Abstract
The high comorbidity rates of posttraumatic stress disorder and chronic pain have been widely reported, but the underlying mechanisms remain unclear. Emerging evidence suggested that an excess of inflammatory immune activities in the hippocampus involved in the progression of both posttraumatic stress disorder and chronic pain. Considering that microglia are substrates underlying the initiation and propagation of the neuroimmune response, we hypothesized that stress-induced activation of hippocampal microglia may contribute to the pathogenesis of posttraumatic stress disorder-pain comorbidity. We showed that rats exposed to single prolonged stress, an established posttraumatic stress disorder model, exhibited persistent mechanical allodynia and anxiety-like behavior, which were accompanied by increased activation of microglia and secretion of pro-inflammatory cytokines in the hippocampus. Correlation analyses showed that hippocampal activation of microglia was significantly correlated with mechanical allodynia and anxiety-like behavior. Our data also showed that both intraperitoneal and intra-hippocampal injection of minocycline suppressed single prolonged stress-induced microglia activation and inflammatory cytokines accumulation in the hippocampus, and attenuated both single prolonged stress-induced mechanical allodynia and anxiety-like behavior. Taken together, the present study suggests that stress-induced microglia activation in the hippocampus may serve as a critical mechanistic link in the comorbid relationship between posttraumatic stress disorder and chronic pain. The novel concept introduces the possibility of cotreating chronic pain and posttraumatic stress disorder.
Keywords: Posttraumatic stress disorder, chronic pain, comorbidity, hippocampus, microglia, stress-induced hyperalgesia
Introduction
Posttraumatic stress disorder (PTSD) is an anxiety disorder that develops after traumatic events like combat, natural disaster, terrorist attack, and other serious accidents.1 As it often shares comorbidity with other disorders,2,3 PTSD brings enormous patient suffering and economic burden.4,5 To date, the comorbidity of PTSD and chronic pain has been widely reported by clinical studies.3,6 Amital et al.7 showed that 45% of the PTSD patients had fibromyalgia syndrome. An investigation on U.S. veterans reported a chronic pain prevalence rate of 66% among patients suffering from PTSD.8 Despite the high PTSD-pain comorbidity, the neurobehavioral mechanisms underlying this phenomenon remain unclear.9
Recently, emerging evidence suggested that stress-related immune dysregulation in the central nervous system (CNS) may play a vital role in the pathogenesis of PTSD. In an animal model of PTSD, rats exhibited elevated pro-inflammatory cytokines in the hippocampus,10 a brain region critically involved in the pathophysiology of PTSD.11 Further study showed that the changes of pro-inflammatory cytokines in the hippocampus paralleled PTSD-like behaviors.12 Moreover, as one of the central components of limbic system, the hippocampus is also implicated in both the processing and modification of nociceptive stimuli.13–15 Inflammatory response in the hippocampus is considered to facilitate the perception of pain.16 Elevated levels of hippocampal cytokines including interleukin-1β (IL-1β) and IL-6 were observed in rodents with neuropathic pain.17,18 Decreasing hippocampal tumor necrosis factor (TNF) by nanomedicine was reported to reduce the neuropathic pain-like behavior.19 Further study indicated that increasing TNF levels solely in the rat hippocampus produced persistent pain-like symptoms.20 Since accumulated pro-inflammatory cytokines in the hippocampus have been implicated in the pathophysiology of both pain and PTSD, it is assumed that stress-induced neuroinflammation within the hippocampus may be a contributor to the pathogenesis of PTSD-pain comorbidity.
In the CNS, microglia are the major source of pro-inflammatory cytokines and have been considered as the substrates underlying the initiation and propagation of the neuroimmune response.21 Under pathological or stress conditions, microglia are the vanguard to respond to the injury or stimulus.22 Through propagation of inflammatory signaling, microglia can directly alter neuroplasticity after stress23 and mediate the pathophysiology of neuropsychiatric disorders.24–26 Although microglia activation has been implicated in the progression of various stress-related disorders, there is no research investigating whether pro-inflammatory activation of microglia underlies the shared neurobiology of comorbid PTSD and chronic pain.
Single prolonged stress (SPS) is an established animal model of PTSD that has recently been reported to induce pain-like behavior, which provided a reliable preclinical model to investigate the mechanism of PTSD-pain comorbidity.27 In this study, we used the rat model of PTSD (SPS) to test the hypothesis that stress-induced activation of hippocampal microglia may underlie the shared neurobiology of comorbid PTSD and chronic pain. The study would provide a novel perspective that targeting this key biological pathway may introduce the possibility of cotreating chronic pain and PTSD.
Materials and methods
Experimental animals
Adult male Sprague–Dawley rats (180–200 g, obtained from the Laboratory Animal Center of Nanjing Drum Tower Hospital) were used in this study. The rats were pair-housed in a vivarium where they were allowed to get food and water ad libitum. The temperature of the vivarium was set to 21 ± 1℃, and the interval of dark/light cycles was set to 12 h. All experimental procedures were conducted under the approval of the Animal Care and Use Committee at Nanjing Drum Tower Hospital and in accordance with the ethical guidelines for the use of experimental animals.28
Drug preparation
Minocycline hydrochloride (Sigma–Aldrich, St. Louis, USA), an inhibitor of microglial activation, was dissolved in 0.9% sterile, isotonic saline. The drug was prepared with a dosage of 30 mg/kg in a volume of 1.5 ml for intraperitoneal injection and with a dosage of 10 µg in a volume of 0.5 μl for intra-hippocampal injection. The dosage of minocycline was chosen referring to previous studies29–31 and based on our preliminary experiment. Meanwhile, a same volume of isotonic saline was used for vehicle treatment.
SPS procedure
The SPS procedure was carried out as previously described by Liberzon et al.32 Briefly, rats were restrained for 2 h inside a plastic animal holder, and then they were forced to swim for 20 min individually in an acrylic water tank (height 60 cm, diameter 25 cm) that was filled with clean water (24℃) of two-thirds of its height. After swimming, the rats were allowed to rest for 15 min, followed by inhalation of anesthetic ether until they lost consciousness. Meanwhile, control rats were maintained in an adjacent room without any treatment.
Stereotaxic surgery and intra-hippocampal injection
Rats were anesthetized intraperitoneally with 10% chloral hydrate (250 mg/kg) and placed in a stereotaxic apparatus with a flat skull position. Two holes were drilled through the skull for the implantation of bilateral stainless steel guide cannulas (22 gauge) targeting the hippocampus (coordinates relative to bregma: A/P −2.8 mm, L/R ± 2.0 mm, D/V −2.6 mm).33,34 Cannulas were secured to the skull with stainless steel screws and dental cement. Each rat was allowed to recover for six days before the experiment.
Intra-hippocampal injections were performed by means of an internal cannula (27 gauge), terminating 1 mm below the tip of the guides and connected by polyethylene tubing to a 0.5 -μl Hamilton syringe. The rats received bilateral injection of 0.25 μl of either vehicle or minocycline (20 µg/μl) over a 60 s period (a total volume of 0.5 μl per rat).31 After injection, the inner cannula was left in place for an additional 60 s to allow diffusion of the solution and to reduce the possibility of reflux.
Experimental design
Experiment 1
Sixteen rats were randomly divided into two groups (n = 8): group control (rats without any treatment) and group SPS (rats underwent an SPS procedure). Pain behavioral tests were performed on the day before (baseline) and on days 1, 2, 3, 5, and 7 after SPS. Anxiety-like behavior was tested in the elevated plus maze (EPM) on day 7 after SPS. After the last behavioral testing, the brain samples of rats were collected either for immunohistochemistry (n = 3) or for enzyme-linked immuno sorbent assay (ELISA) and Western blot analysis (n = 5).
Experiment 2
Thirty-two rats were randomly divided into four groups (n = 8): group control + vehicle i.p. (rats were administered vehicle); group SPS + vehicle i.p. (rats were administered vehicle and underwent an SPS procedure); group control + minocycline i.p. (rats were administered minocycline hydrochloride); group SPS + minocycline i.p. (rats were administered minocycline hydrochloride and underwent an SPS procedure). Minocycline hydrochloride (i.p. 30 mg/kg) or vehicle was injected once daily for eight days (at 30 min before and on days 1–7 after SPS). Pain behavioral tests were performed on the day before (baseline) and on days 1, 2, 3, 5, 7 after SPS. Anxiety-like behavior was tested in the EPM on day 7 after SPS. After the last behavioral testing, the brain samples of rats were collected either for Immunohistochemistry (n = 3) or for ELISA and Western blot analysis (n = 5).
Experiment 3
Thirty-two rats were randomly divided into four groups (n = 8): group control + vehicle IH (rats were intra-hippocampally administered vehicle); group SPS + vehicle IH (rats were intra-hippocampally administered vehicle and underwent an SPS procedure); group control + minocycline IH (rats were intra-hippocampally administered minocycline hydrochloride); group SPS + minocycline IH (rats were intra-hippocampally administered minocycline hydrochloride and underwent an SPS procedure). All the rats underwent a stereotaxic surgery to implant cannulas into the hippocampus. On the day after SPS, vehicle or minocycline hydrochloride (10 µg) was injected into the hippocampus of rats via the cannulas. Pain behavioral tests were performed at 1 h before and at 1, 2, 4, 8, 12 h after intra-hippocampal injection. Another 32 rats were randomly divided into four groups in the same way (i.e. group control + vehicle IH, group SPS + vehicle IH, group control + minocycline IH, and group SPS + minocycline IH; n = 8). Anxiety-like behavior was tested in the EPM at 2 h after intra-hippocampal injection, and the brain samples of rats were collected immediately after the behavioral testing for ELISA and Western blot analysis (n = 5).
Pain sensitivity tests
In this study, we examined the changes of nociceptive responses to mechanical stimuli after SPS with an electronic von Frey Anaesthesiometer (IITC, Woodland Hills, USA). Before each test, rats were placed in transparent Plexiglas compartments (length 20 cm, width 25 cm, height 15 cm) individually for 30 min to be habituated. The compartments were on a metal mesh floor, which allowed the tip of anaesthesiometer to stimulate the mid-plantar aspect of the right hind paw. The test was repeated five times and the interval between each stimulation was at least 10 min. The paw withdrawal mechanical threshold (PWMT) was recorded as the tolerance level in grams, and the mean PWMT of each hind paw was calculated by averaging the latter three tests. The data of PWMT were also expressed as hyperalgesic index, which was represented by the area under the curve (i.e. percent change from baseline × day), to define the magnitude of hyperalgesia.35
EPM test
The EPM was utilized to determine anxiety-like behavior,36 a hallmark of PTSD phenotype. The EPM apparatus consists of two opposed open arms (60 cm × 10 cm) and two opposed closed arms (60 cm × 10 cm × 40 cm) with 40-cm-high opaque walls on sides. The four arms are crossed at right angle and delimit a central area of 10 cm2 where the arms cross. The maze was elevated 40 cm above the floor and placed in a room with dim light. Each rat was placed on the central platform facing an open arm at the start of testing. The behavior was recorded with a video camera for 5 min and analyzed by an investigator who was blind to treatment conditions. The following behavioral parameters were calculated: number of entries into open and closed arms, duration of exploration in open and closed arms, and total arm entries and total time on all arms. Arm entry was defined as placing all four paws in the arm. Percentage (%) of open-arm entries was calculated as percentage of open-arm entries to the total arm entries. Percentage (%) of time spent on open arms was defined as percentage (%) of time on open arms in reference to total time on all arms. Anxiety index was also calculated as previously reported: 1 − [(time spent in open arm/total time on the maze)/2 + (number of entries to the open arms/total exploration on the maze)/2].37
Immunohistochemistry
Rats were transcardially perfused with heparinized normal saline under deep anesthesia by intraperitoneal injection of pentobarbital sodium (150 mg/kg) and then the rats were fixed by perfusion fixative (i.e. 4% paraformaldehyde dissolved in 0.1 M phosphate buffer). Whole brains were quickly removed, post-fixed in the same fixative for 24 h, and then paraffin embedded. The prepared tissues were cut transversely into 5 -µm-thick sections using a microtome and mounted on glass slides. The sections were deparaffinized in xylenes and rehydrated in alcohols. Antigen retrieval procedure was conducted by boiling the sections in citrate buffer (pH 6) for 10 min. Sections were treated with in 3% H2O2/methanol for 15 min to eliminate endogenous peroxidase. After washed with phosphate buffer solution (PBS), the sections were blocked with 10% rabbit serum for 30 min at room temperature, and then they were incubated in PBS containing the primary antibody against Iba1 (1:2000; Abcam, Cambridge, UK) overnight at 4℃. The brain sections were washed in PBS and then incubated with HRP-labeled rabbit anti-goat IgG secondary antibody (1:250; Abcam, Cambridge, UK) for 2 h at room temperature. For visualization, an ABC kit and a DAB peroxidase substrate kit were used. The section was then counterstained with haematoxylin and coverslipped. Images were captured using a light microscope.
Glial activation was quantified by counting the numbers of immunoreaction-positive cells. For each group, 10 sections of lumbar spinal cord from three rats were randomly selected for cell counting. With Image J software (NIH, Washington, USA) being used, we manually counted the number of Iba1-positive cell bodies in the hippocampus.38
Western blotting
The brain tissues of rats were removed after anesthesia. The hippocampus was dissected and then weighed and homogenized in lysis buffer. After centrifugation of the tissue homogenates at 12,000 r/min for 20 min at 4℃, the supernatants were collected. Protein concentration of each supernatant was determined using the bicinchoninine acid assay method. Protein samples were separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred onto a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). The filter membranes were blocked with 5% non-fat milk for 1 h at room temperature and incubated with the primary antibody against Iba1 (1:500; Abcam, Cambridge, UK) for a night at 4℃. After washed with Tris-buffered saline with Tween 20 buffer, the membranes were incubated with the rabbit anti-goat IgG secondary antibody (1:5000; Abcam, Cambridge, UK) for 2 h at room temperature. The protein bands were visualized in enhanced chemiluminescence solution for 1 min and exposed to hyperfilms (Amersham Biosciences, Piscataway, NJ, USA) for 1 to 10 min. β-actin (1:1000; Abcam, Cambridge, UK) was used as the loading and blotting control. The gray value of each band was measured with a computer-assisted imaging analysis system (IPLab software, Scanalytics, Fairfax, VA, USA) and quantified by NIH ImageJ software (Bethesda, MD, USA).
ELISA
The concentrations of IL-1β and TNF-α in supernatants of tissue homogenates were analyzed using ELISA kits (Senbeijia, Nanjing, China) according to the manufacturer’s instructions. Hippocampal cytokine levels were expressed in pg/mg wet tissue.
Statistical analysis
All data were expressed as the mean ± standard deviation (SD). A repeated measures analysis of variance (ANOVA) followed by the Bonferroni post hoc test was performed to assess the changes of PWMT over time. For the data of hyperalgesic index and data from EPM testing, immunohistochemistry, Western blot, and ELISA experiments, a two-tailed Student’s t test (in Experiment 1) or a two-way ANOVA with the Bonferroni post hoc test (in Experiments 2 and 3) was used to compare the differences between/among groups. Correlation between mechanical allodynia, anxiety index, and microglia activation was analyzed by Pearson’s coefficients. The statistical analysis was conducted using SPSS 19.0 software (IBM, Armonk, USA), and a P value < 0.05 was used as the criterion of significance.
Results
SPS exposure produced persistent mechanical allodynia and anxiety-like behavior
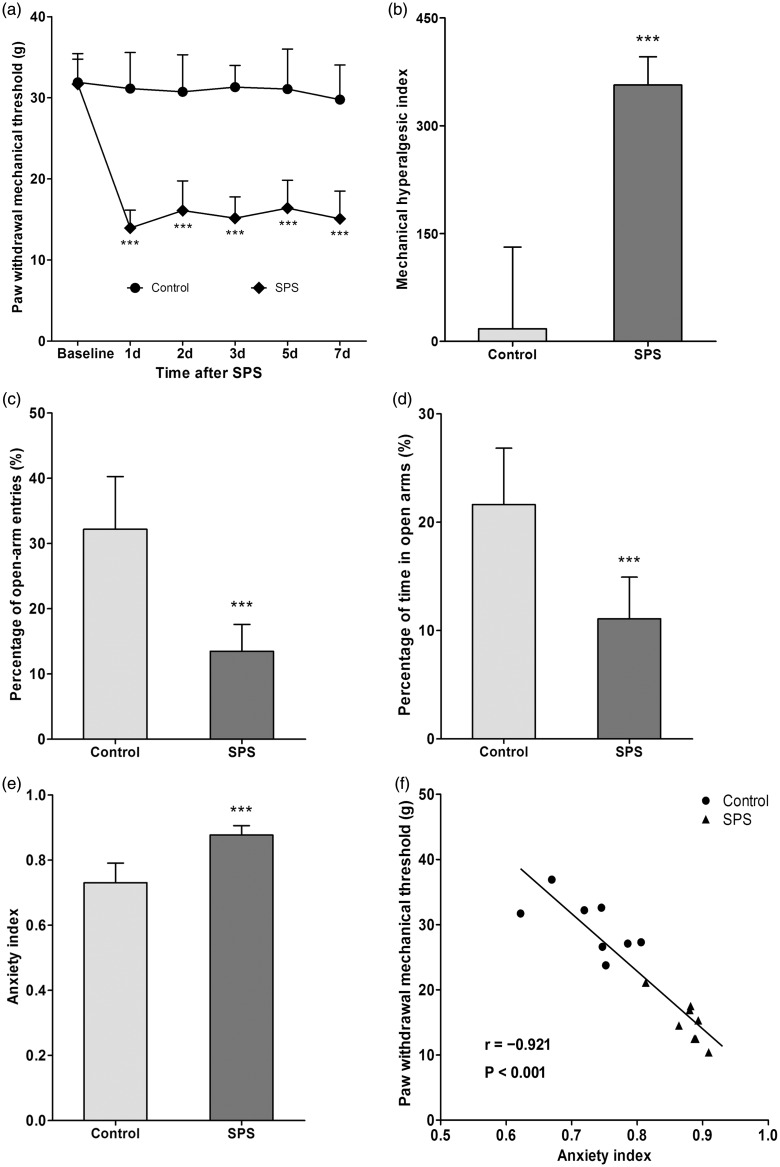
Changes of pain sensitivity after SPS exposure were determined by measuring PWMTs on the day before SPS (baseline) and on days 1, 2, 3, 5, 7 after SPS. The PWMTs at baseline were similar in rats of group control and group SPS (P > 0.05, n = 8 for each group, Figure 1(a)). However, decreased PWMTs were exhibited in SPS-exposed rats on day 1 after SPS compared to control rats, and the allodynia was sustained for at least seven days (P < 0.001, n = 8 for each group, Figure 1(a)). Moreover, SPS-exposed rats also showed higher hyperalgesic index (P < 0.001, n = 8 for each group, Figure 1(b)). The results indicated that SPS exposure produced persistent mechanical allodynia.
Figure 1.
Single prolonged stress (SPS) exposure induced mechanical allodynia and anxiety-like behavior. (a) Changes of pain sensitivity after SPS exposure were determined by measuring PWMT on the day before SPS (baseline) and on days 1, 2, 3, 5, 7 after SPS. (b) The data of PWMT were also expressed as the hyperalgesic index. Anxiety-like behavior was tested in the EPM on day 7 after SPS. Percentage (%) of open-arm entries (c) and time spent in the open arms (d) as well as anxiety index (e) were calculated. (f) Correlation analysis between PWMT and anxiety index on day 7 was conducted. Data were expressed as the mean ± standard deviation; n = 8 in each group. ***P < 0.001 compared with control group.
Anxiety-like behavior was tested in the EPM on day 7 after SPS. SPS-exposed rats showed lower open-arm exploration (P < 0.001, n = 8 for each group, Figure 1(c) and (d)) and higher anxiety index (P < 0.001, n = 8 for each group, Figure 1(e)), which suggested anxiety-like behavior was induced after SPS exposure.
Correlation analysis between mechanical allodynia and anxiety-like behavior was conducted. The results revealed that there was a significant correlation between the PWMT and the anxiety index on day 7 (P < 0.001, n = 8 for each group, Pearson’s correlation coefficient (r) = −0.921) (Figure 1(f)).
SPS induced activation of microglia and accumulation of pro-inflammatory cytokines in the hippocampus
After the last behavioral testing, the brain samples of rats were collected for further determination of microglia activation and pro-inflammatory cytokines levels.
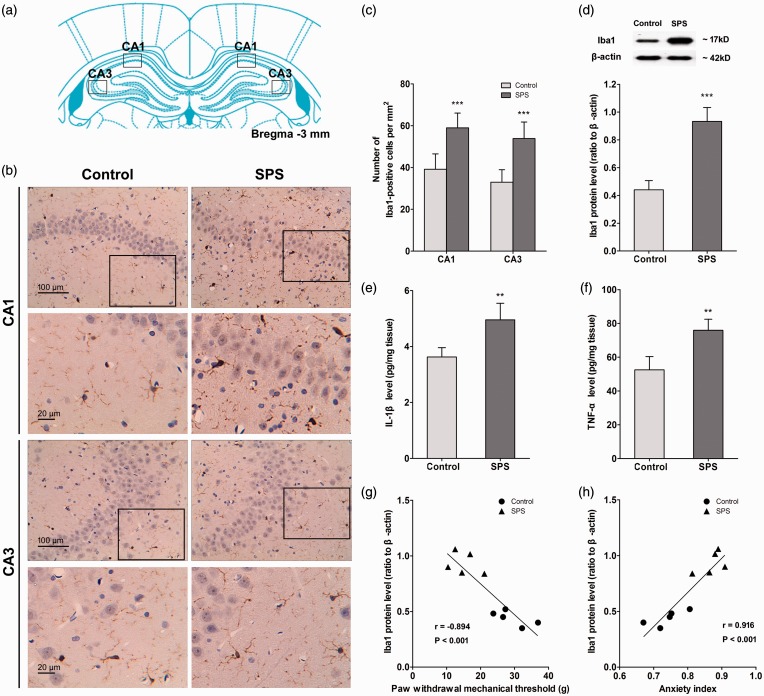
Immunohistochemistry was used to visualize the morphological changes of microglia after SPS exposure. Figure 2(b) illustrates the representative micrographs of immunostaining for Iba1 in the CA1 and CA3 regions of rat hippocampus in each group. The number of positive stained cells of Iba1 was quantified and summarized in Figure 2(c). As it shows, SPS-exposed rats presented an increased expression of activated microglia in the hippocampus (P < 0.001, n = 10 sections of three rats for each group). The Western blot data further confirmed the results. Compared with control rats, stressed rats presented significantly higher Iba1 levels in the hippocampus (P < 0.001, n = 5 for each group, Figure 2(d)).
Figure 2.
Single prolonged stress (SPS) induced activation of microglia and accumulation of pro-inflammatory cytokines in the hippocampus. After the last behavioral testing on day 7 after SPS, the brain samples of rats were collected. Hippocampal microglia were visualized using Immunohistochemistry, and images were captured under a light microscope. (a) The location of image capture.34 (b) The images were captured in the CA1 and CA3 regions of the hippocampus. (c) The number of Iba1-positive cell bodies was counted using ImageJ software (n = 10 sections of three rats for each group). (d) Iba1 protein levels in the hippocampus were measured using Western blot analysis, and hippocampal levels of IL-1β (e) and TNF-α (f) were measured using enzyme-linked immuno sorbent assay analysis (n = 5 in each group). Correlation analysis between hippocampal Iba1 expression level and PWMT (g) as well as anxiety index (h) on day 7 was conducted (n = 5 in each group). Data were expressed as the mean ± standard deviation. **P < 0.01, ***P < 0.001 compared with control group.
The hippocampal levels of pro-inflammatory cytokines, including IL-1β and TNF-α, were determined by ELISA. Coincident with the changes of hippocampal microglia activation, SPS-exposed rats showed a significant increase in the levels of IL-1β (P < 0.01, n = 5 for each group, Figure 2(e)) and TNF-α (P < 0.01, n = 5 for each group, Figure 2(f)) in the hippocampus compared with control rats. Together, the above-mentioned results indicated that SPS exposure induced morphological and functional activation of hippocampal microglia.
We further conducted correlation analyses on the relationship between hippocampal activation of microglia and mechanical allodynia as well as anxiety-like behavior. The results showed that the Iba1 expression level was negatively correlated with the PWMT (P < 0.001, Pearson’s correlation coefficient (r) = −0.894, n = 5 for each group, Figure 2(g)) but positively correlated with the anxiety index (P < 0.001, Pearson’s correlation coefficient (r) = 0.916, n = 5 for each group, Figure 2(h)) on day 7.
Intraperitoneal injection of minocycline attenuated SPS-induced mechanical allodynia and anxiety-like behavior
To further confirm the role of hippocampal activation of microglia in the pathogenesis of PTSD-pain comorbidity, minocycline was used to inhibit microglia in the following experiment. Minocycline hydrochloride (i.p. 30 mg/kg) or vehicle was injected once daily for eight days (at 30 min before and on days 1–7 after SPS) to control and SPS-exposed rats.
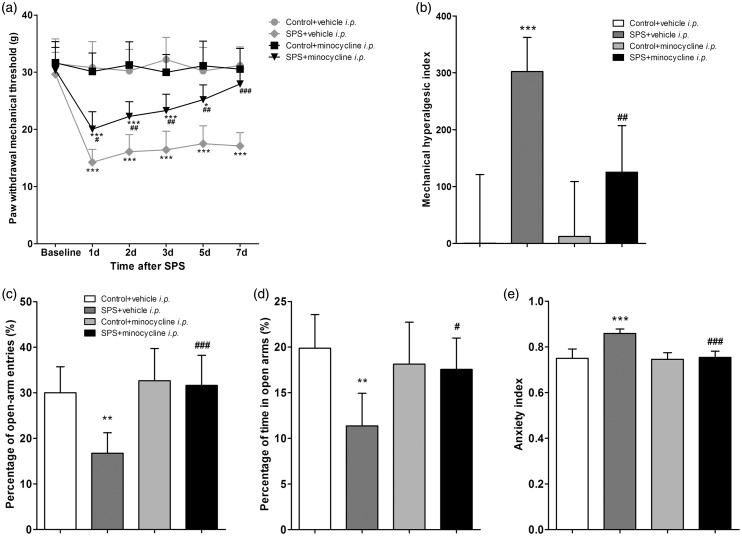
PWMTs were measured at various time points (baseline and days 1, 2, 3, 5, 7 after SPS). Compared to vehicle, minocycline (i.p.) exerted anti-hyperalgesic effects as early as 1 day after SPS (P < 0.05, n = 8 for each group, Figure 3(a)), and the effects lasted for seven days until totally blocked the mechanical allodynia induced by SPS (Figure 3(a)). Minocycline also decreased the hyperalgesic index in SPS-exposed rats (P < 0.01, n = 8 for each group, Figure 3(b)). However, minocycline had no effect on pain behaviors in control rats (P > 0.05, n = 8 for each group, Figure 3(a) and (b)).
Figure 3.
Intraperitoneal (i.p.) injection of minocycline attenuated single prolonged stress (SPS)-induced mechanical allodynia and anxiety-like behavior. (a) Pain sensitivity was determined by measuring paw withdrawal mechanical threshold (PWMT) on the day before SPS (baseline) and on days 1, 2, 3, 5, 7 after SPS. (b) The data of PWMT was also expressed as the hyperalgesic index. Anxiety-like behavior was tested in the EPM on day 7 after SPS. Percentage (%) of open-arm entries (c) and time spent in the open arms (d) as well as anxiety index (e) were calculated. Data were expressed as the mean ± standard deviation; n = 8 in each group. *P < 0.05, **P < 0.01, ***P < 0.001 compared with group control + vehicle i.p.; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with group SPS + vehicle i.p.
Anxiety-like behavior was tested in the EPM on day 7 after SPS. Compared with vehicle, minocycline (i.p.) resulted in higher open-arm exploration (P < 0.001 for percentage of open-arm entries and P < 0.05 for percentage of time in open arms, n = 8 for each group, Figure 3(c) and (d)) and lower anxiety index (P < 0.001, n = 8 for each group, Figure 3(e)) in SPS-exposed rats. The above data indicated that minocycline (i.p.) attenuated both SPS-induced mechanical allodynia and anxiety-like behavior.
The anti-hyperalgesic and anxiolytic effects of minocycline were achieved by suppression of SPS-induced microglia activation and pro-inflammatory cytokines accumulation in the hippocampus
The brain samples of rats were collected after the last behavioral testing on day 7 after SPS.
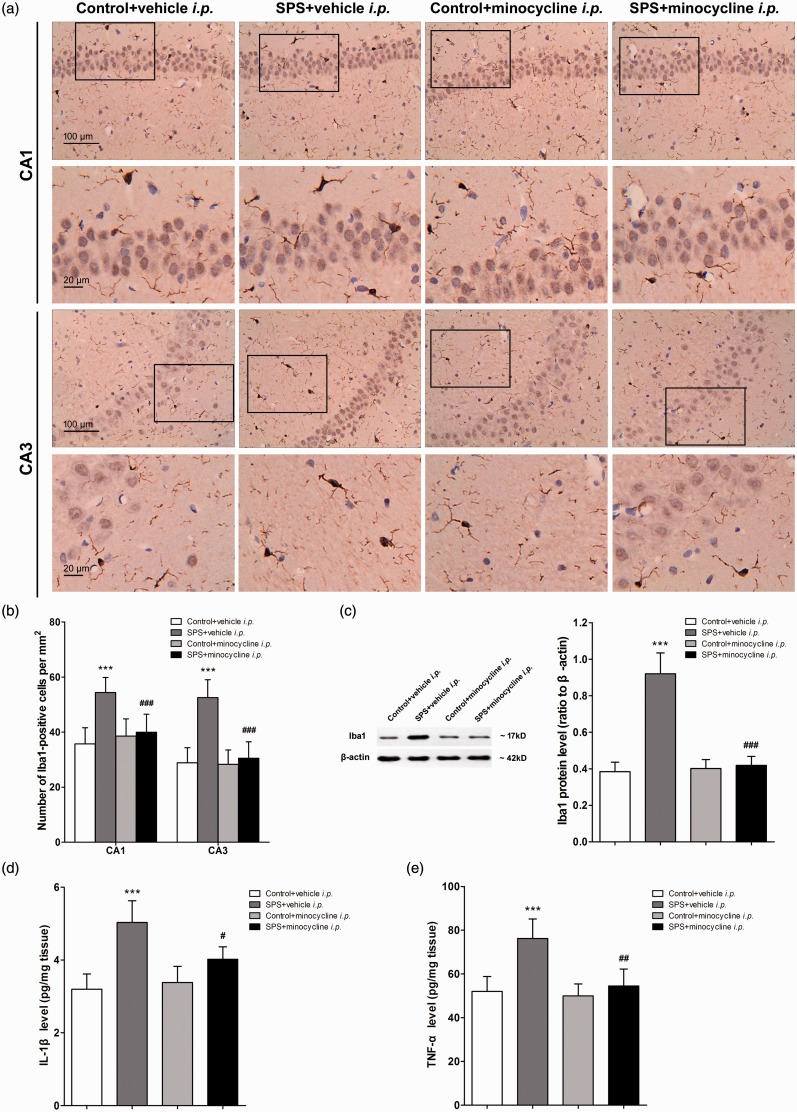
The morphological changes of microglia were visualized by immunohistochemistry. Figure 4(a) illustrates the representative micrographs of immunostaining for Iba1 in the CA1 and CA3 regions of rat hippocampus in each group. The number of positive stained cells of Iba1 was quantified and summarized in Figure 4(b). As it shows, minocycline (i.p.) suppressed the elevated activation of hippocampal microglia induced by SPS (P < 0.001, n = 10 sections of three rats for each group). The Western blot data further showed that minocycline decreased the Iba1 levels in the hippocampus of SPS-exposed rats (P < 0.001, n = 5 for each group, Figure 4(c)). However, minocycline (i.p.) had no effect on Iba1 expression in control rats (P > 0.05, n = 5 for each group, Figure 4 (b) and (c)).
Figure 4.
Intraperitoneal (i.p.) injection of minocycline suppressed hippocampal microglia activation and inflammatory cytokine accumulation in rats exposed to single prolonged stress (SPS). After the last behavioral testing on day 7 after SPS, the brain samples of rats were collected. Hippocampal microglia were visualized using immunohistochemistry. (a) The images were captured in the CA1 and CA3 regions of the hippocampus. (b) The number of Iba1-positive cell bodies was counted using ImageJ software (n = 10 sections of three rats for each group). (c) Iba1 protein levels in the hippocampus were measured using Western blot analysis, and hippocampal levels of IL-1β (d) and TNF-α (e) were measured using enzyme-linked immuno sorbent assay analysis (n = 5 in each group). Data were expressed as the mean ± standard deviation. ***P < 0.001 compared with group control + vehicle i.p.; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with group SPS + vehicle i.p.
Minocycline also suppressed the elevated accumulation of pro-inflammatory cytokines in the hippocampus induced by SPS. The levels of hippocampal IL-1β and TNF-α were determined by ELISA. Compared to vehicle, minocycline (i.p.) decreased the hippocampal levels of IL-1β (P < 0.05, n = 5 for each group, Figure 4(d)) and TNF-α (P < 0.01, n = 5 for each group, Figure 4(e)) in SPS-exposed rats, but had no effects on control rats (P > 0.05, n = 5 for each group, Figure 4(d) and (e)). Together, the above data suggested that the anti-hyperalgesic and anxiolytic effects of minocycline (i.p.) were achieved by suppression of SPS-induced microglia activation and pro-inflammatory cytokines accumulation in the hippocampus
Intra-hippocampal injection of minocycline suppressed SPS-induced microglia activation and pro-inflammatory cytokines accumulation and attenuated SPS-induced mechanical allodynia and anxiety-like behavior
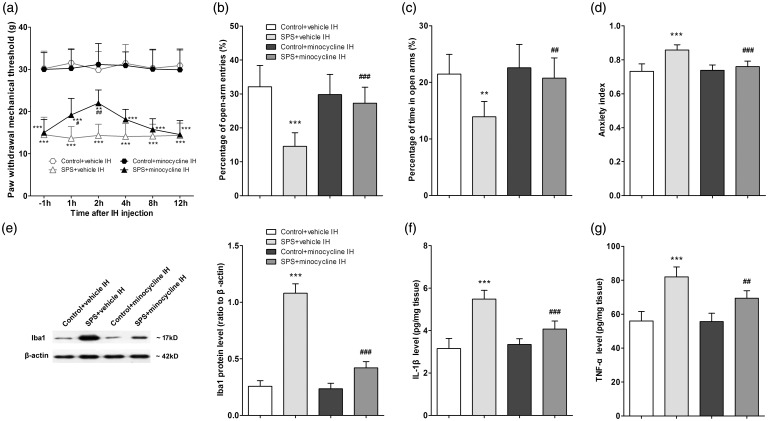
Minocycline (10 µg) or vehicle was intra-hippocampally injected to rats on the day after SPS. PWMTs were measured at 1 h before and at 1, 2, 4, 8, 12 h after injection. Compared to vehicle, local injection of minocycline exerted anti-hyperalgesic effects at 1 h after injection (P < 0.05, n = 8 for each group, Figure 5(a)), up to a maximal effect at 2 h after treatment (P < 0.01, n = 8 for each group, Figure 5(a)). A second cohort of rats received hippocampal minocycline 2 h before they were tested in the EPM on the day after SPS. Compared with vehicle, local injection of minocycline resulted in higher open-arm exploration (P < 0.001 for percentage of open-arm entries and P < 0.01 for percentage of time in open arms, n = 8 for each group, Figure 5(b) and (c)) and lower anxiety index (P < 0.001, n = 8 for each group, Figure 5(d)) in SPS-exposed rats. These data indicated that intra-hippocampal minocycline attenuated both mechanical allodynia and anxiety-like behavior induced by SPS.
Figure 5.
Intra-hippocampal (IH) injection of minocycline suppressed single prolonged stress (SPS)-induced hippocampal microglia activation and attenuated SPS-induced mechanical allodynia and anxiety-like behavior. (a) Pain sensitivity was determined by measuring paw withdrawal mechanical threshold (PWMT) at 1 h before and at 1, 2, 4, 8, and 12 h after IH injection on the day after SPS (n = 8 in each group). Anxiety-like behavior was tested in the EPM at 2 h after IH injection on the day after SPS. Percentage (%) of open-arm entries (b) and time spent in the open arms (c) as well as anxiety index (d) were calculated (n = 8 in each group). (e) Iba1 protein levels in the hippocampus were measured using Western blot analysis, and hippocampal levels of IL-1β (f) and TNF-α (g) were measured using enzyme-linked immuno sorbent assay analysis (n = 5 in each group). Data were expressed as the mean ± standard deviation. **P < 0.01, ***P < 0.001 compared with group control + vehicle IH; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with group SPS + vehicle IH.
The brain samples of rats were collected after the EPM testing on the day after SPS. Hippocampal levels of Iba1 and pro-inflammatory cytokines were determined using Western blot and ELISA analysis, respectively. Compared with vehicle, local injection of minocycline decreased the hippocampal levels of Iba1 (P < 0.001, n = 5 for each group, Figure 5(e)), IL-1β (P < 0.001, n = 5 for each group, Figure 5(f)), and TNF-α (P < 0.01, n = 5 for each group, Figure 5(g)) in SPS-exposed rats but had no effects in control rats.
Discussion
The comorbidity of PTSD and chronic pain was demonstrated to negatively impact the course of both disorders. Compared with either one disorder, co-occurrence of PTSD and chronic pain results in more severe pain, affective distress, and disability.39,40 Moreover, the coprevalence of the two disorders is very high, with approximately one-half to three-quarters of PTSD patients being diagnosed with chronic pain.4,7,8 The high rate of comorbidity between PTSD and pain suggests that both disorders may underlie shared pathophysiological processes.9 However, few laboratory studies were conducted to investigate the underlying mechanisms. Recently, emerging evidence suggested that inflammatory response in the CNS was involved in the progression of both PTSD and chronic pain,16,17,41 indicating neuroinflammation may be implicated in the shared neurobiology of comorbid PTSD and chronic pain. In this study, we tested whether microglia, one of the most important immune cells in the CNS, could serve as a critical mechanistic link in the comorbid relationship between PTSD and chronic pain. The experiments were conducted in a rat model of PTSD (SPS), which could closely mimic the comorbidity of PTSD and chronic pain. Our data revealed that SPS exposure induced mechanical allodynia and anxiety-like behavior that were accompanied by increased activation of microglia and secretion of pro-inflammatory cytokines (i.e. IL-1β and TNF-α) in the hippocampus. Both intraperitoneal and intra-hippocampal injection of minocycline, an inhibitor of microglia activation, suppressed SPS-induced microglial pro-inflammatory activation and attenuated SPS-induced mechanical allodynia and anxiety-like behavior. The results indicated that stress-induced hippocampal activation of microglia may play a critical role in regulating the comorbid PTSD and chronic pain.
In the CNS, microglia are considered as the primary responders to harmful stimuli, including insults and stress.22 Although their responses are mainly organized to eliminate pathogens and cellular debris,21 emerging evidence revealed that microglia were also involved in the shaping of neuronal networks via diverse pathways,42,43 one of which was through propagation of inflammatory signaling.44 After stress exposure, microglia are activated and secrete various pro-inflammatory cytokines, such as IL-1β and TNF-α, which promote the inflammatory state and alter neuronal plasticity.23 Elevated neuroinflammatory signaling after stress was reported to influence neuronal functions by inducing neuronal hyperexcitability and altering metabolism of neurotrophins and neurotransmitters.22,42 A growing body of literature has demonstrated the crucial role of pro-inflammatory activation of microglia in mediating neuropsychiatric disorders.24–26 Recently, many clinical studies suggested that PTSD was also associated with an excess of inflammatory immune activities.41,45 Animal studies showed that hippocampal levels of pro-inflammatory cytokines were elevated in PTSD rats.10,12 A recent study reported that minocycline was effective in preventing PTSD-like behavior in rats exposed to predator scent stress.46 Although these studies suggested the potential role of microglia in the pathogenesis of PTSD, the present study further provided direct evidence that microglia were morphologically and functionally activated in the related brain regions in rats exhibiting PTSD-like behaviors. Compared with control rats, more activated microglia were present in the hippocampus of PTSD rats, accompanied by elevated pro-inflammatory cytokines. However, when we inhibited the microglia activation using minocycline, the pro-inflammatory cytokines expression was suppressed and the PTSD-like behavior, i.e. anxiety, was attenuated. Our study firstly demonstrated the contribution of hippocampal activation of microglia to the pathogenesis of PTSD.
The present study also showed that exposure to SPS resulted in increased nociceptive sensitivity demonstrated by decreased paw withdrawal thresholds to mechanical stimuli. Indeed, many stressors were reported to be associated with allodynia or hyperalgesia.47–49 The phenomenon that exposure to physical or psychological stressors could enhance nociception and pain sensitivity was described as stress-induced hyperalgesia (SIH).50 Recently, microglia-mediated neuroinflammation was considered as a potential contributor to SIH. Our previous study has demonstrated the vital role of spinal microglia activation in the progression of SIH.51 Nevertheless, whether microglia are involved in the supraspinal mechanisms of SIH has rarely been studied. At supraspinal level, the hippocampus is one of the central components of limbic system that plays a vital role in modulating the emotional component of pain.13–15 Studies have revealed that inflammatory response in the hippocampus contributed to the facilitation and modulation of the hyper-responsive pain states.16 For example, elevated levels of cytokines including IL-1β and IL-6 were present in the hippocampus of rodents with neuropathic pain, and the levels of these cytokines were negatively correlated with pain threshold.17,18 Intracerebroventricular infusion of a TNF antibody adjacent to the hippocampus completely alleviated neuropathic pain.52 Martuscello et al.20 showed that increasing TNF levels solely in the rat hippocampus produced persistent pain-like symptoms. In the present study, we showed that SPS-induced mechanical allodynia was also associated with accumulation of pro-inflammatory cytokines in the hippocampus, which was accompanied by elevated microglia activation. Inhibition of microglia activation in the hippocampus was shown to attenuate the SPS-induced mechanical allodynia and exert anti-hyperalgesic effects. The results indicated that microglia activation in the hippocampus was implicated in the pathogenesis of SIH by facilitating pain perception via pro-inflammatory cytokines.
Pain is an unpleasant experience that does not only consist of the sensory component but also encompasses the cognitive and emotional components.15 Clinically, patients suffering from chronic pain are often diagnosed with mood disorders, such as anxiety and depression.53 Evidence from animal studies also showed that persistent nociception could cause anxiety- and/or depression-like behaviors.54,55 On the other hand, such mood disorders could also induce altered nociceptive sensitivity.13,56 The comorbid relationship between pain and affective disorders suggests that there may be shared neuroanatomy mediating these disorders.9 In the present study, our results revealed that hippocampus, a brain region critically implicated in modulating the emotional component of pain and also particularly vulnerable to stress, might be the shared neuroanatomy underlying comorbid PTSD and chronic pain. In this study, we measured the anxiety-like and pain-like behaviors on day 1 and day 7 after SPS, the results showed that these behaviors occurred simultaneously after SPS exposure. Further data showed that hippocampal activation of microglia was positively correlated with both anxiety-like and pain-like behaviors. To confirm the role of microglia activation in the pathogenesis of PTSD-pain comorbidity, minocycline, a microglia inhibitor, was injected intraperitoneally and intra-hippocampally to rats. The results showed that suppression of microglia activation in the hippocampus attenuated both SPS-induced mechanical allodynia and anxiety-like behavior. These data indicated that stress-induced microglia activation might serve as a critical mechanistic link in the comorbid relationship between PTSD and chronic pain.
The present study has several limitations. First, minocycline, the drug we used in the study to suppress microglial activation, is non-specific. Although minocycline is widely reported to be an inhibitor of microglial activation,29,31 recent studies reported that it could directly act on other CNS cells, for example neurons,57 and revealed anti-inflammatory effects. The direct action on other CNS cells of minocycline cannot be ruled out in our study. The results, therefore, should be interpreted with caution, and also need to be confirmed by further researches using specific inhibitory strategy for microglia including microglia ablation.58 Second, we did not determine whether other glial cells in the hippocampus or microglia in other brain regions contributed to the pathogenesis of PTSD-pain comorbidity. Further studies are needed to address these issues. Hopefully, our findings would promote more studies to explore the underlying mechanisms of PTSD-pain comorbidity.
In conclusion, the present study indicated that stress-induced microglia activation in the hippocampus may serve as a critical mechanistic link in the comorbid relationship between PTSD and chronic pain. Although this novel concept needs to be confirmed by further researches, our study provides clinical implications as the results demonstrated that inhibition of microglia may introduce the possibility of cotreating chronic pain and PTSD.
Author contributions
RS and ZZ contributed equally to this work. RS and ZM conceived and designed the experiments. RS, ZZ, YL, CL, HR, and YL performed the experiment. RS, YS, and WZ analyzed the data. ZZ and YL prepared the figures. RS drafted the manuscript. ZM and XG edited and revised the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Natural Science Foundation of China (81471129, 81171048, 81171047) and the grant from the Department of Health of Jiangsu Province of China (XK201140, RC2011006).
References
- 1.Yehuda R. Post-traumatic stress disorder. N Engl J Med 2002; 346: 108–114. [DOI] [PubMed] [Google Scholar]
- 2.Cavalcanti-Ribeiro P, Andrade-Nascimento M, Morais-de-Jesus M, et al. Post-traumatic stress disorder as a comorbidity: impact on disease outcomes. Expert Rev Neurother 2012; 12: 1023–1037. [DOI] [PubMed] [Google Scholar]
- 3.Moeller-Bertram T, Keltner J, Strigo IA. Pain and post traumatic stress disorder – review of clinical and experimental evidence. Neuropharmacology 2012; 62: 586–597. [DOI] [PubMed] [Google Scholar]
- 4.Sareen J. Posttraumatic stress disorder in adults: impact, comorbidity, risk factors, and treatment. Can J Psychiatry 2014; 59: 460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorrington S, Zavos H, Ball H, et al. Trauma, post-traumatic stress disorder and psychiatric disorders in a middle-income setting: prevalence and comorbidity. Br J Psychiatry 2014; 205: 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennstuhl MJ, Tarquinio C, Montel S. Chronic pain and PTSD: evolving views on their comorbidity. Perspect Psychiatr Care 2015; 51: 295–304. [DOI] [PubMed] [Google Scholar]
- 7.Amital D, Fostick L, Polliack ML, et al. Posttraumatic stress disorder, tenderness, and fibromyalgia syndrome: are they different entities? J Psychosom Res 2006; 61: 663–669. [DOI] [PubMed] [Google Scholar]
- 8.Shipherd JC, Keyes M, Jovanovic T, et al. Veterans seeking treatment for posttraumatic stress disorder: what about comorbid chronic pain? J Rehabil Res Dev 2007; 44: 153–166. [DOI] [PubMed] [Google Scholar]
- 9.Scioli-Salter ER, Forman DE, Otis JD, et al. The shared neuroanatomy and neurobiology of comorbid chronic pain and PTSD: therapeutic implications. Clin J Pain 2015; 31: 363–374. [DOI] [PubMed] [Google Scholar]
- 10.Wilson CB, McLaughlin LD, Nair A, et al. Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. PLoS One 2013; 8: e76146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitman RK, Rasmusson AM, Koenen KC, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 2012; 13: 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson CB, McLaughlin LD, Ebenezer PJ, et al. Valproic acid effects in the hippocampus and prefrontal cortex in an animal model of post-traumatic stress disorder. Behav Brain Res 2014; 268: 72–80. [DOI] [PubMed] [Google Scholar]
- 13.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013; 14: 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu MG, Chen J. Roles of the hippocampal formation in pain information processing. Neurosci Bull 2009; 25: 237–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simons LE, Elman I, Borsook D. Psychological processing in chronic pain: a neural systems approach. Neurosci Biobehav Rev 2014; 39: 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fasick V, Spengler RN, Samankan S, et al. The hippocampus and TNF: common links between chronic pain and depression. Neurosci Biobehav Rev 2015; 53: 139–159. [DOI] [PubMed] [Google Scholar]
- 17.del Rey A, Apkarian AV, Martina M, et al. Chronic neuropathic pain-like behavior and brain-borne IL-1beta. Ann N Y Acad Sci 2012; 1262: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.del Rey A, Yau HJ, Randolf A, et al. Chronic neuropathic pain-like behavior correlates with IL-1beta expression and disrupts cytokine interactions in the hippocampus. Pain 2011; 152: 2827–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerard E, Spengler RN, Bonoiu AC, et al. Chronic constriction injury-induced nociception is relieved by nanomedicine-mediated decrease of rat hippocampal tumor necrosis factor. Pain 2015; 156: 1320–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martuscello RT, Spengler RN, Bonoiu AC, et al. Increasing TNF levels solely in the rat hippocampus produces persistent pain-like symptoms. Pain 2012; 153: 1871–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michell-Robinson MA, Touil H, Healy LM, et al. Roles of microglia in brain development, tissue maintenance and repair. Brain 2015; 138: 1138–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kettenmann H, Hanisch UK, Noda M, et al. Physiology of microglia. Physiol Rev 2011; 91: 461–553. [DOI] [PubMed] [Google Scholar]
- 23.Delpech JC, Madore C, Nadjar A, et al. Microglia in neuronal plasticity: influence of stress. Neuropharmacology 2015; 96: 19–28. [DOI] [PubMed] [Google Scholar]
- 24.Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 2012; 37: 137–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blank T, Prinz M. Microglia as modulators of cognition and neuropsychiatric disorders. Glia 2013; 61: 62–70. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Gandhi PR, Standifer KM. Increased nociceptive sensitivity and nociceptin/orphanin FQ levels in a rat model of PTSD. Mol Pain 2012; 8: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 29.Rojewska E, Korostynski M, Przewlocki R, et al. Expression profiling of genes modulated by minocycline in a rat model of neuropathic pain. Mol Pain 2014; 10: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi S, De Chiara V, Musella A, et al. Chronic psychoemotional stress impairs cannabinoid-receptor-mediated control of GABA transmission in the striatum. J Neurosci 2008; 28: 7284–7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang G, Zhao BX, Hua R, et al. Hippocampal microglial activation and glucocorticoid receptor down-regulation precipitate visceral hypersensitivity induced by colorectal distension in rats. Neuropharmacology 2016; 102: 295–303. [DOI] [PubMed] [Google Scholar]
- 32.Liberzon I, Krstov M, Young EA. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology 1997; 22: 443–453. [DOI] [PubMed] [Google Scholar]
- 33.Ren J, Li X, Zhang X, et al. The effects of intra-hippocampal microinfusion of D-cycloserine on fear extinction, and the expression of NMDA receptor subunit NR2B and neurogenesis in the hippocampus in rats. Prog Neuropsychopharmacol Biol Psychiatry 2013; 44: 257–264. [DOI] [PubMed] [Google Scholar]
- 34.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 4th ed London: Academic Press, 1998. [Google Scholar]
- 35.Gregus AM, Doolen S, Dumlao DS, et al. Spinal 12-lipoxygenase-derived hepoxilin A3 contributes to inflammatory hyperalgesia via activation of TRPV1 and TRPA1 receptors. Proc Natl Acad Sci USA 2012; 109: 6721–6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lapiz-Bluhm MD, Bondi CO, Doyen J, et al. Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. J Neuroendocrinol 2008; 20: 1115–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serova LI, Tillinger A, Alaluf LG, et al. Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience 2013; 236: 298–312. [DOI] [PubMed] [Google Scholar]
- 38.Kallenborn-Gerhardt W, Schroder K, Del Turco D, et al. NADPH oxidase-4 maintains neuropathic pain after peripheral nerve injury. J Neurosci 2012; 32: 10136–10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bosco MA, Gallinati JL, Clark ME. Conceptualizing and treating comorbid chronic pain and PTSD. Pain Res Treat 2013; 2013: 174728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geisser ME, Roth RS, Bachman JE, et al. The relationship between symptoms of post-traumatic stress disorder and pain, affective disturbance and disability among patients with accident and non-accident related pain. Pain 1996; 66: 207–214. [DOI] [PubMed] [Google Scholar]
- 41.Gill JM, Saligan L, Woods S, et al. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care 2009; 45: 262–277. [DOI] [PubMed] [Google Scholar]
- 42.Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron 2013; 77: 10–18. [DOI] [PubMed] [Google Scholar]
- 43.Wu Y, Dissing-Olesen L, MacVicar BA, et al. Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol 2015; 36: 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patterson SL. Immune dysregulation and cognitive vulnerability in the aging brain: interactions of microglia, IL-1beta, BDNF and synaptic plasticity. Neuropharmacology 2015; 96: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gola H, Engler H, Sommershof A, et al. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry 2013; 13: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levkovitz Y, Fenchel D, Kaplan Z, et al. Early post-stressor intervention with minocycline, a second-generation tetracycline, attenuates post-traumatic stress response in an animal model of PTSD. Eur Neuropsychopharmacol 2015; 25: 124–132. [DOI] [PubMed] [Google Scholar]
- 47.Tramullas M, Finger BC, Moloney RD, et al. Toll-like receptor 4 regulates chronic stress-induced visceral pain in mice. Biol Psychiatry 2014; 76: 340–348. [DOI] [PubMed] [Google Scholar]
- 48.Bradesi S, Svensson CI, Steinauer J, et al. Role of spinal microglia in visceral hyperalgesia and NK1R up-regulation in a rat model of chronic stress. Gastroenterology 2009; 136: 1339–1348. e1331–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Hou B, Zhang W, et al. The activation of spinal astrocytes contributes to preoperative anxiety-induced persistent post-operative pain in a rat model of incisional pain. Eur J Pain 2015; 19: 733–740. [DOI] [PubMed] [Google Scholar]
- 50.Jennings EM, Okine BN, Roche M, et al. Stress-induced hyperalgesia. Prog Neurobiol 2014; 121: 1–18. [DOI] [PubMed] [Google Scholar]
- 51.Sun R, Zhao Z, Feng J, et al. Glucocorticoid-potentiated spinal microglia activation contributes to preoperative anxiety-induced postoperative hyperalgesia. Mol Neurobiol. Epub ahead of print 23 June 2016. DOI: 10.1007/s12035-016-9976-1. [DOI] [PubMed]
- 52.Reynolds JL, Ignatowski TA, Sud R, et al. Brain-derived tumor necrosis factor-alpha and its involvement in noradrenergic neuron functioning involved in the mechanism of action of an antidepressant. J Pharmacol Exp Ther 2004; 310: 1216–1225. [DOI] [PubMed] [Google Scholar]
- 53.Nicolson SE, Caplan JP, Williams DE, et al. Comorbid pain, depression, and anxiety: multifaceted pathology allows for multifaceted treatment. Harv Rev Psychiatry 2009; 17: 407–420. [DOI] [PubMed] [Google Scholar]
- 54.Yalcin I, Barthas F, Barrot M. Emotional consequences of neuropathic pain: insight from preclinical studies. Neurosci Biobehav Rev 2014; 47: 154–164. [DOI] [PubMed] [Google Scholar]
- 55.Liu MG, Chen J. Preclinical research on pain comorbidity with affective disorders and cognitive deficits: challenges and perspectives. Prog Neurobiol 2014; 116: 13–32. [DOI] [PubMed] [Google Scholar]
- 56.Ploghaus A, Narain C, Beckmann CF, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci 2001; 21: 9896–9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang WC, Qiao Y, Xu L, et al. Direct protection of cultured neurons from ischemia-like injury by minocycline. Anat Cell Biol 2010; 43: 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng J, Gu N, Zhou L, et al. Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat Commun 2016; 7: 12029. [DOI] [PMC free article] [PubMed] [Google Scholar]