Abstract
Type 2 diabetes (T2D) is a major worldwide public health concern. Despite a large armamentarium of T2D medications, a large proportion of patients fail to achieve recommended treatment goals for glycemic control. Weight loss has profound beneficial effects on the metabolic abnormalities involved in the pathogenesis of T2D. Accordingly, bariatric surgery, which is the most effective available weight loss therapy, is also the most effective therapy for treating patients with T2D. Surgical procedures that bypass the upper gastrointestinal (UGI) tract are particularly effective in achieving partial and even complete remission of T2D, suggesting that UGI bypass has weight loss-independent effects on glycemic control. Although a number of hypotheses (e.g. a role for multi-organ insulin sensitivity, β-cell function, incretin response, the gut microbiome, bile acid metabolism, intestinal glucose metabolism, and browning of adipose tissue) have been proposed to explain the potential unique effects of UGI tract bypass surgery, none has yet been adequately evaluated to determine therapeutic importance in patients with T2D. Here, we review the efficacy of UGI bypass surgery in treating T2D and the mechanisms that have been proposed to explain its potential weight loss-independent therapeutic effects.
Introduction
Type 2 diabetes (T2D) is a major public health problem worldwide, because of its high and increasing prevalence and profound effects on health and quality of life [1]. Despite the large number and variety of medications available to treat T2D, ~50% of patients fail to achieve American Diabetes Association treatment goals [2]. Bariatric surgery is the most effective therapy for achieving glycemic control in patients with T2D. Data from a series of randomized clinical trials have demonstrated the superiority of bariatric surgery over intensive medical therapy in the management of T2D [3–11]. Surgical procedures that bypass the upper gastrointestinal (UGI) tract are particularly effective in achieving partial and complete remission of T2D. This observation has led to the concept that UGI tract bypass surgery has therapeutic weight loss-independent effects on glucose homeostasis. The purpose of this review is to summarize current knowledge of: (i) the regulation of glucose metabolism and pathogenesis of T2D; (ii) the effectiveness of UGI tract bypass surgery in the treatment of T2D; and (iii) the potential mechanisms responsible for weight loss-independent effects of UGI tract bypass on glucose metabolism.
Pathophysiology and pathogenesis of T2D
The diagnosis of T2D is based on fasting plasma glucose concentration, plasma glucose after an oral glucose load, or glycosylated plasma hemoglobin A1c (HbA1c) [12]. Multiple tissues are responsible for maintaining blood glucose levels within a narrow range by regulating endogenous glucose production (via gluconeogenesis and/or glycogenolyis) and its removal from the circulation (via oxidative or non-oxidative glucose disposal).
During postabsorptive conditions, the liver and kidneys produce glucose, which is secreted into the circulation for delivery to other organs. In healthy lean adults, the total endogenous glucose production is ~2 mg/kg body weight/min, of which the kidneys account for 20% [13, 14]. About half of the glucose released into the circulation (~1 mg/kg body weight/min) is taken up by the brain [15, 16], while ~0.5 mg/kg body weight/min [13] ( or ~1–1.5 μmol/100 g tissue/min [17, 18]) is taken up by the liver and gastrointestinal tract, ~0.4 mg/kg body weight/min by the kidneys [14], and ~1 μmol/100g tissue/min by skeletal muscle and adipose tissue [19]. During hyperinsulinemia, skeletal muscle is the major tissue responsible for glucose clearance (~5.5 mg/kg body weight/min [13] or 6 μmol/100 g tissue/min [19]), while insulin-stimulated glucose uptake is ~3 μmol/100 g small intestine/min [18], ~5 μmol/100 g brown adipose tissue (BAT)/min [19], and ~2.5 μmol/100 g white adipose tissue (WAT)/min [19].
The pathogenesis of T2D involves a constellation of metabolic derangements involving multiple organs that cause: (i) inadequate insulin secretion from pancreatic β-cells [20, 21]; (ii) increased glucose production by the liver because of impaired insulin-mediated suppression of hepatic glucose production [22, 23] and increased pancreatic α-cell glucagon secretion [24–27]; (iii) decreased plasma glucose clearance, because of impaired insulin-stimulated glucose disposal in many tissues, particularly skeletal muscle [22, 28]; and (iv) increased renal glucose reabsorption [29].
Bariatric surgery and remission of T2D
Bariatric surgery is an extraordinarily effective therapy for patients with T2D. Data from a series of randomized controlled clinical trials have demonstrated that bariatric surgery results in better glycemic control and greater rates of T2D remission than intensive medical/lifestyle therapy [3–11]. The remission rate reported in different studies varies from 20% to 100%, depending on the patient population, type of surgical procedure, duration of follow-up, and the amount of weight loss achieved. The criteria used to define remission are also a critical determinant of reported remission rates and confound the interpretation of results among studies [30–32]. In 2009, an expert panel developed criteria to determine ‘partial’ and ‘complete’ remission of T2D [33]. Partial remission was defined as HbA1c <6.5%, fasting glucose 100–125 mg/dL (5.6–6.9 mmol/L) of at least 1 year’s duration in the absence of active pharmacological therapy, or ongoing procedures; complete remission was defined as HbA1c in the normal range, fasting glucose <100 mg/dL (5.6 mmol/L) of at least 1 year’s duration in the absence of active pharmacological therapy, or ongoing procedures. However, these criteria have not been universally accepted, and the definition of T2D remission after bariatric surgery continues to vary between studies [4, 8].
Several factors, which reflect the severity of T2D, have been identified that are associated with failure to achieve complete remission of T2D after bariatric surgery, including: (i) poor baseline glycemic control [34–38]; (ii) long duration of T2D [36–39]; and (iii) poor β-cell function [35, 40, 41]. In addition, percent weight loss is associated with remission after surgery, and those who lose more weight are more likely to achieve remission than those who lose less weight [11, 35, 38, 42, 43]. In general, ~50% of patients who achieve remission of T2D will relapse within 5–10 years [4, 44–47]. The most important predictor of relapse is weight regain [44, 46–48]. Preoperative factors associated with relapse include age, poor glycemic control, insulin use, and long duration of T2D [44, 46, 48].
The rate of remission of T2D after bariatric surgery differs among surgical procedures. Data from prospective randomized and non-randomized clinical trials support the notion that bypass of the UGI tract has weight loss-independent therapeutic effects on remission of T2D, and that the length of the bypass influences outcome [3, 7, 10, 49, 50]. The results of randomized controlled trials that evaluated the effect of UGI tract bypass surgery on inducing remission of T2D are presented in Table 1. The results from two randomized clinical trials showed that, at similar relative amounts of weight loss, patients who had Roux-en Y gastric bypass (RYGB) tended to have better glycemic control than those who had sleeve gastrectomy at 1 [7] and 3 years [3] after surgery, and those who had biliopancreatic diversion (BPD) had significantly greater diabetes remission rates than those who had RYGB at 2 [51] and 5 years [4] after surgery. However, it is not known whether all predictors, particularly β-cell function, were the same among groups, which could have influenced the outcomes.
Table 1.
Randomized controlled trials that evaluated the effect of upper gastrointestinal bypass surgery in inducing remission of type 2 diabetes
| Author | Follow- up |
Intervention | Weight loss (%) | Remission criteria | Remission (%) |
|---|---|---|---|---|---|
| Ikramuddin et al. 2013 [5] |
1 year | RYGB: n = 60 MLT: n = 60 |
RYGB: 26. MLT: 7.9 |
HbA1c ≤6.0% | RYGB: 44 MLT: 9 |
| Courcoulas et al. 2014 [6] |
1 year | RYGB: n = 20 LAGB: n = 21 MLT: n = 20 |
RYGB: 27.0 LAGB: 17.3 MLT: 10.2 |
HbA1c <6.5% FPG <126 mg/dL No T2D medication |
RYGB: 50 LAGB: 27 MLT: 0 |
| Schauer et al. 2012 [7] |
1 year | RYGB: n = 50 SG: n = 50 MLT: n = 50 |
RYGB: 27.5 SG: 25.0 MLT: 5.2 |
HbA1c ≤6.0% No T2D medication |
RYGB: 42 SG: 27 MLT: 0 |
| Halperin et al. 2014 [8] |
1 year | RYGB: n = 19 MLT: n = 19 |
BMI decreasea: RYGB: ~9.5 kg/m2 MLT: ~2.2 kg/m2 |
HbA1c <6% FPG <100 mg/dL |
RYGB: 32 MLT: 0 |
| Schauer et al. 2014 [3] |
3 years | RYGB: n = 48 SG: n = 49 MLT: n = 40 |
RYGB: 24.5 SG: 21.1 MLT: 4.2 |
HbA1c ≤6.0% No T2D medication |
RYGB: 35 SG: 20 MLT: 0 |
| Kehagias et al. 2011 [132] |
3 years | RYGB: n = 30, T2D: n = 5 SG: n = 30, T2D n = 5 |
EWLa: RYGB: 62 SG: 68 |
FPG <126 mg/dL 2-h OGTT <200 mg/dL No T2D medication |
RYGB: 80 SG: 80 |
| Risstad et al. 2015 [133] |
5 years | RYGB: n = 31, T2D n = 5 BPD-DS: n = 29, T2D n = 5 |
RYGB: 25.6 BPD-DS: 40.6 |
FPG <100 mg/dL No T2D medication |
RYGB: 80 BPD-DS: 100 |
| Mingrone et al. 2015 [4] |
5 years | RYGB: n = 20 BPD: n = 20 MLT: n = 20 |
RYGB: 28.4 BPD: 31.1 MLT: 7.0 |
HbA1c <6.5% FPG <100 mg/dL No T2D medication |
RYGB: 37 BPD: 63 MLT: 0 |
BMI, body mass index; BPD, biliopancreatic diversion; BPD-DS, biliopancreatic diversion with duodenal switch; EWL, excess weight loss; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin A1c; LAGB. laparoscopic adjustable gastric banding; MLT, medical and/or lifestyle therapy; OGTT, oral glucose tolerance test; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy; T2D, type 2 diabetes.
Alternative measures of weight loss documented in the original manuscripts.
The beneficial long-term effects of bariatric surgery are not limited to remission of T2D. Data from the Swedish Obese Subjects study, which included more than 4000 patients who were followed for up to 20 years, demonstrated that bariatric surgery improves long-term survival, and decreases the incidence ofT2D, myocardial infarction, stroke, and cancer [52]. Nonetheless, bariatric surgery is also associated with complications. Most complications are those that occur with any gastrointestinal surgical procedure, including death, pneumonia, deep vein thrombosis, pulmonary embolism, anastomotic leak with peritonitis, wound infection, gastrointestinal bleeding, and incisional hernias, whereas other complications, such as dumping syndrome, hypoglycemia, and nutritional deficiencies, are related to specific bariatric surgical procedures [4, 52].
Potential weight loss-independent effects of RYGB
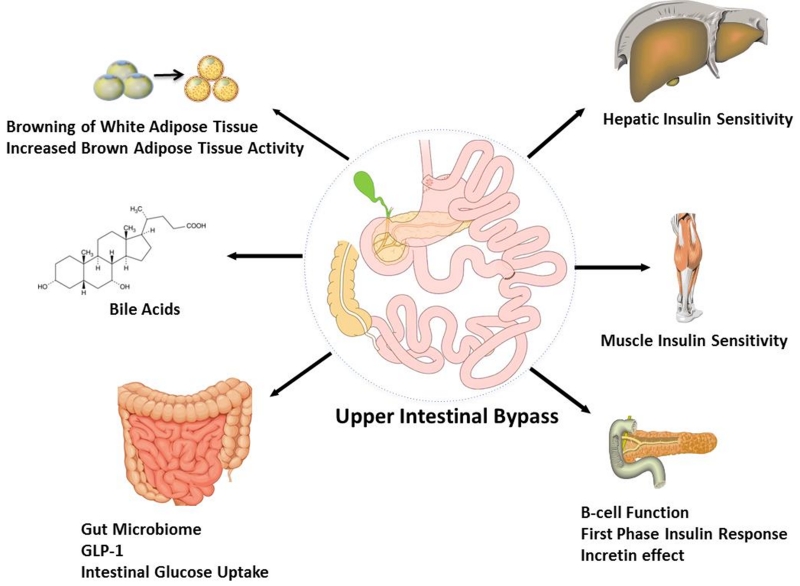
It has been proposed that anatomical bypass of the UGI tract and functional remodeling of the intestine has therapeutic weight loss-independent effects on glycemic control by a number of potential mechanisms (Fig. 1), which can interact with each other, including: (i) insulin sensitivity; (ii) first-phase insulin secretion and incretin effects; (iii) intestinal glucose metabolism; (iv) bile acid (BA) physiology; (v) the gut microbiome; and (vi) browning of adipose tissue.
Fig. 1.
Purported mechanisms involved in the remission of type 2 diabetes in patients who have had upper gastrointestinal bypass surgery. GLP-1, glucagon-like peptide-1.
Insulin sensitivity
Resistance to the action of insulin is a major component of the pathogenesis of T2D [53]. Acute caloric restriction (negative energy balance) rapidly improves hepatic insulin sensitivity [54] due to reduced hepatic glycogen content and glucose production rate [54, 55], whereas a moderate 5% weight loss improves insulin sensitivity in liver, adipose tissue, and skeletal muscle in obese individuals without T2D [56]. The profound effects of calorie restriction and weight loss on insulin sensitivity make it difficult to determine whether UGI tract bypass surgery has weight loss-independent effects on insulin action, because this evaluation requires matching energy intake and weight loss in the comparator group with values in the intestinal bypass group. In addition, differences in the amount of weight loss achieved and the method used to assess insulin sensitivity can affect the results and confound the interpretation of results between studies. Data from most studies do not support a weight loss-independent effect of RYGB on insulin sensitivity. The early improvement in insulin sensitivity, assessed using the homeostasis model assessment of insulin resistance [57] or the intravenous glucose tolerance test [58], after 2–8% weight loss within the first 21 days after RYGB surgery was the same in subjects who had surgery as in control subjects matched on calorie intake and weight loss [59, 60]. In addition, the improvement in insulin sensitivity, assessed using the hyperinsulinemic-euglycemic clamp procedure [61], was the same after ~20% weight loss in subjects who had laparoscopic adjustable gastric banding (LAGB) as in those who had RYGB surgery [10, 62–65]. By contrast, BPD seems to have unique effects on insulin sensitivity, manifested by rapid improvement in insulin sensitivity, assessed using the hyperinsulinemic-euglycemic clamp procedure, after <10% weight loss [66].
First-phase insulin secretion and incretin effect
The ingestion of glucose induces a biphasic secretion of insulin: a rapid first phase followed by a sustained second phase. The first phase of insulin secretion represents the rapid release of insulin from a ‘readily releasable pool’ present in secretory granules within the β-cell and occurs within the first 10 min of glucose ingestion; this phase limits the increase in postprandial glucose concentrations by promoting the systemic clearance of the prandial load and suppressing hepatic glucose production. In addition, oral glucose ingestion causes a much greater increase in plasma insulin concentration than a matched increase in plasma glucose induced by an intravenous glucose infusion [67]. This phenomenon is known as the ‘incretin effect’ because it is believed to be caused by the secretion of the incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotopic polypeptide, released from entero-endocrine L and K cells, respectively [68, 69]. Both the first-phase insulin response and the incretin effect are reduced or absent in individuals with T2D and contribute to postprandial hyperglycemia [70, 71].
The rapid delivery of nutrients into the upper intestine after RYGB surgery causes an early and pronounced increase in plasma glucose, insulin, and GLP-1 concentrations after ingestion of a mixed meal or glucose [65, 72–77]. Moreover, RYGB causes a large increase in the in cretin effect of an oral glucose load [78]. Both the increase in plasma glucose and increase in GLP-1 contribute to the early and high plasma insulin concentrations observed after consumption of a meal or glucose in patients who have had RYGB surgery [79, 80]. Although it seems logical that the improved first-phase insulin response and incretin effect would contribute to achieving remission of T2D after RYGB, this hypothesis has not yet been proven in patients. Pharmacological GLP-1 receptor blockade with exendin (9–39) in patients with T2D who had sleeve gastrectomy resulted in impaired insulin secretion without a deterioration in oral glucose tolerance [81], which underscores the need for similar studies in patients who have had RYGB and who have experienced remission, no remission, relapse, or no relapse of T2D.
Intestinal glucose metabolism
The intestine takes up glucose during both basal conditions and hyperinsulinemia [13, 17, 18]. Data from studies conducted in rodent models have shown that RYGB induces villus hyperplasia and increases villus height in the Roux limb [82–84], and increases intestinal glucose uptake from plasma [85]. In humans, RYGB surgery causes a small decrease in the total amount of glucose delivered to the systemic circulation after ingestion of a mixed meal, but the magnitude of intestinal glucose retention is small and does not cause an improvement in postprandial glycemic control [86]. It was recently found that insulin-stimulated jejunal glucose uptake, assessed using [(18)F]fluoro-2-deoxyglucose positron emission tomography-computed tomography in conjunction with the hyperinsulinemic-euglycemic clamp procedure, increased 6 months after RYGB compared with preoperative baseline values in patients with and without T2D [18]. However, this study did not include a matched diet-induced weight loss group, so it is uncertain whether these results are attributable to weight loss or UGI tract bypass per se.
BA physiology
BAs are synthesized from cholesterol in the liver and are then secreted into the gallbladder, where they are stored. After ingestion of a meal, contraction of the gallbladder results in secretion of BAs into the duodenum, which are subsequently absorbed primarily in the terminal ileum and transported back to the liver via the portal vein, while small amounts are delivered to the colon and excreted in feces [87]. This enterohepatic circulation of BAs occurs many times a day and is important for tightly regulating the BA pool with a minimal amount of de novo synthesis. The primary BAs are those synthesized by the liver and include cholic acid and chenodeoxycholic acid, which can be conjugated to either taurine (taurocholic acid and taurochenodeoxycholic acid) or glycine (glycocholic acid and glycochenodeoxycholic acid) to form BA salts [88, 89]. Primary BAs are dehydroxylated by gut bacteria into secondary BAs (e.g. deoxycholic acid from cholic acid and lithocholic acid from chenodeoxycholic acid).
Although BAs have canonical roles in dietary lipid absorption and in regulating cholesterol metabolism, they also act as hormones by binding to the farnesoid X receptor (FXR) and G protein-coupled bile acid receptor 5 (TGR5), which are highly expressed in hepatocytes and enterocytes and affect glucose, lipid, and energy metabolism [90–92]. BAs can regulate glucose metabolism through FXR-mediated pathways that increase glycolysis, decrease gluconeogenesis, stimulate hepatic glycogen synthesis, and increase insulin sensitivity and glucose disposal [93, 94]. BAs can also activate TGR5 in L cells to stimulate GLP-1 secretion [95]. The effect of BAs on metabolic function is extraordinarily complex, because of the large number of different BAs and differences in their ability to activate FXR and TGR5 receptors [96–98]. Therefore, in order to understand the potential metabolic effect of alterations of BAs induced by bariatric surgery it is important to understand the changes in individual BAs, not simply alterations in total serum BA concentration.
Weight loss induced by RYGB surgery increases both fasting and postprandial serum BA levels [89, 99–103]. Although this observation has led to the hypothesis that increased circulating levels of BAs contribute to the beneficial effects of RYGB surgery on β-cell function and hepatic and muscle insulin sensitivity, there is no direct evidence demonstrating a causal relationship between BAs and improved metabolic function in patients who have had RYGB surgery. Moreover, the relationship between serum BAs and glucose control after RYGB is unclear because of conflicting data from different studies, with some studies demonstrating a dissociation between an increase in serum BAs and an improvement in metabolic outcomes [101, 104, 105]. For example, the results from one study showed a direct correlation between the change in postprandial serum BAs and peak GLP-1 concentrations after matched weight loss induced by either LAGB or RYGB surgery, but improvement in β-cell function and insulin sensitivity was the same in both groups even though basal and postprandial serum BA concentrations decreased after LAGB but increased after RYGB surgery [103]. In two other studies that evaluated subjects before and at 1 week, 3 months, and 1 year after RYGB surgery it was found that the increase in postprandial GLP-1 concentration and improvements in β-cell function, oral glucose tolerance and insulin sensitivity occurred before there was an increase in postprandial BA concentration [101, 104]. The complexity of the relationship between BAs and metabolic function makes it difficult to attribute changes in plasma BA concentrations and composition to changes in metabolic function.
Gut microbiome
Alterations in the composition and diversity of the gut microbiome are associated with obesity and T2D in humans [106–108]. The potential causal relationship between the gut microbiome and metabolic function is supported by data from studies demonstrating that transplantation of fecal microbiota from metabolically abnormal individuals can transmit the abnormal phenotype to gnotobiotic mice [109] and transplantation of fecal microbiota from healthy lean donors to those with metabolic syndrome improves their insulin sensitivity [109].
Few studies have evaluated the effect of RYGB surgery on the human gut microbiome. The available data demonstrate that weight loss induced by RYGB is associated with an increase in the microbial diversity/richness (number of different types of bacteria). There is also a change in the relative amounts of specific bacterial phyla and species, but these changes are not consistent among studies [110–113]. The mechanism responsible for the change in gut bacteria after RYGB is not known, but is likely to involve a combination of factors, such as changes in body weight, dietary intake, nutrient flow through the intestine, gut motility, intraluminal pH, and bile flow [114–117]. Although it has been proposed that bypass of the UGI tract causes unique changes in the gut microbiome that contribute to the beneficial metabolic effects of RYGB, similar changes in gut microbiome are also found after vertical banded gastroplasty [118]. Moreover, it is not known whether the changes in the microbiome are simply associated with, or contribute to, the metabolic benefits of surgery. Transfer of the fecal microbiota from patients who had RYGB or vertical banded gastroplasty to germ-free mice resulted in reduced accumulation of body fat in recipient mice, but the weight-independent effects on metabolic function were not evaluated [118]. Together, data from studies conducted in humans demonstrate that profound alterations in the gut microbiome are caused by bariatric surgery-induced weight loss. Additional studies are needed to determine whether these changes are specific to surgery itself or diet-related weight loss, and whether they confer weight loss-independent therapeutic effects on glucose homeostasis.
Browning of subcutaneous WAT
BAT is primarily located in the supraclavicular adipose tissue depot in adults [119–121]. Brown adipocytes contain high numbers of mitochondria, rich in uncoupling protein 1, which uncouples oxidative phosphorylation from ATP production leading to heat generation [122]. When activated (by cold, insulin, or other stimuli), brown adipocytes can increase their glucose uptake 12-fold [19], suggesting they might be important in whole-body glucose homeostasis [123]. In fact, there is evidence that BAT increases whole-body insulin sensitivity with respect to glucose metabolism [123, 124]. A low amount of BAT in individuals is associated with excess adiposity and T2D [119, 125], whereas weight loss induced by a very low-calorie diet [126], gastric banding [127], or RYGB [128] increases BAT activity. Although RYGB surgery causes an increase in plasma concentrations of BAs and GLP-1, which can increase BAT metabolic activity and cause browning of WAT [129–131], it is not known whether RYGB-induced weight loss causes a greater increase in BAT than calorie restriction alone.
Conclusion
Bariatric surgery is currently the most effective therapy for T2D and results in remission in many patients. Bypass of the UGI tract has profound effects on the metabolic response to ingestion of glucose or a mixed meal and on BA physiology. The greater rate of complete remission of T2D after RYGB than after similar weight loss induced by sleeve gastrectomy, and the greater rate of remission after BPD than after similar weight loss induced by RYGB, suggest that bypass of the UGI tract and the length of the bypass have weight loss-independent effects on glycemic control. Although several hypotheses have been proposed to try to explain the potential unique effects of RYGB surgery in achieving remission of T2D, none has yet been adequately evaluated in studies conducted in patients.
Acknowledgments
This study was supported by National Institutes of Health grants DK101578, DK94483, DK 56341 (Nutrition Obesity Research Center), DK20579 (Diabetes Research Center), and RR024992 (Clinical and Translational Science Award).
Footnotes
Conflict of interest statement
S.K. is a shareholder of Aspire Bariatrics and has served on scientific advisory boards for Takeda Pharmaceuticals and NovoNordisk.
References
- 1.World Health Organization . Global report on diabetes. Geneva: 2016. [Google Scholar]
- 2.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care. 2013;36:2271–9. doi: 10.2337/dc12-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002–13. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386:964–73. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 5.Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309:2240–9. doi: 10.1001/jama.2013.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA surgery. 2014;149:707–15. doi: 10.1001/jamasurg.2014.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–76. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA surgery. 2014;149:716–26. doi: 10.1001/jamasurg.2014.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wentworth JM, Playfair J, Laurie C, et al. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: a randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2:545–52. doi: 10.1016/S2213-8587(14)70066-X. [DOI] [PubMed] [Google Scholar]
- 10.Kashyap SR, Bhatt DL, Wolski K, et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care. 2013;36:2175–82. doi: 10.2337/dc12-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 12.Standards of Medical Care in Diabetes—2016: Summary of Revisions. Diabetes Care. 2016;39:S4–S5. doi: 10.2337/dc16-S003. [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667–87. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 14.Stumvoll M, Chintalapudi U, Perriello G, Welle S, Gutierrez O, Gerich J. Uptake and release of glucose by the human kidney. Postabsorptive rates and responses to epinephrine. J Clin Invest. 1995;96:2528–33. doi: 10.1172/JCI118314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinmuth OM, Scheinberg P, Bourne B. Total cerebral blood flow and metabolism: A new method for the repeated serial measurement of total cerbral blood flow using iodoantipyrine (1131) with a report of determination in normal human beings of blood flow, oxygen consumption, glucose utilization and respiratory quotient of the whole brain. Arch Neurol. 1965;12:49–66. [Google Scholar]
- 16.Huang SC, Phelps ME, Hoffman EJ, Sideris K, Selin CJ, Kuhl DE. Noninvasive determination of local cerebral metabolic rate of glucose in man. Am J Physiol. 1980;238:E69–82. doi: 10.1152/ajpendo.1980.238.1.E69. [DOI] [PubMed] [Google Scholar]
- 17.Immonen H, Hannukainen JC, Iozzo P, et al. Effect of bariatric surgery on liver glucose metabolism in morbidly obese diabetic and non-diabetic patients. J Hepatol. 2014;60:377–83. doi: 10.1016/j.jhep.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Makinen J, Hannukainen JC, Karmi A, et al. Obesity-associated intestinal insulin resistance is ameliorated after bariatric surgery. Diabetologia. 2015;58:1055–62. doi: 10.1007/s00125-015-3501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orava J, Nuutila P, Lidell ME, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14:272–9. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH. Sequential changes in serum insulin concentration during development of non-insulin-dependent diabetes. Lancet. 1989;1:1356–9. doi: 10.1016/s0140-6736(89)92804-3. [DOI] [PubMed] [Google Scholar]
- 21.Zimmet P, Whitehouse S, Alford F, Chisholm D. The relationship of insulin response to a glucose stimulus over a wide range of glucose tolerance. Diabetologia. 1978;15:23–7. doi: 10.1007/BF01219323. [DOI] [PubMed] [Google Scholar]
- 22.Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84:205–13. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrannini E, Simonson DC, Katz LD, et al. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism. 1988;37:79–85. doi: 10.1016/0026-0495(88)90033-9. [DOI] [PubMed] [Google Scholar]
- 24.Baron AD, Schaeffer L, Shragg P, Kolterman OG. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes. 1987;36:274–83. doi: 10.2337/diab.36.3.274. [DOI] [PubMed] [Google Scholar]
- 25.Cervera A, Wajcberg E, Sriwijitkamol A, et al. Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab. 2008;294:E846–52. doi: 10.1152/ajpendo.00030.2008. [DOI] [PubMed] [Google Scholar]
- 26.Matsuda M, Defronzo RA, Glass L, Consoli A, Giordano M, Bressler P, Delprato S. Glucagon dose-response curve for hepatic glucose production and glucose disposal in type 2 diabetic patients and normal individuals. Metabolism. 2002;51:1111–9. doi: 10.1053/meta.2002.34700. [DOI] [PubMed] [Google Scholar]
- 27.Unger RH, Aguilar-Parada E, Muller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970;49:837–48. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeFronzo RA, Gunnarsson R, Björkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985;76:149–55. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54:3427–34. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 30.Blackstone R, Bunt JC, Cortes MC, Sugerman HJ. Type 2 diabetes after gastric bypass: remission in five models using HbA1c, fasting blood glucose, and medication status. Surg Obes Relat Dis. 2012;8:548–55. doi: 10.1016/j.soard.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Pournaras DJ, Aasheim ET, Sovik TT, et al. Effect of the definition of type II diabetes remission in the evaluation of bariatric surgery for metabolic disorders. Br J Surg. 2012;99:100–3. doi: 10.1002/bjs.7704. [DOI] [PubMed] [Google Scholar]
- 32.Mas-Lorenzo A, Benaiges D, Flores-Le-Roux JA, et al. Impact of different criteria on type 2 diabetes remission rate after bariatric surgery. Obes Surg. 2014;24:1881–7. doi: 10.1007/s11695-014-1282-2. [DOI] [PubMed] [Google Scholar]
- 33.Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32:2133–5. doi: 10.2337/dc09-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunn JP, Abumrad NN, Breitman I, et al. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care. 2012;35:137–42. doi: 10.2337/dc11-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dutia R, Brakoniecki K, Bunker P, et al. Limited recovery of beta-cell function after gastric bypass despite clinical diabetes remission. Diabetes. 2014;63:1214–23. doi: 10.2337/db13-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee W-J, Hur KY, Lakadawala M, et al. Predicting success of metabolic surgery: age, body mass index, C-peptide, and duration score. Surgery for Obesity and Related Diseases. 2013;9:379–84. doi: 10.1016/j.soard.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Dixon JB, Chuang LM, Chong K, et al. Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care. 2013;36:20–6. doi: 10.2337/dc12-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panunzi S, Carlsson L, De Gaetano A, et al. Determinants of Diabetes Remission and Glycemic Control After Bariatric Surgery. Diabetes Care. 2016;39:166–74. doi: 10.2337/dc15-0575. [DOI] [PubMed] [Google Scholar]
- 39.Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311:2297–304. doi: 10.1001/jama.2014.5988. [DOI] [PubMed] [Google Scholar]
- 40.Lund MT, Hansen M, Skaaby S, et al. Preoperative beta-cell function in patients with type 2 diabetes is important for the outcome of Roux-en-Y gastric bypass surgery. J Physiol. 2015;593:3123–33. doi: 10.1113/JP270264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khanna V, Malin SK, Bena J, et al. Adults with long-duration type 2 diabetes have blunted glycemic and beta-cell function improvements after bariatric surgery. Obesity (Silver Spring) 2015;23:523–6. doi: 10.1002/oby.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kadera BE, Lum K, Grant J, Pryor AD, Portenier DD, DeMaria EJ. Remission of type 2 diabetes after Roux-en-Y gastric bypass is associated with greater weight loss. Surg Obes Relat Dis. 2009;5:305–9. doi: 10.1016/j.soard.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–84. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 84-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013;23:93–102. doi: 10.1007/s11695-012-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DiGiorgi M, Rosen DJ, Choi JJ, et al. Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis. 2010;6:249–53. doi: 10.1016/j.soard.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 46.Chikunguwo SM, Wolfe LG, Dodson P, et al. Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010;6:254–9. doi: 10.1016/j.soard.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Brethauer SA, Aminian A, Romero-Talamas H, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013;258:628–36. doi: 10.1097/SLA.0b013e3182a5034b. discussion 36-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jimenez A, Casamitjana R, Flores L, Viaplana J, Corcelles R, Lacy A, Vidal J. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012;256:1023–9. doi: 10.1097/SLA.0b013e318262ee6b. [DOI] [PubMed] [Google Scholar]
- 49.Abbatini F, Rizzello M, Casella G, Alessandri G, Capoccia D, Leonetti F, Basso N. Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg Endosc. 2010;24:1005–10. doi: 10.1007/s00464-009-0715-9. [DOI] [PubMed] [Google Scholar]
- 50.Yska JP, van Roon EN, de Boer A, et al. Remission of Type 2 Diabetes Mellitus in Patients After Different Types of Bariatric Surgery: A Population-Based Cohort Study in the United Kingdom. JAMA surgery. 2015;150:1126–33. doi: 10.1001/jamasurg.2015.2398. [DOI] [PubMed] [Google Scholar]
- 51.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–85. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 52.Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–34. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 53.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136:1552–60. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rothman DL, Magnusson I, Katz LD, Shulman RG, Shulman GI. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science. 1991;254:573–6. doi: 10.1126/science.1948033. [DOI] [PubMed] [Google Scholar]
- 56.Magkos F, Fraterrigo G, Yoshino J, et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016 doi: 10.1016/j.cmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 58.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456–67. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Isbell JM, Tamboli RA, Hansen EN, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33:1438–42. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jackness C, Karmally W, Febres G, et al. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and beta-cell Function in type 2 diabetic patients. Diabetes. 2013;62:3027–32. doi: 10.2337/db12-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen KT, Billington CJ, Vella A, et al. Preserved Insulin Secretory Capacity and Weight Loss Are the Predominant Predictors of Glycemic Control in Patients With Type 2 Diabetes Randomized to Roux-en-Y Gastric Bypass. Diabetes. 2015;64:3104–10. doi: 10.2337/db14-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bradley D, Magkos F, Eagon JC, et al. Matched weight loss induced by sleeve gastrectomy or gastric bypass similarly improves metabolic function in obese subjects. Obesity (Silver Spring) 2014;22:2026–31. doi: 10.1002/oby.20803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peterli R, Wolnerhanssen B, Peters T, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250:234–41. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- 65.Bradley D, Conte C, Mittendorfer B, et al. Gastric bypass and banding equally improve insulin sensitivity and beta cell function. J Clin Invest. 2012;122:4667–74. doi: 10.1172/JCI64895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bradley D, Magkos F, Klein S. Effects of bariatric surgery on glucose homeostasis and type 2 diabetes. Gastroenterology. 2012;143:897–912. doi: 10.1053/j.gastro.2012.07.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perley M, Kipnis DM. Plasma insulin responses to glucose and tolbutamide of normal weight and obese diabetic and nondiabetic subjects. Diabetes. 1966;15:867–74. doi: 10.2337/diab.15.12.867. [DOI] [PubMed] [Google Scholar]
- 68.Meier JJ, Holst JJ, Schmidt WE, Nauck MA. Reduction of hepatic insulin clearance after oral glucose ingestion is not mediated by glucagon-like peptide 1 or gastric inhibitory polypeptide in humans. Am J Physiol Endocrinol Metab. 2007;293:E849–56. doi: 10.1152/ajpendo.00289.2007. [DOI] [PubMed] [Google Scholar]
- 69.Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199–206. doi: 10.1152/ajpendo.00545.2003. [DOI] [PubMed] [Google Scholar]
- 70.Nauck M, Stockmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- 71.Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest. 2011;121:2118–25. doi: 10.1172/JCI45680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jorgensen NB, Jacobsen SH, Dirksen C, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303:E122–31. doi: 10.1152/ajpendo.00073.2012. [DOI] [PubMed] [Google Scholar]
- 73.Guidone C, Manco M, Valera-Mora E, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55:2025–31. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- 74.Basso N, Capoccia D, Rizzello M, et al. First-phase insulin secretion, insulin sensitivity, ghrelin, GLP-1, and PYY changes 72 h after sleeve gastrectomy in obese diabetic patients: the gastric hypothesis. Surg Endosc. 2011;25:3540–50. doi: 10.1007/s00464-011-1755-5. [DOI] [PubMed] [Google Scholar]
- 75.Nguyen NQ, Debreceni TL, Bambrick JE, et al. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity (Silver Spring) 2014;22:2003–9. doi: 10.1002/oby.20791. [DOI] [PubMed] [Google Scholar]
- 76.Dirksen C, Hansen DL, Madsbad S, Hvolris LE, Naver LS, Holst JJ, Worm D. Postprandial diabetic glucose tolerance is normalized by gastric bypass feeding as opposed to gastric feeding and is associated with exaggerated GLP-1 secretion: a case report. Diabetes Care. 2010;33:375–7. doi: 10.2337/dc09-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chambers AP, Smith EP, Begg DP, et al. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab. 2014;306:E424–32. doi: 10.1152/ajpendo.00469.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laferrere B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–85. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shah M, Law JH, Micheletto F, et al. Contribution of endogenous glucagon-like peptide 1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes. 2014;63:483–93. doi: 10.2337/db13-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jorgensen NB, Dirksen C, Bojsen-Moller KN, et al. Exaggerated glucagon-like peptide 1 response is important for improved beta-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62:3044–52. doi: 10.2337/db13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jimenez A, Mari A, Casamitjana R, Lacy A, Ferrannini E, Vidal J. GLP-1 and glucose tolerance after sleeve gastrectomy in morbidly obese subjects with type 2 diabetes. Diabetes. 2014;63:3372–7. doi: 10.2337/db14-0357. [DOI] [PubMed] [Google Scholar]
- 82.Hansen CF, Bueter M, Theis N, et al. Hypertrophy dependent doubling of L-cells in Roux-en-Y gastric bypass operated rats. PloS one. 2013;8:e65696. doi: 10.1371/journal.pone.0065696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mumphrey MB, Patterson LM, Zheng H, Berthoud HR. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol Motil. 2013;25:e70–9. doi: 10.1111/nmo.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stearns AT, Balakrishnan A, Tavakkolizadeh A. Impact of Roux-en-Y gastric bypass surgery on rat intestinal glucose transport. Am J Physiol Gastrointest Liver Physiol. 2009;297:G950–7. doi: 10.1152/ajpgi.00253.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341:406–10. doi: 10.1126/science.1235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Magkos F, Bradley D, Eagon JC, Patterson BW, Klein S. Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on gastrointestinal metabolism of ingested glucose. Am J Clin Nutr. 2015 doi: 10.3945/ajcn.115.116111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma H, Patti ME. Bile acids, obesity, and the metabolic syndrome. Best Practice & Research Clinical Gastroenterology. 2014;28:573–83. doi: 10.1016/j.bpg.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chiang JY. Regulation of bile acid synthesis. Front Biosci. 1998;3:d176–93. doi: 10.2741/a273. [DOI] [PubMed] [Google Scholar]
- 89.Simonen M, Dali-Youcef N, Kaminska D, et al. Conjugated Bile Acids Associate with Altered Rates of Glucose and Lipid Oxidation after Roux-en-Y Gastric Bypass. Obes Surg. 2012;22:1473–80. doi: 10.1007/s11695-012-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pols TWH, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The Bile Acid Membrane Receptor TGR5: A Valuable Metabolic Target. Dig Dis. 2011;29:37–44. doi: 10.1159/000324126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–91. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 92.Jiang C, Xie C, Lv Y, et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun. 2015;6:10166. doi: 10.1038/ncomms10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Y, Lee FY, Barrera G, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103:1006–11. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cariou B, van Harmelen K, Duran-Sandoval D, et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem. 2006;281:11039–49. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 95.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–90. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 96.Song P, Rockwell CE, Cui JY, Klaassen CD. Individual bile acids have differential effects on bile acid signaling in mice. Toxicol Appl Pharmacol. 2015;283:57–64. doi: 10.1016/j.taap.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–8. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 98.Sato H, Macchiarulo A, Thomas C, et al. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J Med Chem. 2008;51:1831–41. doi: 10.1021/jm7015864. [DOI] [PubMed] [Google Scholar]
- 99.Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1671–7. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ferrannini E, Camastra S, Astiarraga B, et al. Increased Bile Acid Synthesis and Deconjugation After Biliopancreatic Diversion. Diabetes. 2015;64:3377–85. doi: 10.2337/db15-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jorgensen NB, Dirksen C, Bojsen-Moller KN, et al. Improvements in glucose metabolism early after gastric bypass surgery are not explained by increases in total bile acids and fibroblast growth factor 19 concentrations. J Clin Endocrinol Metab. 2015;100:E396–406. doi: 10.1210/jc.2014-1658. [DOI] [PubMed] [Google Scholar]
- 102.Sachdev S, Wang Q, Billington C, et al. FGF 19 and Bile Acids Increase Following Roux-en-Y Gastric Bypass but Not After Medical Management in Patients with Type 2 Diabetes. Obes Surg. 2015 doi: 10.1007/s11695-015-1834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98:E708–12. doi: 10.1210/jc.2012-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Steinert RE, Peterli R, Keller S, Meyer-Gerspach AC, Drewe J, Peters T, Beglinger C. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity (Silver Spring) 2013;21:E660–8. doi: 10.1002/oby.20522. [DOI] [PubMed] [Google Scholar]
- 105.Sachdev S, Wang Q, Billington C, et al. FGF 19 and Bile Acids Increase Following Roux-en-Y Gastric Bypass but Not After Medical Management in Patients with Type 2 Diabetes. Obes Surg. 2016;26:957–65. doi: 10.1007/s11695-015-1834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Karlsson FH, Tremaroli V, Nookaew I, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 107.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 108.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106:2365–70. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Graessler J, Qin Y, Zhong H, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13:514–22. doi: 10.1038/tpj.2012.43. [DOI] [PubMed] [Google Scholar]
- 112.Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–57. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kong LC, Tap J, Aron-Wisnewsky J, et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98:16–24. doi: 10.3945/ajcn.113.058743. [DOI] [PubMed] [Google Scholar]
- 114.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Santacruz A, Marcos A, Warnberg J, et al. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity (Silver Spring) 2009;17:1906–15. doi: 10.1038/oby.2009.112. [DOI] [PubMed] [Google Scholar]
- 116.Smith CD, Herkes SB, Behrns KE, Fairbanks VF, Kelly KA, Sarr MG. Gastric acid secretion and vitamin B12 absorption after vertical Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 1993;218:91–6. doi: 10.1097/00000658-199307000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ishida RK, Faintuch J, Paula AM, et al. Microbial flora of the stomach after gastric bypass for morbid obesity. Obes Surg. 2007;17:752–8. doi: 10.1007/s11695-007-9139-6. [DOI] [PubMed] [Google Scholar]
- 118.Tremaroli V, Karlsson F, Werling M, et al. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015;22:228–38. doi: 10.1016/j.cmet.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 121.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 122.Porter C, Chondronikola M, Sidossis LS. The Therapeutic Potential of Brown Adipocytes in Humans. Front Endocrinol (Lausanne) 2015;6:156. doi: 10.3389/fendo.2015.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chondronikola M, Volpi E, Borsheim E, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–99. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee P, Smith S, Linderman J, et al. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014;63:3686–98. doi: 10.2337/db14-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–31. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Orava J, Nuutila P, Noponen T, et al. Blunted metabolic responses to cold and insulin stimulation in brown adipose tissue of obese humans. Obesity (Silver Spring) 2013;21:2279–87. doi: 10.1002/oby.20456. [DOI] [PubMed] [Google Scholar]
- 127.Vijgen GH, Bouvy ND, Teule GJ, Brans B, Hoeks J, Schrauwen P, van Marken Lichtenbelt WD. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2012;97:E1229–33. doi: 10.1210/jc.2012-1289. [DOI] [PubMed] [Google Scholar]
- 128.Rachid B, van de Sande-Lee S, Rodovalho S, et al. Distinct regulation of hypothalamic and brown/beige adipose tissue activities in human obesity. Int J Obes (Lond) 2015;39:1515–22. doi: 10.1038/ijo.2015.94. [DOI] [PubMed] [Google Scholar]
- 129.Beiroa D, Imbernon M, Gallego R, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63:3346–58. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- 130.Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–9. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 131.Broeders Evie PM, Nascimento Emmani BM, Havekes B, et al. The Bile Acid Chenodeoxycholic Acid Increases Human Brown Adipose Tissue Activity. Cell Metab. 22:418–26. doi: 10.1016/j.cmet.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 132.Kehagias I, Karamanakos SN, Argentou M, Kalfarentzos F. Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI < 50 kg/m2. Obes Surg. 2011;21:1650–6. doi: 10.1007/s11695-011-0479-x. [DOI] [PubMed] [Google Scholar]
- 133.Risstad H, Sovik TT, Engstrom M, et al. Five-year outcomes after laparoscopic gastric bypass and laparoscopic duodenal switch in patients with body mass index of 50 to 60: a randomized clinical trial. JAMA surgery. 2015;150:352–61. doi: 10.1001/jamasurg.2014.3579. [DOI] [PubMed] [Google Scholar]