ABSTRACT
Microbes and human cells possess mechanisms of mutagenesis activated by stress responses. Stress-inducible mutagenesis mechanisms may provide important models for mutagenesis that drives host-pathogen interactions, antibiotic resistance, and possibly much of evolution generally. In Escherichia coli, repair of DNA double-strand breaks is switched to a mutagenic mode, using error-prone DNA polymerases, via the SOS DNA damage and general (σS) stress responses. We investigated small RNA (sRNA) clients of Hfq, an RNA chaperone that promotes mutagenic break repair (MBR), and found that GcvB promotes MBR by allowing a robust σS response, achieved via opposing the membrane stress (σE) response. Cells that lack gcvB were MBR deficient and displayed reduced σS-dependent transcription but not reduced σS protein levels. The defects in MBR and σS-dependent transcription in ΔgcvB cells were alleviated by artificially increasing σS levels, implying that GcvB promotes mutagenesis by allowing a normal σS response. ΔgcvB cells were highly induced for the σE response, and blocking σE response induction restored both mutagenesis and σS-promoted transcription. We suggest that GcvB may promote the σS response and mutagenesis indirectly, by promoting membrane integrity, which keeps σE levels lower. At high levels, σE might outcompete σS for binding RNA polymerase and so reduce the σS response and mutagenesis. The data show the delicate balance of stress response modulation of mutagenesis.
IMPORTANCE Mutagenesis mechanisms upregulated by stress responses promote de novo antibiotic resistance and cross-resistance in bacteria, antifungal drug resistance in yeasts, and genome instability in cancer cells under hypoxic stress. This paper describes the role of a small RNA (sRNA) in promoting a stress-inducible-mutagenesis mechanism, mutagenic DNA break repair in Escherichia coli. The roles of many sRNAs in E. coli remain unknown. This study shows that ΔgcvB cells, which lack the GcvB sRNA, display a hyperactivated membrane stress response and reduced general stress response, possibly because of sigma factor competition for RNA polymerase. This results in a mutagenic break repair defect. The data illuminate a function of GcvB sRNA in opposing the membrane stress response, and thus indirectly upregulating mutagenesis.
INTRODUCTION
Bacterial (1–7), yeast (8), and human cancer (9, 10) cells possess mechanisms of mutagenesis upregulated by stress responses. Stress-inducible mutation mechanisms may accelerate adaptation specifically when cells are poorly adapted to their environments, i.e., when stressed. Modeling studies indicate that stress-inducible mutagenesis can be selected on the basis of acceleration of adaptation even in asexual bacterial populations, in which deleterious mutations generated cannot be purged by recombination (11, 12). Stress-inducible mutation mechanisms drive evolution of antibiotic resistance (13–15) and cross-resistance (16), antifungal drug resistance (8, 17) and possibly much of evolution generally.
In Escherichia coli, repair of DNA double-strand breaks (DSBs) by homologous recombination is switched to a mutagenic mode using error-prone DNA polymerases under the control of the SOS DNA damage response and the general stress response (1, 2, 6, 18–20). Mutagenic break repair (MBR) requires proteins that perform DSB repair via homologous recombination (21–23), low-fidelity DNA polymerases (Pols) IV (18, 19, 24), V (19, 25), and II (26), and the activators of the general/starvation stress response (RpoS/σS) (18, 19, 27, 28), the SOS DNA damage response (29, 30), and the RpoE/σE membrane stress response (31). The σE response drives mutagenesis by promoting spontaneous DNA breakage (31) at some genomic locations, as do RNA/DNA hybrids (R-loops) caused by transcription (32). The SOS response promotes mutagenesis via its 10-fold transcriptional upregulation of Pol IV (33) and by allowing production of Pol V. The general stress response is activated by the σS transcriptional activator, a sigma factor of RNA polymerase, in response to starvation, antibiotics (16), and many other stresses (34, 35). The general stress response directly and indirectly up- and downregulates transcription of more than 500 E. coli genes (34, 35) and promotes mutagenesis by allowing the use of, or errors made by, the error-prone DNA Pols in DSB repair, by an as-yet-unknown mechanism (18, 19, 26). Thus, even in cells with a DSB, an activated SOS response and the resulting 10-fold-higher levels of Pol IV, DSB repair remains relatively nonmutagenic, using high-fidelity DNA Pol III (36), unless the general stress response is also activated either by stress or artificially (18, 19, 26). That is, σS-inducing stress is not itself needed for mutagenesis during DSB repair; artificial activation of the σS response is sufficient to make repair mutagenic even in growing cells (18, 19). The MBR mechanism, in which DNA Pol IV initiates mutagenic DNA synthesis from a D-loop (intermediate in recombinational repair), has been recapitulated in solution with purified proteins (37). Further, the mutation signatures of σS-promoted mutagenesis are overrepresented in extant bacterial genomes, suggesting that MBR is widespread in bacterial mutagenesis in the wild (38).
Mutagenic break repair in E. coli is promoted by a large network of more than 93 genes, mutations in any of which decrease mutagenesis (39). More than half of MBR network genes promote mutagenesis by sensing stress and transducing signals that lead to activation of the σS, SOS, and/or σE stress responses (39), indicating the importance of stress response control of mutagenesis to E. coli. Among the genes discovered in this screen for MBR-defective mutants is hfq, which encodes the Hfq RNA chaperone (39). Hfq is required for MBR in E. coli (39).
Hfq was discovered as a bacterial host factor required for synthesis of bacteriophage Qβ RNA (40) and is part of the conserved family of Sm-like RNA-modulating proteins found in eukaryotes, archaea, and eubacteria (41). Hfq is required for virulence of several bacterial species (42–49). Acting as an RNA chaperone, Hfq facilitates base pairing of a collection of small RNAs (sRNAs) to specific mRNA molecules, which allows the sRNAs to up- or-downregulate translation from the mRNAs (50, 51). sRNAs are approximately 100 bp long and downregulate translation of some mRNAs by base pairing that blocks ribosome-binding sites (52). sRNAs also upregulate translation by melting mRNA secondary structures such as hairpins that would otherwise prevent ribosome recognition (53). Several sRNAs are upregulated during stress, including DsrA and RprA, both of which promote translation of the rpoS mRNA to σS protein (54). Of the approximately 100 sRNAs known in E. coli (55–58), 30 sRNAs require Hfq to function (59). Although the means by which Hfq promotes MBR is unknown, the fact that it does so suggests that one or more of the Hfq client sRNAs may promote mutagenesis. In this study, we examined nine sRNA clients of Hfq that are not encoded within protein-coding genes and that showed expression patterns potentially relevant to starvation stress (59). We report below that cells that lack the GcvB sRNA are MBR defective.
Found in diverse bacteria, GcvB is an Hfq-chaperoned sRNA that up- or downregulates translation of amino acid biosynthesis and transport proteins (60–63). In Salmonella enterica, GcvB is a master regulator of amino acid metabolism and directly up- or downregulates translation of ∼1% of all mRNAs (64). GcvB regulates a network of mRNAs by inhibiting or enabling translation based on cellular environment. E. coli ΔgcvB mutant cells are acid sensitive, possibly caused partly by reduced σS levels, shown with a σS-LacZ fusion protein (65).
Many sRNAs in E. coli promote membrane integrity and do so by regulating outer membrane protein genes (66–68). The levels of various sRNAs are increased under different stresses (56, 69), and many are upregulated by the σE membrane stress response (70–72). sRNAs, such as MicA, RybB, and MicL, are induced by cell envelope stress, and then they downregulate translation of outer membrane porins and lipoproteins, aiding membrane integrity (73–77). Transcription of rpoE and σE-dependent promoter use is increased in an hfq mutant, supporting the roles of sRNAs in averting the σE response and promoting membrane integrity (78). Here, we show that the GcvB sRNA is required for MBR. We find that GcvB promotes MBR by allowing a robust σS response. We report that ΔgcvB mutant cells display reduced σS-regulated promoter activity and MBR but not reduced σS protein levels. Artificial upregulation of σS restored σS-regulated promoter activity and MBR, implying that normal quantities of σS are insufficient to activate the general stress response in ΔgcvB cells. We provide evidence that the MBR and σS response deficiency in ΔgcvB cells result from hyperactivation of the σE membrane stress response. We suggest that GcvB may promote the σS response, and so also MBR, indirectly by keeping membrane stress low enough for σS to compete successfully with σE for RNA polymerase. The data illuminate a function of GcvB in opposing the membrane stress response, and thus indirectly upregulating mutagenesis.
MATERIALS AND METHODS
Strains and materials.
E. coli K-12 strains and plasmids used are shown in Table 1. Strains were constructed using phage lambda Red-mediated recombineering as described previously (79) and phage P1-mediated transduction as described previously (80). M9 minimal medium (80) had carbon sources added at 0.1% and vitamin B1 (B1) at 10 μg/ml. LBH medium was as described previously (81). Antibiotics and other additives were used at the following final concentrations: ampicillin, 100 μg/ml; chloramphenicol, 25 μg/ml; kanamycin, 50 μg/ml; tetracycline (Tet), 10 μg/ml; rifampin, 100 μg/ml; and 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal), 40 μg/ml.
TABLE 1.
Escherichia coli K-12 strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or description | Reference(s), source, or construction |
|---|---|---|
| Strains | ||
| CAG45114 | MG1655 (λ rpoHP3-lacZ) | 106 |
| ENZ280 | Δ(srlR-recA)306::Tn10 [mini-F recA+] | 107, 108 |
| FC29 | Δ(lac-proB)XIII thi ara [F′128 proAB+ lacIq] | 29 |
| FC40 | Δ(lac-proB)XIII thi ara Rifr [F′128 proAB+ lacIq lacI33-lacZ] | 29 |
| JW3677 | BW25113 ΔrecF::FRT-Kanr-FRT | 109 |
| JW5437 | BW25113 ΔrpoS::FRT-Kanr-FRT | 109 |
| SP874 | MC4100 ΔrpoE::cat | T. Silhavy (Princeton) |
| SMR820 | FC40 lexA3(Ind−) | 24 |
| SMR3856 | SMR4562 Lac+ day 5 | 86 |
| SMR4562 | Independent construction of FC40 | 30 |
| SMR5236 | SMR4562 rpoE2072::Tn10dCam | 31 |
| SMR5535 | SMR4562 ΔrecA | SMR4562 × P1 (ENZ280) |
| SMR5833 | SMR4562(pKD46) | SMR4562 × pKD46 |
| SMR8842 | CAG45114 rpoE2072::Tn10dCam | CAG45114 × P1 (SMR5236) |
| SMR10336 | SMR4562 ΔrpoS::FRT-Kanr-FRT | SMR4562 × P1 (JW5437) |
| SMR10582 | SMR4562 yiaG-yfp FRT-cat-FRT | 39 |
| SMR10777 | SMR4562 Δzie3920.5::3Chi-Kan-I-SceI cut site | 19 |
| SMR10808 | FC36 ΔaraBAD567 Δattλ::PBADI-SceI tet2 FRT | 19 |
| SMR10823 | FC36 ΔaraBAD567 Δattλ::PBADI-SceI tet2 FRT rpoE2072::Tn10dCam | SMR10808 × P1 (SMR5236) |
| SMR10832 | FC36 ΔaraBAD567 Δattλ::PBADI-SceI tet2 FRT ΔrpoS::FRT | 19 |
| SMR10854 | FC36 ΔaraBAD567 Δattλ::PBADI-SceI tet2 FRT Δzie3920.5::3Chi-Kan-I-SceI cut site rpoE2072::Tn10dCam | SMR10823 × P1 (SMR10777) |
| SMR10862 | FC36 ΔaraBAD567 Δattλ::PBADI-SceI tet2 FRT Δzie3920.5::3Chi-Kan-I-SceI cut site ΔrpoS::FRT | 19 |
| SMR10866 | FC36 ΔaraBAD567 Δattλ::PBADI-SceI tet2 FRT Δzie3920.5::3Chi-Kan-I-SceI cut site | 19 |
| SMR12566 | SMR4562 ΔrssB::Tetr | 39 |
| SMR12661 | SMR4562 ΔrpoS746::FRT-Kanr-FRT yiaG-yfp FRT-cat-FRT | 39 |
| SMR12692 | SMR4562 ΔrssB::Tetr yiaG-yfp FRT-cat-FRT | 39 |
| SMR12848 | SMR4562 yiaG-yfp FRT-cat-FRT | SMR4562 × P1 (SMR10582) |
| SMR13014 | SMR4562 ΔrpoS::FRT | SMR10336 × pCP20 |
| SMR13096 | SMR4562 yiaG-yfp FRT | 39 |
| SMR17962 | MG1655 Δattλ::PsulAmCherry FRT-cat-FRT | 83 |
| SMR17966 | MG1655 Δattλ::PsulAmCherry FRT lexA3(Ind−) malB::Tn9 | 83 |
| SMR20177 | SMR5833 ΔoxyS::FRT-cat-FRT | This work |
| SMR20181 | SMR5833 ΔrprA::FRT-cat-FRT | This work |
| SMR20183 | SMR5833 ΔdsrA::FRT-cat-FRT | This work |
| SMR20185 | SMR5833 ΔrybB::FRT-cat-FRT | This work |
| SMR20201 | SMR5833 ΔmicF::FRT-cat-FRT | This work |
| SMR20203 | SMR5833 Δspf::FRT-cat-FRT | This work |
| SMR20205 | SMR5833 ΔryhB::FRT-cat-FRT | This work |
| SMR20207 | SMR5833 ΔgcvB::FRT-cat-FRT | This work |
| SMR20219 | SMR4562 ΔoxyS::FRT-cat-FRT | SMR4562 × P1 (SMR20177) |
| SMR20220 | SMR4562 ΔdsrA::FRT-cat-FRT | SMR4562 × P1 (SMR20181) |
| SMR20221 | SMR4562 ΔrybB::FRT-cat-FRT | SMR4562 × P1 (SMR20185) |
| SMR20230 | SMR4562 ΔrprA::FRT-cat-FRT | SMR4562 × P1 (SMR20181) |
| SMR20232 | SMR4562 ΔmicF::FRT-cat-FRT | SMR4562 × P1 (SMR20201) |
| SMR20234 | SMR4562 Δspf::FRT-cat-FRT | SMR4562 × P1 (SMR20203) |
| SMR20236 | SMR4562 ΔryhB::FRT-cat-FRT | SMR4562 × P1 (SMR20205) |
| SMR20238 | SMR4562 ΔgcvB::FRT-cat-FRT | SMR4562 × P1 (SMR20207) |
| SMR20290 | SMR4562 ΔcyaR::FRT-cat-FRT | SMR4562 × P1 (SMR20179) |
| SMR21332 | SMR3856 ΔgcvB::FRT-cat-FRT | SMR3856 × P1 (SMR20207) |
| SMR21361 | SMR4562 ΔgcvB::FRT-cat-FRT ΔrssB::Tetr | SMR12566 × P1 (SMR20207) |
| SMR21448 | SMR4562 ΔgcvB::FRT | SMR20238 × pCP20 |
| SMR21450 | SMR4562 ΔmicF::FRT | SMR20232 × pCP20 |
| SMR21467 | SMR4562 ΔrybB::FRT | SMR20221 × pCP20 |
| SMR21471 | SMR4562 ΔgcvB::FRT yiaG-yfp FRT-cat-FRT | SMR21448 × P1 (SMR12848) |
| SMR21553 | SMR21641 ΔgcvB::FRT-cat-FRT | SMR21641 × P1 (SMR20207) |
| SMR21633 | SMR10866 ΔgcvB::FRT-cat-FRT | SMR10866 × P1 (SMR20207) |
| SMR21641 | SMR4562 Δattλ::PsulAmCherry FRT-cat-FRT | SMR4562 × P1 (SMR17962) |
| SMR21725 | SMR4562 ΔrpoS::FRT Δattλ::PsulAmCherry FRT-cat-FRT | SMR13014 × P1 (SMR17962) |
| SMR21728 | SMR4562 Δattλ::PsulAmCherry FRT-cat-FRT ΔrecF::FRT-Kanr-FRT | SMR21641 × P1 (JW3677) |
| SMR21909 | SMR4562 ΔrssB::FRT ΔgcvB::FRT | SMR21361 × pCP20 |
| SMR21933 | SMR21553 ΔrssB::FRT | SMR21909 × P1 (SMR17962) |
| SMR21934 | SMR4562 ΔgcvB::FRT ΔrssB::Tetr yiaG-yfp FRT-cat-FRT | SMR21909 × P1 (SMR12848) |
| SMR21996 | SMR4562 ΔgcvB::FRT rpoE2072::Tn10dCam | SMR21448 × P1 (SMR5296) |
| SMR21998 | SMR4562 ΔgcvB::FRT yiaG-yfp FRT | SMR21471 × pCP20 |
| SMR22047 | SMR4562 ΔgcvB::FRT rpoE2072::Tn10dCam yiaG-yfp FRT | SMR21998 × P1 (SMR5236) |
| SMR22064 | SMR4562 yiaG-yfp FRT rpoE2072::Tn10dCam | SMR13096 × P1 (SMR5236) |
| SMR22066 | SMR10866 ΔgcvB::FRT | SMR21633 × pCP20 |
| SMR22074 | SMR21633 rpoE2072::Tn10dCam | SMR22066 × P1 (SMR5236) |
| SMR22216 | CAG45114ΔgcvB::FRT-cat-FRT | CAG45114 × P1 (SMR20207) |
| SMR22296 | CAG45114ΔgcvB::FRT | SMR22216 × pCP20 |
| SMR22310 | SMR22296 rpoE2072::Tn10dCam | SMR22296 × P1 (SMR5296) |
| SMR22549 | SMR4562 ΔdsrA::FRT | SMR20220 × pCP20 |
| SMR22551 | SMR4562 ΔrprA::FRT | SMR20230 × pCP20 |
| SMR22554 | SMR4562 ΔdsrA::FRT-cat-FRT ΔrprA::FRT | SMR22551 × P1 (SMR20220) |
| SMR22556 | SMR4562 ΔcyaR::FRT | SMR20290 × pCP20 |
| SMR22558 | SMR4562 ΔoxyS::FRT | SMR20219 × pCP20 |
| SMR22560 | SMR4562 ΔryhB::FRT | SMR20236 × pCP20 |
| SMR22562 | SMR4562 Δspf::FRT | SMR20234 × pCP20 |
| SMR22936 | SMR3856 ΔrprA::FRT-cat-FRT | SMR3856 × P1 (SMR20181) |
| SMR22940 | SMR10866 ΔrprA::FRT-cat-FRT | SMR10808 × P1 (SMR20181) |
| SMR22950 | SMR3856 ΔrprA::FRT | SMR22936 × pCP20 |
| SMR22954 | SMR10866 ΔrprA::FRT | SMR22940 × pCP20 |
| SMR22960 | SMR3856 ΔrprA::FRT ΔdsrA::FRT-cat-FRT | SMR22950 × P1 (SMR20183) |
| SMR22964 | SMR10866 ΔrprA:FRT ΔdsrA::FRT-cat-FRT | SMR22954 × P1 (SMR20183) |
| Plasmids | ||
| pCP20 | Temperature-inducible yeast Flp recombinase gene controlled by λcIts857 in a temperature-sensitive replicon | 110 |
| pKD3 | Source of FRT-cat-FRT | 79 |
| pKD46 | ori101 repA101ts PBADgam-bet-exo Ampr | 79 |
Each of nine nonpolar deletions of sRNA genes was constructed by recombineering using pKD3 as the PCR template (79). The nucleotides deleted for each new deletion allele are shown in Table S1 in the supplemental material.
Quantitative Lac mutagenesis assays with spontaneous DSBs.
The Lac assay (29) for stress-inducible MBR measures reversion of an F′-borne lacI-Z gene with a +1-bp frameshift allele during starvation stress, and the assay was performed as described previously (23). Viable Lac− starving cells on the lactose-containing plates were measured daily throughout the experiments as described previously (29) and varied less than 2-fold during the days of the experiments reported. Lac+ revertant CFU (indel mutants) are counted to day 5. The mutation rates (Lac+ CFU per 108 CFU per day) shown are means ± standard errors of the means (SEM) from four separate experiments with four independent cultures for each strain and were calculated as described previously (28) by subtracting the number of colonies counted on day 3 from the number of colonies counted on day 5 and dividing by 2.
Chromosomal Tet reversion assay with I-SceI-induced DSBs in plasmid-free cells.
The Tet reversion assay of Shee et al. (19, 20, 39) was performed as described previously. A chromosomally encoded arabinose-inducible, glucose-repressible I-SceI endonuclease, produced weakly by leaky expression, cleaves a chromosomal I-SceI cut site (I-site) near a tet mutation reporter gene in liquid-starved plasmid-free cells (19). The chromosomal tet gene with a +1-bp frameshift allele resides 8.5 kb from the I-site (I-site A, tet2) (19). Cells are grown for 12 h to saturation in liquid, starved for 72 further hours, then rescued from starvation, plated on LBH solid medium containing glucose and tetracycline (LBH glucose tetracycline solid medium), and incubated at 37°C to select tet+ revertant tetracycline-resistant (Tetr) colonies, which are counted the following day, as are the total viable CFU assayed on medium without tetracycline. Mutant frequencies are the titers of Tetr mutant CFU per milliliter on LBH glucose tetracycline medium divided by those of total CFU/ml from LBH glucose plates. Data presented are the values (means ± SEM) from 10 independent experiments with three cultures for each strain.
Flow cytometric assays for σS and SOS response-regulated promoter activity.
σS and SOS response activation for σS activity were quantified by flow cytometry as described previously (39) for σS. The method of Pennington and Rosenberg (82) as modified by Nehring et al. (83) was used for SOS. The chromosomal yiaG-yfp σS response reporter gene (39) and Δattλ::PsulAmCherry SOS reporter gene (83) were used in separate cells. Strains were grown at 37°C for 48 h with aeration in liquid M9 medium containing vitamin B1 and glycerol (liquid M9 B1 glycerol). σS and SOS response-dependent promoter activity was quantified in two ways. First, for the SOS response, in which only a small subpopulation of growing cells is induced spontaneously relative to negative-control, SOS-off mutant cells (82), we set gates by the method of Pennington and Rosenberg (82). Gates were calibrated using negative-control SOS-off lexA(Ind−) cells, and SOS response “on” was scored as the fluorescence intensity shown by the most fluorescent 1% of events observed in wild-type cultures. Cells that fell below this gate (less fluorescence) were scored as negative. The values (percent positive cells; means ± SEM) from five independent SOS activity experiments with three independent cultures for each strain are given. Second, for both SOS and σS responses, we report the mean fluorescence intensity per cell, a measure more useful for responses and mutants in which cells display a unimodal distribution of fluorescence intensities, and a majority or all of the cells in the population of mutants examined are shifted relative to the wild-type strain or the negative-control strains.
Western blot analyses of σS and σE protein levels.
Western blot analyses for quantification of σS and σE protein levels in stationary cultures were performed by the methods of Galhardo et al. (33) and Gibson et al. (31), respectively. The optical density at 600 nm (OD600) was taken of 5-ml samples from 48-h cultures grown in M9 B1 glycerol, and the concentrations were adjusted to standardize the different strains. The cells were pelleted and resuspended in 1 ml of lysis buffer/loading sample (62.5 mm Tris [pH 6.8], 25% glycerol, 2% SDS, 0.01% bromophenol blue, 0.5% β-mercaptoethanol) and boiled. Fifteen-microliter portions of each sample were electrophoresed on 13% SDS–polyacrylamide gels, and the proteins were transferred to Hybond-LFP polyvinylidene difluoride (PVDF) membranes (Amersham Biosciences). The membranes were blocked with 2% blocking buffer and probed with 1:700 dilution of polyclonal mouse anti-σS antibody (Neoclone) (84) or 1:5,000 dilution of polyclonal rabbit anti-σE antibody (85) (gift of Carol Gross, University of California at San Francisco [UCSF]). Goat anti-mouse and anti-rabbit secondary antibodies conjugated to Cy5 fluorescent dye (Amersham Biosciences) were used at a 1:5,000 dilution to detect σS and σE proteins, respectively. Fluorescence was assessed on a Typhoon scanner with a photomultiplier voltage (PMT) of 500 and a 670-nm bandpass (670BP) 30Cy5 emission filter, and the bands were quantified using ImageJ software (NIH). Quantifications from four separate Western blots for σS and σE are reported, each with band intensities normalized to isogenic wild-type control strain SMR4562 and means ± 1 SEM are shown.
Reconstruction experiments.
Reconstruction experiments were used to demonstrate that Lac+ ΔgcvB and Lac+ ΔdsrA ΔrprA cells form colonies normally under selective assay conditions in the presence of neighbor cells, using assays described previously (86) as reviewed in reference 87, such that their defect in producing Lac+ mutant colonies reflects reduced mutagenesis, not impaired colony formation. We quantified the timing of colony appearance and fraction of known numbers of ΔgcvB and ΔdsrA ΔrprA CFU that formed colonies under precise reconstructions of experimental conditions and compared these with those of isogenic nonmutant strains.
UV light sensitivity assays.
Saturated liquid M9 B1 glycerol cultures were starved as in the Lac mutagenesis assays described above, diluted, plated at various concentrations onto LBH solid medium, and exposed to various doses of UVC light in a Stratalinker (Artisan Technology Group). CFU titers were quantified and graphed. Data are normalized to viable-cell titers with no UVC irradiation. lexA(Ind−) mutant cells, which produce an uncleavable LexA transcriptional repressor of the SOS genes, and so are SOS response defective, were used as a positive control for SOS response deficiency. ΔrecA cells have stronger UV sensitivity and were also used as a positive control.
Semiquantitative SDS-EDTA sensitivity assay for σE response deficiency.
σE response-defective cells are sensitive to SDS-EDTA, which disrupts the membrane (88). Strains grown to saturation and starved, per Lac mutagenesis experiments, were diluted, and 10-μl spots containing ∼30 and ∼300 CFU deposited onto solid M9 B1 glycerol medium with and without 0.01% SDS and 0.25 mM EDTA, incubated for 48 h at 30°C (the permissive temperature for a ΔrpoE control strain [89]) and scored. We used control isogenic cells carrying the rpoE2072::Tn10dCam separation-of-function allele, which confers σE response deficiency but maintains the essential function of σE (31), and are SDS-EDTA sensitive (31). Although rpoE is an essential gene (89), the ΔrpoE mutant is viable because of acquisition of compensatory extragenic “suppressor” mutations that permit viability (90).
Catalase colony assays for σS response activity.
Catalase colony assays for σS response activity were performed as described previously (91). Wild-type control, isogenic ΔgcvB, and ΔrpoS strains were grown into colonies on M9 B1 glycerol medium for 48 h at 37°C. Three microliters of 30% hydrogen peroxide was dropped onto each colony, and the time elapsed before bubbles appeared was measured. Six colonies were tested in four independent experiments, and the times to bubbling (means ± SEM) (in seconds) were reported. P values compared with the values for wild-type colonies were determined using two-tailed Student's t test.
β-Galactosidase assay for σE activity.
Because the rpoH P3 promoter is σE dependent, the rpoHP3-lacZ fusion gene is a reporter for σE-dependent transcription (92), which is measured as β-galactosidase activity in liquid cultures. β-Galactosidase assays of saturated M9 B1 glycerol cultures were performed as described previously (31). The values (means ± SEM) from three experiments and four independent cultures for each strain are reported.
Acid sensitivity assays.
Acid sensitivity assays were performed as described previously (65). Saturated overnight cultures of wild-type control and isogenic ΔgcvB, rpoE2072::Tn10dCam (rpoE::Tn), and ΔgcvB rpoE::Tn cells were diluted 1:50 in LB medium and grown at 37°C with aeration for 5 h. Acid challenge was performed by adding 3 volumes of acidified LB medium (pH 1.9) to cultures, resulting in a final pH of 2.0. Cells were grown in acidified liquid culture for 30 min. The challenge was interrupted with the addition of 3 volumes of alkalinized LB medium (pH 9.3), resulting in a final pH of 7.0. The optical density at 600 nm was taken after 3 h of recovery. The values (means ± SEM) for three experiments containing three independent cultures per strain are reported.
RESULTS
MBR deficiency caused by deletion of Hfq client genes gcvB and dsrA or rprA.
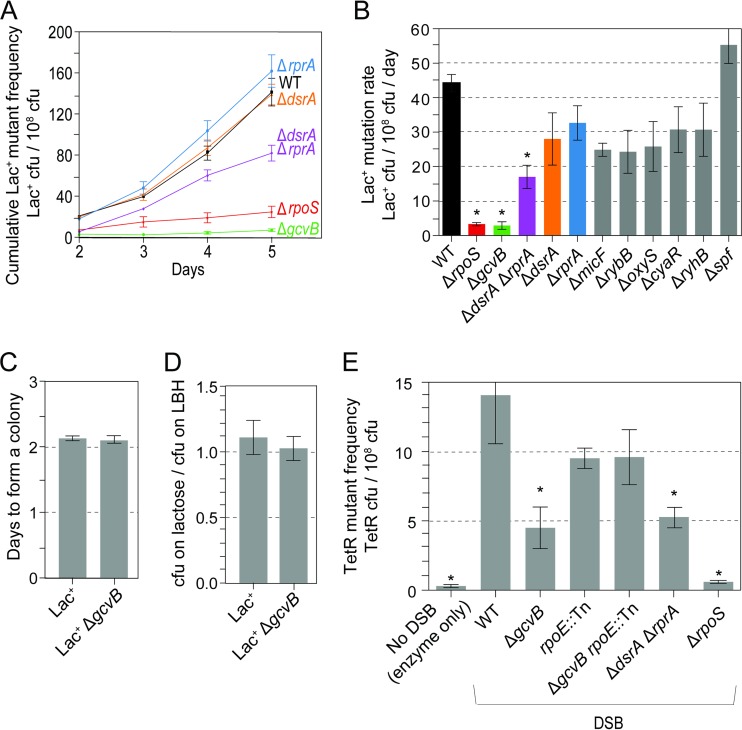
We deleted nine genes that encode sRNA clients of Hfq (see Table S1 in the supplemental material), genes that are not embedded in another gene and that showed expression patterns potentially relevant to starvation stress (59). We assayed the deletion mutants for MBR proficiency/deficiency using the Lac MBR assay (29). The Lac assay quantifies reversion via MBR of a conjugative-plasmid-borne lac gene with a +1-bp frameshift allele during prolonged starvation for days on minimal lactose solid medium (1–3, 6, 7). Colonies visible on day 2 are roughly 50% preexisting generation-dependent Lac+ reversion mutants, and colonies from day 3 onward are DSB-, DinB/Pol IV-, SOS-, σS-dependent, MBR-generated revertants. Δhfq cells show a strong 16-fold ± 2-fold deficiency in MBR in the Lac assay (39). We found that ΔgcvB cells showed a significant 12-fold ± 2-fold defect in Lac+ MBR revertant accumulation (Fig. 1A and B, mean ± SEM mutation rate compared with the WT), a defect smaller than that of the Δhfq mutant. The double mutant ΔgcvB Δhfq was inviable and could not be tested. In addition, the ΔdsrA ΔrprA double mutant showed a smaller but significant 2-fold ± 0.4-fold reduction in the accumulation of Lac+ revertants (Fig. 1A and B). Because neither the ΔdsrA nor ΔrprA single mutant showed reduction, the data imply that either DsrA or RprA can function in MBR (they are redundant functions), such that loss of neither sRNA singly reduces accumulation of revertants. Both sRNAs promote translation of σS (54). Here, we follow up the role of sRNA GcvB.
FIG 1.
sRNA GcvB promotes mutagenic break repair in chromosomal and F′-based MBR assays. (A) Lac MBR assay. Lac+ CFU are revertants of a conjugative-plasmid-borne lac frameshift allele during starvation on solid medium. The results of a representative experiment are shown. (B) Quantification of Lac+ MBR mutation rates as described in Materials and Methods. The values are means ± standard errors of the means (SEM) (error bars) from three experiments for each strain. Asterisks indicate values that are significantly different from the value for the isogenic wild-type (WT) control strain (P = 0.0002 for the ΔrpoS strain, P = 0.0004 for the ΔgcvB strain, and P = 0.01 for the ΔdsrA ΔrprA strain; two-tailed Student's t test used in all comparisons here). From left to right, isogenic strains are SMR4562, SMR10336, SMR21448, SMR22554, SMR22549, SMR22551, SMR21450, SMR21467, SMR22558, SMR22556, SMR22560, and SMR22562. (C and D) Reconstruction experiments show that, when constructed, ΔgcvB mutant Lac+ cells are proficient at colony formation (Materials and Methods). (C) Normal speed of colony formation by ΔgcvB cells under MBR assay conditions. (D) Similar efficiencies of colony formation under selective conditions, compared with CFU on rich (LBH) medium without neighbor cells. (E) Chromosomal Tet MBR assay in plasmid-free cells. ΔgcvB cells display a MBR defect that is relieved by the rpoE::Tn separation-of-function mutation, which blocks the membrane stress response (31). Asterisks indicate values that are significantly different from the value for the isogenic wild-type (WT) control strain (P = 0.04 for the ΔgcvB strain, P = 0.04 for the ΔdsrA ΔrprA strain, P = 0.001 for the ΔrpoS strain, and P = 0.001 for “No DSB” [I-SceI enzyme present with no cut site]). Values are means ± SEM from seven experiments with positive controls. From left to right, the strains are SMR10808, SMR10866, SMR21633, SMR10854, SMR22074, SMR22964, and SMR10862. TetR, tetracycline resistant.
GcvB and RprA or DsrA are required for mutagenesis, not mutant colony formation.
We show that the failure of ΔgcvB cells to produce Lac+ revertant colonies (Fig. 1A and B) is not merely the inability of Lac+ revertants carrying a ΔgcvB mutation to form colonies under experimental conditions. We performed reconstruction experiments in which a functional Lac+ allele is moved into ΔgcvB cells, and their efficiency and speed of colony formation under precise reconstructions of experimental conditions are measured (∼100 Lac+ ΔgcvB cells mixed with ∼109 Δlac nonrevertible neighbor cells on selective plates). The data show that ΔgcvB cells form colonies normally and do not have decreased viability or growth rate under experimental conditions (Fig. 1C and D). Similar reconstruction experiments showed that ΔdsrA ΔrprA mutant cells also do not have decreased ability to form colonies under experimental conditions (see Fig. S1 in the supplemental material). We conclude that GcvB is required for mutagenesis, not outgrowth of mutant cells into colonies, as are DsrA or RprA.
GcvB and either DsrA or RprA are required for MBR in the chromosomal Tet assay.
We confirmed the MBR deficiency of ΔgcvB and ΔdsrA ΔrprA cells using the chromosomal Tet MBR assay (19) in which a chromosomal tet gene in plasmid-free cells reverts by indel mutation during prolonged starvation in liquid and then the cells are rescued from starvation and selected for tetracycline-resistant (Tetr) mutant CFU. A chromosomally encoded I-SceI endonuclease is weakly induced and cleaves an I-SceI cut site near the tet gene, provoking repair, presumably with a sister chromosome (present in ∼40% of stationary-phase E. coli [93]). The tet reversions that result are dependent on DSBs, DSB repair protein, SOS, DinB, and σS (19), all as observed in the Lac assay (18). We found that GcvB and either DsrA or RprA promoted a significant 69% ± 11% and 43% ± 9% of MBR, respectively, in the chromosomal Tet assay (Fig. 1E). We conclude that GcvB promotes much of stress-inducible MBR in E. coli, generally.
GcvB promotes MBR other than or in addition to by promoting SOS, DSB repair, the σE response, or spontaneous DNA breakage.
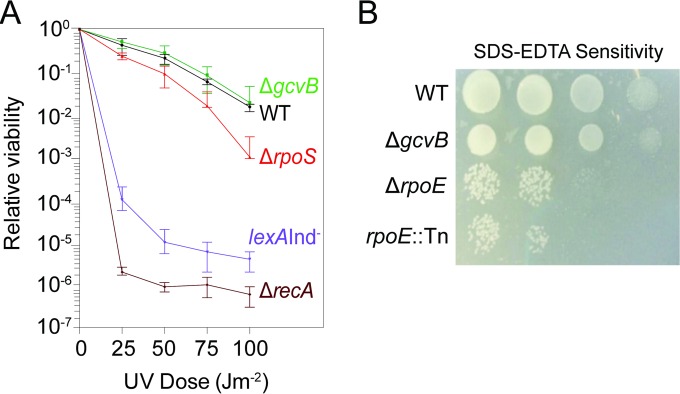
We tested ΔgcvB cells for possible defects in several known components of MBR reactions. Cells defective for homologous recombinational (HR) DSB repair or the SOS response both show sensitivity to UV light (94, 95) [Fig. 2A, lexA(Ind−) and ΔrecA positive-control strains]. We found that ΔgcvB cells were as UV resistant as isogenic gcvB+ cells (“wild-type” [WT] cells in Fig. 2A), indicating that they have neither SOS nor HR defects. Two lines of evidence show that a defective σE membrane stress response does not underlie the MBR deficiency of ΔgcvB cells. First, cells with defects in the σE membrane stress response show sensitivity to SDS-EDTA, which disrupts the cell membrane (88). We found that ΔgcvB cells were as resistant to SDS-EDTA as the wild-type isogenic control (Fig. 2B). Second, σE promotes MBR in the Lac assay by promoting spontaneous DNA breakage (31); thus, σE is not required in the Tet assay in which DSBs are provided by I-SceI endonuclease (19, 39). Our finding that GcvB also promotes MBR in the Tet assay (Fig. 1E) indicates that GcvB promotes MBR other than or in addition to by allowing a σE response and other than or in addition to by promoting spontaneous DNA breakage. Because ΔgcvB cells showed a greater reduction of MBR in the Lac assay than in the Tet assay, it remains possible that GcvB plays two roles: one that affects spontaneous DNA breakage and another DSB-/σE-independent role.
FIG 2.
ΔgcvB cells show normal recombinational DNA repair and activation of the SOS and σE stress responses. (A) UV sensitivity assay. Values are means ± SEM from three experiments with two cultures for each experiment. The UV resistance of ΔgcvB cells indicates homologous recombinational (HR) repair and SOS response proficiency. ΔrecA HR- and SOS-defective cells and SOS-uninducible lexA(Ind−) cells are UV sensitive (P < 0.02 compared with the value for the WT; Student's two-tailed paired t test for each UV dose). The mild UV sensitivity of rpoS-null cells was observed previously (105). The lack of UV sensitivity of ΔgcvB cells indicates that their σS response impairment (Fig. 3) is not as severe as in σS-null cells (Fig. 3B). From top to bottom, the strains are SMR20238, SMR4562, SMR10336, SMR820, and SMR5535. (B) ΔgcvB cells are SDS-EDTA resistant, indicating a functional σE response. Shown is a representative image of cultures spotted onto solid medium containing membrane-disrupting detergent (SDS) and EDTA at ∼300 CFU per spot (left two spots) and ∼30 CFU per spot (right two spots). SDS and EDTA retard growth of σE-response-defective ΔrpoE (88) and rpoE::Tn10dCam (31) mutant cells. Although rpoE is an essential gene (89), the ΔrpoE mutant is viable because of acquisition of compensatory extragenic “suppressor” mutations that permit viability (90). From top to bottom, the strains are SMR4562, SMR20238, MC4100, and SMR5236.
Decreased σS response but normal σS protein levels in cells that lack GcvB.
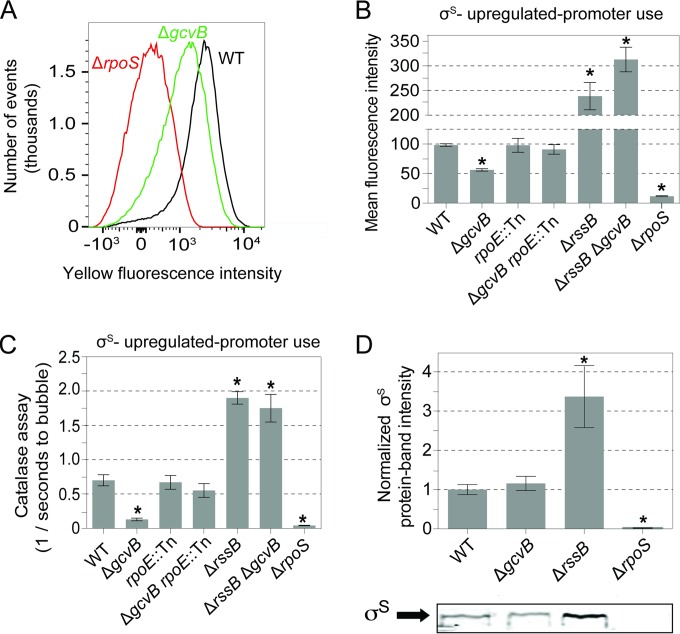
We found that ΔgcvB cells display reduced activity of σS-upregulated promoters in two assays (Fig. 3). First, cells defective for the σS response have decreased katE transcription and thus decreased catalase activity and a defect in metabolizing hydrogen peroxide (H2O2) (96). When a drop of hydrogen peroxide is placed on an E. coli colony, H2O2 is metabolized to H2O and O2, and bubbles appear on the colony. The rapidity of onset of bubbling indicates σS-upregulated promoter activity (96). For wild-type and ΔrpoS strains, bubbles appeared after 2 ± 0.3 and 22 ± 1 s, respectively (Fig. 3C, plotted as 1/time to bubbling). ΔgcvB cells take 11 ± 1 s (mean ± SEM) to produce bubbles, which is significantly different from the wild-type cells and not significantly different from the σS-null ΔrpoS strain data (Fig. 3C).
FIG 3.
Reduced σS-upregulated transcription, but not σS protein levels, in stationary-phase ΔgcvB cells and its dependence on the σE response. In all experiments, measurements and assays are from stationary-phase cells, grown under experimental conditions as for mutagenesis experiments. (A) Reduced σS-regulated promoter activity in ΔgcvB cells. A flow cytometric fluorescence assay of stationary-phase starved cultures shows σS-dependent yellow fluorescence (in arbitrary fluorescence units) from the yiaG-yfp σS response reporter gene (39). The results of a representative experiment are shown. (B) Quantification of mean fluorescence intensities per cell from five independent experiments. Fluorescence intensity is shown in arbitrary fluorescence units. Values are means ± SEM. The rpoE::Tn σE-response-defective mutation (31) restored σS response activity to ΔgcvB cells, indicating that the σS response reduction in ΔgcvB cells is σE response dependent. Asterisks indicate values that are significantly different from the value for the WT strain (P = 1 × 10−6 for the ΔgcvB strain, P = 4 × 10−8 for the ΔrssB strain, P = 9 × 10−8 for the ΔrssB ΔgcvB strain, and P = 2 × 10−10 for the ΔrpoS strain) by Student's two-tailed t test. The value for the ΔgcvB rpoE::Tn double mutant is significantly different from the value for the ΔgcvB single mutant (P = 4 × 10−3). There is no significant difference between the values for the ΔrssB mutant and ΔrssB ΔgcvB mutant (P = 0.56 by Student's two-tailed t test). From left to right, the strains are SMR10582, SMR21471, SMR22064, SMR22047, SMR12692, SMR21934, and SMR12661. (C) σE-response-dependent reduction of σS response activity in ΔgcvB cells by the catalase colony assay (Materials and Methods). Asterisks indicate values that are significantly different from the value for the WT strain (P = 8 × 10−5 for the ΔgcvB strain, P = 4 × 10−13 for the ΔrpoS strain, P = 2 × 10−4 for the ΔrssB strain, and P = 0.01 for the ΔrssB ΔgcvB strain. Values are means ± SEM from four experiments with five colonies per experiment. The strains are the same strains used in panel B. (D) Western blot analyses show σS protein levels in stationary phase unaffected by the ΔgcvB mutation. (Top) Results from three quantified immunoblots normalized to the WT value. Values are means ± SEM. Asterisks indicate values that are significantly different from the value for the WT strain (P = 1 × 10−3 for the ΔrpoS strain and P = 7 × 10−4 for the ΔrssB strain). (Bottom) Representative immunoblot. From left to right, the strains are SMR4562, SMR20238, SMR12566, and SMR10336.
Second, we measured activity of the σS-upregulated yiaG promoter using flow cytometry of cells carrying a chromosomal yiaG-yfp reporter gene (39), which exploits the σS specificity of the yiaG promoter (Fig. 3A). We found that ΔgcvB cells showed a significant 1.6-fold ± 0.08-fold decrease in mean yellow fluorescence intensity (per cell) compared with the WT control (Fig. 3B). The decreased production of yellow fluorescent protein (YFP) from PyiaG is not as great as in σS-null ΔrpoS cells (Fig. 3A and B), implying that σS-regulated promoter activity is reduced but not abolished in ΔgcvB cells. The reductions in expression of σS-upregulated genes in both assays were reversed by artificial upregulation of σS via deletion of rssB (Fig. 3B and C). RssB is a protein chaperone that brings σS to the ClpXP protease for degradation (97), such that its removal causes artificially high σS levels (97).
Although σS-upregulated promoter activity is reduced in ΔgcvB cells, we found that σS protein levels are not detectably reduced relative to isogenic gcvB+ (“wild-type”) cells. A representative Western blot is shown in Fig. 3D. Quantification from multiple Western blots shows that the relative levels of σS protein in ΔgcvB cells compared to wild-type cells are 1.1 ± 0.1 and 1.0 ± 0.1, respectively (Fig. 3D, wild-type normalized to 1.0, mean ± SEM from four experiments, the SEM on the WT is normalized proportionally to 1 to show the day-to-day variability in measurement). These data imply that GcvB does not regulate σS production or stability in stationary cells. We conclude that σS is present in ΔgcvB cells but that σS -upregulated promoter activity is decreased.
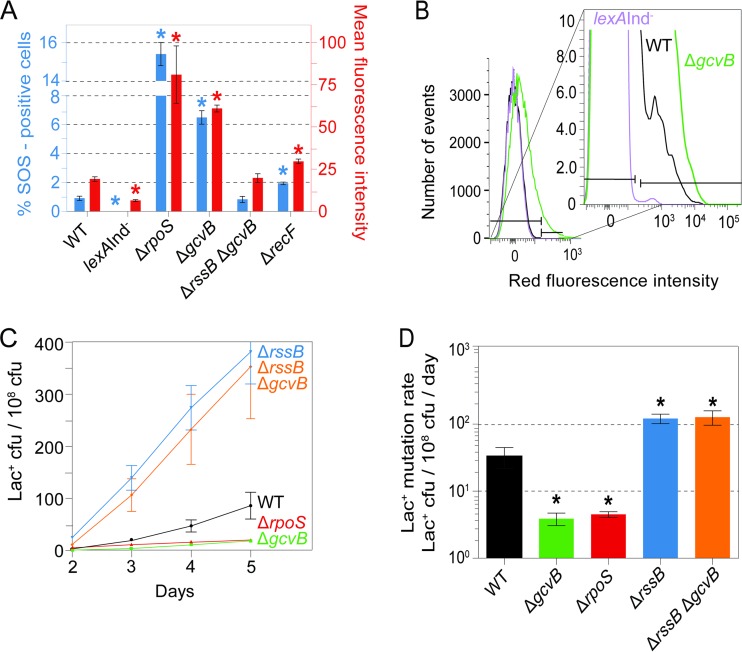
Mutagenesis and high spontaneous SOS induction in ΔgcvB cells are relieved by artificial upregulation of σS.
We observed previously that ΔrpoS cells, which lack a functional σS response, display increased spontaneous induction of the SOS response (98) (Fig. 4A) for unknown reasons. Perhaps damaged cellular components and/or high levels of reactive oxygen species, normally reduced by the σS response, cause DNA damage. We also found increased spontaneous SOS induction in ΔgcvB cells (Fig. 4A and B). We found that both the mutagenesis defect and the high spontaneous SOS response in ΔgcvB cells were reversed by deletion of rssB, which upregulates σS by reducing ClpXP-mediated proteolytic degradation of σS (97). Deletion of rssB increases the general stress response (97), and it also increases MBR (39). rssB deletion fully restored mutagenesis to ΔgcvB cells, elevated mutagenesis to the greater-than-wild-type levels seen in ΔrssB cells (Fig. 4C and D; see also Table S2 in the supplemental material), and ameliorated the ΔgcvB high-SOS phenotype (Fig. 4A). The data indicate that GcvB promotes MBR by allowing a robust σS response, such that in ΔgcvB cells, MBR is reduced via σS response deficiency.
FIG 4.
Artificial upregulation of σS substitutes for GcvB in mutagenesis and suppression of spontaneous SOS induction. (A) Increased spontaneous SOS response in ΔrpoS or ΔgcvB cells and its reversibility in ΔgcvB cells by the ΔrssB mutation, which promotes σS stability. SOS activity was measured by the method of Pennington and Rosenberg (82) as modified by Nehring et al. (83) by flow cytometry of strains with a chromosomal SOS-upregulated fluorescence reporter transgene, Δattλ::PsulAmCherry. This assay quantifies single cells with spontaneous DNA damage that triggers the SOS response (not spurious promoter firing, shown in reference 82). On the left-hand y axis, the percentage of SOS-positive cells quantifies the fraction of cells in a subpopulation with higher fluorescence than the main population. Gates (horizontal brackets shown in panel B) are set per Materials and Methods for the bimodal distribution in wild-type cells, also shown in panel B. The right-hand y axis shows the mean fluorescence intensity (in arbitrary fluorescence units per cell) of all single cells assayed. The mean fluorescence intensity reports on shifts that affect most of the cells in the population, as seen in ΔgcvB cells (shown also in panel B). The data imply that the σS response prevents some spontaneous DNA damage. Asterisks indicate values that are significantly different from the value for the WT strain [P = 2 × 10−6 for the lexA(Ind−) strain, P = 4 × 10−10 for the ΔgcvB strain, P = 5 × 10−4 for the ΔrpoS strain, and P = 5 × 10−6 for the ΔrecF strain]. Values are means ± SEM from four experiments. From left to right, the strains are SMR21641, SMR17966, SMR21725, SMR21553, SMR21933, and SMR21728. (B) Representative flow cytometry analysis of spontaneous SOS induction showing the small cell subpopulation with spontaneous SOS-activating DNA damage, as described previously (82), in arbitrary fluorescence units. (C) The ΔgcvB MBR-deficient phenotype is suppressed by ΔrssB, a mutation that increases σS protein levels by reducing σS proteolytic degradation (97), and increases MBR as shown here and shown previously (39, 98). The results of a representative experiment are shown. (D) Quantification of mutation rates from three experiments. Values are means ± SEM. Asterisks indicate values that are significantly different from the value for the WT strain (P = 0.05 for the ΔgcvB strain, P = 0.05 for the ΔrpoS strain, P = 0.006 for the ΔrssB strain, and P = 0.04 for the ΔgcvB ΔrssB strain) by Student's two-tailed t test. From left to right, the strains are SMR4562, SMR20238, SMR10336, SMR12566, and SMR21361.
Blocking the σE membrane stress response restores mutagenesis and σS response activity to ΔgcvB cells.
We tested the hypothesis that reduced transcription of σS-upregulated genes and reduced MBR in ΔgcvB cells (Fig. 1, 3, and 4) might result from hyperinduction of the σE membrane stress response. The σE response promotes MBR by promoting spontaneous DNA DSBs by as yet unknown means (31). Although σE is an essential protein, encoded by rpoE, we previously isolated a separation-of-function rpoE mutation, rpoE2072::Tn10dCam (rpoE::Tn), that retains the σE essential function but is incapable of mounting a σE stress response (31). Cells carrying this special rpoE::Tn mutation show a ≥10-fold reduction in spontaneous MBR but no reduction if DSBs are supplied by I-SceI double-strand endonuclease (31). Because the Tet MBR assay measures mutagenesis activated by I-SceI cleavage at a nearby tet gene (19), MBR in the Tet assay is σE independent. We used the Tet MBR assay to test whether a σE response interferes with σS-dependent promoter activity and thus MBR. We found that the rpoE::Tn mutation restored normal levels of MBR to ΔgcvB cells (Fig. 1E, ΔgcvB rpoE::Tn compared with WT) and normal σS-dependent-promoter activity measured with flow cytometric and colony/catalase assays (Fig. 3B and C, ΔgcvB rpoE::Tn compared with WT). We conclude that the σE role in the membrane stress response underlies the defects in σS-dependent promoter activity and MBR in ΔgcvB cells. This might result from hyperinduction of the σE response in ΔgcvB cells, in which excessive σE molecules titrate RNA polymerase (RNAP), decreasing normal levels of σS-RNAP enzyme in favor of the σE-RNAP enzyme, thus reducing the σS response. Whereas rpoE::Tn increased σS-dependent promoter activity and MBR in ΔgcvB cells (Fig. 1E and 3B and C), rpoE::Tn did not increase σS protein levels in ΔgcvB cells (see Fig. S2 in the supplemental material), supporting the σE/σS competition hypothesis.
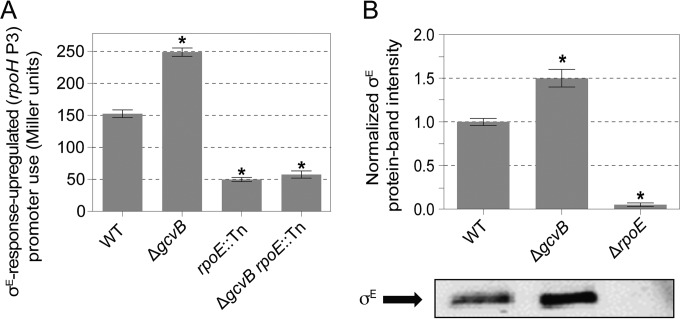
σE activity and protein levels are increased in ΔgcvB cells.
We measured σE activity using the rpoHP3-lacZ promoter fusion reporter gene (92), which reports on σE stress response-dependent transcription as β-galactosidase activity (92). We found a significant 1.6-fold ± 0.04-fold increase in σE-dependent β-galactosidase activity in ΔgcvB cells relative to their isogenic gcvB+ parent cells (Fig. 5A). We also found that σE protein levels were increased 1.5-fold ± 0.1-fold in ΔgcvB cells (mean ± SEM for four Western blots [a representative blot shown in Fig. 5B]). The data imply that normally, GcvB plays a role that suppresses the σE stress response. GcvB might simply function in some way that promotes membrane integrity by, for example, maintaining proper levels of membrane proteins such that upon its removal, the σE response is induced.
FIG 5.
Hyperactivation of the σE membrane stress response in cells that lack sRNA GcvB. (A) Increased activation of the σE-dependent rpoH P3 promoter in stationary-phase ΔgcvB cells measured as β-galactosidase activity from the rpoHP3-lacZ fusion gene. Values are means ± SEM from three experiments. Asterisks indicate values that are significantly different from the value for the WT strain (P = 4 × 10−3 for the ΔgcvB strain, P = 2 × 10−3 for the rpoE::Tn strain, and P = 3 × 10−3 for the ΔgcvB rpoE::Tn strain). From left to right, the strains are SMR8841, SMR22216, SMR8842, and SMR22310. (B) σE protein levels are increased in cells that lack GcvB. (Top) Quantified Western immunoblots normalized to WT bands. Values are means ± SEM from three experiments. Asterisks indicate values that are significantly different from the value for the WT strain (P = 7 × 10−4 for the ΔgcvB strain and P = 5 × 10−6 for the ΔrpoE strain). (Bottom) Representative immunoblot. From left to right, the strains are SMR4562, SMR20238, and MC4100.
The σE response is required for acid resistance.
We attempted to test whether the acid sensitivity of the E. coli ΔgcvB mutant (65) might, like MBR, result from hyperinduction of the σE response. Surprisingly, we found that cells that carry the rpoE2072::Tn allele, which blocks the σE stress response without impairing the σE essential function (31), were also acid sensitive, and more acid sensitive than ΔgcvB cells were (Fig. 6). We conclude that a functional σE response is required for acid resistance. This is not incompatible with the possibility that the acid sensitivity of the E. coli ΔgcvB cells (65) results from a hyperinduced σE response, implying that both too much of a σE response and too little result in acid sensitivity. Further experiments would be needed to establish that specific mechanism.
FIG 6.

The σE response is required for acid resistance. The rpoE2072::Tn mutation, which ablates the σE response without affecting the σE essential function (31), caused strong acid sensitivity, indicating that the σE response is required for acid resistance. Because ΔgcvB cells display a hyper-σE response (Fig. 5) and rpoE::Tn cells have no σE response (31), the data suggest that both too much and too little σE response activity may result in acid sensitivity and that the σE response must occur at just the right level for resistance. The OD600 of cultures after 3-h recovery from a 30-min acid challenge was measured. Values are means ± SEM from three experiments with three independent cultures for each strain. Asterisks indicate values that are significantly different from the value for the wild-type (WT) control strain (P = 0.01 for the ΔgcvB strain, P = 5 × 10−8 for the rpoE::Tn strain, and P = 3 × 10−9 for the ΔgcvB rpoE::Tn strain) by Student's two-tailed paired t test. From left to right, isogenic strains are SMR4562, SMR20238, SMR5236, and SMR21996.
DISCUSSION
We found that GcvB, an sRNA client of Hfq, promotes mutagenic break repair (MBR) during starvation stress in E. coli in two different MBR assays (Fig. 1) and presented evidence that it does so by allowing a robust σS general/starvation stress response, apparently by suppressing the σE membrane stress response. First, MBR proficiency was restored to ΔgcvB cells by artificial upregulation of σS using an rssB mutation, which blocks σS protein degradation (Fig. 4C and D), implying that the ΔgcvB MBR defect is caused by failure to mount a robust σS response. Moreover, we found that (i) cells that lack GcvB showed decreased σS-regulated gene expression in two assays (Fig. 3A to C) and that (ii) the σS-dependent reduction in gene expression was also reversible by σS upregulation (Fig. 3B and C) but that (iii) σS protein levels were not reduced in ΔgcvB cells (Fig. 3D).
Second, blocking the σE membrane stress response, but not the σE essential function, with an rpoE::Tn separation-of-function mutation (31) restored σS-regulated promoter activity (Fig. 3B and C) and MBR (Fig. 1E) to ΔgcvB cells without increasing σS protein levels (see Fig. S2 in the supplemental material). The data imply that a too-active σE response inhibits the σS response and MBR, possibly via sigma factor competition for RNA polymerase (RNAP) (model below). Supporting this possibility, σE protein levels and activity were abnormally high in ΔgcvB cells (Fig. 5). We suggest that GcvB may promote membrane integrity and thus avert membrane stress response hyperinduction.
Our data demonstrate that GcvB promotes stress-inducible MBR and suggest a possible function for GcvB in E. coli in membrane maintenance. These data reinforce the importance and delicacy of stress response regulation of mutagenesis (39; for recent reviews, see references 2, 6, and 10).
Model in which sigma factor competition reduces MBR in ΔgcvB mutant cells.
In Fig. 7 we outline a possible model in which sigma factor competition for RNAP could promote the delicate regulatory balance between the σS and σE responses and thus modulate MBR in response to the presence or absence of GcvB sRNA. Sigma factor competition for RNAP has been implicated in shifting transcriptional patterns under various circumstances, and it is affected by both the number of sigma factors present in the cell and their affinity for RNAP (99, 100). σE has higher affinity for RNAP than σS has (101). Several bacterial sRNAs promote membrane integrity (67) (see the introduction), making it a reasonable hypothesis that GcvB suppresses the σE response (Fig. 5) because it, too, is needed for integrity of the cell membrane. GcvB would thus indirectly suppress hyper-σE response induction. We suggest that normally GcvB promotes membrane integrity and that when σS is induced during starvation, σS-RNAP complexes can form, allowing activation of the σS response (Fig. 7B). We found previously that there is some σE response induction in E. coli under MBR starvation conditions (31), so we infer that normally during starvation both σS and σE responses are activated (Fig. 7B). We suggest that in ΔgcvB cells, membrane stress-promoted hyperinduction of σE blocks σS access to RNAP via competition (Fig. 7C). This model is supported by our findings that loss of σE response induction capability restores MBR and σS-regulated promoter activity to ΔgcvB cells (Fig. 1E and 3B and C) without increasing σS protein levels (see Fig. S2 in the supplemental material). This model might also explain some of the acid sensitivity reported for ΔgcvB cells (65). Perhaps a hyperinduced σE response contributes to σS response depression and acid sensitivity of ΔgcvB cells in addition to the reduced production of σS (a σS-LacZ fusion protein) observed in that study (65). We found that GcvB and a functional σE response both promote acid resistance (Fig. 6). The data suggest that both too much and too little σE response activity may result in sensitivity, and the data are not incompatible with the possibility that some of the acid sensitivity of E. coli ΔgcvB cells (65) results from a hyper-σE response. Other models are possible.
FIG 7.
Model in which σE competition for RNA polymerase reduces the σS response and mutagenic break repair in ΔgcvB mutant cells. The observed σE-dependent reductions of both MBR and σS-dependent gene expression in ΔgcvB cells could result from competition of σE with σS for RNA polymerase (RNAP) (gray arc shapes) in cells that lack GcvB. (B) We suggest that sRNA GcvB is required for normal membrane integrity and thus keeps the σE response low in σS-response-induced stationary cells. (C) In the absence of GcvB, excessive membrane stress is proposed to hyperactivate the σE response, producing increased σE-RNAP complexes, at the expense of σS-RNAP complexes. Reduced σS-RNAP complexes could cause a reduced general stress response, which causes MBR deficiency (18, 19, 27, 28). The pink and blue lines represent σS- and σE-dependent transcripts, respectively.
Which GcvB target gene(s) may affect membrane integrity under MBR starvation conditions is not known. A list of known and predicted targets of the GcvB sRNA is given in Table S3 in the supplemental material. An outer membrane protein gene, ompF, is an experimentally implicated GcvB target (102), and it might contribute to destabilization of the membrane when upregulated due to loss of GcvB. GcvB regulation of other genes on, and possibly not on, the lists in Table S3 might contribute to membrane integrity additionally or alternatively.
sRNAs play many and various roles in bacterial biology and across the tree of life, yet the functions of many sRNAs remain obscure, even in E. coli (68, 103, 104). GcvB is now implicated in membrane integrity and demonstrated to regulate mutagenesis. Other possible roles and the specific mechanism(s) of action of GcvB await future exploration.
Supplementary Material
ACKNOWLEDGMENTS
We thank Susan Gottesman for guidance throughout this study, including suggesting that the GcvB role in mutagenesis might be to promote membrane integrity, averting an extreme σE response, and so tilt σ factor competition for RNA polymerase in favor of σS. We thank Carol Gross for the gift of anti-σE antibody, J. Sederstrom for expert assistance in flow cytometry, and Susan Gottesman, P. J. Hastings, and anonymous reviewers for improving the manuscript.
Funding Statement
This work was supported by National Institutes of Health (NIH) grant R01-GM53158 (S.M.R.), NIH postdoctoral fellowship F32-GM095267 (R.L.F.), Cancer Prevention and Research Institute of Texas, Baylor College of Medicine Comprehensive Cancer Training Program Postdoctoral Fellowship RP160283 (D.M.F.), and the Cytometry and Cell Sorting Core at Baylor College of Medicine with funding from NIH grants P30-AI036211, S10 RR024574, and P30 CA125123 to the Dan L. Duncan Comprehensive Cancer Center.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00555-16.
REFERENCES
- 1.Rosenberg SM, Shee C, Frisch RL, Hastings PJ. 2012. Stress-induced mutation via DNA breaks in Escherichia coli: a molecular mechanism with implications for evolution and medicine. Bioessays 34:885–892. doi: 10.1002/bies.201200050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers E, Correa R, Barreto B, Bravo Núñez MA, Minnick PJ, Vera Cruz D, Xia J, Hastings PJ, Rosenberg SM. 2016. Double-strand-break repair, mutagenesis, and stress, p 185–195. In de Bruijn FJ. (ed), Stress and environmental control of gene expression and adaptation in bacteria. Wiley & Sons Publisher, Hoboken, NJ. [Google Scholar]
- 3.Foster PL. 2007. Stress-induced mutagenesis in bacteria. Crit Rev Biochem Mol Biol 42:373–397. doi: 10.1080/10409230701648494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robleto EA, Yasbin R, Ross C, Pedraza-Reyes M. 2007. Stationary phase mutagenesis in B. subtilis: a paradigm to study genetic diversity programs in cells under stress. Crit Rev Biochem Mol Biol 42:327–339. doi: 10.1080/10409230701597717. [DOI] [PubMed] [Google Scholar]
- 5.Saint-Ruf C, Pesut J, Sopta M, Matic I. 2007. Causes and consequences of DNA repair activity modulation during stationary phase in Escherichia coli. Crit Rev Biochem Mol Biol 42:259–270. doi: 10.1080/10409230701495599. [DOI] [PubMed] [Google Scholar]
- 6.Rogers E, Bravo Núñez MA, Hastings PJ, Rosenberg SM. 2016. How a large gene network couples mutagenic DNA break repair to stress in Escherichia coli, p 570–576. de Bruijn FJ. (ed), Stress and environmental control of gene expression and adaptation in bacteria. Wiley & Sons Publisher, Hoboken, NJ. [Google Scholar]
- 7.Galhardo RS, Hastings PJ, Rosenberg SM. 2007. Mutation as a stress response and the regulation of evolvability. Crit Rev Biochem Mol Biol 42:399–435. doi: 10.1080/10409230701648502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shor E, Fox CA, Broach JR. 2013. The yeast environmental stress response regulates mutagenesis induced by proteotoxic stress. PLoS Genet 9:e1003680. doi: 10.1371/journal.pgen.1003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bindra RS, Crosby ME, Glazer PM. 2007. Regulation of DNA repair in hypoxic cancer cells. Cancer Metastasis Rev 26:249–260. doi: 10.1007/s10555-007-9061-3. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald DM, Hastings PJ, Rosenberg SM. 16 September 2016. Stress-induced mutagenesis: implications in cancer and drug resistance. Annu Rev Cancer Biol 1:6.1–6.22. doi: 10.1146/annurev-cancerbio-050216-121919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ram Y, Hadany L. 2012. The evolution of stress-induced hypermutation in asexual populations. Evolution 66:2315–2328. doi: 10.1111/j.1558-5646.2012.01576.x. [DOI] [PubMed] [Google Scholar]
- 12.Ram Y, Hadany L. 2014. Stress-induced mutagenesis and complex adaptation. Proc Biol Sci 281:20141025. doi: 10.1098/rspb.2014.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjedov I, Tenaillon O, Gerard B, Souza V, Denamur E, Radman M, Taddei F, Matic I. 2003. Stress-induced mutagenesis in bacteria. Science 300:1404–1409. doi: 10.1126/science.1082240. [DOI] [PubMed] [Google Scholar]
- 14.Cirz RT, Chin JK, Andes DR, de Crecy-Lagard V, Craig WA, Romesberg FE. 2005. Inhibition of mutation and combating the evolution of antibiotic resistance. PLoS Biol 3:e176. doi: 10.1371/journal.pbio.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cirz RT, Romesberg FE. 2007. Controlling mutation: intervening in evolution as a therapeutic strategy. Crit Rev Biochem Mol Biol 42:341–354. doi: 10.1080/10409230701597741. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez A, Laureti L, Crussard S, Abida H, Rodriguez-Rojas A, Blazquez J, Baharoglu Z, Mazel D, Darfeuille F, Vogel J, Matic I. 2013. Beta-lactam antibiotics promote bacterial mutagenesis via an RpoS-mediated reduction in replication fidelity. Nat Commun 4:1610. doi: 10.1038/ncomms2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forche A, Abbey D, Pisithkul T, Weinzierl MA, Ringstrom T, Bruck D, Petersen K, Berman J. 2011. Stress alters rates and types of loss of heterozygosity in Candida albicans. mBio 2:e00129–11. doi: 10.1128/mBio.00129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponder RG, Fonville NC, Rosenberg SM. 2005. A switch from high-fidelity to error-prone DNA double-strand break repair underlies stress-induced mutation. Mol Cell 19:791–804. doi: 10.1016/j.molcel.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Shee C, Gibson JL, Darrow MC, Gonzalez C, Rosenberg SM. 2011. Impact of a stress-inducible switch to mutagenic repair of DNA breaks on mutation in Escherichia coli. Proc Natl Acad Sci U S A 108:13659–13664. doi: 10.1073/pnas.1104681108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shee C, Gibson JL, Rosenberg SM. 2012. Two mechanisms produce mutation hotspots at DNA breaks in Escherichia coli. Cell Rep 2:714–721. doi: 10.1016/j.celrep.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris RS, Longerich S, Rosenberg SM. 1994. Recombination in adaptive mutation. Science 264:258–260. doi: 10.1126/science.8146657. [DOI] [PubMed] [Google Scholar]
- 22.Foster PL, Trimarchi JM, Maurer RA. 1996. Two enzymes, both of which process recombination intermediates, have opposite effects on adaptive mutation in Escherichia coli. Genetics 142:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris RS, Ross KJ, Rosenberg SM. 1996. Opposing roles of the Holliday junction processing systems of Escherichia coli in recombination-dependent adaptive mutation. Genetics 142:681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKenzie GJ, Lee PL, Lombardo MJ, Hastings PJ, Rosenberg SM. 2001. SOS mutator DNA polymerase IV functions in adaptive mutation and not adaptive amplification. Mol Cell 7:571–579. doi: 10.1016/S1097-2765(01)00204-0. [DOI] [PubMed] [Google Scholar]
- 25.Petrosino JF, Galhardo RS, Morales LD, Rosenberg SM. 2009. Stress-induced beta-lactam antibiotic resistance mutation and sequences of stationary-phase mutations in the Escherichia coli chromosome. J Bacteriol 191:5881–5889. doi: 10.1128/JB.00732-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frisch RL, Su Y, Thornton PC, Gibson JL, Rosenberg SM, Hastings PJ. 2010. Separate DNA Pol II- and Pol IV-dependent pathways of stress-induced mutation during double-strand-break repair in Escherichia coli are controlled by RpoS. J Bacteriol 192:4694–4700. doi: 10.1128/JB.00570-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Layton JC, Foster PL. 2003. Error-prone DNA polymerase IV is controlled by the stress-response sigma factor, RpoS, in Escherichia coli. Mol Microbiol 50:549–561. doi: 10.1046/j.1365-2958.2003.03704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lombardo M-J, Aponyi I, Rosenberg SM. 2004. General stress response regulator RpoS in adaptive mutation and amplification in Escherichia coli. Genetics 166:669–680. doi: 10.1534/genetics.166.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cairns J, Foster PL. 1991. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics 128:695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKenzie GJ, Harris RS, Lee PL, Rosenberg SM. 2000. The SOS response regulates adaptive mutation. Proc Natl Acad Sci U S A 97:6646–6651. doi: 10.1073/pnas.120161797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibson JL, Lombardo MJ, Thornton PC, Hu KH, Galhardo RS, Beadle B, Habib A, Magner DB, Frost LS, Herman C, Hastings PJ, Rosenberg SM. 2010. The sigma(E) stress response is required for stress-induced mutation and amplification in Escherichia coli. Mol Microbiol 77:415–430. doi: 10.1111/j.1365-2958.2010.07213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wimberly H, Shee C, Thornton PC, Sivaramakrishnan P, Rosenberg SM, Hastings PJ. 2013. R-loops and nicks initiate DNA breakage and genome instability in non-growing Escherichia coli. Nat Commun 4:2115. doi: 10.1038/ncomms3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galhardo RS, Do R, Yamada M, Friedberg EC, Hastings PJ, Nohmi T, Rosenberg SM. 2009. DinB upregulation is the sole role of the SOS response in stress-induced mutagenesis in Escherichia coli. Genetics 182:55–68. doi: 10.1534/genetics.109.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Battesti A, Majdalani N, Gottesman S. 2011. The RpoS-mediated general stress response in Escherichia coli. Annu Rev Microbiol 65:189–213. doi: 10.1146/annurev-micro-090110-102946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hengge-Aronis R. 2002. Signal transduction and regulatory mechanisms involved in control of the sigma(S) (RpoS) subunit of RNA polymerase. Microbiol Mol Biol Rev 66:373–395. doi: 10.1128/MMBR.66.3.373-395.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Motamedi MR, Szigety SK, Rosenberg SM. 1999. Double-strand-break repair recombination in Escherichia coli: physical evidence for a DNA replication mechanism in vivo. Genes Dev 13:2889–2903. doi: 10.1101/gad.13.21.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pomerantz RT, Kurth I, Goodman MF, O'Donnell ME. 2013. Preferential D-loop extension by a translesion DNA polymerase underlies error-prone recombination. Nat Struct Mol Biol 20:748–755. doi: 10.1038/nsmb.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maharjan R, Ferenci T. 2015. Mutational signatures indicative of environmental stress in bacteria. Mol Biol Evol 32:380–391. doi: 10.1093/molbev/msu306. [DOI] [PubMed] [Google Scholar]
- 39.Al Mamun AA, Lombardo MJ, Shee C, Lisewski AM, Gonzalez C, Lin D, Nehring RB, Saint-Ruf C, Gibson JL, Frisch RL, Lichtarge O, Hastings PJ, Rosenberg SM. 2012. Identity and function of a large gene network underlying mutagenic repair of DNA breaks. Science 338:1344–1348. doi: 10.1126/science.1226683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kajitani M, Ishihama A. 1991. Identification and sequence determination of the host factor gene for bacteriophage Q beta. Nucleic Acids Res 19:1063–1066. doi: 10.1093/nar/19.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brennan RG, Link TM. 2007. Hfq structure, function and ligand binding. Curr Opin Microbiol 10:125–133. doi: 10.1016/j.mib.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Chao Y, Vogel J. 2010. The role of Hfq in bacterial pathogens. Curr Opin Microbiol 13:24–33. doi: 10.1016/j.mib.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Sittka A, Pfeiffer V, Tedin K, Vogel J. 2007. The RNA chaperone Hfq is essential for the virulence of Salmonella typhimurium. Mol Microbiol 63:193–217. doi: 10.1111/j.1365-2958.2006.05489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simonsen KT, Nielsen G, Bjerrum JV, Kruse T, Kallipolitis BH, Moller-Jensen J. 2011. A role for the RNA chaperone Hfq in controlling adherent-invasive Escherichia coli colonization and virulence. PLoS One 6:e16387. doi: 10.1371/journal.pone.0016387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christiansen JK, Larsen MH, Ingmer H, Sogaard-Andersen L, Kallipolitis BH. 2004. The RNA-binding protein Hfq of Listeria monocytogenes: role in stress tolerance and virulence. J Bacteriol 186:3355–3362. doi: 10.1128/JB.186.11.3355-3362.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geng J, Song Y, Yang L, Feng Y, Qiu Y, Li G, Guo J, Bi Y, Qu Y, Wang W, Wang X, Guo Z, Yang R, Han Y. 2009. Involvement of the post-transcriptional regulator Hfq in Yersinia pestis virulence. PLoS One 4:e6213. doi: 10.1371/journal.pone.0006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding Y, Davis BM, Waldor MK. 2004. Hfq is essential for Vibrio cholerae virulence and downregulates sigma expression. Mol Microbiol 53:345–354. doi: 10.1111/j.1365-2958.2004.04142.x. [DOI] [PubMed] [Google Scholar]
- 48.Sonnleitner E, Hagens S, Rosenau F, Wilhelm S, Habel A, Jager KE, Blasi U. 2003. Reduced virulence of a hfq mutant of Pseudomonas aeruginosa O1. Microb Pathog 35:217–228. doi: 10.1016/S0882-4010(03)00149-9. [DOI] [PubMed] [Google Scholar]
- 49.Wang MC, Chien HF, Tsai YL, Liu MC, Liaw SJ. 2014. The RNA chaperone Hfq is involved in stress tolerance and virulence in uropathogenic Proteus mirabilis. PLoS One 9:e85626. doi: 10.1371/journal.pone.0085626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valentin-Hansen P, Eriksen M, Udesen C. 2004. The bacterial Sm-like protein Hfq: a key player in RNA transactions. Mol Microbiol 51:1525–1533. doi: 10.1111/j.1365-2958.2003.03935.x. [DOI] [PubMed] [Google Scholar]
- 51.Vogel J, Luisi BF. 2011. Hfq and its constellation of RNA. Nat Rev Microbiol 9:578–589. doi: 10.1038/nrmicro2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Lay N, Schu DJ, Gottesman S. 2013. Bacterial small RNA-based negative regulation: Hfq and its accomplices. J Biol Chem 288:7996–8003. doi: 10.1074/jbc.R112.441386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frohlich KS, Vogel J. 2009. Activation of gene expression by small RNA. Curr Opin Microbiol 12:674–682. doi: 10.1016/j.mib.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 54.McCullen CA, Benhammou JN, Majdalani N, Gottesman S. 2010. Mechanism of positive regulation by DsrA and RprA small noncoding RNAs: pairing increases translation and protects rpoS mRNA from degradation. J Bacteriol 192:5559–5571. doi: 10.1128/JB.00464-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen S, Lesnik EA, Hall TA, Sampath R, Griffey RH, Ecker DJ, Blyn LB. 2002. A bioinformatics based approach to discover small RNA genes in the Escherichia coli genome. Biosystems 65:157–177. doi: 10.1016/S0303-2647(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 56.Wassarman KM, Repoila F, Rosenow C, Storz G, Gottesman S. 2001. Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev 15:1637–1651. doi: 10.1101/gad.901001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogel J, Bartels V, Tang TH, Churakov G, Slagter-Jager JG, Huttenhofer A, Wagner EG. 2003. RNomics in Escherichia coli detects new sRNA species and indicates parallel transcriptional output in bacteria. Nucleic Acids Res 31:6435–6443. doi: 10.1093/nar/gkg867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Argaman L, Hershberg R, Vogel J, Bejerano G, Wagner EG, Margalit H, Altuvia S. 2001. Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr Biol 11:941–950. doi: 10.1016/S0960-9822(01)00270-6. [DOI] [PubMed] [Google Scholar]
- 59.Zhang A, Wassarman KM, Rosenow C, Tjaden BC, Storz G, Gottesman S. 2003. Global analysis of small RNA and mRNA targets of Hfq. Mol Microbiol 50:1111–1124. doi: 10.1046/j.1365-2958.2003.03734.x. [DOI] [PubMed] [Google Scholar]
- 60.Urbanowski ML, Stauffer LT, Stauffer GV. 2000. The gcvB gene encodes a small untranslated RNA involved in expression of the dipeptide and oligopeptide transport systems in Escherichia coli. Mol Microbiol 37:856–868. doi: 10.1046/j.1365-2958.2000.02051.x. [DOI] [PubMed] [Google Scholar]
- 61.Pulvermacher SC, Stauffer LT, Stauffer GV. 2009. Role of the Escherichia coli Hfq protein in GcvB regulation of oppA and dppA mRNAs. Microbiology 155:115–123. doi: 10.1099/mic.0.023432-0. [DOI] [PubMed] [Google Scholar]
- 62.Pulvermacher SC, Stauffer LT, Stauffer GV. 2009. The small RNA GcvB regulates sstT mRNA expression in Escherichia coli. J Bacteriol 191:238–248. doi: 10.1128/JB.00915-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pulvermacher SC, Stauffer LT, Stauffer GV. 2009. Role of the sRNA GcvB in regulation of cycA in Escherichia coli. Microbiology 155:106–114. doi: 10.1099/mic.0.023598-0. [DOI] [PubMed] [Google Scholar]
- 64.Sharma CM, Papenfort K, Pernitzsch SR, Mollenkopf HJ, Hinton JC, Vogel J. 2011. Pervasive post-transcriptional control of genes involved in amino acid metabolism by the Hfq-dependent GcvB small RNA. Mol Microbiol 81:1144–1165. doi: 10.1111/j.1365-2958.2011.07751.x. [DOI] [PubMed] [Google Scholar]
- 65.Jin Y, Watt RM, Danchin A, Huang JD. 2009. Small noncoding RNA GcvB is a novel regulator of acid resistance in Escherichia coli. BMC Genomics 10:165. doi: 10.1186/1471-2164-10-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vogel J, Papenfort K. 2006. Small non-coding RNAs and the bacterial outer membrane. Curr Opin Microbiol 9:605–611. doi: 10.1016/j.mib.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 67.Guillier M, Gottesman S, Storz G. 2006. Modulating the outer membrane with small RNAs. Genes Dev 20:2338–2348. doi: 10.1101/gad.1457506. [DOI] [PubMed] [Google Scholar]
- 68.Gottesman S, Storz G. 2011. Bacterial small RNA regulators: versatile roles and rapidly evolving variations. Cold Spring Harb Perspect Biol 3:a003798. doi: 10.1101/cshperspect.a003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tjaden B, Saxena RM, Stolyar S, Haynor DR, Kolker E, Rosenow C. 2002. Transcriptome analysis of Escherichia coli using high-density oligonucleotide probe arrays. Nucleic Acids Res 30:3732–3738. doi: 10.1093/nar/gkf505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Papenfort K, Pfeiffer V, Mika F, Lucchini S, Hinton JC, Vogel J. 2006. SigmaE-dependent small RNAs of Salmonella respond to membrane stress by accelerating global omp mRNA decay. Mol Microbiol 62:1674–1688. doi: 10.1111/j.1365-2958.2006.05524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thompson KM, Rhodius VA, Gottesman S. 2007. SigmaE regulates and is regulated by a small RNA in Escherichia coli. J Bacteriol 189:4243–4256. doi: 10.1128/JB.00020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johansen J, Rasmussen AA, Overgaard M, Valentin-Hansen P. 2006. Conserved small non-coding RNAs that belong to the sigmaE regulon: role in down-regulation of outer membrane proteins. J Mol Biol 364:1–8. doi: 10.1016/j.jmb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 73.Rasmussen AA, Eriksen M, Gilany K, Udesen C, Franch T, Petersen C, Valentin-Hansen P. 2005. Regulation of ompA mRNA stability: the role of a small regulatory RNA in growth phase-dependent control. Mol Microbiol 58:1421–1429. doi: 10.1111/j.1365-2958.2005.04911.x. [DOI] [PubMed] [Google Scholar]
- 74.Udekwu KI, Darfeuille F, Vogel J, Reimegard J, Holmqvist E, Wagner EG. 2005. Hfq-dependent regulation of OmpA synthesis is mediated by an antisense RNA. Genes Dev 19:2355–2366. doi: 10.1101/gad.354405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coornaert A, Lu A, Mandin P, Springer M, Gottesman S, Guillier M. 2010. MicA sRNA links the PhoP regulon to cell envelope stress. Mol Microbiol 76:467–479. doi: 10.1111/j.1365-2958.2010.07115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gogol EB, Rhodius VA, Papenfort K, Vogel J, Gross CA. 2011. Small RNAs endow a transcriptional activator with essential repressor functions for single-tier control of a global stress regulon. Proc Natl Acad Sci U S A 108:12875–12880. doi: 10.1073/pnas.1109379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo MS, Updegrove TB, Gogol EB, Shabalina SA, Gross CA, Storz G. 2014. MicL, a new sigmaE-dependent sRNA, combats envelope stress by repressing synthesis of Lpp, the major outer membrane lipoprotein. Genes Dev 28:1620–1634. doi: 10.1101/gad.243485.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guisbert E, Rhodius VA, Ahuja N, Witkin E, Gross CA. 2007. Hfq modulates the sigmaE-mediated envelope stress response and the sigma32-mediated cytoplasmic stress response in Escherichia coli. J Bacteriol 189:1963–1973. doi: 10.1128/JB.01243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller JH. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Plainview, NY. [Google Scholar]
- 81.Torkelson J, Harris RS, Lombardo MJ, Nagendran J, Thulin C, Rosenberg SM. 1997. Genome-wide hypermutation in a subpopulation of stationary-phase cells underlies recombination-dependent adaptive mutation. EMBO J 16:3303–3311. doi: 10.1093/emboj/16.11.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pennington JM, Rosenberg SM. 2007. Spontaneous DNA breakage in single living Escherichia coli cells. Nat Genet 39:797–802. doi: 10.1038/ng2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nehring RB, Gu F, Lin HY, Gibson JL, Blythe MJ, Wilson R, Bravo Nunez MA, Hastings PJ, Louis EJ, Frisch RL, Hu JC, Rosenberg SM. 2016. An ultra-dense library resource for rapid deconvolution of mutations that cause phenotypes in Escherichia coli. Nucleic Acids Res 44:e41. doi: 10.1093/nar/gkv1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nguyen LH, Jensen DB, Thompson NE, Gentry DR, Burgess RR. 1993. In vitro functional characterization of overproduced Escherichia coli katF/rpoS gene product. Biochemistry 32:11112–11117. doi: 10.1021/bi00092a021. [DOI] [PubMed] [Google Scholar]
- 85.Ades SE, Connolly LE, Alba BM, Gross CA. 1999. The Escherichia coli sigma(E)-dependent extracytoplasmic stress response is controlled by the regulated proteolysis of an anti-sigma factor. Genes Dev 13:2449–2461. doi: 10.1101/gad.13.18.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McKenzie GJ, Lombardo MJ, Rosenberg SM. 1998. Recombination-dependent mutation in Escherichia coli occurs in stationary phase. Genetics 149:1163–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosenberg SM. 2001. Evolving responsively: adaptive mutation. Nat Rev Genet 2:504–515. doi: 10.1038/35080556. [DOI] [PubMed] [Google Scholar]
- 88.Rouviere PE, De Las Penas A, Mecsas J, Lu CZ, Rudd KE, Gross CA. 1995. rpoE, the gene encoding the second heat-shock sigma factor, sigma E, in Escherichia coli. EMBO J 14:1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.De Las Penas A, Connolly L, Gross CA. 1997. SigmaE is an essential sigma factor in Escherichia coli. J Bacteriol 179:6862–6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alba BM, Gross CA. 2004. Regulation of the Escherichia coli sigma-dependent envelope stress response. Mol Microbiol 52:613–619. doi: 10.1111/j.1365-2958.2003.03982.x. [DOI] [PubMed] [Google Scholar]
- 91.Loewen PC. 1984. Isolation of catalase-deficient Escherichia coli mutants and genetic mapping of katE, a locus that affects catalase activity. J Bacteriol 157:622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mecsas J, Rouviere PE, Erickson JW, Donohue TJ, Gross CA. 1993. The activity of sigma E, an Escherichia coli heat-inducible sigma-factor, is modulated by expression of outer membrane proteins. Genes Dev 7:2618–2628. doi: 10.1101/gad.7.12b.2618. [DOI] [PubMed] [Google Scholar]
- 93.Akerlund T, Nordstrom K, Bernander R. 1995. Analysis of cell size and DNA content in exponentially growing and stationary-phase batch cultures of Escherichia coli. J Bacteriol 177:6791–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Radman M. 1975. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis, p 355–367. In Hanawalt P, Setlow RB (ed), Molecular mechanisms for repair of DNA. Plenum Press, New York, NY. [DOI] [PubMed] [Google Scholar]
- 95.Witkin EM. 1976. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev 40:869–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vijayakumar SR, Kirchhof MG, Patten CL, Schellhorn HE. 2004. RpoS-regulated genes of Escherichia coli identified by random lacZ fusion mutagenesis. J Bacteriol 186:8499–8507. doi: 10.1128/JB.186.24.8499-8507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hryckowian AJ, Battesti A, Lemke JJ, Meyer ZC, Welch RA. 2014. IraL is an RssB anti-adaptor that stabilizes RpoS during logarithmic phase growth in Escherichia coli and Shigella. mBio 5:e01043–14. doi: 10.1128/mBio.01043-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gonzalez C. 2009. A hypermutable cell subpopulation in stress-induced mutagenesis. PhD thesis Baylor College of Medicine, Houston, TX. [Google Scholar]
- 99.Malik S, Zalenskaya K, Goldfarb A. 1987. Competition between sigma factors for core RNA polymerase. Nucleic Acids Res 15:8521–8530. doi: 10.1093/nar/15.20.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kolesky S, Ouhammouch M, Brody EN, Geiduschek EP. 1999. Sigma competition: the contest between bacteriophage T4 middle and late transcription. J Mol Biol 291:267–281. doi: 10.1006/jmbi.1999.2953. [DOI] [PubMed] [Google Scholar]
- 101.Maeda H, Fujita N, Ishihama A. 2000. Competition among seven Escherichia coli sigma subunits: relative binding affinities to the core RNA polymerase. Nucleic Acids Res 28:3497–3503. doi: 10.1093/nar/28.18.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Melamed S, Peer A, Faigenbaum-Romm R, Gatt YE, Reiss N, Bar A, Altuvia Y, Argaman L, Margalit H. 2016. Global mapping of small RNA-target interactions in bacteria. Mol Cell 63:884–897. doi: 10.1016/j.molcel.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Waters LS, Storz G. 2009. Regulatory RNAs in bacteria. Cell 136:615–628. doi: 10.1016/j.cell.2009.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Livny J, Waldor MK. 2007. Identification of small RNAs in diverse bacterial species. Curr Opin Microbiol 10:96–101. doi: 10.1016/j.mib.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 105.Ivanova AB, Glinsky GV, Eisenstark A. 1997. Role of rpoS regulon in resistance to oxidative stress and near-UV radiation in delta oxyR suppressor mutants of Escherichia coli. Free Radic Biol Med 23:627–636. doi: 10.1016/S0891-5849(97)00013-0. [DOI] [PubMed] [Google Scholar]
- 106.Ades SE, Grigorova IL, Gross CA. 2003. Regulation of the alternative sigma factor E during initiation, adaptation, and shutoff of the extracytoplasmic heat shock response in Escherichia coli. J Bacteriol 185:2512–2519. doi: 10.1128/JB.185.8.2512-2519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dri AM, Rouviere-Yaniv J, Moreau PL. 1991. Inhibition of cell division in hupA hupB mutant bacteria lacking HU protein. J Bacteriol 173:2852–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dutreix M, Moreau PL, Bailone A, Galibert F, Battista JR, Walker GC, Devoret R. 1989. New recA mutations that dissociate the various RecA protein activities in Escherichia coli provide evidence for an additional role for RecA protein in UV mutagenesis. J Bacteriol 171:2415–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006–0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cherepanov PP, Wackernagel W. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9–14. doi: 10.1016/0378-1119(95)00193-A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.