).
Assessment of oxidative stress parameters: A marked increase in MDA levels was observed in levetiracetam group (P<0.01) and topiramate group (P<0.05) compared to control group. There was no significant difference among the different drug treated groups (Fig. 3A).
Fig. 3.
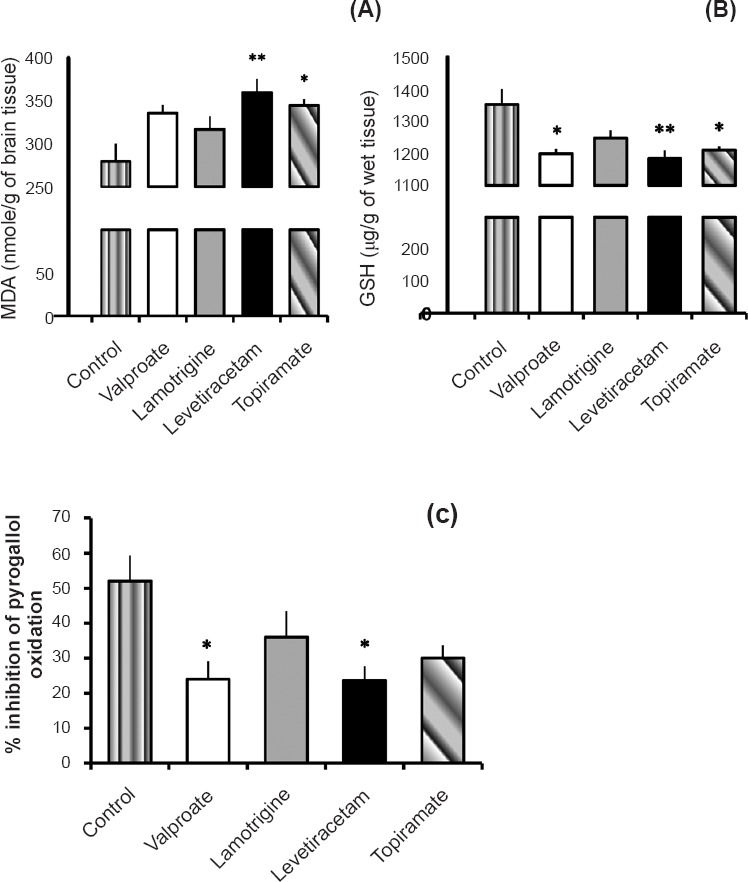
Effect of valproate, lamotrigine, levetiracetam and topiramate on (A) malondialdehyde (MDA) levels, (B) reduced glutathione (GSH) levels, and (C) superoxide dismutase (SOD) activity (expressed as % inhibition of pyrogallol oxidation) in brain tissue. Values are expressed as the mean ± SEM (n=6). P *<0.05 **<0.01 compared to control group.
There was a marked decrease in GSH level in the levetiracetam group (1185.1 ± 25.12 μg/g of brain tissue) (P<0.01) as compared to the control group (1354.0 ± 48.06 μg/g). Topiramate group (1210.8 ± 12.12 μg/g of brain tissue) and valproate group (1199.6 ± 14.85 μg/g of brain tissue) also showed significant (P<0.05) decrease in glutathione activity, but lamotrigine group showed no significant change as compared to control group (Fig. 3B).
There was a marked decrease in SOD activity in levetiracetam and valproate groups in comparison with control group (P<0.05). No significant difference was observed among the various drugs treated group (Fig. 3C).
Linear discriminant analysis: Tests of equality of group means through Wilks’ lambda test revealed that the following variables contributed significance (P<0.01) in discriminant functions: Morris water maze test (days 3, 4 and 5 latency time; and time spent on platform zone), passive avoidance test (retention latency time), and oxidative stress parameters (MDA, GSH, and SOD levels). LDA highlighted the differences among drug formulations with a discriminant model with one significant (P<0.05 for the Wilks’ lambda test) discriminant function. The first function mainly separated control group from levetiracetam group, the second function demarcated valproate group from levetiracetam group. Combined group scatter plot for canonical discriminant functions (with functions 1 and 2) of different drug groups in accordance with their neurocognitive functions and role in oxidative stress is shown in Fig. 4.
Fig. 4.
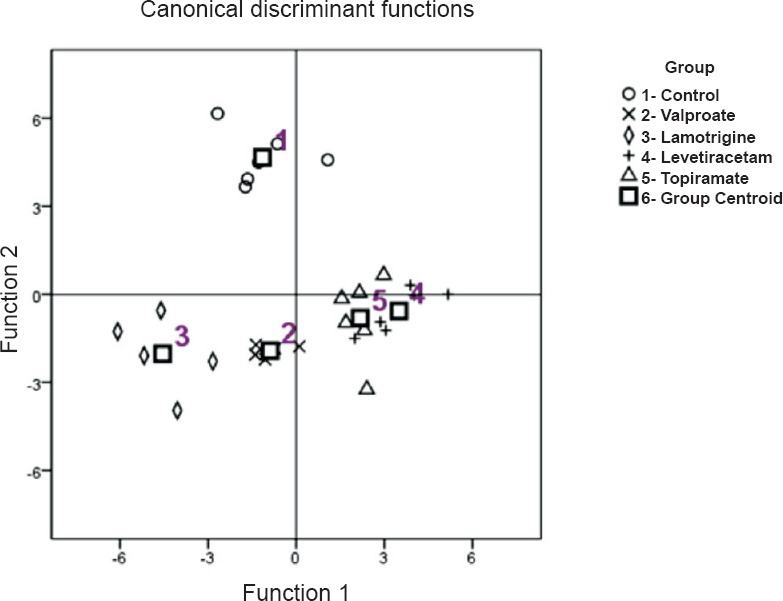
Combined group scatter plot for Canonical discriminant functions of different drug groups in accordance with their neurocognitive functions and role in oxidative stress.
Discussion
Approximately 50 per cent patients experience one or more ADRs during the first line AEDs treatment2. Simultaneously, the prevalence of drug resistant epilepsy is upto 20-30 per cent among newly diagnosed epilepsy patients2. In the present study we investigated the effects of commonly used first line AEDs which included newer antiepileptics like lamotrigine, levetiracetam and topiramate in comparison with the control group and conventional antiepileptic valproate in terms of neurobehavioural function and oxidative stress in rat brain tissue. Anxiety response studied through elevated plus maze and locomotor and motor coordination responses assessed by rotarod test did not show significant variation among the different groups. In some previous studies lamotrigine exposure led to impaired rotarod performance, whereas there was no effect of lamotrigine on anxiety behaviour in elevated plus maze test in adult rats20 and kindled mice21.
Levetiracetam and topiramate treated rats showed significantly less retention of memory as compared to lamotrigine and control groups. There was no significant difference between the control group and those treated with valproate and lamotrigine. Some studies have demonstrated spatial memory enhancing effect of lamotrigine22, and topiramate23, and fear learning enhancement with levetiracetam24, while others have shown decreased learning performance with levetiracetam25, valproate26, and topiramate21. No effect on learning and memory has been demonstrated with treatment of valproate25, levetiracetam24,26, and lamotrigine23. It is difficult to pinpoint which of the numerous differences between the drugs may account for the divergent behavioural outcomes after long term exposure. These drugs belong to distinct structural classes (valproate is a fatty acid, lamotrigine is a phenyltriazine, levetiracetam a pyrrolidine, and topiramate is a fructose derivative), vary in mechanism of anti-seizure activity, generate diverse metabolites, alter trace elements status and demonstrate non-overlapping sets of side effects10,11,27.
In our study, lipid peroxidation was significantly higher in the levetiracetam and topiramate treated groups compared to controls; the GSH content was significantly lowered by treatment of valproate, levetiracetam and topiramate; the SOD level was significantly lower in the valproate and levetiracetam groups. In a previous study, epilepsy patients after two months of treatment with mono- or poly-therapy of AEDs including valproate, carbamazepine and levetiracetam showed decreased serum antioxidant activity and increased oxidant activity3. Increase in MDA and decrease in glutathione levels were observed in a previous study after topiramate administration to kindled as well as non-kindled animals, but no significant alteration was seen after administration of lamotrigine and oxcarbazepine21. Forcelli et al20 opined that GABAergic mechanism of lamotrigine was involved in the protective effect against 3-nitropropionic acid induced neurotoxicity and oxidative stress. In some studies (in vitro and non-epilepsy conditions like diabetes and ischaemic retina), antiepileptic drugs like levetiracetam, topiramate and valproate have been shown to reduce the oxidative stress28,29,30.
This study was performed in healthy rodents, which is a major limitation of this study. It would have been of more translational value in doing this study in true epilepsy models for instance, animal mutants or transgenic animals with spontaneously recurrent seizures, which are more closely related to human epilepsy than conventional seizure models like MES (maximal electroshock seizure) and PTZ (pentylenete trazol) induced seizure models.
In conclusion, the findings revealed that long-term administration of AEDs at maximum therapeutic dose had differential effect on neurobehavioural functions mainly learning and memory, and oxidative stress parameters. Levetiracetam and topiramate significantly impaired the retention memory as compared to the control group, which was not observed in case of valproate and lamotrigine treatment. All AEDs except lamotrigine significantly affected oxidative stress. As all these drugs are widely used in clinical practice, stringent pharmacovigilance is required to establish safety profile of these antiepileptic drugs.
Acknowledgment
Authors thank Yajnaseni Dash, Research Student, Center for Atmospheric Sciences, IIT Delhi, for her help in statistical analysis.
Footnotes
Conflicts of Interest: None.
References
- 1.Indian Epilepsy Society. Guidelines for Management of Epilepsy in India (GEMIND) 2008. [accessed on April 20, 2014]. Available from: http://www.epilepsyindia.org/ies/GUIDELINES/Gemind_Combine.pdf .
- 2.Schmidt D, Schachter SC. Drug treatment of epilepsy in adults. BMJ. 2014;348:g254. doi: 10.1136/bmj.g254. [DOI] [PubMed] [Google Scholar]
- 3.Varoglu AO, Yildirim A, Aygul R, Gundogdu OL, Sahin YN. Effects of valproate, carbamazepine, and levetiracetam on the antioxidant and oxidant systems in epileptic patients and their clinical importance. Clin Neuropharmacol. 2010;33:155–7. doi: 10.1097/WNF.0b013e3181d1e133. [DOI] [PubMed] [Google Scholar]
- 4.Lopez MR, Cheng JY, Kanner AM, Carvalho DZ, Diamond JA, Wallace DM. Insomnia symptoms in South Florida military veterans with epilepsy. Epilepsy Behav. 2013;27:159–64. doi: 10.1016/j.yebeh.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Robins A, Patel M, Azim A. A case of physical and mental adverse drug reactions associated with levetiracetam in post-stroke epilepsy. J Am Geriatr Soc. 2012;60:159–60. doi: 10.1111/j.1532-5415.2011.03749.x. [DOI] [PubMed] [Google Scholar]
- 6.Nadkarni S, Devinsky O. Psychotropic effects of antiepileptic drugs. Epilepsy Curr. 2005;5:176–81. doi: 10.1111/j.1535-7511.2005.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veiby G, Daltveit AK, Engelsen BA, Gilhus NE. Fetal growth restriction and birth defects with newer and older antiepileptic drugs during pregnancy. J Neurol. 2014;261:579–88. doi: 10.1007/s00415-013-7239-x. [DOI] [PubMed] [Google Scholar]
- 8.Leon AC, Solomon DA, Li C, Fiedorowicz JG, Coryell WH, Endicott J, et al. Antiepileptic drugs for bipolar disorder and the risk of suicidal behavior: a 30-year observational study. Am J Psychiatry. 2012;169:285–91. doi: 10.1176/appi.ajp.2011.11060948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baf MH, Subhash MN, Lakshmana KM, Rao BS. Sodium valproate induced alterations in monoamine levels in different regions of the rat brain. Neurochem Int. 1994;24:67–72. doi: 10.1016/0197-0186(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 10.Lowenstein DH. In: Seizures and Epilepsy. Harrison's principles of internal medicine. 18th ed. Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo JL, editors. New York: McGraw-Hill; 2012. pp. 6454–6. [Google Scholar]
- 11.Porter RJ, Meldrum BS. In: Antiseizure Drugs. Basic & clinical pharmacology. 12th ed. Katzung BG, Masters SB, Trevor AJ, editors. New Delhi: Tata McGraw-Hill; 2012. pp. 409–19. [Google Scholar]
- 12.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 13.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–8. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28:2060–5. doi: 10.1161/01.str.28.10.2060. [DOI] [PubMed] [Google Scholar]
- 15.Sharma M, Gupta YK. Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment. Life Sci. 2001;68:1021–9. doi: 10.1016/s0024-3205(00)01005-5. [DOI] [PubMed] [Google Scholar]
- 16.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 17.Ohkawa H, Ohisi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 19.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 20.Forcelli PA, Kozlowski R, Snyder C, Kondratyev A, Gale K. Effects of neonatal antiepileptic drug exposure on cognitive, emotional, and motor function in adult rats. J Pharmacol Exp Ther. 2012;340:558–66. doi: 10.1124/jpet.111.188862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal NB, Agarwal NK, Mediratta PK, Sharma KK. Effect of lamotrigine, oxcarbazepine and topiramate on cognitive functions and oxidative stress in PTZ-kindled mice. Seizure. 2011;20:257–62. doi: 10.1016/j.seizure.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Kumar P, Kalonia H, Kumar A. Possible GABAergic mechanism in the neuroprotective effect of gabapentin and lamotrigine against 3-nitropropionic acid induced neurotoxicity. Eur J Pharmacol. 2012;674:265–74. doi: 10.1016/j.ejphar.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 23.Mikati MA, Daderian R, Zeinieh M, Leonard AS, Azzam D, Kurdi R. Potential neuroprotective effects of continuous topiramate therapy in the developing brain. Epilepsy Behav. 2011;20:597–601. doi: 10.1016/j.yebeh.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Celikyurt IK, Ulak G, Mutlu O, Akar FY, Mulayim S, Erden F, et al. Positive impact of levetiracetam on emotional learning and memory in naive mice. Life Sci. 2012;90:185–9. doi: 10.1016/j.lfs.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Brandt C, Glien M, Gastens AM, Fedrowitz M, Bethmann K, Volk HA, et al. Prophylactic treatment with levetiracetam after status epilepticus: lack of effect on epileptogenesis, neuronal damage, and behavioral alterations in rats. Neuropharmacology. 2007;53:207–21. doi: 10.1016/j.neuropharm.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Eddy CM, Rickards HE, Cavanna AE. The cognitive impact of antiepileptic drugs. The Adv Neurol Disord. 2011;4:385–407. doi: 10.1177/1756285611417920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarangi SC, Tripathi M, Kakkar AK, Gupta YK. Effect of antiepileptic therapy on trace elements status in Indian population in a tertiary care hospital from northern India: A cross sectional study. Epilepsy Res. 2014;108:917–27. doi: 10.1016/j.eplepsyres.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Stettner M, Dehmel T, Mausberg A AK, Köhne A, Rose CR, Kieseier BC. Levetiracetam exhibits protective properties on rat Schwann cells in vitro . J Peripher Nerv Syst. 2011;16:250–60. doi: 10.1111/j.1529-8027.2011.00355.x. [DOI] [PubMed] [Google Scholar]
- 29.Price TO, Eranki V, Banks WA, Ercal N, Shah GN. Topiramate treatment protects blood-brain barrier pericytes from hyperglycemia-induced oxidative damage in diabetic mice. Endocrinology. 2012;153:362–72. doi: 10.1210/en.2011-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z, Tong N, Gong Y, Qiu Q, Yin L, Lv X, et al. Valproate protects the retina from endoplasmic reticulum stress-induced apoptosis after ischemia-reperfusion injury. Neurosci Lett. 2011;504:88–92. doi: 10.1016/j.neulet.2011.09.003. [DOI] [PubMed] [Google Scholar]


