Abstract
BACKGROUND
Intraindividual blood pressure (BP) fluctuates dynamically over time. Previous studies suggested an adverse link between greater visit-to-visit variability (VVV) in systolic blood pressure (SBP) and various outcomes. However, these studies have significant limitations, such as a small size, inclusion of selected populations, and restricted outcomes.
OBJECTIVES
We investigated the association of increased VVV and all-cause mortality, cardiovascular events, and end-stage renal disease (ESRD) in a large cohort of U.S. veterans.
METHODS
From among 3,285,684 U.S. veterans with and without hypertension and normal estimated glomerular filtration rates (eGFR) during 2005 and 2006, we identified 2,865,157 patients who had 8 or more outpatient BP measurements. SBP variability (SBPV) was measured using the SD of all SBP values (normally distributed) in 1 individual. Associations of SD quartiles (<10.3, 10.3 to 12.7, 12.7 to 15.6, and ≥15.6 mm Hg) with all-cause mortality, incident coronary heart disease (CHD), stroke, and ESRD was examined using Cox models adjusted for sociodemographic characteristics, baseline eGFR, comorbidities, body mass index, SBP, diastolic BP, and antihypertensive medication use.
RESULTS
Several sociodemographic variables (older age, male sex, African-American race, divorced or widowed status) and clinical characteristics (lower baseline eGFR, higher SBP and DBP), and comorbidities (presence of diabetes, hypertension, cardiovascular disease, and lung disease) were all associated with higher intraindividual SBPV. The multivariable adjusted hazard ratios and 95% confidence intervals for SD quartiles 2 through 4 (compared to the first quartile) associated with all-cause mortality, CHD, stroke, and ESRD were incrementally higher.
CONCLUSIONS
Higher SBPV in individuals with and without hypertension was associated with increased risks of all-cause mortality, CHD, stroke, and ESRD. Further studies are needed to determine interventions that can lower SBPV and their impact on adverse health outcomes.
Keywords: hypertension, outcomes, visit-to-visit variability
Elevated blood pressure (BP) is the most common chronic medical disease observed in a variety of populations (1). It has been consistently demonstrated that higher baseline BP - both untreated and treated - is associated with a higher risk for all-cause mortality, cardiovascular morbidity and mortality, and end-stage renal disease (ESRD) (2–8). However, BP does not remain steady but instead fluctuates continually, within a 24-h period, from day to day, and from month to month (9). Furthermore, these fluctuations are not random and tend to remain consistent within patients (10,11). Therefore, the traditional correlation between baseline BP and outcomes of interest has a potential to underestimate the true risk of elevated BP. This phenomenon, known as “regression dilution,” arises from the combined effects of measurement errors and both short-term (diurnal and seasonal) and long-term (changes in BP with aging, antihypertensive medication use, and adherence to antihypertensive medications) within-individual variability of BP (12). A large meta-analysis from the Prospective Study Group unequivocally demonstrated that the mean or “usual” BP, corrected for regression dilution, was strongly and linearly related to increased risk of mortality from coronary heart disease (CHD), stroke, and other vascular causes (13). However, even averaging BP over longer periods of follow-up may not fully address risks associated with BP variations. Visit-to-visit variability (VVV) of BP is being increasingly considered as a newer method to evaluate intraindividual BP fluctuations.
Higher systolic BP variability (SBPV) has been shown to be a better predictor of all-cause and cardiovascular mortality (14–16), stroke (17,18), and cardiac disease (5,19–21), compared to average systolic blood pressure (SBP). Nevertheless, a strong adverse association between increased SBPV and cardiovascular disease (CVD) and stroke was not confirmed in all studies (21,22); additionally, some studies found associations between higher SBPV with all-cause mortality, but not with stroke or coronary events (20). Furthermore, these previous clinical observations have important limitations, such as being restricted to very specific or high-risk populations, having a small number of BP measurements and a small sample size, or assessing only selected outcomes. Therefore, we conducted a large cohort study involving 2,865,157 U.S. veterans who had at least 8 outpatient BP measurements to examine the prognostic significance of increased VVV of SBP on all-cause mortality, CHD, stroke, and ESRD.
Methods
We used data from a historic cohort study examining risk factors in patients with incident chronic kidney disease consisting of 3,582,478 patients with an estimated glomerular filtration rate (eGFR) of ≥60 ml/min/1.73 m2, based on serum creatinine measurements performed during October 1, 2004, to September 30, 2006 in any U.S. Department of Veterans Affairs (VA) facility (23–25). Of the 3,492,943 patients with any available SBP measurements, we excluded 5,466 with only inpatient SBP measurements and 622,320 patients who had ≤7 SBP records during follow-up. Our final analytic sample consisted of 2,865,157 patients (Online Figure 1).
Sociodemographic Characteristics and Comorbidities
Cohort entry was defined as the date of the first eGFR measurement of ≥60 ml/min/1.73 m2 during October 1, 2004, to September 30, 2006. Information about baseline age, race, sex, marital status, per capita income, comorbid conditions, body mass index (BMI), service connectedness (indicating whether comorbidities were directly caused by military service, resulting in certain privileges such as preferential access to care and lower copayments), and receipt of influenza vaccinations during the cohort entry period, and frequency of health care encounters (defined as the number of health care visits/year throughout the entire follow-up period) were obtained from national VA research data files, as previously described (26–28). Race was determined by combining information from VA sources with those obtained from Medicare through the VA-Medicare data merge project (29). In case of discrepancies, we used the race determination from Medicare due to its more accurate nature (30). Comorbidities and clinical events were assessed from the VA Inpatient and Outpatient Medical SAS Datasets using International Classification of Diseases, Ninth Revision (ICD-9) diagnostic and procedure codes and Current Procedural Terminology (CPT) codes (23). Baseline (prevalent) comorbidities were defined as the presence of relevant ICD-9 and CPT codes recorded during the cohort entry period. In addition to VA data, we extracted select socioeconomic indicators using 2004 county typology codes (housing stress, low education, low employment, and persistent poverty) based on patients’ home addresses, obtained from the Area Health Resources Files (AHRF) system. Geographic variation was examined by grouping Veterans Integrated Service Networks (VISNs) into 4 regions: Northeast (VISNs 1, 2, 4), Midwest (VISNs 10, 12, 15, 23), South (VISNs 5, 6, 7, 8, 9, 16, 17), and West (VISNs 19, 20, 21, 22) (31).
Blood Pressure and Medication Use
Information about BP was collected from the date of cohort entry until the end of follow-up (death, last VA contact, or July 26, 2013). All BP values measured during outpatient clinical encounters in any VA facility throughout follow-up were recorded. In the case of multiple BP measurements taken on the same day, we selected only the last BP value. We expressed visit-to-visit SBP variability as the SD of the longitudinal intraindividual SBP measurements in each patient. VA pharmacy dispensation records (32) were used to assess baseline exposure to different classes of antihypertensive medications and to statin type of cholesterol-lowering medications.
Outcomes
Outcomes of interest were all-cause mortality, CHD, incident ischemic strokes, and ESRD. Deaths were identified from the VA Vital Status Files, the sensitivity and specificity of which (using the U.S. National Death index as gold standard) are 98.3% and 99.8% respectively (33). Incident CHD was defined as the composite of a first occurrence of an ICD-9 or CPT code for acute myocardial infarction, percutaneous angioplasty, or coronary artery bypass grafting after October 1, 2006, in patients without such diagnoses prior to this date. Incident ischemic stroke was defined as the first occurrence of an ICD-9 code for ischemic stroke following the date of October 1, 2006, in patients without such diagnosis prior to this date. Information on ESRD (defined as initiation of renal replacement therapy) was obtained from the United States Renal Data System.
Statistical Analysis
Data were expressed as mean ± SD, median (interquartile range), and proportions, and examined across SD quartiles. Due to the large sample size, traditional statistical testing of differences in baseline characteristics was not carried out. The start of the follow-up period was the date of cohort entry for analyses of mortality and ESRD, and October 1, 2006, for incident CHD and stroke. Patients were followed until death or were censored at the date of the last health care or administrative VA encounter, or on July 26, 2013, for mortality, CHD and stroke, and December 31, 2011, for ESRD.
The association of SBP SD categories with the outcomes of interest was examined in crude (model 1) and multivariable adjusted Cox models. Models were adjusted sequentially based on a priori considerations for baseline values of age, sex, race, and baseline eGFR (model 2); baseline comorbidities such as diabetes mellitus, hypertension, CVD, congestive heart failure [CHF], cerebrovascular disease, peripheral arterial disease, chronic lung disease, dementia, rheumatoid diseases, peptic ulcer disease, chronic liver disease, hemiplegia, malignancies, human immunodeficiency virus/acquired immunodeficiency syndrome , depression, and BMI (model 3); per capita income, marital status, service connectedness, receipt of statins and influenza vaccination(s), frequency of health care visits, and living in areas with high housing stress, low education, low employment and persistent poverty, and medical diagnosis of non-adherence (the V15.81 code from ICD-9) (model 4); and baseline SBP and diastolic BP, and receipt of different antihypertensive medication classes at baseline (renin-angiotensin system inhibitors, beta-blockers, alpha blockers, vasodilators, thiazide diuretics, loop diuretics, potassium-sparing diuretics and centrally-acting alpha agonists), and geographic region (model 5). A total of 2,322,759 patients (81% of the total sample) had complete data for analysis in the final multivariable models. We used unimputed (complete data) analyses and we substituted missing data by performing multiple imputations. Sensitivity analyses were performed to examine the association of SBP SD with all outcomes of interest in subgroups of patients with different number of SBP measurements available for SD calculation and in various subgroups. Adherence to antihypertensive medications was estimated by proportion of days covered (PDC, defined as percentage of days a subject had medication available) (33–35). PDC was calculated in a subgroup of individuals prescribed antihypertensive medications (n = 611,249). Good adherence was considered as PDC ≥80% (n = 487,886; 79.8%) and inadequate adherence as <80% (n = 123,363; 20.2%). The results of multivariable adjusted models including adjustment for PDC among antihypertensive drug users were similar to the main analyses (Online Table 1). We performed a sensitivity analysis, where VVV of SBP was assessed as the SD of at least 3 longitudinal intraindividual SBP measurements in each patient during the initial 12-month period of their follow-up. Statistical analyses were performed using STATA MP Version 13 and 14 (STATA Corporation, College Station, Texas). The study protocol was approved by the Research and Development Committees at the Memphis VA Medical Center and Long Beach VA Medical Center.
Results
The mean age of the cohort was 60 ± 13 years, 94% were male, 78% white, and 18% African American. Patients had a median of 24 (15 to 42) SBP measurements. The mean baseline SBP was 133 ± 18 mm Hg overall, and 127 ± 12, 130 ± 15, 134 ± 17, and 142 ± 22 mm Hg for SD quartiles 1, 2, 3, and 4, respectively. Baseline characteristics of the entire cohort, as well as the patients categorized by SD quartiles, are shown in Table 1. Patients with higher SBPV were older, more likely to be male, African-American and unmarried, had lower income and higher prevalence of comorbid conditions, and more frequently used all classes of antihypertensive medications.
Table 1.
Baseline Characteristics
| Variable | Overall (N = 2,865,157) | SD of Systolic BP (mm Hg) | ≥15.6 (n = 716,289) | |||
|---|---|---|---|---|---|---|
| <10.3 (n = 716,288) | 10.3–12.7 (n = 716,292) | 12.8–15.5 (n = 716,288) | ||||
| Age, yrs | 59.3 ± 13.1 | 56.4 ± 14.5 | 58.6 ± 13.1 | 61.1 ± 12.1 | 63.7 ± 11.6 | |
| Male | 2,690,181 (93.9) | 655,512 (91.5) | 668,327 (93.3) | 679,509 (94.9) | 686,833 (95.9) | |
| Race | White | 2,082,512 (77.8) | 516,298 (79.8) | 526,070 (78.8) | 528,409 (78.0) | 511,735 (74.9) |
| African-American | 474,403 (17.7) | 98,760 (15.2) | 111,589 (16.7) | 120,584 (17.8) | 143,470 (21.0) | |
| Hispanic | 62,965 (2.4) | 16,137 (2.5) | 15,547 (2.3) | 15,223 (2.2) | 16,058 (2.3) | |
| Other | 56,173 (2.1) | 15,848 (2.5) | 14,721 (2.2) | 13,342 (2.0) | 12,262 (1.8) | |
| Marital status | Married | 1,520,945 (55.0) | 424,870 (61.4) | 391,081 (56.5) | 367,192 (53.1) | 337,802 (48.8) |
| Single | 296,923 (10.7) | 80,835 (11.7) | 76,783 (11.1) | 71,985 (10.4) | 67,320 (9.7) | |
| Divorced | 748,305 (27.0) | 152,402 (22.0) | 181,822 (26.3) | 198,426 (28.7) | 215,655 (31.2) | |
| Widowed | 201,784 (7.3) | 33,696 (4.9) | 41,877 (6.1) | 54,359 (7.8) | 71,579 (10.3) | |
| Service connection | 1,251,338 (43.7) | 329,960 (46.1) | 328,662 (45.9) | 310,563 (43.4) | 283,153 (39.4) | |
| Number of provider visits, per month | ≤1 | 152,792 (40.3) | 417, 836 (58.4) | 290,438 (40.6) | 234,298 (32.8) | 210,221 (29.4) |
| 2–3 | 1,515,625 (53.0) | 281,819 (29.4) | 386,018 (54.0) | 420,156 (58.7) | 427,632 (59.8) | |
| ≥4 | 192,594 (6.7) | 15,707 (2.2) | 38,903 (5.4) | 60,772 (8.5) | 77,212 (10.8) | |
| Influenza vaccination | 917,194 (32.0) | 195,106 (27.2) | 226,001 (31.6) | 245,198 (34.2) | 250,889 (35.0) | |
| Housing stress | 960, 116 (34.9) | 230,642 (33.7) | 240,875 (35.0) | 244,386 (35,5) | 244,213 (35.5) | |
| Low education | 303,339 (11.0) | 71,083 (10.4) | 75,049 (10.9) | 77,527 (11.3) | 79,680 (11.6) | |
| Low employment | 267,074 (9.7) | 64,100 (9.4) | 66,915 (9.7) | 67,489 (9.8) | 68,570 (10.0) | |
| Persistent poverty | 140, 288 (5.1) | 34,861 (5.1) | 35,268 (5.1) | 34,902 (5.1) | 35,257 (5.1) | |
| eGFR, ml/min/1.73 m2 | 83.9 ± 15.4 | 85.1 ± 15.7 | 84.5 ± 15.4 | 83.5 ± 15.2 | 82.3 ± 15.2 | |
| BMI, kg/m2 | 29.3 ± 5.7 | 29.1 ± 5.1 | 29.5 ± 5.6 | 29.5 ± 6.0 | 29.3 ± 6.2 | |
| Income, $ | 22,362 (11,659–34,347) | 25,026 (12,480–40,380) | 23,285 (11,998–35,022) | 21,893 (11,568–35,022) | 19,666 (11,022–31,640) | |
| Geographic region | Northeast | 416,946 (15.2) | 105,240 (15.4) | 105,377 (15.3) | 106,218 (15.5) | 100,129 (14.7) |
| Midwest | 478,204 (17.4) | 113,820 (16.6) | 115,653 (16.9) | 120,275 (17.6) | 128,456 (18.8) | |
| South | 1,269,442 (46.3) | 325,926 (47.5) | 318,743 (46.5) | 313,356 (45.7) | 311,417 (45.6) | |
| West | 574,972 (20.1) | 140,716 (20.5) | 145,906 (21.3) | 145,247 (21.2) | 143,103 (21.0) | |
| Systolic BP, mm Hg | 133.1 ± 17.6 | 127.1 ± 12.2 | 129.9 ± 14.5 | 133.8 ± 16.7 | 141.5 ± 21.9 | |
| Diastolic BP, mm Hg | 77.5 ± 11.8 | 75.7 ± 10.1 | 76.6 ± 10.8 | 77.7 ± 11.7 | 79.8 ± 13.7 | |
| Diabetes | 731,960 (25.6) | 124,361 (17.4) | 164,563 (23.0) | 201,526 (28.2) | 241,510 (33.8) | |
| Hypertension | 1,797,718 (62.9) | 317,750 (44.5) | 401,786 (56.2) | 491,009 (68.7) | 587,173 (82.2) | |
| Cardiovascular disease | 347,084 (12.1) | 53,368 (7.5) | 74,240 (10.4) | 97,064 (13.6) | 122,412 (17.1) | |
| Cerebrovascular disease | 182,696 (6.4) | 22,291 (3.1) | 33,496 (4.7) | 48,857 (6.8) | 78,052 (10.9) | |
| Peripheral artery disease | 167,480 (5.9) | 18,896 (2.6) | 29,653 (4.2) | 45,332 (6.3) | 73,599 (10.3) | |
| Chronic lung disease | 551,927 (19.3) | 99,195 (13.9) | 129,819 (18.2) | 153,778 (21.5) | 169,135 (23.7) | |
| Dementia | 21,795 (0.8) | 1,964 (0.3) | 3,403 (0.5) | 5,932 (0.8) | 10,496 (1.5) | |
| Rheumatologic disease | 42,874 (1.5) | 8,301 (1.2) | 10,970 (1.5) | 11,934 (1.7) | 11,669 (1.6) | |
| Liver disease | 36,231 (1.3) | 4,537 (0.6) | 7,919 (1.1) | 10,978 (1.5) | 12,797 (1.8) | |
| Malignancies | 296,585 (10.4) | 51,299 (7.2) | 66,268 (9.3) | 81,934 (11.5) | 97,084 (13.6) | |
| AIDS/HIV | 20,161 (0.7) | 5,037 (0.7) | 5,844 (0.8) | 5,115 (0.7) | 4,165 (0.6) | |
| Depression | 298,440 (10.4) | 59,148 (8.3) | 79,292 (11.1) | 83,343 (11.7) | 76,657 (10.7) | |
| Nonadherence diagnosis | 206,211 (7.2) | 24,452 (3.4) | 40,336 (5.6) | 57,771 (8.1) | 83,652 (11.7) | |
| Statin | 435,410 (15.2) | 99,458 (13.9) | 106,106 (14.8) | 112,568 (15.7) | 117,278 (16.4) | |
| ACEI or ARB | 667,744 (23.3) | 111,395 (15.6) | 142,578 (19.9) | 180,323 (25.2) | 233,448 (32.6) | |
| Beta-blockers | 487,408 (17.0) | 81,270 (11.4) | 104,238 (14.6) | 131.218 (18.3) | 170,681 (23.8) | |
| CCB | 328,115 (11.5) | 56,824 (7.9) | 68,547 (9.6) | 86,156 (12.0) | 116,588 (16.3) | |
| Alpha blockers | 208,646 (7.3) | 38,810 (5.4) | 46,815 (6.5) | 55,640 (7.8) | 67,381 (9.4) | |
| Vasodilators | 6,577 (0.2) | 549 (0.1) | 816 (0.1) | 1,471 (0.2) | 3,741 (0.5) | |
| Thiazide diuretics | 292,421 (10.2) | 48,143 (6.7) | 61,960 (8.7) | 78,569 (11.0) | 103,722 (14.5) | |
| Loop diuretics | 102,995 (3.6) | 14,010 (2.0) | 21,113 (3.0) | 29,310 (4.1) | 38,562 (5.4) | |
| Potassium-sparing diuretic | 73,762 (2.6) | 13,783 (1.9) | 16,881 (2.4) | 19,826 (2.8) | 23,272 (3.3) | |
| Central alpha agonist | 26,165 (0.9) | 2,014 (0.3) | 3,429 (0.5) | 5,890 (0.8) | 14,832 (2.1) | |
| Adherence ≥80% among antihypertensive drug users | 487,886 (79.8) | 63,202 (84.3) | 98,899 (82.3) | 137,672 (80.5) | 188,110 (76.8) | |
Values are mean ± SD, n (%), or median (interquartile range).
ACEI = angiotensin-converting enzyme inhibitor; AIDS/HIV = acquired immunodeficiency syndrome/human immunodeficiency virus; ARB = angiotensin receptor blocker; BMI = body mass index; BP = blood pressure; CCB = calcium channel blockers; eGFR = estimated glomerular filtration rate.
All-Cause Mortality
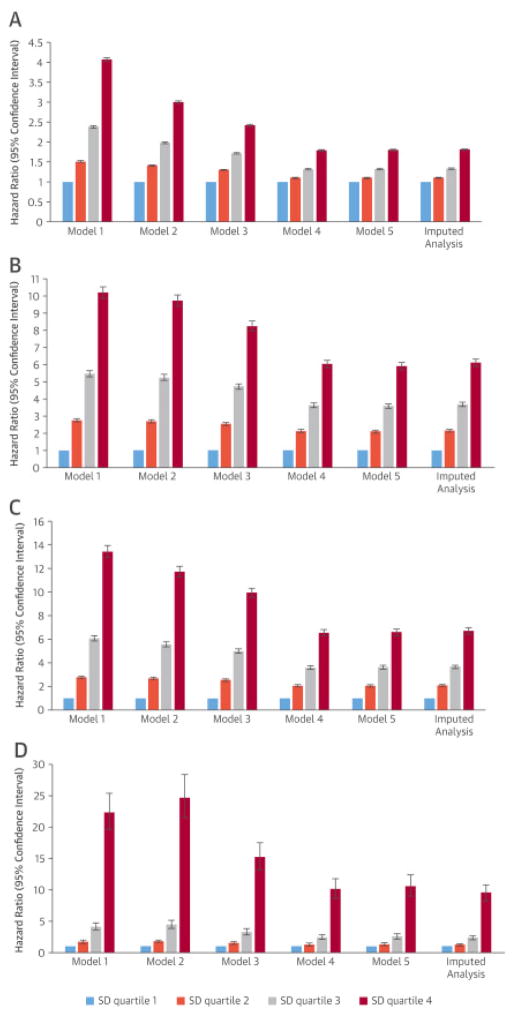
There were 484,887 deaths during a median follow-up of 8 years (16.9%; mortality rate: 22.87 [22.80 to 22.94] per 1,000 patient-years) in the entire cohort. An incrementally higher all-cause mortality was observed with higher SD quartiles in unadjusted (Figure 1A) and adjusted models. In the fully-adjusted model, SD quartiles 2 through 4 (compared to the first quartile) were associated with all-cause mortality hazard ratios (HR) of 1.10 (95% confidence interval [CI]: 1.08 to 1.11), 1.32 (95% CI: 1.30 to 1.34), and 1.80 (95% CI:c1.78 to 1.82), respectively. The results remained essentially the same when missing values were imputed and in analyses using SBPV calculated from measurements performed during the first year of follow-up (Online Table 2).
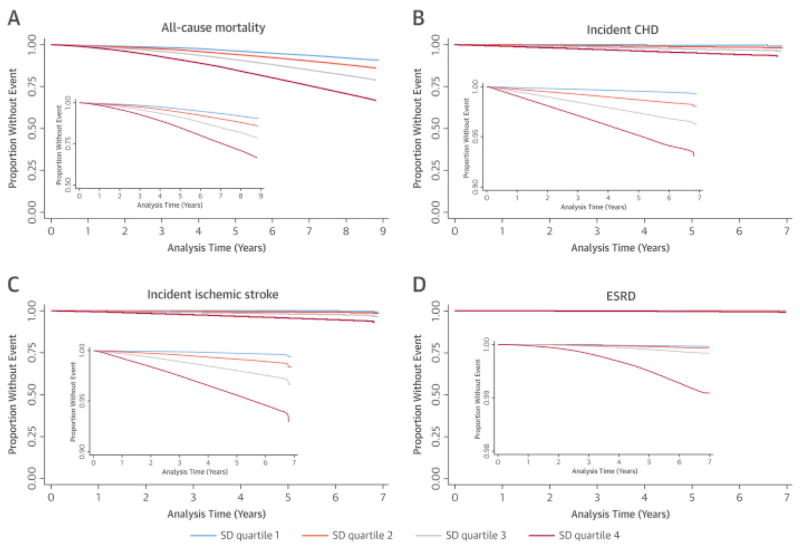
Figure 1. Kaplan-Meier curves of various clinical outcomes associated with different SD quartiles of mean SBP.
Increasing systolic blood pressure (SBP) variability, as measured in SD quartiles, was associated with corresponding increase in all-cause mortality in (A) unadjusted and (B) all adjusted models. Composition of the models is outlined in the Statistical Analysis section.
Other Outcomes
There were 67,227 CHD events during a median follow-up of 8 years (2.7%; incident rate: 3.63 [3.60–3.66] per 1,000 patient-years). Higher SBPV was associated with significantly higher risk of incident CHD in unadjusted (Figure 1B) and all adjusted models (Figure 2B). In the fully-adjusted model, HRs of CHD for SD quartiles 2 through 4 (compared to SD quartile 1) were: 2.11 (95% CI: 2.02 to 2.19); 3.59 (95% CI: 3.45 to 3.72), and 5.92 (95% CI: 5.70 to 6.14), respectively. Similar trends were seen in imputed analysis (Figure 2B) as well as in analyses using SBPV calculated from measurements performed during the first year of follow-up (Online Table 2).
Figure 2. Hazard ratios and 95% confidence intervals of outcome of interest associated with different SD quartiles of mean SBP in unadjusted and various adjusted analyses.
Increasing SBP variability, as measured in SD quartiles, was associated with corresponding increase in incident coronary heart disease (CHD) in (A) unadjusted and (B) all adjusted models. Composition of the models is outlined in the Statistical Analysis section. Abbreviations as in Figure 1.
There were 62,523 incident stroke events during a median follow-up of 8 years (2.3%; incident rate: 3.16 [3.14 to 3.19] per 1,000 patient-years). Higher SBPV was associated with significantly higher risk of incident stroke in all models (Figures 1C and 2C). In the fully-adjusted model, HRs of stroke for SD quartiles 2 through 4 (compared to SD quartile 1) were 2.05 (95% CI: 1.95–2.14), 3.63 (95% CI: 3.47 to 3.79), and 6.60 (95% CI: 6.32 to 6.89), respectively. The results remained similar in imputed analysis (Figure 2C), and in analyses using SBPV calculated from measurements performed during the first year of follow-up (Online Table 2).
There were 6,710 new ESRD cases during a median follow-up of 4.9 years (0.23%; incident rate: 0.39 [0.38 to 0.40] per 1,000 patient-years). Similar to the previous outcomes, higher SBPV was associated with higher risk of ESRD in unadjusted (Figure 1D) and all adjusted analyses (Figure 2D). In the fully-adjusted model, HRs of ESRD for SD quartiles 2 through 4 (compared to SD quartile 1) were: 1.33 (95% CI: 1.10 to 1.60), 2.56 (95% CI: 2.16 to 3.03), and 10.59 (95% CI: 9.02 to 12.43), respectively. The association between higher SBPV and ESRD was similar in imputed analysis and in analyses using SBPV calculated from measurements performed during the first year of follow-up (Online Table 2).
Subgroup Analyses
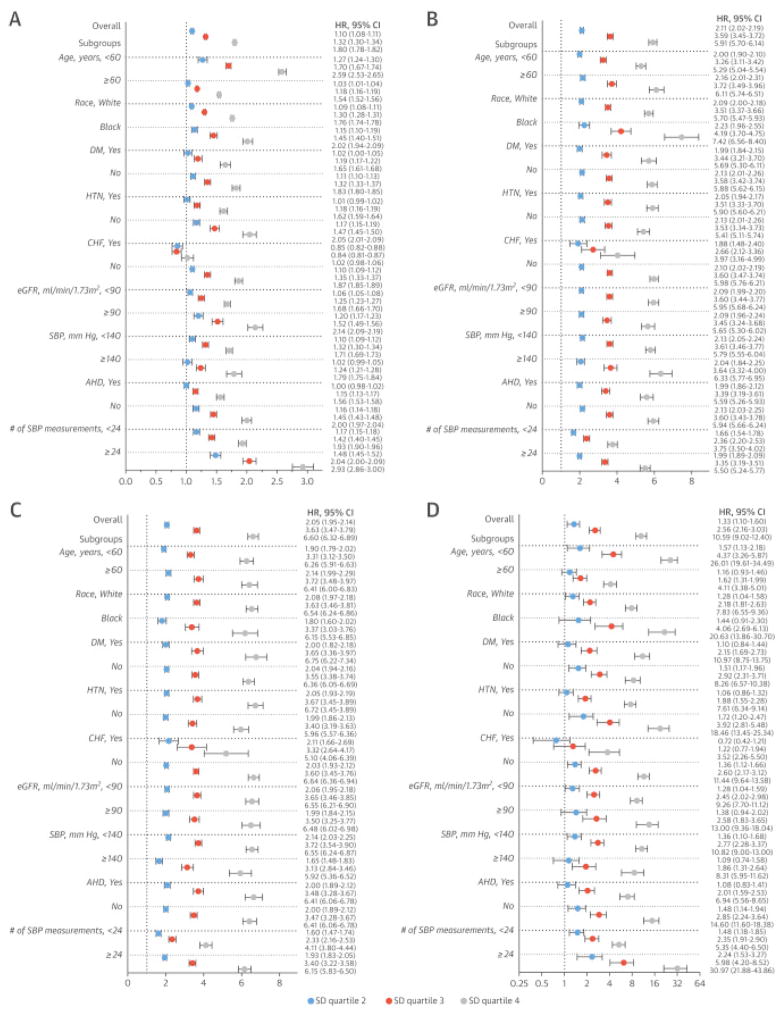
The results of fully-adjusted analyses in various subgroups are shown in Figure 3. Higher SBPV remained predictive of worse survival (Figure 3A) and increased risk of incident CHD (Figure 3B), stroke (Figure 3C), and ESRD (Figure 3D) in all studied subgroups, including different geographic regions (Online Figure 2). The exception was patients with CHF (4.7% of the whole cohort). In this subgroup, no increased mortality was observed in association with higher SD quartiles. However, similarly to the whole cohort, the adverse associations between higher SD quartiles and other outcomes (CHD, stroke, and ESRD) were present in patients with CHF (Figures 3A through 3D).
Figure 3. Adjusted Hazard ratios and 95% confidence intervals of outcomes of interest associated with different SD quartiles of mean SBP in various subgroups.
Increasing SBP variability, as measured in SD quartiles, was associated with corresponding increase in incident ischemic stroke in (A) unadjusted and (B) all adjusted models. Composition of the models is outlined in the Statistical Analysis section. Abbreviations as in Figure 1.
Discussion
To the best of our knowledge, this is the largest study to date examining the association between long-term visit-to-visit systolic blood pressure variability and all-cause mortality and cardiovascular and renal outcomes. Increased SBPV, measured via higher SDs of intraindividual SBP assessed over 8 years, was associated with a graded increase in the risks of all-cause mortality, incident CHD, stroke, and ESRD independent from baseline sociodemographic characteristics and comorbidities, including hypertension, BP, and antihypertensive medication use.
These findings confirmed and strengthened the importance of long-term variability in SBP for health-related outcomes. Previously, higher VVV in SBP was linked to increased all-cause mortality among 956 enrollees of the third National Health and Nutrition Examination Survey (14) and among 8,811 participants of the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation) study (36). The ADVANCE study also demonstrated a higher risk of macrovascular and microvascular outcomes with higher VVV in SBP. Rothwell et al. showed that among 2 cohorts of patients (2,006 and 18,530 patients, respectively) with previous transient ischemic attack, higher VVV in SBP was associated with higher risk of subsequent stroke independent of mean SBP (17). The risk of stroke was similarly higher during 5.4 years of follow-up with the higher VVV in SBP among 58,228 women enrolled in the Women’s Health Initiative (18). Increased SBPV was also linked to adverse renal outcomes. One SD higher SBPV was associated with 15% (95% CI: 11% to 20%) higher incidence in renal dysfunction defined as new proteinuria on dipstick, eGFR <60 ml/min1.73 m2, or rapid eGFR decline (≥3 ml/min/1.73 m2 per year) in a nationwide Japanese study including 48,587 participants (37). A post hoc analysis of the Irbesartan Diabetic Nephropathy Trial and the Reduction of End Points in Non-Insulin-Dependent Diabetes with the Angiotensin II Antagonist Losartan study that included 2,739 individuals with baseline nephropathy demonstrated that greater baseline VVV in SBP was associated with higher risk of the composite outcome of doubling serum creatinine, ESRD, or death, but not with cardiovascular outcomes (38). In a subset of 21,245 patients from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attach Trial, higher VVV in SBP was also associated with increased risk of ESRD, 50% decline of eGFR, or both (39).
The methods for assessing long-term or VVV in BP are not standardized. It has been argued that SD of SBPV is directly related to BP levels; therefore, high SD would be seen in patients with higher BP. However, it has been shown in several reports that the SD of intervisit SBP measurements correlates with other more complex methods of VVV assessment, which are independent from mean SBP, such as variation independent of mean, average successive variability (17), and SDreg-, the SD about the participant’s regression line with regressed SBP across visits (18).
Visit-to-visit variability is more than a measurement error as VVV was observed even in trials that aggressively standardized BP determination techniques and monitored adherence. Moreover, VVV derived even from routine office “unstandardized” BP values was well reproducible (11). The reasons behind increases in VVV are unclear but several mechanisms were proposed, such as nonadherence to BP medications (40), changes in the elastic properties of blood vessels and aortic distensibility (41), and disturbed baroreflex function leading to exaggerated pressor response to physical and emotional stimuli, as well as social and lifestyle factors (42). In turn, increased VVV caused greater stress on blood vessels and endothelial dysfunction promoting early target-organ damage (43). Visit-to-visit variability was shown to correlate positively with pulse wave velocity and negatively with ankle-brachial index, suggesting a link between impaired vascular function and VVV (19). Interestingly, limited data suggested that antihypertensive drug classes are associated with differential effects on VVV. A meta-analysis of 389 randomized controlled trials of antihypertensive medications demonstrated that when antihypertensive drug classes were compared with each other, calcium channel blockers (CCB) and nonloop diuretics were associated with respective reductions of 19% (p < 0.001) and 13% (p = 0.007) in VVV in SBP; whereas angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and beta-blockers were associated with increases in SBPV of 8% (p = 0.008), 16% (p < 0.001), and 17% (p < 0.001), respectively (44). Only CCB, as an antihypertensive drug class, was associated with a reduction in SBPV compared with placebo (44). Future studies are needed to better understand the effects of antihypertensive medications on SBPV as a potential intervention to reduce the risks associated with high visit-to-visit variability in BP.
Study Limitations
The strengths of our study included its large sample size of almost 3 million individuals, the use of a large number of BP measurements to calculate SBPV variability, and its representativeness of veterans across the United States. However, several limitations of our analysis need to be recognized. This was an observational study; therefore, we only reported associations and cannot make inferences about the causality of SBPV. Although we used adjusted analyses accounting for numerous baseline patient characteristics, we cannot exclude the effect of potential unmeasured confounders on our results. Additionally, our cohort consisted of predominantly male veterans; thus, these results may not be generalizable to women or to general populations. Previous studies have shown some discrepancies in BP variation between men and women as well as, perhaps, different levels of prognostic significance between men and women (22). Not all potential confounders affecting outcomes were included such as smoking status and baseline proteinuria, as the data on these variables were not available in sufficient numbers for analysis. We studied SBPV, but did not examine separately diastolic BP variability; however, it has been shown in several previous studies that VVV variability in diastolic BP had poor correlation with stroke (17) and all-cause mortality (14).
Conclusions
Greater SBPV was associated with higher risk of all-cause mortality, CHD, stroke, and ESRD. In addition to being an important prognostic indicator, SBPV may also become a therapeutic target. Future studies are needed to examine the effects of interventions that lower SBPV on clinical outcomes.
Supplementary Material
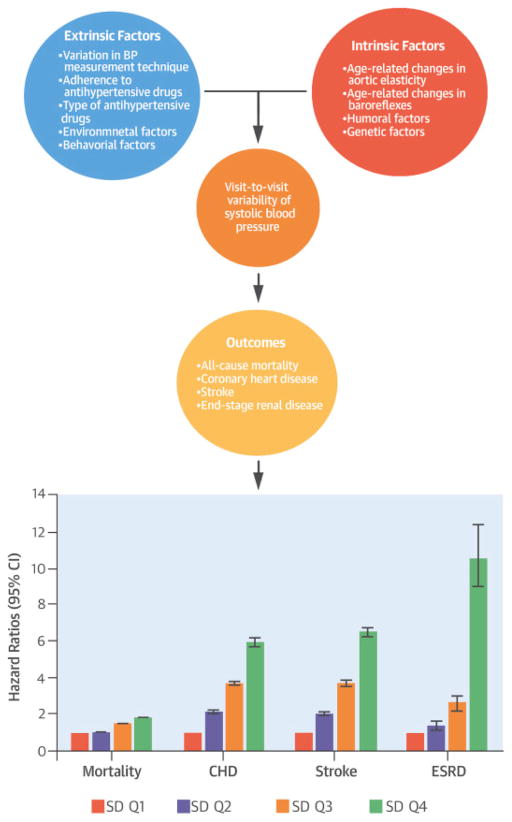
Figure 4. Central Illustration. Systolic Blood Pressure Variability: Clinical Outcomes.
A variety of intrinsic and extrinsic factors influence visit-to-visit systolic blood pressure variability (SBPV), which was measured using SDs of normally distributed SBP values. As SD quartiles rose, so too did risk of all-cause mortality, incident coronary heart disease (CHD), stroke, and end-stage renal disease (ESRD). CI = confidence interval.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Greater long-term systolic blood pressure variability is associated with increased risks of all-cause mortality, coronary disease, stroke, and end-stage renal disease, independent of diagnosis or treatment of hypertension.
TRANSLATIONAL OUTLOOK
Additional research is needed to understand the impact on clinical outcomes of interventions that reduce SBPV.
Acknowledgments
EOG, KKZ, and CPK are employees of the US Department of Veterans Affairs. Opinions expressed in this paper are those of the authors’ and do not necessarily represent the opinion of the Department of Veterans Affairs.
Funding: This study was supported by grant R01DK096920 to CPK and KKZ and is the result of work supported with resources and the use of facilities at the Memphis VA Medical Center and the Long Beach VA Medical Center. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
ABBREVIATIONS AND ACRONYMS
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence interval
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- HR
hazard ratio
- SBP
systolic blood pressure
- SBPV
systolic blood pressure variability
- VVV
visit-to-visit variability
Footnotes
Conflict of interest: None of the authors have relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.James PA, Oparil S, Carter BL, et al. 2014 Evidence-Based Guidelines for the Management of High Blood Pressure in Adults. JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 3.Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–38. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 4.Perry HM, Jr, Miller JP, Fornoff JR, et al. Early predictors of 15-year end-stage renal disease in hypertensive patients. Hypertension. 1995;25:587–94. doi: 10.1161/01.hyp.25.4.587. [DOI] [PubMed] [Google Scholar]
- 5.Stokes J, 3rd, Kannel WB, Wolf PA, D'Agostino RB, Cupples LA. Blood pressure as a risk factor for cardiovascular disease. The Framingham Study--30 years of follow-up. Hypertension. 1989;13:I13–8. doi: 10.1161/01.hyp.13.5_suppl.i13. [DOI] [PubMed] [Google Scholar]
- 6.Kovesdy CP, Bleyer AJ, Molnar MZ, et al. Blood pressure and mortality in U.S. veterans with chronic kidney disease: a cohort study. Ann Intern Med. 2013;159:233–42. doi: 10.7326/0003-4819-159-4-201308200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gosmanov AR, Lu JL, Sumida K, et al. Synergistic association of combined glycemic and blood pressure level with risk of complications in US veterans with diabetes. J Hypertens. 2016;34:907–13. doi: 10.1097/HJH.0000000000000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovesdy CP, Alrifai AAZ, Gosmanova EO, et al. Age and Outcomes Associated with Blood Pressure in Patients with Incident CKD. Clin J Am Soc Nephrol. 2016;11:821–31. doi: 10.2215/CJN.08660815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parati G, Ochoa JE, Salvi P, Lombardi C, Bilo G. Prognostic value of blood pressure variability and average blood pressure levels in patients with hypertension and diabetes. Hypertension. 2013;36:312–23. doi: 10.2337/dcS13-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howard SC, Rothwell PM. Reproducibility of measures of visit-to-visit variability in blood pressure after transient ischaemic attack or minor stroke. Cerebrovasc Dis. 2009;28:331–40. doi: 10.1159/000229551. [DOI] [PubMed] [Google Scholar]
- 11.Muntner P, Joyce C, Levitan E, et al. Reproducibility of visit-to-visit variability of blood pressure measured as part of routine clinical care. J Hypertens. 2011;29:2332–8. doi: 10.1097/HJH.0b013e32834cf213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–53. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 13.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 14.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population. Hypertension. 2011;57:160–6. doi: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 15.McMullan CJ, Bakris GL, Phillips RA, Forman JP. Association of BP Variability with Mortality among African Americans with CKD. Clin J Am Soc Nephrol. 2013;8:731–8. doi: 10.2215/CJN.10131012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selvarajah V, Pasea L, Ojha S, Wilkinson IB, Tomlinson LA. Pre-Dialysis Systolic Blood Pressure-Variability Is Independently Associated with All-Cause Mortality in Incident Haemodialysis Patients. PLOS ONE. 2014;9:e86514. doi: 10.1371/journal.pone.0086514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 18.Shimbo D, Newman J, Aragaki A, et al. Association between Annual Visit-to-Visit Blood Pressure Variability and Stroke in Postmenopausal Women: Data from the Women's Health Initiative. Hypertension. 2012;60:625–30. doi: 10.1161/HYPERTENSIONAHA.112.193094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okada H, Fukui M, Tanaka M, et al. Visit-to-visit variability in systolic blood pressure is correlated with diabetic nephropathy and atherosclerosis in patients with type 2 diabetes. Atherosclerosis. 2012;220:155–9. doi: 10.1016/j.atherosclerosis.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 20.Poortvliet RK, Ford I, Lloyd SM, et al. Blood Pressure Variability and Cardiovascular Risk in the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER) PLOS ONE. 2012;7:e52438. doi: 10.1371/journal.pone.0052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancia G, Facchetti R, Parati G, Zanchetti A. Visit-to-Visit Blood Pressure Variability, Carotid Atherosclerosis, and Cardiovascular Events in the European Lacidipine Study on Atherosclerosis. Circulation. 2012;126:569–78. doi: 10.1161/CIRCULATIONAHA.112.107565. [DOI] [PubMed] [Google Scholar]
- 22.Schutte R, Thijs L, Liu Y-P, et al. Within-Subject Blood Pressure Level - Not Variability - Predicts Fatal and Nonfatal Outcomes in a General Population. Hypertension. 2012;60:1138–47. doi: 10.1161/HYPERTENSIONAHA.112.202143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovesdy CP, Norris KC, Boulware LE, et al. Association of Race With Mortality and Cardiovascular Events in a Large Cohort of US Veterans. Circulation. 2015;132:1538–48. doi: 10.1161/CIRCULATIONAHA.114.015124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu JL, Molnar MZ, Naseer A, Mikkelsen MK, Kalantar-Zadeh K, Kovesdy CP. Association of age and BMI with kidney function and mortality: a cohort study. Lancet Diabetes Endocrinol. 2015;3:704–14. doi: 10.1016/S2213-8587(15)00128-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molnar MZ, Alhourani HM, Wall BM, et al. Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology. 2015;61:1495–502. doi: 10.1002/hep.27664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George LK, Molnar MZ, Lu JL, Kalantar-Zadeh K, Koshy SK, Kovesdy CP. Association of Pre-Operative Albuminuria with Post-Operative Outcomes after Coronary Artery Bypass Grafting. Sci Rep. 2015;5:16458. doi: 10.1038/srep16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grams ME, Sang Y, Coresh J, et al. Acute Kidney Injury After Major Surgery: A Retrospective Analysis of Veterans Health Administration Data. Am J Kidney Dis. 2016;67:872–80. doi: 10.1053/j.ajkd.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molnar MZ, Mucsi I, Novak M, et al. Association of incident obstructive sleep apnoea with outcomes in a large cohort of US veterans. Thorax. 2015;70:888–95. doi: 10.1136/thoraxjnl-2015-206970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stroupe KT, Tarlov E, Zhang Q, et al. Use of Medicare and DOD data for improving VA race data quality. Journal of rehabilitation research and development. 2010;47:781–95. doi: 10.1682/jrrd.2009.08.0122. [DOI] [PubMed] [Google Scholar]
- 30.Sohn MW, Zhang H, Arnold N, et al. Transition to the new race/ethnicity data collection standards in the Department of Veterans Affairs. Popul Health Metr. 2006;4:7. doi: 10.1186/1478-7954-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hajjar I, Kotchen T. Regional variations of blood pressure in the United States are associated with regional variations in dietary intakes: the NHANES-III data. J Nutr. 2003;133:211–4. doi: 10.1093/jn/133.1.211. [DOI] [PubMed] [Google Scholar]
- 32.Smith MW, Joseph GJ. Pharmacy data in the VA health care system. Med Care Res Rev. 2003;60:92S–123S. doi: 10.1177/1077558703256726. [DOI] [PubMed] [Google Scholar]
- 33.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gosmanova EO, Lu JL, Streja E, et al. Association of medical treatment nonadherence with all-cause mortality in newly treated hypertensive US veterans. Hypertension. 2014;64:951–7. doi: 10.1161/HYPERTENSIONAHA.114.03805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gosmanova EO, Molnar MZ, Alrifai A, et al. Impact of Non-Adherence on Renal and Cardiovascular Outcomes in US Veterans. Am J Nephrol. 2015;42:151–7. doi: 10.1159/000440685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hata J, Arima H, Rothwell PM, et al. Effects of Visit-to-Visit Variability in Systolic Blood Pressure on Macrovascular and Microvascular Complications in Patients With Type 2 Diabetes Mellitus. Circulation. 2013;128:1325–34. doi: 10.1161/CIRCULATIONAHA.113.002717. [DOI] [PubMed] [Google Scholar]
- 37.Yano Y, Fujimoto S, Kramer H, et al. Long-Term Blood Pressure Variability, New-Onset Diabetes Mellitus, and New-Onset Chronic Kidney Disease in the Japanese General Population. Hypertension. 2015;66:30–6. doi: 10.1161/HYPERTENSIONAHA.115.05472. [DOI] [PubMed] [Google Scholar]
- 38.McMullan CJ, Lambers Heerspink HJ, Parving HH, Dwyer JP, Forman JP, de Zeeuw D. Visit-to-visit variability in blood pressure and kidney and cardiovascular outcomes in patients with type 2 diabetes and nephropathy: a post hoc analysis from the RENAAL study and the Irbesartan Diabetic Nephropathy Trial. Am J Kidney Dis. 2014;64:714–22. doi: 10.1053/j.ajkd.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Whittle J, Lunch AI, Tanner RM, et al. Visit-to-visit variability of BP and CKD outcomes: Results of the ALLHAT. Clin J Am Soc Nephrol. 2016;11:471–80. doi: 10.2215/CJN.04660415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muntner P, Levitan EB, Joyce C, et al. Association between antihypertensive medication adherence and visit-to-visit variability of blood pressure. J Clin Hypertens (Greenwich) 2013;15:112–7. doi: 10.1111/jch.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimbo D, Shea S, McClelland RL, et al. Associations of Aortic Distensibility and Aarterial Elasticity with Long-Term Visit-to-Visit Blood Pressure Variability: The Multi-Ethnic Study of Atherosclerosis (MESA) Am J Hypertens. 2013;26:896–902. doi: 10.1093/ajh/hpt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eguchi K, Hoshide S, Schwartz JE, et al. Visit-to-visit and Ambulatory Blood Pressure Variability as Predictors of Incident Cardiovascular Events in Patients with Hypertension. J Hypertens. 2012;30:1556–63. doi: 10.1038/ajh.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eto M, Toba K, Akishita M, et al. Reduced endothelial vasomotor function and enhanced neointimal formation after vascular injury in a rat model of blood pressure lability. Hypertens Res. 2003;26:991–8. doi: 10.1291/hypres.26.991. [DOI] [PubMed] [Google Scholar]
- 44.Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375:906–15. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.