Abstract
In many taxa, sex chromosomes are heteromorphic and largely non-recombining. Evolutionary models predict that spread of recombination suppression on the Y chromosome is fueled by the accumulation of sexually antagonistic alleles in close linkage to the sex determination region. However, empirical evidence for the existence of sexually antagonistic alleles is scarce. In the mosquito Aedes aegypti, the sex-determining chromosomes are homomorphic. The region of suppressed recombination, which surrounds the male-specific sex-determining gene, remains very small, despite ancient origin of the sex chromosomes in the Aedes lineage. We conducted a genetic analysis of the A. aegypti chromosome region tightly linked to the sex locus. We used a strain with an enhanced green fluorescent protein (EGFP)-tagged transgene inserted near the male-determining gene to monitor crossing-over events close to the boundary of the sex-determining region (SDR), and to trace the inheritance pattern of the transgene in relation to sex. In a series of crossing experiments involving individuals with a recombinant sex chromosome we found developmental abnormalities leading to 1:2 sex biases, caused by lethality of half of the male or female progeny. Our results suggest that various factors causing sex-specific lethal effects are clustered within the neighborhood of the SDR, which in the affected sex are likely lost or gained through recombination, leading to death. These may include genes that are recessive lethal, vital for development and/or sexually antagonistic. The sex chromosome fragment in question represents a fascinating test case for the analysis of processes that shape stable boundaries of a non-recombining region.
Introduction
Evolutionary theory predicts that selection will favor mechanisms that reduce recombination between the primary sex determination gene and genes with sexually antagonistic alleles arising near the sex locus (Fisher, 1931; Charlesworth and Charlesworth, 1980; Bull, 1983; Rice, 1987). In the XY systems with the Y carrying the primary sex determiner, it will promote the accumulation, near the male-determining locus, of genes that are beneficial to males, but detrimental to females. Tight enough linkage to the sex locus would allow accumulation on the Y, and spread in a population, of alleles with selective advantage in males, even if they are highly deleterious or lethal to females (Rice, 1987). Complete recombination suppression between the X and Y in the region involved would ensue, creating the sex-determining region (SDR). Close linkage to the SDR would promote the accumulation of sexually antagonistic alleles in the regions adjacent to the SDR boundary, fueling expansion of a non-recombining portion of the Y chromosome. This process could continue until the X and Y fail to recombine over their entire lengths, leading to mutation-driven erosion of Y-linked genes, accumulation of repetitive sequences and, eventually, heteromorphism of sex chromosomes (Bachtrog, 2013). However, in many taxa cessation of recombination in sex chromosomes has not gone to completion. In eutherian mammals sex chromosomes are largely non-recombining and heteromorphic, but homologous pairing and exchange of chromosomal arms has been maintained within the pseudoautosomal regions for over 140 million years (Veyrunes et al., 2008). In some groups, such as ratite birds and boid snakes, the SDR has not expanded and the sex-determining chromosomes remain homomorphic, despite being nearly as ancient as those in Eutheria (Bachtrog et al., 2011). The question of why such homomorphic chromosomes persist remains unresolved. Attempts to explain it include lack of sexually antagonistic mutations in some species and resolution of sexual conflict by sex-specific or sex-biased expression (Vicoso et al., 2013).
All but one of mosquito species studied have three pairs of chromosomes; the only known exception, Chagasia bathana, has four pairs (Kumar and Rai, 1993). In Anopheles, the karyotype comprises two pairs of freely recombining autosomes and a pair of non-recombining heteromorphic sex chromosomes, with males being heterogametic (XY) and females homogametic (XX). In culicines, such as Aedes and Culex, chromosomes are homomorphic and each pair, traditionally referred to as chromosomes 1, 2 and 3, undergoes recombination. In Culex and Aedes, chromosome 1 is sex determining; its p and q arms are largely syntenic to, respectively, the X chromosome and chromosomal arm 2R of Anopheles gambiae (Nene et al., 2007; Arensburger et al., 2010). In Aedes aegypti males, one chromosome of the chromosome 1 pair (hereafter called the M-chromosome, as opposed to the non-sex-specific m-chromosome) carries the SDR that does not recombine and harbors a dominant male-determining gene Nix (Hall et al., 2015). Thus, similar to anophelines, the A. aegypti males are heterogametic (Mm) and females homogametic (mm). The SDR, located in that species in the chromosomal region 1q21 (Timoshevskiy et al., 2013), appears to be very short. Its physical location was delineated using two A. aegypti transgenic strains (called ‘sensor' and J2), each tagged with a different fluorescent marker integrated into the m-chromosome in the vicinity of the sex locus (‘sensor' in the ribosomal DNA array) (Hall et al., 2014). Crossing experiments yielded males with the M-chromosome that sequentially acquired both transgenes, each on the opposite flanks of the SDR, through a low rate recombination (‘sensor': 0.4%, J2: 1.24%). Very little is known about the content of the SDR and its neighborhoods. In addition to Nix, a male-specifically expressed gene myo-sex was identified between the two transgenic markers (Hall et al., 2014). However, myo-sex can be transferred together with the J2 transgene onto the m-chromosome through recombination, and thus it is not located within the SDR. Fluorescence in situ hybridization to mitotic chromosome spreads showed that in double recombinant males the hybridization signal from the transgenes practically colocalizes with the signal from Nix and myo-sex (Hall et al., 2014, 2015). An estimated resolution of fluorescence in situ hybridization probe mapping to mitotic chromosomes (Timoshevskiy et al., 2013) indicates that the SDR may be <1 Mb. Despite a short non-recombining region, the Aedes sex-determining chromosomes likely have an ancient origin. Aedes may have diverged from Culex not earlier than 170 Mya (Reidenbach et al., 2009), yet genetic markers that flank the maleness locus are conserved in both taxa (Mori et al., 1999), suggesting that their SDRs may share the same ancestry.
A. aegypti is known for naturally occurring departures from equal sex ratio toward excess of males (Craig et al., 1961). In some laboratory strains the proportion of females can vary from 15 to 30%, whereas for some interstrain crosses it is close to 0%. Skewed sex ratios are caused by a system of segregation distorter genes of unknown nature. In these cases the levels of distortion are not faithfully inherited between generations or by individuals of the same family (see Hickey and Craig, 1966 and references therein). Stably inherited 1:2 sex biases are also known. Wood (1975) reported 1:2 sex biases toward either females or males in broods from single pairs of individuals drawn from a population with an overall parity between the sexes; the distorted sex ratios were hypothesized to result from the action of recessive lethal genes. McGivern and Rai (1974) observed the 1♂:2♀ ratio in a progeny of wild-type females crossed to males carrying a large irradiation-induced translocation of a segment of chromosome 2 onto the M-chromosome and a large paracentric inversion embedded within the translocated region. The sex bias was attributed to a single crossover within the inversion loop during male meiosis that led to a loss of half of the M-chromosomes in dicentric bridges and to an inviability of the affected gametes.
Here we conducted a genetic analysis of the chromosome 1 region tightly linked to the SDR in A. aegypti. We generated a transgenic strain, in which insertion of a transgene close to the SDR boundary resulted in a predominantly male inheritance of an eye promoter-controlled enhanced green fluorescent protein (EGFP) tag. Rare crossing-over events during male meiosis break the linkage between the marker and the sex locus. We observed developmental abnormalities leading to 1:2 sex ratios caused by lethality of either males or females in families derived from individuals carrying a recombinant chromosome 1. These results indicate that within the SDR neighborhood there are several factors that in the affected sex are likely lost or gained through recombination. The A. aegypti genome is not assembled in that chromosomal region; that complicates identification of the molecular background of these intriguing phenomena.
Materials and methods
Mosquitoes
A. aegypti wild-type Rockefeller/UGAL strain was reared at 27 °C and 80% humidity. The 3-day-old previtellogenic females were fed on anesthetized rats. At 3 days after blood meal the eggs were collected and prepared for microinjection as described previously (Bian et al., 2005). All mosquitoes used in this study were maintained at the same insectary conditions and their females fed either on rats or on expired human transfusion blood using the Hemotek membrane feeding system (Discovery Workshops, Accrington, UK).
Molecular construct and transformation
The pBac[3xP3-EGFP, afm] vector containing an EGFP transformation marker under the control of the 3xP3 eye-specific promoter (Horn and Wimmer, 2000) was used to subclone a DNA fragment containing the vitellogenin (Vg) gene promoter linked to the cecropin A (CecA) gene complementary DNA and SV40 polyadenylation element (Kokoza et al., 2010). The resulting pBac[3xP3-EGFP afm, Vg-CecA] donor plasmid was mixed with the phsp-pBac helper plasmid at final concentrations of 0.35 and 0.25 μg ml−1, respectively, in a 5 mM KCl and 0.1 mM NaH2PO4 (pH 6.8) buffer. Injections with the above plasmid mixture into preblastoderm A. aegypti Rockefeller/UGAL strain embryos and development of transgenics were performed as previously described (Kokoza et al., 2001).
Microscopy
Larvae and pupae of transgenic mosquitoes were screened for EGFP fluorescence in their eyes using a Leica MZ FLIII stereo fluorescence microscope (Leica, Wetzlar, Germany) equipped with a GFP-B filter (GFP Band pass, cat no. C/6455, Ex 470/40 DM 495 BA 525/50). The intensity of EGFP fluorescence was used to score progeny of line B-derived females as hetero- or homozygotes. Images of larvae were captured with a Nikon DXM2100 camera (Nikon Inc., Melville, NY, USA) on a Leica MZ FLIII microscope. Emerging mosquitoes were photographed using Canon D1000 camera (Canon, Tokyo, Japan) with a close-up lens.
Genetic analysis
To determine the linkage group of the piggyBac insertion, individual EGFP-positive males were crossed with 2–3 females of A. aegypti RED strain, in which a recessive mutation associated with a distinct visible marker is present on each of the three chromosomes: the red-eye (re) locus on chromosome 1, the spot-abdomen (s) locus on chromosome 2 and the black-tarsus (blt) locus on chromosome 3 (Severson et al., 1993). The resulting male EGFP-positive progeny were similarly backcrossed with RED strain females. The F2 segregating populations were scored for the RED phenotype and the presence of EGFP expression.
To evaluate the frequency of crossing-over between the sex-determining locus and the transgene integration site, the F1 progeny of the EGFP-positive males and EGFP-negative females from either the C42 transgenic strain (generated in this study) or the RED strain were produced by single pair matings or mass matings and scored for gender and EGFP expression at the pupal stage.
Sex ratios were evaluated in families represented by progeny of individual females mated with single males. Up to 10 females were used for crosses with the same male individual, if no more males were available. For families indicating excess of males or females, χ2 analysis was used to test for statistical significance of deviation from the expected 1:1 ratio.
To evaluate mortality in the postembryonic stages, numbers of newly hatched larvae were compared with the numbers of pupae in 10 families (progeny of 10 single females). This procedure was done for the families with the 1:2 sex ratio bias, as well as the families of the C42 strain and a wild-type Rockefeller strain mosquitoes.
Molecular analysis
DNA was extracted from individual mosquitoes using DNeasy Blood & Tissue Kit (Qiagen, Manchester, UK) according to the manufacturer's protocol. PCR was performed in 50 μl containing 50 mM KCl, 10 mM Tris-HCl, pH 8.3, 1.5 mM MgCl2, 0.2 mM each dNTP, 2.5 U Platinum Taq polymerase (Invitrogen, Carlsbad, CA, USA), 10–25 pmol each primer and 1 μl template DNA (1/100th of the DNA extracted from a single mosquito). PCR thermal cycling included 3 min of initial denaturation at 94 °C, followed by 35 cycles of 40 s at 94 °C, 45 s at 52–65 °C and 30–240 s at 72 °C, and a final elongation for 10 min at 72 °C. Inverse PCR was conducted using either Platinum Taq or LA Taq polymerase (Takara Bio, Otsu, Japan) and, as templates, genomic DNA of EGFP-positive males digested singly with selected restriction endonucleases and then circularized by ligation. The endonucleases and sequences of the corresponding primers used in inverse PCR are listed in Supplementary Table S1; the primer sequences were either published earlier or designed manually and screened for a negligible potential to form dimers and hairpin structures using OligoAnalyzer (http://eu.idtdna.com/analyzer/Applications/OligoAnalyzer/). PCR products were purified using QIAquick Gel Extraction Kit (Qiagen), ligated into pGEM-T Easy vectors (Promega Corporation, Madison, WI, USA), and electroporated into Escherichia coli ElectroMAX DH10B cells (Invitrogen). Cloned templates were PCR amplified and sequenced using ABI BigDye terminator chemistry (PE Applied Biosystems, Foster City, CA, USA) on an ABI 3130xl Genetic Analyzer (PE Applied Biosystems). The sequences were used as queries to search GenBank and A. aegypti genome databases using BLAST (Altschul et al., 1990).
Integrity of the junctions between genomic DNA and the transgene was tested by PCR using the following primers: 5′ junction, near_pBac5'_2 (5′-CTTCGATGTCGGCTCTTCCT-3′) located in the genomic flank and pBc5′_invR2 (5′-CCTCGTGCGCTCTCCTGTTC-3′) located within the transgene; the 3′ junction, pBac3′_invFN (5′-CAGTAGGAAGACGAATAGGTGG-3′) located within the transgene and near_pBac3′_2 (5′-GTTGTCTTCCATTGAATACGCA-3′) located within the genomic flank. Loss of the transgene in males and gain thereof in females as a consequence of crossing over during male meiosis was tested by PCR using primers listed above, and primers EGFP_end (5′-CTTGTACAGCTCGTCCATGCC-3′) and EGFP_invF (5′-GGGCATCGACTTCAAGGAGGAC-3′) targeting a portion of the EGFP gene. Presence of the myo-sex gene in the recombinant and non-recombinant individuals of the C42 strain was tested by PCR using primers Myo_1_F (5′-CCTTCAAGCACACCGTTACA-3′) and Myo_1_R (5′-TCACTATGCAGGAGTTGTTTCG-3′). In addition, to evaluate quality of the DNA templates, PCR was done using primers AmsF2 (5′-TTCGAGACGCTCAAGTACGA-3′) and AmsR2 (5′-CTCACGGTCCTTTTCGATGT-3′) targeting a fragment of the Ams gene that is located in supercontig 1.64 mapped to chromosome 3 (Krzywinska and Krzywinski, 2009; Timoshevskiy et al., 2014).
For Southern blot analysis, genomic DNA from single males and females of the C42 strain and from males of the wild-type Rockefeller strain mosquitoes was digested with the respective restriction endonucleases. The DNA fragments were separated by electrophoresis on a 0.8% agarose gel and transferred by capillary blotting onto Hybond-N+ membranes (Amersham Biosciences, Buckinghamshire, UK) in 10 × standard saline citrate buffer (Sambrook et al., 1998). Southern blots were hybridized overnight as described previously (Severson, 1997) with a 653 bp long EGFP probe radioactively labeled using the gene-specific primers. Membranes were washed twice in 2 × standard saline citrate, 0.1% SDS and then three times at high stringency in 0.1 × standard saline citrate, 0.1% SDS at 65 °C for 15 min each.
Results
Generation of a transgenic line
Approximately 1000 injected preblastoderm embryos yielded 100 G0 adult individuals that were crossed with the wild-type mosquitoes. Out of 43 resulting families, one with a G0 male founder was used to establish a transgenic strain (C42), in which only males expressed EGFP, as indicated by screening of randomly selected individuals from several consecutive generations. The C42 males were shown to carry a single transposon insertion in their genomes (Figures 1a and b). Females lacked the EGFP expression altogether (Supplementary Figure S1); no transgene sequence was detected in their genome by PCR (Figure 2) or Southern blot analysis (data not shown). The transgene construct has remained stably integrated into the male genome for more than 60 generations. The insertion seemed not to markedly affect fitness of the C42 males.
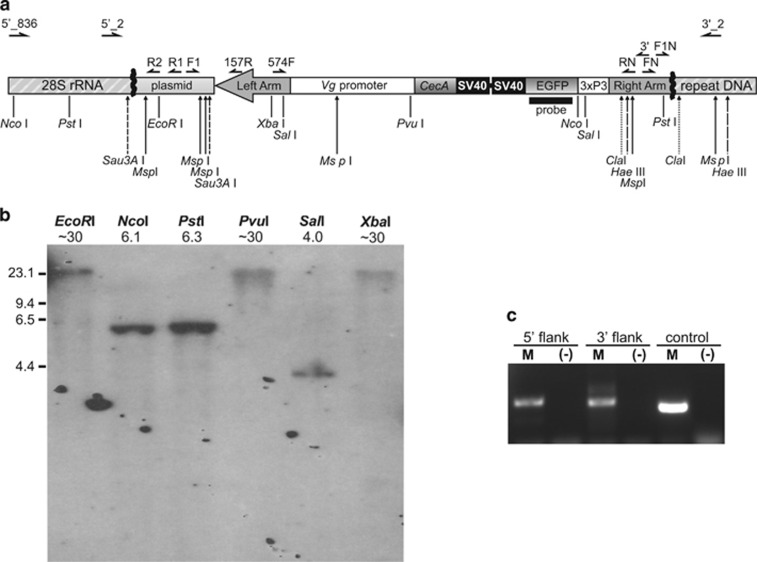
Figure 1.
The piggyBac transgene integration into the A. aegypti C42 strain genome. (a) A map of the construct flanked by the identified genomic DNA (not drawn to scale). Approximate positions of inverse PCR primers used to isolate the junctions between the transgene and flanking genomic DNA (thick zigzag lines) are marked directly above the map. Primer names are abbreviated for clarity; see Supplementary Table S1 for complete names and sequences of the primers. Target sites of the restriction enzymes used to generate templates for inverse PCR are marked as vertical bars below the map. Primers used to verify integrity of the junctions are shown at the top of the figure. Target sites of the endonucleases used for Southern blot analysis are marked by long vertical arrows below the map; for clarity target sites for different enzymes are represented by different arrow styles. Solid horizontal line represents a DNA fragment used as a probe in Southern blot analysis. (b) Southern blot analysis of the C42 males carrying the EGFP tag. Names of the restriction enzymes used for DNA digestion and sizes of the hybridizing fragments (in kb) are shown above the lanes. The position of the HindIII digested Lambda DNA fragments used as a high-molecular-weight marker is indicated on the left. (c) A PCR confirming integrity of the junctions in the EGFP-positive individuals. See Materials and methods for primer combinations used. Quality of the template DNA was evaluated using the EGFP-specific primers (control).
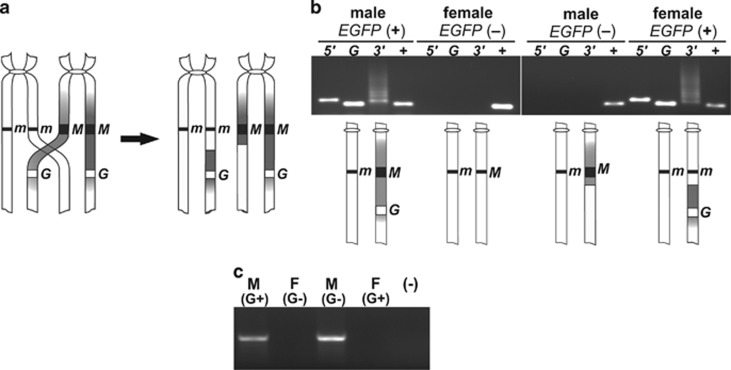
Figure 2.
Loss of linkage between the maleness locus and the EGFP tag during male meiosis. (a) A diagram of a crossover between two non-sister chromatids during meiotic prophase, giving rise to recombinant chromosomes (only pericentromeric fragments of chromosomes 1 are presented). The sex locus (black line or square denoted either m or M) and the transgene (white square denoted G) are shown. (b) A PCR confirming integrity of the junctions in the EGFP-positive individuals and indicating loss of the transposon in males and gain thereof in females in a generation following the recombination. The phenotypes of individuals analyzed are given at the top. For each phenotype, lanes are marked as follows: 5′, a product spanning the 5′ junction; G, a fragment of the EGFP gene; 3′, a product spanning the 3′ junction; +, a fragment of the Aams gene (positive control of DNA quality). A smeary ladder-like pattern of the PCR product spanning the 3′ junction results from binding of the near_pBac3′_2 primer to multiple target sites within a tandemly repeated flanking DNA. Combinations of chromosome 1 pairs corresponding to each phenotype are depicted below the gel image. (c) Test for presence of myo-sex in the EGFP-positive and EGFP-negative individuals, denoted as G+ or G−. M, male; F, female; (−), negative control.
Characterization of the insertion site
Junctions between the transgene and the mosquito genomic DNA were isolated using inverse and standard PCR approaches (Figure 1a; see also Supplementary Information). The sequence identified at the 5′ junction contained a nearly 2 kb long fragment of flanking genomic DNA, an unexpected 873 bp-long fragment of a donor plasmid and a piggyBac inverted terminal repeat. The fragment isolated at the 3′ junction consisted of an unexpectedly truncated transposon arm (missing 488 bp from the 3′ piggyBac terminus) abutting a 367 bp long flanking genomic DNA. Thus, the integration of the element occurred either by a non-canonical transposition or by an illegitimate recombination independent of a transposase activity. Integrity of the transgene and its contiguity with genomic flanks was confirmed by PCR (Figure 1c). Unlike in other dipterans, piggyBac transposons integrated into the A. aegypti genome may not be able to remobilize (Palavesam et al., 2013). Fortuitous truncation of the 3′ arm only strengthens that notion; it made the insertion highly stable, because remobilization of piggyBac would require both arms intact (Li et al., 2001).
The genomic DNA flanking the transgene from the 5′ end has several, periodically occurring, perfect matches to genomic supercontig 1.836 (Genbank GI number: 78216866) and represents a fragment of the 28S ribosomal RNA (rRNA) gene that forms a part of the rRNA array of cistrons. In A. aegypti the rRNA genes are tandemly repeated ~500 times and located on chromosome 1 in a cluster adjacent to the SDR (Gale and Crampton, 1989; Kumar and Rai, 1990; Timoshevskiy et al., 2013). The genomic sequence flanking the 3′ end of the transgene represents a class of noncoding DNA organized in short tandem repeats, with matches to sequences irregularly scattered between clusters of rRNA genes within supercontig 1.836. Similar to the C42 transgene, the ‘sensor' transgene is also inserted within the array of rRNA genes (Hall et al., 2014).
Using primers targeting both genomic flanks, we isolated by PCR and sequenced a region from the wild-type genome (Rockefeller and Liverpool strains of A. aegypti), into which piggyBac apparently became inserted in the C42 strain. Sequences identical or similar to two parts (corresponding to each flank of the C42 transgene) of such an ‘empty' site are interspersed in supercontig 1.836, but rather than forming an expected contiguous DNA stretch, they are spaced by a minimum 4 kb. Thus, the supercontig, or other currently available A. aegypti genome data (Nene et al., 2007), do not provide information on a larger genomic context of the transgene integration, perhaps because of instability of shotgun clones containing tandemly repeated sequences (Song et al., 2001) and/or inadequate genome sampling during the A. aegypti genome project. Lack of the SDR-linked contigs within the A. aegypti genome has been highlighted earlier (Hall et al., 2014).
A comparison of the ‘empty' site sequence with the sequences flanking the transgene revealed a deletion in a repetitive DNA fragment that likely occurred during the transposon integration process (Supplementary Figure S2). The deficiency is apparently small and inconsequential for the viability and fertility of mosquitoes, because the C42 strain males mated with the RED, Liverpool or non-recombinant C42 strain females produce progeny with balanced sex ratios and in numbers comparable to the progeny of the wild-type males (Table 1, and data not shown).
Table 1. Results of crossing experiments to establish linkage group of the transgene insertion in the C42 strain.
| Cross |
Male |
Female |
||||||
|---|---|---|---|---|---|---|---|---|
|
EGFP(+) |
EGFP(−) |
EGFP(+) |
EGFP(−) |
|||||
| Black | Red | Black | Red | Black | Red | Black | Red | |
| RED ♀ X C42 ♂ | 652 | — | 3a | — | 1a | — | 695 | — |
| RED ♀ X F1 (RED ♀ X C42 ♂) ♂ | 878 | 20b | — | 3a | 2a | — | 25b | 787 |
Abbreviations: EGFP, enhanced green fluorescent protein; +, EGFP positive; −, EGFP negative.
Figures represent numbers of progeny individuals with a given phenotype. Mosquitoes were scored at the pupal stage for sex, EGFP expression and eye color. The RED strain individuals are homozygous for a recessive red eye color mutation. Black denotes a wild-type eye phenotype.
Individuals carrying a recombinant chromosome 1 with the breakpoint between the sex locus and the transgene.
Individuals carrying a recombinant chromosome 1 with the breakpoint between the sex locus and the re locus.
piggyBac integration into chromosome 1 and linkage to the sex locus
During the strain maintenance we noted a rare occurrence of males lacking EGFP expression. To explore the EGFP inheritance pattern and to confirm chromosomal location of the transgene, we conducted crossing experiments between the EGFP-positive males and females of the RED strain that carries visible recessive markers on each chromosome. Segregation of the phenotypes in the back-cross F2 generation was consistent with the integration of the transgene in chromosome 1 and its linkage to the re locus (Table 1) that itself is linked to the sex locus (McClelland, 1966). Therefore, an almost exclusive presence of the EGFP tag in males is a consequence of the transgene integration into the M-chromosome, close to the SDR. A small number of EGFP-positive females and EGFP-negative males among F1 and F2 individuals indicates rarely occurring recombination between the M factor and the transgene, and transfer of the transgene onto the non-sex-specific m-chromosome during male meiosis (Figure 2). The low proportion of recombinants (0.29%) in the crossing experiments described above and in the progeny from mass crosses between the EGFP-tagged males and the EGFP-negative females (>8000 randomly selected F1 individuals screened) indicates that the transgene is tightly linked to the SDR. Slightly higher recombination rate (0.4%) was observed between the M-locus and the ‘sensor' transgene (Hall et al., 2014).
We used PCR to follow the inheritance pattern of the myo-sex gene, which is closely linked to the SDR, in the recombinant and non-recombinant C42 strain individuals. The tests revealed that regardless of possessing the transgene or not, myo-sex was absent in females and present in males (Figure 2c). This result indicates distal (telomeric side) location of the C42 transgene relative to Nix, and is consistent with the suggestion that myo-sex is located closer to the centromere than Nix (Hall et al., 2014).
Developmental abnormalities revealed by crossing experiments
We randomly selected two females, which acquired the EGFP marker through independent recombination events, to establish mosquito lines A and B. According to the Mendelian inheritance, half of such females' progeny should be EGFP positive, regardless of sex, and both sexes should be present in approximately equal proportions, as is the case with broods between the non-recombinant C42 strain individuals. However, the observed results deviated from the expectations.
Line A females produced progeny with a distorted sex ratio (1♂:2♀). Whereas half of the F1 females carried the transgene, only 2 out of over 1000 F1 males did so (Table 2, crosses 1 and 2; see also Supplementary Figure S3), indicating that nearly all the EGFP-positive males were inviable. The same inheritance pattern was reproduced in consecutive generations, wherever line A females were used for crosses (data not shown). In each generation, larval and pupal mortality was low and comparable to mortality in the wild-type or C42 strain mosquitoes reared under the same conditions. Thus, the EGFP-positive males failed to develop past the embryonic stage. The observed lethal effect must have been caused by a factor on the maternal recombinant (transgene-carrying) m-chromosome. Yet, from crosses between EGFP-positive mothers and fathers lacking EGFP we recovered two males that were EGFP positive (Table 2, crosses 1 and 2). We suggest that the two surviving males acquired the transgene on the m-chromosome that had undergone a secondary crossing-over during female meiosis (cf. Supplementary Figure S3), during which the male-lethal factor had been purged, and which rescued male viability. A very low number of these GFP-positive males is consistent with a notion of a secondary recombination. Mutations inactivating the lethal factor (assuming that the lethality is caused by gene expression) could have the same effect; however, such mutations would occur with a frequency orders of magnitude lower than the frequency of the EGFP-positive males or other ‘unexpected' rare phenotypes observed during this study (cf. Supplementary Figure S3).
Table 2. Summary of crossing experiments using recombinant chromosome 1-carrying female lines and their derivatives (see also Supplementary Figures S3 and S4).
| Cross | Genotypea | Families with 1:2 sex biasb |
Male |
Female |
||
|---|---|---|---|---|---|---|
| EGFP(+) | EGFP(−) | EGFP(+) | EGFP(−) | |||
| Line A females | ||||||
| 1 | C42A EGFP ♀ X wt ♂ | 22/22 F | 1 (3) c | 458 | 452 | 394 |
| 2 | C42A EGFP ♀ X C42 (−) ♂ | 26/26 F | 1 | 623 | 636 | 644 |
| Line A-derived recombinants | ||||||
| 3 | C42A EGFP ♀ X Cross 2 F1 EGFP ♂ | 9/9 F | 2 (4) | 196 | 417 (4) | 2 |
| 4 | Cross 3 F1 EGFP ♀ X Cross 3 F1 EGFP ♂ | 3/3 M | 54 | 38 | 31 (5) | — |
| 5 | Cross 4 F1 EGFP ♀ X C42 EGFP ♂ | 1/12 F | 499 (6) | 1 | 282 | 239 (6) |
| 6 | Cross 5 F1 (−) ♀ X Cross 5 F1 EGFP ♂ | 20/22 M | 761 (7, 9) d | 1 | 1 | 398 (7, 8) d |
| 7 | Cross 6 F1 (−) ♀ X Cross 6 F1 EGFP ♂ | 21/40 M | 1468 | 1 | 3 | 981 |
| 8 | Cross 6 F1 (−) ♀ X wt ♂ | 15/35 M | — | 1492 | — | 1055 |
| 9 | wt ♀ X Cross 6 F1 EGFP ♂ | 0/18 | 569 | 4 | 1 | 579 |
| Line B females | ||||||
| i | C42B EGFP ♀ X C42 (−) ♂ | 0/3 | 53 (ii, iv) | 43 | 46 (ii, iii) | 55 |
| ii | wt ♀ X Cross i F1 EGFP ♂ | 5/13 M | 1 | 422 | 306 | 3 |
| iii | Cross i F1 EGFP ♀ X C42 EGFP ♂ | 0/10 | 585e | — | 271 | 269 |
| iv | Cross i F1 EGFP ♀ X Cross i F1 EGFP ♂ | 0/9 | 159 | 147 | 274f | — |
Abbreviations: EGFP, enhanced green fluorescent protein; F, female; M, male; wt, wild type; +, EGFP positive; −, EGFP negative.
Figures represent cumulative numbers of progeny from a given cross, counted at the pupal stage. The observed 1:2 sex biases reveal sex-specific developmental abnormalities mediated by different factors linked to the sex locus.
The EGFP-positive and the EGFP-negative individuals used in crosses are denoted as EGFP (+) and (−), respectively. The C42 (−) males are the C42 strain derivatives that inherited a paternal M allele-bearing chromosome 1 that lost EGFP through recombination (cf. Figure 2). The wt individuals were from the Rockefeller strain.
Number of families with the sex bias out of the total number of families studied; F and M following the numbers denotes excess of either females or males in the affected families.
Superscript numbers in parentheses indicate cross, for which individuals from a given phenotype were taken.
Only individuals from families with sex bias were taken for crosses.
Of the 303 male pupae examined, 151 were scored as homozygotes and 152 as heterozygotes for transgene insertion; 8% and 3% of those, respectively, died during eclosion.
Of the 186 female pupae examined, 97 were scored as homozygotes and 89 as heterozygotes for transgene insertion; 89% and 4% of those, respectively, died during eclosion. Small difference in fluorescence intensity can make distinction of homo- and heterozygotes ambiguous; therefore, females from the former group that survived to adulthood are likely heterozygotes incorrectly scored as homozygotes.
Availability of viable males carrying the m-linked EGFP marker created an opportunity to produce a line homozygous for transgene insertion. The attempts to do so were unsuccessful, but they provided further insight into the SDR neighborhood (Table 2 and Supplementary Figure S3). Among cross 4 progeny we expected equal sex ratios and homozygosity for transgene insertion in half of the females. Instead, there was an excess of males and apparently there were no homozygous females, because none of the females taken from cross 4 progeny produced EGFP-only broods (Table 2; probability of drawing 12 heterozygotes in a row for cross 5 from an equal mixture of homo- and heterozygotes, P=0.0002). Female deficiency in cross 4 progeny was caused by embryonic lethality, similar to the male bias described above. Furthermore, the expectation of equal proportion of homo- and heterozygotes among male progeny from cross 5 was not met. None of the males sampled from that pool (for cross 6) produced EGFP-only females, indicating that all tested males were heterozygous, with the transgene linked to the M-chromosome (Table 2, cross 6; probability of drawing 22 heterozygotes in a row for cross 6 from an equal mixture of homo- and heterozygotes P=2.4 × 10−7). Intriguingly, in the majority of cross 6 families there was 2♂:1♀ sex bias caused by female embryo lethality. When individuals from families with the biased sex ratios were inbred (Table 2, cross 7), or females from such families were crossed with wild-type males (Table 2, cross 8), approximately half of the resulting families exhibited 2♂:1♀ bias. In contrast, none of the males from the affected families, when crossed with wild-type females, sired progeny deviating from parity between the sexes (Table 2, cross 9).
In contrast to line A females, the progeny of line B females consisted of all expected phenotypes, including males with the inherited m-linked EGFP marker (Table 2 and Supplementary Figure S4). Crossing such males with the wild-type females yielded an excess of males, resulting from the 2♂:1♀ sex ratio caused by female embryonic lethality in 5 out of 13 analyzed families (Table 2, females cross ii). We used the EGFP-positive progeny of line B females in a further attempt to establish a line homozygous for the transgene insertion. It proved unsuccessful, but revealed another phenotypic effect. Whereas homozygous males developed normally and survived to adulthood (Table 2, cross iii), homozygous females could not complete emergence and, almost invariably, died while attempting to leave pupal exuvium (Table 2, cross iv; Supplementary Figure S5). They usually had protracted larval development, with up to twice the length of the fourth instar larva period, compared with heterozygotes or wild-type females (data not shown).
Discussion
Availability of an A. aegypti strain with an EGFP-tagged transgene inserted near the SDR allowed us to easily detect crossing-over events close to the SDR boundary and to trace the inheritance pattern of the fluorescent marker in relation to sex. The initial crosses involving rarely occurring females possessing a recombinant EGFP-positive m-chromosome produced broods lacking one of the anticipated phenotypes. Prompted by this surprising result, we conducted further crossing experiments and identified additional phenotypic effects, with a stable 1:2 sex bias driven by lethality of either males or females in all or half of the families analyzed.
Our results have clear parallels with an earlier report of naturally occurring 1:2 sex biases (Wood, 1975). However, in that study the skewed sex ratios were caused by larval mortality, and the male-lethal locus was at a considerable distance (5–10 cM) from the sex locus (Wood, 1975), indicating that different lethal factors were involved. The lethality observed in our study is, in most cases, attributable to the recombinant EGFP-positive m-chromosome. To explain these phenomena, we suggest that within the SDR neighborhood there are several factors that in the affected sex are likely lost or gained through recombination, leading to death (Figure 3). These may include genes (or groups of tightly linked genes) vital for sex-specific development, genes carrying recessive lethal mutations or genes that are sexually antagonistic, causing highly deleterious conflict that has been resolved by tight linkage with the sex locus. The hypothetical scenarios of lethality are presented in more detail below. Other, more complex schemes are possible, but not discussed in this study. The scenarios presented here assume that within the sex locus and its close neighborhoods encompassing the recombination region, the M- and the m-chromosomes diverged sufficiently to share no functional alleles of the lethal factors considered in this study.
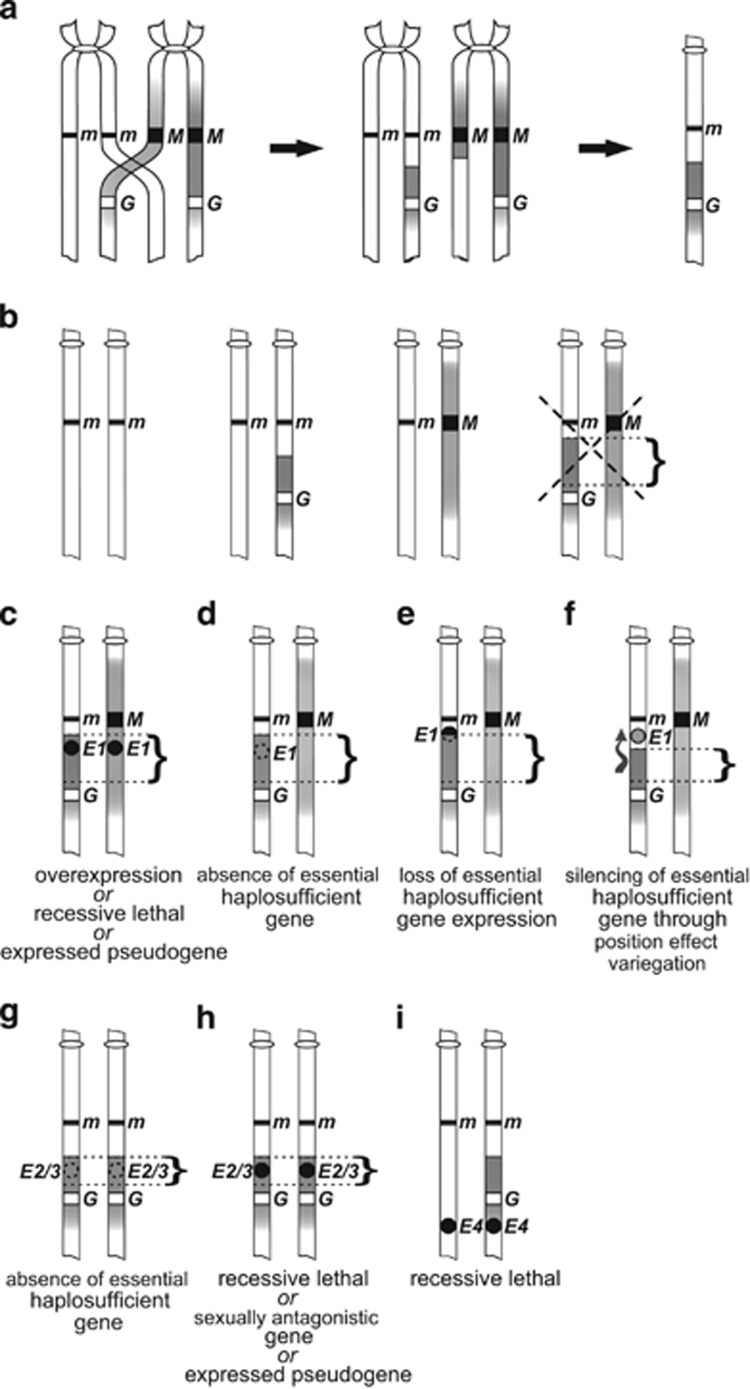
Figure 3.
Hypothetical mechanisms of sex-specific lethality observed in this study. (a) Crossing-over during male meiosis results in decoupling of the transgene from the SDR. Following assortment of gametes, the recombinant chromosomes are transmitted to the F1 generation, in which females inherit EGFP along with a portion of the M-chromosome (shown on the right). (b) Females carrying a recombinant m-chromosome produce progeny with four potential karyotypes (pictured), including a lethal karyotype (crossed; for clarity karyotypes sired by wild-type males are shown). Individuals with the lethal karyotype carry on the m-chromosome a duplicated portion of the M-chromosome region (marked by a curly bracket) that is tightly linked to the SDR and normally present exclusively in a single copy in males. (c–f) Potential mechanisms of male lethality. (c) If the duplicated region harbors a male-specifically expressed embryonic gene E1, the male lethality (Table 2, line A females, crosses 1 and 2) may be caused by a dosage-dependent deleterious effect (overexpression from two copies). Alternatively, the male lethality may result from a recessive lethal gene E1, or from expressed pseudogenes. (d) The EGFP-positive males inheriting the transgene on the m-chromosome lack a non-sex-specific chromosomal region located close to the m-locus. If the missing region carries a haplosufficient gene essential for embryo development (missing gene E1), individuals with such a chromosomal arrangement would be inviable. (e) If the essential haplosufficient gene E1 is disrupted by the recombination breakpoint, it would not be transcribed or it would produce incomplete transcripts. (f) Positioning of the m-linked haplosufficient gene E1 under the influence (depicted as a wavy arrow) of the heterochromatin from the SDR neighborhood could lead to silencing due to position effect variegation. (g–i) Potential mechanisms of female lethality. (g) Lack of non-sex-specific region and a concomitant lack of haplosufficient genes E2/3 residing in that region may drive lethality of homozygous females at embryo (E2) or late (E3) stage (cf. Table 2, LINE A-derived recombinants cross 4 and line B females cross iv). (h) The SDR-linked region recombined into the m-chromosome could contain female-specific recessive lethal genes, sexually antagonistic genes or deleterious expressed pseudogenes E2/3. (i) A recessive lethal m-linked gene E4 located close to the sex locus may drive female-specific embryonic lethality not related to recombination (cf. Table 2, line B females cross ii). Presence or absence of hypothetical genes that may cause lethal effects is denoted by black solid circles and dashed line circles, respectively.
Male lethality
The 1♂:2♀ bias in line A female crosses (Table 2, crosses 1 and 2) may be due to either a male-specifically expressed or recessive lethal gene normally effectively male specific because of its close linkage to the SDR, but transferred through recombination onto the m-chromosome. Female carriers of such a recombinant chromosome would be viable and fertile. However, half of their male progeny could be inviable for three potential reasons. (1) If the exchanged portion of the chromosome harbors a gene expressed only in male embryos, the lethality may be caused by a dosage-dependent deleterious effect. In males inheriting the recombinant m-chromosome, the gene would be present in a double dose (one copy on the M-chromosome and one copy on the m-chromosome; Figure 3c) that would lead to its overexpression and, in effect, embryonic lethality. A study on Drosophila, in which duplication of two short X chromosome regions led to male embryonic lethality (Venken et al., 2010), lends support to this scenario. (2) If a recessive embryonic-lethal gene is involved, males inheriting the maternal recombinant chromosome would be homozygous and inviable (Figure 3c). Loci closely linked to the SDR are kept effectively heterozygous. Consequently, recessive lethal mutations arising in such SDR-linked regions would be sheltered by the wild-type alleles on the m-chromosome, and they may be readily fixed in a population (Muller, 1932; Nei, 2013). Linkage to the SDR would result in male-lethal effect, even if the recessive lethal gene was non-sex-specifically expressed. It is possible that expressed pseudogenes, which are a product of sequence degeneration around the M-factor, are involved, if two copies are sufficient to cause deleterious effects. There is growing evidence for the causal link of pseudogene expression with disease (Poliseno et al., 2015). (3) Lethality could be caused by recombination-mediated loss of a gene, loss of gene expression or gene inactivation. Males inheriting a recombinant m-chromosome would have a segment of the M-chromosome duplicated, but would lack a portion of the m-chromosome located close to the sex locus. If the missing non-sex-specific region carries a gene essential and haplosufficient for embryo development, individuals lacking the region would die (Figure 3d). The same lethal effect would occur if the essential haplosufficient gene was disrupted by the recombination breakpoint and, in result, was not transcribed or produced incomplete transcripts (Figure 3e). Alternatively, the m-linked haplosufficient gene could be silenced because of position effect variegation (Schotta et al., 2003), provided the recombination brought the gene close to and under the influence of the heterochromatin from the SDR neighborhood (Figure 3f).
Female lethality
The female lethality observed in this study involves likely three different factors and at least two alternative mechanisms. The 2♂:1♀ bias in cross 4 progeny (Table 2; line A-derived recombinants) can be explained by scenario (3) described above, with homozygous females possessing two copies of an M-chromosome segment, and thus lacking a corresponding m-linked region carrying a haplosufficient gene essential for female embryonic development (Figure 3g). Similarly, lethality can be caused by inheriting two copies of a nonfunctional haplosufficient gene (analogous to situation depicted in Figures 3e and f). A lack of a haplosufficient gene or inheritance of nonfunctional gene copies could also have driven late lethality (during eclosion) of line B-derived homozygous females (Table 2, line B females cross iv). Alternatively, the SDR-linked female-specific recessive lethal genes recombined into the m-chromosome could also be responsible (Figure 3h). Similarly, the SDR-linked sexually antagonistic genes could produce observed developmental abnormalities if sexual conflict was not strong in females heterozygous for the antagonistic genes, but sufficiently deleterious to lead to death of homozygous females (Figure 3h). Such an explanation is consistent with the prediction that the region around the maleness gene may be highly detrimental if recombined into females (Jordan and Charlesworth, 2012). In this context we tested whether the myo-sex gene residing close to the SDR and strongly expressed in male pupae might be responsible for the female late lethality detected in our study. We found that myo-sex segregates with maleness, rather than with the C42 transgene, consistent with its location on the opposite site of the SDR relative to the transgene. As such, myo-sex cannot be a female-lethal factor in this case. Finally, as mentioned earlier, it is possible that deleterious effects are exerted by expressed pseudogenes (Figure 3h).
A different mechanism must have led to female deficiency in some line B-derived cross ii families (Table 2). In that case, inheritance of a recessive female-lethal gene on the m-chromosome from a wild-type heterozygous mother and on the paternal recombinant m-chromosome could have led to inviability of the resulting homozygous females (Figure 3i). A similar or a different interaction could have led to 2♂:1♀ bias in the line A-derived families from crosses 6–8 (Table 2), in which the inviable females inherited the EGFP-less, apparently non-recombinant first chromosomes from both parents. These interactions may have involved epigenetic phenomena, such as paramutations (Hollick, 2010), that lead to heritable altered gene expression states, whose toxic effects could result in non-Mendelian inheritance patterns. Currently, we do not have sufficient data to offer a plausible interpretation of these results, but they indicate the existence within the sex locus neighborhood of additional loci affecting female development.
Implausible scenarios of sex-specific lethality
Sex-specific embryonic lethality in Drosophila is almost exclusively linked to misregulation of dosage compensation machinery (Cline and Meyer, 1996). Similar mechanisms of lethality are rather unlikely to be behind the phenomena described here, because dosage compensation appears not to exist in A. aegypti (Hall et al., 2015). Similarly, abnormalities in chromosomal transmission caused by transgene-linked inversions (cf. McGivern and Rai, 1974) lend no plausible explanation. In our study, sex bias was observed in the progeny of the EGFP-positive females (but not in the C42 strain males), and was associated with inheriting the recombinant maternal (non-sex-specific) m-chromosome. Thus, even if an inversion was present on such an m-chromosome, a crossover in the inversion loop during female meiosis would not yield detectable sex bias, because loss of gametes would equally affect male and female progeny. Moreover, published evidence argues against existence of any larger inversions encompassing the SDR that might cause loss of one type of gametes, because individuals carrying a recombinant sex chromosome, with a recombination breakpoint close to either side of the SDR, produce progeny with all expected phenotypes, when crossed to wild-type mosquitoes (Hall et al., 2014).
Genes within the sex locus neighborhood
The proposed scenarios dictate that the line A-derived viable males possessing the m-chromosome-linked transgene could have originated only after secondary crossing-over events during female meiosis that eliminated the male embryonic-lethal factor from the neighborhood of the transgene. A male lacking such a lethal factor subsequently produced embryonic-lethal homozygous females (Table 2, cross 4). Thus, the male embryonic-lethal gene must be different from the female embryonic-lethal gene. In addition, lethality observed at two distinct developmental stages suggests that at least two factors are responsible for developmental abnormalities in females. We propose that all the loci involved are located on chromosome 1 pair in the following linear order: myo-sex, Nix, male embryonic-lethal E1 (likely M-linked), female embryonic-lethal E2 (m-linked, or sexually antagonistic M-linked), late development female-lethal E3 (m-linked, or sexually antagonistic M-linked), the transgene, female embryonic-lethal E4 (Supplementary Figure S6). Independent recombination events within the sex locus neighborhood, each with a single chromosomal breakpoint between different loci, could have led to assortment or elimination of different lethal factors in the progeny, eventually resulting in the observed alternative lethal phenotypes. This model implies a persistent significant sequence similarity between the M- and m-haplotypes to promote recombination that may be mediated by repetitive elements.
Evolutionary persistence of a short SDR
The primary model for the evolution of sex chromosomes implicates genes with sexually antagonistic properties as drivers of selection for suppressed recombination around the primary sex-determining gene (Fisher, 1931; Charlesworth and Charlesworth, 1980; Rice, 1987). Numerous studies regarding theoretical considerations on sexual conflict and sex chromosomes sharply contrast with a very limited empirical evidence for the existence of sexually antagonistic loci. Among few well-documented cases are genes increasing male reproductive success but affecting survival, such as those encoding coloration in guppies and Lake Malawi cichlids (Lindholm and Breden, 2002; Roberts et al., 2009). However, virtually nothing is known about sexually antagonistic effects caused by genes with sex differences in development. Our data suggest that such genes are likely present in Aedes.
Our study shows that the SDR neighborhood in Aedes is a fascinating test case for the analysis of processes that shape boundaries of a non-recombining region stably maintained for long evolutionary times. According to the models, spread of recombination suppression is fueled by the availability of alleles with sexually antagonistic properties in the vicinity of the sex locus. However, the process may cease if the junctions with the SDR have high recombination rates per physical distance (Otto et al., 2011), or if the non-recombining region abuts a sequence of sufficient recombinational length that fails to provide genetic variation for sexually antagonistic traits (Rice, 1987). Indeed, an extensive array of the ribosomal RNA genes adjacent to the SDR in Aedes and Culex may constitute a sufficient barrier that prevents expansion of the non-recombining region toward the telomere. Conversely, a cluster of lethal, likely sexually antagonistic, genes positioned close to the boundary of the non-recombining region may effectively guard against recombination between the neighborhoods of the sex locus and prevent homogenization of the corresponding areas of the M- and m-chromosomes in a population to protect the very small SDR from shrinking that might be detrimental to the male sex. The A. aegypti genome is not assembled in that region and no genetic markers located between Nix and the transgene are currently available. Therefore, further considerable work is needed to reveal the molecular identity of the lethal factors involved.
Acknowledgments
Akio Mori provided the A. aegypti RED strain mosquitoes for genetic analysis. Kanchana Panaram helped with scoring the phenotypes in some of the initial crosses. Luke Alphey, Gareth Lycett and Dave Weetman provided valuable comments on the manuscript. We acknowledge financial assistance for this work to JK from UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) (Grant A60345) and to ASR from the National Institutes of Health (Grant R37 AI24716).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Heredity website (http://www.nature.com/hdy)
Supplementary Material
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. (1990). Basic local alignment search tool. J Mol Biol 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Arensburger P, Megy K, Waterhouse RM, Abrudan J, Amedeo P, Antelo B et al. (2010). Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science 330: 86–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtrog D. (2013). Y-chromosome evolution: emerging insights into processes of Y-chromosome degeneration. Nat Rev Genet 14: 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtrog D, Kirkpatrick M, Mank JE, McDaniel SF, Pires JC, Rice W et al. (2011). Are all sex chromosomes created equal? Trends Genet 27: 350–357. [DOI] [PubMed] [Google Scholar]
- Bian G, Shin SW, Cheon HM, Kokoza V, Raikhel AS. (2005). Transgenic alteration of Toll immune pathway in the female mosquito Aedes aegypti. Proc Natl Acad Sci USA 102: 13568–13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull J. (1983) Evolution of Sex Determining Mechanisms. Benjamin Cummings: Menlo Park, CA, USA. [Google Scholar]
- Charlesworth D, Charlesworth B. (1980). Sex differences in fitness and selection for centric fusions between sex-chromosomes and autosomes. Genet Res 35: 205–214. [DOI] [PubMed] [Google Scholar]
- Cline TW, Meyer BJ. (1996). Vive la difference: males vs females in flies vs worms. Annu Rev Genet 30: 637–702. [DOI] [PubMed] [Google Scholar]
- Craig Jr JB, Vandehey RC, Hickey WA. (1961). Genetic variability in populations of Aedes aegypti. Bull World Health Organ 24: 527–539. [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. (1931). The evolution of dominance. Biol Rev 6: 345–368. [Google Scholar]
- Gale K, Crampton J. (1989). The ribosomal genes of the mosquito, Aedes aegypti. Eur J Biochem 185: 311–317. [DOI] [PubMed] [Google Scholar]
- Hall AB, Basu S, Jiang X, Qi Y, Timoshevskiy VA, Biedler JK et al. (2015). A male-determining factor in the mosquito Aedes aegypti. Science 348: 1268–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AB, Timoshevskiy VA, Sharakhova MV, Jiang X, Basu S, Anderson MA et al. (2014). Insights into the preservation of the homomorphic sex-determining chromosome of Aedes aegypti from the discovery of a male-biased gene tightly linked to the M-locus. Genome Biol Evol 6: 179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey WA, Craig GB Jr. (1966). Genetic distortion of sex ratio in a mosquito, Aedes aegypti. Genetics 53: 1177–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollick JB. (2010). Paramutation and development. Annu Rev Cell Dev Biol 26: 557–579. [DOI] [PubMed] [Google Scholar]
- Horn C, Wimmer EA. (2000). A versatile vector set for animal transgenesis. Dev Genes Evol 210: 630–637. [DOI] [PubMed] [Google Scholar]
- Jordan CY, Charlesworth D. (2012). The potential for sexually antagonistic polymorphism in different genome regions. Evolution 66: 505–516. [DOI] [PubMed] [Google Scholar]
- Kokoza V, Ahmed A, Wimmer EA, Raikhel AS. (2001). Efficient transformation of the yellow fever mosquito Aedes aegypti using the piggyBac transposable element vector pBac[3xP3-EGFP afm]. Insect Biochem Mol Biol 31: 1137–1143. [DOI] [PubMed] [Google Scholar]
- Kokoza V, Ahmed A, Woon Shin S, Okafor N, Zou Z, Raikhel AS. (2010). Blocking of Plasmodium transmission by cooperative action of Cecropin A and Defensin A in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci USA 107: 8111–8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywinska E, Krzywinski J. (2009). Analysis of expression in the Anopheles gambiae developing testes reveals rapidly evolving lineage-specific genes in mosquitoes. BMC Genomics 10: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Rai KS. (1990). Chromosomal localization and copy number of 18S+28S ribosomal RNA genes in evolutionarily diverse mosquitoes (Diptera, Culicidae). Hereditas 113: 277–289. [DOI] [PubMed] [Google Scholar]
- Kumar A, Rai KS. (1993). Molecular organization and evolution of mosquito genomes. Comp Biochem Physiol B 106: 495–504. [DOI] [PubMed] [Google Scholar]
- Li X, Lobo N, Bauser CA, Fraser MJ Jr. (2001). The minimum internal and external sequence requirements for transposition of the eukaryotic transformation vector piggyBac. Mol Genet Genomics 266: 190–198. [DOI] [PubMed] [Google Scholar]
- Lindholm A, Breden F. (2002). Sex chromosomes and sexual selection in poeciliid fishes. Am Nat 160 (Suppl 6): S214–S224. [DOI] [PubMed] [Google Scholar]
- McClelland GA. (1966). Sex-linkage at two loci affecting eye pigment in the mosquito Aedes aegypti (Diptera: Culicidae). Can J Genet Cytol 8: 192–198. [DOI] [PubMed] [Google Scholar]
- McGivern JJ, Rai KS. (1974). Sex-ratio distortion and directed alternate segregation of interchange complexes in a mosquito. J Hered 65: 71–77. [DOI] [PubMed] [Google Scholar]
- Mori A, Severson DW, Christensen BM. (1999). Comparative linkage maps for the mosquitoes (Culex pipiens and Aedes aegypti based on common RFLP loci. J Hered 90: 160–164. [DOI] [PubMed] [Google Scholar]
- Muller HJ. (1932). Further studies on the nature and causes of gene mutations. In: Jones DF (ed). Proceedings of the Sixth International Congress of Genetics. Brooklyn Botanic Gardens: Ithaca, NY. pp 213–255.
- Nei M. (2013) Mutation-Driven Evolution. Oxford University Press: Oxford. [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ et al. (2007). Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316: 1718–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto SP, Pannell JR, Peichel CL, Ashman TL, Charlesworth D, Chippindale AK et al. (2011). About PAR: the distinct evolutionary dynamics of the pseudoautosomal region. Trends Genet 27: 358–367. [DOI] [PubMed] [Google Scholar]
- Palavesam A, Esnault C, O'Brochta DA. (2013). Post-integration silencing of piggyBac transposable elements in Aedes aegypti. PLoS One 8: e68454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliseno L, Marranci A, Pandolfi PP. (2015). Pseudogenes in Human Cancer. Front Med (Lausanne) 2: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidenbach KR, Cook S, Bertone MA, Harbach RE, Wiegmann BM, Besansky NJ. (2009). Phylogenetic analysis and temporal diversification of mosquitoes (Diptera: Culicidae) based on nuclear genes and morphology. BMC Evol Biol 9: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice WR. (1987). The accumulation of sexually antagonistic genes as a selective agent promoting the evolution of reduced recombination between primitive sex chromosomes. Evolution 41: 911–914. [DOI] [PubMed] [Google Scholar]
- Roberts RB, Ser JR, Kocher TD. (2009). Sexual conflict resolved by invasion of a novel sex determiner in Lake Malawi cichlid fishes. Science 326: 998–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. (1998) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA. [Google Scholar]
- Schotta G, Ebert A, Dorn R, Reuter G. (2003). Position-effect variegation and the genetic dissection of chromatin regulation in Drosophila. Semin Cell Dev Biol 14: 67–75. [DOI] [PubMed] [Google Scholar]
- Severson DW. (1997). RFLP analysis of insect genomes In: Crampton JM, Beaty, BJ, Louis C (eds). The Molecular Biology of Insect Disease Vectors. Chapman & Hall: London. pp 309–320. [Google Scholar]
- Severson DW, Mori A, Zhang Y, Christensen BM. (1993). Linkage map for Aedes aegypti using restriction fragment length polymorphisms. J Hered 84: 241–247. [DOI] [PubMed] [Google Scholar]
- Song J, Dong F, Lilly JW, Stupar RM, Jiang J. (2001). Instability of bacterial artificial chromosome (BAC) clones containing tandemly repeated DNA sequences. Genome 44: 463–469. [PubMed] [Google Scholar]
- Timoshevskiy VA, Kinney NA, deBruyn BS, Mao C, Tu Z, Severson DW et al. (2014). Genomic composition and evolution of Aedes aegypti chromosomes revealed by the analysis of physically mapped supercontigs. BMC Biol 12: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timoshevskiy VA, Severson DW, Debruyn BS, Black WC, Sharakhov IV, Sharakhova MV. (2013). An integrated linkage, chromosome, and genome map for the yellow fever mosquito Aedes aegypti. PLoS Negl Trop Dis 7: e2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken KJ, Popodi E, Holtzman SL, Schulze KL, Park S, Carlson JW et al. (2010). A molecularly defined duplication set for the X chromosome of Drosophila melanogaster. Genetics 186: 1111–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyrunes F, Waters PD, Miethke P, Rens W, McMillan D, Alsop AE et al. (2008). Bird-like sex chromosomes of platypus imply recent origin of mammal sex chromosomes. Genome Res 18: 965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicoso B, Emerson JJ, Zektser Y, Mahajan S, Bachtrog D. (2013). Comparative sex chromosome genomics in snakes: differentiation, evolutionary strata, and lack of global dosage compensation. PLoS Biol 11: e1001643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RJ. (1975). Lethal genes on the sex chromosomes concealed in a population of the mosquito Aedes aegypti L. Genetica 46: 49–66. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.