Abstract
Fibroblast growth factor (FGF) signaling is an essential regulator of lens epithelial cell proliferation and survival, as well as lens fiber cell differentiation. However, the identities of these FGF factors, their source tissue and the genes that regulate their synthesis are unknown. We have found that Chx10-Cre;Lhx2lox/lox mice, which selectively lack Lhx2 expression in neuroretina from E10.5, showed an early arrest in lens fiber development along with severe microphthalmia. These mutant animals showed reduced expression of multiple neuroretina-expressed FGFs and canonical FGF-regulated genes in neuroretina. When FGF expression was genetically restored in Lhx2-deficient neuroretina of Chx10-Cre;Lhx2lox/lox mice, we observed a partial but nonetheless substantial rescue of the defects in lens cell proliferation, survival and fiber differentiation. These data demonstrate that neuroretinal expression of Lhx2 and neuroretina-derived FGF factors are crucial for lens fiber development in vivo.
KEY WORDS: FGF, Genetics, Lens, Mouse, Retina, Transcription factor
Summary: The LIM homeodomain transcription factor Lhx2 regulates FGF3, FGF9 and FGF15 and is essential for lens cell proliferation, survival and differentiation in mice.
INTRODUCTION
Vertebrate lens development has long been a model system for studying the role of inductive signaling in tissue patterning and cell specification (Gunhaga, 2011). During embryogenesis, the surface ectoderm adjacent to the optic vesicle thickens and invaginates to give rise to the lens vesicle. Subsequently, cells in the anterior lens vesicle become a monolayer of lens epithelial cells, while cells in the posterior half of the vesicle elongate and differentiate to form primary lens fibers, which fill the lens vesicle. After this distinctive architecture has been established, lens growth continues in a spatially restricted manner maintaining lens polarity. Lens epithelial cells proliferate in the germinative zone, the region just above the lens equator. The progeny then migrate or are displaced below the equator into the transitional zone where they exit the cell cycle, elongate and differentiate into secondary fiber cells (Lovicu and Robinson, 2004; McAvoy et al., 1999).
Previous work has shown that the differentiation of lens epithelial cells into fiber cells is dependent on diffusible signals from the neuroretina (Coulombre and Coulombre, 1963; McAvoy and Fernon, 1984; Yamamoto, 1976). However, the identity of these retinal-derived factors remains unclear. Multiple growth factors have been implicated in control of lens fiber differentiation (Lovicu et al., 2011; Lovicu and McAvoy, 2005; Wang et al., 2010). FGFs have long been a top candidate among these, following landmark studies demonstrating that treatment of cultured lens epithelial cells with FGF1 and FGF2 was sufficient to induce differentiation into lens fiber cells (Chamberlain and McAvoy, 1987, 1989). Subsequent studies have provided compelling evidence that FGFs promote lens epithelial cell proliferation and fiber cell differentiation in a dose-dependent manner (Lovicu and McAvoy, 2005; McAvoy and Chamberlain, 1989; Schulz et al., 1993). Although this stimulated speculation that FGFs might be the long-sought neuroretina-derived signal, targeted mutation studies carried out over recent decades have yet to identify any specific FGFs that are necessary, either individually or in combination, for lens cell proliferation or differentiation (Robinson, 2006). More recently, targeted deletion of three FGF receptor genes (Fgfr1, Fgfr2 and Fgfr3) in lens pit/vesicle have been shown to disrupt lens cell survival and fiber cell differentiation. However, individual or pairwise deletion of FGF receptor genes still resulted in grossly normal lens formation (Zhao et al., 2008). This functional redundancy of FGF receptors suggests that multiple FGFs act in concert to promote lens fiber differentiation. The identities and source of these factors, however, remain obscure.
Lhx2 is a LIM-homeodomain transcription factor essential for eye development. A previous study has shown that lens development arrests at, or just prior to, lens placode formation in Lhx2 germline mutant animals. FGF15, BMP4, BMP7 and phosphorylated SMAD1, SMAD5 and SMAD8 (pSMAD1/5/8), an indicator of BMP signaling, are all downregulated in the Lhx2−/− optic neuroepithelium and lens-forming region of the surface ectoderm. Restoration of BMP4 and BMP7 led to upregulation of FGF15 in the optic neuroepithelium and induction of the lens placode marker Sox2 in the surface ectoderm of Lhx2−/− animals. But BMP treatment does not rescue the morphology of Lhx2−/− mutant optic vesicle or surface ectoderm. These findings suggest that BMP signaling partially mediates Lhx2-dependent regulation of optic vesicle and lens vesicle induction (Yun et al., 2009). However, whether Lhx2 is required for lens development beyond lens placode formation is not known.
We recently observed that selective deletion of Lhx2 in neuroretina after lens placode induction led to severe microphthalmia and a near-total loss of the expression of many retinal progenitor-specific genes (Roy et al., 2013). Interestingly, we also noticed severe disruptions of lens development in these animals. This suggested that Lhx2 may regulate the expression of neuroretina-derived diffusible factors that are necessary for lens development beyond the lens placode stage. To address this possibility, we set out to identify candidate factors whose expression was disrupted in Lhx2-deficient neuroretina, and to determine whether restoring the activity of these factors was sufficient to rescue lens cell proliferation and fiber differentiation.
RESULTS
Selective deletion of Lhx2 in embryonic neuroretina disrupts lens development
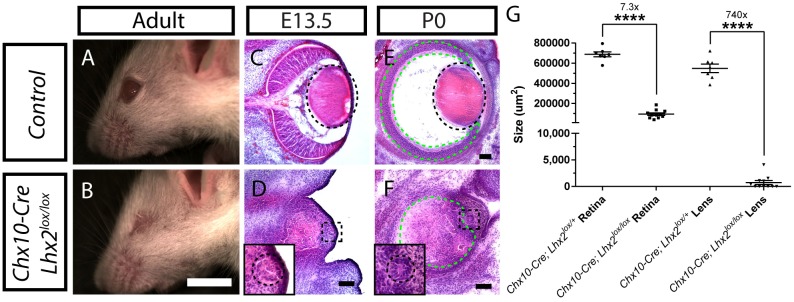
We observed that selective deletion of Lhx2 in neuroretina using Chx10-Cre (Vsx2-Cre) resulted in severe microphthalmia in adult mice (Fig. 1A,B). Close examination of Chx10-Cre;Lhx2lox/lox eye sections revealed severe defects in lens development at embryonic day (E)13.5, as well as postnatal day (P)0.5 (Fig. 1C-F). The lens was either missing altogether, or detectable as only a vestigial lumen rudiment (Fig. 1D,F). Lens size was reduced to a substantially greater extent (740-fold smaller) than retinal size (7.3-fold smaller) at P0.5 (Fig. 1G). Analysis of Chx10-Cre;Ai9(Rosa26LSL−tdTomato) mice confirmed previous reports (Rowan and Cepko, 2004) that this Cre line is selectively active in neuroretina and not active in other ocular tissues, including lens (Fig. S1A-D). Immunostaining for Lhx2 showed selective deletion of Lhx2 in neuroretina starting at E10.5 with the expression almost entirely lost in neuroretina by E11.5 (Fig. S1E-L). Even though some Lhx2 staining was observed in both control and mutant lenses, signals were never detected in the nuclei of lens cells. Moreover, Lhx2 mRNA was also never detected in either control or Chx10-Cre;Lhx2lox/lox lenses (insets in Fig. 2B-G). As previous work has shown that Lhx2 expression is absent in the lens at all stages of ocular development (Hägglund et al., 2011), we conclude that the Lhx2 signal observed in the lens here represents non-specific background staining. Furthermore, as the Cre is active only in the neuroretina of our animals, we conclude that the disruption of lens development observed in our Chx10-Cre;Lhx2lox/lox animals must have resulted from disrupted signaling by secreted retinal-derived factors.
Fig. 1.
Loss of function of Lhx2 in neuroretina led to microphthalmia and lens development defects. (A,B) Lateral view of control (A) and Chx10-Cre;Lhx2lox/lox animals (B) indicating microphthalmia in Chx10-Cre;Lhx2lox/lox animals. (C-F) Hematoxylin and Eosin staining of eye sections from E13.5 (C,D) and P0.5 (E,F) of control (C,E) and Chx10-Cre;Lhx2lox/lox (D,F) mice. Dotted green and black outlines mark the retinas and lenses, respectively (C-F) and insets (D,F) are digital zooms of the boxed regions. (G) Graph indicating average neuroretinal area and lens area in sections of control and Chx10-Cre;Lhx2lox/lox mice at P0.5. Data were analyzed using unpaired two-tailed t-test; n=7 for Chx10-Cre;Lhx2lox/+; n=11 for Chx10-Cre;Lhx2lox/lox; ****P<0.0001. Scale bars: 5 mm in A,B; 100 µm in C-F.
Fig. 2.
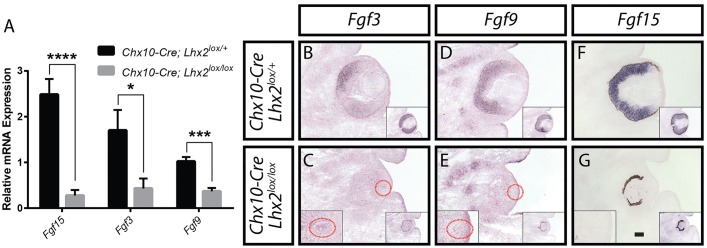
Fgf3, Fgf9 and Fgf15 were downregulated in Chx10-Cre;Lhx2lox/lox retinas. (A) Real-time quantitative PCR analysis of Fgf3, Fgf9 and Fgf15 mRNA expression levels in Chx10-Cre;Lhx2lox/lox retinas compared with Chx10-Cre;Lhx2lox/+ controls at E13.5. Data represent mean normalized to Gapdh values±s.e.m. Data were analyzed using unpaired two-tailed t-test; n=3; *P<0.05; ***P<0.001; ****P<0.0001. (B-G) In situ hybridization of Fgf3, Fgf9 and Fgf15 mRNA expression levels in Chx10-Cre;Lhx2lox/+ (B,D,F) and Chx10-Cre;Lhx2lox/lox (C,E,G) eyes at E13.5. (C,E) Dotted red circles mark the lenses shown at higher magnification in the left-hand insets. (B-G) Lens Lhx2 expression in adjacent sections is shown in right-hand insets. Lhx2 expression in RPE is maintained and outlines the neural retina in unpigmented animals (right-hand insets in C,E). Scale bar: 100 µm.
FGF signaling is downregulated in Lhx2-deficient eyes
To identify candidate retinal-derived factors whose expression was altered in Lhx2-deficient neuroretina, we analyzed previously reported microarray data obtained from retinas of control and Chx10-Cre;Lhx2lox/lox mice at E13.5 (Roy et al., 2013; Accession number GSE87707). These data indicated that multiple different FGFs and FGF-regulated genes showed altered expression in Chx10-Cre;Lhx2lox/lox retina. Fgf3, Fgf9 and Fgf15 – all of which have previously been reported to be expressed in embryonic neuroretinal progenitors (Colvin et al., 1999; Kurose et al., 2004; Wilkinson et al., 1989) – were dramatically downregulated (−5.0-, −3.2- and −11.9-fold, respectively). The FGF-regulated genes Etv5 and Spry2 were also downregulated (−11.4- and −2.2-fold, respectively), whereas the FGF receptor gene Fgfr3 was upregulated (3.1-fold). Loss of Fgf3, Fgf9 and Fgf15 expression in Chx10-Cre;Lhx2lox/lox retinas at E13.5 was confirmed using qRT-PCR and in situ hybridization analysis (Fig. 2). In situ hybridization analysis at E13.5 showed a reduction in the expression of the FGF-regulated genes Etv5, Etv1 and Spry2 in the lenses of Chx10-Cre;Lhx2lox/lox mice (Fig. 3I,K,M,O,Q,S). Taken together, these data suggested that loss of expression of multiple different FGF genes in Chx10-Cre;Lhx2lox/lox retinas resulted in a global loss of FGF signaling in the lenses.
Fig. 3.
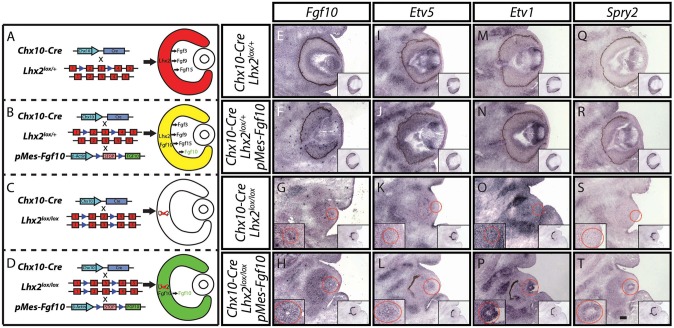
Cre-mediated induction of Fgf10 expression in Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 retinas restored expression of FGF-regulated genes in lens. (A-D) Mouse genetics and anticipated retinal-derived FGF expression for (A) Chx10-Cre;Lhx2lox/+, (B) Chx10-Cre;Lhx2lox/+; pMes-Fgf10, (C) Chx10-Cre;Lhx2lox/lox and (D) Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 eyes. (E-T) In situ hybridization demonstrates Fgf10 induction in neuroretina (E-H), and induction of expression of FGF-regulated genes, including Etv5 (I-L), Etv1 (M-P) and Spry2 (Q-T). Right-hand insets indicate Lhx2 expression in adjacent sections. Lhx2 expression in RPE is maintained and outlines the neural retina in unpigmented animals (insets in G,K,O,S,H,T). Dotted red circles mark the lenses shown at higher magnification in the left-hand insets (G-T). Scale bar: 100 µm.
Forced expression of FGF10 in neuroretina induces FGF-regulated genes in the lens
As FGFs typically signal through multiple different FGF receptors (Ornitz and Itoh, 2015), and previous work had shown considerable redundancy in FGF receptor action in control of lens fiber development (Lovicu and Overbeek, 1998; Zhao et al., 2008), we hypothesized that selectively activating the expression of any individual FGF in Lhx2-deficient retina might rescue lens development. We accomplished this by using pMes-Fgf10 transgenic mice, a previously described line in which Cre-dependent excision of a transcriptional stop cassette leads to expression of full-length mouse Fgf10 under the control of the chick β-actin promoter (Song et al., 2013). Using this line, we aimed to induce Fgf10 expression selectively in neuroretina of both control and Chx10-Cre;Lhx2lox/lox mice (Fig. 3B,D). We confirmed that Fgf10 expression could be robustly and selectively induced in neuroretina of E13.5 Chx10-Cre;Lhx2lox/+;pMes-Fgf10 as well as Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 mice (Fig. S2A; Fig. 3F,H). Furthermore, we observed that the FGF target genes Etv5, Etv1 and Spry2 were substantially upregulated in lenses of Chx10-Cre;Lhx2lox/+;pMes-Fgf10 mice relative to Chx10-Cre;Lhx2lox/+ controls (Fig. 3). Strikingly, we also observed robust induction of these same FGF target genes in the lenses of Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 mice relative to Chx10-Cre;Lhx2lox/lox mutants (Fig. 3). By E17.5, Fgf10 expression in Chx10-Cre;Lhx2lox/+;pMes-Fgf10 mice was absent from the central retinal, and became restricted to a small subset of cells at the neuroretinal periphery (Fig. S3C). By contrast, Fgf10 expression was still seen in Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 mice at both E17.5 and P0.5 (Fig. S3E,J).
Retinal overexpression of Fgf10 leads to the persistence of lens stalk
Overexpression of Fgf10 on a control background did not lead to any gross defects in lens development. The morphology and marker expression of Chx10-Cre;Lhx2lox/+;pMes-Fgf10 lenses were comparable with those of Chx10-Cre;Lhx2lox/+ controls (Figs 4, 5; Fig. S5). However, Chx10-Cre;Lhx2lox/+;pMes-Fgf10 lenses were slightly but significantly larger than control lenses at all time points examined (Fig. S2B). In addition, the anterior pole of the lens remained tethered to the surface ectoderm (Fig. S4B,D,F,H) and this persistent lens stalk led to the development of corneal opacification in some adult mice (Fig. S4J).
Fig. 4.
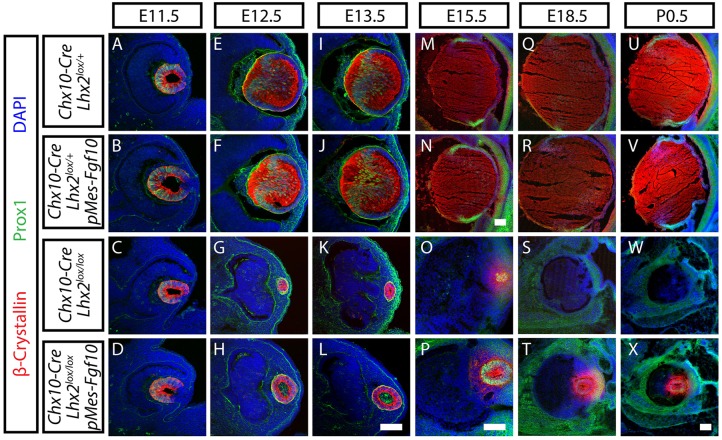
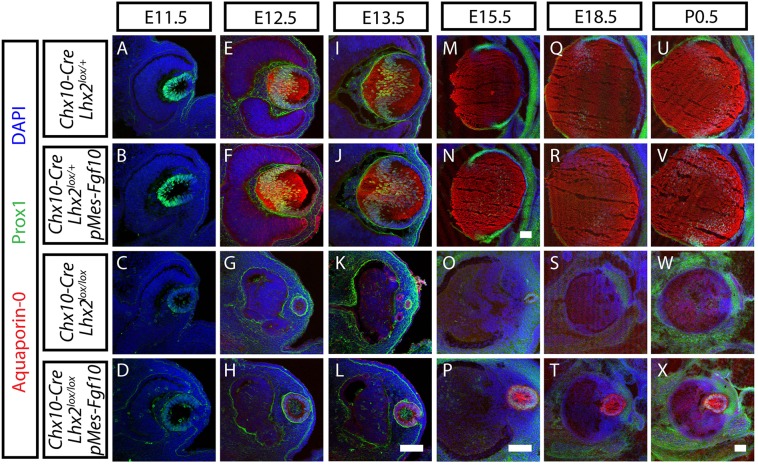
Overexpression of Fgf10 in Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 animals rescued lens fiber development. Developmental time-course of immunohistochemical staining for Prox1 (green) and β-crystallin (red) in lenses at E11.5 (A-D), E12.5 (E-H), E13.5 (I-L), E15.5 (M-P), E18.5 (Q-T) and P0.5 (U-X) of Chx10-Cre;Lhx2lox/+ (A,E,I,M,Q,U), Chx10-Cre;Lhx2lox/+;pMes-Fgf10 (B,F,J,N,R,V), Chx10-Cre;Lhx2lox/lox (C,G,K,O,S,W) and Chx10-Cre;Lhx2lox/lox; pMes-Fgf10 (D,H,L,P,T,X) animals . Nuclei are counterstained with DAPI (blue). Scale bars: 100 µm.
Fig. 5.
Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 animals expressed the mature lens fiber marker Aquaporin-0. Developmental time-course of immunohistochemistry for Prox1 (green) and Aquaporin-0 (red) expression in lenses at E11.5 (A-D), E12.5 (E-H), E13.5 (I-L), E15.5 (M-P), E18.5 (Q-T) and P0.5 (U-X) of Chx10-Cre;Lhx2lox/+ (A,E,I,M,Q,U), Chx10-Cre;Lhx2lox/+;pMes-Fgf10 (B,F,J,N,R,V), Chx10-Cre;Lhx2lox/lox (C,G,K,O,S,W) and Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 (D,H,L,P,T,X) animals. Nuclei are counterstained with DAPI (blue). Scale bars: in N, 100 µm for M,N; in L, 100 µm for A-L; in P, 100 µm for O,P; in X, 100 µm for Q-X.
Forced expression of FGF10 in Lhx2-deficient neuroretina rescues lens fiber differentiation
To investigate whether restoration of retinal FGF expression was able to rescue lens development in Lhx2 knockout animals, we conducted a detailed characterization of the expression of lens markers at multiple time points. At E11.5, prior to the formation of distinct lens fiber cells, no clear difference in lens size or the expression of Prox1 or β-crystallin was observed among Chx10-Cre;Lhx2lox/+, Chx10-Cre;Lhx2lox/lox and Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 mice (Fig. S2B; Fig. 4A-D). This may reflect that fact that Lhx2 deletion from neuroretina is not complete until E11.5, thus allowing lens development to proceed normally prior to this point. However, by E12.5, after the onset of lens fiber differentiation, a dramatic reduction in lens size was seen in both Chx10-Cre;Lhx2lox/lox and Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 relative to Chx10-Cre;Lhx2lox/+ mice (Fig. S2B). However, lens size in Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 mice was significantly larger than that of Chx10-Cre;Lhx2lox/lox mice, with Prox1-positive differentiating lens fiber cells observed only in Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 lenses (Fig. 4G,H). This difference in lens size and lens fiber differentiation became more prominent as development proceeded. By E15.5, Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 mice showed well-defined lenses filled with β-crystallin-positive lens fiber cells (Fig. 4P). By P0.5, more than half of all Chx10-Cre;Lhx2lox/lox mice no longer had a visible lens rudiment. When a lens rudiment was detectable, it was extremely small (Fig. 4W; Fig. S2B). However, all Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 mice examined at P0.5 showed well-defined, though often small, lenses that expressed Prox1 and β-crystallin. Comparisons of eyes for the presence or absence of lenses in Chx10-Cre;Lhx2lox/lox and Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 mice at P0.5 indicated that the maintenance of lenses from FGF10 overexpression in Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 mice was statistically significant (Fig. S2C). Interestingly, at no point did overexpression of Fgf10 rescue defects in neuroretinal size (Fig. S2D). Expression of β-crystallin in lens epithelial cells through P0.5 was observed in lens epithelial cells of both Chx10-Cre;Lhx2lox/lox and Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 mice (Fig. 4G,H,K,P,T,X). By contrast, β-crystallin expression was restricted to lens fiber cells in Chx10-Cre;Lhx2lox/+ controls and Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 by E12.5 (Fig. 4E,F).
To further characterize the rescue of lens development observed following neuroretinal-specific overexpression of Fgf10 in Lhx2-deficient mice, we analyzed the expression of additional lens markers, a selective marker for lens epithelial cells, E-cadherin, and two markers for lens fiber cells, N-cadherin and Aquaporin-0 (originally known as main intrinsic polypeptide, MIP) (Xu et al., 2002; Yancey et al., 1988). We observed that Chx10-Cre;Lhx2lox/lox animals initially formed E-cadherin-positive lens vesicle but failed to differentiate to form lens fiber cells that are positive for N-cadherin or Aquaporin-0 at E12.5 (Fig. S5G; Fig. 5G). As development progressed, these E-cadherin-positive lens vesicles became smaller and were no longer detectable in most animals by E18.5 (Fig. S5S). By contrast, Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 mice continued to express E-cadherin robustly at E13.5, and begin to show a progressive increase in N-cadherin expression from this point through P0.5 (Fig. S5L,P,T,X). We also observed that by E15.5, Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 mice showed robust expression of Aquaporin-0, a mature lens fiber cell marker, although the onset of Aquaporin-0 expression was delayed relative to controls (Fig. 5). However, the Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 rescue lens failed to develop normal lens polarity. E-cadherin and Prox1-positive cells persisted in the posterior pole of the lens at all time points examined (Figs 4, 5; Fig. S5).
FGF10 overexpression in Lhx2-deficient neuroretina promotes lens cell proliferation and survival
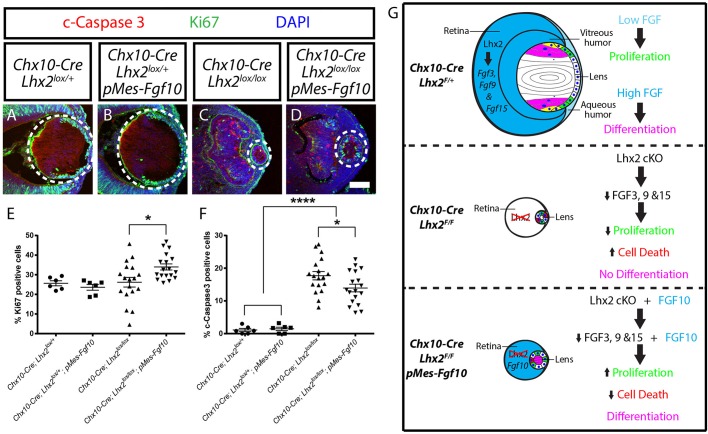
Exogenous FGFs have been reported to both promote the proliferation and survival of lens epithelial cells, in addition to driving lens fiber cell differentiation. To determine whether lens epithelial cell proliferation and survival were altered by overexpression of Fgf10, we examined the expression of Ki67 (Mki67 – Mouse Genome Informatics) and activated caspase 3 (c-caspase 3), respectively, at E12.5 (Fig. 6). We observed a significant increase in lens epithelial cell proliferation in Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 relative to Chx10-Cre;Lhx2lox/lox mutant lens (Fig. 6E). Cell death was increased in both Chx10-Cre;Lhx2lox/lox mutants and Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 rescue mice relative to the Chx10-Cre;Lhx2lox/+ and Chx10-Cre;Lhx2lox/+;pMes-Fgf10 controls, but was decreased in rescue mice relative to mutants (Fig. 6F). We thus conclude that overexpression of Fgf10 in Lhx2-deficient neuroretina promotes lens cell proliferation, survival and differentiation.
Fig. 6.
Fgf10 overexpression rescued defects in lens cell proliferation and cell death. (A-D) Immunostaining of E12.5 eye sections for Ki67 (green) and activated caspase 3 (c-Caspase3, red). White dotted circles mark the lenses. Scale bars: 100 µm. (E) Graph indicating the percentage of Ki67-positive cells relative to all DAPI-positive cells in the lenses. (F) Graph indicating the percentage of c-Caspase3-positive cells relative to all DAPI-positive cells in the lenses. Data were analyzed using one-way ANOVA followed by Sidak's test; n=6 for Chx10-Cre;Lhx2lox/+ and Chx10-Cre;Lhx2lox/+;pMes-Fgf10; n=18 for Chx10-Cre;Lhx2lox/lox and Chx10-Cre;Lhx2lox/lox;pMes-Fgf10, *P<0.05; ****P<0.0001. Error bars indicate s.e.m. (G) An diagram of the findings of this study.
Loss of Lhx2 expression in neuroretina also disrupts BMP signaling in the lens
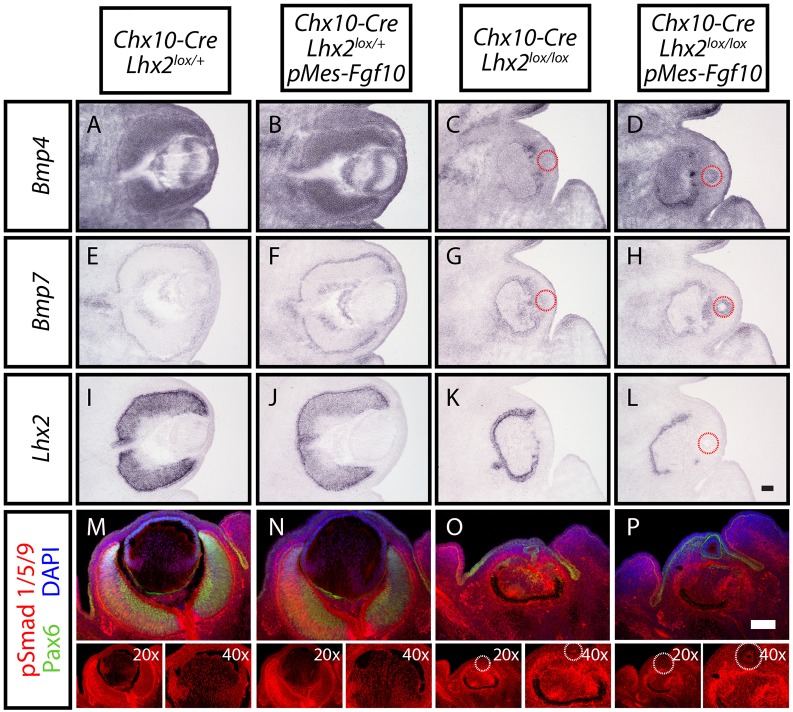
A previous study has shown that Lhx2 regulates lens placode formation through BMP signaling (Yun et al., 2009), whereas other studies have shown an essential role of BMP signaling in lens induction as well as fiber differentiation (Boswell et al., 2008; Furuta and Hogan, 1998; Jarrin et al., 2012; Murali et al., 2005; Wawersik et al., 1999). To investigate whether BMP signaling was altered in either Lhx2-deficient or Fgf10-overexpressing eyes, we performed in situ hybridization for Bmp4 and Bmp7, and immunostaining for phosphorylated SMAD1, SMAD5 and SMAD9 (pSmad1/5/9) at E13.5 (Fig. 7). In both Chx10-Cre;Lhx2lox/+ and Chx10-Cre;Lhx2lox/+;pMes-Fgf10 controls, broad Bmp4 expression was observed in neuroretina and RPE, and in lens epithelial and differentiating fiber cells, whereas more restricted Bmp7 expression was detected in a subset of neuroretinal cells, RPE and lens epithelial cells (Fig. 7A,B,E,F). Loss of neuroretinal Lhx2 expression led to a dramatic reduction of Bmp4 expression in Chx10-Cre;Lhx2lox/lox mutant neuroretina and lens, whereas forced Fgf10 expression partially rescued Bmp4 expression in Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 neuroretina and lens (Fig. 7C,D). Bmp7 expression was not altered in both Chx10-Cre;Lhx2lox/lox mutants and Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 rescue mice in agreement with a previous study by Hägglund et al. (2011) (Fig. 7G,H). Robust pSmad1/5/9 expression was observed in the neuroretina of both Chx10-Cre;Lhx2lox/+ and Chx10-Cre;Lhx2lox/+;pMes-Fgf10 controls in the dorsoventral gradient. The expression was also observed in the nuclei of differentiating lens fiber cells (Fig. 7M,N). By contrast, even though some pSmad1/5/9 staining was observed the neuroretina of both Chx10-Cre;Lhx2lox/lox and Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 animals, pSmad1/5/9 expression was not detected in the lens of both lines (Fig. 7O,P). We conclude that loss of neuroretinal Lhx2 disrupts Bmp4 expression and severely reduces BMP signaling in the lens.
Fig. 7.
Selective deletion of Lhx2 in neuroretina led to downregulation of BMP signaling in the neuroretina and lens. (A-L) In situ hybridization of Bmp4 (A-D), Bmp7 (E-H) and Lhx2 (I-L) mRNA expression levels in Chx10-Cre;Lhx2lox/+ (A,E,I), Chx10-Cre;Lhx2lox/+; pMes-Fgf10 (B,F,J), Chx10-Cre;Lhx2lox/lox (C,G,K) and Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 (D,H,L) eyes at E13.5. Dotted red circles mark the lenses (C,D,G,H,L). (M-P) Immunohistochemical staining for Pax6 (green) and pSmad1/5/9 (red) in lenses of Chx10-Cre;Lhx2lox/+ (M), Chx10-Cre;Lhx2lox/+;pMes-Fgf10 (N), Chx10-Cre;Lhx2lox/lox (O) and Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 animals (P) at E13.5. Nuclei are counterstained with DAPI (blue). The images of pSmad1/5/9 staining in single channel and at higher magnification are included underneath. Dotted white circles mark the lenses (O,P). Scale bars: 100 µm.
DISCUSSION
In this study, we showed that Lhx2 regulated the expression of Bmp4 and multiple FGF genes – Fgf3, Fgf9 and Fgf15 – in neuroretina during early stages of retinal neurogenesis. The downregulation of these retinal-derived FGFs in Lhx2 knockout animals contributed to severe defects in lens cell proliferation, survival and differentiation. Restoring FGF expression in neuroretina, however, led to a partial rescue of lens development defects, despite continued retinal development defects. These findings demonstrate the central role of Lhx2 in regulating lens development, and strongly suggest that FGFs do indeed constitute a major component of the long-sought retinal-derived signals that control lens maturation.
Previous work has shown that Lhx2 expression in the optic neuroepithelium is essential for lens placode specification (Yun et al., 2009). Our findings demonstrate the continued requirement of Lhx2 in the neuroretina past lens vesicle formation in regulating lens cell proliferation, survival and differentiation. The present study examined later stages of lens development than this earlier work, and identified a number of key differences in the relative contribution of BMP and FGF signaling. The previous study showed that loss of Lhx2 from optic vesicle severely disrupted Bmp4/7 expression, but only modestly reduced FGF signaling, as evidenced by continued expression of Fgf8, Etv5 (Erm) and phosphorylated ERK1/2 (pERK) in the optic vesicle and surface ectoderm. This same study showed that adding BMP7 or the combination of BMP4 and BMP7 to Lhx2−/− head cultures was sufficient to induce Sox2 expression in surface ectoderm (Yun et al., 2009). Other studies have also demonstrated an essential role for BMP4/7 signaling in early stages of lens development (Furuta and Hogan, 1998; Murali et al., 2005; Pandit et al., 2015; Wawersik et al., 1999). By contrast, our study indicated FGF signaling as perhaps the major mediator in Lhx2 regulation of lens maturation. Loss of neuroretinal Lhx2 led to reduced retinal Bmp4 expression, and severely disrupted BMP signaling in the lens; these changes were not rescued by overexpression of Fgf10 (Fig. 7). Nonetheless, lens fiber development was substantially, though not completely, rescued, suggesting that this process is at least partially independent of BMP signaling. This difference in the relative contribution of FGF and BMP signaling may reflect differences in the roles of neuroretina-derived BMP and FGF signaling during earlier and later stages of lens development. However, the lack of complete rescue may thus result from the well-documented synergistic role of BMP and FGF signaling in promoting lens fiber differentiation (Boswell et al., 2008; Boswell and Musil, 2015; Jarrin et al., 2012). The extent to which restoring BMP signaling can rescue lens development in Lhx2-deficient mice awaits further investigation.
Our finding that Lhx2 regulates neuroretinal FGFs is supported by a previous study from our lab that showed direct binding of Lhx2 to cis-regulatory regions of Fgf15 in neonatal mouse retina (de Melo et al., 2016). Although neuroretina-specific loss of function of other retinal progenitor-expressed transcription factors disrupts retinal progenitor cell proliferation and differentiation, and leads to microphthalmia (Burmeister et al., 1996; Marquardt et al., 2001; Taranova et al., 2006), only loss of Lhx2 significantly disrupts lens development. It is likely that these other mutant lines maintain neuroretinal expression of one or more FGFs, leading to normal lens development even in the face of a hypoplastic neuroretina.
Previous cell culture-based studies demonstrate that FGF signaling regulates multiple aspects of lens cell development and maturation (McAvoy and Chamberlain, 1989; Schulz et al., 1993). This study provides in vivo evidence for the importance of FGF signaling in lens cell proliferation, survival and differentiation. Our findings identify FGF3, FGF 9 and FGF15 as the three neuroretinal-derived FGFs that regulate lens development. Previous studies show that FGF3 can activate FGFR2b and FGFR1b; FGF9 can activate FGFR3c, FGFR2c, FGFR1c, FGFR3b and FGFR4; and FGF15 can activate FGFR1c, FGFR2c, FGFR3c and FGFR4 (Ornitz et al., 1996; Zhang et al., 2006). Neuroretinal expression of these three different FGFs, each of which can activate different subsets of FGF receptors, likely accounts for the observation that only Fgfr1/Fgfr2/Fgfr3 triple mutants produced severe defects in lens development (Zhao et al., 2008). This also likely accounts for the failure thus far to observe defects in lens fiber development in individual or pairwise combinations of targeted Fgf gene mutations (Robinson, 2006). Our ability to rescue lens fiber development by overexpression of Fgf10, which is expressed at only low levels in developing retina, agrees with previous findings of redundant roles for FGF3 and FGF10 in regulating cardiovascular and inner ear development (Alvarez et al., 2003; Urness et al., 2011).
Retinal overexpression of Fgf10 in animals that are not deficient for Lhx2 leads to the persistent close apposition of lens and surface ectoderm at the anterior lens pole (Fig. S3). This phenotype was also observed in a number of other mutant mouse lines, including a transgenic mouse line that expressed a dominant-negative FGF receptor in the presumptive lens ectoderm (Faber et al., 2001; Wolf et al., 2009; Zhao et al., 2012). It is possible that a tightly controlled level of FGF signaling activities is required for the lens vesicle to detach completely from the surface ectoderm. Alternatively, the spatial expression pattern of FGFs in neuroretina might influence lens vesicle detachment. At E13.5, when lens vesicle detachment from the surface ectoderm is complete, Fgf3, Fgf9 and Fgf15 are all predominantly expressed in central but not peripheral retina (Fig. 2). By contrast, Fgf10 at this stage is expressed across the whole retina in Chx10-Cre;Lhx2lox/+;pMes-Fgf10 animals (Fig. 3), and Fgf10 secretion from peripheral retina may interfere with the complete detachment of the lens vesicle.
The rescue of lens development that we observe following Fgf10 overexpression is incomplete, with lenses being quite small and lacking normal anterior-posterior polarity (Fig. 4; Figs S3 and 4). Moreover, lens differentiation is delayed, as seen by the persistent co-expression of β-crystallin and E-cadherin in lens epithelial cells (Fig. 4) and the delayed onset of Aquaporin-0 expression in lens fiber cells (Fig. 5). Several factors may account for this lack of complete rescue. First, the retina remains very small and severely disorganized. These persistent defects may physically limit lens growth. Furthermore, in normal eyes, different regions of the lens are exposed to different ocular environments, with the anterior part of the lens bathed in aqueous humor while the posterior part is in vitreous humor. As suggested by previous studies (Chamberlain and McAvoy, 1997), this distinct ocular architecture allows different parts of the lens to be exposed to different levels of FGFs, promoting the establishment of lens polarity. However, all regions of our rescued lenses were likely exposed to similar concentrations of FGF10. As FGFs show dose-dependent effects on proliferation of lens epithelial cells and their differentiation into lens fiber cells (McAvoy and Chamberlain, 1989; Schulz et al., 1993), the disruption of the normal gradient of FGF signaling in our rescue animals probably accounts for the persistence of lens epithelial cells at the posterior pole. In addition, as mentioned above, overexpression of Fgf10 in neuroretina did not rescue BMP signaling in the lens in Lhx2-deficient eyes. A schematic figure summarizing our results is shown in Fig. 6G.
MATERIALS AND METHODS
Animals
Lhx2lox/lox (Mangale et al., 2008) mice were obtained from Dr Edwin Monuki (University of California, Irvine, CA, USA). Chx10-Cre (Rowan and Cepko, 2004) mice were a gift from Dr Connie Cepko (Harvard, Boston, MA, USA). pMes-Fgf10 (Song et al., 2013) mice were generously provided by Dr Yang Chai (University of Southern California, Los Angeles, CA, USA). Ai9 (R26-CAG-lox-stop-lox-tdTomato) (Madisen et al., 2010) mice were a gift from Dr Xinzhong Dong (Johns Hopkins, Baltimore, MD, USA). Chx10-Cre;Lhx2lox/+, Chx10-Cre;Lhx2lox/+;pMes-Fgf10, Chx10-Cre;Lhx2lox/lox, Chx10-Cre;Lhx2lox/lox;pMes-Fgf10 and Chx10-Cre;Ai9 mice were generated by breeding with subsequent backcrossing. Immunohistochemistry or in situ hybridization was performed to confirm Cre-mediated inactivation of Lhx2. Only animals with successful inactivation of Lhx2 expression were included in the analysis. All experimental animal procedures were preapproved by the Johns Hopkins University Institutional Animal Care and Use Committee.
Quantitative RT-PCR
The cDNAs of E13.5 dissected retinas were synthesized from total RNAs using SuperScript III First-Strand Synthesis System (Invitrogen). Quantitative RT-PCR was performed using Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent) on a 7300 Real-Time PCR System (Applied Biosystems) following the manufacturers' recommended protocols. Primer sets for genes examined were as follows: fibroblast growth factor 3 (Fgf3) forward, TGCGCTACCAAGTACCACC and Fgf3 reverse, CACCGCAGTAATCTCCAGGAT; Fgf9 forward, ATGGCTCCCTTAGGTGAAGTT and Fgf9 reverse, TCCGCCTGAGAATCCCCTTT; Fgf10 forward, GCAGGCAAATGTATGTGGCAT and Fgf10 reverse, ATGTTTGGATCGTCATGGGGA; Fgf15 forward, GGTCCCTATGTCTCCAACTGC and Fgf15 reverse, CTTGATGGCAATCGTCTTCAGA; Gapdh forward, AGGTCGGTGTGAACGGATTTG and Gapdh reverse, TGTAGACCATGTAGTTGAGGTCA.
In situ hybridization
Chromogenic in situ hybridization experiments were performed as described previously with minor changes (Blackshaw et al., 2004). Briefly, fresh frozen sections were fixed by 4% paraformaldehyde and hybridized with digoxigenin-labeled probes at 70°C overnight. Excess probes were washed out, followed by rinsing in RNase buffer (0.5 M NaCl, 10 mM Tris pH 7.5, 5 mM EDTA) at 37°C and treatment with RNaseA diluted in RNase buffer to 2 μg/ml for 30 min at 37°C. Slides were then washed in RNase buffer, and 2×SSC prior to being blocked with sheep serum and overnight incubation in anti-digoxigenin antibodies conjugated to alkaline phosphatase (1:5000) at 4°C. Color was developed with combinations of the chromogens nitroblue tetrazolium (NBT) and 5-bromo, 4-chloro, 3-indolylphosphate (BCIP). Fgf3, Fgf9 and Fgf10 constructs were obtained from Marysia Placzek (University of Sheffield, UK). Bmp4 and Bmp7 probes were obtained from Dr Jane Dodd (Columbia University, New York, NY, USA) and Dr Jeanette C. Perron (St John's University, Jamaica, NY, USA). RNA probes were generated using the following EST sequences as templates: Etv5 (GenBank accession #BE996421), Etv1 (GenBank accession #AI852622), Spry2 (GenBank accession #BC095983) and Fgf15 (GenBank accession #BE952015). Lhx2 probe template was amplified from retinal cDNA. The sequences of the primers used for amplification were: forward, ACCATGCCGTCCATCAGC; reverse, GGCGTTGTAAGCTGCCAG.
Histology and immunohistochemistry
Haematoxylin and Eosin staining and fluorescence immunohistochemistry were performed as previously described (de Melo et al., 2012; Roy et al., 2013). The following primary antibodies were used: rabbit anti-Lhx2 [1:1000; generated for our lab by Covance (de Melo et al., 2012)], mouse anti-Pax6 (1:200; AB_528427, DSHB), rabbit anti-β-crystallin (1:500; a generous gift from Dr Jeremy Nathans, Johns Hopkins University, Baltimore, MD, USA), mouse anti-Prox1 (1:200; MAB5654, Millipore), rabbit anti-E-Cadherin (1:200; 3195, Cell Signaling), mouse anti-N-Cadherin (1:200; 333900, Novex), rabbit anti-Aquaporin 0 (1:200; AB3071, Millipore), rabbit anti-c-caspase 3 (1:200; 9664, Cell Signaling), mouse anti-Ki67 [1:200; 550609 (Clone B56), BD Pharmingen], rat anti-RFP (1:1000; ABIN334653, Chromotek) and rabbit anti-pSmad1/5/9 (1:300; 13820, Cell Signaling). Secondary antibodies used were as follows: FITC-conjugated donkey anti-mouse IgG (1:500; 715-095-150, Jackson ImmunoResearch), Alexa Fluor 488-conjugated goat anti-mouse IgG, Fcγ subclass 1 specific (1:500; 115-545-205, Jackson ImmunoResearch), FITC-conjugated goat anti-mouse IgG (subclasses 1+2a+2b+3), Fcγ fragment (1:500; 115-095-164, Jackson ImmunoResearch), Alexa Fluor 594-conjugated donkey anti-rabbit IgG (1:500; 711-585-152, Jackson ImmunoResearch) and Alexa Fluor 555-conjugated goat anti-rat IgG (1:500; A-21434, Invitrogen). Hematoxylin and Eosin sections were imaged using a Zeiss Axioskop 2 Mot Plus Microscope. Immunohistochemical sections were imaged on a Zeiss Meta 510 LSM confocal microscope.
Cell quantification
The total number of DAPI-, Ki67- and c-caspase 3-positive cells on each lens section was quantified and the percentage of Ki67- and cleaved caspase 3-positive cells was calculated (n=6 for Chx10-Cre;Lhx2lox/+ and Chx10-Cre;Lhx2lox/+;pMes-Fgf10; n=18 for Chx10-Cre;Lhx2lox/lox and Chx10-Cre;Lhx2lox/lox;pMes-Fgf10). Lens and retinal sizes were measured using ImageJ (n≥4 for E11.5; n≥9 for E12.5; n≥6 for E13.5; n≥7 for P0.5.) All cell counts and measurements were repeated blindly by a student researcher.
Statistical analysis
Data are presented as mean±s.e.m. Statistical analysis was performed using GraphPad Prism Software. Comparisons of means between two groups were evaluated using Student's t-test. Comparison of means among multiple groups was performed by one-way ANOVA followed by Tukey's and Sidak's multiple comparison tests. Categorical data was analyzed using Fisher's exact test. P<0.05 was considered to be statistically significant.
Acknowledgements
We thank Dr Marysia Placzek (University of Sheffield, UK) for Fgf3, Fgf9 and Fgf10 probes; Drs Carol Mason (Columbia University, New York, NY, USA) and Jeanette Perron (St Johns University, Jamaica, NY, USA) for Bmp4 and Bmp7 probes; and Dr Yang Chai (University of Southern California, Los Angeles, CA, USA) for the pMes-Fgf10 transgenic mouse line.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptulalization: T.T., J.d.M. and S.B.; Methodology: T.T., J.d.M., C.Z., B.S.C., F.J. and S.B.; Investigation: T.T., J.d.M., C.Z., B.S.C. and S.B.; Writing – Original Draft: T.T and S.B.; Writing – Review and Editing: T.T., J.d.M., C.Z., B.S.C. and S.B. Funding Acquisition: J.d.M. and S.B.; Resources: S.B.; Supervision: S.B.
Funding
This work was supported by the National Institutes of Health [R01EY020560 and R01EY017015 to S.B and F32EY024201 to B.S.C], by a grant from the Knights Templar Eye Foundation (to J.d.M.). S.B. was awarded a W. M. Keck Distinguished Young Scholar in Medical Research. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.137760.supplemental
References
- Alvarez Y., Alonso M. T., Vendrell V., Zelarayan L. C., Chamero P., Theil T., Bösl M. R., Kato S., Maconochie M., Riethmacher D. et al. (2003). Requirements for FGF3 and FGF10 during inner ear formation. Dev. Camb. Engl. 130, 6329-6338. 10.1242/dev.00881 [DOI] [PubMed] [Google Scholar]
- Blackshaw S., Harpavat S., Trimarchi J., Cai L., Huang H., Kuo W. P., Weber G., Lee K., Fraioli R. E., Cho S.-H. et al. (2004). Genomic analysis of mouse retinal development. PLoS Biol. 2, E247 10.1371/journal.pbio.0020247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell B. A. and Musil L. S. (2015). Synergistic interaction between the fibroblast growth factor and bone morphogenetic protein signaling pathways in lens cells. Mol. Biol. Cell 26, 2561-2572. 10.1091/mbc.E15-02-0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell B. A., Overbeek P. A. and Musil L. S. (2008). Essential role of BMPs in FGF-induced secondary lens fiber differentiation. Dev. Biol. 324, 202-212. 10.1016/j.ydbio.2008.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister M., Novak J., Liang M. Y., Basu S., Ploder L., Hawes N. L., Vidgen D., Hoover F., Goldman D., Kalnins V. I., Roderick T. H., Taylor B. A., Hankin M. H. and McInnes R. R. (1996). Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat. Genet. 12, 376-384. 10.1038/ng0496-376 [DOI] [PubMed] [Google Scholar]
- Chamberlain C. G. and McAvoy J. W. (1987). Evidence that fibroblast growth factor promotes lens fibre differentiation. Curr. Eye Res. 6, 1165-1168. 10.3109/02713688709034890 [DOI] [PubMed] [Google Scholar]
- Chamberlain C. G. and McAvoy J. W. (1989). Induction of lens fibre differentiation by acidic and basic fibroblast growth factor (FGF). Growth Factors 1, 125-134. 10.3109/08977198909029122 [DOI] [PubMed] [Google Scholar]
- Chamberlain C. G. and McAvoy J. W. (1997). Fibre differentiation and polarity in the mammalian lens: a key role for FGF. Prog. Retin. Eye Res. 16, 443-478. 10.1016/S1350-9462(96)00034-1 [DOI] [Google Scholar]
- Colvin J. S., Feldman B., Nadeau J. H., Goldfarb M. and Ornitz D. M. (1999). Genomic organization and embryonic expression of the mouse fibroblast growth factor 9 gene. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 216, 72-88. [DOI] [PubMed] [Google Scholar]
- Coulombre J. L. and Coulombre A. J. (1963). Lens development: fiber elongation and lens orientation. Science 142, 1489-1490. 10.1126/science.142.3598.1489 [DOI] [PubMed] [Google Scholar]
- de Melo J., Miki K., Rattner A., Smallwood P., Zibetti C., Hirokawa K., Monuki E. S., Campochiaro P. A. and Blackshaw S. (2012). Injury-independent induction of reactive gliosis in retina by loss of function of the LIM homeodomain transcription factor Lhx2. Proc. Natl. Acad. Sci. USA 109, 4657-4662. 10.1073/pnas.1107488109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo J., Zibetti C., Clark B. S., Hwang W., Miranda-Angulo A. L., Qian J. and Blackshaw S. (2016). Lhx2 is an essential factor for retinal gliogenesis and Notch signaling. J. Neurosci. 36, 2391-2405. 10.1523/JNEUROSCI.3145-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber S. C., Dimanlig P., Makarenkova H. P., Shirke S., Ko K. and Lang R. A. (2001). Fgf receptor signaling plays a role in lens induction. Dev. Camb. Engl. 128, 4425-4438. [DOI] [PubMed] [Google Scholar]
- Furuta Y. and Hogan B. L. M. (1998). BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 12, 3764-3775. 10.1101/gad.12.23.3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunhaga L. (2011). The lens: a classical model of embryonic induction providing new insights into cell determination in early development. Philos. Trans. R. Soc. B Biol. Sci. 366, 1193-1203. 10.1098/rstb.2010.0175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägglund A.-C., Dahl L. and Carlsson L. (2011). Lhx2 is required for patterning and expansion of a distinct progenitor cell population committed to eye development. PLoS ONE 6, e23387 10.1371/journal.pone.0023387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrin M., Pandit T. and Gunhaga L. (2012). A balance of FGF and BMP signals regulates cell cycle exit and Equarin expression in lens cells. Mol. Biol. Cell 23, 3266-3274. 10.1091/mbc.E12-01-0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurose H., Bito T., Adachi T., Shimizu M., Noji S. and Ohuchi H. (2004). Expression of Fibroblast growth factor 19 (Fgf19) during chicken embryogenesis and eye development, compared with Fgf15 expression in the mouse. Gene Expr. Patterns 4, 687-693. 10.1016/j.modgep.2004.04.005 [DOI] [PubMed] [Google Scholar]
- Lovicu F. J. and McAvoy J. W. (2005). Growth factor regulation of lens development. Dev. Biol. 280, 1-14. 10.1016/j.ydbio.2005.01.020 [DOI] [PubMed] [Google Scholar]
- Lovicu F. J. and Overbeek P. A. (1998). Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Dev. Camb. Engl. 125, 3365-3377. [DOI] [PubMed] [Google Scholar]
- Lovicu F. J. and Robinson M. L. (Ed.). (2004). Development of the Ocular Lens. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Lovicu F. J., McAvoy J. W. and de Iongh R. U. (2011). Understanding the role of growth factors in embryonic development: insights from the lens. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 1204-1218. 10.1098/rstb.2010.0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Zwingman T. A., Sunkin S. M., Oh S. W., Zariwala H. A., Gu H., Ng L. L., Palmiter R. D., Hawrylycz M. J., Jones A. R. et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133-140. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangale V. S., Hirokawa K. E., Satyaki P. R. V., Gokulchandran N., Chikbire S., Subramanian L., Shetty A. S., Martynoga B., Paul J., Mai M. V. et al. (2008). Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science 319, 304-309. 10.1126/science.1151695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy J. W. and Chamberlain C. G. (1989). Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Dev. Camb. Engl. 107, 221-228. [DOI] [PubMed] [Google Scholar]
- McAvoy J. W. and Fernon V. T. P. (1984). Neural retinas promote cell division and fibre differentiation in lens epithelial explants. Curr. Eye Res. 3, 827-834. 10.3109/02713688409000795 [DOI] [PubMed] [Google Scholar]
- Marquardt T., Ashery-Padan R., Andrejewski N., Scardigli R., Guillemot F. and Gruss P. (2001). Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105, 43-55. 10.1016/S0092-8674(01)00295-1 [DOI] [PubMed] [Google Scholar]
- McAvoy J. W., Chamberlain C. G., de Longh R. U., Hales A. M. and Lovicu F. J. (1999). Lens development. Eye 13, 425-437. 10.1038/eye.1999.117 [DOI] [PubMed] [Google Scholar]
- Murali D., Yoshikawa S., Corrigan R. R., Plas D. J., Crair M. C., Oliver G., Lyons K. M., Mishina Y. and Furuta Y. (2005). Distinct developmental programs require different levels of Bmp signaling during mouse retinal development. Dev. Camb. Engl. 132, 913-923. 10.1242/dev.01673 [DOI] [PubMed] [Google Scholar]
- Ornitz D. M. and Itoh N. (2015). The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 4, 215-266. 10.1002/wdev.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D. M., Xu J., Colvin J. S., McEwen D. G., MacArthur C. A., Coulier F., Gao G. and Goldfarb M. (1996). Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 271, 15292-15297. 10.1074/jbc.271.25.15292 [DOI] [PubMed] [Google Scholar]
- Pandit T., Jidigam V. K., Patthey C. and Gunhaga L. (2015). Neural retina identity is specified by lens-derived BMP signals. Dev. Camb. Engl. 142, 1850-1859. 10.1242/dev.123653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. L. (2006). An essential role for FGF receptor signaling in lens development. Semin. Cell Dev. Biol. 17, 726-740. 10.1016/j.semcdb.2006.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan S. and Cepko C. L. (2004). Genetic analysis of the homeodomain transcription factor Chx10 in the retina using a novel multifunctional BAC transgenic mouse reporter. Dev. Biol. 271, 388-402. 10.1016/j.ydbio.2004.03.039 [DOI] [PubMed] [Google Scholar]
- Roy A., de Melo J., Chaturvedi D., Thein T., Cabrera-Socorro A., Houart C., Meyer G., Blackshaw S. and Tole S. (2013). Lhx2 is necessary for the maintenance of optic identity and for the progression of optic morphogenesis. J. Neurosci. Off. J. Soc. Neurosci. 33, 6877-6884. 10.1523/JNEUROSCI.4216-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz M. W., Chamberlain C. G., de Iongh R. U. and McAvoy J. W. (1993). Acidic and basic FGF in ocular media and lens: implications for lens polarity and growth patterns. Dev. Camb. Engl. 118, 117-126. [DOI] [PubMed] [Google Scholar]
- Song Z., Liu C., Iwata J., Gu S., Suzuki A., Sun C., He W., Shu R., Li L., Chai Y et al. . et al. (2013). Mice with Tak1 deficiency in neural crest lineage exhibit cleft palate associated with abnormal tongue development. J. Biol. Chem. 288, 10440-10450. 10.1074/jbc.M112.432286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taranova O. V., Magness S. T., Fagan B. M., Wu Y., Surzenko N., Hutton S. R. and Pevny L. H. (2006). SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 20, 1187-1202. 10.1101/gad.1407906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urness L. D., Bleyl S. B., Wright T. J., Moon A. M. and Mansour S. L. (2011). Redundant and dosage sensitive requirements for Fgf3 and Fgf10 in cardiovascular development. Dev. Biol. 356, 383-397. 10.1016/j.ydbio.2011.05.671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., McAvoy J. W. and Lovicu F. J. (2010). Growth factor signaling in vitreous humor-induced lens fiber differentiation. Invest. Ophthalmol. Vis. Sci. 51, 3599-3610. 10.1167/iovs.09-4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawersik S., Purcell P., Rauchman M., Dudley A. T., Robertson E. J. and Maas R., (1999). BMP7 acts in murine lens placode development. Dev. Biol. 207, 176-188. 10.1006/dbio.1998.9153 [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S. and McMahon A. P. (1989). Expression pattern of the FGF-related proto-oncogene int-2 suggests multiple roles in fetal development. Dev. Camb. Engl. 105, 131-136. [DOI] [PubMed] [Google Scholar]
- Wolf L. V., Yang Y., Wang J., Xie Q., Braunger B., Tamm E. R., Zavadil J. and Cvekl A. (2009). Identification of pax6-dependent gene regulatory networks in the mouse lens. PLoS ONE 4, e4159 10.1371/journal.pone.0004159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Overbeek P. A. and Reneker L. W. (2002). Systematic analysis of E-, N- and P-cadherin expression in mouse eye development. Exp. Eye Res. 74, 753-760. 10.1006/exer.2002.1175 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y. (1976). Growth of lens and ocular environment: role of neural retina in the growth of mouse lens as revealed by an implantation experiment. Dev. Growth Differ. 18, 273-278. 10.1111/j.1440-169X.1976.00273.x [DOI] [PubMed] [Google Scholar]
- Yancey S. B., Koh K., Chung J. and Revel J. P. (1988). Expression of the gene for main intrinsic polypeptide (MIP): separate spatial distributions of MIP and beta-crystallin gene transcripts in rat lens development. J. Cell Biol. 106, 705-714. 10.1083/jcb.106.3.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S., Saijoh Y., Hirokawa K. E., Kopinke D., Murtaugh L. C., Monuki E. S. and Levine E. M. (2009). Lhx2 links the intrinsic and extrinsic factors that control optic cup formation. Dev. Camb. Engl. 136, 3895-3906. 10.1242/dev.041202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Ibrahimi O. A., Olsen S. K., Umemori H., Mohammadi M. and Ornitz D. M. (2006). Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J. Biol. Chem. 281, 15694-15700. 10.1074/jbc.M601252200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Yang T., Madakashira B. P., Thiels C. A., Bechtle C. A., Garcia C. M., Zhang H., Yu K., Ornitz D. M., Beebe D. C. et al. (2008). Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev. Biol. 318, 276-288. 10.1016/j.ydbio.2008.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Kawai K., Wang H., Wu D., Wang M., Yue Z., Zhang J. and Liu Y.-H. (2012). Loss of Msx2 function down-regulates the FoxE3 expression and results in anterior segment dysgenesis resembling Peters anomaly. Am. J. Pathol. 180, 2230-2239. 10.1016/j.ajpath.2012.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]