Abstract
Background and Objectives
Many laboratory tests have normal ranges that change with age. While people older than 85 years of age are the fastest growing age group, distributions for such tests at extreme old ages are unknown.
Design
Cross-sectional cohort study.
Settings
International cohort study.
Participants
Participants of the Long Life Family Study (LLFS, n=~5,000, age range 25 to 110 years, median age 67, 45% males).
Measurements
Serum biomarkers were selected based on association with aging-related diseases and included: complete blood count, lipids (triglycerides, HDL-c, LDL-c and total cholesterol), 25-OH vitamin D2 and D3, and vitamin D epi-isomer, diabetes related biomarkers (adiponectin, insulin, insulin-like growth factor 1 (IGF1), glucose, hemoglobin A1C, soluble receptor for advanced glycation end-product (sRAGE)), kidney disease related biomarkers (albumin, creatinine, cystatin), endocrine biomarkers (dehydroepiandrosterone (DHEA), and sex-hormone binding globulin (SHBG), testosterone) markers of inflammation (interleukin 6 (IL6), high-sensitive C-reactive protein, NT-proBNP), ferritin, and transferrin.
Results
Out of 38 measured biomarkers, 34 were significantly correlated with age. Summary statistics were generated for all biomarkers according to sex and 5-year age increments, spanning 50 to 100+ years, after exclusion of participants with diseases and treatments that were associated with biomarkers. We also generated a biomarker dataset that will be useful for other investigators seeking to compare biomarker levels across studies.
Conclusion
Levels of several biomarkers change with older age in healthy individuals. The descriptive statistics that we have identified herein will be useful in future studies and, if replicated in additional studies, might become useful also in clinical practice. The availability of the reference dataset will facilitate appropriate calibration of biomarkers measured in different laboratories.
Keywords: Healthy aging, Long Life Family Study, serum biomarkers, reference values
Introduction
Many circulating biomarkers change with age independently of disease (1). Characterizing age-adjusted distributions of these biomarkers in healthy older adults would therefore be important to increase specificity of diagnosis, inform treatment and prevention, and limit unnecessary procedures and treatments. Despite the fact that people over the age of 85 are the fastest growing age segment of our population (2), characterization of many circulating biomarkers for this age group remain incomplete, and changes at extreme old ages are predicted from mathematical models (3). Furthermore, in those studies where data are available for this age range, such as those reported by the NHANES (National Health and Nutrition Examination Survey) (4), and by the Mayo Clinic, the data for age 85 and older are aggregated, rather than differentiated into additional age subgroups. For example, the NHANES and Mayo Clinic reported normal range for circulating levels of NT-proBNP (5) is truncated at age 83 (median age of survival for women of the 1940 birth year cohort (6)). Although elevated NT-proBNP can be indicative of systolic dysfunction, atrial fibrillation and some other age-related diseases (7), NT-proBNP levels can increase with age alone (8) and therefore normal ranges specific for older ages in healthy individuals may be useful as a reference marker for healthy aging. As another example, Mayo Clinic provides normal levels of adiponectin corrected for body mass index (9), but this biomarker also increases substantially with older age in people without heart disease, diabetes, or osteoporosis (10). Similarly, the Mayo Clinic reports a normal range for testosterone for men older than 18 years (11), confounding the interpretation of normal values of testosterone in older men (12). Similar concerns exist for other commonly assessed biomarkers, a number of which we were able to address in this study.
The lack of known distribution of established and emerging biomarkers in people of older ages was the rationale for determining summary statistics of 38 biomarkers according to sex and 5-year age increments spanning 50 to 100+ years, in participants of the Long Life Family Study (LLFS). The selected study participants were free of specific age-related diseases at the time of blood collection and considered “clinically healthy” (13). The 38 biomarkers included commonly used laboratory data such as cholesterol, but also emerging biomarkers like soluble receptor for advanced glycation end-product (sRAGE). We also generated a biomarker data set that will be useful for calibration of biomarkers measured in different laboratories.
Material and Methods
Study population
The LLFS is a family-based, longitudinal study of healthy aging and longevity that enrolled approximately 5,000 subjects between 2006 and 2009 via three American and one Danish field centers. Probands were screened for inclusion using the Family Longevity Selection Score (FLoSS), which scored the degree of familial longevity using sex and birth-year cohort survival probabilities of the proband and their siblings (14). Eligibility of sibships for the study was based on a FLoSS score >7, lack of detectable cognitive impairment in the proband, and having at least one living sibling. Socio-demographic, medical history data, and current medical conditions and medications were collected via in-person visits for all subjects as described in (13, 15). A sensitive diagnosis of cognitive impairment suggestive of Alzheimer’s disease was based on the algorithm described in (16). Medications were grouped into categories including anti-hypertensives, anti-anginals, oral hypoglycemics and insulin, and lipid-lowering drugs as described in (13). All data are available from dbGaP (dbGaP Study Accession: phs000397.v1.p1). All participants underwent informed consent.
Biomarkers
Fasting blood samples were obtained following a standardized venipuncture protocol by staff at the LLFS baseline visit. Approximately 50 mL of blood specimens were collected according to the standardized protocol (13). The serum tubes were kept at room temperature for 30–45 minutes prior to centrifugation to allow for clotting and centrifuged on site at 3000 × g for 10 minutes. The centrifuged serum tubes along with the other unprocessed blood tubes were shipped to the Advanced Diagnostics and Research Laboratory (ARDL) at the University of Minnesota. To evaluate peripheral blood mononuclear cells, the anticoagulated EDTA tubes were centrifuged at 3000 × g for 30 minutes at 15°C. An unprocessed EDTA tube was used for measurement of complete blood counts. All serum, plasma aliquots and RNA samples were stored at -80°C until analysis. The central laboratory maintains a biorepository of plasma, serum, genomic DNA, and RNA for future analysis. Description of the assays and their accuracy is available from https://dsgweb.wustl.edu/llfs/.
The biomarkers were selected based on known or hypothetical association with aging-related diseases and include: complete blood count, lipids (triglycerides, HDL-c, LDL-c and total cholesterol), 25-OH vitamin D2 and D3, and vitamin D epi-isomer, diabetes related biomarkers (adiponectin, insulin, insulin-like growth factor 1 (IGF1), glucose, hemoglobin A1C, soluble receptor for advanced glycation end-product (sRAGE, adiponectin)), kidney disease related biomarkers (albumin, creatinine, cystatin), sex hormone markers (dehydroepiandrosterone (DHEA), sex-hormone binding globulin (SHBG), testosterone) markers of inflammation (interleukin 6 (IL6), high-sensitive C-reactive protein, NT-proBNP), ferritin, and transferrin.
Statistical analysis
38 biomarkers were included in the analysis. Undetectable levels of IGF1R, DHEA, ferritin, hsCRP, insulin, vitamin D2 were imputed using uniform distributions between 0 and the lower detection level. Significant correlation of each biomarker with age at enrollment was tested using Pearson correlation coefficient and t-test. The results are summarized in Table 1. Associations of each individual biomarker with age-related diseases collected at the enrollment, fasting status (whether or not individuals fasted ≥ 8 hours before blood draw), and medications for heart disease, hypertension, diabetes, or elevated LDL or total cholesterol, were tested using age and sex standardized biomarkers as outcomes of linear regression models. Age standardization was conducted in a model-free way, using 5 year-age intervals <50, 50–54, 55–59,…, 96–99 and >=100, based on enrollment ages. Specifically, for each biomarker we computed the standardized data as where μage, sex and σage, sex are age-strata and sex specific mean and standard deviation using 5 year-age intervals <50, 50–54, 55–59,…, 96–99 and >=100, based on enrollment ages. This model-free standardization was used to avoid using a linear dependency of biomarker data with age that is often not satisfied. Some biomarkers were log-transformed (adiponectin, creatinine, hsCRP, cystatin C, HBA1C, ferritin, IGF1, IL6, insulin, NT-proBNP, sRAGE, transferrin) or cubic-root transformed (Absolute basophils, eosinophils, monocyte counts, vitamin D2, DHEA, SHBGE) before standardization. Supplement Table 1 summarizes the results. This preliminary analysis identified the diseases and conditions that could affect the steady-state level of each biomarker and, for each biomarker, summary statistics of the raw data were computed on the subset of LLFS participants who did not present with those diseases or conditions at enrollment and represented the “healthy cohort” in LLFS. Summary statistics included sample size, range, 2.5th, 50th, and 97.5th percentiles. Distribution of biomarkers was displayed using boxplots for sex and 5-years strata (Table 2, Figure 1, Supplement Table 2 and Supplement Figures). The data used to generate the reference summary statistics are available from https://dsgweb.wustl.edu/llfs/. All analyses were conducted with the statistical software R v3.0 using in-house scripts.
Table 1.
List of Biomarkers with significant correlation with age at enrollment
| Biomarker | Correlation | Pvalue |
|---|---|---|
| Abs. eosinophil count | 0.0812 | 6.05E-08 |
| Abs monocyte counts | 0.2058 | <1E-253 |
| Abs. neutrophil count | 0.2437 | <1E-253 |
| Adiponectin | 0.3178 | <1E-253 |
| Albumin | −0.3615 | 3.35E-145 |
| Creatine | 0.3073 | <1E-253 |
| High sensitive CRP | 0.1489 | <1E-253 |
| Cystatin | 0.6294 | <1E-253 |
| Vitamin D3 | −0.1086 | 8.23E-14 |
| Vitamin D2 | 0.0731 | 6.05E-07 |
| Vitamin D (epi-isomer) | −0.0945 | 8.33E-11 |
| Total Choleststerol | −0.1832 | 9.04E-37 |
| HDL Cholesterol | −0.0989 | 1.09E-11 |
| LDL Cholesterol | −0.1688 | 2.10E-31 |
| Ferritin | 0.0438 | 0.003347 |
| DHEA | −0.4766 | 9.06E-253 |
| Glucose | 0.0775 | 1.11E-07 |
| Hemoglobin | −0.3427 | 1.06E-129 |
| HA1C | 0.2161 | <1E-253 |
| IL6 | 0.2101 | <1E-253 |
| Igf1 | −0.1586 | 1.27E-26 |
| Hematocrit | −0.2770 | 1.38E-83 |
| MCV | 0.1790 | <1E-253 |
| MCH | 0.0314 | 0.031319 |
| MCHC | −0.1603 | 1.86E-28 |
| NT-proBNP | 0.3793 | <1E-253 |
| Platelets | −0.1030 | 1.42E-12 |
| RBC count | −0.3477 | 9.22E-134 |
| Red cell distribution width % | 0.3191 | <1E-253 |
| SHBG | 0.2835 | <1E-253 |
| SRAGE | 0.2622 | <1E-253 |
| Testosterone | −0.0677 | 3.32E-06 |
| Transferrin receptor | 0.1377 | <1E-253 |
| WBC count | 0.1855 | <1E-253 |
| Abs. basophil counts | 0.0174 | 0.25747 |
| Abs. lymphocyte counts | 0.0040 | 0.78653 |
| Insulin | −0.0197 | 0.17969 |
| Triglycerides | −0.0142 | 0.33107 |
Pearson correlation coefficient and the p-value from the t statistic to test the null hypothesis of no correlation with age. The 4 biomarkers at the bottom of the table did not show a significant correlation with age at enrollment.
Table 2.
Example of reference values for 2 biomarkers.
| NT-proBNP pg/mL | ||||||||||
| Men | Women | |||||||||
| age | n | 2.5th | median | 97.5th | range | n | 2.5th | median | 97.5th | range |
| <50 | 78 | 5 | 24 | 93.2 | 5 ; 115 | 105 | 10.2 | 54 | 414 | 5 ; 578 |
| 50–54 | 110 | 5 | 25 | 88.8 | 5 ; 183 | 151 | 7 | 46 | 158.2 | 5 ; 200 |
| 55–59 | 156 | 5 | 29 | 153.28 | 5 ; 844 | 216 | 11.3 | 53 | 234.7 | 5 ; 332 |
| 60–64 | 182 | 9 | 36 | 269.57 | 5 ; 1121 | 205 | 13.03 | 62 | 283.35 | 5 ; 684 |
| 65–69 | 132 | 6.55 | 55.5 | 791.67 | 5 ; 1570 | 134 | 16.65 | 80.5 | 286.9 | 5 ; 850 |
| 70–74 | 58 | 10.4 | 61.5 | 660.12 | 5 ; 693 | 58 | 35.85 | 120.5 | 400.45 | 19 ; 3650 |
| 75–79 | 32 | 16.77 | 103 | 1247.32 | 16 ; 1741 | 33 | 28.6 | 168 | 1110.4 | 19 ; 2812 |
| 80–84 | 25 | 56.8 | 150 | 1061.8 | 40 ; 1276 | 35 | 33.8 | 159 | 588.6 | 44 ; 4141 |
| 85–89 | 31 | 60.5 | 264 | 1112.25 | 50 ; 1263 | 35 | 51.65 | 213 | 701.1 | 53 ; 5244 |
| 90–94 | 77 | 75.4 | 341 | 3001.4 | 44 ; 3165 | 53 | 82.2 | 311 | 1904.8 | 72 ; 8398 |
| 95–99 | 40 | 75.7 | 315.5 | 2154.32 | 25 ; 2401 | 44 | 104.3 | 360 | 2251.67 | 32 ; 8837 |
| 100+ | 9 | 287 | 644 | 3581.4 | 263 ; 3986 | 13 | 196.92 | 863 | 2449.17 | 79 ; 4307 |
| SRAGE pg/mL | ||||||||||
| Men | Women | |||||||||
| age | n | 2.5th | median | 97.5th | range | n | 2.5th | median | 97.5th | range |
| <50 | 87 | 210.15 | 399 | 794.95 | 72 ; 1910 | 130 | 238.35 | 479 | 1082.97 | 234 ; 1796 |
| 50–54 | 126 | 189.5 | 415 | 831.12 | 166 ; 2256 | 205 | 224.27 | 472 | 1225.45 | 165 ; 3411 |
| 55–59 | 209 | 202.12 | 418.5 | 866.62 | 100 ; 2104 | 319 | 214.6 | 495 | 1251 | 134 ; 2959 |
| 60–64 | 248 | 193 | 414 | 998.75 | 127 ; 3198 | 312 | 208.45 | 512.5 | 1431.15 | 28 ; 2665 |
| 65–69 | 199 | 208.1 | 421.5 | 1120.17 | 178 ; 5992 | 233 | 214 | 490 | 1561.6 | 161 ; 4776 |
| 70–74 | 104 | 205.3 | 423 | 885.32 | 148 ; 1416 | 103 | 218.65 | 511 | 1224.8 | 186 ; 1853 |
| 75–79 | 50 | 242.57 | 455.5 | 1347.65 | 217 ; 1789 | 72 | 212.17 | 566.5 | 1208.75 | 174 ; 3669 |
| 80–84 | 57 | 209.6 | 545 | 1390.2 | 170 ; 1443 | 88 | 220.5 | 603 | 1819.75 | 145 ; 5714 |
| 85–89 | 73 | 240 | 541 | 1638.6 | 156 ; 2307 | 108 | 274.9 | 687.5 | 1879.77 | 209 ; 5214 |
| 90–94 | 175 | 265.4 | 695 | 1745.7 | 172 ; 4837 | 164 | 263.5 | 661 | 1680.05 | 229 ; 4439 |
| 95–99 | 104 | 254.28 | 689 | 1990.77 | 189 ; 7944 | 159 | 325.8 | 763.5 | 1871.75 | 213 ; 6314 |
| 100+ | 13 | 307.1 | 727 | 2287.2 | 275 ; 2643 | 45 | 351.9 | 865 | 1784.47 | 245 ; 2864 |
The sample sizes per age groups vary because different diseases and treatment groups were found associated with nt-proBNP and SRAGE.
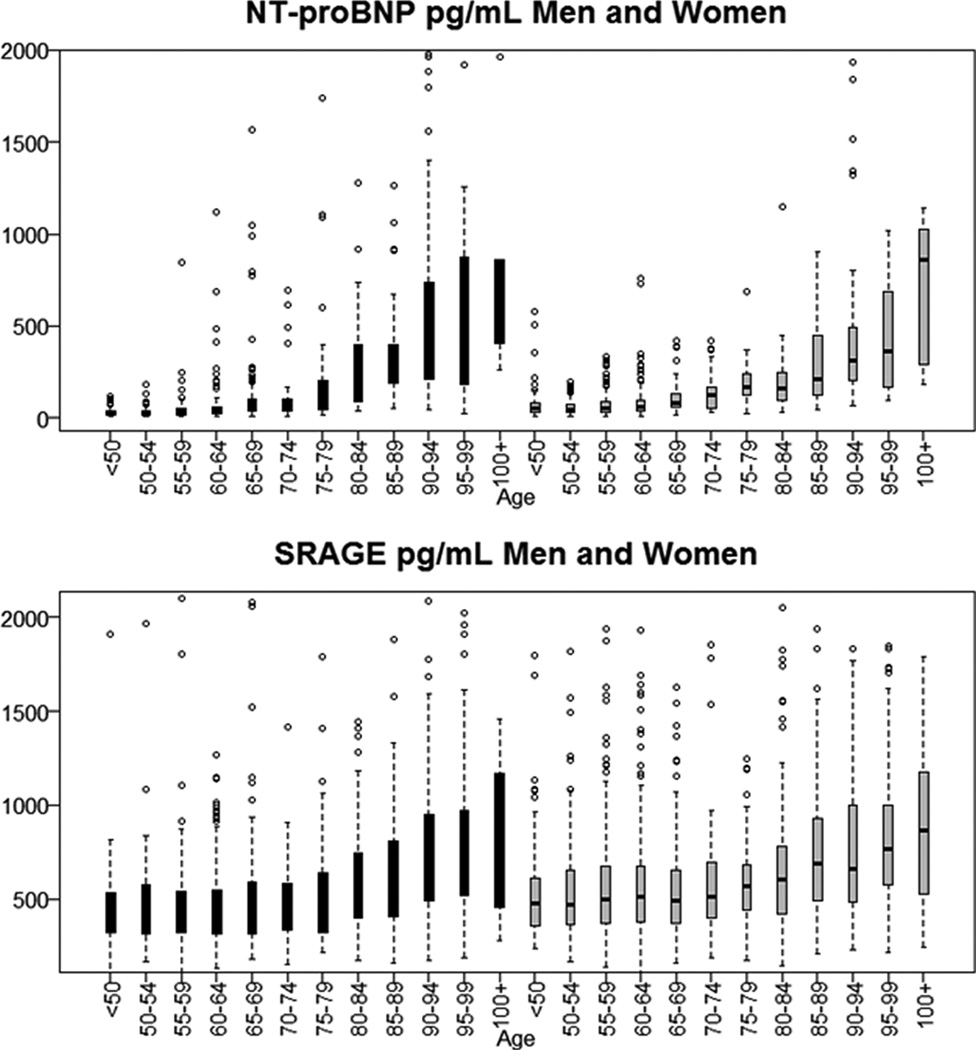
Figure 1.
Distribution of N-terminal prohormone of brain natriuretic peptide (NT proBNP) and soluble receptor for advanced glycation end product (SRAGE) by sex (black: men grey: women) and age group in healthy aging individuals from the Long Life Family Study. Side-by-side boxplots show the interquartile range and medians. Whiskers extend to 1.5 times the interquartile range.
Results
LLFS participants have been described at length in several manuscripts, see for example (13, 15). The study enrolled participants from families with evidence of longevity, and 4704 participants had blood samples collected at enrollment. These participants included approximately 45% males, 99.3% Caucasians, and had detailed information about medical history, medications, and major health conditions at the time of enrollment.
Thirty eight biomarkers were measured on all LLFS participants based on known or hypothetical association with aging-related diseases. Thirty four of these biomarkers displayed a statistically significant association with age at enrollment (Table 1), while absolute basophil and lymphocyte counts, triglycerides, and insulin did not show significant changes with older age. Correlations with age ranged between −0.4 (DHEA) and 0.6 (Cystatin) and were very small for some biomarkers suggesting either small changes with age (for example ferritin) or non-linear changes (for example Vitamin D3). All biomarkers were analyzed for association with fasting status (whether participants were fasting for more than 8 hours at time of blood collection), with each of the 4 categories of medications, and with age-related diseases as reported at enrollment. For this analysis, data on different types of cancer (excluding prostate cancer) were aggregated, because of the small prevalence of individual cancers (Supplement Table 1). This analysis identified the disease set and treatments associated with each biomarker. To generate summary values for healthy aging, we removed values for individuals with reported diseases that we found associated with individual biomarkers. We also removed measurements taken in participants who were not fasting for at least 8 hours before the blood draw, or treated with medications that were associated with significantly different biomarker levels. Participants’ selection was done for each biomarker, so that the number of healthy individuals available for each biomarker varied slightly. As an example, Table 2 shows summary statistics for NT-proBNP and SRAGE, and Figure 1 displays the distribution of the two biomarkers, stratified by sex and 5-year age groups.
Summary statistics by age groups and sex and graphical displays of additional biomarkers with >10% correlation with age at enrollment are available in Supplement Table 2. Summaries of all 38 biomarkers are distributed from the LLFS web site together with the companion dataset (https://dsgweb.wustl.edu/llfs/). The plots in Figure 1 and Supplement Figures show that the distributions of some biomarkers changed substantially over a range of 50 years, in both sexes. For example median levels of cystatin or DHEA differed by more than 2 fold, while median levels of NT-proBNP changed by more than 10 folds. Some of the most evident changes in distributions occurred at late ages (for example the distribution of adiponectin changed after age 75) while for other biomarkers the changes occurred continuously with older ages. Some biomarkers were characterized by smaller changes in the range of 1.5 folds. The distributions of hematocrit, hemoglobin, CRP, IL6, RBC, and RDW in males aged 100+ were very different from the other age groups, but only a small number of males were available to calculate the summary statistics of those biomarkers (n=2 to 4).
Discussion
In this study, we generated age-based summary statistics for 38 circulating biomarkers in healthy older adults enrolled in the LLFS. These data integrate existing information about biomarkers commonly used to monitor the health of older individuals by providing descriptive statistics ranging from <50 up to age 100 and older, stratified by age and sex. We also provide descriptive statistics for emerging biomarkers of aging. While there is a plethora of epidemiological studies of aging that report reference values in the 50–75 years range (17), very little data is available for older ages and virtually no data is available for ages 90 and older. The summary statistics that we generated highlight the importance of using appropriate age-specific reference values for very old individuals, because their values can vary substantially at extreme ages, even in healthy people. For example Figure 1 shows the steady increase in levels of NT-proBNP in both males and females with increasing older age, and emphasizes the only partial overlapping of interquartile range observed for example for the age groups 80–84 and 90–94.
In the clinical laboratory medicine literature, it is commonly assumed that calculation of reference intervals requires a minimum of 120 individuals. Since most clinical laboratories do not have the resources to establish a reference range for their analytes, most laboratories verify the reference range supplied by the manufacturer by running 30–40 samples. The age group “100 and older” includes a smaller number of participants compared to other age groups (Table 2 and Supplement Table 2). Rather than combining males and females together and reporting aggregate data, we present the data stratified by sex with a note of caution that the sample sizes are small thus reducing the reliability of these reference values. Results are presented for all races combined. Exclusion of the 17 subjects of African ancestry did not change the summary statistics substantively.
One potential weakness of this study is that we selected individuals to be included in the analysis based on the lack of disease as reported at enrollment. Although there may be LLFS participants who did not report diseases at enrollment, we hypothesize that this rate of undetected cases is low because the prevalence of aging-related diseases in LLFS is low. LLFS investigators have shown that this cohort is enriched of healthy agers and has a lower prevalence of aging-related diseases compared to other studies (13, 15, 16). Furthermore, the summary statistics that are provided in Supplement Table 2 include different ranges based on quartiles, deciles, and percentiles so that more conservative ranges of values can be used if needed.
It is important to emphasize that, while this report provides novel and useful data to study aging, these results are not yet usable in a clinical setting. There are several aspects of LLFS bio-specimen collection protocol and processing that are unique to this study, for example overnight shipping of samples and processing and freezing of samples 24–48 hours after blood collection. These issues limit the applicability of this study to clinical laboratories with standard operating procedures. Also, about 75% of LLFS members have evidence for familial longevity and tend to be healthier than participants in other studies (13, 15), hence these values may not reflect what might be observed in the general population. It is an open question whether the calculated statistics in LLFS represent what healthy aging individuals should be compared to or aim for, or whether for example genetic variants associated with longevity may actually affect the distribution of these biomarker data. The analysis also includes family members who may have more similar biomarker data because of shared environment and genetics. Since the analyses are presented stratified by age groups and sex, the number of related people per stratum is typically small (approximately 10% on average and essentially null in the oldest age strata) so we ignored corrections for relatedness. Wider ranges of values could be observed in healthy agers randomly selected from the population and only access to larger datasets will provide comprehensive data.
Additionally, the companion dataset that is distributed from https://dsgweb.wustl.edu/llfs/ includes data of all 38 biomarkers, for the sex and age groups used in the manuscript and will be useful to the scientific community in assessing and comparing biomarker levels across multiple studies. A challenge of comparing biomarkers from different studies of aging is that such comparisons can be confounded by laboratory-specific biases that are not easy to account for without access to the raw data. The companion data set can be used to calibrate biomarker data across different studies by using appropriate statistical analysis of the biomarker data aggregated from different studies to produce estimates of laboratory effects that can be used to remove lab-specific biases.
Finally, this manuscript did not attempt to characterize underlying changes in biomarker levels and their implication on disease prognostics/forecasting, health span and lifespan. We showed that the distribution of many circulating biomarkers are different in various age groups in a large sample of individuals who are clinically healthy, and we provided summary statistics that describe these differences over a wide range of ages. More work is needed to understand the biological mechanisms underlying these differences and the implication on health-span and lifespan. By showing that the distributions of many biomarkers vary with age and sex in apparently healthy people, we expect that this data will be an important reference for future studies of biomarkers of aging and for the generation of biomarker signatures that characterize specific aging-related processes or diseases.
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Aging (NIA cooperative agreements U01-AG023712, U01-AG23744, U01-AG023746, U01-AG023749, and U01-AG023755)
Conflicts of Interest
Nicole Schupfs: (1) Employment: Professor of Epidemiology at CUMC. Columbia University Medical Center, New York, NY 10032 (2) Grants. From the NIH and from the Alzheimer’s Association
LSH receives some NIH funding for research, and this project is one such project which receives NIH funding
Bharat Thyagarajan is an employee of the University of Minnesota and has received several grants/contracts from the NIH to support research projects.
Monty Montano is a founder and consultant, and has stock in MyoSyntax.
LLFS grant
Sponsor’s Role
The sponsor, the NIA, played no role in any of the above aspects of this paper.
Footnotes
Author Contributions
Paola Sebastiani designed the study, conducted the analyses, generated all the results and wrote the manuscript.
Stephanie Cosentino assisted with data analysis and revision of manuscript.
Fangui Sun assisted with data analysis.
Nicole Schupfs: acquisition of data and preparation of manuscript
LSH: acquisition of subjects and/or data, analysis and interpretation of data, and preparation of manuscript
BT: generated biomarker data and contributed to interpretation of data and writing of manuscript
Thomas Perls assisted with acquisition of subjects and data and preparation of manuscript
ABN Contributed to the study concept and design, acquisition of subjects and/or data, and critical revisions of the manuscript.
Contributor Information
Paola Sebastiani, Email: sebas@bu.edu.
Bharat Thyagarajan, Email: thya0003@umn.edu.
Fangui Sun, Email: jennysun@bu.edu.
Lawrence S. Honig, Email: lh456@columbia.edu.
Nichole Schupf, Email: ns24@columbia.edu.
Stephanie Cosentino, Email: sc2460@cumc.columbia.edu.
Mary F Feitosa, Email: mfeitosa@wustl.edu.
Mary Wojczynski, Email: mwojczynski@dsgmail.wustl.edu.
Anne B Newman, Email: newmana@edc.pitt.edu.
Monty Montano, Email: MMONTANO@bwh.harvard.edu.
Thomas T Perls, Email: thperls@bu.edu.
References
- 1.Kubota K, Kadomura T, Ohta K, Koyama K, Okuda H, Kobayashi M, et al. Analyses of laboratory data and establishment of reference values and intervals for healthy elderly people. J Nutr Health Aging. 2012;16(4):412–416. doi: 10.1007/s12603-011-0355-3. [DOI] [PubMed] [Google Scholar]
- 2.Colby S, Ortman JM. In: Projections of the size and composition of U.S. population: 2014 to 2060. Bureau UC, editor. Washington, DC: 2014. pp. P25–P1143. [Google Scholar]
- 3.Arbeev KG, Ukraintseva SV, Akushevich I, Kulminski AM, Arbeeva LS, Akushevich L, et al. Age trajectories of physiological indices in relation to healthy life course. Mech Ageing Dev. 2011;132(3):93–102. doi: 10.1016/j.mad.2011.01.001. PMCID: 3064744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat 1. 2013;(56):1–37. [PubMed] [Google Scholar]
- 5.PBNP: NT-Pro B-Type Natriuretic Peptide (BNP) Serum [database on the Internet] 2015 Available from: http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/84291. [Google Scholar]
- 6.Bell F, Miller M. Life Tables for the United States Social Security Area 1900–2100. Actuarial Study No 116. 2005 [Google Scholar]
- 7.Kim H-N, Januzzi JL. Natriuretic Peptide Testing in Heart Failure. Circulation. 2011;123(18):2015–2019. doi: 10.1161/CIRCULATIONAHA.110.979500. [DOI] [PubMed] [Google Scholar]
- 8.Bay M, Kirk V, Parner J, Hassager C, Nielsen H, Krogsgaard K, et al. NT-proBNP: a new diagnostic screening tool to differentiate between patients with normal and reduced left ventricular systolic function. Heart. 2003;89(2):150–154. doi: 10.1136/heart.89.2.150. PMCID: 1767525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adiponectin [database on the Internet] 2015 Available from: http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/91378. [Google Scholar]
- 10.Adamczak M, Rzepka E, Chudek J, Wiȩcek A. Ageing and plasma adiponectin concentration in apparently healthy males and females. Clinical endocrinology. 2005;62(1):114–118. doi: 10.1111/j.1365-2265.2004.02182.x. [DOI] [PubMed] [Google Scholar]
- 11.Testosteron [database on the Internet] 2015 Available from: http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/8533. [Google Scholar]
- 12.Perls T, Handelsman DJ. Disease Mongering of Age-Associated Declines in Testosterone and Growth Hormone Levels. Journal of the American Geriatrics Society. 2015;63(4):809–811. doi: 10.1111/jgs.13391. [DOI] [PubMed] [Google Scholar]
- 13.Newman AB, Glynn NW, Taylor CA, Sebastiani P, Perls TT, Mayeux R, et al. Health and function of participants in the Long Life Family Study: A comparison with other cohorts. Aging (Albany NY) 2011;3(1):63–76. doi: 10.18632/aging.100242. PMCID: 3047140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sebastiani P, Hadley EC, Province M, Christensen K, Rossi W, Perls TT, et al. A family longevity selection score: ranking sibships by their longevity, size, and availability for study. Am J Epidemiol. 2009;170(12):1555–1562. doi: 10.1093/aje/kwp309. PMCID: 2800272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sebastiani P, Sun FX, Andersen SL, Lee JH, Wojczynski MK, Sanders JL, et al. Families Enriched for Exceptional Longevity also have Increased Health-Span: Findings from the Long Life Family Study. Front Public Health. 2013;1:38. doi: 10.3389/fpubh.2013.00038. PMCID: 3859985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cosentino S, Schupf N, Christensen K, Andersen SL, Newman A, Mayeux R. Reduced prevalence of cognitive impairment in families with exceptional longevity. JAMA Neurol. 2013;70(7):867–874. doi: 10.1001/jamaneurol.2013.1959. PMCID: 4151346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrucci L. The Baltimore Longitudinal Study of Aging (BLSA): a 50-year-long journey and plans for the future. J Gerontol A Biol Sci Med Sci. 2008;63(12):1416–1419. doi: 10.1093/gerona/63.12.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.