Abstract
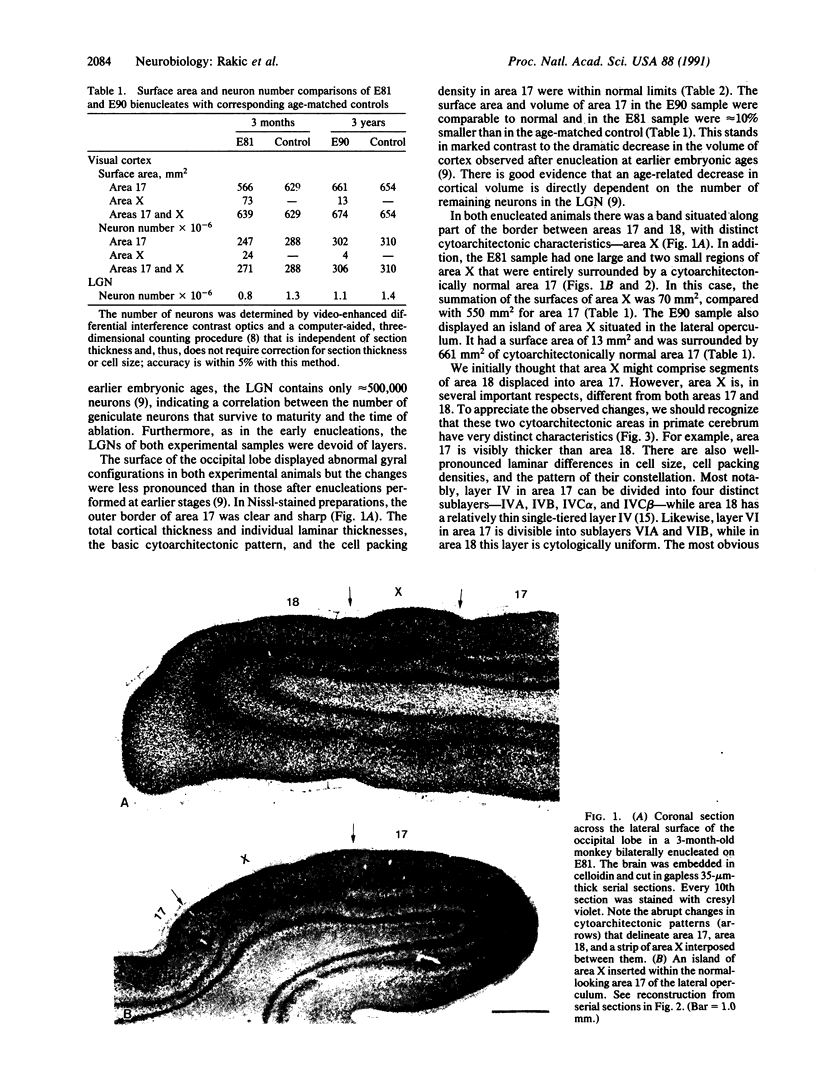
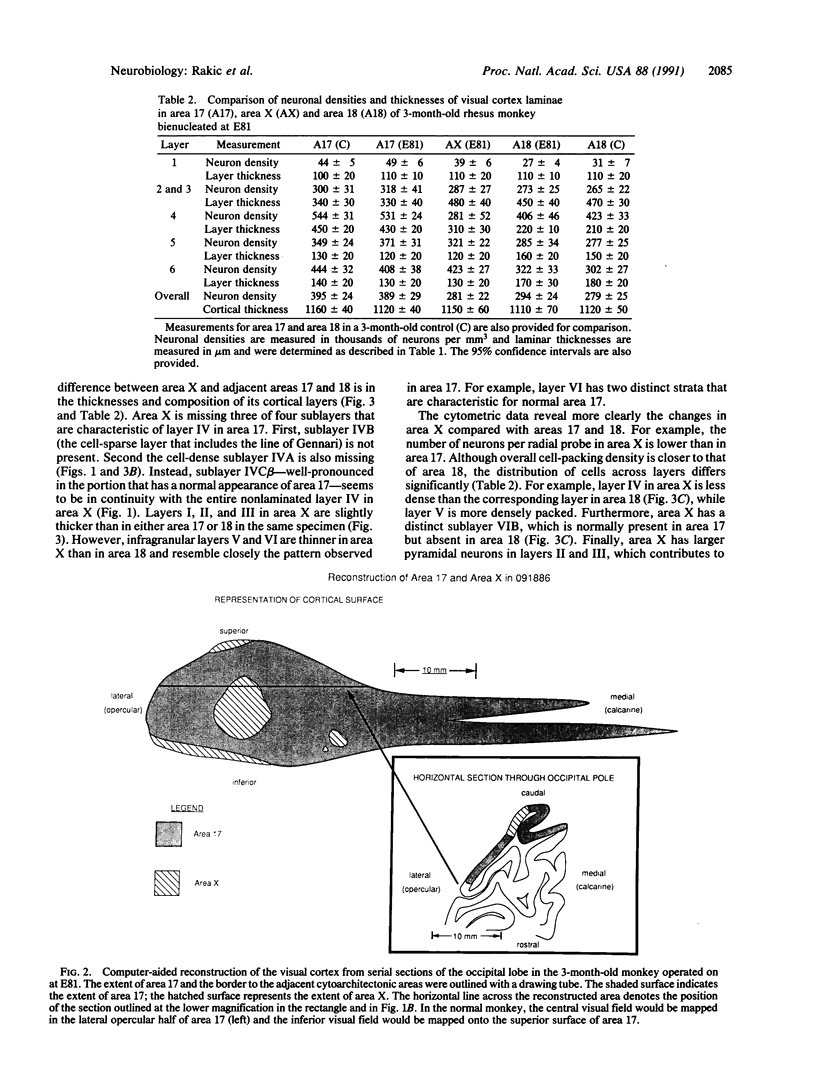
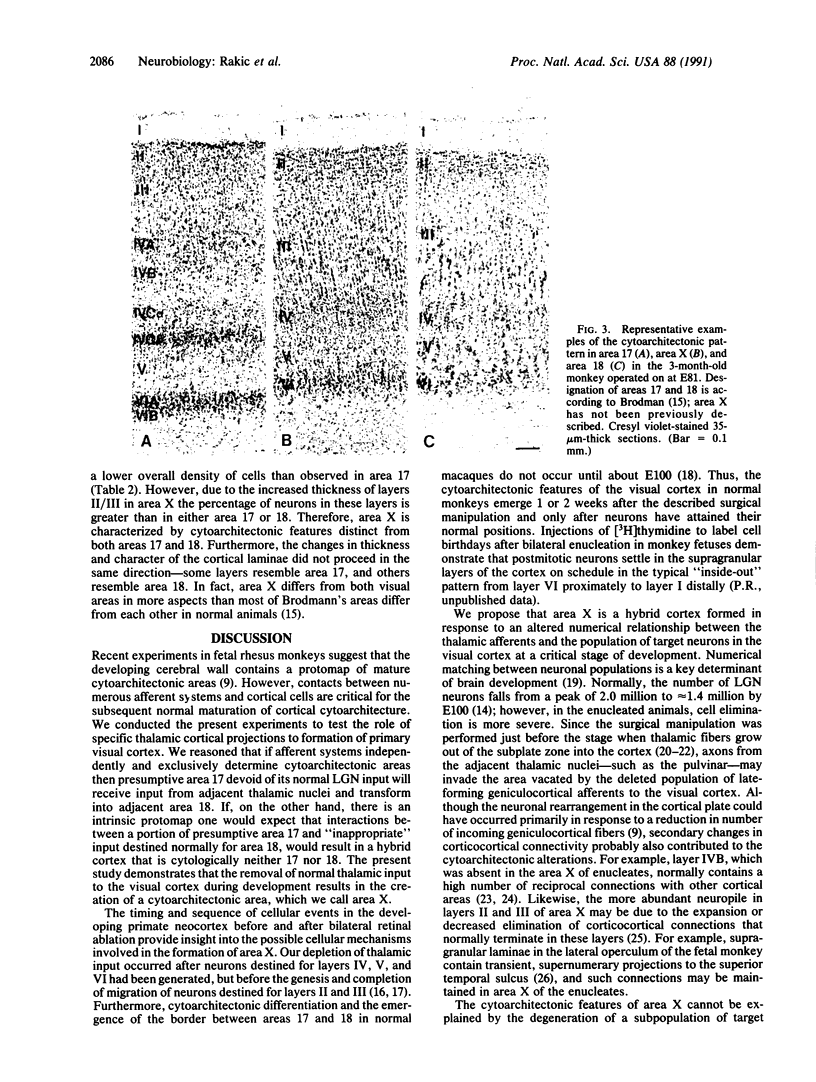
The cerebral cortex is divisible into a number of cytoarchitectonic areas, but developmental mechanisms that regulate their number and size remain unknown. Here we provide evidence that reducing the population of selected thalamic fibers projecting into the primary visual cortex (area 17) of monkeys during midgestation induces the formation of a novel cytoarchitectonic area situated along the border of and embedded within area 17. This region, termed area X, differs cytoarchitectonically from both area 17 and the adjacent secondary visual cortex (area 18). We propose that an aberrant combination of thalamic and cortical connections acting on a portion of prospective area 17 deprived of its normal thalamic input may result in formation of a hybrid cortex. Our results support the protomap hypothesis of cortical parcellation and suggest how during evolution new cytoarchitectonic regions may arise by cell-cell interactions that depend on a unique combination of intrinsic properties of cortical neurons and afferent fibers.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunquell P. J., Papale J. H., Horton J. C., Williams R. S., Zgrabik M. J., Albert D. M., Hedley-Whyte E. T. Sex-linked hereditary bilateral anophthalmos. Pathologic and radiologic correlation. Arch Ophthalmol. 1984 Jan;102(1):108–113. doi: 10.1001/archopht.1984.01040030092044. [DOI] [PubMed] [Google Scholar]
- Burkhalter A., Bernardo K. L. Organization of corticocortical connections in human visual cortex. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1071–1075. doi: 10.1073/pnas.86.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall J. E., Herrup K. Patterns of cell lineage in the cerebral cortex reveal evidence for developmental boundaries. Exp Neurol. 1990 Jul;109(1):131–139. doi: 10.1016/s0014-4886(05)80014-7. [DOI] [PubMed] [Google Scholar]
- Dehay C., Horsburgh G., Berland M., Killackey H., Kennedy H. Maturation and connectivity of the visual cortex in monkey is altered by prenatal removal of retinal input. Nature. 1989 Jan 19;337(6204):265–267. doi: 10.1038/337265a0. [DOI] [PubMed] [Google Scholar]
- Finlay B. L., Slattery M. Local differences in the amount of early cell death in neocortex predict adult local specializations. Science. 1983 Mar 18;219(4590):1349–1351. doi: 10.1126/science.6828866. [DOI] [PubMed] [Google Scholar]
- Frost D. O., Metin C. Induction of functional retinal projections to the somatosensory system. Nature. 1985 Sep 12;317(6033):162–164. doi: 10.1038/317162a0. [DOI] [PubMed] [Google Scholar]
- Haberland C., Perou M. Primary bilateral anophthalmia. J Neuropathol Exp Neurol. 1969 Apr;28(2):337–351. [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Kennedy H., Bullier J., Dehay C. Transient projection from the superior temporal sulcus to area 17 in the newborn macaque monkey. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8093–8097. doi: 10.1073/pnas.86.20.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuljis R. O., Rakic P. Hypercolumns in primate visual cortex can develop in the absence of cues from photoreceptors. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5303–5306. doi: 10.1073/pnas.87.14.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary D. D. Do cortical areas emerge from a protocortex? Trends Neurosci. 1989 Oct;12(10):400–406. doi: 10.1016/0166-2236(89)90080-5. [DOI] [PubMed] [Google Scholar]
- Rakic P. Geniculo-cortical connections in primates: normal and experimentally altered development. Prog Brain Res. 1983;58:393–404. doi: 10.1016/S0079-6123(08)60042-4. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974 Feb 1;183(4123):425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal development of the visual system in rhesus monkey. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):245–260. doi: 10.1098/rstb.1977.0040. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976 Jun 10;261(5560):467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988 Jul 8;241(4862):170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rockland K. S., Virga A. Organization of individual cortical axons projecting from area V1 (area 17) to V2 (area 18) in the macaque monkey. Vis Neurosci. 1990 Jan;4(1):11–28. doi: 10.1017/s095252380000273x. [DOI] [PubMed] [Google Scholar]
- Steindler D. A., Cooper N. G., Faissner A., Schachner M. Boundaries defined by adhesion molecules during development of the cerebral cortex: the J1/tenascin glycoprotein in the mouse somatosensory cortical barrel field. Dev Biol. 1989 Jan;131(1):243–260. doi: 10.1016/s0012-1606(89)80056-9. [DOI] [PubMed] [Google Scholar]
- Sur M., Garraghty P. E., Roe A. W. Experimentally induced visual projections into auditory thalamus and cortex. Science. 1988 Dec 9;242(4884):1437–1441. doi: 10.1126/science.2462279. [DOI] [PubMed] [Google Scholar]
- Veraart C., De Volder A. G., Wanet-Defalque M. C., Bol A., Michel C., Goffinet A. M. Glucose utilization in human visual cortex is abnormally elevated in blindness of early onset but decreased in blindness of late onset. Brain Res. 1990 Feb 26;510(1):115–121. doi: 10.1016/0006-8993(90)90735-t. [DOI] [PubMed] [Google Scholar]
- Welker E., Van der Loos H. Is areal extent in sensory cerebral cortex determined by peripheral innervation density? Exp Brain Res. 1986;63(3):650–654. doi: 10.1007/BF00237487. [DOI] [PubMed] [Google Scholar]
- Williams R. W., Rakic P. Elimination of neurons from the rhesus monkey's lateral geniculate nucleus during development. J Comp Neurol. 1988 Jun 15;272(3):424–436. doi: 10.1002/cne.902720310. [DOI] [PubMed] [Google Scholar]
- Williams R. W., Rakic P. Three-dimensional counting: an accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol. 1988 Dec 15;278(3):344–352. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]









